Abstract
Increasingly targeted in drug discovery, protein-protein interactions challenge current high throughput screening technologies in the pharmaceutical industry. Developing an effective and efficient method for screening small molecules or compounds is critical to accelerate the discovery of ligands for enzymes, receptors and other pharmaceutical targets. Here, we report developments of methods to increase the signal-to-noise ratio (SNR) for screening protein-protein interactions using atomic force microscopy (AFM) force spectroscopy. We have demonstrated the effectiveness of these developments on detecting the binding process between focal adhesion kinases (FAK) with protein kinase B (Akt1), which is a target for potential cancer drugs. These developments include optimized probe and substrate functionalization processes and redesigned probe-substrate contact regimes. Furthermore, a statistical-based data processing method was developed to enhance the contrast of the experimental data. Collectively, these results demonstrate the potential of the AFM force spectroscopy in automating drug screening with high throughput.
Keywords: Index Terms, Bionanotechnology, Nanosensors, Force spectroscopy, Atomic force microscopy, Protein-protein interactions
I. INTRODUCTION
The productivity of drug discovery has been an increasing concern in the past decade. Its limitations challenge the current business model of the pharmaceutical industry, and hinder the development of drugs for orphan diseases and diseases prevalent in undeveloped countries [1–3]. In the early stage of drug discovery, screening is applied to identify the “hits” from a library or from newly designed candidate drugs [4, 5]. The screening efficiency and effectiveness significantly affect the total productivity of drug discovery [6, 7]. To address this problem, many assays have been developed to screen compounds for pharmaceutical purposes. Biophysically, nuclear magnetic resonance (NMR), surface plasmon resonance (SPR), and isothermal titration calorimetry have been widely used [8]. Many biochemical assays, such as enzyme-linked immunosorbent assays (ELISA), fluorescence polarization, and fluorescence resonance energy transfer (FRET) are also common for this purpose [8].
Protein molecules mostly assemble together as a supramolecular complex to fulfill their physiological functions [9]. Playing a central role in physiological and pathological processes [10, 11], protein-protein interactions thus provide a rich source of potential therapeutic targets in drug discovery [12, 13]. Compared with traditional therapeutic targets, such as enzymes and G protein-coupled receptors, the interface of protein-protein interactions is large, often highly hydrophobic or charged. These unique characteristics of protein-protein interactions challenge the widely used high-throughput screening technologies mentioned above [14]. Each of these technologies has its own strengths and weaknesses, but some weaknesses are common to many. Some of the methods require fluorescence labelling, which changes the biophysical characteristics of the target proteins [15]. In addition, these methods have high false positive rates in testing protein-protein interactions [16]. Thus, an urgent and significant need exists for developing an effective and efficient method for screening protein-protein interactions [3, 17].
Since its emergence, nanotechnologies have been impacting screening in drug discovery [18, 19]. Among them, atomic force microscopy (AFM) was one of the earliest to be applied to drug discovery [20–23]. Not only can AFM image protein molecules in situ to investigate their morphology[24], but it also capable of measuring protein-protein interactions with pico-Newton (pN) resolution [25–29] to study their nanomechanical and adhesion properties [28–30]. This method was also used to analyse protein unfolding [29] and its structu characteristics [31]. AFM based force spectroscopy, quantifying the binding strength of protein affinity, has been considered as one of the promising candidates for drug screening [7, 32]. Using this method, the fluorescence staining process can be avoided, and the low false positive rate can achieved. Moreover, it can be equipped with other capabilities to achieve the high throughput needed for screening. A high-throughput drug discovery screening technique based on AFM force spectroscopy requires parallel and automated operations as well as integration with other biosensing technologies. To run the screening in parallel, the AFM system can be upgraded from a single cantilever to a cantilever array. In addition, th protein samples can also be patterned into arrays using state-ofthe-art patterning methods [33]. At present, several of these capabilities have been realized [34–36].
The application of AFM force spectroscopy on drug discovery suffers from low signal to noise ratio (SNR) as compared with other methods (Table I). Since drug screening aims to test whether the compounds promote or inhibit protein-protein interactions, the SNR in screening large number interactions is more important than accuracy. Though AFM force spectroscopy measures single molecular interactions with high resolution, a sufficient analysis requires hundreds of for curve measurements due to this low SNR.
Table I. SNR OF DRUG SCREEN METHODS.
Hence in this study, as initial steps toward developing an efficient screening method for inhibitors of protein-protein interactions, the process of AFM force spectroscopy was optimized to improve SNR. The characterization of Akt1 binding to FAK was selected as a prototypical example because of the clinical relevance of this molecular pair. Cancer cells upregulate their adhesiveness in response to physical forces, such as increased extracellular pressure and shear stress [44]. In this pathway, the binding of protein kinase B (Akt1) to focal adhesion kinase (FAK) is a critical step [45]. This biochemical response occurs in a variety of cancer types, including adenocarcinomas [45], squamous cell carcinomas [46], and even sarcomas [47]. Indeed, perturbing the interaction between FAK and Akt1 can substantially improve tumour-free survival in a mouse model [48]. Functionalizing FAK to an AFM probe and immobilizing Akt1 to a substrate, we first sought to improve the chemical functionalization method to minimize non-specific bindings. In addition, the AFM tip-substrate contact regimes were redesigned to ensure a robust interaction between the target molecules, including tip-substrate contact level and time, as well as the tip moving speed. Furthermore, we developed a novel data processing method based on statistical analysis to enhance SNR and the contrast between control and experimental samples. Equipped with an AFM based nanorobotics platform, this development will pave the way for high throughput screening in drug discovery.
II. MATERIALS AND METHODS
A. Materials and reagents:
APTES ((3-Aminopropyl) triethoxysilane), N,N-Diisopropylethylamine (DIEA), triethylamine (TEA) and chloroform were purchased from Sigma Aldrich (St. Louis, MO). NHS-PEG-MAL was purchased from JenKem Technology (Plano, TX). SATP (N-succinimidyl-S-acetylthiopropionate) and secondary antibody for fluorescence detecting were from Thermo Fisher Scientific (Waltham, MA). Akt1 molecules were purchased from Origene (Rockville, MD). PD-10 columns were purchased from GE Healthcare Life sciences (Pittsburgh, PA). The AFM system used in this research was a Bioscope from the Bruker (Santa Barbara, CA). AFM probes were also purchased from the Bruker.
B. AFM probe functionalization
To measure molecular interactions, the two proteins of interest were coated onto an AFM probe and a flat substrate, respectively. In this study, silicon nitride cantilevers with a spring constant of ~0.06 N/m were used. Before each experiment, the spring constant of the cantilevers was measured using the thermal tuning method [49]. The tip functionalization method was developed by H Schindler et al. [10].
First, the AFM probe was cleaned in chloroform for an hour. Cleaned probes were rinsed in fresh chloroform and blown dry by argon gas. Probes were then processed in an oxygen plasma cleaner to enhance the hydroxyl group density on the silicon nitride surface. APTES was subsequently coated onto the plasma treated AFM probes via gas phase deposition in a chamber filled with argon gas (APTES, 45 μl, DIEA, 15 μl). The APTES coated probes were then functionalized with PEG linker protein with its two ends grafted by NHS-and MAL-groups (NHS-PEG-MAL). The PEG linker molecule-coated AFM probes were incubated in SATP-functionalized FAK (target protein) molecules for 2–3 hours. The functionalized probes were rinsed three times with phosphate buffered saline (PBS), and then stored in PBS at 4°C before use. To graft the FAK molecule with SATP, SATP solution was mixed with FAK solution, with the molar concentration of SATP 10 times higher than that of FAK to ensure that all FAK molecules were functionalized. After incubating the SATP-FAK mixture for 15 minutes, the solution was then eluted through a PD-10 column. 500 μl of SATP-FAK solution was dipped into a PD-10 column each time for 9 times. The 7th and 8th eluates were collected for usage.
C. Substrate functionalization
Akt1 molecules were directly deposited onto polystyrene (PS) substrate via hydrophobic interactions [50, 51]. Before each experiment, Akt1 molecules were incubated on the surface of a petri dish for 2 hours, and the dish was rinsed with PBS. Due to the hydrophobicity of polystyrene substrate, the Akt1 molecules were coated onto a local region of the petri dish surface. Regions without coating in the same dish were used as a negative control. In another experimental condition, the Akt1 molecules were deposited via APTES linker molecule. Fresh mica substrates were first functionalized by APTES, by the same procedure described in the AFM probe functionalization section above. The Akt1 molecules then bind to the amino groups of APTES molecules.
D. Immunofluorescence staining
To visualized substrate functionalization, FAK molecules were deposited on to APTES coated mica substrate and polystyrene substrate. After coating, the samples were blocked in 5% BSA solution for 1 hour. Following this, the samples were incubated in secondary fluorescence antibody solution for 1 hour in dark. The secondary antibody was Goat anti-Mouse Secondary Antibody with Alexa Fluor 488 from Invitrogen. The samples were rinsed with PBS for three times, and five minutes each time. After that, the samples were imaged with Nikon fluorescence microscope TE1000 (Nikon Instruments Inc., Melville, NY, the USA). The images were captured by CoolSNAP HQ2 CCD Camera (Photometrics, Tucson, AZ), and processed using ImageJ (NIH, Bethesda, MD)
E. AFM based single molecular measurement
In this research, FAK molecules were fixed onto an AFM tip, while Akt1 was deposited onto a flat substrate. In the force spectroscopy experiment, forces rupturing the FAK-akt1 binding were measured by the deflection of the AFM cantilever. The rupture force of molecular pairs relaxed the AFM cantilever deflection during the retraction process, as represented by the sudden drop in measured force. To reduce the influence of capillary forces, and maintain the native conformation of protein molecules, AFM force spectroscopy experiments were performed in PBS. The moving range of the AFM probe was confined within 200 nm. The force spectroscopy was set to trigger mode with a triggering threshold of 5 nm. This parameter was also optimized ranging from 1 nm to 10 nm. For each experimental condition, a 20 by 20 array of spots was probed with a 500 nm pitch size. The tip moving velocity and contact level of the AFM tip were optimized in this study. The binding force was calculated using a customized script in MATLAB (MathWorks Inc.).
III. RESULT
A. Optimizing substrate functionalization to reduce non-specific bindings
APTES has been widely used as a linker molecule to functionalize the surface of mica, silicon or silicon nitride substrates [52–54]. In aqueous solutions, however, APTES molecules aggregate [55]. This uneven distribution will be translated to the non-uniform distribution of the protein molecules of interest, Akt1 (Fig. 1 (a)). In addition, APTES molecules are positively charged under physiological conditions, which increases their electrostatic interactions with the negative charged protein molecules on the AFM probe. Both factors increase the probability of undesired non-specific bindings. On APTES-coated substrates, the measured binding force between FAK molecules and the substrate increased significantly compared with data from substrates without APTES coating (Mica-APTES, Fig. 2 (a)). Depositing Akt1 molecules onto APTES coated mica substrates changed the distribution of binding force (Mica-APTES-AKT, Fig. 2 (b)). The 10 pN peak rose to more than 60% in magnitude, signifying that non-specific binding events were amplified. The Akt1 molecules grafted on top of APTES significantly suppressed the binding probability of FAK to APTES. The peak located at around 80 pN (in Mica-APTES, Fig. 2(b)), representing the binding force between FAK and APTES molecules, disappeared. Meanwhile, the FAK-Akt1 interactions require specific orientations of the two molecules. Thus, the binding probability of FAK-Akt1 is much lower than that of FAKAPTES. This increases the percentage of non-specific bindings, the peak at 10 pN. Further, due to the large binding force of FAK-APTES, the two distributions overlap significantly, making it difficult to distinguish the two interactions (Fig. 2 (b)).
Fig. 1.
Directly depositing Akt1 molecules on polystyrene substrate improves the distribution of molecules on the substrate. (a) Fluorescence imaging of Akt1 molecules deposited onto an APTES functionalized mica substrate. (b) Fluorescence image of Akt1 molecules deposited onto a fresh polystyrene substrate. Scale bar: 10 μm.
Fig. 2.
Optimization of substrate functionalization method to reduce non-specific bindings. The Y axis, frequency, defines the ratio of data points in a specific binding force range and the total data points plotted. (a) Histogram of binding forces between FAK and APTES functionalized mica, and betwee FAK and fresh mica. (b) Histogram of binding forces between FAK and APTES functionalized mica, and between FAK and Akt1 on APTES functionalized mica. (c) Histograms of binding forces between FAK and PS (polystyrene), and between FAK and APTES functionalized mica. (d, and e) BSA blocking inhibits non-specific binding between FAK and Akt1 molecules. Histogram of binding forces between FAK and Akt1 molecules without (d) and with (e) BSA blocking on PS substrate.
To avoid the undesired bindings caused by APTES, Akt1 molecules were then deposited onto a fresh polystyrene (PS) substrate directly. The hydrophobic interactions of Akt1 with the PS substrate are much stronger than the specific binding between FAK and Akt1. Akt1 molecules that are directly deposited onto fresh PS substrates exhibit significantly improved uniformity compared with Akt1 molecules binding to APTES functionalized PS substrates (Fig. 1 (b)). In addition, non-specific binding forces between the substrate and the FAK molecules increased from 10 pN in mica-APTES substrate to 30 pN in fresh PS substrate. However, the strong interactions between FAK and APTES were avoided (Fig. 2 (c)). Thus, it is easy to distinguish the interactions of FAK-Akt1 and FAK-PS. Binding forces in FAK-PS were mainly restricted in the range smaller than 80 pN (PS, Fig. 2 (d)), while a large portion of FAK-Akt1 binding forces were greater than 80 pN. (AKT, Fig. 2 (d)).
In this drug discovery scenario, the signal here means interaction between FAK and Akt1 via specific interactive binding sites. Besides the strong non-specific binding forces from APTES, the non-specific binding between FAK and Akt1, and FAK and substrate also limited the SNR. Bovine serum albumin (BSA) has been widely used in immunofluorescence staining and western blotting to reduce the non-specific binding. In the context of AFM force spectroscopy, BSA coating to PS substrates increased the percentage of the non-specific binding forces at 30 pN in the experimental group from 45% (PS, Fig. 2 (d)) to 80% (PS-BSA, Fig. 2 (e)). However, BSA blocking also reduced the possibilities of FAK-Akt1 interactions in experimental group, which decreased their binding forces (Fig. 2 (e)). The peak in the histogram is at 75 pN. In both cases, the non-specific binding maintained as ~30 pN. Overall, the SNR was improved dramatically by coating the protein uniformly onto substrate and blocking with BSA.
B. Optimizing AFM tip-substrate interaction
The molecules on AFM tip interact with their counterparts on the substrate when they contact with each other. The characteristics of their contact regime, along with the mechanism of molecular interactions, determines the measured inter molecular forces. To improve the SNR, three parameters of the contact regime were optimized: level and time of AFM tip-substrate contact, and AFM tip moving speed during tip-substrate separation. The values used during experiments are provided in Table II.
TABLE II. PARAMETERS OF CONTACT REGIME.
| Parameter | Contact level | Contact time | Tip moving speed |
|---|---|---|---|
| Contact level (nm) | 1, 3, 5, 10 | 5 | 5 |
| Contact time (ms) | No delay | 500, 1000 | 1000 |
| Tip moving speed (nm/s) | 100 | 100 | 100, 200, 400 |
| Tip moving distance (nm) | 200 | 200 | 200 |
Each column is an experimental condition during optimization.
1). AFM tip-substrate contact level
AFM tip-substrate contact level affects the distribution of the binding forces between FAK-Akt1 molecular pairs. AFM tip contact level is related to the size of interface between surface molecules on the AFM probe and the substrate. Enlarging this interface will increase the number of FAK-Akt1 molecular pairs, which tends to increase the measured binding forces. To test this hypothesis, four contact levels were tested: 1 nm, 3 nm, 5 nm, and 10 nm. In FAK-PS negative control groups, tip contact depth affected the binding force minimally. As the depth increased from 1 nm to 10 nm, larger binding forces emerged; however, the majority of the measured binding force is concentrated at 30 pN, which can be interpreted as noise (FAK-PS in Fig. 3 (e–h)). On the other hand, measured binding forces between FAK-Akt1 molecular pairs increased significantly with the increased contact levels. The peaks of the distribution were located at around 50 pN and 130 pN for 1 nm contact level, around 70 pN and 150 pN for 3 nm contact level, around 80 pN and 110 pN for 5 nm contact level, and around 80 pN, 144 pN and 215 pN for 10 nm contact level (Fig. 3 (a–d)).
Fig. 3.
Optimization of the AFM tip contact level: increasing tip contact level increases the binding forces in FAK-Akt1 and FAK-PS, respectively. Histogram of binding forces of FAK-Akt1 at different contact levels: 1 nm (a), 3 nm (b), 5 nm (c), 10 nm (d). Histogram of binding forces of FAK-PS at different contact levels: 1 nm (e), 3 nm (f), 5 nm (g), 10 nm (h).
2). AFM tip-substrate contact time
Forming specific binding requires a particular orientation of the molecular pairs. Considering the time cost of orienting molecules, forming a specific binding might require a much longer time than forming a non-specific binding [56]. We therefore evaluated the effect of tip-substrate contact time on the measured binding force. Two tip-substrate contact times were tested: 500 ms and 1000 ms. In FAK-PS negative control group, the binding force histogram distribution maintained the same pattern with the two contact times: 98% and 93% of measured binding force is concentrated around peaks at 24 pN and 36 pN for contact times of 500 ms and 1000 ms, respectively (FAK-PS, Fig. 4). In FAK-Akt1 experimental groups, the binding forces of FAK-Akt1 interactions are increased significantly. Two peaks occurred at 50 pN and 70 pN when the contact time increasing from 500 ms to 1000 ms (Fig. 4 (b)).
Fig. 4.
Optimization of the tip-substrate contact time. (a-b) Histogram o inding forces between FAK-Akt1 (blue bars) and FAK-PS (gray bars) with tip-substrate contact time as 500 ms (a) and 1000 ms (b).
3). AFM tip moving speed
It has been reported that the moving speed of AFM tip affected the measured binding force significantly [57–59]. Increasing the tip moving speed increases the SNR [38]. Three moving speeds of the AFM tip were tested: 100 nm/s, 200 nm/s, and 400 nm/s. In the FAK-PS case, the non-specific binding force increased as the moving speed of AFM tip is increased (FAK-PS, Fig. 5 (a–c)). However, this influence is minimal, as the binding forces mainly distributed in the range from 20 pN to 40 pN; 97%, 94%, and 87% for tip moving speeds of 100 nm/s, 200 nm/s, and 400 nm/s, respectively, which is consistent with previous reports [58, 60]. In the FAK-Akt1 case, however, the best measurement occurred at a medium speed level, 200 nm/s (FAK-AKT, Fig. 5 (a–c)). At the speed of 200 nm/s, the measured FAK-Akt1 interactions exhibited a Gaussian distribution (Fig. 5 (b)). The peaks of the binding force are located at 60 pN and 80 pN for tip moving at 100 nm/s and 200 nm/s. Two distribution peaks existed at 50 pN and 90 pN for tip moving speed at 400 nm/s. At the speed of 400 nm/s, the binding force at 30 pN, which interpreted as non-specific binding, increased to more than 50% compared (Fig. 5 (c)) with 30% and less than 5% at the tip moving speed at 100 nm/s and 200 nm/s (Fig. 5 (a and b)).
Fig. 5.
Optimization of tip moving speed. Histogram of FAK-Akt1 and FAKPS binding forces with tip moving speeds at 100 nm/s (a), 200 nm/s (b), and 400 nm/s (c).
C. Statistical analysis-based data processing
Besides optimizing the experimental conditions, data processing and visualization methods also affects the perceived SNR. Assuming that the molecules on AFM tip has the same probabilities to form molecular pairs with the molecules on the substrate, the measured AFM force spectroscopy data follow a Poisson distribution [61–63]. After mathematical manipulation, the binding force of single molecular pair can be estimated by the following equation [61]:
| (1) |
| (2) |
In this equation, μm and are the mean and standard deviation of the measured binding forces, Fi is the binding force is the mean number of molecular of a single molecular pair, μn is the sum of all non-specific bindings. A linear regression analysis of the measured data reveals the binding force of a single molecular pair, Fi. Assuming Fi does not change during the experiment, the experimental data with larger measured binding force will also have a larger standard deviation. Considering the binding force in experimental groups (FAK-Akt1) is much larger than that in negative control groups, the standard deviation should increase much faster in FAK-Akt1 than that in FAK-PS groups. Here we presented the mean value and standard deviation of the experimental data in different experimental conditions: the level and time of tip-substrate contact and AFM tip moving speeds. In all experimental conditions, the mean value of experimental and control groups is approximately 63.1 ± 24.7 pN, and 27.5 ± 2.8 pN (mean ± SD), respectively (Fig. 6 (a, c, and e)). However, the standard deviation varies 10 to 100 times between these two groups (Fig. 6 (b, d, and f)). On average, the standard deviations of FAK-Akt1 and FAK-PS binding forces are 2311.1 ± 1226.1 pN2 and 70.7 ± 75.5 pN2, respectively. Thus, the standard deviation is a better parameter to enhance the contrast between experimental and negative control groups in screening drug candidates, as shown in Fig. 6.
Fig. 6.
Standard deviation (SD) of binding force data presents the FAK-Akt1 and FAK-PS binding force with high contrast. (a, c and e) the mean of FAKAkt1 and FAK-PS binding forces with different: (a) tip-substrate contac levels, (c) tip-substrate contact times, and (e) tip moving speeds. (b, d and f) the mean of FAK-Akt1 and FAK-PS binding forces with different: (b) tip-substrate contact levels (d) tip indentation times, and (f) tip movin speeds.
IV. DISCUSSION
In this research, the substrate functionalization method and tip-substrate contact regime have been optimized to improve the SNR when measuring protein-protein interactions. Three factors in tip-substrate contact regime were optimized: the level and time of tip-substrate contact, and the tip moving speed before and after tip-substrate contact.
Directly depositing protein molecules onto PS substrates via hydrophobic interactions generates a uniform protein layer. One concern that might be raised is whether a monolayer of Akt1 molecules were deposited or not. We did not specifically prove that this deposition process creates a monolayer of molecules. However, we are ultimately trying to model FAKAkt1 interaction in living cells, where there are neither monolayers nor multilayers of Akt1, but where multiple different molecules may interact in suspension within the cytosol or in multi-protein complexes.
The measured protein-protein interactions with the new substrate functionalization method is in the same range reported previously. Specifically, there have been reports showing that non-specific binding forces below 10 pN [22]. Although, we have not been able to find publications reporting the binding force between FAK and Akt1 molecules, with repeated experiments showing the peak binding forces at 80 pN, we have no reason to believe it is not the interaction force between FAK and Akt1. In fact, this force falls in the same range as other binding forces between a pair of protein molecules with affinity [22, 27].
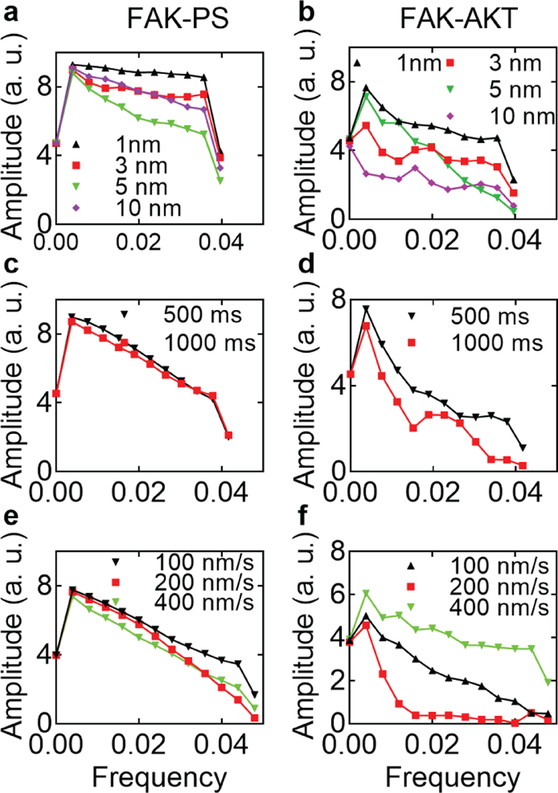
Discrete Fourier transform, implemented by Fast Fourier transform (FFT), illuminates the periodic characters in discrete input data sequence as well as the relative strengths of each periodic component. The experimental data were analyzed by FFT to reveal their frequency distributions. Increasing tip-substrate contact levels narrowed the frequency distribution in negative control groups, FAK-PS. (Fig. 7 (a)). Larger binding forces emerged in deeper contact levels and expanded the range of measured binding forces. This enlarged range of force distributions decreases the energy at high frequencies. In the FAK-Akt1 group, increasing tip contact depth changed the distribution dramatically. As the contact depth increased, the relative amplitude strengths of higher frequencies increased. This means that more peaks with a small period exist. This is consistent with the analysis of the distribution. These results indicate that increasing contact depth increased the observed binding forces by forming more interacting molecular pairs. Together with the histogram data, FFT analysis results revealed that contact depth affects binding forces in the FAK-Akt1 experimental groups than that in the FAK-PS negative control groups. Furthermore, compared with other contact levels, the contact level of 5 nm has unique characteristics. In both FAKAkt1 and FAK-PS groups, the amplitude at high frequencies keep at low level. This character also presented as a single peak in the histogram (Fig. 3 (c and g)). Based on these results, we selected a depth of 5 nm for further experiment.
Fig. 7.
Fast Fourier transform (FFT) analysis of the histogram data from measured binding forces in various experimental conditions. (a, b) FFT of the histogram data of binding forces between FAK-Akt1 (a) and FAK-PS (b) at different tip-substrate contact levels. (c, d) FFT of the histogram data binding forces between FAK-Akt1 (c) and FAK-PS (d) with different tip-substrate contact times. (e, f) FFT of the histogram data of binding forces between FAK-Akt1 (e) and FAK-PS (f) with the three tip moving speeds.
Regarding the tip-substrate contact time, FFT analysis further confirmed the minimal effect of contact time for negative control groups, with two curves almost overlapping with each other. (Fig. 7 (c)). This result is consistent with previous report [56]. In the FAK-Akt1 groups, however, increasing tip-substrate contact time elevated the measured binding force significantly. The frequencies at larger binding forces increased at longer contact time. Binding and unbinding of FAK-Akt1 molecular pairs are dynamic processes, which require specific orientation of the target molecules. A longer reaction time permits the formation of more binding events before the reaction reaches equilibrium. FFT analysis presented a peak at 0.027 pN−1 (Fig. 7 (d)), which represent that a high component of period of 37 pN. This is consistent with histogram analysis.
Regarding the tip moving speeds during tip-substrate contact, in the FAK-PS group, as the moving speed increased, the percentage of binding forces at 20 pN is transferred to 30 pN and 40 pN. The FFT analysis also confirmed that the tip moving speed affected the binding force minimally in the negative control groups (Fig. 7 (e)). In the FAK-Akt1 group, FFT results revealed that most of the energy is distributed in low frequency ranges with tip moving speed at 200 nm/s, which is consistent with the single Gaussian distribution (Fig. 7 (e)). At 200 nm/s, the SNR improved dramatically, as the overlap area between FAK-PS and FAK-AKT1 groups was significantly reduced. The average of FAK-AKT1 binding force is around 3 times larger than that of the FAK-PS group (Fig. 6(e)). Considering the standard deviation of the binding forces as the signal (Fig. 6(f)), the signal is 80 times larger than that of the noise, as the standard deviation of the FAK-PS group. These results demonstrated that the methods improved the SNR significantly compared with previous reports [37, 38].
The existence of an optimal tip moving speed differs from the theoretical analysis in the literature which suggests that faster tip moving speed induces stronger binding forces [58, 59]. This change might be due to the differences in experimental conditions. In previous studies, molecules were fixed to the substrate via a linker molecule or on gold surface [29, 64]. The Akt1 molecules in the current study were directly deposited onto the substrate via hydrophobic interactions. The structure of a complex organic molecule will change during forming and breaking a molecular bond, which affects the number of available hydrophobic sites. The high rates of non-specific bindings in experimental group with 400 nm/s tip moving speed, indicated that the Akt1 molecules might be released from PS substrate. Collectively, these results demonstrated that an optimum moving speed exists for measuring the binding force in current experimental condition. Further mechanistic analysis is needed to fully understand this process.
This statistical analysis method for presenting the data also reveals new insights for the influences of different parameters on the measured binding forces. Increasing the tip-substrate contact level increases the mean value, 23.3% and 33% in FAKAkt1 and FAK-PS groups, respectively (Fig. 6 (a, b)). However, the standard deviation increases 94% and 85.4%, respectively. This means that contact depths increase the noises more than the signals. Tip-substrate contact time minimally affects negative control groups but increases the mean and standard deviation in the experimental group 29.1% and 14.7%, respectively (Fig. 6 (c, d)). This means that tip-substrate contact time mainly amplifies the signal with little effect on the noise level. The tip moving speed increases the mean value of the measured binding forces but does not affect their standard deviation in FAK-Akt1 groups. This is consistent with the data in the histogram. Moving speed mainly shifts the peak right and does not affect the range (Fig. 6 (e, f)). According to (2), this can be interpreted as the tip moving speed elevating the measured binding forces of a single molecular pair, consistent with previous models [59].
Certainly, there is more work to be done to combine all these advances to utilize AFM force spectroscopy as a high throughput screening technology. However, throughput may be a big hurdle if large numbers of replicates are required. We therefore sought here to improve the efficiency and effectiveness of the screening process itself by enhancing the SNR. In this way, the total number of force curves required to generate an identifiable contrast between experimental and control groups will be significantly reduced, thus increasing the throughput of this screening method.
V. CONCLUSION AND FUTURE PERSPECTIVES
In sum, toward developing an effective and efficient high throughput screening method for drug discovery using AFM force spectroscopy, the experimental settings were optimized to improve the SNR in screening protein-protein interactions. New substrate functionalization method was developed to reduce the noises. Experimental parameters, including the level and time of tip-substrate contact and tip moving speeds, were optimized towards this application to enhance the SNR. A new data processing method based on statistical analysis was also developed to enhance the contrast between experimental and control groups. Collectively, these techniques may facilitate the development of an AFM based high throughput screening system.
Further, besides the high sensitivity of AFM force spectroscopy, it can also integrate with other biosensing or sample handling technologies. Integrating with microfluidics, the molecules involved in the screening process can be in small volume and be changed in a fast manner [65]. To minimize the size of the device and to simplify the design, the complicated laser based position sensing device can be replace by piezoelectric sensing device [66]. Fluorescence based biosensor can also be integrated with AFM force spectroscopy to characterize the protein-protein interaction both at single molecular and large volume level. These progresses, parallelizing and automating the system operation, integrating with other biosensing and system-enabling technologies, and optimizing interaction regimes presented in this paper, altogether formed a concrete foundation to develop an AFM force spectroscopy-enabled high-throughput screening of protein-protein interactions for drug discovery.
ACKNOWLEDGMENT
We, the authors, thank Dr. Mi Li for his help in experimental techniques. We thank Dr. Jing Yu for her help on arranging the experiments.
R. Yang acknowledges the funding from the Nebraska Center for Integrated Biomolecular Communication (NIH National Institutes of General Sciences P20 GM113126), from Nebraska Center for Nanomedicine (GM127200), from NSF award 1826135.
Contributor Information
Yongliang Yang, Department of Electrical and Computer Engineering, Michigan State University, East Lansing, MI, 48823, USA.; Department of Biomedical Engineering, Michigan State University.
Bixi Zeng, Departments of Surgery and Biomedical Sciences, University of North Dakota, Grand Forks, ND, 58202, USA..
Zhiyong Sun, Department of Electrical and Computer Engineering, Michigan State University, East Lansing, MI, 48823, USA.; Department of Industrial and Manufacturing Systems Engineering, The University of Kong, Hong Kong, China.
Amir Monemian Esfahani, Department of Mechanical and Materials Engineering, University of Nebraska Lincoln, NE 68588 USA..
Jing Hou, School of Information and Control Engineering, Shenyang Jianzhu University, Shenyang 110168, China..
Liangliang Chen, Department of Electrical and Computer Engineering, Michigan State University, East Lansing, MI, 48823, USA..
Marc D Basson, Departments of Surgery and Biomedical Sciences, University of North Dakota, Grand Forks, ND, 58202, USA..
Lixin Dong, Department of Electrical and Computer Engineering, Michigan State University, East Lansing, MI, 48823, USA..
Ruiguo Yang, Department of Mechanical and Materials Engineering, University of Nebraska Lincoln, NE 68588 USA..
Ning Xi, Department of Electrical and Computer Engineering, Michigan State University, East Lansing, MI, 48823, USA.; Department of Industrial and Manufacturing Systems Engineering, The University of Kong, Hong Kong, China.
REFERENCES
- [1].Shih H-P, Zhang X, and Aronov AM, “Drug discovery effectiveness from the standpoint of therapeutic mechanisms and indications,” Nature Reviews Drug Discovery, vol. 17, pp. 19, 10/27/online, 2017. [DOI] [PubMed] [Google Scholar]
- [2].Paul SM, Mytelka DS, Dunwiddie CT, Persinger CC, Munos BH, Lindborg SR, and Schacht AL, “How to improve R&D productivity: the pharmaceutical industry’s grand challenge,” Nature Reviews Drug Discovery, vol. 9, pp. 203, 2010. [DOI] [PubMed] [Google Scholar]
- [3].Pammolli F, Magazzini L, and Riccaboni M, “The productivity crisis in pharmaceutical R&D,” Nature Reviews Drug Discovery, vol. 10, pp. 428, 06/01/online, 2011. [DOI] [PubMed] [Google Scholar]
- [4].Hood JD, and Cheresh DA, “Role of integrins in cell invasion and migration,” Nature Reviews Cancer, vol. 2, no. 2, pp. 91-+, February, 2002. [DOI] [PubMed] [Google Scholar]
- [5].Blanchoin L, Boujemaa-Paterski R, Sykes C, and Plastino J, “ACTIN DYNAMICS, ARCHITECTURE, AND MECHANICS IN CELL MOTILITY,” Physiological Reviews, vol. 94, no. 1, pp. 235–263, January, 2014. [DOI] [PubMed] [Google Scholar]
- [6].Bleicher KH, Böhm H-J, Müller K, and Alanine AI, “Hit and lead generation: beyond high-throughput screening,” Nature Reviews Drug Discovery, vol. 2, pp. 369, 05/01/online, 2003. [DOI] [PubMed] [Google Scholar]
- [7].Renaud JP, Chung CW, Danielson UH, Egner U, Hennig M, Hubbard RE, and Nar H, “Biophysics in drug discovery: impact, challenges and opportunities,” Nature Reviews Drug Discovery, vol. 15, no. 10, pp. 679–698, October, 2016. [DOI] [PubMed] [Google Scholar]
- [8].Zhou M, Li Q, and Wang R, “Current Experimental Methods for Characterizing Protein–Protein Interactions,” ChemMedChem, vol. 11, no. 8, pp. 738–756, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bai Y, Luo Q, and Liu J, “Protein self-assembly via supramolecular strategies,” Chemical Society Reviews, vol. 45, no. 10, pp. 2756–2767, 2016. [DOI] [PubMed] [Google Scholar]
- [10].Arkin MR, and Wells JA, “Small-molecule inhibitors of protein– protein interactions: progressing towards the dream,” Nature Reviews Drug Discovery, vol. 3, pp. 301, 04/01/online, 2004. [DOI] [PubMed] [Google Scholar]
- [11].Rone MB, Fan J, and Papadopoulos V, “Cholesterol transport in steroid biosynthesis: Role of protein–protein interactions and implications in disease states,” Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids, vol. 1791, no. 7, pp. 646–658, 2009/07/01/, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Scott DE, Bayly AR, Abell C, and Skidmore J, “Small molecules, big targets: drug discovery faces the protein–protein interaction challenge,” Nature Reviews Drug Discovery, vol. 15, pp. 533, 04/11/online, 2016. [DOI] [PubMed] [Google Scholar]
- [13].Thiel P, Kaiser M, and Ottmann C, “Small-Molecule Stabilization of Protein–Protein Interactions: An Underestimated Concept in Drug Discovery?,” Angewandte Chemie International Edition, vol. 51, no. 9, pp. 2012–2018, 2012/02/27, 2012. [DOI] [PubMed] [Google Scholar]
- [14].Mullard A, “Protein–protein interaction inhibitors get into the groove,” Nature Reviews Drug Discovery, vol. 11, pp. 173, 03/01/online, 2012. [DOI] [PubMed] [Google Scholar]
- [15].Cooper MA, “Optical biosensors in drug discovery,” Nature Reviews Drug Discovery, vol. 1, pp. 515, 07/01/online, 2002. [DOI] [PubMed] [Google Scholar]
- [16].Curpan RF, Simons PC, Zhai D, Young SM, Carter MB, Bologa CG, Oprea TI, Satterthwait AC, Reed JC, Edwards BS, and Sklar LA, “High-Throughput Screen for the Chemical Inhibitors of Antiapoptotic Bcl-2 Family Proteins by Multiplex Flow Cytometry,” ASSAY and Drug Development Technologies, vol. 9, no. 5, pp. 465–474, 2011/10/01, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Arkin MR, Tang Y, and Wells JA, “Small-molecule inhibitors of protein-protein interactions: progressing toward the reality,” Chem Biol, vol. 21, no. 9, pp. 1102–14, September 18, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wakabayashi N, Shin S, Slocum SL, Agoston ES, Wakabayashi J, Kwak M-K, Misra V, Biswal S, Yamamoto M, and Kensler TW, “Regulation of Notch1 Signaling by Nrf2: Implications for Tissue Regeneration,” Science Signaling, vol. 3, no. 130, pp. ra52–ra52, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cialfi S, Palermo R, Manca S, De Blasio C, Romero PV, Checquolo S, Bellavia D, Uccelletti D, Saliola M, D’Alessandro A, Zolla L, Gulino A, Screpanti I, and Talora C, “Loss of Notch1-dependent p21(Waf1/Cip1) expression influences the Notch1 outcome in tumorigenesis,” Cell Cycle, vol. 13, no. 13, pp. 2046–2055, July 1, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hsiao P-J, Jao J-C, Tsai J-L, Chang W-T, Jeng K-S, and Kuo K-K, “Inorganic arsenic trioxide induces gap junction loss in association with the downregulation of connexin43 and E-cadherin in rat hepatic “stem-like” cells,” The Kaohsiung Journal of Medical Sciences, vol. 30, no. 2, pp. 57–67, 2014/02/01/, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nriagu J, Lin T-S, Mazumder DG, and Chatterjee D, “E-cadherin polymorphisms and susceptibility to arsenic-related skin lesions in West Bengal, India,” Science of The Total Environment, vol. 420, pp. 65–72, 2012. [DOI] [PubMed] [Google Scholar]
- [22].Patterson Kevin C., Yang R, Zeng B, Song B, Wang S, Xi N, and Basson Marc D., “Measurement of Cationic and Intracellular Modulation of Integrin Binding Affinity by AFM-Based Nanorobot,” Biophysical Journal, vol. 105, no. 1, pp. 40–47, 2013/07/02/, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yang Y, Yu J, Esfahani AM, Seiffert-Sinha K, Xi N, Lee I, Sinha AA, Chen L, Sun Z, and Yang R, “Single-Cell Membrane Drug Delivery using Porous Pen Nanodeposition,” Nanoscale, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Scaramuzzo FA, Salvati R, Paci B, Generosi A, Rossi-Albertini V, Latini A, and Barteri M, “Nanoscale In Situ Morphological Study of Proteins Immobilized on Gold Thin Films,” The Journal of Physical Chemistry B, vol. 113, no. 48, pp. 15895–15899, 2009/12/03, 2009. [DOI] [PubMed] [Google Scholar]
- [25].Stroh C, Wang H, Bash R, Ashcroft B, Nelson J, Gruber H, Lohr D, Lindsay SM, and Hinterdorfer P, “Single-molecule recognition imaging microscopy,” Proceedings of the National Academy of Sciences of the United States of America, vol. 101, no. 34, pp. 12503–12507, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lin L, Wang H, Liu Y, Yan H, and Lindsay S, “Recognition Imaging with a DNA Aptamer,” Biophysical Journal, vol. 90, no. 11, pp. 4236–4238, 2006/06/01/, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Senapati S, Manna S, Lindsay S, and Zhang P, “Application of Catalyst-Free Click Reactions in Attaching Affinity Molecules to Tips of Atomic Force Microscopy for Detection of Protein Biomarkers,” Langmuir, vol. 29, no. 47, pp. 14622–14630, 2013/11/26, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fahs A, and Louarn G, “Plant protein interactions studied using AFM force spectroscopy: nanomechanical and adhesion properties,” Physical Chemistry Chemical Physics, vol. 15, no. 27, pp. 11339–11348, 2013. [DOI] [PubMed] [Google Scholar]
- [29].McAllister C, Karymov MA, Kawano Y, Lushnikov AY, Mikheikin A, Uversky VN, and Lyubchenko YL, “Protein Interactions and Misfolding Analyzed by AFM Force Spectroscopy,” Journal of Molecular Biology, vol. 354, no. 5, pp. 1028–1042, 2005/12/16/, 2005. [DOI] [PubMed] [Google Scholar]
- [30].Hu W, Li CM, Cui X, Dong H, and Zhou Q, “In Situ Studies of Protein Adsorptions on Poly(pyrrole-co-pyrrole propylic acid) Film by Electrochemical Surface Plasmon Resonance,” Langmuir, vol. 23, no. 5, pp. 2761–2767, 2007/02/01, 2007. [DOI] [PubMed] [Google Scholar]
- [31].Oesterhelt F, Rief M, and Gaub HE, “Single molecule force spectroscopy by AFM indicates helical structure of poly(ethyleneglycol) in water,” New Journal of Physics, vol. 1, no. 1, pp. 6, 1999. [Google Scholar]
- [32].Leake MC, “The physics of life: one molecule at a time,” Philosophical Transactions of the Royal Society B-Biological Sciences, vol. 368, no. 1611, February 5, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lee K-B, Park S-J, Mirkin CA, Smith JC, and Mrksich M, “Protein Nanoarrays Generated By Dip-Pen Nanolithography,” Science, vol. 295, no. 5560, pp. 1702, 2002. [DOI] [PubMed] [Google Scholar]
- [34].Bosshart Patrick D., Frederix Patrick L. T. M., and Engel A, “Reference-Free Alignment and Sorting of Single-Molecule Force Spectroscopy Data,” Biophysical Journal, vol. 102, no. 9, pp. 2202–2211, 2012/05/02/, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ignacio C, and Simon S, “Automated setpoint adjustment for biological contact mode atomic force microscopy imaging,” Nanotechnology, vol. 21, no. 3, pp. 035104, 2010. [DOI] [PubMed] [Google Scholar]
- [36].Jens S, Reiner W, Mirko L, Joao N, Harald J, Ulrich G, Gerd H, Torsten J, and Daniel JM, “Fully automated single-molecule force spectroscopy for screening applications,” Nanotechnology, vol. 19, no. 38, pp. 384020, 2008. [DOI] [PubMed] [Google Scholar]
- [37].Baumgartner W, Hinterdorfer P, and Schindler H, “Data analysis of interaction forces measured with the atomic force microscope,” Ultramicroscopy, vol. 82, no. 1, pp. 85–95, 2000/02/01/, 2000. [DOI] [PubMed] [Google Scholar]
- [38].Calderon CP, Chen W-H, Lin K-J, Harris NC, and Kiang C-H, “Quantifying DNA melting transitions using single-molecule force spectroscopy,” Journal of Physics: Condensed Matter, vol. 21, no. 3, pp. 034114, 2008/12/17, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Machleidt T, Woodroofe CC, Schwinn MK, Méndez J, Robers MB, Zimmerman K, Otto P, Daniels DL, Kirkland TA, and Wood KV, “NanoBRET—A Novel BRET Platform for the Analysis of Protein–Protein Interactions,” ACS Chemical Biology, vol. 10, no. 8, pp. 1797–1804, 2015/08/21, 2015. [DOI] [PubMed] [Google Scholar]
- [40].Collins BC, Hunter CL, Liu Y, Schilling B, Rosenberger G, Bader SL, Chan DW, Gibson BW, Gingras A-C, Held JM, Hirayama-Kurogi M, Hou G, Krisp C, Larsen B, Lin L, Liu S, Molloy MP, Moritz RL, Ohtsuki S, Schlapbach R, Selevsek N, Thomas SN, Tzeng S-C, Zhang H, and Aebersold R, “Multi-laboratory assessment of reproducibility, qualitative and quantitative performance of SWATH-mass spectrometry,” Nature Communications, vol. 8, no. 1, pp. 291, 2017/08/21, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Abumazwed A, Kubo W, Shen C, Tanaka T, and Kirk AG, “Projection method for improving signal to noise ratio of localized surface plasmon resonance biosensors,” Biomedical Optics Express, vol. 8, no. 1, pp. 446–459, 2017/01/01, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Beuwer MA, Prins MWJ, and Zijlstra P, “Stochastic Protein Interactions Monitored by Hundreds of Single-Molecule Plasmonic Biosensors,” Nano Letters, vol. 15, no. 5, pp. 3507–3511, 2015/05/13, 2015. [DOI] [PubMed] [Google Scholar]
- [43].Garner AL, “cat-ELCCA: catalyzing drug discovery through click chemistry,” Chemical Communications, vol. 54, no. 50, pp. 6531–6539, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gayer CP, and Basson MD, “The effects of mechanical forces on intestinal physiology and pathology,” Cellular Signalling, vol. 21, no. 8, pp. 1237–1244, August, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wang SY, and Basson MD, “Akt directly regulates focal adhesion kinase through association and serine phosphorylation: implication for pressure-induced colon cancer metastasis,” American Journal of Physiology-Cell Physiology, vol. 300, no. 3, pp. C657–C670, March, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Conway WC, Van J Zyp der Voort van, Thamilselvan V, Walsh MF, Crowe DL, and Basson MD, “Paxillin modulates squamous cancer cell adhesion and is important in pressure-augmented adhesion,” Journal of Cellular Biochemistry, vol. 98, no. 6, pp. 1507–1516, 2006. [DOI] [PubMed] [Google Scholar]
- [47].Mitra SK, Hanson DA, and Schlaepfer DD, “Focal adhesion kinase: In command and control of cell motility,” Nature Reviews Molecular Cell Biology, vol. 6, no. 1, pp. 56–68, January, 2005. [DOI] [PubMed] [Google Scholar]
- [48].Germolec DR, Yoshida T, Gaido K, Wilmer JL, Simeonova PP, Kayama F, Burleson F, Dong W, Lange RW, and Luster MI, “Arsenic induces overexpression of growth factors in human keratinocytes,” Toxicology and Applied Pharmacology, vol. 141, no. 1, pp. 308–318, 1996/11/01/, 1996. [DOI] [PubMed] [Google Scholar]
- [49].Hutter JL, and Bechhoefer J, ĀCalibration of atomicϋforce microscope tips,ā Review of Scientific Instruments, vol. 64, no. 7, pp. 1868–1873, 1993. [Google Scholar]
- [50].Kasemo B, “Biological surface science,” Surface science, vol. 500, no. 1, pp. 656–677, 2002. [Google Scholar]
- [51].Yang Y, Volmering J, Junkin M, and Wong PK, “Comparative assembly of colloidal quantum dots on surface templates patterned by plasma lithography,” Soft matter, vol. 7, no. 21, pp. 10085–10090, 2011. [Google Scholar]
- [52].Kumar R, Ramakrishna SN, Naik VV, Chu Z, Drew ME, Spencer ND, and Yamakoshi Y, “Versatile method for AFM-tip functionalization with biomolecules: fishing a ligand by means of an in situ click reaction,” Nanoscale, vol. 7, no. 15, pp. 6599–6606, 2015/04/21/, 2015. [DOI] [PubMed] [Google Scholar]
- [53].Awsiuk K, Budkowski A, Psarouli A, Petrou P, Bernasik A, Kakabakos S, Rysz J, and Raptis I, “Protein adsorption and covalent bonding to silicon nitride surfaces modified with organo-silanes: Comparison using AFM, angle-resolved XPS and multivariate ToF-SIMS analysis,” Colloids and Surfaces B: Biointerfaces, vol. 110, pp. 217–224, 2013/10/01/, 2013. [DOI] [PubMed] [Google Scholar]
- [54].Crampton N, Bonass WA, Kirkham J, and Thomson NH, “Formation of Aminosilane-Functionalized Mica for Atomic Force Microscopy Imaging of DNA,” Langmuir, vol. 21, no. 17, pp. 7884–7891, 2005/08/01, 2005. [DOI] [PubMed] [Google Scholar]
- [55].Gartmann N, Schutze C, Ritter H, and Bruhwiler D, “The Effect of Water on the Functionalization of Mesoporous Silica with 3-Aminopropyltriethoxysilane,” Journal of Physical Chemistry Letters, vol. 1, no. 1, pp. 379–382, January, 2010. [Google Scholar]
- [56].Celik E, and Moy VT, “Nonspecific interactions in AFM force spectroscopy measurements,” Journal of Molecular Recognition, vol. 25, no. 1, pp. 53–56, 2012. [DOI] [PubMed] [Google Scholar]
- [57].Schlierf M, and Rief M, “Single-molecule unfolding force distributions reveal a funnel-shaped energy landscape,” Biophysical Journal, vol. 90, no. 4, pp. L33–L35, February, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Dudko OK, Hummer G, and Szabo A, “Theory, analysis, and interpretation of single-molecule force spectroscopy experiments,” Proceedings of the National Academy Of Sciences, vol. 105, no. 41, pp. 15755–15760, October 14, 2008, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Bullerjahn JT, Sturm S, and Kroy K, “Theory of rapid force spectroscopy,” Nature communications, vol. 5, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Lo Y-S, Zhu Y-J, and Beebe TP, “Loading-Rate Dependence of Individual Ligand-Receptor Bond-Rupture-Rupture Forces Studied by Atomic Force Microscopy,” Langmuir, vol. 17, no. 12, pp. 3741–3748, 2001/06/01, 2001. [Google Scholar]
- [61].Williams JM, Han T, and Beebe TP, “Determination of Single-Bond Forces from Contact Force Variances in Atomic Force Microscopy,” Langmuir, vol. 12, no. 5, pp. 1291–1295, 1996/01/01, 1996. [Google Scholar]
- [62].Stevens F, Lo Y-S, Harris JM, and Beebe TP, “Computer Modeling of Atomic Force Microscopy Force Measurements: Comparisons of Poisson, Histogram, and Continuum Methods,” Langmuir, vol. 15, no. 1, pp. 207–213, 1999/01/01, 1999. [Google Scholar]
- [63].Wenzler LA, Moyes GL, Olson LG, Harris JM, and Beebe TP, “Single-Molecule Bond-Rupture Force Analysis of Interactions between AFM Tips and Substrates Modified with Organosilanes,” Analytical Chemistry, vol. 69, no. 14, pp. 2855–2861, 1997/07/01, 1997. [Google Scholar]
- [64].Neuert G, Albrecht C, Pamir E, and Gaub HE, “Dynamic force spectroscopy of the digoxigenin–antibody complex,” FEBS Letters, vol. 580, no. 2, pp. 505–509, 2006. [DOI] [PubMed] [Google Scholar]
- [65].Kim S, Kihm KD, and Thundat T, “Fluidic applications for atomic force microscopy (AFM) with microcantilever sensors,” Experiments in Fluids, vol. 48, no. 5, pp. 721–736, 2010/05/01, 2010. [Google Scholar]
- [66].Gonzalez L, Rodrigues M, Benito AM, Pérez-García L, Puig-Vidal M, and Otero J, “Piezoelectric tuning fork biosensors for the quantitative measurement of biomolecular interactions,” Nanotechnology, vol. 26, no. 49, pp. 495502, 2015. [DOI] [PubMed] [Google Scholar]