Abstract
Background
Activities of daily living (ADLs) and instrumental activities of daily living (IADLs) are essential for independent living and are predictors of morbidity and mortality in older populations. Older adults who are dependent in ADLs and IADLs are also more likely to have poor muscle measures defined as low muscle mass, muscle strength, and physical performance, which further limit their ability to perform activities. The aim of this systematic review and meta‐analysis was to determine if muscle measures are predictive of ADL and IADL in older populations.
Methods
A systematic search was conducted using four databases (MEDLINE, EMBASE, Cochrane, and CINAHL) from date of inception to 7 June 2018. Longitudinal cohorts were included that reported baseline muscle measures defined by muscle mass, muscle strength, and physical performance in conjunction with prospective ADL or IADL in participants aged 65 years and older at follow‐up. Meta‐analyses were conducted using a random effect model.
Results
Of the 7760 articles screened, 83 articles were included for the systematic review and involved a total of 108 428 (54.8% female) participants with a follow‐up duration ranging from 11 days to 25 years. Low muscle mass was positively associated with ADL dependency in 5/9 articles and 5/5 for IADL dependency. Low muscle strength was associated with ADL dependency in 22/34 articles and IADL dependency in 8/9 articles. Low physical performance was associated with ADL dependency in 37/49 articles and with IADL dependency in 9/11 articles. Forty‐five articles were pooled into the meta‐analyses, 36 reported ADL, 11 reported IADL, and 2 reported ADL and IADL as a composite outcome. Low muscle mass was associated with worsening ADL (pooled odds ratio (95% confidence interval) 3.19 (1.29–7.92)) and worsening IADL (1.28 (1.02–1.61)). Low handgrip strength was associated with both worsening ADL and IADL (1.51 (1.34–1.70); 1.59 (1.04–2.31) respectively). Low scores on the short physical performance battery and gait speed were associated with worsening ADL (3.49 (2.47–4.92); 2.33 (1.58–3.44) respectively) and IADL (3.09 (1.06–8.98); 1.93 (1.69–2.21) respectively). Low one leg balance (2.74 (1.31–5.72)), timed up and go (3.41 (1.86–6.28)), and chair stand test time (1.90 (1.63–2.21)) were associated with worsening ADL.
Conclusions
Muscle measures at baseline are predictors of future ADL and IADL dependence in the older adult population.
Keywords: Muscle mass, Muscle strength, Handgrip strength, Physical performance, Activities of daily living, Aged
Introduction
Dependence in activities of daily living (ADLs), the basic tasks required of an individual to maintain their independence at home, is associated with increased risk of morbidity and mortality.1, 2 Individuals that are dependent in ADL are also likely to be dependent in instrumental activities of daily living (IADLs), the tasks required of an individual to maintain their independence in the community.3, 4, 5 The prevalence of ADL and IADL disability for at least one activity is 34.6% and 53.5%, respectively, in adults aged 65 years and older,6 and this prevalence increases with age.7 Those with lower muscle measures, defined by muscle mass, muscle strength, and physical performance,8 are more likely to be dependent in ADL and/or IADL.9, 10, 11 The more difficult tasks are for an individual, the more effort and demand they require relative to their muscle's maximum capacity.12
Older adults that develop ADL dependence are less likely to recover function, stressing the need for strategies that can prevent or delay the onset of ADL dependence.13 Higher muscle strength is protective against declining below the threshold where dependence in ADL and IADL occurs.14 In community‐dwelling older adults, physical performance measured by gait speed has been shown to be a strong predictor of ADL disability.15, 16 Similarly, low muscle mass, muscle strength, and gait speed have all been associated with an impaired ability to perform ADL and IADL.17, 18 Currently, there are no systematic review and/or meta‐analyses that quantifies the association between muscle mass, muscle strength, and physical performance as predictors of ADL and IADL dependence. By determining which muscle measures are predictive of ADL and IADL dependence allows for the identification of individuals at high risk of decline as well as the development and implementation of strategies that can prevent or delay the onset of dependence.
The aim of this systematic review and meta‐analysis was to determine if muscle mass, muscle strength, or physical performance are predictors of ADL and/or IADL at follow‐up in older populations.
Methods
Search strategy
This systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta‐analyses and registered with the PROSPERO International Prospective Register of Systematic Reviews (CRD42019125666).19 The following four electronic databases were screened for potential relevance from date of inception to 7 June 2018: MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials, and Cumulative Index of Nursing and Allied Health Literature. The search strategy was developed in consultation with a senior tertiary librarian from The University of Melbourne, with expertise in research and search strategies. The following terms were used in the search strategy: ‘muscle mass’, ‘fat free mass’, ‘lean mass’, ‘muscle loss’/atrophy, ‘muscle strength’, ‘physical performance’/mobility/fitness/endurance, ‘activities of daily living’, ‘functional decline’/disability, and aged/elderly/older. The full search strategy can be found in Supporting Information, Table S1 .
Eligibility criteria
Inclusion criteria consisted of prospective longitudinal cohorts of older adults with a reported mean/median age of 65 years and older at follow‐up and reporting at least one of the following measurements: muscle mass, muscle strength, or physical performance in conjunction with a follow‐up outcome of ADL or IADL.
Exclusion criteria included cross‐sectional and case–control studies; anthropometric measurements as measures of muscle mass such as body mass index, hip‐waist ratio, waist or calf circumference measurements, and skin‐fold thickness; populations that suffered from cancer, muscular dystrophy, genetically inherited diseases, and HIV/AIDS; cohorts that received an intervention other than usual care or placebo; and non‐English articles. Articles including participants of the same cohort more than once were excluded in a hierarchical manner: (i) if there was no statistical analysis conducted regarding odds ratio (OR), hazards ratio, or relative risk and lacking data to calculate the OR; (ii) if their primary research question was not exploring the association between muscle measures with ADL or IADL; or (iii) an article had a smaller sample size.
Article selection
Articles obtained through the search strategy had their title and abstract screened followed by full‐text screening for eligibility independently by two authors (D. W., Y. Z., or J. Y.) using Covidence (Covidence Systematic Review Software, Veritas Health Innovation, Melbourne, Australia). The decision made by authors was compared, and conflicts were settled by a third reviewer (E. M. R.).
Data extraction and quality assessment
Data extraction was performed by two authors (D. W., Y. Z., or J. Y.). The following data were extracted from included articles: author, year, study/cohort, setting, country/region, demographical information [sample size, age (mean/median), and sex (% female)], and follow‐up duration. Muscle mass, muscle strength, physical performance, ADL, and IADL were extracted based on the measurement method, unit, and cut‐offs applied in analyses. Effect sizes were extracted from text, tables, or figures if not described elsewhere.
The quality of the included articles was assessed by a modified version of the Newcastle–Ottawa Scale (NOS) for cohort studies.20 Quality assessment was performed based on the following categories: selection, comparability, and outcome. Articles were deemed to be high quality with the following criteria: (i) the population was representative of the 65 years and older at follow‐up; (ii) in the case of a dichotomized sarcopenic cohort (low muscle mass, muscle strength, and physical performance), both cohorts were recruited from the same population; (iii) the technique used to measure muscle mass, muscle strength, or physical performance; (iv) the analysis was controlled for age and/or sex, as well as other factors; (v) ADL or IADL was measured with a validated method or a study designed questionnaire or survey; (vi) follow‐up duration was ≥3 months; and (vii) subjects were all followed up, accounted for or the number lost to follow‐up was unlikely to introduce bias (≤20%). The adapted version of the NOS can be found in Supporting Information, Supplementary Material 2 . Studies above the median score (7/7 and 8/8) were the cut‐off point for articles of high quality.21
Data synthesis and analysis
Effect sizes were extracted and reported where associations were made between baseline muscle measures and follow‐up ADL and/or IADL. Inclusion into the meta‐analysis required articles to report an OR, hazards ratio, relative risk, or if the article provided sufficient information to calculate an OR for the association between baseline muscle measures and follow‐up ADL and/or IADL. Forest plots were generated for the graphical representation of the meta‐analysis. A random effect model was adopted to account for differences between articles.22 Articles were presented in order from the shortest to longest follow‐up duration to determine if follow‐up duration impacts effect size, i.e. longer follow‐up duration showing greater effect size. Articles that reported sex‐stratified results or ADL and IADL were entered in the meta‐analysis separately. The least adjusted statistical model consisting of age and sex was used in the meta‐analysis, followed by the next least adjusted model then the unadjusted model. Heterogeneity was measured using the I‐squared (I 2) test. Low heterogeneity was defined as an I 2 ≤ 25%, moderate as 25–75%, and high as ≥75%.23 The P‐value for significance was set at <0.05, and the P‐value for a trend was set at 0.05 < P < 0.01. Meta‐analyses were performed separately according to the unit of gait speed, ADL, and IADL. The meta‐analysis was performed using Comprehensive Meta‐analysis (version 3.3; Biostat Inc., Englewood, NK).
Results
Figure 1 presents the Preferred Reporting Items for Systematic Reviews and Meta‐analyses flow diagram of selected articles. The four databases yielded 12 950 potential articles to which an additional 13 were added by snowballing. After removing duplicate articles, 7760 progressed to title and abstract screening. Of these, 7535 articles were excluded resulting in 225 articles for full‐text screening. Ultimately, 83 articles were included in the systematic review and 45 articles in the meta‐analysis.
Figure 1.
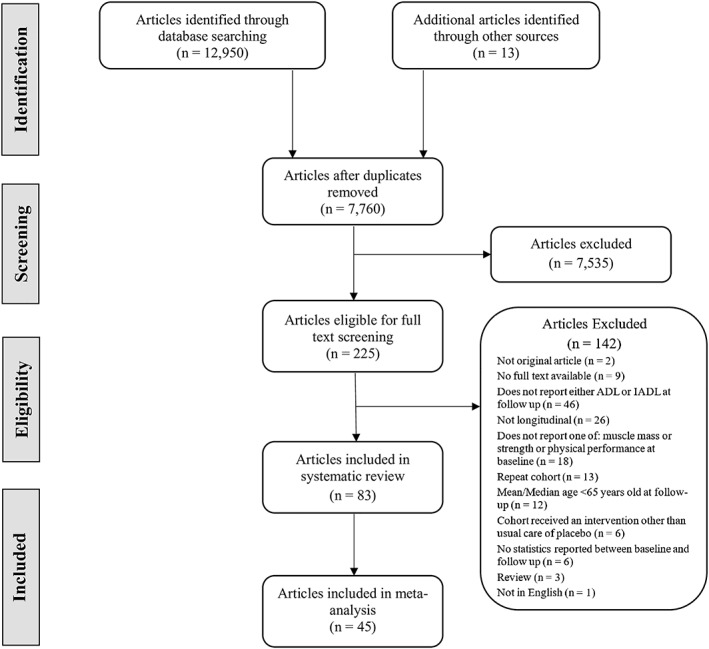
Preferred Reporting Items for Systematic Reviews and Meta‐analyses flow chart for the study selection process.
Characteristics of included articles
Table 1 presents the study characteristics of included articles. The majority of the articles were conducted on community‐dwelling participants (n = 65/83). The mean or median age of the study ranged from 54 to 86 years at baseline. The number of participants in each study ranged from 41 to 8000 and included a total of 108 428 (54.8% female) participants. Follow‐up duration ranged from 11 days to 25 years. ADL was measured in 73 articles that examined physical motor tasks, 30 of which had developed an adapted questionnaire to assess the motor component of ADL (Supporting Information, Table S3 ) and 21 articles using the Katz Index or a modified version. Of the articles reporting ADL, 63 articles used a dichotomous cut‐off reporting a worsening ADL score (≥1 points loss, i.e. more dependency) at follow‐up compared with using a continuous ADL score (n = 11). IADL was measured in 35 articles with the Lawton–Brody IADL scale being used in 14 articles, three of which were modified. A total of 26 articles included measures of both ADL and IADL.
Table 1.
Characteristics of included studies and measured activities of daily living and instrumental activities of daily living
| Study characteristics | Participants | FU | ADL | IADL | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First author (year) [ref] | Cohort name | Setting | Country | N | Age (years) | F (%) | Measure | Cut‐offs | Measure | Cut‐offs | ||
| Abete (2017) 24 | — | CD | ITA | 907 | 81.3 ± 6.5 | 56.7 | 2 y | Katz | ≥1 loss | — | — | |
| Al snih (2004) 25 | HEPESE | CD | USA | 2493 | 72.4 ± 6.2a | 57.9 | 7 y | M Katz | ≥1 loss | — | — | |
| Albert (2015) 26 | SITE | CD | USA | 375 | 78.9 ± 5.8 | 68.9 | 2 y | — | — | AMPS | Cont | |
| Alexandre (2012) 27 | SABE | CD | BRA | 1634 | 68.6 ± 0.4 | 57.1 | 6 y | M Katz | ≥1 loss | — | — | |
| Amigues (2013) 28 | EPIDOS | CD | FRA | 975 | 79.9 ± 3.5 | 100.0 | 4 y | — | — | LB | ≥1 loss | |
| Arnau (2016) 29 | — | OP | ESP | 252 | 81.7 ± 4.6 | 58.7 | 1 y | BI | ≥10 loss | M LB | ≥1 loss | |
| Artaud (2015) 30 | 3C | CD | FRA | 3814 | 73.2 ± 4.6 | 60.9 | 11 y | M Katz | ≥1 loss | LB | ≥1 loss | |
| Basic (2017) 31 | — | IP | AUS | 1693 | 81.9 ± 7.5 | 61.5 | 11 d | M BI | ≥1 loss | — | — | |
| Baumgartner (2004) 32 | NMAPS | CD | USA | 451 | 72.7 ± 6.3a | 61.9 | 8 y | — | — | Own Q | ≥1 loss | |
| Beauchamp (2015) 33 | Boston RISE | PB | USA | 360 | 76.6 ± 7.0 | 68.0 | 2 y | LLFDI‐FC | Cont | — | — | |
| Beloosesky (2009) 34 | — | OP | ISR | 93 | 81.2 ± 7.2 | 69.5 | 6 m | Own Q | Cont | — | — | |
| Bianchi (2015) 35 | InCHIANTI | CD | ITA | 538 | 77.1 ± 5.5 | 53.5 | 9 y | — | — | LB | ≥1 loss | |
| Broadwin (2001) 36 | — | CD | USA | 1051 | 70.7a | 60.3 | 4 y | Own Q | ≥1 loss | — | — | |
| Carriere (2005) 37 | EPIDOS | CD | FRA | 545 | 79 (76–81) | 100 | 7 y | — | — | LB | ≥1 loss | |
| Cesari (2015) 38 | InCHIANTI | CD | ITA | 991 | 73.9 ± 6.7 | 57.0 | 9 y | Katz | ≥1 loss | LB | Cont | |
| Chan (2014) 39 | ISCOPE | CD | USA | 764 | 83 (79–87) | 68.2 | 1 y | GARS | Cont | GARS | Cont | |
| Chaudhry (2010) 40 | CHS | CD | USA | 5888 | 72.4a | 57.6 | 7 y | Own Q | ≥1 loss | — | — | |
| Chu (2006) 41 | — | CD | HKG | 1419 | 73.1 ± 6.2 | 49.5 | 1 y | M BI | ≥1 loss | LB | ≥1 loss | |
| Cooper (2011) 42 | LASA | CD | NLD | 1532 | 70.0 ± 8.5 | 54.8 | 3 y | Own Q | ≥1 loss | — | — | |
| Corsonello (2012) 43 | PVC | IP | ITA | 506 | 80.1 ± 5.9 | 54.3 | 1 y | M Katz | ≥1 loss | — | — | |
| Costanzo (2018) 44 | InCHIANTI | CD | ITA | 709 | 73.4 ± 6.5 | 56.3 | 6 y | Katz | ≥1 loss | — | — | |
| Den Ouden (2013) 45 | PROFIEL | CD | NLD | 625 | 62.3 ± 8.9 | 49.0 | 10 y | M Katz | ≥1 loss | — | — | |
| Denkinger (2010) 46 | IRIE | CD | DEU | 161 | 82 (58–93) | 72.7 | 3 w | BI | Cont | — | — | |
| Di Monaco (2015) 47 | — | CD | ITA | 193 | 80.0 ± 7.7 | 100 | 6 m | BI | ≥15 loss | — | — | |
| Donoghue (2014) 48 | TILDA | CD | IRL | 1819 | 72.8 ± 6.1 | 52.6 | 2 y | Own Q | ≥1 loss | Own Q | ≥1 loss | |
| Duchowny (2018) 49 | HRS | CD | USA | 8467 | 74.6 ± 7.0a | 57.0 | 2 y | Own Q | ≥1 loss | — | — | |
| Fantin (2007) 50 | — | CD | ITA | 159 | 71.4 ± 2.3a | 61.0 | 6 y | Own Q | ≥1 loss | Own Q | ≥1 loss | |
| Femia (1997) 51 | OCTO Project | CD | SWE | 95 | 86.8 ± 2.3 | 74.0 | 4 y | Own Q | Cont | Own Q | Cont | |
| Fujiwara (2016) 52 | TMIG‐LISA | CD | JPN | 981 | 71.5 ± 5.2 | 58.1 | 8 y | Own Q | ≥1 loss | — | — | |
| Giampaoli (1999) 53 | FINE | CD | ITA | 140 | 76.5 ± 3.4a | 0 | 4 y | WHO scale | ≥1 loss | WHO scale | ≥1 loss | |
| Gill (1996) 54 | PS | CD | USA | 775 | 79.1 ± 5.0 | 74 | 3 y | M Katz | ≥1 loss | — | — | |
| Gill (2009) 55 | PEP | CD | USA | 722 | 78.4 ± 5.2 | 62.4 | 11 y | Own Q | ≥1 loss | — | — | |
| Guralnik (2000) 56 | EPESE | CD | USA | 2478 | — | — | 6 y | Own Q | ≥1 loss | — | — | |
| Hansen (1999) 57 | — | PB | USA | 73 | 80.4 ± 7.0 | 66.0 | 1 m | Katz | ≥1 loss | M LB | ≥1 loss | |
| Heiland (2016) 58 | SNAC‐K | CD | SWE | 3060 | 73.7 ± 10.8 | 63.7 | 6 y | Own Q | ≥1 loss | — | — | |
| Hirani (2015) 59 | CHAMP | CD | AUS | 1819 | 77.3 ± 5.8a | 0 | 5 y | M Katz | ≥1 loss | — | — | |
| Hirani (2017) 60 | CHAMP | CD | AUS | 1685 | 76.9 ± 5.5 | 0 | 5 y | M Katz | ≥1 loss | LB | ≥1 loss | |
| Hoeymans (1996) 61 | Zitphen Elderly | CD | NLD | 303 | 75.8 ± 5.4 | 0 | 3 y | M WHO | ≥1 loss | M WHO | ≥1 loss | |
| Hong (2016) 62 | — | CD | KOR | 8000 | 72.5 ± 5.5 | 59.4 | 3 y | — | — | KIADL | ≥1 loss | |
| Idland (2013) 63 | — | CD | NOR | 113 | 79.4 ± 2.9 | 100 | 9 y | M A PADL‐H scale | ≥1 loss | — | — | |
| Ishizaki (2000) 64 | LISA | CD | JPN | 583 | 70.9 ± 4.9 | 55.9 | 3 y | Own Q | ≥1 loss | TMIG IC | ≥1 loss | |
| Janssen (2006) 65 | CHS | CD | USA | 3694 | 73.5a | 53.2 | 8 y | Own Q | ≥1 loss | — | — | |
| Jonkman (2018) 16 | InCHIANTI, LASA | CD | ITA, NLD | 798 | 67.5 ± 2.1a | 53.8 | 9 y | Own Q | ≥1 loss | Own Q | ≥1 loss | |
| Kempen (1998) 66 | GLAS | CD | NLD | 557 | 72.4 ± 7.7a | 74.7 | 2 y | GARS | Cont | — | — | |
| Kozicka (2016) 67 | — | CD | POL | 41 | 69.8 ± 9.0 | 41.5 | 1 y | Katz | Cont | LB | Cont | |
| Kwon (2012) 68 | — | IP | USA | 204 | 71.1 ± 5.3 | 57.8 | 1 y | HAQ | ≥1 loss | — | — | |
| Legrand (2014) 69 | BFc80+ | CD | BEL | 431 | 84.4 ± 3.5 | 63.0 | 34 m | Own Q | ≥3 loss | — | — | |
| Lopez‐Teros (2014) 70 | Coyoacan | CD | MEX | 133 | 75.5 ± 4.7 | 53.4 | 1 y | Own Q | ≥1 loss | Own Q | ≥1 loss | |
| McGrath (2018) 71 | HEPESE | CD | USA | 672 | 81.7 ± 4.1 | 64.6 | 2 y | M Katz | ≥1 loss | OARS and RB | ≥1 loss | |
| Minneci (2015) 72 | ICARe Dicomano | PB, HF | ITA | 561 | 72.9 ± 7.1a | 57.6 | 3 y | Own Q | ≥1 loss | — | — | |
| Moen (2018) 73 | — | PD | NOR | 115 | 86.0 ± 5.9 | 55.0 | 3 w | Nor BI | Cont | — | — | |
| Onder (2005) 74 | WHAS | CD | USA | 884 | 78.7 ± 8.0 | 100 | 3 y | Own Q | ≥1 loss | — | — | |
| Ostir (1998) 75 | EPESE | CD | USA | 1342 | 73.3 | 53.0 | 2 y | Own Q | ≥1 loss | — | — | |
| Peel (2014) 76 | — | TCP | AUS | 351 | 79.0 ± 8.8 | 65.8 | 6 m | interRAC HC | ≥1 loss | — | — | |
| Pisters (2012) 77 | — | IP | NLD | 216 | 66.1 ± 8.5 | 72.2 | 5 y | WOMAC | Cont | — | — | |
| Purser (2005) 78 | VA | IP | USA | 1388 | 74 ± 6.0 | 2.0 | 1 y | Katz | Cont | LB | Cont | |
| Rajan (2012) 79 | CNDS | CD | USA | 5317 | 73.2 ± 6.4 | 61.0 | 8 y | Katz | ≥1 loss | — | — | |
| Rantanen (1999) 80 | HPP, HAAS | CD | USA | 6089 | 54.0 ± 5.5 | 0 | 25 y | Own Q | ≥1 loss | — | — | |
| Rantanen (2002) 81 | NORA75 | CD | DNK, SWE, FIN | 567 | 75+, NR | 60.0 | 5 y | Own Q | ≥1 loss | — | — | |
| Rodriguez‐Pascual (2017) 82 | — | PD, HF | ESP | 497 | 85.2 ± 7.3 | 61.0 | 1 y | Katz | ≥1 loss | — | — | |
| Rothman (2008) 83 | PEP | CD | USA | 754 | 78.4 ± 5.3 | 64.6 | 8 y | Own Q | ≥1 loss | — | — | |
| Sakamoto (2016) 84 | TLAS | CD | JPN | 188 | 80.2 ± 3.9a | 65.4 | 2 y | Own Q | ≥1 loss | — | — | |
| Sanchez‐Martinez (2016) 85 | Penagrade cohort | CD | ESP | 607 | 77.0 ± 7.6 | 50.9 | 4 y | Own Q | ≥1 loss | — | — | |
| Sanchez‐Rodrigeuz (2014) 86 | — | IP | ESP | 99 | 84.6 ± 6.6 | 61.6 | 3 m | BI | Cont | — | — | |
| Sarkisian (2000) 87 | SOF | CD | USA | 6632 | 73.0 ± 4.9 | 100 | 4 y | Own Q | ≥1 loss | Own Q | ≥1 loss | |
| Sarkisian (2001) 88 | SOF | CD | USA | 89 | 72.4 ± 4.5a | 100 | 4 y | NHIS | ≥1 loss | — | — | |
| Schoenenberg (2013) 89 | — | IP, TAVI | CHE | 119 | 83.4 ± 4.6 | 55.5 | 6 m | Katz | ≥1 loss | LB | ≥1 loss | |
| Seidel (2011) 90 | SHARE | CD | EU | 6841 | 72 ± 6.0 | 52.5 | 2 y | — | — | Own Q | ≥1 loss | |
| Shimada (2010) 91 | E‐SAS project | CD | JPN | 436 | 79.2 ± 6.8 | 72.5 | 1 y | — | — | TMIG IC | ≥1 loss | |
| Shimada (2015) 92 | OSHPE | CD | JPN | 4081 | 71.7 ± 5.3 | 51.6 | 2 y | LTIC | ≥1 loss | — | — | |
| Shinkai (2000) 93 | TMIG‐LISA | CD | JPN | 748 | NR | NR | 6 y | Own Q | ≥1 loss | — | — | |
| Shinkai (2003) 94 | TMIG‐LISA | CD | JPN | 601 | 73.0 ± 5.3 | 65 | 4 y | Own Q | ≥1 loss | TMIG IC | ≥1 loss | |
| Sourdet (2012) 95 | REAL.FR | CD, AD | FRA | 632 | 77.8 ± 7.0a | 72.2 | 2 y | M Katz | ≥0.5 loss | — | — | |
| Stenholm (2014) 96 | InCHIANTI | CD | ITA | 724 | 67.1 ± 15.0a | 54.3 | 9 y | Own Q | ≥1 loss | — | — | |
| Taekema (2010) 18 | Leiden 85‐plus | CD | NLD | 555 | NR | 65.0 | NR | GARS | ≥1 loss | GARS | ≥1 loss | |
| Takuhiro (2017) 97 | Hizen‐Oshima | CD | JPN | 104 | 69.3 ± 3.0 | 100 | 9 y | Composite | ≥3 loss | — | — | |
| Tanimoto (2013) 98 | — | CD | JPN | 716 | 73.2 ± 6.1a | 65.8 | 2 y | M Katz | ≥1 loss | — | — | |
| Terhorst (2017) 99 | — | OP | USA | 256 | 78.9 ± 5.1 | 100 | 6 m | PASS | ≥1 loss | PASS | ≥1 loss | |
| Tinetti (2005) 100 | PEP, PS | CD | USA | 1471 | 78.8 ± 5.2a | 70.8 | 3 y | — | — | LB | ≥1 loss | |
| Volpato (2011) 101 | — | IP | ITA | 87 | 77.4 ± 6.5a | 49.0 | 3 m | Own Q | Cont | M LB | Cont | |
| Wennie Huang (2010) 102 | — | CD | USA | 110 | 80.3 ± 7.0 | 70.9 | 18 m | NHIS | ≥1 loss | — | — | |
| Zhang (2013) 103 | InCHIANTI | CD | ITA | 562 | 71.4 ± 5.7 | 47.9 | 3 y | Own Q | ≥1 loss | Own Q | ≥1 loss | |
| Zoico (2007) 104 | — | CD | ITA | 145 | 71.7 ± 2.3a | 58.6 | 2 y | Composite | ≥1 loss | — | — | |
Cohort: 3C, Three City Study; BFc80+, BELFRAIL; Boston RISE, Boston Rehabilitative Impairment Study of the Elderly; CHAMP, The Concord Health and Ageing in Men Project; CHS, Cardiovascular Health Study; CNDS, Chicago Neighbourhood and Disability Study; Coyoacan, Mexica Study of Nutritional and Psychosocial Markers of Frailty among Community‐dwelling Elderly; EPESE, Established Populations for the Epidemiological Study for the Elderly; EPIDOS, epidemiology of osteoporosis; E‐SAS project, Elderly Status Assessment Set; FINE, Finland, Italy, Netherlands Elderly; GLAS, Groningen Longitudinal Ageing Study; HAAS, Honolulu Asia Aging Study; HEPESE, Hispanic Established Populations for the Epidemiological Study for the Elderly; HPP, Honolulu Heart Program; HRS, Health and Retirement Study; ICARe Dicomano, Insufficienza Cardiaca negi Anziani Residenti a Dicomano; InCHIANTI, Invecchiare in Chianti; ISCOPE, Integrated Systematic Care for Older People; LASA, Longitudinal Aging Study Amsterdam; NMAPS, New Mexico Aging Process Study; NORA75, Nordic Research on Aging 75 study; OSHPE, Obu Study of Health Promotion for the Elderly; PEP, Precipitating Events Project; PS, Project Safety; PVC, PharmacosurVeillance in the elderly Care; REAL.FR, Reseau sur la maladie Alzheimer Francais; SABE, Saude, Bem‐Estar e Envelhecimento; SITE, Sources of Independence in the Elderly; SHARE, The Survey of Health, Ageing and Retirement in Europe; SNAC‐K, Swedish National study on Aging and Care in Kungsholemn; SOF, Study of Osteoporotic Fractures; TILDA, The Irish Longitudinal Study of Ageing; LISA, Longitudinal Interdisciplinary Study on Aging; TLAS, Tosa Longitudinal Aging Study; TMIG‐LISA, Tokyo Metropolitan Institute of Gerontology Longitudinal Interdisciplinary Study on Ageing; VA, Department of Veterans Affairs; WHAS, Women's Health and Aging Study. Setting: AD, Alzheimer's disease; CD, community‐dwelling; OP, outpatients; IP, inpatients; TAVI, transcatheter aortic valve implantation; TCP, transitional care program; PB, population based; PD, post‐discharge; HF, heart failure. Country: AUS, Australia; BEL, Belgium; BRA, Brazil; CHE, Switzerland; DEU, Germany; DNK, Denmark; ESP, Spain; EU, Europe; FIN, Finland; FRA, France; HKG, Hong Kong; IRL, Ireland; ISR, Israel; ITA, Italy; JPN, Japan; KOR, Korea; MEX, Mexico; NLD, Netherlands; NOR, Norway; POL, Poland; SWE, Sweden; USA, United States. Age: presented as mean ± SD or median (range) or (IQR); range, percentage; —, not applicable or reported; F, female; FU, follow‐up duration; D, day(s); M, month(s); Y, year(s). ADL: —, not applicable or reported; BI, Barthel Index; Cont, continuous; GARS, Groningen Activities Restriction Scale; HAQ, Stanford Health Assessment Questionnaire; interRAC HC, interRAC Home Care; Nor BI, Norwegian Barthel Index; M A PADL‐H scale, modified Avlund Physical ADL‐H scale; M Katz, modified Katz Index; M BI, modified Barthel Index; M WHO, modified World Health Organization scale; LLFDI‐FC, Functional Component of the Late‐Life Function and Disability Instrument; LTCI, long‐term care insurance system; PASS, Performance Assessment of Self‐Care Skills; WHO, World Health Organization; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index. IADL: —, not applicable or reported; AMPS, assessment of motor and process skills; GARS, Groningen Activities Restriction Scale; KIADL, Korean IADL; LB, Lawton and Brody; M LB, modified Lawton and Brody; M WHO, modified WHO scale; NHIS, National Health Interview Survey; OARS, Older Americans Resources and Services; RB, Rosow‐Breslau scale; TMIG‐IC, Tokyo Metropolitan Institute of Gerontology Index of Competence.
Calculated mean ± SD or mean from information provided.
Qualitative analysis
Muscle mass was reported in 13 articles,28, 32, 35, 36, 37, 38, 50, 59, 60, 65, 86, 98, 104 six using dual‐energy X‐ray absorptiometry, five using bioelectrical impedance analysis, one using computed tomography, and one did not report the measurement method (Table 2). Low muscle mass was positively associated with worsening ADL in 5/9 articles and IADL in 5/5 at follow‐up.
Table 2.
Muscle mass as predictor of activities of daily living or instrumental activities of daily living
| First author (year) [ref] | N | Tool | Measure | Units | Cut‐offs | AM | MA |
|---|---|---|---|---|---|---|---|
| Amigues (2013) 28 | 975 | DXA | SMI | kg/m2 | SD (NR) | A | Y |
| Quartilea | A | N | |||||
| 975 | DXA | LM | kg | SD (NR) | A | N | |
| Baumgartner (2004) 32 | 451 | DXA | SMI | kg/m2 | M: 7.26. F: 5.45 | A | N |
| Bianchi (2015) 35 | 538 | BIA | SMI | kg/m2 | M: 8.87. F: 6.42 | A | Y |
| Broadwin (2001) 36 | 1051 | BIA | FFM | % | Quintileb | A | N |
| Carriere (2005) 37 | 545 | — | LM/BM | — | <0.54, 0.54–0.63, ≥0.63 | A | N |
| Cesari (2015) 38 | 991 | CT | Muscle density | mg/cm3 | SD (M: 3.32. F: 3.60) | A | N |
| Fantin (2007) 50 | 159 | DXA | FFM | kg | Continuous | U | N |
| Hirani (2015) 59 | 1819 | DXA | ALM | kg | <19.75 | A | N |
| Hirani (2017) 60 | 1685 | DXA | ALM/BMI | — | <0.789 | A | Y |
| Janssen (2006) 65 | 3694 | BIA | MM | kg | Quartile (NR) | A | N |
| 3694 | BIA | SMI | kg/m2 | M: <10.75. F: <6.75 | A | Y | |
| Sanchez‐Rodrigeuz (2014) 86 | 99 | BIA | FFM | kg | Continuous | A | N |
| 99 | BIA | LBM | kg | Continuous | A | N | |
| Tanimoto (2013) 98 | 716 | BIA | AMI | kg/m2 | M: <7.0. F: <5.8 | U | Y |
| Zoico (2007) 104 | 145 | DXA | AMI | kg/m2 | <7.6 | A | N |
Measure: —, not applicable or reported; ALM, appendicular lean mass; AMI, appendicular mass index; BIA, bioelectrical impedance analysis; BM, body mass; CT, computed tomography; DXA, dual‐energy X‐ray absorptiometry; FFM, fat free mass; LBM, lean body mass; LM, lean mass; MM, muscle mass; SMI, skeletal muscle index. Cut‐off: NR, not reported; SD, standard deviation. Expressed as either dichotomous, ranges for specific tertiles, quartiles, quintiles, or categories or per unit or score. AM, adjustment model denotes whether the model in the meta‐analysis was: A, adjusted; or U, unadjusted. MA, meta‐analysis; N, no; Y, yes.
≥6.72, 6.30–6.72, 5.82–6.30, <5.82.
M: 35.5–75.6, 75.7–78.0, 78.1–80.2, 80.3–82.8, 82.9–93.0. F: 45.6–67.0, 67.1–69.4, 69.5–71.8, 71.9–74.7, 74.8–88.0.
Muscle strength was investigated in 4118, 24, 25, 27, 28, 34, 35, 37, 38, 39, 40, 44, 45, 47, 49, 51, 53, 55, 59, 64, 67, 68, 69, 70, 71, 72, 73, 74, 77, 80, 81, 82, 83, 86, 87, 88, 90, 93, 94, 98, 102 articles as a predictor of ADL and IADL (Table 3). Handgrip strength was used as a measure of muscle strength in 40 articles, five using quadriceps or knee strength and each of the following were used in single articles: shoulder strength, ankle and torso flexion, and extension. Low muscle strength was positively associated with worsening ADL in 22/34 and IADL in 8/9 at follow‐up.
Table 3.
Muscle strength as predictor of activities of daily living or instrumental activities of daily living
| First author (year) [ref] | N | Measure | Units | Cut‐offs | AM | MA |
|---|---|---|---|---|---|---|
| Abete (2017) 24 | 907 | HGS | NR | Dich (NR) | A | Y |
| 907 | SS | NR | Dich (NR) | A | N | |
| Al snih (2004) 25 | 2493 | HGS | kg | Continuous | U | Y |
| Quartilea | U | Y | ||||
| Alexandre (2012) 27 | 1634 | HGS | kg | Continuous | A | Y |
| Amigues (2013) 28 | 975 | HGS | kPa | SD (NR) | A | N |
| Beloosesky (2009) 34 | 93 | HGS | kg | Continuous | U | N |
| Bianchi (2015) 35 | 538 | HGS | kg | BMI specificb | A | N |
| Carriere (2005) 37 | 545 | HGS | kPa | <47 | A | Y |
| 545 | QS | N/cm | <3.52, 3.52–4.95, ≥4.95 | A | N | |
| Cesari (2015) 38 | 991 | HGS | kg | SD (M: 10.11. F: 7.49) | A | N |
| 991 | AE | kg | SD (M: 9.79. F: 8.35) | A | N | |
| Chan (2014) 39 | 570 | HGS | kg | Continuous | A | N |
| 570 | QS | kg | Continuous | A | N | |
| Chaudhry (2010) 40 | 5888 | HGS | kg | Lowest quintile for sex and BMI (NR) | A | Y |
| Costanzo (2018) 44 | 709 | HGS | — | Lowest quintile for sex and BMI (NR) | U | Y |
| Den Ouden (2013) 45 | 625 | HGS | kg | Per 10 | A | N |
| 625 | QS | Nm | Per 10 | A | N | |
| Di Monaco (2015) 47 | 193 | HGS | kg | SD (5.7) | A | N |
| Duchowny (2018) 49 | 8467 | HGS | kg | WM: <35, BM: <40, WW: <22, BW: <31 | A | Y |
| Femia (1997) 51 | 95 | HGS | kPa | Continuous | U | N |
| Giampaoli (1999) 53 | 140 | HGS | kPa | Continuous | A | N |
| Gill (2009) 55 | 722 | HGS | kg | BMI specificc | A | Y |
| Hirani (2015) 59 | 1819 | HGS | kg | <26 | A | N |
| Ishizaki (2000) 64 | 468 | HGS | kg | Continuous | A | Y |
| Kozicka (2016) 67 | 41 | HGS | kg | Continuous | U | N |
| Kwon (2012) 68 | 204 | HGS | kg | BMI specificd | A | N |
| Legrand (2014) 69 | 309 | HGS | kg | Tertilee | A | Y |
| Lopez‐Teros (2014) 70 | 133 | HGS | kg | Continuous | A | N |
| McGrath (2018) 71 | 672 | HGS | kg | Continuous | A | N |
| Minneci (2015) 72 | 453 | HGS | kg | Continuous | A | Y |
| Moen (2018) 73 | 115 | HGS | kg | Continuous | A | N |
| Onder (2005) 74 | 458 | HGS | kg | SD (5.9) | A | N |
| Pisters (2012) 77 | 216 | KE | N/kg | SD (0.6) | A | N |
| Rantanen (1999) 80 | 6089 | HGS | kg | Tertilef | A | N |
| Rantanen (2002) 81 | 553 | HGS | N | M: <392. F: <225 | U | Y |
| 554 | AF | N | M: <274, <348. F: <159, <198 | A | N | |
| 550 | KE | N | M: <363, <449. F: <225, <287 | A | N | |
| 546 | TE | N | M: <542, <631. F: <271, <393 | A | N | |
| 538 | TF | N | M: <472, <571. F: <231, <330 | A | N | |
| Rodriguez‐Pascual (2017) 82 | 277 | HGS | kg | Lowest quintile for sex and BMI (NR) | A | Y |
| Rothman (2008) 83 | 754 | HGS | kg | BMI specificg | A | Y |
| Sanchez‐Rodrigeuz (2014) 86 | 99 | HGS | kg | Continuous | U | N |
| Sarkisian (2000) 87 | 6632 | HGS | kg | Lowest Quintile (NR) | A | Y |
| Sarkisian (2001) 88 | 89 | HGS | kg | Decile (NR) | A | N |
| Seidel (2011) 90 | 6670 | HGS | kg | <26 | U | Y |
| Shinkai (2000) 93 | 513 | HGS | kg | Age specifich | U | Y |
| Shinkai (2003) 94 | 601 | HGS | kg | Quartile decrease | A | Y |
| Taekema (2010) 18 | 555 | HGS | kg | Continuous | A | N |
| Tanimoto (2013) 98 | 716 | HGS | kg | Lowest quartile (NR) | U | N |
| Wennie Huang (2010) 102 | 65 | HGS | kg | Continuous | A | Y |
Measure: —: not applicable or reported; AE, ankle extension; AF, arm flexion; BMI, body mass index; HGS, handgrip strength; KE, knee extension; QS, quadriceps strength; SS, shoulder strength; TE, trunk extension; TF, trunk flexion. Cut‐offs: NR, not reported; SD, standard deviation. Expressed as either dichotomous, ranges for specific tertiles, quartiles, quintiles, or categories or per unit or score. AM, adjustment model denotes whether the model in the meta‐analysis was: A, adjusted; or U, unadjusted. MA, meta‐analysis; N, no; Y, yes.
M: <22.00, 21.01–30.00, 30.01–35.00, ≥35.01. F: <14.00, 14.01–18.20, 18.21–22.50, ≥22.51.
M: ≤24: ≤29, 24.1–28: ≤30, >30: ≤32. F: ≤23: ≤17, 23.1–26: ≤17.3, 26.1–29: ≤21.
M: ≤24: ≤29, 24.1–26: ≤30, 26.1–28: ≤30, >28: ≤32. F: ≤23: ≤17, 23.1–26: ≤17.3, 26.1–29: ≤18, >29: ≤21.
M: <25, 25.0–29.9, 30.0–39.9, >40. F: <15, 15.0–19.9, 20.0–24.9, >25.
M: <25.3, 25.4–33.2, >33.3. F: <15.0, 15.1–20.0, >20.1.
<37.0, 37.0–42.0, >42.0.
M: ≤24: ≤29, 24.1–26: ≤30, 26.1–28: ≤30, >28: ≤32. F: ≤23: ≤17, 23.1–26: ≤17.3, 26.1–29: ≤18, >29: ≤21.
M: 65–74: <37, ≥75: <30. F: 65–74: <22, ≥75: <20.
A total of 62 articles16, 26, 27, 28, 29, 30, 31, 33, 35, 37, 38, 40, 41, 42, 43, 44, 45, 46, 48, 49, 52, 54, 55, 56, 57, 58, 59, 61, 62, 63, 66, 68, 69, 70, 71, 72, 73, 74, 75, 76, 78, 79, 82, 83, 84, 85, 87, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103 reported physical performance as a predictor of ADL and IADL (Table 4). Gait speed was the most reported measure, with a total of 37 articles, followed by the composite test short physical performance battery (SPPB), which was reported in 14 articles. Other measures of physical performance included a variety of balance (n = 15), chair stand (n = 10), timed up and go (n = 8), and functional reach tests (n = 2) as well as tests that involve multiple assessments (n = 3). Poor physical performance was positively associated with worsening ADL in 37/49 articles and IADL in 9/11 at follow‐up: SPPB (10/13, 2/2), gait speed (27/33, 7/8), one leg balance (3/4, 0/0), and chair stand test (4/9, 0/1).
Table 4.
Physical performance as predictor of activities of daily living or instrumental activities of daily living
| First author (year) [ref] | N | Measure | Units | Cut‐offs | AM | MA |
|---|---|---|---|---|---|---|
| Albert (2015) 26 | 347 | Gait | m/s | Quartilea | A | N |
| Alexandre (2012) 27 | 1634 | OLB | s | Continuous | A | N |
| 1634 | CST | s | Continuous | A | N | |
| Amigues (2013) 28 | 975 | Gait | m/s | SD (0.22) | A | N |
| 974 | Balance | s | Tertileb | A | N | |
| Arnau (2016) 29 | 252 | SPPB | points | <7 | U | Y |
| Artaud (2015) 30 | 3814 | Fast Gait | m/s | SD (0.22) | A | N |
| 3814 | Change in FG | m/s | SD (0.013) | A | N | |
| Basic (2017) 31 | 1693 | TUG | s | Continuous | U | N |
| Beauchamp (2015) 33 | 430 | SPPB | points | Continuous | U | N |
| 430 | Gait | m/s | Continuous (0.1) | U | N | |
| 428 | 400 m walk | min | Continuous | U | N | |
| 413 | Stair climb | watts | Continuous | U | N | |
| Bianchi (2015) 35 | 538 | Gait | m/s | <0.8 | A | N |
| Carriere (2005) 37 | 545 | Gait | m/s | <0.78 | A | Y |
| 545 | Standing balance | — | Tertilec | A | N | |
| 545 | Dynamic balance | — | Cat (3) | A | N | |
| 545 | CST | s | <13 s | A | Y | |
| 545 | Foot tapping | — | Tertile | A | N | |
| Cesari (2015) 38 | 991 | Gait | m/s | SD (M: 0.24. F: 0.23) | A | Y |
| Chaudhry (2010) 40 | 5888 | Gait | m/s | Lowest quintile for sex and height (NR) | A | Y |
| Chu (2006) 41 | 1338 | Gait | m/s | <0.65 m/s | A | Y |
| Cooper (2011) 42 | 1425 | Timed walk test | — | Continuous | A | N |
| 1425 | Tandem stand | — | A | N | ||
| 1425 | Cardigan test | s | A | N | ||
| 1425 | CST | s | A | N | ||
| Corsonello (2012) 43 | 506 | SPPB | points | Continuous | A | Y |
| <9 | U | Y | ||||
| Costanzo (2018) 44 | 709 | Gait | m/s | Lowest quintile for sex and height (NR) | U | Y |
| 709 | Balance | s | Lowest quintile for sex and height (NR) | U | N | |
| Den Ouden (2013) 45 | 625 | SPPB | points | Continuous | A | Y |
| Denkinger (2010) 46 | 161 | Change in gait | m/s | Continuous | A | N |
| Donoghue (2014) 48 | 1391 | Gait | m/s | Continuous (0.1) | U | N |
| 1391 | TUG | s | Continuous | U | N | |
| Duchowny (2018) 49 | 8467 | Gait | m/s | <0.8 | A | Y |
| Fujiwara (2016) 52 | 981 | Gait | m/s | Tertile (NR) | A | Y |
| Gill (1996) 54 | 775 | Own test | — | Quartile (25%) | U | N |
| Gill (2009) 55 | 722 | SPPB | points | Continuous | A | Y |
| 722 | RGT | s | ≤10 | U | N | |
| 722 | Gait and balance | points | Continuous | U | N | |
| 722 | CST | s | Dich (NR) | U | Y | |
| 722 | Chair | — | Dich | U | N | |
| 722 | Manual dexterity | s | Quartiled | U | N | |
| 722 | GMC | s | Quartilee | U | N | |
| Guralnik (2000) 56 | 2542 | SPPB | points | <10 | U | Y |
| Hansen (1999) 57 | 73 | TUG | s | Tertilef | U | N |
| 73 | Tinetti balance | points | Tertileg | U | N | |
| Heiland (2016) 58 | 1971 | Gait | m/s | <0.8 | A | Y |
| 1971 | OLB | s | <5 | A | Y | |
| Hirani (2015) 59 | 1819 | Gait | m/s | ≤0.8 | A | N |
| Hoeymans (1996) 61 | 303 | Gait | m/s | <0.73 | U | N |
| 303 | CST | s | ≤17.2 | U | N | |
| Hong (2016) 62 | 8000 | Gait | m/s | <0.6 | A | Y |
| Idland (2013) 63 | 113 | Gait | m/s | Cont (1) | A | Y |
| 113 | FRT | cm | Cont (1) | A | N | |
| 113 | Step climb test | cm | Per 10 | A | N | |
| Jonkman (2018) 16 | 798 | Gait | m/s | Dich (NR) | A | N |
| 798 | Tandem stand | s | <10 s | A | N | |
| Kempen (1998) 66 | 557 | Walk turn walk | s | Cont | A | N |
| 557 | CST | s | Cont | A | N | |
| 557 | CST | s | Cont | A | N | |
| 557 | Jacket | s | Cont | A | N | |
| Kwon (2012) 68 | 204 | Gait | m/s | Quartileh | A | N |
| 204 | Balance | s | FT10, FT1‐9, ST10, StS10 | A | N | |
| 204 | CST | s | Quartilei | A | N | |
| Legrand (2014) 69 | 308 | SPPB | points | M: <10. F: <8 | U | Y |
| Lopez‐Teros (2014) [54] | 133 | Gait | m/s | Continuous (1) | A | Y |
| McGrath (2018) 71 | 672 | Gait | m/s | Lowest quintile for sex and height (NR) | A | Y |
| Minneci (2015) 72 | 453 | Gait | m/s | Continuous (1) | A | Y |
| 453 | SPPB | points | Continuous | A | Y | |
| 453 | 6MWT | m | Continuous | A | N | |
| Moen (2018) 73 | 75 | TUG | s | Continuous | A | N |
| Onder (2005) 74 | 458 | Balance | s | SD (10.2) | A | N |
| 458 | CST | s | SD (8.4) | A | N | |
| 458 | Gait | m/s | SD (0.31) | A | Y | |
| 458 | LE comp | — | SD (0.69) | A | N | |
| 458 | Blouse | s | SD (72) | A | N | |
| 458 | Purdue | s | SD (10.5) | A | N | |
| 458 | UE comp | — | SD (0.49) | A | N | |
| Ostir (1998) 75 | 1342 | SPPB | points | <9 | U | Y |
| 1328 | Gait | m/s | <0.8 | U | Y | |
| 1342 | CST | s | <10.9 | U | Y | |
| 1006 | Balance | s | FT10, FT2‐10, StS10 | U | N | |
| Peel (2014) 76 | 280 | Gait | m/s | Continuous (0.1) | A | Y |
| Purser (2005) 78 | 1388 | Gait | m/s | Continuous (0.1) | A | N |
| 1388 | Change in gait | m/s | Continuous (0.1) | A | N | |
| Rajan (2012) 79 | 5317 | M SPPB | points | Continuous | A | Y |
| Rodriguez‐Pascual (2017) 82 | 218 | Gait | m/s | Lowest quintile for sex (NR) | A | Y |
| Rothman (2008) 83 | Gait | m/s | <0.3 | A | N | |
| Sakamoto (2016) 84 | 188 | TUG | s | <15 s | A | Y |
| 188 | FRT | cm | <20 | A | N | |
| Sanchez‐Martinez (2016) 85 | 607 | SPPB | points | <8 | A | N |
| 607 | Gait | m/s | <0.8 | A | N | |
| Sarkisian (2000) 87 | 6632 | Gait | m/s | Lowest quintile (NR) | A | N |
| Schoenenberg (2013) 89 | 119 | TUG | s | <20 s | U | Y |
| Seidel (2011) 90 | 1804 | Gait | m/s | <0.4 | U | Y |
| Shimada (2010) 91 | 436 | TUG | s | <12 | A | Y |
| Shimada (2015) 92 | 4081 | Gait | — | <1.0 | A | N |
| Shinkai (2000) 93 | 513 | UWS | m/s | Tertilej | U | Y |
| 513 | MWS | m/s | Age and sex specific quartilesk | A | N | |
| 513 | OLB | s | Tertilel | U | Y | |
| Shinkai (2003) 94 | 601 | Gait | m/s | Quartile lower | A | N |
| 601 | Fast gait | m/s | Quartile lower | A | N | |
| 601 | OLB | s | Quartile lower | A | N | |
| Sourdet (2012) 95 | 583 | OLB | s | <5 | A | N |
| Stenholm (2014) 96 | 727 | Gait | m/s | Continuous (0.1) | A | Y |
| 727 | SPPB | points | Continuous | U | Y | |
| Takuhiro (2017) 97 | 104 | RWS | m/s | SD (0.24) | A | Y |
| Tanimoto (2013) 98 | 716 | Gait | m/s | Lowest quartile (NR) | U | N |
| Terhorst (2017) 99 | 256 | Balance | — | NR | U | N |
| 256 | Forward reach | — | NR | U | N | |
| Tinetti (2005) 100 | 1042 | CST | s | Tertilem | U | N |
| Volpato (2011) 101 | 74 | SPPB | points | <8 | U | Y |
| Wennie Huang (2010) 102 | 65 | SPPB | points | Continuous | A | Y |
| 65 | Gait | m/s | Continuous (1) | A | Y | |
| 65 | BBS | points | Continuous | A | N | |
| 65 | TUG | s | Continuous | A | N | |
| Zhang (2013) 103 | 504 | CST | s | <11.2 | A | Y |
Physical Performance: —, not applicable or reported; 6MWT, 6 min walk test; BBS, Berg Balance Scale; CST, chair stand test; OLB, one leg balance; FRT, functional reach test; FT, full tandem; MWS, maximum walking speed; POMA, performance oriented mobility assessment; PPT, physical performance test; RGT, rapid gait test; RWS, regular walking speed; SPPB, short physical performance battery; ST, semi tandem; StS, side to side; TUG, timed up and go; UWS, usual walking speed; WS, walking speed. Cut‐offs: NR, not reported; SD, standard deviation. Expressed as either dichotomous, ranges for specific tertiles, quartiles, quintiles, or categories or per unit or score unless otherwise stated in brackets. AM, adjustment model denotes whether the model in the meta‐analysis was: A, adjusted; or U, unadjusted. MA, meta‐analysis; N, no; Y, yes.
≥1, 0.74–0.99, 0.57–0.73, <0.57.
<2, 3–9, ≥10.
Full tandem, semi tandem, side to side.
<21.8, 21.8–24.3, 24.4–27.5, ≥27.6.
<8.8, 8.8–10.3, 10.4–12.4, ≥12.5.
<20, 20–40, ≥40.
15–27, 28–38, 39–41.
M: >4.5, 4.0–4.5, 3.0–3.9, <3. F: >5, 4.0–5.0, 3.0–3.9, <3.
M: >20, 17.0–19.0, 11–16.9. F: >21, 18.0–20.9, 12.0–17.9, <12.
M: ≤1.08, ≥75: ≥0.82. F: ≤0.9, ≥75: ≤0.69.
M: 65–74: ≤1.81, 1.82–2.10, 2.11–2.36, ≥2.37. ≥75: ≤1.34, 1.35–1.64, 1.65–1.99, ≥2.00. F: 65–75: ≤1.45, 1.46–1.70, 1.71–1.98, ≥1.97. ≥75: ≤1.08, 1.09–1.34, 1.35–1.62, ≤1.63.
M: ≤18, ≥75: ≤5. F: ≤7, ≥75: ≤16.
<9, 9–14, >14.
Quality assessment
A complete breakdown of the NOS can be found in Table 5. The majority of the articles were of high quality (46/83).
Table 5.
Quality assessment of included studies using a modified Newcastle–Ottawa Scale (NOS)
| First author (year) [ref] | Selection | Comp | Outcome | Total score | ||||
|---|---|---|---|---|---|---|---|---|
| Q1 | Q2a | Q3 | Q1 | Q1 | Q2 | Q3 | ||
| Abete (2017) 24 | 1 | 1 | 1 | 1 | 1 | 1 | 6/7 | |
| Al snih (2004) 25 | 1 | 1 | 2 | 1 | 1 | 1 | 7/7 | |
| Albert (2015) 26 | 1 | 1 | 0 | 1 | 1 | 1 | 5/7 | |
| Alexandre (2012) 27 | 1 | 1 | 0 | 1 | 1 | 1 | 5/7 | |
| Amigues (2013) 28 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8/8 |
| Arnau (2016) 29 | 1 | 1 | 2 | 1 | 1 | 1 | 7/7 | |
| Artaud (2015) 30 | 1 | 1 | 2 | 1 | 1 | 1 | 7/7 | |
| Basic (2017) 31 | 1 | 1 | 0 | 1 | 0 | 0 | 3/7 | |
| Baumgartner (2004) 32 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8/8 |
| Beauchamp (2015) 33 | 1 | 1 | 0 | 1 | 1 | 0 | 4/7 | |
| Beloosesky (2009) 34 | 0 | 1 | 0 | 1 | 1 | 1 | 4/7 | |
| Bianchi (2015) 35 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8/8 |
| Broadwin (2001) 36 | 1 | 1 | 2 | 1 | 1 | 1 | 7/7 | |
| Carriere (2005) 37 | 1 | 1 | 2 | 1 | 1 | 1 | 7/7 | |
| Cesari (2015) 38 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8/8 |
| Chan (2014) 39 | 1 | 1 | 1 | 1 | 1 | 1 | 6/7 | |
| Chaudhry (2010) 40 | 1 | 1 | 2 | 1 | 1 | 1 | 7/7 | |
| Chu (2006) 41 | 1 | 1 | 2 | 1 | 1 | 1 | 7/7 | |
| Cooper (2011) 42 | 1 | 1 | 2 | 1 | 1 | 1 | 7/7 | |
| Corsonello (2012) 43 | 0 | 1 | 2 | 1 | 1 | 1 | 6/7 | |
| Costanzo (2018) 44 | 1 | 1 | 0 | 1 | 1 | 1 | 5/7 | |
| Den Ouden (2013) 45 | 1 | 1 | 2 | 1 | 1 | 1 | 7/7 | |
| Denkinger (2010) 46 | 1 | 1 | 2 | 1 | 0 | 1 | 6/7 | |
| Di Monaco (2015) 47 | 1 | 1 | 2 | 1 | 1 | 1 | 7/7 | |
| Donoghue (2014) 48 | 1 | 1 | 0 | 1 | 1 | 1 | 5/7 | |
| Duchowny (2018) 49 | 1 | 1 | 2 | 1 | 1 | 1 | 7/7 | |
| Fantin (2007) 50 | 1 | 1 | 0 | 1 | 1 | 1 | 5/7 | |
| Femia (1997) 51 | 1 | 1 | 0 | 1 | 1 | 1 | 5/7 | |
| Fujiwara (2016) 52 | 1 | 1 | 2 | 1 | 1 | 1 | 7/7 | |
| Giampaoli (1999) 53 | 1 | 1 | 2 | 1 | 1 | 1 | 7/7 | |
| Gill (1996) 54 | 1 | 1 | 2 | 1 | 1 | 1 | 7/7 | |
| Gill (2009) 55 | 1 | 1 | 2 | 1 | 1 | 1 | 7/7 | |
| Guralnik (2000) 56 | 1 | 1 | 2 | 1 | 1 | 1 | 7/7 | |
| Hansen (1999) 57 | 1 | 1 | 0 | 1 | 0 | 1 | 4/7 | |
| Heiland (2016) 58 | 1 | 1 | 2 | 1 | 1 | 1 | 7/7 | |
| Hirani (2015) 59 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8/8 |
| Hirani (2017) 60 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8/8 |
| Hoeymans (1996) 61 | 1 | 1 | 1 | 1 | 1 | 1 | 6/7 | |
| Hong (2016) 62 | 1 | 1 | 2 | 1 | 1 | 1 | 7/7 | |
| Idland (2013) 63 | 1 | 1 | 2 | 1 | 1 | 1 | 7/7 | |
| Ishizaki (2000) 64 | 1 | 1 | 2 | 1 | 1 | 1 | 7/7 | |
| Janssen (2006) 65 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8/8 |
| Jonkman (2018) 16 | 1 | 1 | 2 | 1 | 1 | 1 | 7/7 | |
| Kempen (1998) 66 | 1 | 1 | 1 | 1 | 1 | 1 | 6/7 | |
| Kozicka (2016) 67 | 1 | 1 | 0 | 1 | 1 | 0 | 4/7 | |
| Kwon (2012) 68 | 0 | 1 | 2 | 1 | 1 | 1 | 6/7 | |
| Legrand (2014) 69 | 1 | 1 | 2 | 1 | 1 | 1 | 7/7 | |
| Lopez‐Teros (2014) 70 | 1 | 1 | 2 | 1 | 1 | 1 | 7/7 | |
| McGrath (2018) 71 | 1 | 1 | 2 | 1 | 1 | 1 | 7/7 | |
| Minneci (2015) 72 | 0 | 1 | 2 | 1 | 1 | 1 | 6/7 | |
| Moen (2018) 73 | 1 | 1 | 2 | 1 | 0 | 1 | 6/7 | |
| Onder (2005) 74 | 1 | 1 | 1 | 1 | 1 | 1 | 6/7 | |
| Ostir (1998) 75 | 1 | 1 | 2 | 1 | 1 | 0 | 6/7 | |
| Peel (2014) 76 | 0 | 1 | 1 | 1 | 1 | 0 | 4/7 | |
| Pisters (2012) 77 | 0 | 1 | 2 | 1 | 1 | 1 | 6/7 | |
| Purser (2005) 78 | 0 | 1 | 2 | 1 | 1 | 1 | 6/7 | |
| Rajan (2012) 79 | 1 | 1 | 2 | 1 | 1 | 1 | 7/7 | |
| Rantanen (1999) 80 | 1 | 1 | 2 | 1 | 1 | 1 | 7/7 | |
| Rantanen (2002) 81 | 1 | 1 | 1 | 1 | 1 | 1 | 6/7 | |
| Rodriguez‐Pascual (2017) 82 | 0 | 1 | 1 | 1 | 1 | 1 | 5/7 | |
| Rothman (2008) 83 | 1 | 1 | 2 | 1 | 1 | 1 | 7/7 | |
| Sakamoto (2016) 84 | 1 | 1 | 2 | 1 | 1 | 1 | 7/7 | |
| Sanchez‐Martinez (2016) 85 | 1 | 1 | 2 | 1 | 1 | 1 | 7/7 | |
| Sanchez‐Rodrigeuz (2014) 86 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 5/8 |
| Sarkisian (2000) 87 | 1 | 1 | 2 | 1 | 1 | 1 | 7/7 | |
| Sarkisian (2001) 88 | 1 | 1 | 2 | 1 | 1 | 1 | 7/7 | |
| Schoenenberg (2013) 89 | 0 | 1 | 0 | 1 | 1 | 1 | 4/7 | |
| Seidel (2011) 90 | 1 | 1 | 2 | 1 | 1 | 1 | 7/7 | |
| Shimada (2010) 91 | 1 | 1 | 2 | 1 | 1 | 1 | 7/7 | |
| Shimada (2015) 92 | 1 | 1 | 2 | 1 | 1 | 0 | 6/7 | |
| Shinkai (2000) 93 | 1 | 1 | 2 | 1 | 1 | 1 | 7/7 | |
| Shinkai (2003) 94 | 1 | 1 | 2 | 1 | 1 | 1 | 7/7 | |
| Sourdet (2012) 95 | 0 | 1 | 2 | 1 | 1 | 1 | 6/7 | |
| Stenholm (2014) 96 | 1 | 1 | 2 | 1 | 1 | 1 | 7/7 | |
| Taekema (2010) 18 | 1 | 1 | 1 | 1 | 1 | 1 | 6/7 | |
| Takuhiro (2017) 97 | 1 | 1 | 0 | 1 | 1 | 1 | 5/7 | |
| Tanimoto (2013) 98 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 8/8 |
| Terhorst (2017) 99 | 1 | 1 | 0 | 1 | 1 | 1 | 5/7 | |
| Tinetti (2005) 100 | 1 | 1 | 0 | 1 | 1 | 1 | 5/7 | |
| Volpato (2011) 101 | 0 | 1 | 0 | 1 | 1 | 1 | 4/7 | |
| Wennie Huang (2010) 102 | 1 | 1 | 2 | 1 | 1 | 0 | 6/7 | |
| Zhang (2013) 103 | 1 | 1 | 2 | 1 | 1 | 1 | 7/7 | |
| Zoico (2007) 104 | 1 | 1 | 2 | 1 | 1 | 0 | 6/7 | |
Comp, comparability.
Only applied to studies that dichotomized sarcopenic cohorts.
Meta‐analysis
Muscle mass
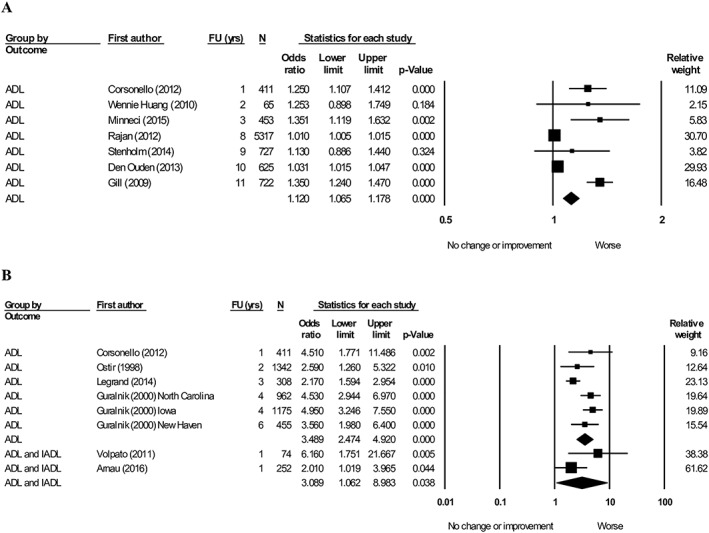
Two articles60, 98 evaluating muscle mass (low vs. high) and its association with ADL were included in the meta‐analysis. Low muscle mass was associated with worsening ADL [OR = 3.19, 95% confidence interval (CI): 1.29–7.92, I 2 = 68.8]. Four articles28, 35, 60, 65 were pooled into a meta‐analysis exploring the effect of muscle mass (low vs. high), which favoured worsening IADL (OR = 1.28, 95% CI: 1.02–1.61, I 2 = 75.8) (Figure 2).
Figure 2.

Forest plot showing the association between baseline muscle mass (low vs. high) with activity of daily living (ADL) and instrumental activity of daily living (IADL) at follow‐up. Heterogeneity (I 2): ADL = 68.8. IADL = 75.8. M, male; F, female. Articles that reported both ADL and IADL were denoted a and b.
Muscle strength
Six articles25, 27, 64, 72, 102, 105 evaluating the association between handgrip strength (per 1 kg lower) and ADL were pooled, favoured worsening ADL (OR = 1.09, 95% CI: 1.05–1.13, I 2 = 87.5) (Figure 3A). Ten articles24, 25, 40, 44, 49, 69, 81, 82, 83, 93 were pooled into meta‐analysis demonstrating the association between low vs. high handgrip strength with ADL (Figure 3B). The pooled result again favoured worsening ADL (OR = 1.51, 95% CI: 1.34–1.70, I 2 = 50.0). Four articles37, 87, 90, 93 were pooled measuring handgrip strength (low vs. high) and IADL favouring worsening ADL (OR = 1.59, 95% CI: 1.04–2.41, I 2 = 94.7) (Figure 3B).
Figure 3.
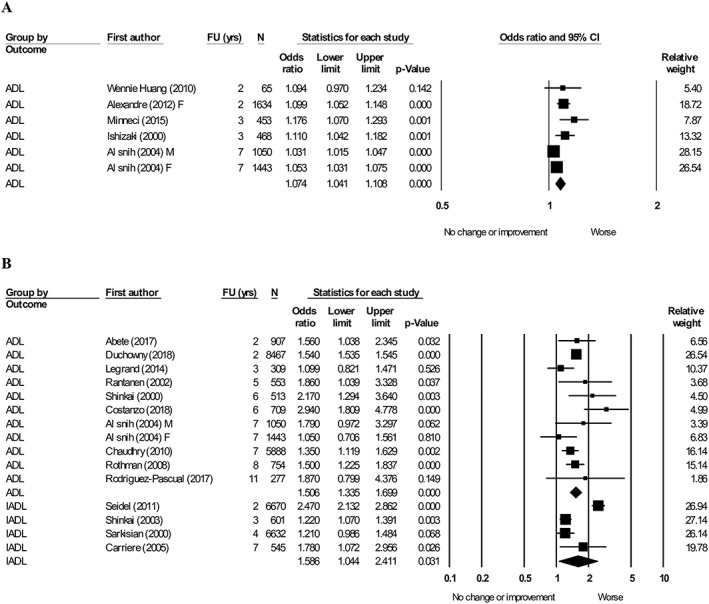
Forest plot showing the association between baseline handgrip strength with activity of daily living (ADL) and instrumental activity of daily living (IADL) at follow‐up. (A) Handgrip strength (per 1 kg lower), heterogeneity (I 2) = 72.8. (B) Handgrip strength (low vs. high), heterogeneity (I 2): ADL = 50.0. IADL: 94.7. M, male; F, female.
Physical performance
Seven articles43, 45, 55, 72, 79, 96, 102 evaluating the association between SPPB and ADL were pooled, which demonstrated that a lower SPPB score (per one point) is associated with worsening ADL (OR = 1.12, 95% CI: 1.07–1.18, I 2 = 91.8) (Figure 4A). Four articles43, 56, 69, 75 were pooled exploring the association between SPPB (low vs. high) and ADL (Figure 4B). The pooled effect favoured worsening ADL (OR = 3.49, 95% CI: 2.47–4.92, I 2 = 63.3). Two articles29, 101 combined ADL and IADL and showed that SPPB score (low vs. high) favoured a worsening of the combined ADL and IADL measure (OR = 3.09, 95% CI: 1.06–8.98, I 2 = 57.6) (Figure 4B).
Figure 4.
Forest plot showing the association between short physical performance battery (SPPB) with activity of daily living (ADL) and/or instrumental activity of daily living (IADL) at follow‐up. (A) SPPB (per 1 point lower), heterogeneity (I 2) = 91.8. (B) SPPB (low vs. high), heterogeneity (I 2): ADL = 63.3. IADL = 57.6.
Seven articles were pooled in a meta‐analysis demonstrating the association of gait speed (per unit increase) with ADL (Figure 5A). After subgrouping for a lower unit (per 0.1 m/s, per 1 m/s and per SD) in gait speed, a lower gait speed of 0.1 m/s76, 96 was not associated with worsening ADL (OR = 1.64, 95% CI: 0.80–3.38, I 2 = 85.0) while a lower gait speed of 1.0 m/s63, 72, 102 and 1 SD38, 74 was associated with worsening ADL (OR = 4.40, 95% CI: 1.34–14.48, I 2 = 52.1; OR = 1.85, 95% CI: 1.44–2.36, I 2 = 62.8). Six articles41, 49, 52, 58, 75, 93 pooling gait speed (low vs. high) with ADL favoured worsening in ADL (OR = 2.33, 95% CI: 1.58–3.44, I 2 = 94.2) (Figure 5B). Four articles37, 41, 62, 90 evaluating the association between gait speed (low vs. high) with IADL demonstrated worsening in IADL (OR = 1.93, 95% CI: 1.69–2.21, I 2 = 0.0) (Figure 5B). Five articles40, 44, 71, 82, 87 were pooled comparing the lowest quintile for gait speed with the upper four quintiles with ADL demonstrated an association in worsening ADL (OR = 3.08, 95% CI: 2.13–4.46, I 2 = 75.7) (Figure 5C).
Figure 5.
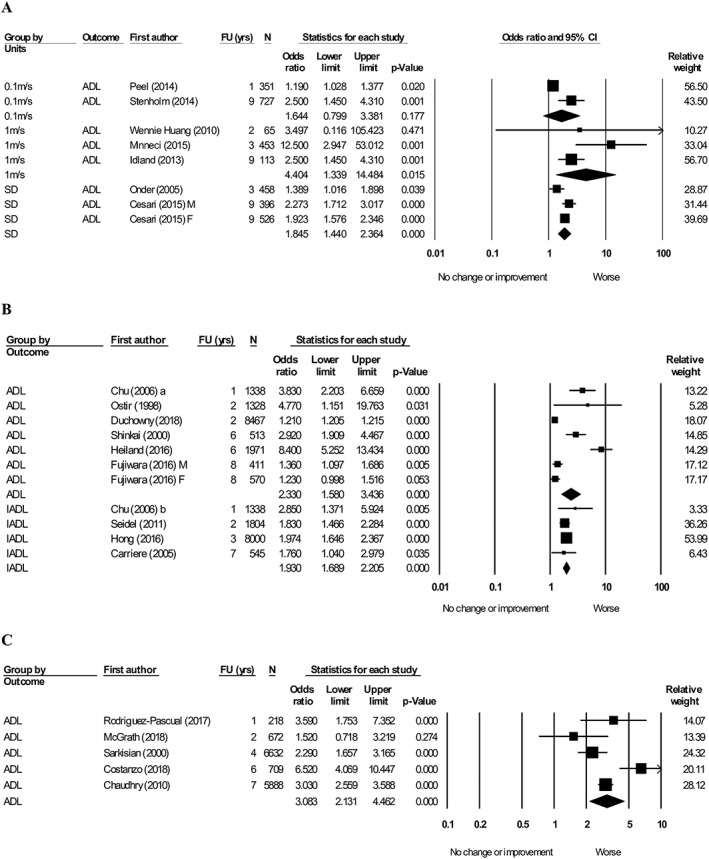
Forest plot showing the association between gait speed with activity of daily living (ADL) and instrumental activity of daily living (IADL) at follow‐up. (A) Gait (per unit lower), heterogeneity (I 2): 0.1 m/s = 85. 1.0 m/s = 52.1. SD = 62.8. (B) Gait (low vs. high), heterogeneity (I 2): ADL = 94.2. IADL = 0.0. (C) Gait (lowest quintile vs. upper four quintiles), heterogeneity (I 2) = 75.7.
Three articles58, 93, 95 were pooled demonstrating a strong association between one leg balance (low vs. high) and a decline in ADL (OR = 2.74, 95% CI: 1.31–5.72, I 2 = 88.5) (Figure 6A). Two articles84, 89 reported timed up and go (slow vs. fast) with ADL (Figure 6B). Slow timed up and go favoured worsening in ADL (OR = 3.41, 95% CI: 1.86–6.28, I 2 = 41.6). Three articles55, 75, 103 explored the effect of chair stand test time (slow vs. fast) with ADL (Figure 6B). Slow chair stand test favoured worsening in ADL (OR = 1.90, 95% CI: 1.63–2.21, I 2 = 0.0). Two articles37, 103 were pooled exploring the effect of chair stand test time (slow vs. fast) was not associated with worsening IADL (OR = 2.10, 95% CI: 0.80–5.48, I 2 = 74.8) (Figure 6C).
Figure 6.
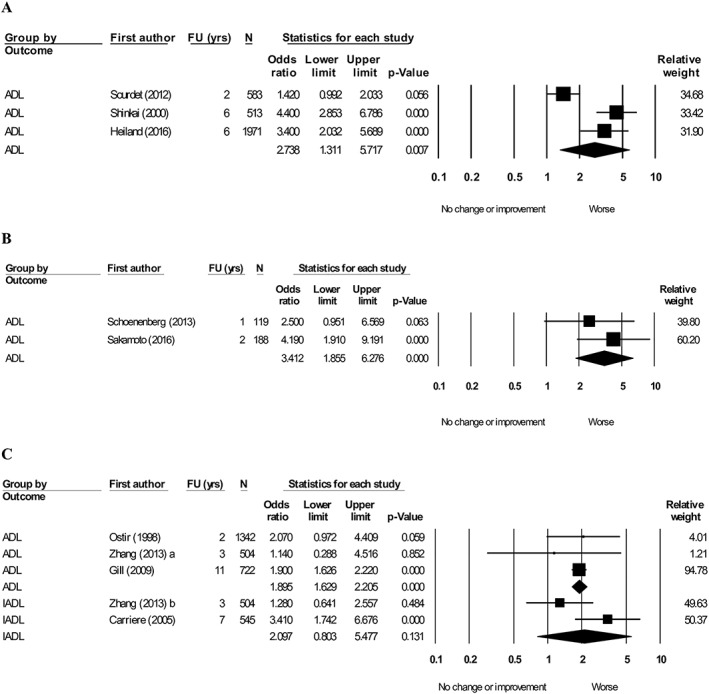
Forest plot showing the association between other physical performance measures with activity of daily living (ADL) at follow‐up. (A) One leg balance time (low vs. high), heterogeneity (I 2) = 88.5. (B) Timed up and go (slow vs. fast), heterogeneity (I 2) = 41.6. (C) Chair stand test time (slow vs. fast), heterogeneity (I 2): ADL = 0.0. Instrumental activity of daily living (IADL) = 74.8. Articles that reported both ADL and IADL were denoted a and b.
All meta‐analyses were ordered by study follow‐up duration from shortest to longest. No pattern was present based on follow‐up duration in all meta‐analyses.
Discussion
Muscle mass was associated with the development of IADL dependence. Muscle strength and physical performance were associated with the development of ADL and IADL dependence at follow‐up in older adults.
Muscle mass
Surprisingly, muscle mass was associated with the development of new IADL dependence, but not ADL dependence at follow‐up in this meta‐analysis. While the association between muscle mass and ADL was not statistically significant, perhaps because of only two studies being included, a trend suggested that it was a clinically significant predictor of worsening ADL. The Concord Health and Ageing in Men Project reported that low muscle mass and sarcopenic obesity were significantly associated with worsening ADL and IADL.60 Additionally, a prospective study reported that low fat free mass was associated with functional disability.36 It has been previously hypothesized that muscle mass plays an important role in the loss of ADL with increasing age 106. Loss of muscle mass could be explained by a variety of different factors including the loss of innervation from alpha‐motor neurons 107, reduced dietary protein intake 108, 109, and less physical activity 110. Individuals with lower muscle mass have more difficulty in performing ADL and IADL 111, 112. Furthermore, it has been suggested that the infiltration of fat into the muscle is a risk factor for low muscle mass, which is associated with worsening ADL and IADL 111. Prior studies exploring the effect of training on muscle mass demonstrate its effectiveness in increasing performance and preservation of ADL and IADL 113, 114. Muscle mass has also been shown to improve in response to resistance exercise training and nutritional interventions, even in frail populations 109, 113, 115, 116, 117.
Muscle strength
The predictive ability of muscle strength at baseline of ADL and IADL decline is consistent with a previous study consisting of 6089 participants, which suggested that having higher muscle strength was protective against the onset of future disability.80 However, the discrepancies in measures and cut‐offs and thus a lack of consensus in measuring muscle strength. Handgrip strength has been used as a measure of upper limb strength in older populations.80, 118 However handgrip strength should not be used as an approximation of overall muscle strength in older adults because of the variation between individuals and the variation in muscle groups within the same individual 119. Although a strong association between muscle mass and muscle strength exists 120, 121, 122, muscle strength may better predict worsening ADL and IADL as muscle mass can also be influenced by factors like disease, muscle use, and muscle morphology 123.
It has previously been hypothesized that there is a minimal amount of muscle strength required to complete ADL 124. Handgrip strength declines by 0.06 kg per year up to the age of 50 with an even steeper decline of 0.37 kg per year after the age of 50 years 125. In healthy older adults, large changes in muscle strength have little effect on ADL while small changes in a frail population have a more profound effect 124. This suggests that the completion of ADL and IADL requires a minimum threshold of strength and that higher muscle strength provides individuals with a protective reserve against the development of ADL and IADL dependence.14 Thus, individuals with lower muscle strength, and therefore a lower protective reserve, are at higher risk of developing new ADL and IADL dependence at follow‐up.14
The prevalence of ADL and IADL dependence increases with age, muscle strength amongst older adults has been shown to decrease.126, 127, 128, 129 Gender has also been suggested to influence muscle strength as hospitalized older adults demonstrated an association between handgrip strength with ADL and IADL in male patients, but not female patients 112. Individuals with high muscle strength at baseline are also likely to preserve their higher handgrip strength at follow‐up 129. It would be expected that the association is stronger with longer follow‐up duration. However, this was not observed in our meta‐analysis. A reason this could be due to the different baseline ages and follow‐up duration between studies. Given that muscle mass decreases at a slower rate compared with muscle strength, it may be easier to preserve and maintain muscle mass, which can also prevent the decline in muscle strength.130
Muscle quality, which refers to the muscle strength or power per unit of muscle mass,131 has been associated with worse ADL in a previous cross‐sectional study.10 As previous studies support the assumption that muscle strength decline occurs faster than muscle mass, it also suggests that muscle quality has likewise declined 130. Physical performance measured by gait speed has previously shown that a higher gait speed to be associated with higher muscle quality in older adults 132, 133. Currently, no studies have explored the association between muscle quality and worsening ADLs; however, as muscle quality has been shown to decline with age 134, 135, 136, it can be expected to observe similar results as muscle mass, strength, and physical performance.
Physical performance
Lower SPPB scores were associated with worsening ADL at follow‐up. The SPPB encompasses three assessments that require strength, balance, dexterity, and cognitive control, which captures functional elements that are required for the completion of ADL and IADL 137, 138, 139. Lower extremity physical performance measured by the SPPB was associated with worse ADL. Furthermore, a difference in score of 1 point on the SPPB was also significantly associated with worse ADLs as shown in this review and prior research 138. Lower SPPB scores are a reflection of impaired skeletal muscle function and structural changes associated with chronic inflammatory processes 140, as well as neurological pathologies that impair gait and balance 141 that are thought to mediate the development of ADL and IADL dependence 142. High heterogeneity was observed between studies that evaluated the SPPB as a continuous variable compared with a dichotomous variable (low vs. high). The findings from this review were consistent with a previous systematic review, where the SPPB was predictive of long‐term disability.143
Gait speed measured by a 4 m walk test is a component of the SPPB and is often used in clinical settings to identify individuals at high risk of adverse health outcomes144 and assist in the diagnosis of sarcopenia.145 Gait speed is both a simple and highly reproducible measure of physical performance and is comparable with the SPPB as a predictor of ADL dependence.56, 146 In community‐dwelling outpatients, the association between gait sped with ADL and IADL was stronger than its association with the other sarcopenia diagnosis criteria.147 While having a higher 0.1 m/s gait speed was not statistically significant in this study, all other analyses showed a lower gait speed favoured a worsening ADL and/or IADL. One study, which evaluated 27 200 community‐dwelling older adults for gait speed, demonstrated its predictive value on the development of disability.148 Other studies reported different cut‐offs for slow and fast gait speed, but no single threshold was evident with the incidence of disability.148 Therapies or preventative interventions targeted at improving or maintaining gait speed should be considered amongst older adults, as these changes are reflective of the progression and development of ADL and IADL dependence.149, 150 The findings of this meta‐analysis also resonate with a previous systematic review, which demonstrated that slow gait speed was associated with worsening ADL in older populations.15, 151
One leg balance was significantly associated with worsening ADL in the follow‐up. The importance of balance is well described controlling both static and dynamic posture while performing a variety of daily activities,152, 153 such that it has been used as a predictor of high‐risk individuals prone to falls.154 Although one previous study reported no statistically significant association between one leg balance and ADL dependence, the three studies included in this meta‐analysis demonstrated a strong association between low one leg balance time and worsening ADLs.155 Timed up and go is a measure of walking, balance, strength, and cognition.156 A descriptive meta‐analysis exploring the cut‐off times of the timed up and go test reported cut‐off values of 8.1, 9.2, and 11.3 s for those aged 60–69, 70–79, and 80–99 years old, respectively.157 All studies included in the meta‐analysis84, 89, 91 had greater cut‐offs for a slow timed up and go time, potentially underestimating the pooled effect size. Timed up and go had a smaller effect size but less heterogeneity in predicting worsening ADLs compared with one leg balance in this meta‐analysis. Prior research comparing different measures of balance reported timed up and go as being a better predictor of ADL in older the community‐dwelling population.158
The chair stand test was significantly associated with worsening ADL but not IADL. Chair stand test has been used as a measure of lower body strength in older adults in community‐dwelling older adults and as part of the SPPB.11, 159 A previous study reported that 22% of community‐dwelling older individuals are unable to complete the chair stand test (5 to 10 rises) without the use of hands and/or arms.11 The 30 s chair stand test may be a better protocol as it is more reliable given that some individuals are unable to complete standard protocols and the floor effect.159 Surprisingly, no studies included in the meta‐analysis reported the 30 s protocol; only the 5× chair stand test was used. High heterogeneity was observed in IADL perhaps because of the different cut‐off points used (11.2 s103 and 13 s160) although this was not the case for ADLs.
Strength and limitations
This systematic review and meta‐analysis explored muscle mass, muscle strength, and physical performance as predictors for ADL and IADL rather than limiting to just a single measure. By evaluating the effects of all three measures, this review presented the most detailed assessment of this topic. To reduce the risk of reverse causation when interpreting the associations reported, only prospective studies were included. There are also limitations to this review. Not all studies were pooled into a meta‐analysis because of differences in measures and cut‐offs of muscle mass, muscle strength, and physical performance, the use of different statistical analyses, or the lack of data required to calculate an OR. Studies that were pooled were also reporting univariate and multivariate analyses, adjusting for different confounders that were inconsistent between studies that may have further led to an over or under estimation of the effect sizes. Studies that did not have a primary aim of exploring the association between baseline muscle measures with ADL and/or IADL may have used less standardized procedures. The follow‐up duration between studies varied significantly, which may have impacted the results.
Future recommendations
There were few pre‐existing studies that explored the association between baseline muscle mass and ADL and/or IADL. Studies should continue to investigate the association of muscle mass alone with ADL and/or IADL. Future studies should also explore the association of muscle quality with ADL and/or IADL. Interventions tailored for specifically increasing muscle measures should be developed to prevent individuals from having worsening ADL and/or IADL in their future. Interventions should be developed with the focus of preserving or increasing muscle measures as we age.
Conclusions
This study quantified the current research available between muscle measures and (I)ADL. Muscle mass is predictive of ADL and IADL decline whereas muscle strength and physical performance are predictive of both ADL and/or IADL decline. Future studies should continue to develop and improve interventions to preserve and improve muscle measures to prevent ADL and IADL dependence.
Conflict of Interest
Daniel X. M. Wang, Jessica Yao, Yasar Zirek, Esmee M. Reijnierse, and Andrea B. Maier declare that they have no conflict of interest.
Supporting information
Table S1. Search Strategy
Data S2. Newcastle–Ottawa Scale
Table S3. Breakdown of the ADL components in articles that created their own questionnaire
Acknowledgements
The authors would like to thank Patrick Condron (senior liaison librarian, Brownless Biomedical Library, Faculty of Medicine, Dentistry & Health Science, The University of Melbourne), who assisted with the development of the search strategy. The authors of this manuscript complied with the principles and ethical guidelines for authorship and publication in the Journal of Cachexia, Sarcopenia and Muscle.161
Wang D. X. M., Yao J., Zirek Y., Reijnierse E. M., and Maier A. B. (2020) Muscle mass, strength, and physical performance predicting activities of daily living: a meta‐analysis, Journal of Cachexia, Sarcopenia and Muscle, 11: 3–25. 10.1002/jcsm.12502.
Jessica Yao and Yasar Zirek contributed equally to the work.
References
- 1. Stuck AE, Walthert JM, Nikolaus T, Bula CJ, Hohmann C, Beck JC. Risk factors for functional status decline in community‐living elderly people: a systematic literature review. Soc Sci Med. 1999;48:445–469. [DOI] [PubMed] [Google Scholar]
- 2. Millan‐Calenti JC, Tubio J, Pita‐Fernandez S, Gonzalez‐Abraldes I, Lorenzo T, Fernandez‐Arruty T, et al. Prevalence of functional disability in activities of daily living (ADL), instrumental activities of daily living (IADL) and associated factors, as predictors of morbidity and mortality. Arch Gerontol Geriatr. 2010;50:306–310. [DOI] [PubMed] [Google Scholar]
- 3. Lawton MP, Brody EM. Assessment of older people: self‐maintaining and instrumental activities of daily living. The gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 4. Velazquez Alva Mdel C, Irigoyen Camacho ME, Delgadillo Velazquez J, Lazarevich I. The relationship between sarcopenia, undernutrition, physical mobility and basic activities of daily living in a group of elderly women of Mexico City. Nutricion hospitalaria. 2013;28:514–521. [DOI] [PubMed] [Google Scholar]
- 5. Sonn U, Asberg KH. Assessment of activities of daily living in the elderly. A study of a population of 76‐year‐olds in Gothenburg, Sweden. Scand J Rehabil Med. 1991;23:193–202. [PubMed] [Google Scholar]
- 6. Millan‐Calenti JC, Tubío J, Pita‐Fernández S, González‐Abraldes I, Lorenzo T, Fernandez‐Arruty T, et al. Prevalence of functional disability in activities of daily living (ADL), instrumental activities of daily living (IADL) and associated factors, as predictors of morbidity and mortality. Arch Gerontol Geriatr. 2010;50:306–310. [DOI] [PubMed] [Google Scholar]
- 7. Berlau DJ, Corrada MM, Kawas C. The prevalence of disability in the oldest‐old is high and continues to increase with age: findings from The 90+ Study. Int J Geriatr Psychiatry. 2009;24:1217–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on sarcopenia in older people. Age and ageing. 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. Journal of the American Geriatrics Society. 2002;50:889–896. [DOI] [PubMed] [Google Scholar]
- 10. Hairi NN, Cumming RG, Naganathan V, Handelsman DJ, Le Couteur DG, Creasey H, et al. Loss of muscle strength, mass (sarcopenia), and quality (specific force) and its relationship with functional limitation and physical disability: the Concord Health and Ageing in Men Project. J Am Geriatr Soc. 2010;58:2055–2062. [DOI] [PubMed] [Google Scholar]
- 11. Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self‐reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. [DOI] [PubMed] [Google Scholar]
- 12. Hortobagyi T, Mizelle C, Beam S, DeVita P. Old adults perform activities of daily living near their maximal capabilities. J Gerontol A Biol Sci Med Sci. 2003;58:M453–M460. [DOI] [PubMed] [Google Scholar]
- 13. Gill TM, Robison JT, Tinetti ME. Predictors of recovery in activities of daily living among disabled older persons living in the community. J Gen Intern Med. 1997;12:757–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rantanen T. Muscle strength, disability and mortality. Scand J Med Sci Sports. 2003;13:3–8. [DOI] [PubMed] [Google Scholar]
- 15. Vermeulen J, Neyens JC, van Rossum E, Spreeuwenberg MD, de Witte LP. Predicting ADL disability in community‐dwelling elderly people using physical frailty indicators: a systematic review. BMC geriatrics. 2011;11:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jonkman NH, Del Panta V, Hoekstra T, Colpo M, van Schoor NM, Bandinelli S, et al. Predicting trajectories of functional decline in 60‐ to 70‐year‐old people. Gerontology. 2018;64:212–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Velazquez Alva MC, Irigoyen Camacho ME, Delgadillo Velazquez J, Lazarevich I. The relationship between sarcopenia, undernutrition, physical mobility and basic activities of daily living in a group of elderly women of Mexico City. Nutricion hospitalaria. 2013;28:514–521. [DOI] [PubMed] [Google Scholar]
- 18. Taekema DG, Gussekloo J, Maier AB, Westendorp RGJ, de Craen AJM. Handgrip strength as a predictor of functional, psychological and social health. A prospective population‐based study among the oldest old. Age Ageing. 2010;39:331–337. [DOI] [PubMed] [Google Scholar]
- 19. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta‐analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. [DOI] [PubMed] [Google Scholar]
- 20. Stang A. Critical evaluation of the Newcastle–Ottawa Scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur J Epidemiol. 2010;25:603–605. [DOI] [PubMed] [Google Scholar]
- 21. Hermont AP, Oliveira PA, Martins CC, Paiva SM, Pordeus IA, Auad SM. Tooth erosion and eating disorders: a systematic review and meta‐analysis. PLoS One. 2014;9:e111123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fleiss JL. The statistical basis of meta‐analysis. Stat Methods Med Res. 1993;2:121–145. [DOI] [PubMed] [Google Scholar]
- 23. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 24. Abete P, Basile C, Bulli G, Curcio F, Liguori I, Della‐Morte D, et al. The Italian version of the “frailty index” based on deficits in health: a validation study. Aging Clin Exp Res. 2017;29:913–926. [DOI] [PubMed] [Google Scholar]
- 25. Al Snih S, Markides KS, Ottenbacher KJ, Raji MA. Hand grip strength and incident ADL disability in elderly Mexican Americans over a seven‐year period. Aging Clin Exp Res. 2004;16:481–486. [DOI] [PubMed] [Google Scholar]
- 26. Albert SM, Bear‐Lehman J, Anderson SJ. Declines in mobility and changes in performance in the instrumental activities of daily living among mildly disabled community‐dwelling older adults. J Gerontol A Biol Sci Med Sci. 2015;70:71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alexandre TS, Corona LP, Nunes DP, Santos JLF, Duarte YAO, Lebrao ML. Gender differences in incidence and determinants of disability in activities of daily living among elderly individuals: SABE study. Arch Gerontol Geriatr. 2012;55:431–437. [DOI] [PubMed] [Google Scholar]
- 28. Amigues I, Schott A‐M, Amine M, Gelas‐Dore B, Veerabudun K, Paillaud E, et al. Low skeletal muscle mass and risk of functional decline in elderly community‐dwelling women: the prospective EPIDOS study. J Am Med Dir Assoc. 2013;14:352–357. [DOI] [PubMed] [Google Scholar]
- 29. Arnau A, Espaulella J, Serrarols M, Canudas J, Formiga F, Ferrer M. Risk factors for functional decline in a population aged 75 years and older without total dependence: a one‐year follow‐up. Arch Gerontol Geriatr. 2016;65:239–247. [DOI] [PubMed] [Google Scholar]
- 30. Artaud F, Singh‐Manoux A, Dugravot A, Tzourio C, Elbaz A. Decline in fast gait speed as a predictor of disability in older adults. J Am Geriatr Soc. 2015;63:1129–1136. [DOI] [PubMed] [Google Scholar]
- 31. Basic D, Ni Chroinin D, Conforti D, Shanley C. Predictors on admission of functional decline among older patients hospitalised for acute care: a prospective observational study. Australas J Ageing. 2017;36:E57–E63. [DOI] [PubMed] [Google Scholar]
- 32. Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res. 2004;12:1995–2004. [DOI] [PubMed] [Google Scholar]
- 33. Beauchamp MK, Jette AM, Ward RE, Kurlinski LA, Kiely D, Latham NK, et al. Predictive validity and responsiveness of patient‐reported and performance‐based measures of function in the Boston RISE study. J Gerontol A Biol Sci Med Sci. 2015;70:616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Beloosesky Y, Weiss A, Manasian M, Salai M. Handgrip strength of the elderly after hip fracture repair correlates with functional outcome. Disabil Rehabil. 2010;32:367–373. [DOI] [PubMed] [Google Scholar]
- 35. Bianchi L, Ferrucci L, Cherubini A, Maggio M, Bandinelli S, Savino E, et al. The predictive value of the EWGSOP definition of sarcopenia: results from the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2016;71:259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Broadwin J, Goodman‐Gruen D, Slymen D. Ability of fat and fat‐free mass percentages to predict functional disability in older men and women. J Am Geriatr Soc. 2001;49:1641–1645. [DOI] [PubMed] [Google Scholar]
- 37. Carriere I, Colvez A, Favier F, Jeandel C, Blain H, Epidos study g . Hierarchical components of physical frailty predicted incidence of dependency in a cohort of elderly women. J Clin Epidemiol. 2005;58:1180–1187. [DOI] [PubMed] [Google Scholar]
- 38. Cesari M, Rolland Y, Abellan Van Kan G, Bandinelli S, Vellas B, Ferrucci L. Sarcopenia‐related parameters and incident disability in older persons: results from the “invecchiare in Chianti” study. J Gerontol A Biol Sci Med Sci. 2015;70:457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chan OYA, van Houwelingen AH, Gussekloo J, Blom JW, den Elzen WPJ. Comparison of quadriceps strength and handgrip strength in their association with health outcomes in older adults in primary care. Age (Dordr). 2014;36:9714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chaudhry SI, McAvay G, Ning Y, Allore HG, Newman AB, Gill TM. Geriatric impairments and disability: the cardiovascular health study. J Am Geriatr Soc. 2010;58:1686–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chu L‐W, Chiu AYY, Chi I. Impact of falls on the balance, gait, and activities of daily living functioning in community‐dwelling Chinese older adults. J Gerontol A Biol Sci Med Sci. 2006;61:399–404. [DOI] [PubMed] [Google Scholar]
- 42. Cooper R, Huisman M, Kuh D, Deeg DJH. Do positive psychological characteristics modify the associations of physical performance with functional decline and institutionalization? Findings from the longitudinal aging study Amsterdam. J Gerontol B Psychol Sci Soc Sci. 2011;66:468–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Corsonello A, Lattanzio F, Pedone C, Garasto S, Laino I, Bustacchini S, et al. Prognostic significance of the short physical performance battery in older patients discharged from acute care hospitals. Rejuvenation Res. 2012;15:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Costanzo L, Pedone C, Cesari M, Ferrucci L, Bandinelli S, Antonelli Incalzi R. Clusters of functional domains to identify older persons at risk of disability. Geriatr Gerontol Int. 2018;18:685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. den Ouden MEM, Schuurmans MJ, Brand JS, Arts IEMA, Mueller‐Schotte S, van der Schouw YT. Physical functioning is related to both an impaired physical ability and ADL disability: a ten year follow‐up study in middle‐aged and older persons. Maturitas. 2013;74:89–94. [DOI] [PubMed] [Google Scholar]
- 46. Denkinger MD, Igl W, Jamour M, Bader A, Bailer S, Lukas A, et al. Does functional change predict the course of improvement in geriatric inpatient rehabilitation? Clin Rehabil. 2010;24:463–470. [DOI] [PubMed] [Google Scholar]
- 47. Di Monaco M, Castiglioni C, De Toma E, Gardin L, Giordano S, Tappero R. Handgrip strength is an independent predictor of functional outcome in hip‐fracture women: a prospective study with 6‐month follow‐up. Medicine (Baltimore). 2015;94:e542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Donoghue OA, Savva GM, Cronin H, Kenny RA, Horgan NF. Using timed up and go and usual gait speed to predict incident disability in daily activities among community‐dwelling adults aged 65 and older. Arch Phys Med Rehabil. 2014;95:1954–1961. [DOI] [PubMed] [Google Scholar]
- 49. Duchowny KA, Clarke PJ, Peterson MD. Muscle weakness and physical disability in older Americans: longitudinal findings from the U.S. Health and Retirement Study. J Nutr Health Aging. 2018;22:501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fantin F, Francesco VD, Fontana G, Zivelonghi A, Bissoli L, Zoico E, et al. Longitudinal body composition changes in old men and women: interrelationships with worsening disability. J Gerontol A Biol Sci Med Sci. 2007;62:1375–1381. [DOI] [PubMed] [Google Scholar]
- 51. Femia EE, Zarit SH, Johansson B. Predicting change in activities of daily living: a longitudinal study of the oldest old in Sweden. J Gerontol B Psychol Sci Soc Sci. 1997;52:P294–P302. [DOI] [PubMed] [Google Scholar]
- 52. Fujiwara Y, Shinkai S, Kobayashi E, Minami U, Suzuki H, Yoshida H, et al. Engagement in paid work as a protective predictor of basic activities of daily living disability in Japanese urban and rural community‐dwelling elderly residents: an 8‐year prospective study. Geriatr Gerontol Int. 2016;16:126–134. [DOI] [PubMed] [Google Scholar]
- 53. Giampaoli S, Ferrucci L, Cecchi F, Noce CL, Poce A, Dima F, et al. Hand‐grip strength predicts incident disability in non‐disabled older men. Age Ageing. 1999;28:283–288. [DOI] [PubMed] [Google Scholar]
- 54. Gill TM, Williams CS, Richardson ED, Tinetti ME. Impairments in physical performance and cognitive status in predisposing factors for functional dependence among nondisabled older persons. J Gerontol A Biol Sci Med Sci. 1996;51A:M283–M288. [DOI] [PubMed] [Google Scholar]
- 55. Gill TM, Murphy TE, Barry LC, Allore HG. Risk factors for disability subtypes in older persons. J Am Geriatr Soc. 2009;57:1850–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hansen K, Mahoney J, Palta M. Risk factors for lack of recovery of ADL independence after hospital discharge. J Am Geriatr Soc. 1999;47:360–365. [DOI] [PubMed] [Google Scholar]
- 58. Heiland EG, Welmer A‐K, Wang R, Santoni G, Angleman S, Fratiglioni L, et al. Association of mobility limitations with incident disability among older adults: a population‐based study. Age Ageing. 2016;45:812–819. [DOI] [PubMed] [Google Scholar]
- 59. Hirani V, Blyth F, Naganathan V, Le Couteur DG, Seibel MJ, Waite LM, et al. Sarcopenia is associated with incident disability, institutionalization, and mortality in community‐dwelling older men: the Concord Health and Ageing in Men Project. J Am Med Dir Assoc. 2015;16:607–613. [DOI] [PubMed] [Google Scholar]
- 60. Hirani V, Naganathan V, Blyth F, Le Couteur DG, Seibel MJ, Waite LM, et al. Longitudinal associations between body composition, sarcopenic obesity and outcomes of frailty, disability, institutionalisation and mortality in community‐dwelling older men: the Concord Health and Ageing in Men Project. Age Ageing. 2017;46:413–420. [DOI] [PubMed] [Google Scholar]
- 61. Hoeymans N, Feskens EJ, van den Bos GA, Kromhout D. Measuring functional status: cross‐sectional and longitudinal associations between performance and self‐report (Zutphen Elderly Study 1990‐1993). J Clin Epidemiol. 1996;49:1103–1110. [DOI] [PubMed] [Google Scholar]
- 62. Hong S, Kim S, Yoo J, Kim BS, Choi HR, Choi SE, et al. Slower gait speed predicts decline in instrumental activities of daily living in community‐dwelling elderly: 3‐year prospective finding from Living Profiles of Older People Survey in Korea. Journal of clinical gerontology & geriatrics. 2016;7:141–145. [Google Scholar]
- 63. Idland G, Pettersen R, Avlund K, Bergland A. Physical performance as long‐term predictor of onset of activities of daily living (ADL) disability: a 9‐year longitudinal study among community‐dwelling older women. Arch Gerontol Geriatr. 2013;56:501–506. [DOI] [PubMed] [Google Scholar]
- 64. Ishizaki T, Watanabe S, Suzuki T, Shibata H, Haga H. Predictors for functional decline among nondisabled older Japanese living in a community during a 3‐year follow‐up. J Am Geriatr Soc. 2000;48:1424–1429. [DOI] [PubMed] [Google Scholar]
- 65. Janssen I. Influence of sarcopenia on the development of physical disability: the Cardiovascular Health Study. J Am Geriatr Soc. 2006;54:56–62. [DOI] [PubMed] [Google Scholar]
- 66. Kempen GI, Ormel J. The impact of physical performance and cognitive status on subsequent ADL disability in low‐functioning older adults. Int J Geriatr Psychiatry. 1998;13:480–483. [DOI] [PubMed] [Google Scholar]
- 67. Kozicka I, Kostka T. Handgrip strength, quadriceps muscle power, and optimal shortening velocity roles in maintaining functional abilities in older adults living in a long‐term care home: a 1‐year follow‐up study. Clin Interv Aging. 2016;11:739–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kwon S, Symons R, Yukawa M, Dasher N, Legner V, Flum DR. Evaluating the association of preoperative functional status and postoperative functional decline in older patients undergoing major surgery. Am Surg. 2012;78:1336–1344. [PMC free article] [PubMed] [Google Scholar]
- 69. Legrand D, Vaes B, Mathei C, Adriaensen W, Van Pottelbergh G, Degryse J‐M. Muscle strength and physical performance as predictors of mortality, hospitalization, and disability in the oldest old. J Am Geriatr Soc. 2014;62:1030–1038. [DOI] [PubMed] [Google Scholar]
- 70. Lopez‐Teros T, Gutierrez‐Robledo LM, Perez‐Zepeda MU. Gait speed and handgrip strength as predictors of incident disability in Mexican older adults. J Frailty Aging. 2014;3:109–112. [DOI] [PubMed] [Google Scholar]
- 71. McGrath R, Robinson‐Lane SG, Peterson MD, Bailey RR, Vincent BM. Muscle strength and functional limitations: preserving function in older Mexican Americans. J Am Med Dir Assoc. 2018;19:391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Minneci C, Mello AM, Mossello E, Baldasseroni S, Macchi L, Cipolletti S, et al. Comparative study of four physical performance measures as predictors of death, incident disability, and falls in unselected older persons: the insufficienza Cardiaca negli Anziani Residenti a Dicomano Study. J Am Geriatr Soc. 2015;63:136–141. [DOI] [PubMed] [Google Scholar]
- 73. Moen K, Ormstad H, Wang‐Hansen MS, Brovold T. Physical function of elderly patients with multimorbidity upon acute hospital admission versus 3 weeks post‐discharge. Disabil Rehabil. 2018;40:1280–1287. [DOI] [PubMed] [Google Scholar]
- 74. Onder G, Penninx BWJH, Ferrucci L, Fried LP, Guralnik JM, Pahor M. Measures of physical performance and risk for progressive and catastrophic disability: results from the Women's Health and Aging Study. J Gerontol A Biol Sci Med Sci. 2005;60:74–79. [DOI] [PubMed] [Google Scholar]
- 75. Ostir GV, Markides KS, Black SA, Goodwin JS. Lower body functioning as a predictor of subsequent disability among older Mexican Americans. J Gerontol A Biol Sci Med Sci. 1998;53:M491–M495. [DOI] [PubMed] [Google Scholar]
- 76. Peel NM, Navanathan S, Hubbard RE. Gait speed as a predictor of outcomes in post‐acute transitional care for older people. Geriatr Gerontol Int. 2014;14:906–910. [DOI] [PubMed] [Google Scholar]
- 77. Pisters MF, Veenhof C, van Dijk GM, Heymans MW, Twisk JWR, Dekker J. The course of limitations in activities over 5 years in patients with knee and hip osteoarthritis with moderate functional limitations: risk factors for future functional decline. Osteoarthritis Cartilage. 2012;20:503–510. [DOI] [PubMed] [Google Scholar]
- 78. Purser JL, Weinberger M, Cohen HJ, Pieper CF, Morey MC, Li T, et al. Walking speed predicts health status and hospital costs for frail elderly male veterans. J Rehabil Res Dev. 2005;42:535–545. [DOI] [PubMed] [Google Scholar]
- 79. Rajan KB, Hebert LE, Scherr P, Dong X, Wilson RS, Evans DA, et al. Cognitive and physical functions as determinants of delayed age at onset and progression of disability. J Gerontol A Biol Sci Med Sci. 2012;67:1419–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rantanen T, Guralnik JM, Foley D, Masaki K, Leveille S, Curb JD, et al. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281:558–560. [DOI] [PubMed] [Google Scholar]
- 81. Rantanen T, Avlund K, Suominen H, Schroll M, Frandin K, Pertti E. Muscle strength as a predictor of onset of ADL dependence in people aged 75 years. Aging Clin Exp Res. 2002;14:10–15. [PubMed] [Google Scholar]
- 82. Rodriguez‐Pascual C, Paredes‐Galan E, Ferrero‐Martinez A‐I, Gonzalez‐Guerrero J‐L, Hornillos‐Calvo M, Menendez‐Colino R, et al. The frailty syndrome is associated with adverse health outcomes in very old patients with stable heart failure: a prospective study in six Spanish hospitals. Int J Cardiol. 2017;236:296–303. [DOI] [PubMed] [Google Scholar]
- 83. Rothman MD, Leo‐Summers L, Gill TM. Prognostic significance of potential frailty criteria. J Am Geriatr Soc. 2008;56:2211–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sakamoto R, Okumiya K, Ishine M, Wada T, Fujisawa M, Imai H, et al. Predictors of difficulty in carrying out basic activities of daily living among the old‐old: a 2‐year community‐based cohort study. Geriatr Gerontol Int. 2016;16:214–222. [DOI] [PubMed] [Google Scholar]
- 85. Sanchez‐Martinez M, Castell MV, Gonzalez‐Montalvo JI, De La Cruz JJ, Banegas JR, Otero A. Transitions in functional status of community dwelling older adults: impact of physical performance. depression and cognition. Eur Geriatr Med. 2016;7:111–116. [Google Scholar]
- 86. Sanchez‐Rodriguez D, Marco E, Miralles R, Fayos M, Mojal S, Alvarado M, et al. Sarcopenia, physical rehabilitation and functional outcomes of patients in a subacute geriatric care unit. Arch Gerontol Geriatr. 2014;59:39–43. [DOI] [PubMed] [Google Scholar]
- 87. Sarkisian CA, Liu H, Gutierrez PR, Seeley DG, Cummings SR, Mangione CM. Modifiable risk factors predict functional decline among older women: a prospectively validated clinical prediction tool. The Study of Osteoporotic Fractures Research Group. J Am Geriatr Soc. 2000;48:170–178. [DOI] [PubMed] [Google Scholar]
- 88. Sarkisian CA, Liu H, Ensrud KE, Stone KL, Mangione CM. Correlates of attributing new disability to old age. Study of Osteoporotic Fractures Research Group. J Am Geriatr Soc. 2001;49:134–141. [DOI] [PubMed] [Google Scholar]
- 89. Schoenenberger AW, Stortecky S, Neumann S, Moser A, Juni P, Carrel T, et al. Predictors of functional decline in elderly patients undergoing transcatheter aortic valve implantation (TAVI). Eur Heart J. 2013;34:684–692. [DOI] [PubMed] [Google Scholar]
- 90. Seidel D, Brayne C, Jagger C. Limitations in physical functioning among older people as a predictor of subsequent disability in instrumental activities of daily living. Age Ageing. 2011;40:463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Shimada H, Sawyer P, Harada K, Kaneya S, Nihei K, Asakawa Y, et al. Predictive validity of the classification schema for functional mobility tests in instrumental activities of daily living decline among older adults. Arch Phys Med Rehabil. 2010;91:241–246. [DOI] [PubMed] [Google Scholar]
- 92. Shimada H, Makizako H, Doi T, Tsutsumimoto K, Suzuki T. Incidence of disability in frail older persons with or without slow walking speed. J Am Med Dir Assoc. 2015;16:690–696. [DOI] [PubMed] [Google Scholar]
- 93. Shinkai S, Watanabe S, Kumagai S, Fujiwara Y, Amano H, Yoshida H, et al. Walking speed as a good predictor for the onset of functional dependence in a Japanese rural community population. Age Ageing. 2000;29:441–446. [DOI] [PubMed] [Google Scholar]
- 94. Shinkai S, Kumagai S, Fujiwara Y, Amano H, Yoshida Y, Watanabe S, et al. Predictors for the onset of functional decline among initially non‐disabled older people living in a community during a 6‐year follow‐up. Geriatr Gerontol Int. 2003;3:S31–S39. [Google Scholar]
- 95. Sourdet S, van Kan GA, Soto ME, Houles M, Cantet C, Nourhashemi F, et al. Prognosis of an abnormal one‐leg balance in community‐dwelling patients with Alzheimer's disease: a 2‐year prospective study in 686 patients of the REAL.FR study. J Am Med Dir Assoc. 2012;13: 407.e1‐6. [DOI] [PubMed] [Google Scholar]
- 96. Stenholm S, Guralnik JM, Bandinelli S, Ferrucci L. The prognostic value of repeated measures of lower extremity performance: should we measure more than once? J Gerontol A Biol Sci Med Sci. 2014;69:894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Takuhiro O, Yasuyo A, Yoshihito T, Satoshi M, Mitsuo K, Kazuhiko A, et al. Age‐specific risk factors for incident disability in activities of daily living among middle‐aged and elderly community‐dwelling Japanese women during an 8–9‐year follow up: The Hizen‐Oshima study. Geriatr Gerontol Int. 2017;17:1096–1101. [DOI] [PubMed] [Google Scholar]
- 98. Tanimoto Y, Watanabe M, Sun W, Tanimoto K, Shishikura K, Sugiura Y, et al. Association of sarcopenia with functional decline in community‐dwelling elderly subjects in Japan. Geriatr Gerontol Int. 2013;13:958–963. [DOI] [PubMed] [Google Scholar]
- 99. Terhorst L, Holm MB, Toto PE, Rogers JC. Performance‐based impairment measures as predictors of early‐stage activity limitations in community‐dwelling older adults. J Aging Health. 2017;29:880–892. [DOI] [PubMed] [Google Scholar]
- 100. Tinetti ME, Allore H, Araujo KL, Seeman T. Modifiable impairments predict progressive disability among older persons. J Aging Health. 2005;17:239–256. [DOI] [PubMed] [Google Scholar]
- 101. Volpato S, Cavalieri M, Sioulis F, Guerra G, Maraldi C, Zuliani G, et al. Predictive value of the short physical performance battery following hospitalization in older patients. J Gerontol A Biol Sci Med Sci. 2011;66:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Wennie Huang W‐N, Perera S, VanSwearingen J, Studenski S. Performance measures predict onset of activity of daily living difficulty in community‐dwelling older adults. J Am Geriatr Soc. 2010;58:844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zhang F, Ferrucci L, Culham E, Metter EJ, Guralnik J, Deshpande N. Performance on five times sit‐to‐stand task as a predictor of subsequent falls and disability in older persons. J Aging Health. 2013;25:478–492. [DOI] [PubMed] [Google Scholar]
- 104. Zoico E, Di Francesco V, Mazzali G, Zivelonghi A, Volpato S, Bortolani A, et al. High baseline values of fat mass, independently of appendicular skeletal mass, predict 2‐year onset of disability in elderly subjects at the high end of the functional spectrum. Aging Clin Exp Res. 2007;19:154–159. [DOI] [PubMed] [Google Scholar]
- 105. Raji MA, Kuo Y‐F, Snih SA, Markides KS, Peek MK, Ottenbacher KJ. Cognitive status, muscle strength, and subsequent disability in older Mexican Americans. J Am Geriatr Soc. 2005;53:1462–1468. [DOI] [PubMed] [Google Scholar]
- 106. Evans WJ. What is sarcopenia? The Journals of Gerontology: Series A. 1995;50A:5–8. [DOI] [PubMed] [Google Scholar]
- 107. Brown WF. A method for estimating the number of motor units in thenar muscles and the changes in motor unit count with ageing. Journal of Neurology, Neurosurgery &amp; Psychiatry. 1972;35:845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Young VR. Amino acids and proteins in relation to the nutrition of elderly people. Age and ageing. 1990;19:S10–S24. [DOI] [PubMed] [Google Scholar]
- 109. Martin‐Cantero A RE, Gill BMT, Maier AB. Factors influencing the efficacy of nutritional interventions on muscle mass in older adults: a systematic review and meta‐analysis. [under review]. [DOI] [PMC free article] [PubMed]
- 110. Westerterp KR. Daily physical activity and ageing. Curr Opin Clin Nutr Metab Care. 2000;3:485–488. [DOI] [PubMed] [Google Scholar]
- 111. Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well‐functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333. [DOI] [PubMed] [Google Scholar]
- 112. Meskers C, Reijnierse E, Numans S, Kruizinga R, Pierik V, van Ancum J, et al. Association of handgrip strength and muscle mass with dependency in (instrumental) activities of daily living in hospitalized older adults—the EMPOWER study. J Nutr Health Aging. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Fiatarone MA, O'Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769–1775. [DOI] [PubMed] [Google Scholar]
- 114. Brown M, Holloszy JO. Effects of walking, jogging and cycling on strength, flexibility, speed and balance in 60‐ to 72‐year olds. Aging (Milano). 1993;5:427–434. [DOI] [PubMed] [Google Scholar]
- 115. Sipilä S, Multanen J, Kallinen M, Era P, Suominen H. Effects of strength and endurance training on isometric muscle strength and walking speed in elderly women. Acta Physiol Scand Suppl. 1996;156:457–464. [DOI] [PubMed] [Google Scholar]
- 116. Frontera WR, Meredith CN, O'Reilly KP, Knuttgen HG, Evans WJ. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol (1985). 1988;64:1038–1044. [DOI] [PubMed] [Google Scholar]
- 117. Kamleh AA RE, Aarden JJ, Daly RM, Andrea BM. The optimal resistance exercise training program for older adults to increase muscle mass: a systematic review and meta‐analysis. [under review].
- 118. Fried LP, Ettinger WH, Lind B, Newman AB, Gardin J. Physical disability in older adults: a physiological approach. Cardiovascular Health Study Research Group. J Clin Epidemiol. 1994;47:747–760. [DOI] [PubMed] [Google Scholar]
- 119. Yeung SS, Reijnierse EM, Trappenburg MC, Hogrel J‐Y, McPhee JS, Piasecki M, et al. Handgrip strength cannot be assumed a proxy for overall muscle strength. J Am Med Dir Assoc. 2018. [DOI] [PubMed] [Google Scholar]
- 120. Reed RL, Pearlmutter L, Yochum K, Meredith KE, Mooradian AD. The relationship between muscle mass and muscle strength in the elderly. J Am Geriatr Soc. 1991;39:555–561. [DOI] [PubMed] [Google Scholar]
- 121. Frontera WR, Hughes VA, Lutz KJ, Evans WJ. A cross‐sectional study of muscle strength and mass in 45‐ to 78‐yr‐old men and women. J Appl Physiol (1985). 1991;71:644–650. [DOI] [PubMed] [Google Scholar]
- 122. Freilich RJ, Kirsner RL, Byrne E. Isometric strength and thickness relationships in human quadriceps muscle. Neuromuscul Disord. 1995;5:415–422. [DOI] [PubMed] [Google Scholar]
- 123. Larsson L, Grimby G, Karlsson J. Muscle strength and speed of movement in relation to age and muscle morphology. J Appl Physiol Respir Environ Exerc Physiol. 1979;46:451–456. [DOI] [PubMed] [Google Scholar]
- 124. Buchner DM, Larson EB, Wagner EH, Koepsell TD, De Lateur BJ. Evidence for a non‐linear relationship between leg strength and gait speed. Age Ageing. 1996;25:386–391. [DOI] [PubMed] [Google Scholar]
- 125. Beenakker KG, Ling CH, Meskers CG, de Craen AJ, Stijnen T, Westendorp RG, et al. Patterns of muscle strength loss with age in the general population and patients with a chronic inflammatory state. Ageing Res Rev. 2010;9:431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Koyano W, Shibata H, Nakazato K, Haga H, Suyama Y, Matsuzaki T. Prevalence of disability in instrumental activities of daily living among elderly Japanese. J Gerontol A Biol Sci Med Sci. 1988;43:S41–S45. [DOI] [PubMed] [Google Scholar]
- 127. Covinsky KE, Palmer RM, Fortinsky RH, Counsell SR, Stewart AL, Kresevic D, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003;51:451–458. [DOI] [PubMed] [Google Scholar]
- 128. Kallman DA, Plato CC, Tobin JD. The role of muscle loss in the age‐related decline of grip strength: cross‐sectional and longitudinal perspectives. J Gerontol. 1990;45:M82–M88. [DOI] [PubMed] [Google Scholar]
- 129. Rantanen T, Masaki K, Foley D, Izmirlian G, White L, Guralnik JM. Grip strength changes over 27 yr in Japanese‐American men. J Appl Physiol (1985). 1998;85:2047–2053. [DOI] [PubMed] [Google Scholar]
- 130. Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health. aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. [DOI] [PubMed] [Google Scholar]
- 131. Barbat‐Artigas S, Rolland Y, Zamboni M, Aubertin‐Leheudre M. How to assess functional status: a new muscle quality index. J Nutr Health Aging. 2012;16:67–77. [DOI] [PubMed] [Google Scholar]
- 132. Shin S, Valentine RJ, Evans EM, Sosnoff JJ. Lower extremity muscle quality and gait variability in older adults. Age Ageing. 2012;41:595–599. [DOI] [PubMed] [Google Scholar]
- 133. Cawthon PM, Fox KM, Gandra SR, Delmonico MJ, Chiou C‐F, Anthony MS, et al. Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J Am Geriatr Soc. 2009;57:1411–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Metter EJ, Lynch N, Conwit R, Lindle R, Tobin J, Hurley B. Muscle quality and age: cross‐sectional and longitudinal comparisons. J Gerontol A Biol Sci Med Sci. 1999;54:B207–B218. [DOI] [PubMed] [Google Scholar]
- 135. Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12‐yr longitudinal study. J Appl Physiol (1985). 2000;88:1321–1326. [DOI] [PubMed] [Google Scholar]
- 136. Trappe S, Gallagher P, Harber M, Carrithers J, Fluckey J, Trappe T. Single muscle fibre contractile properties in young and old men and women. The Journal of physiology. 2003;552:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Wolfson L, Wei X, Hall CB, Panzer V, Wakefield D, Benson RR, et al. Accrual of MRI white matter abnormalities in elderly with normal and impaired mobility. J Neurol Sci. 2005;232:23–27. [DOI] [PubMed] [Google Scholar]
- 138. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower‐extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Wright AA, Cook CE, Baxter GD, Garcia J, Abbott JH. Relationship between the Western Ontario and McMaster Universities Osteoarthritis Index Physical Function Subscale and physical performance measures in patients with hip osteoarthritis. Arch Phys Med Rehabil. 2010;91:1558–1564. [DOI] [PubMed] [Google Scholar]
- 140. Evans WJ, Paolisso G, Abbatecola AM, Corsonello A, Bustacchini S, Strollo F, et al. Frailty and muscle metabolism dysregulation in the elderly. Biogerontology. 2010;11:527–536. [DOI] [PubMed] [Google Scholar]
- 141. Inzitari M, Pozzi C, Ferrucci L, Chiarantini D, Rinaldi LA, Baccini M, et al. Subtle neurological abnormalities as risk factors for cognitive and functional decline, cerebrovascular events, and mortality in older community‐dwelling adults. Arch Intern Med. 2008;168:1270–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Corsonello A, Lattanzio F, Pedone C, Garasto S, Laino I, Bustacchini S, et al. Prognostic significance of the short physical performance battery in older patients discharged from acute care hospitals. Rejuvenation research. 2012;15:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Gawel J, Vengrow D, Collins J, Brown S, Buchanan A, Cook C. The short physical performance battery as a predictor for long term disability or institutionalization in the community dwelling population aged 65 years old or older. Physical Therapy Reviews. 2012;17:37–44. [Google Scholar]
- 144. Taekema DG, Gussekloo J, Westendorp RG, De Craen AJ, Maier AB. Predicting survival in oldest old people. Am J Med. 2012;125:1188–94. e1. [DOI] [PubMed] [Google Scholar]
- 145. Cesari M, Kritchevsky SB, Penninx BW, Nicklas BJ, Simonsick EM, Newman AB, et al. Prognostic value of usual gait speed in well‐functioning older people—results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53:1675–1680. [DOI] [PubMed] [Google Scholar]
- 146. Woolley D, Studenski S, Perera S, Rogers N. Feasibility and reproducibility of walking speed as a geriatric vitalsign incommunity practice. J Am Geriatr Soc. 2004;52:S195. [Google Scholar]
- 147. Bahat G, Tufan A, Kilic C, Karan MA, Cruz‐Jentoft AJ. Prevalence of sarcopenia and its components in community‐dwelling outpatient older adults and their relation with functionality. Aging Male. 2018;1–7. [DOI] [PubMed] [Google Scholar]
- 148. Perera S, Patel KV, Rosano C, Rubin SM, Satterfield S, Harris T, et al. Gait speed predicts incident disability: a pooled analysis. J Gerontol A Biol Sci Med Sci. 2016;71:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Perera S, Studenski S, Newman A, Simonsick E, Harris T, Schwartz A, et al. Are estimates of meaningful decline in mobility performance consistent among clinically important subgroups? (Health ABC Study). J Gerontol A Biol Sci Med Sci. 2014;69:1260–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Cummings SR, Studenski S, Ferrucci L. A diagnosis of dismobility—giving mobility clinical visibility: a Mobility Working Group recommendation. JAMA. 2014;311:2061–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Clark DO, Stump TE, Hui SL, Wolinsky FD. Predictors of mobility and basic ADL difficulty among adults aged 70 years and older. J Aging Health. 1998;10:422–440. [DOI] [PubMed] [Google Scholar]
- 152. Manchester D, Woollacott M, Zederbauer‐Hylton N, Marin O. Visual, vestibular and somatosensory contributions to balance control in the older adult. J Gerontol. 1989;44:M118–M127. [DOI] [PubMed] [Google Scholar]
- 153. Pasma J, Engelhart D, Schouten A, Van der Kooij H, Maier A, Meskers C. Impaired standing balance: the clinical need for closing the loop. Neuroscience. 2014;267:157–165. [DOI] [PubMed] [Google Scholar]
- 154. Vellas BJ, Wayne SJ, Romero L, Baumgartner RN, Rubenstein LZ, Garry PJ. One‐leg balance is an important predictor of injurious falls in older persons. J Am Geriatr Soc. 1997;45:735–738. [DOI] [PubMed] [Google Scholar]
- 155. Gill TM, Williams CS, Tinetti ME. Assessing risk for the onset of functional dependence among older adults: the role of physical performance. J Am Geriatr Soc. 1995;43:603–609. [DOI] [PubMed] [Google Scholar]
- 156. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. [DOI] [PubMed] [Google Scholar]
- 157. Bohannon RW. Reference values for the timed up and go test: a descriptive meta‐analysis. J Geriatr Phys Ther. 2006;29:64–68. [DOI] [PubMed] [Google Scholar]
- 158. Lin MR, Hwang HF, Hu MH, Wu HDI, Wang YW, Huang FC. Psychometric comparisons of the timed up and go, one‐leg stand, functional reach, and Tinetti balance measures in community‐dwelling older people. Journal of the American Geriatrics Society. 2004;52:1343–1348. [DOI] [PubMed] [Google Scholar]
- 159. Jones CJ, Rikli RE, Beam WC. A 30‐s chair‐stand test as a measure of lower body strength in community‐residing older adults. Res Q Exerc Sport. 1999;70:113–119. [DOI] [PubMed] [Google Scholar]
- 160. Carriere I, Colvez A, Favier F, Jeandel C, Blain H, group Es . Hierarchical components of physical frailty predicted incidence of dependency in a cohort of elderly women. J Clin Epidemiol. 2005;58:1180–1187. [DOI] [PubMed] [Google Scholar]
- 161. von Haehling S, Morley JE, Coats AJ, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017. J Cachexia Sarcopenia Muscle. 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Search Strategy
Data S2. Newcastle–Ottawa Scale
Table S3. Breakdown of the ADL components in articles that created their own questionnaire


