Abstract
Background
Cancer cachexia is a multidimensional wasting syndrome and a reduced dietary intake is both common and strongly correlated with degree of weight loss. Many patients with cachexia do not achieve recommended dietary intake even after nutritional counselling. Prior reports suggest this is likely due to barrier symptoms, but other potential contributory factors have not been studied in detail.
Methods
Dietitian‐assigned barriers to successful nutritional intervention were recorded at each visit in all patients attending a multidisciplinary clinic for management of cancer cachexia. The barriers were grouped into 15 categories and classified as either symptom‐related or not symptom‐related. In addition, symptom scores, dietary intake, and weight change were recorded.
Results
Data on 94 new patients showed that 89% of patients had at least one major barrier. Four of the five most common barriers and 65% of all barriers identified were not symptom‐related. Over sequential visits the specific barrier(s) in any one patient changed approximately 50% of the time. However, the presence of barriers did not render patients refractory to nutritional intervention and with intervention from the CNR‐JGH team, mean dietary intake increased significantly.
Conclusions
In advanced cancer patients with cachexia, non‐symptom‐related barriers to nutritional intervention are more common than symptom‐related. Barriers are dynamic, and repeated careful evaluation over time is required to achieve optimal impact with nutritional intervention in cancer cachexia. Members of the multidisciplinary team need appropriate expertise to address the barriers identified and achieve optimal results with nutritional intervention.
Keywords: Cancer cachexia, Nutrition counselling, Dietary energy intake, Nutrition‐impact symptoms
Background
Weight loss is common in patients with cancer and is the primary defining feature of cancer cachexia. Weight loss is more severe and more frequent in those with advanced disease, but it is a strong poor prognostic sign at all stages of disease and is associated with reduced treatment tolerance and increased morbidity and mortality.1, 2, 3, 4, 5, 6 Low dietary energy intake is clearly correlated with weight loss, and recent data from our group have shown that over 80% of patients referred to our clinic for cancer cachexia were not consuming recommended levels of protein and energy.7 Using a multidisciplinary approach, including dietitian‐led nutritional counselling, mean dietary intake was increased, and overall weight stabilized in patients attending the clinic. However, despite the improvements in mean dietary intake, 54% of patients did not attain recommended levels of dietary intake by the third clinic visit.7 Furthermore, many patients who did increase intake to the recommended levels required repeated sessions of nutritional counselling in conjunction with other interventions offered by the team, before they were able to increase or optimize nutritional intake.
Not only is there evidence that nutritional counselling can increase dietary intake in patients with cancer‐related weight loss,7, 8, 9 there is also some data that those who adhere to nutritional counselling advice have better health outcomes, including increased muscle mass and progression‐free survival.10, 11 However, many patients are unable or unwilling to follow nutritional advice. In one study, only half of the patients with cancer complied with nutritional advice,12 and another study of inpatients with cancer reported that only 61% were willing to follow dietary advice given by a dietitian.13 The reasons for poor adherence were not reported in these studies, but the presence of symptoms has been correlated with reduced food intake in cancer patients. Such ‘nutrition‐impact' symptoms include early satiety, nausea, vomiting, diarrhoea, constipation, altered taste, pain, dysphagia, mouth sores, xerostomia, dental problems, difficulty chewing, depression, and anxiety.14, 15 Moreover, in patients with head and neck cancer, a survey of experts (physicians, nutritionists, and speech language pathologists) listed additional non‐symptom‐related factors that were also seen as barriers to successful implementation of nutrition care.16 These non‐symptom‐related factors included patients' preferences and motivation as well as social issues (e.g. food security, alcoholism, and social isolation).
Currently, it is difficult to develop strategies to address the barriers to poor adherence to nutritional advice in cancer cachexia as the relative frequency and importance of both nutrition‐impact symptoms and non‐symptom‐related factors have not been defined. To address this gap in knowledge, a survey of barriers to successful nutritional intervention was performed in sequential patients attending the McGill Cancer Nutrition Rehabilitation clinic at the Jewish General Hospital (CNR‐JGH) in Montreal. The CNR‐JGH clinic has a multidisciplinary team focused on the management of cancer cachexia, and interventions include nutritional counselling by a registered dietitian. The individual nutritional barriers identified in the CNR‐JGH clinic were classified as symptom‐related or non‐symptom‐related. In addition, the impact of these different types of barriers on success of nutritional intervention was assessed.
Material and methods
Retrospective chart review was performed for all patients attending the CNR‐JGH clinic between June 2016 and January 2017. This study was approved by the Research Ethics committee of the Jewish General Hospital (Approval # CODIM‐MBM‐CR17‐35).
The CNR‐JGH clinic is based in the Segal Cancer Centre at the Jewish General Hospital, a McGill University Teaching Hospital. All oncologists or other health care professionals are encouraged to refer patients with cancer who are suffering weight loss, anorexia, or generalized functional decline to the CNR‐JGH. The CNR‐JGH team comprises a physician, nurse, physiotherapist, and a dietitian. At each visit, patients are evaluated by each professional, and an inter‐disciplinary intervention plan is developed to address any barrier symptoms and optimize both dietary intake and functional status using individualized nutritional counselling and exercise training programmes. At each CNR‐JGH clinic visit, patients are evaluated, and their intervention plan is adjusted as needed. Where appropriate, a follow‐up visit is scheduled, and these occur typically at 6 week intervals, but this can be adjusted depending on clinical need and patients' availability.
Patient‐reported symptoms are collected at the start of each clinic visit using a customized questionnaire. The severity of 10 symptoms is recorded using a Likert‐like scale questionnaire (0 none–10 maximal symptom): pain, strength, loss of appetite, nausea, vomiting, diarrhoea, constipation, fatigue, shortness of breath, and depression. Six additional yes/no response questions are asked about the presence of mouth sores, dry mouth, swallowing difficulties, early satiety, and taste and smell disturbances. Patient‐reported weight or weight loss are recorded at the first visit and corroborated using medical records where available. At each visit, the following data are recorded: weight (to nearest 0.1 kg without shoes using calibrated digital scale), height (to nearest 0.5 cm using stadiometer), age, performance status, cancer stage, anti‐cancer treatment, and other medical conditions.
Dietary intake is determined by the CNR‐JGH dietitian using the patient's report of intake over the last 24 h period (24 h recall), or in selected cases where this approach was difficult, an estimate of current usual intake in 24 hr was used. Portion sizes are validated by use of examples or food models, and diet records are analysed by using commercial software (the Food Processor®, ESHA, 2009) with Canadian food composition tables. Nutritional advice given by CNR‐JGH dietitians aims to achieve target intakes of at least 30 kcal/kg body weight and 1.3 g protein/kg body weight.17, 18 Primary dietary recommendations typically include the use of energy and protein‐dense foods and beverages and increasing meal frequency, with or without the use of liquid nutritional supplements. Dietary recommendations take account of each individual's usual diet, personal eating patterns, manageable food consistency, and medical conditions. In addition, nutritional advice is frequently adapted to respond to specific symptoms (e.g. dysphagia, constipation, diarrhoea, altered taste, and temperature sensitivity). Follow up of patients continues between clinic visits to support and adjust dietary changes to achieve and maintain target intakes, using face‐to‐face interviews if patients are attending other appointments in the hospital (for chemotherapy or outpatient physiotherapy) or by telephone. The dietitian is also available to respond to questions outside the scheduled clinic times.
Immediately after each scheduled clinic visit during the study period, the CNR‐JGH dietitian who had evaluated the patient recorded what they perceived to be the main barrier(s) impeding patients from adhering to nutritional advice. Up to three different dietitian‐reported barriers were identified in this way for each patient. The initial clinical recording of barriers was not structured into pre‐defined categories in an attempt to make the process as comprehensive as possible. When data retrieval was complete at the end of the study, the barriers recorded were collated and consensus categories were formed after discussion between authors, to help describe the range and type of barriers reported. These categories were then classified into two groups, namely, symptom‐related or not symptom‐related.
Analysis
For the purposes of this study, only data from Visits 1 and 2 are reported. The prevalence of barriers to adherence to dietary advice in new patients (Visit 1) is likely most representative of the true range and frequency of different barriers that patients experience and avoids the risk of potential confounding effect of CNR‐JGH team interventions. In addition, analysis of the barriers at Visit 2 is included to evaluate the changes in frequency of barriers both at the individual and group levels over time. Data are presented as N (%) or mean (SD) as indicated. Significance testing was performed to compare means and was performed using Student's t‐test and analysis of variance (ANOVA) with Tukey's honestly significant difference test to correct for multiple significance testing as appropriate. A P value of <0.05 was taken as significant. Analysis was performed with R19 using R Studio (version 1.1.383), and plots were prepared using ggplot2. Dietary intake was categorized as follows: a poor diet likely inadequate even for healthy individuals (<20 kcal/kg energy and <0.8 g/kg protein), a sufficient diet meeting current guidelines for cancer patients (≥30 kcal/kg energy and ≥1.3 g/kg protein) and an intermediate diet likely sufficient for healthy adults but not adequate for cancer patients (20–30 kcal/kg energy and/or 0.8–1.3 g/kg protein). A subset of patients with only minor symptoms (‘low symptom group') were defined as those with scored <6 on all scaled questions and ‘no' to all direct symptom questions. A second subset of patients with moderate–severe symptoms (‘high‐symptom group') was defined as all who scored >5 on one scaled symptom question and ‘yes' for one direct symptom questions or ‘yes' on at least two symptom questions or >5 on at least two scaled symptom questions.
Results
One hundred fourteen individuals were seen at least once in the CNR‐JGH clinic during the 7 month study period. Data on dietitian‐reported barriers was missing for three individuals, including two of the 96 new patients seen during the study period. Of the 94 new patients for whom data were available, 51 returned for a second visit during the study period.
Baseline clinical characteristics and nutritional status of the new patients (Table 1) of this cohort of patients at their first CNR‐JGH clinic are very similar to a previously published data from the CNR‐JGH clinic.7 A majority (56.4%) of the patients had either gastrointestinal or lung cancers, and most patients (77.6%) were advanced stage disease (Stage 3 or 4); 69.2% were at an early stage in their cancer treatment having received a maximum of one line of treatment, and only a minority (29.8%) had markedly impaired performance status (Karnofsky performance score20 <70) (Table 1).
Table 1.
Clinical characteristics and nutritional status of patients attending the CNR‐JGH clinic (N = 94)
| Age, mean (SD) | 66.4(13) |
| Female, N (%) | 44 (47%) |
| Cancer type | |
| Gastrointestinal | 33 (35.1) |
| Lung | 20 (21.3) |
| Haematological | 14 (14.9) |
| Urogenital | 11 (11.7) |
| Breast | 7 (7.4) |
| Other | 9 (9.6) |
| Cancer stage | |
| 3 | 13 (13.8) |
| 4 | 60 (63.8) |
| Other | 17 (18.1) |
| NA | 4 (4.3) |
| Cancer treatment line | |
| 0 | 25 (26.6) |
| 1 | 40 (42.6) |
| 2 | 12 (12.8) |
| ≥3 | 17 (18.1) |
| Performance status (Karnofsky)[20] | |
| 50 | 9 (9.6) |
| 60 | 19 (20.2) |
| 70 | 28 (29.8) |
| 80 | 27 (28.7) |
| 90 | 11 (11.7) |
| Cachexia code [21] | |
| None | 3 (3.2) |
| Pre‐cachexia | 19 (20.2) |
| Cachexia | 72 (76.6) |
| Cancer weight loss grade [22] | |
| 0–2 | 28 (29.8) |
| 3 | 34 (36.2) |
| 4 | 31 (33.0) |
| NA | 1 (1.1) |
| Modified Glasgow Prognostic Score [23] | |
| 0 | 54 (57.4) |
| 1 | 16 (17.0) |
| 2 | 22 (23.4) |
| NA | 2 (2.1) |
| Diet categorya | |
| Poor | 29 (30.9) |
| Intermediate | 52 (53.2) |
| Sufficient | 13 (13.8) |
| NA | 2 (2.1) |
Diet Categories were assigned based on 24 hr recall at Visit 1: Poor (<20 kcal/kg energy and <0.8 g/kg protein), Sufficient (≥30 kcal/kg energy and ≥1.3 g/kg protein), and Intermediate (20–30 kcal/kg energy and/or 0.8–1.3 g/kg protein).
Most patients had cachexia (76.6%) or pre‐cachexia (20.2%)21 (Table 1). Furthermore, when classified using a combined weight loss and body mass index prognostic grading system for cancer patients,22 most patients were in the two worst categories: Grade 3 (36.2%) or 4 (33.0%) (Table 1). However, using the inflammation‐based modified Glasgow Prognostic Score,23 17.0% had score 1 (CRP >10 mg/L) and 23.4% had the worst prognostic score of 2, with both raised CRP and reduced albumin (Table 1). Only 2/94 (2%) and 2/51 (4%) patients had clinically significant oedema or ascites at Visits 1 and 2, respectively. These patients were excluded from the analysis of weight change. Mean (SD) body mass index for all patients at Visit 1 was 24.2 (4.9) kg/m2 with a mean (SD) weight loss of 1.6 (3.8) kg in the preceding 6 weeks. Finally, only 13.8% were consuming recommended levels of dietary energy and protein, whereas 32.3% of patients were consuming a diet with both inadequate energy (<20 kcal/kg) and protein (<0.8 g/kg) content (Table 1). Seventy‐eight (83%) of patients were in the high‐symptom group with at least two moderate or severe symptoms (see Methods), 11 (12%) reported s single moderate or severe symptom, and only five (5%) were in the low symptom group with no moderate or severe symptoms.
Many patients have more than one barrier
Dietitian‐reported barriers were collated and grouped by similarity into 15 categories (Table 2). Furthermore, the barrier categories were classified as either symptom‐related or not symptom‐related. In addition to examples of each non‐symptom barrier category (Table 2), four clinical vignettes detailing the impact of selected non‐symptom barriers on adherence to nutritional counselling are included (Table 3). Only 10 patients (11%) had no barriers, 50 (53%) had one barrier, 29 (31%) had two barriers, and 5 (5%) had three barriers (Figure 1A).
Table 2.
Categories for dietitian‐identified barriers to adherence with nutritional counselling advice
| Class | Category | Examples |
|---|---|---|
| Non‐symptom‐related | ||
| 1. Medical/oncology | Low fat, salt, sugar diet because of metabolic syndrome, diabetes, hypertension; food allergies; fluid and dietary restrictions to control ileostomy or colostomy output; texture restrictions due to gastrointestinal strictures or previous obstruction; dementia; and direct effects of oncology or other medical treatment | |
| 2. Conflicting advice | Following advice from external sources, for example, internet, other health care or complementary health professionals, or influenced by family members' beliefs that conflict with CNR‐JGH advice | |
| 3. Poor motivation | Happy about weight loss, for example, if previously obese, and not interested in nutritional advice | |
| 4. Social barriers | Financial constraints; unstable living arrangements; social isolation; responsibilities as carer for others with dietary restrictions; alcohol, cigarette, substance abuse; lack of access to recommended foods; and limited time or opportunity for shopping and food preparation | |
| 5. Food preference restrictions | Strong dislikes or intolerance of dietary supplements or food items; and cultural, religious culinary customs or restrictions | |
| 6. Communication difficulties | Does not listen to advice, poor understanding due to language barriers, and poor historian | |
| Symptom‐related | ||
| 7. Lower gastrointestinal symptoms | Diarrhoea, constipation, bloating, and discomfort | |
| 8. Swallowing difficulties | ||
| 9. Fatigue | ||
| 10. Anorexia or early satiety | ||
| 11. Nausea and vomiting | ||
| 12. Taste changes and dry mouth | ||
| 13. Pain | ||
| 14. Anxiety and depression | ||
| 15. Dental problems | Caries or missing, loose, or painful dentition |
Table 3.
Four illustrative clinical vignettes of cases of patients with non‐symptom‐related barriers
| Patient 1. Poor motivation: A 57‐year‐old female with metastatic breast cancer and leptomeningeal disease. She was obese (BMI of 40) despite being 47 kg (33%) below her usual body weight and had markedly reduced muscle mass and strength. Her reported dietary energy intake was very poor (15 kcal/kg) which was thought to be largely related to very poor appetite. Nutritional intervention in the CNR‐JGH clinic focused on explaining the importance of maintaining weight stability and correcting low muscle mass and recommended increases in her calorie and protein intake. However, at subsequent visits, dietary energy intake remained the same, and protein intake halved. Attempts were made to address a variety of other physical symptoms impeding oral intake (e.g. abdominal pain and nausea), but by the 4th visit, it became clear that she was not motivated to gain weight. The patient finally expressed feeling conflicted between her desire for further weight loss and her understanding that stabilizing her weight was associated with better health outcomes. With this information, the dietitian was able to refocus the goals of nutritional counselling away from her physical size and weight towards improving body composition. The patient agreed that maintaining muscle mass was important to her, and with the aid of a food log, an appropriate rehabilitation programme, and support, she increased her protein intake and stabilized her weight. |
| Patient 2. Conflicting advice: A 44‐year‐old male with metastatic renal cell cancer presented with a history of severe weight loss and was 20% below his usual body weight. He had invested large amounts of time searching the Internet to find the best diet to combat cancer and was struggling to resolve the conflicting information he had found. He was following a ketogenic diet (low carbohydrate, 20 g) but had adopted other restrictions including low‐fat, limited dairy products, and gluten‐free diet. Despite CNR‐JGH nutritional counselling advice to achieve a more balanced and sufficient diet to stabilize or regain weight, he continued to follow a restrictive diet. The patient admitted that whilst valuing the CNR‐JGH advice and assessment of the scientific evidence, he was only prepared to make minor changes. He felt that the only things he could control through his cancer journey were his diet and emotions, and he exercised this limited control by following a strict restrictive diet. Even though he felt hungry, he was convinced that if he avoided eating this would contribute to ‘starve' the cancer. Over the course of several discussions with the CNR‐JGH dietitians, he began to re‐incorporate some food groups and was able to gain weight (2.5 kg) at the third and subsequent clinic visits. |
| Patient 3. Conflicting advice: A 53‐year‐old male recently diagnosed with Stage 4 non‐small cell lung cancer. At the first CNR‐JGH evaluation, he had anorexia, early satiety, and dysphagia and had lost 10 kg (13% of his usual weight) over the previous 2 months. In addition, the patient was following a vegan diet and including various natural health products, avoiding sugar (including fruits) and juicing (vegetables). He did not enjoy these dietary restrictions but had adopted them in an attempt to favourably impact his prognosis. It became clear that his belief system around food was the main barrier to increasing his dietary intake and stabilizing his weight. However, a further significant feature was that his wife was a major driver and influence on his dietary habits as she wanted to do everything she could to improve outcomes for him and held very strong beliefs about the potential benefits of a vegan diet. The conclusions that the patient's wife drew from her extensive internet research into optimal diet were frequently in conflict with the nutritional advice from the CNR‐JGH team dietitians. This made it very difficult for the patient to decide how best to proceed and the protracted process of discussion and attempts to synthesize advice from his wife and that of the professionals was quite burdensome for the patient. This situation was further compounded by his lack of enjoyment of the vegan, low‐sugar diet. Sadly, despite the CNR‐JGH team's attempts to find a workable solution, the discussion was never fully resolved even after seven visits to the CNR‐JGH clinic. |
| Patient 4. Medical or oncology: A 72‐year‐old man with Stage 4 pancreatic cancer and prior Type 2 diabetes and orthostatic hypotension complained of poor appetite and early satiety when he was first seen in the CNR‐JGH clinic and had lost 6.4% of his body weight in 1 month prior to his first visit. It became clear over the initial interactions that, despite the metastatic cancer diagnosis, the patient and his family were most concerned about carbohydrate intake and blood sugar control. In this case, the greatest barrier to effectively manage his nutritional needs was his strong dietary beliefs about the best nutritional management of his diabetes. It was difficult for the patient to accept advice to adjust his nutritional priorities to reverse his rapid weight loss and stabilize his weight by increasing dietary energy intake including increasing carbohydrates. |
Figure 1.
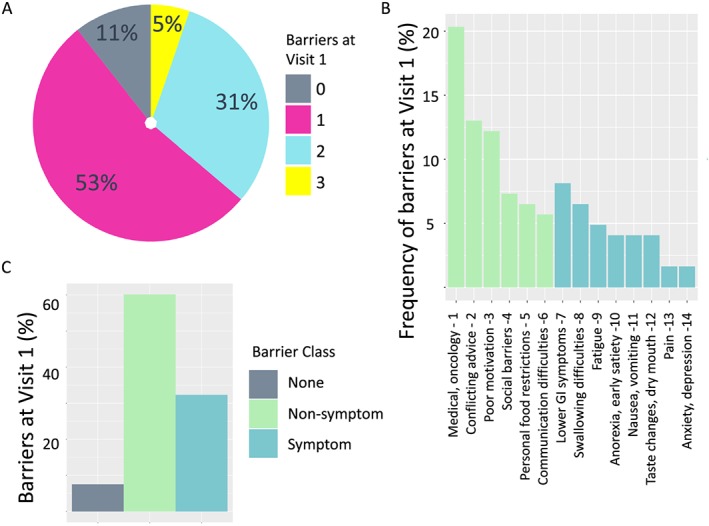
Frequency and type of dietitian‐identified barriers to adherence with nutritional counselling advice in patients with cancer cachexia. The frequency distribution of number of barriers to nutritional intervention in 94 patients attending their first visit to the CNR‐JGH clinic (A) along with frequency of each category of dietitian‐reported barriers (B) and the relative proportions symptom and non‐symptom‐related barriers (C).
Non‐symptom‐related barriers are more prevalent than symptom‐related barriers
The frequency of each dietitian‐reported barrier in all 84 patients at first visit who had at least one barrier identified is shown in Figure 1B. Five barriers accounted for around 61% of all dietitian‐reported barriers identified. Furthermore, four of these five most prevalent barriers were not symptom‐related. These included a variety of issues related to medical conditions or oncology treatment, for example, restrictions due to prior diabetes or dementia (20.3%), conflicting nutritional advice (13.0%), poor motivation (12.2%), and social barriers (7.3%). In contrast, the most prevalent symptom‐related barrier category was lower gastrointestinal symptoms such as diarrhoea or bloating (8.1%) (Table 2). In fact, six non‐symptom‐related barrier categories accounted for the majority (65%) of all barriers identified (Figure 1B).
Barriers are dynamic
The proportions of patients that had 0, 1, 2, or 3 dietitian‐reported barriers to adherence with nutritional counselling advice were very similar at Visits 1 and 2 (Figure 2A). However, for individual patients, the type of barriers varied considerably across successive visits. This can be demonstrated most clearly in the subset of 60 patients with 0 or 1 barrier at Visit 1. For these individuals, only 35–40% were unchanged at Visit 2 (i.e. remained without barrier or same barrier as Visit 1). Thus, for example, amongst the patients with only one major barrier at Visit 1, 50% had a different barrier at Visit 2, 35% continued to have the same barrier, and in 15%, no barriers were evident at the second visit (Figure 2B).
Figure 2.
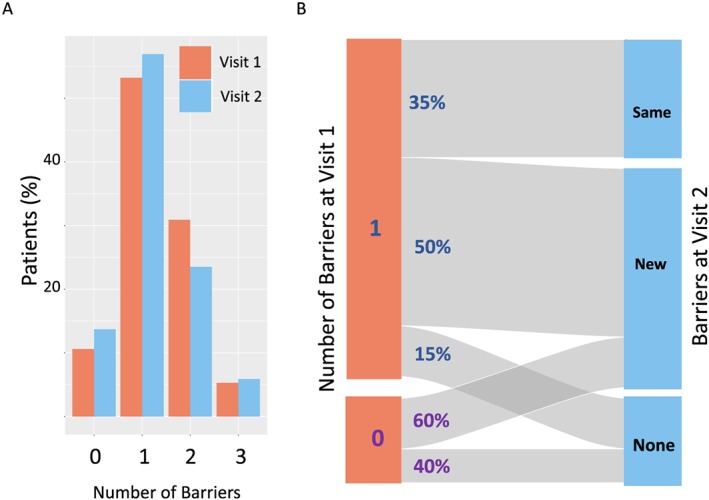
Comparison of dietitian‐identified barriers to adherence with nutritional counselling advice between Visits 1 and 2 in patients attending the CNR‐JGH clinic. To determine if there were differences in profile of dietitian‐identified barriers between successive visits, patients attending both Visit 1 and 2 were selected (N = 51). The frequency distribution of the number of barriers identified was very similar between Visits 1 and 2 (A). However, when the changes in patients with 0 or 1 barrier at Visit 1 were evaluated (N = 34), only a minority (35–40%) had the same type and number of barriers identified (B) at Visit 2.
Symptom screening does not substitute for dietitian assessment of nutritional barriers
Higher scores for anorexia correlated with lower dietary energy intake (R = −0.32, P = 0.001) and greater weight loss (R = 0.27, P = 0.01), and this is consistent with other reports.7 However, not all patients reporting severe anorexia find this a barrier to increasing nutritional intake. In this study, 36 patients reported high levels of anorexia (symptom severity >6/10), but in only four (11%) of these patients did the CNR‐JGH dietitians feel this was a major barrier to adherence with nutritional counselling advice. Of course, other symptoms have also been reported as barriers to adherence with nutritional counselling, including fatigue.15 However, although 40 patients reported severe fatigue, only five (12.5%) were assessed to have fatigue as a major barrier to adhering to nutritional advice. More generally, in the high‐symptom group, all of whom had two or more moderate–severe symptoms (see Material and methods section), 7/78 (9%) had no barriers, whereas 3/5 (60%) of patients in the low‐symptom group (see Material and methods section) had one or two barriers identified. Thus, frequently reported symptoms do not constitute a barrier to increasing nutritional intake, but the presence or absence of symptoms alone is not a reliable and specific indicator of clinically important barriers to adherence with nutritional advice.
Presence of barriers does not indicate patients are refractory to nutritional intervention
On average, patients had lost 1.6 (3.8) kg in 6 weeks prior to Visit 1. The mean weight change prior to Visit 1 for those with no barriers was less than those who did have barriers, but this did not reach statistical significance [−0.5 (4.0) vs. −1.7 (3.8) kg, P = 0.40]. When patients with only 0 or 1 barriers at Visit 1 and no oedema were selected (N = 59), there was no difference in weight loss in 6 weeks prior to the first CNR‐JGH clinic visit between those with none (−0.5 kg), symptom (−1.4 kg), and non‐symptom (−1.1 kg) barriers (ANOVA, P = 0.83).
In contrast, those patients with no barriers had a significantly greater dietary energy intake than those with barriers at Visit 1: 33.1 (8.0) vs. 20.5 (9.8) kcal/kg (P = 0.0005). Furthermore, there was an inverse correlation between the number of barriers and the number of dietary energy intake (R = −0.30, P = 0.004) (Figure 3A). Indeed, the average energy intake for the small number of patients with three barrier symptoms was only 13.9 (2.6) kcal/kg. When only patients with one barrier at Visit 1 were selected (N = 49), there was no difference between patients with symptom barriers and patients with non‐symptom barriers (20.2 vs. 20.8 kcal/kg, P = 0.99), but both groups had significantly lower energy intake than patients with no barriers (33.1 kcal/kg, ANOVA, P < 0.001).
Figure 3.
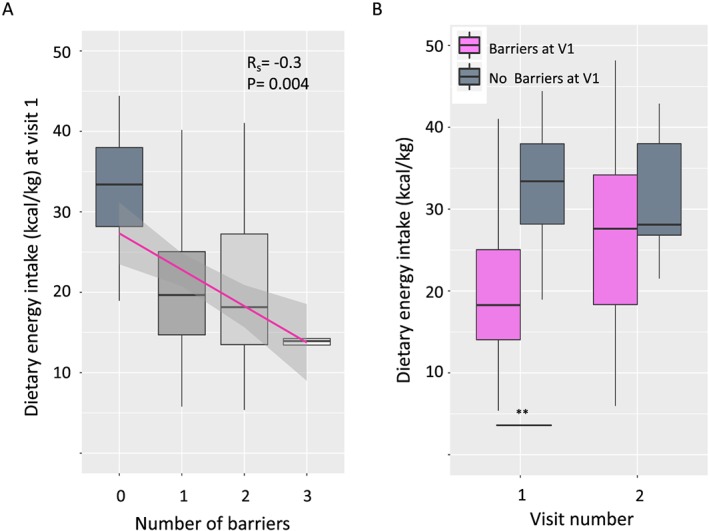
The number of dietitian‐identified barriers to adherence with nutritional counselling advice correlates with dietary energy intake at Visit 1 in patients attending CNR‐JGH. There was a significant negative correlation between the number of dietitian‐identified barriers to adherence with nutritional counselling advice and dietary energy intake at Visit 1 (Spearman's correlation coefficient, R s = −0.3, P = 0.004) (A). However, patients with barriers at Visit 1 were still able to increase their dietary energy intake, and by the time they attended for Visit 2, there was no difference in dietary intake between the two groups (**barriers vs. no barriers, t‐test, P = 0.0005).
Overall, patients that came to two visits (N = 51) remained weight stable between Visits 1 and 2 [mean (SD) weight change: 0.2 (2.0) kg]. There was also no significant difference in weight change between patients who did have barriers and did not have barriers at Visit 1 [no barriers vs. with barriers: 0.8 (2.6) vs. 0.1 (1.9) kg P = 0.57]. However, although both groups remained weight stable at Visit 2, patients with barriers at Visit 1 increased their dietary intake more than patients with no barriers (change in energy intake; barriers vs. no barriers: 379 (723) vs. −39 (299) kcal, P = 0.03). Thus, by Visit 2, both groups had similar energy intake: barriers vs. no barriers, 27.3 (10.2) vs. 31.5 (8.7) kcal/kg, P = 0.37 (Figure 3B).
Patients who came to two visits and had a maximum of one barrier at Visit 1 (N = 34) were categorized as (i) none, (ii) non‐symptom barrier, or (iii) symptom barrier. Between Visits 1 and 2, there was no significant difference in weight gain between these three groups (none, non‐symptom, and symptom barriers: 0.82, 0.06, 0.5 kg; P = 0.79). As mentioned earlier, those with no barriers at Visit 1 had little change in their intake at visit 2 [−39 (299) kcal]. However, those with a single non‐symptom‐related barrier at Visit 1 increased their intake by 406 (670) kcal at Visit 2, whereas for those patients with symptom‐related barriers, there was a small, non‐statistically significant reduction in their intake of −120 (501) kcal (ANOVA with Tukey's honestly significant difference, P = 0.08).
Conclusions
This is the first study to report on dietitian‐identified barriers to implementing nutritional advice in a cancer cachexia clinic. Around 89% of patients had at least one major barrier to successful nutritional intervention and 36% had two or more barriers (Figure 1). Whereas other authors have focused on the prevalence and negative impact of symptoms likely to impair dietary intake and hinder attempts to improve nutritional status in cancer cachexia,14, 15 the current study shows that in fact, non‐symptom‐related barriers are more prevalent. Thus, six non‐symptom‐related barriers accounted for 65% of all barriers identified (Figure 1).
The overall pattern of prevalence of barriers to nutrition was similar at both Visits 1 and 2, but at the individual patient level, nutritional barriers changed frequently. At Visit 2 (approximately 6 weeks later), around 50% of patients had a change where new barriers became evident or old ones resolved (Figure 2). Whilst screening patients for self‐reported symptoms has many merits, this approach alone lacks specificity and sensitivity to correctly identify genuine barriers to nutritional intervention. Thus, in <13% of patients reporting high levels of anorexia or fatigue was this symptom thought to be a major barrier to nutritional intervention. In contrast, 3/5 (60%) of patients reporting no nutrition‐related symptoms were assessed as having a barrier to successful nutritional intervention. At Visit 1, those patients with barriers had a significantly lower dietary energy intake than those without barriers (Figure 3). However, for patients attending the CNR‐JGH clinic, those with barriers at Visit 1 (either symptom‐related or non‐symptom‐related) were not refractory to nutritional intervention and, in fact, as a group, they achieved a significantly greater mean increase in dietary energy intake (than those with no barriers) and stabilized their weight. There were insufficient patients to draw detailed conclusions about the impact of each specific barrier, but there was a trend to suggest that attempts to increase dietary energy intake were more successful in those with non‐symptom‐related barriers.
We acknowledge the limitations of this observational study including the fact that the patients were drawn from a specialized cancer cachexia clinic. Referrals to the CNR‐JGH clinic are not confined to one hospital, and there is anecdotal evidence that many patients within the catchment area with weight loss are not referred. Thus, we cannot exclude the possibility of referral bias, for example, perhaps patients with more severe or refractory weight loss and related symptoms are more likely to be referred by their treating oncology teams. Whilst it is certainly possible that the frequency or severity of barriers identified in this study cohort is not truly representative of all advanced cancer patients with cachexia, the demographic, nutritional, and oncological characteristics of this patient group were very similar to other larger cohorts that we have reported on over the last 5 years,24, 25 suggesting that the results are at least representative of the wider CNR‐JGH patient population. The patients studied include a mix of cancer types with a predominance of lung and gastrointestinal cancers. It seems likely that similar surveys of more selected populations (e.g. head and neck or breast cancer) may show differences in the prevalence of specific barriers or highlight the importance of disease‐specific symptoms not identified in the current study. Furthermore, it is frequently the case that other members of the multidisciplinary team discover additional pertinent information that was not available to the dietitian when making their evaluation of the main barriers to adherence with nutritional advice. Hence, it is possible that discussion with other members of the team could have made the assessment of barriers even more complete or balanced.
The dietitians on the CNR‐JGH team calculated nutritional intake as part of their evaluation and collated information on likely barriers to nutritional intervention using all available information. This information included the dietitian's knowledge of the patient's medical and oncological history and current treatment, patient‐reported symptom questionnaire data, their weight loss history and the dietitian's own interaction with the patient and their family or carer. Whilst this was a practical approach, the decision about which problems constituted the main barriers at each visit was inherently subjective. Nevertheless, this professional evaluation of the likely key barrier(s) to adherence with nutritional advice in each individual is still an improvement on prior reports that have simply looked at correlations between reported symptoms and nutritional intake.14, 15 The dietitian was not blinded to nutritional intake data. However, it does not appear that this led to attribution of greater significance to symptoms in patients with poorer dietary intake; for example, of patients who reported severe anorexia, the proportion in whom anorexia was considered a barrier to adherence to nutritional advice was similar in those consuming poor vs. intermediate or sufficient diets (e.g. poor vs. other: 3/18 vs. 1/19, χ 2 test, P = 0.56).
Although it was not the primary purpose of this study, it is worth highlighting the challenges in proving that any given barrier is the limiting factor in adherence to nutritional advice. Sometimes, there are profound difficulties in determining the real barriers impeding behaviour change and the barrier category assigned at Visit 1 may prove to be incorrect (Table 3). Furthermore, if multiple barriers are identified that change over time (Figure 2B), it is also challenging to measure the relative contribution of each one in impeding adherence to nutritional counselling. The CNR‐JGH intervention is multidisciplinary, and this study did not include a control group who underwent the same evaluation but then received no nutritional counselling intervention. Hence, it is not possible to demonstrate the independent impact of the CNR‐JGH team intervention or to show what aspects of the CNR‐JGH intervention contributed to success in any one patient. Nevertheless, the range of barriers to adherence with nutritional advice described (Table 2) is broad and implies that maximizing adherence to nutritional advice requires a careful and comprehensive evaluation. A multidisciplinary team is needed, and input from other professionals including specialists in, for example, symptom‐control, dentistry, psycho‐social counselling, and social work, may each have a role in addressing the many different barriers to nutritional intervention identified.
Clearly, successful deployment of a nutritional intervention is impossible if the barriers to adherence to adequate nutrition are not identified and addressed. However, data on this critical aspect of nutritional care are often missing from interventional studies in cancer cachexia.7, 26, 27 The data from the current study are consistent with clinical experience in the CNR‐JGH clinic that supports an iterative approach to evaluation and planning nutritional management in cancer cachexia. Barriers change over time, and the nutritional interventions or approaches need to be adjusted to address these effectively. The true nature of the barriers to nutritional intervention may only become clear after several visits (Table 3, Patient 1), and even when the barriers are clear, building an effective therapeutic relationship can take time (Table 3, Patient 1).
Barrier symptoms may be difficult to overcome in some patients with advanced cancer, but in motivated patients, these do not necessarily impede their ability to follow nutritional recommendations. However, this study illustrates that there are frequently many additional factors at play that can impact on a patient's adherence to nutritional counselling. Thus, even in the absence of barrier symptoms, finding ways to address different perspectives about nutrition, to negotiate a mutually agreeable approach to achieve adequate energy and protein intake in weight‐losing patients and to effect the necessary behaviour change, is not always possible (Table 3, Patients 3 and 4). New tools and approaches are needed to help clinicians address this problem.
In summary, in this study of advanced cancer patients with cachexia, non‐symptom‐related issues are frequent and more commonly cause difficulties in adhering to nutritional advice than symptoms. To achieve optimal results with nutritional intervention for treatment or prevention of cancer cachexia, evaluation of patients should include assessment of both symptoms and non‐symptom‐related barriers.
Author contributions
R.T.J. and R.N. conceived the study. R.N. and C.V.D.B. collected the data on dietary barriers and performed the nutritional assessments. M.K. administered the questionnaires, processed and calculated the data from symptom questionnaires, and assembled all additional data. R.T.J. and R.N. performed the data analysis. R.T.J., RN., and C.V.D.B. wrote the initial manuscript. All authors contributed to editing and correcting the final manuscript.
Conflict of interest
None declared.
Funding
R.T.J. received salary support from the Peter Brojde Lung Cancer Centre and from the Backler Foundation, Jewish General Hospital Foundation. The CNR‐JGH received financial support from funds raised by the Angel Ball, Stephen and Lillian Vineberg, and the Lila Sigal Hockey Marathon.
Ethics statement
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle 28
Acknowledgements
The authors would like to thank their colleagues and other members of the McGill Cancer Nutrition Rehabilitation Program team at the Jewish General Hospital who were involved in the clinical care of the patients who are the subject of this report.
Nasrah R., Van Der Borch C., Kanbalian M., and Jagoe R. T. (2020) Defining barriers to implementation of nutritional advice in patients with cachexia, Journal of Cachexia, Sarcopenia and Muscle, 11: 69–78. 10.1002/jcsm.12490.
References
- 1. Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, et al. Prognostic effect of weight loss prior tochemotherapy in cancer patients. Am J Med 1980;69:491–497. [DOI] [PubMed] [Google Scholar]
- 2. Di Fiore F, Lecleire S, Pop D, Rigal O, Hamidou H, Paillot B, et al. Baseline nutritional status is predictive of response to treatment and survival in patients treated by definitive chemoradiotherapy for a locally advanced esophageal cancer. Am J Gastroenterol 2007;102:2557–2563. [DOI] [PubMed] [Google Scholar]
- 3. Nourissat A, Vasson M, Merrouche Y, Bouteloup C, Goutte M, Mille D, et al. Relationship between nutritional status and quality of life in patients with cancer. Eur J Cancer 2008;44:1238–1242. [DOI] [PubMed] [Google Scholar]
- 4. Odelli C, Burgess D, Bateman L, Hughes A, Ackland S, Gillies J, et al. Nutrition support improves patient outcomes, treatment tolerance and admission characteristics in oesophageal cancer. Clin Oncol 2005;17:639–645. [DOI] [PubMed] [Google Scholar]
- 5. Ross P, Ashley S, Norton A, Priest K, Waters J, Eisen T, et al. Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br J Cancer 2004;90:1905–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andreyev H, Norman A, Oates J, Cunningham D. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer 1998;34:503–509. [DOI] [PubMed] [Google Scholar]
- 7. Nasrah R, Kanbalian M, Van Der Borch C, Swinton N, Wing S, Jagoe R. Defining the role of dietary intake in determining weight change in patients with cancer cachexia. Clin Nutr 2018;37:235–241. [DOI] [PubMed] [Google Scholar]
- 8. Bourdel‐Marchasson I, Blanc‐Bisson C, Doussau A, Germain C, Blanc J‐F, Dauba J, et al. Nutritional advice in older patients at risk of malnutrition during treatment for chemotherapy: a two‐year randomized controlled trial. PLoS ONE 2014;9:e108687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bauer JD, Capra S. Nutrition intervention improves outcomes in patients with cancer cachexia receiving chemotherapy—a pilot study. Support Care Cancer 2005;13:270–274. [DOI] [PubMed] [Google Scholar]
- 10. Kabarriti R, Ohri N, Bontempo A, Romano M, Modi C, Viswanathan S, et al. The impact of dietary regimen compliance and sarcopenia in head and neck cancer patients treated with definitive radiation therapy. Int J Radiat Oncol Biol Phys 2015;93:E332–E333. [Google Scholar]
- 11. Hopanci Bicakli D, Ozkaya Akagunduz O, Meseri Dalak R, Esassolak M, Uslu R, Uyar M, et al. The effects of compliance with nutritional counselling on body composition parameters in head and neck cancer patients under radiotherapy. J Nutr Metab 2017;2017:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baldwin C, Spiro A, McGough C, Norman A, Gillbanks A, Thomas K, et al. Simple nutritional intervention in patients with advanced cancers of the gastrointestinal tract, non‐small cell lung cancers or mesothelioma and weight loss receiving chemotherapy: a randomised controlled trial. J Hum Nutr Diet 2011;24:431–440. [DOI] [PubMed] [Google Scholar]
- 13. Tung A‐C, Wen C‐F, Li P‐R. An evaluation on satisfaction with nutrition counseling for cancer inpatients. FASEB J 2016;30:676. [Google Scholar]
- 14. Kubrak C, Olson K, Jha N, Jensen L, McCargar L, Seikaly H, et al. Nutrition impact symptoms: key determinants of reduced dietary intake, weight loss, and reduced functional capacity of patients with head and neck cancer before treatment. Head Neck‐J Sci Spec 2010;32:290–300. [DOI] [PubMed] [Google Scholar]
- 15. Omlin A, Blum D, Wierecky J, Haile SR, Ottery FD, Strasser F. Nutrition impact symptoms in advanced cancer patients: frequency and specific interventions, a case–control study. J Cachexia Sarcopenia Muscle 2013;4:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martin L, de van der Schueren MA, Blauwhoff‐Buskermolen S, Baracos V, Gramlich L. Identifying the barriers and enablers to nutrition care in head and neck and esophageal cancers: an international qualitative study. JPEN J Parenter Enter Nutr 2016;40:355–366. [DOI] [PubMed] [Google Scholar]
- 17. Arends J, Bodoky G, Bozzetti F, Fearon K, Muscaritoli M, Selga G, et al. ESPEN guidelines on enteral nutrition: non‐surgical oncology. Clin Nutr 2006;25:245–259. [DOI] [PubMed] [Google Scholar]
- 18. Bauer JD, Ash S, Davidson WL, Hill JM, Brown T, Isenring EA, et al. Evidence based practice guidelines for the nutritional management of cancer cachexia. Nutr Diet 2006;63:S3–S32. [Google Scholar]
- 19. R Development Core , Team R. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2010. [Google Scholar]
- 20. Schag CC, Heinrich RL, Ganz P. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol 1984;2:187–193. [DOI] [PubMed] [Google Scholar]
- 21. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 22. Martin L, Senesse P, Gioulbasanis I, Antoun S, Bozzetti F, Deans C, et al. Diagnostic criteria for the classification of cancer‐associated weight loss. J Clin Oncol 2014;33:90–99. [DOI] [PubMed] [Google Scholar]
- 23. McMillan DC. The systemic inflammation‐based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev 2013;39:534–540. [DOI] [PubMed] [Google Scholar]
- 24. Parmar MP, Swanson T, Jagoe RT. Weight changes correlate with alterations in subjective physical function in advanced cancer patients referred to a specialized nutrition and rehabilitation team. Support Care Cancer 2013;21:2049–2057. [DOI] [PubMed] [Google Scholar]
- 25. Parmar MP, Vanderbyl BL, Kanbalian M, Windholz TY, Tran A‐T, Jagoe RT. A multidisciplinary rehabilitation programme for cancer cachexia improves quality of life. BMJ Support Palliat Care 2017;7:441–449. [DOI] [PubMed] [Google Scholar]
- 26. Temel JS, Abernethy AP, Currow DC, Friend J, Duus EM, Yan Y, et al. Anamorelin in patients with non‐small‐cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double‐blind, phase 3 trials. Lancet Oncol 2016;17:519–531. [DOI] [PubMed] [Google Scholar]
- 27. Fearon KC, Barber MD, Moses AG, Ahmedzai SH, Taylor GS, Tisdale MJ, et al. Double‐blind, placebo‐controlled, randomized study of eicosapentaenoic acid diester in patients with cancer cachexia. J Clin Oncol 2006;24:3401–3407. [DOI] [PubMed] [Google Scholar]
- 28. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017. J Cachexia Sarcopenia Muscle 2017;8: 1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]


