Abstract
Introduction:
BRAF V600E and V600K melanomas have distinct clinicopathologic features, and V600K appear to be less responsive to BRAFi+/−MEKi. We investigated mechanisms for this and explored whether genotype affects response to immunotherapy.
Methods:
Pre-treatment formalin-fixed paraffin-embedded (FFPE) tumors from patients treated with BRAFi+/−MEKi underwent gene expression profiling and DNA sequencing. Molecular results were validated using TCGA (The Cancer Genome Atlas) data. An independent cohort of V600E/K patients treated with anti-PD-1 immunotherapy was examined.
Results:
Baseline tissue and clinical outcome with BRAFi+/−MEKi were studied in 93 patients (78 V600E, 15 V600K). V600K patients had numerically less tumour regression (median −31% vs −52%, p=0.154) and shorter progression-free survival (PFS, median 5.7 vs 7.1 months, p=0.15) compared with V600E. V600K melanomas had lower expression of the ERK pathway feedback regulator DUSP6, confirmed with TCGA data (116 V600E, 17 V600K). Pathway analysis showed V600K had lower expression of ERK and higher expression of PI3K-AKT genes than V600E. Higher mutational load was observed in V600K, with a higher proportion of mutations in PIK3R1 and tumour suppressor genes. In patients treated with anti-PD-1, V600K (n=19) had superior outcomes than V600E (n=84), including response rate (53% vs 29%, p=0.059), PFS (median 19 vs 2.7 months, p=0.049) and overall survival (20.4 vs 11.7 months, p=0.081).
Conclusions:
BRAF V600K melanomas appear to benefit less from BRAFi+/−MEKi than V600E, potentially due to less reliance on ERK pathway activation and greater use of alternative pathways. In contrast, these melanomas have higher mutational load and respond better to immunotherapy.
Keywords: Melanoma, BRAF, V600K, V600E, targeted therapy, immunotherapy, gene expression, mutational load
Introduction
The identification of BRAF driver mutations in melanoma has led to the development of specific inhibitors targeting the extracellular-signal-regulated kinase (ERK) pathway, which have high response rates and improve survival in patients with BRAF-mutant advanced melanoma and those at high risk of recurrence1,2,3,4. BRAF mutations are present in approximately 40% of cutaneous melanomas and the majority occur at codon 600 (90%)5. Among these, 70–80% are V600E (codon GTG>GAG), 20–30% are V600K (GTG>AAG), and rarer mutations including V600R (GTG>AGG), V600D (GTG>GAT), V600E2 (GTG>GAA), V600G (GTG>GGG), V600M (GTG>ATG), V600A (GTG>GCG) and V600L (GTG>TTG) 1,6,7.
Previous studies have shown that V600E and V600K BRAF-mutant metastatic melanoma have distinct clinicopathologic features, suggesting different etiology8,9. V600K melanomas occur more frequently in older people, in the head and neck region, and are associated with chronic sun damage8,9. Furthermore, even though individual phase III targeted therapy trials have not performed a direct comparison between V600E and V600K-mutant melanomas, in three separate trials V600K melanomas had numerically lower response rate and shorter median progression-free survival with BRAFi compared to V600E melanomas, and two pooled analyses of BRAFi+/−MEKi showed shorter progression-free survival in multivariate analysis10,11,12,13,14,15. As far as we are aware, there are no published data on the potential mechanisms responsible for these features, nor whether the BRAF-mutant genotype influences response to immunotherapy.
In this study, we sought to identify potential mechanisms for these apparent clinicopathologic differences, by comparing gene expression and mutational profiles of V600E and V600K melanomas from patients treated with BRAFi+/−MEKi, validated on the Skin Cutaneous Melanoma TCGA (The Cancer Genome Atlas) dataset. We also explored whether the specific BRAF-mutant genotype affects response to immunotherapy.
Methods
Patients and Outcome
Consecutive patients with V600E/K BRAF-mutant metastatic melanoma enrolled on clinical trials of BRAF+/−MEK inhibitors at two Melanoma Institute Australia treatment facilities (The Poche Centre, North Sydney and Westmead Hospital - ethical approval from the Sydney Local Health District Human Research Ethics Committee, Protocol Number X15–0454 and HREC/11/RPAH/444) between July/2009 and July/2013 were included (BRAFi+/−MEKi cohort)2,3,12,13,16,17,18. This study was conducted in accordance with Declaration of Helsinki, and written informed consented was obtained from all patients. Demographic and clinical data at the time of BRAFi+/−MEKi treatment commencement were recorded for each patient, including gender, age, mutation status, lactate dehydrogenase (LDH) level, Eastern Cooperative Oncology Group (ECOG) performance status, AJCC v.7 M-stage and treatment (BRAFi +/− MEKi). RECIST response, progression-free (PFS) and overall survival (OS) data were collected prospectively (data cut November 2016). An independent cohort of V600E/K BRAF-mutant metastatic melanoma patients treated with anti-PD-1 immunotherapy (pembrolizumab or nivolumab) were examined to explore response to anti-PD-1 immunotherapy by genotype (Immunotherapy cohort)19.
Tumor samples and molecular testing
Tumor genomic DNA and RNA were extracted from formalin-fixed, paraffin-embedded (FFPE) melanoma tissue pre-treatment samples from the 93 patients (BRAFi+/−MEKi cohort). Two samples were from primary melanomas, 25 were from lymph nodes, and the remaining 66 were from distant metastatic sites. Samples were macrodissected prior to nucleic acid extraction. DNA was extracted using QIAamp DNA FFPE Tissue Kit (Qiagen, #56404) and RNA was extracted using the High Pure RNA Micro Kit (Roche, #04823125001).
NanoString RNA gene expression analysis:
A custom-designed NanoString panel of 814 probes was used (Supplementary Table 1). Of these, 5 genes (HPRT1, GUSB, CLTC, PGK1, SDHA) were built-in as housekeeping genes, 8 probes were negative controls and 6 were positive controls. All samples had mutant BRAF detected with adequate quality. Expression data were imported and normalized by the NanoStringQCPro package, following the manufacturer’s recommendations20.
Next-generation DNA sequencing:
A pan-cancer 88-gene Next-generation sequencing (NGS) panel to detect DNA coding mutations was performed on all 93 samples as has been previously described21. Briefly, 963 amplicons targeting 88 genes were enriched for by Access Array (Fluidigm) and sequenced (2×108 bp). FASTQ reads were aligned to the human reference genome (NCBI Build 37) using GSNAP22,23. Duplicate reads in the resulting BAM file were marked using PicardTools, and indels realigned using the GATK IndelRealigner tool. The median sequencing depth was 2822x (range 4–3415x), and a minimum of 150x was required for analysis. Synonymous coding mutations (as defined by Ensembl) were discarded and mutational load was calculated as number of mutations per sample (Supplementary Table 2).
TCGA data:
The Cancer Genome Atlas (TCGA) skin cutaneous melanoma (SKCM)24 RNA sequencing (RNAseq) and whole exome sequencing (WES) datasets for BRAF V600E (N=116) and V600K (n=17) samples were downloaded on 6th of February 2017 using R package TCGAbiolinks25 from the GDC portal. All alignments were performed using the human reference genome GRCh38.d1.vd1. Aligned and co-cleaned BAM files were processed through the Somatic Mutation Calling Workflow as tumour-normal pairs. Variant calling was performed using the mutect226 pipeline. Synonymous variants were discarded and then mutational load was calculated as number of mutations per sample. Sample with more than 2000 mutations were filtered out and a Mann-Whitney test was performed between V600E and V600K samples. Raw RNA-Seq read count data were downloaded with the option HTSeq-count. Genes with less than 20 counts were filtered out.
Statistical methods
Gene expression analysis:
On both the NanoString platform and the TCGA data27, a differential expression analysis was performed between the V600E and V600K cohorts and a gene was defined as differentially expressed when its FDR-adjusted p-value was less than 0.05. On the NanoString platform, the differential analysis was performed using a moderated t-test implemented in the R28 package limma29 and on the TCGA data, the differential analysis was performed on raw counts using a negative binomial model and the associated Wald significant test implemented in the DESeq2 package30.
On both the TCGA and the NGS datasets, the Mann-Whitney U test was performed to compare the mutational load between V600E and V600K samples. Statistical significant was defined by p-value less than 0.05. Visualization of genes expression levels and subsequent pathway analysis used log-transformed FPKM value.
Gene set enrichment analysis:
Selected genesets were downloaded from Molecular Signatures Database (MSigDB) version 6.0. We created a ERK pathway gene set by using 12 genes common to the two expert derived Pratilas31 and Nazarian32 gene sets. A PI3K-AKT pathway gene set was compiled by using 112 genes common to the AKT_UP.V1_UP and AKT_UP_MTOR_DN.V1_UP genesets in MSigDB33,34. To determine the significance of a pathway, a Hotelling T2 test with a shrinkage estimator was used based on implementation in the Hotelling package35.
Survival analysis:
Kaplan-Meier plots and the log-rank test implemented in the survival package36 in R were used for survival analysis.
Results
Patient Characteristics (BRAFi+/−MEKi cohort)
Ninety-three consecutive patients with V600E (n=78) or V600K (n=15) BRAF-mutant metastatic melanoma treated with BRAFi+/−MEKi on clinical trials at two Melanoma Institute Australia treatment facilities (The Poche Centre, North Sydney and Westmead Hospital) were examined (Table 1). The cohort was typical for a metastatic melanoma clinical trial population and there were no statistical differences between genotypes (Table 1). Although not statistically different, likely due to small sample size for the V600K group, the median age was numerically higher in V600K compared to V600E melanoma patients (med 58 versus 56.5 years-old). Forty percent of patients in both groups had an ECOG (Eastern Cooperative Oncology Group) performance status ≥1, 80% had AJCC (American Joint Committee on Cancer) M1c disease and LDH (lactate dehydrogenase) was elevated in 27% (V600E) and 40% (V600K) of patients. Only 20% of the V600E patients were treated with combination BRAF+MEK inhibitors, while 33% of the V600K patients were treated with combination therapy.
Table 1:
Baseline characteristics of patients with V600E versus V600K metastatic melanoma (BRAFi+/−MEKi cohort)
| Characteristics | V600E (n=78) | V600K (n=15) | P-value |
|---|---|---|---|
| Age (years) | |||
| Median | 56.5 | 58.0 | 0.418 |
| Gender (N, %) | 0.5786 | ||
| Female | 33 (42%) | 5 (33%) | |
| Male | 45 (58%) | 10 (67%) | |
| AJCC stage1 (N, %) | 1 | ||
| IIIC-M1b | 19 (24%) | 3 (20%) | |
| M1c | 59 (76%) | 12 (80%) | |
| LDH (N, %) | 0.3563 | ||
| Normal | 57 (73%) | 9 (60%) | |
| Elevated | 21 (27%) | 6 (40%) | |
| ECOG (N, %) | 1 | ||
| 0 | 47 (60%) | 9 (60%) | |
| ≥1 | 31 (40%) | 6 (40%) | |
| Treatment (N, %) | 0.3162 | ||
| BRAFi | 62 (80%) | 10 (67%) | |
| BRAFi + MEKi | 16 (20%) | 5 (33%) |
AJCC v7 anatomic staging, excluding LDH and brain metastases
V600K melanomas have inferior response and progression-free survival with BRAFi+/−MEKi, compared with V600E melanomas
Few direct comparisons of response rates and survival between V600E and V600K melanomas have been performed previously, however phase III clinical trials and two pooled analyses suggest that V600K melanomas may have a lower response rate and shorter progression-free survival with BRAFi+/−MEKi compared to V600E melanomas, and yet have similar overall survival10,11,12,13,14,15. In this study which included a small number of V600K melanomas, despite the fact more patients with V600K melanoma were treated with combination BRAF+MEK inhibitors, patients with V600K melanoma had a numerically lower median degree of response to therapy (−31% vs −52%, p=0.15) and a lower rate of complete response (0% vs 10%) (Figure 1.A). While not statistically significant, V600K patients also had a median 5.7 months progression-free survival and no patient remained progression-free beyond 9 months, while median progression-free survival was 7.1 months in V600E patients and approximately 20% remained progression-free at 5 years (Figure 1.B). Despite these numerical differences in response rates and progression-free survival, overall survival was similar (Figure 1.C).
Figure 1.
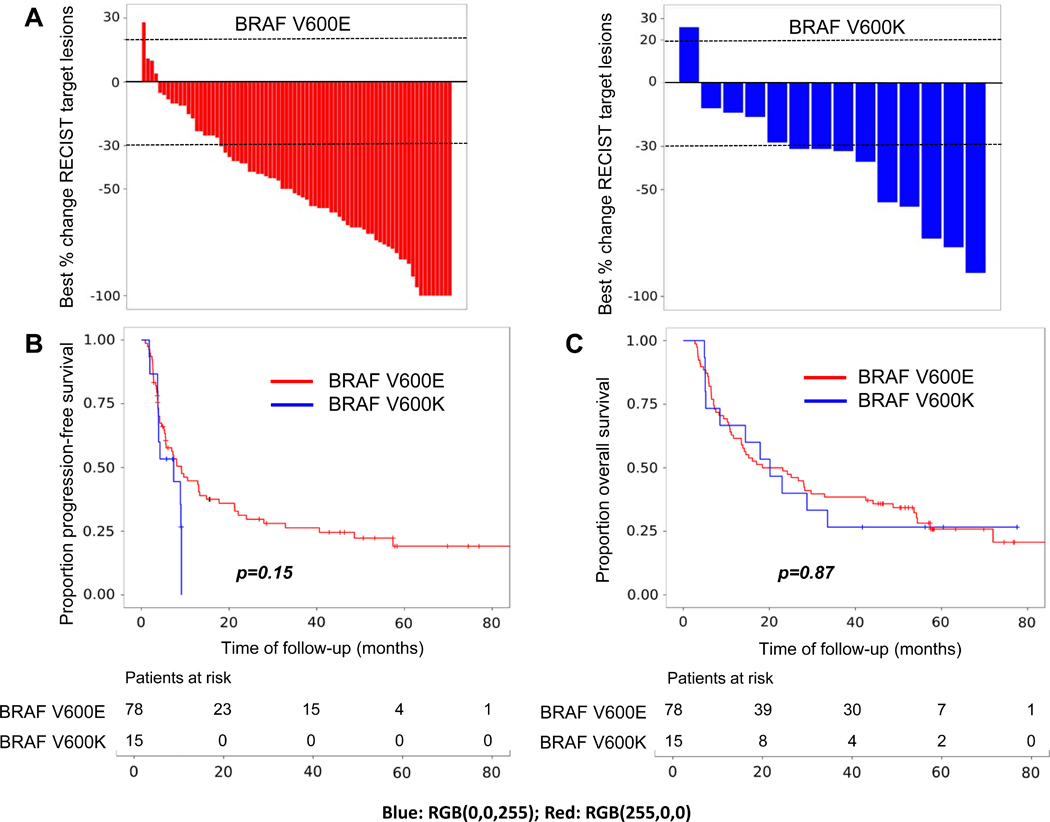
Clinical outcomes after treatment with BRAFi+/−MEKi in V600E (red) and V600K (blue) metastatic melanoma. A Best change in RECIST target lesions (%); B Progression-free survival; and C Overall survival.
V600K melanomas have less ERK but more PI3K pathway activation
To investigate potential mechanisms for these clinicopathologic differences, including a possible smaller benefit with BRAFi+/−MEKi in V600K melanomas, we sought to identify differences in gene expression between both groups. Nanostring data revealed that expression of DUSP6 (Dual Specificity Phosphatase 6), a transcriptional target of the ERK pathway involved in feedback regulation and reflective of ERK activation, was the most significant differentiated gene, with lower expression in V600K melanoma (p=0.024, Figure 2.A). This was confirmed using RNA sequencing data from the TCGA, including V600E (n=116) and V600K (n=17) tumors (n=133, p=0.003; Figure 2.B). Moreover, besides DUSP6, other genes related to transcriptional output of MEK/ERK pathway including ETV4, DUSP4 and SPRY2 were within the top 30 differentially expressed genes, with higher expression in V600E melanomas, while PIK3CB expression was higher in V600K melanomas (Supplementary table 1). We then performed pathway analyses assessing the expression of ERK and PI3K (an alternative survival pathway) pathway gene set signatures using the TCGA dataset. Interestingly, the expression of ERK pathway genes was lower in V600K melanomas (p=0.0003), while the expression of PI3K-AKT pathway genes, was significantly higher in V600K melanoma compared to V600E (p=0.005) (Supplementary Figure 1). These data support the hypothesis that V600K melanomas are less dependent on the ERK pathway and more dependent on alternative pathways, specifically the PI3K-AKT pathway.
Figure 2.
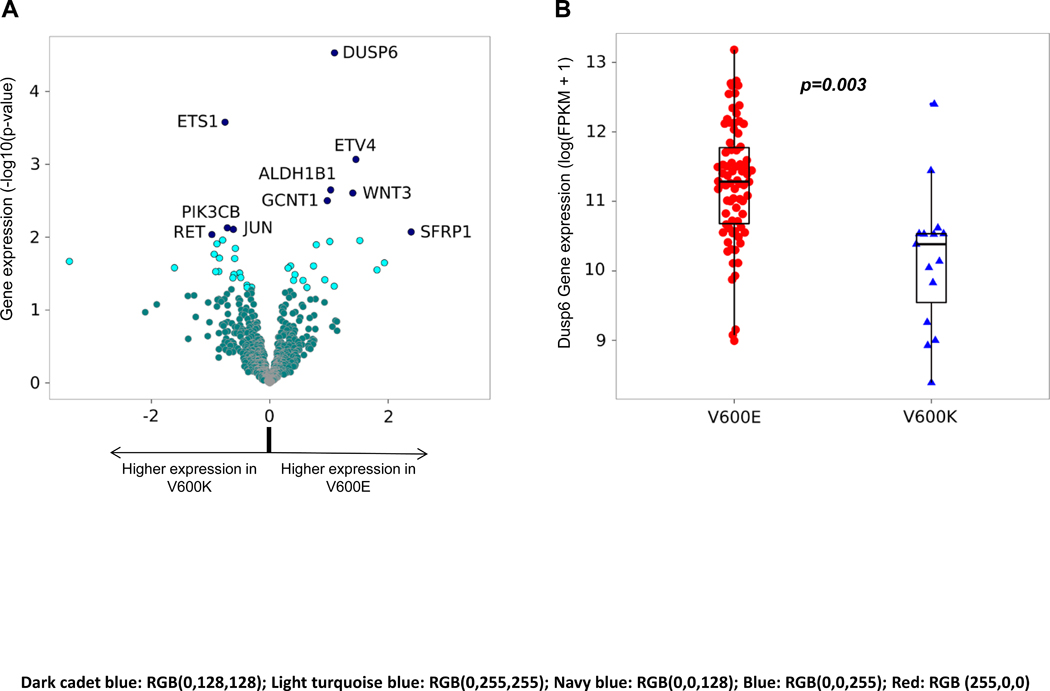
Differences in gene expression between V600E and V600K melanoma. A. Differentially expressed genes (Nanostring data from patient cohort). Unadjusted p-values: <=0.05 (light turquoise blue) and >0.05 (dark cadet blue). The top 10 differentially expressed genes are labeled (navy blue). B. Validation of increased DUSP6 expression in V600E melanomas (TCGA data). DESeq2 P−value: 1.329e−05. DESeq2 adj.P−value: 0.002843. Each dot/triangle represents one single sample.
V600K melanomas have a higher mutational load
In an attempt to explore whether mutational data may explain differences in both pathway expression and the clinical phenotype of V600E and V600K melanoma, targeted NGS data of genomic DNA were examined. As expected given the clinical phenotype, V600K melanomas had a numerically but insignificantly higher mutational load in our BRAFi+/−MEKi cohort using the 88-gene panel (p=0.1375) (Figure 3.A), however, whole exome sequencing data from the TCGA cohort revealed a significantly higher mutational load in V600K melanomas (p=0.0003) (Figure 3.B). The genes that were more frequently mutated in V600K melanoma on NGS fitted mainly into three groups: PI3K-AKT pathway genes (PIK3R1), tumor suppressor genes (SMARCA4, NF2, RB1, FBXW7, TP53 and APC) and proto-oncogenes (KIT, RET and KRAS) (Supplementary Figure 2 and Supplementary table 2). In the TCGA dataset, however, the frequency of mutations in these genes was very low, precluding further conclusions (Supplementary Figure 3). Given prior associations with mutational load, immune expression and immunotherapy response, we then looked at the expression of immune genes, by checking iPRES signature37 in V600E versus V600K mutant melanoma in the TCGA dataset, but no significant difference was detected between these two genotypes (Supplementary Figure 4). We further explored whether there was a subset of V600E melanomas that behave like V600K melanomas. Analyses of DUSP6 expression, mutational load, age and response to therapy did not identify a clear subset of V600E melanomas behaving like V600K melanoma, that is, having lower DUSP6 expression, with higher mutation burden, in older patients with less response to BRAFi+/−MEKi (Supplementary Figure 5).
Figure 3.
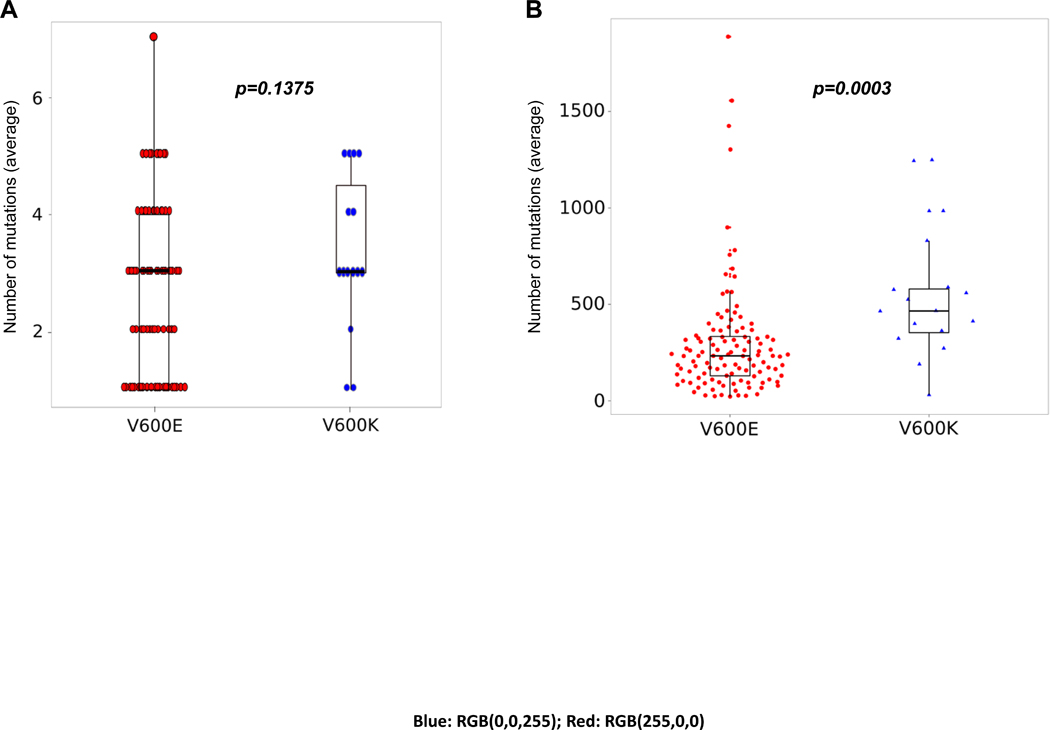
Mutational load in V600E (red) and V600K (blue) melanoma. NGS data from BRAFi+/−MEKi cohort (A) and TCGA (B) data. Each dot/triangle represents one single sample.
V600K melanomas have superior response and survival with immunotherapy
Finally, based on the higher mutational load seen in V600K melanomas, we hypothesized that they should respond better to immunotherapy than V600E melanoma. We examined an independent cohort of 103 BRAF V600E/K patients treated with anti-PD-1 immunotherapy (V600E, n=84; V600K, n=19)19 (Table 2). Patients with V600K were significantly older than those with V600E melanoma (61.5 vs 50 year-old, p=0.0358) and were more often male (84% vs 51%, p=0.01) (Table 2). No significant difference was found between groups regarding known prognostic factors including AJCC stage, LDH level or ECOG PS, and a similar percentage of patients from both groups had received prior treatment with BRAFi+/−MEKi (42% vs 44%, p=0.8042). With a median 31.7 months follow-up, there was a trend toward a higher response rate to immunotherapy in patients with V600K compared to V600E melanomas (53% vs 29%, P=0.059) (Figure 4.A). Moreover, progression-free survival was longer in V600K melanoma patients (median 19 vs 2.7 months, p=0.049) (Figure 4.B), while the prolonged overall survival for V600K did not reach statistical significance (20.4 vs 11.7 months, p=0.081) (Figure 4.C).
Table 2.
Baseline characteristics of patients with V600E vs V600K metastatic melanoma (immunotherapy cohort).
| Characteristics | V600E (n=84) | V600K (n=19) | P-value |
|---|---|---|---|
| Age (years) | |||
| Median | 51 | 61.5 | 0.0358 |
| Gender (N, %) | 0.001 | ||
| Female | 41 (49%) | 3 (16%) | |
| Male | 43 (51%) | 16 (84%) | |
| AJCC stage2 (N, %) | 0.7571 | ||
| IIIC-M1b | 18 (21%) | 3 (16%) | |
| M1c | 66 (79%) | 16 (84%) | |
| LDH (N, %) | 0.1195 | ||
| Normal | 49 (58%) | 15 (79%) | |
| Elevated | 35 (42%) | 4 (21%) | |
| ECOG (N, %) | 0.2037 | ||
| 0 | 42 (50%) | 13 (68%) | |
| ≥1 | 42 (50%) | 6 (32%) | |
| Prior MAPKi (N, %) | 0.8042 | ||
| No | 47 (56%) | 11 (58%) | |
| Yes | 37 (44%) | 8 (42%) | |
| PD1 therapy (N, %) | 0.0229 | ||
| Nivolumab | 22 | 2 | |
| Pembrolizumab | 62 | 17 |
AJCC v7 anatomic staging, excluding LDH and brain metastases
Figure 4.
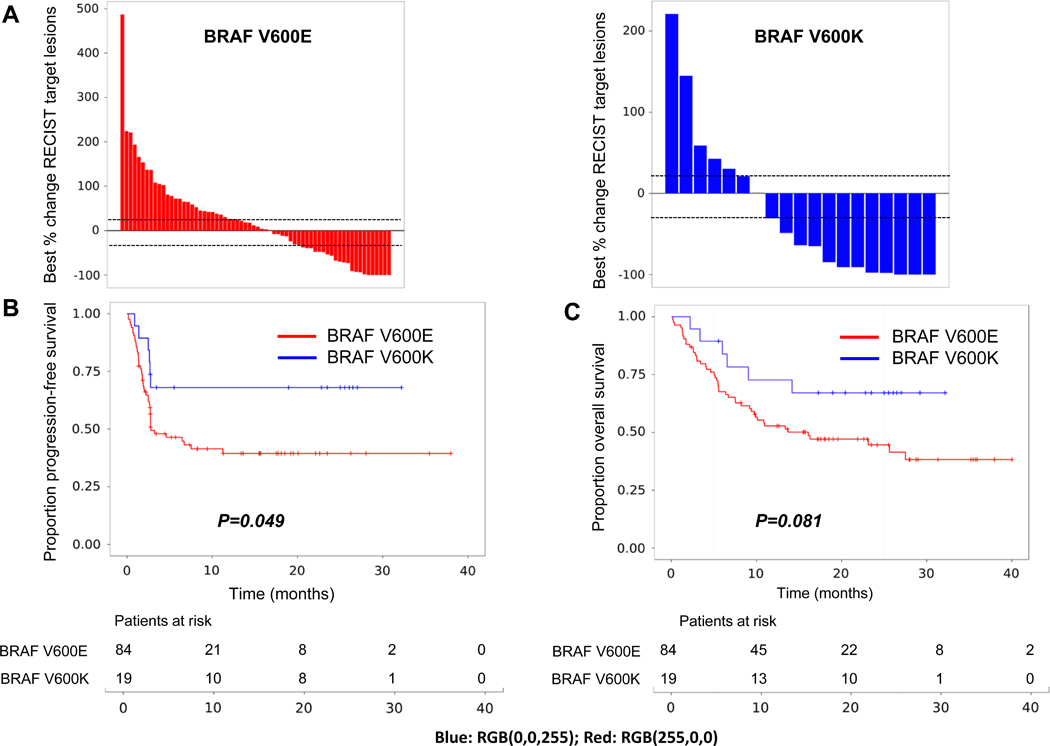
Clinical outcomes after treatment with anti-PD-1 immunotherapy in V600E (red) and V600K (blue) metastatic melanoma. A. Best change in RECIST targets (%); B. Progression-free survival; and C. Overall survival.
Discussion
This study demonstrates that V600E and V600K BRAF-mutant melanomas are biologically distinct subtypes of melanoma, not only having different clinical phenotypes, but also different molecular features and differing responses to systemic therapies. V600K melanomas, most frequent in older patients with chronic sun damage, have less activation of the ERK pathway than V600E melanomas, potentially explaining their relative resistance to BRAFi+/−MEKi, but in contrast, they have a higher mutational load and respond better to immunotherapy.
BRAF is a serine/threonine protein kinase that activates the ERK signaling pathway38. BRAF V600 mutations induce RAS-independent activation of the ERK pathway, with consequent dysregulation of ERK signaling (high expression and activation of ERK), driving cell proliferation and survival39. Moreover, the normal feedback regulation through the Sprouty and dual specificity phosphatase (DUSP) families is insufficient to down-regulate pathway activation in the presence of constitutively activated BRAF40. In this study we demonstrated in pre-treatment biopsies from melanoma patients that the negative feedback regulator of the ERK pathway, DUSP641, is expressed higher in V600E melanomas compared to V600K. This relative increase in ERK pathway activation, confirmed using gene set expression analysis from the TCGA dataset, is consistent with cell line data demonstrating higher fold BRAF-kinase activity in V600E mutant cell lines compared to V600K42. Furthermore, we demonstrated that an alternative proliferation and survival pathway, the PI3K-AKT pathway, is more activated in V600K melanoma. Together these data suggest differential activity of pathway signaling in V600E and V600K melanomas, potentially explaining the relative resistance of V600K melanoma to BRAFi+/−MEKi despite similar potency for inhibition with dabrafenib (IC50 0.65 V600E and 0.5 V600K, respectively)43. This possible disadvantage of V600K melanoma with BRAFi+/−MEKi may be seen in the clinic, with this data set as well as clinical trials suggesting that V600K patients have lower response rate and shorter progression-free survival compared to V600E patients10,11,12,13,14,15. Few direct comparisons have been performed in large datasets from clinical trials, and given the small V600K sample size in the study, results require validation in larger studies.
While V600 BRAF-mutant melanomas typically occur in younger patients whose tumors arise on skin without chronic sun-induced damage5, these associations are driven by the V600E genotype, which is the most frequent (70–80% BRAF-mutant melanomas). In contrast, V600K melanomas (20–30%) typically occur in older patients, on the head and neck, and in chronic sun damaged skin8. Consistent with these clinicopathologic differences, our data demonstrate a higher mutational load in V600K melanomas, and that several groups of genes, notably tumor suppressor genes and proto-oncogenes, are more frequently mutated in this genotype. Our data also show that BRAF V600K melanomas respond significantly better than V600E melanomas to anti-PD-1 immunotherapy, with a higher response rate, and longer progression-free and overall survival. Such results are consistent with several studies that have shown an association with tumor mutational load and response to immunotherapy44,45, but confirmation of these clinical findings should be validated in larger cohorts, such as a post-hoc analysis of the CheckMate-067 and Keynote-006 clinical trials46,47.
Despite the large number of melanoma tissue samples, a limitation of this study is the size of the V600K cohort, which diminished the power to analyze associations between the molecular findings and clinical outcomes. This limitation could explain the lack of significant difference in the expression of immune markers between these two genotypes. We did not see a difference in the expression of immune markers in TCGA cohort either, as differently from our BRAFi+/−MEKi cohort where we have macro-dissected the tumors, TCGA analyzed the highest tumor content areas, and may have missed the tumor-stroma interface. Limited DNA sequencing and RNA expression panels were run from formalin-fixed paraffin-embedded tumors, with less data obtained than that gained from whole exome/genome and RNA sequencing studies in fresh-frozen tumors. Such data were available in the TCGA cohort, however, and validation of our FFPE-based molecular data using a similarly size TCGA dataset strengthens the findings of this study. Prospective samples from stage III and IV patients have been collected in order to further characterize immune signatures and correlate these with response to immunotherapy in the adjuvant and metastatic settings, and the respective mutant BRAF genotype.
For patients with BRAF-mutant metastatic melanoma, the selection of therapy to give the highest chance of long-term durable control, either immunotherapy or targeted therapy, remains a challenge. There are no trial data to suggest the most appropriate sequence, and there are no biomarkers that can accurately identify patients likely to derive long-term benefit with either treatment. This is now also a dilemma in the adjuvant setting4,48,49. Our data, along with the published BRAF+/−MEKi clinical trials, suggest that V600K melanomas may be best served with adjuvant immunotherapy. In addition to exploring clinical trial data in the metastatic setting, data from the adjuvant clinical trials should also be explored by BRAF mutation genotype4,48,49. If our results are validated, the best treatment for patients with BRAF mutant melanoma may well differ based on V600E and V600K genotypes.
The differences in gene expression and mutational load between V600E and V600K BRAF-mutant melanomas provide translational data that explain clinically meaningful differences in response to BRAFi+/−MEKi and immunotherapy, and provide further rationale for the selection of one therapy over the other. Such findings should lead to the development of alternative treatment approaches for specific genotypes of BRAF-mutant melanoma.
Supplementary Material
Statement of translational relevance.
This study demonstrates that V600E and V600K BRAF-mutant melanomas are biologically distinct subtypes, not only having different clinical phenotypes, but also different molecular features and differing responses to systemic therapies. V600K melanomas, most frequent in older patients with chronic sun damage, have less activation of the ERK pathway than V600E melanomas, what might explain their potential lower benefit with BRAFi −/+ MEKi inhibitors, but in contrast, they have a higher mutational load and respond better to immunotherapy. The differences in gene expression and mutational load between V600E and V600K BRAF-mutant melanomas provide mechanistic data that translate to clinically significant differences in response to BRAFi −/+ MEKi inhibitors and immunotherapy. Such findings should lead to the development of alternative treatment approaches for specific subtypes of BRAF-mutant melanoma.
Acknowledgements:
The authors thank the assistance of Jess Hyman, Hazel Burke, Alex Guminski and Raghwa Sharma.
JWS is supported by an NHMRC Research Fellowship.
JLM is supported by an ASCO/CCF Career Development Award, a Melanoma SPORE Developmental Research Program Award, and an NIH T32 Training Grant CA009666.
RAS is supported by a NHMRC Practitioner Fellowship.
JYY is supported by NHMRC CDF and ARC Discovery Project grant (DP170100654)
GVL is supported by a NHMRC Practitioner Fellowship and the University of Sydney Medical Foundation.
AMM is supported by a Cancer Institute NSW Fellowship.
This work was supported by a Pfizer Australia grant (WS2345795 to AMM), and a Cancer Council NSW grant (RG17–04 to JH, JSW and AMM).
This research was also supported by an Australian National Health and Medical Research Council program grant. Assistance from other colleagues at Melanoma Institute Australia and the Royal Prince Alfred Hospital is also gratefully acknowledged.
Footnotes
Disclosures:
JH: Founder – MetabloQ Pharmaceuticals
MW: Genentech employee; stock ownership in Roche and ARIAD Pharmaceuticals
YY: Genentech employee
DBJ: Advisory board – Array Biopharma, BMS, Incyte, Merck, Novartis.
MSC Advisory board – BMS, MSD, Novartis, Amgen, Pierra-Fabre
GVL: Advisory board - Amgen, Array, BMS, MSD, Novartis, Pierre-Fabre, Roche and Incyte
AMM: Advisory board – BMS, MSD, Novartis, Roche, Pierra-Fabre.
References
- 1.Hodis E, Watson IR, Kryukov GV., et al. A landscape of driver mutations in melanoma. Cell. 2012;150(2):251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapman PB, Hauschild A, Robert C, et al. Improved Survival with Vemurafenib in Melanoma with BRAF V600E Mutation. Vol 364; 2011. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hauschild A, Grob JJ, Demidov LV., et al. Dabrafenib in BRAF-mutated metastatic melanoma: A multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380(9839):358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 4.Long GV, Hauschild A, Santinami M, et al. Adjuvant Dabrafenib plus Trametinib in Stage III BRAF-Mutated Melanoma. N Engl J Med. 2017;377(19):1813–1823. doi: 10.1056/NEJMoa1708539. [DOI] [PubMed] [Google Scholar]
- 5.Ascierto PA, Kirkwood JM, Grob J-J, et al. The role of BRAF V600 mutation in melanoma. J Transl Med. 2012;10:85. doi: 10.1186/1479-5876-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29(10):1239–1246. doi: 10.1200/JCO.2010.32.4327. [DOI] [PubMed] [Google Scholar]
- 7.Lyle M, Haydu LE, Menzies AM, et al. The molecular profile of metastatic melanoma in Australia. Pathology. 2016;48(2):188–193. doi: 10.1016/j.pathol.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Menzies AM, Haydu LE, Visintin L, et al. Distinguishing clinicopathologic features of patients with V600E and V600K BRAF-mutant metastatic melanoma. Clin Cancer Res. 2012;18(12):3242–3249. doi: 10.1158/1078-0432.CCR-12-0052. [DOI] [PubMed] [Google Scholar]
- 9.Bucheit AD, Syklawer E, Jakob JA, et al. Clinical characteristics and outcomes with specific BRAF and NRAS mutations in patients with metastatic melanoma. Cancer. 2013;119(21):3821–3829. doi: 10.1002/cncr.28306. [DOI] [PubMed] [Google Scholar]
- 10.McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 2014;15(3):323–332. doi: 10.1016/S1470-2045(14)70012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larkin J, Ascierto PA, Dréno B, et al. Combined Vemurafenib and Cobimetinib in BRAF -Mutated Melanoma. N Engl J Med. 2014;371(20):1867–1876. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- 12.Robert C, Karaszewska B, Schachter J, et al. Improved Overall Survival in Melanoma with Combined Dabrafenib and Trametinib. N Engl J Med. 2014;372(1):141116004513004. doi: 10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]
- 13.Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK Inhibition versus BRAF Inhibition Alone in Melanoma. N Engl J Med. 2014;371(20):1877–1888. doi: 10.1056/NEJMoa1406037. [DOI] [PubMed] [Google Scholar]
- 14.Schadendorf D, Long GV., Stroiakovski D, et al. Three-year pooled analysis of factors associated with clinical outcomes across dabrafenib and trametinib combination therapy phase 3 randomised trials. Eur J Cancer. 2017;82:45–55. doi: 10.1016/j.ejca.2017.05.033. [DOI] [PubMed] [Google Scholar]
- 15.Long GV, Grob J-J, Nathan P, et al. Factors predictive of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: a pooled analysis of individual patient data from randomised trials. Lancet Oncol. 2016;17(12):1743–1754. doi: 10.1016/S1470-2045(16)30578-2. [DOI] [PubMed] [Google Scholar]
- 16.Flaherty KT, Robert C, Hersey P, et al. Improved Survival with MEK Inhibition in BRAF-Mutated Melanoma. N Engl J Med. 2012;367(2):107–114. doi: 10.1056/NEJMoa1203421. [DOI] [PubMed] [Google Scholar]
- 17.Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(11):1087–1095. doi: 10.1016/S1470-2045(12)70431-X. [DOI] [PubMed] [Google Scholar]
- 18.Ascierto PA, McArthur GA, Dréno B, et al. Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016;0(0):949–954. doi: 10.1016/S1470-2045(16)30122-X. [DOI] [PubMed] [Google Scholar]
- 19.Rai R, et al. Safety and efficacy of anti-PD-1 antibodies in elderly patients with metastatic melanoma. Ann Oncol 27 379–400 101093/annonc/mdw379. [Google Scholar]
- 20.Nickles D, Sandmann T, Ziman R, Bourgon R. NanoStringQCPro: Quality metrics and data processing methods for NanoString mRNA gene expression data. R Packag version 1100. 2017. [Google Scholar]
- 21.Bourgon R, Lu S, Yan Y, et al. High-throughput detection of clinically relevant mutations in archived tumor samples by multiplexed PCR and next-generation sequencing. Clin Cancer Res. 2014;20(8):2080–2091. doi: 10.1158/1078-0432.CCR-13-3114. [DOI] [PubMed] [Google Scholar]
- 22.Wu TD, Nacu S. Fast and SNP-tolerant detection of complex variants and splicing in short reads. Bioinformatics. 2010;26(7):873–881. doi: 10.1093/bioinformatics/btq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu TD, Reeder J, Lawrence M, Becker G, Brauer MJ. GMAP and GSNAP for genomic sequence alignment: Enhancements to speed, accuracy, and functionality. In: Methods in Molecular Biology. Vol 1418; 2016:283–334. doi: 10.1007/978-1-4939-3578-9_15. [DOI] [PubMed] [Google Scholar]
- 24.Guan J, Gupta R, Filipp FV. Cancer systems biology of TCGA SKCM: efficient detection of genomic drivers in melanoma. Sci Rep. 2015;5:7857. doi: 10.1038/srep07857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colaprico A, Silva TC, Olsen C, et al. TCGAbiolinks: An R/Bioconductor package for integrative analysis of TCGA data. Nucleic Acids Res. 2016;44(8):e71. doi: 10.1093/nar/gkv1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cibulskis K, Lawrence MS, Carter SL, et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol. 2013;31(3):213–219. doi: 10.1038/nbt.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Network TCGA. Genomic Classification of Cutaneous Melanoma. Cell. 2015;161(7):1681–1696. doi: 10.1016/j.cell.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. R Found Stat Comput Vienna, Austria: 2017. [Google Scholar]
- 29.Ritchie ME, Phipson B, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. 2015:1–13. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pratilas CA, Taylor BS, Ye Q, et al. V600EBRAF is associated with disabled feedback inhibition of RAF-MEK signaling and elevated transcriptional output of the pathway. Proc Natl Acad Sci. 2009;106(11):4519–4524. doi: 10.1073/pnas.0900780106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nazarian R, Shi H, Wang Q, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468(7326):973–977. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci. 2005;102(43):15545 LP-15550. http://www.pnas.org/content/102/43/15545.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liberzon A, Birger C, Thorvaldsdóttir H, Ghandi M, Mesirov JP, Tamayo P. The Molecular Signatures Database Hallmark Gene Set Collection. Cell Syst. 2015;1(6):417–425. doi: 10.1016/j.cels.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Curran J Hotelling: Hotelling’s T^2 Test and Variants. 2017:R package version 1.0–4. [Google Scholar]
- 36.Therneau TM. A Package for Survival Analysis in S. 2015. [Google Scholar]
- 37.Hugo W, Zaretsky JM, Sun L, et al. Genomic and Transcriptomic Features of Response to Anti-PD-1 Therapy in Metastatic Melanoma. Cell. 2016;165(1):35–44. doi: 10.1016/j.cell.2016.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26(22):3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 39.Poulikakos PI, Rosen N. Mutant BRAF melanomas-dependence and resistance. Cancer Cell. 2011;19(1):11–15. doi: 10.1016/j.ccr.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 40.Montero-Conde C, Ruiz-Llorente S, Dominguez JM, et al. Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their antitumor effects in BRAF -mutant thyroid carcinomas. Cancer Discov. 2013;3(5):520–533. doi: 10.1158/2159-8290.CD-12-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kidger AM, Keyse SM. The regulation of oncogenic Ras/ERK signalling by dual-specificity mitogen activated protein kinase phosphatases (MKPs). Semin Cell Dev Biol. 2016;50:125–132. doi: 10.1016/j.semcdb.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wan PTC, Garnett MJ, Roe SM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116(6):855–867. doi: 10.1016/S0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 43.Menzies AM, Long GV., Murali R. Dabrafenib and its potential for the treatment of metastatic melanoma. Drug Des Devel Ther. 2012;6:391–405. doi: 10.2147/DDDT.S38998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Snyder A, Makarov V, Merghoub T, et al. Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma. N Engl J Med. 2014;371(23):2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Danilova L, Wang H, Sunshine J, et al. Association of PD-1/PD-L axis expression with cytolytic activity, mutational load, and prognosis in melanoma and other solid tumors. Proc Natl Acad Sci U S A. 2016;113(48):E7769-E7777. doi: 10.1073/pnas.1607836113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus Ipilimumab in Advanced Melanoma. N Engl J Med. 2013;369(2):122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372(26):2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 48.Weber J, Mandala M, Del Vecchio M, et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N Engl J Med. 2017:NEJMoa1709030. doi: 10.1056/NEJMoa1709030. [DOI] [PubMed] [Google Scholar]
- 49.Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N Engl J Med. 2018;378(19):1789–1801. doi: 10.1056/NEJMoa1802357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


