Abstract
Idiopathic calcium oxalate (CaOx) stone formers form stones that are commonly attached to calcium phosphate (CaP) deposits in the renal tissue, known as Randall’s plaques (RP). Plaques are suggested to originate in the renal tubular basement membrane, where they exhibit a morphology of concentrically laminated apatitic spherules, while in the interstitial regions, the collagen fibrils and vesicles become mineralized. We hypothesize that these minerals might form by non-classical crystallization mechanisms, such as via amorphous precursors, some of which might originate from a polymer-induced liquid-precursor (PILP) process. Thus, our goal is to identify mineralogical ‘signatures’ of various stone formation mechanisms. To do this for idiopathic CaOx stones, we are developing a two stage model system of CaP-CaOx composite stones, consisting of Stage 1) CaP mineralized plaque, followed by Stage 2) CaOx overgrowth into a stone. For the studies presented here, decellularized porcine kidneys were mineralized with CaP using polyaspartic acid or the protein osteopontin (OPN) to induce the PILP process and create biomimetic RP. Analysis of the PILP-mineralized tissues shows features that resemble the native plaques, including mineral spherules and collagen with intrafibrillar mineral. In contrast, the classical crystallization produced large apatitic spherulites, which is a very different morphology, but one which is also found in some stones. An alternative hypothesis regarding Randall’s plaque, and if or when it becomes pathological, is discussed.
Keywords: Randall’s plaque, Urolithiasis, Kidney Stones, Osteopontin, Biomimetic model system, PILP
Introduction:
Idiopathic calcium oxalate (CaOx) stone formers make up the majority of those who form kidney stones, yet prevention or treatment of this painful recurring disease is difficult given the lack of understanding on how or why stones are formed [1–4]. Consistently, however, in these particular patients, stones are commonly attached to calcium phosphate (CaP) deposits, known as Randall’s plaques (RP), which are located at the renal papillary surface [5]. Investigations of RP have shown that the initial nanodeposits are spherically shaped and found in the basement membrane beneath cells of the thin loops of Henle [6–11]. The spherules then appear to grow and fuse, eventually spreading throughout the interstitial spaces, with deposits that appear to be closely associated with the collagen bundles [6,7,10,12]. This CaP plaque was shown to be in the amorphous or apatitic phase [6–9,12–18]. More extensive plaque deposits were found to reach the suburothelial space and to penetrate the urothelium [6–9,12]. These finding led Evan et. al. to develop the RP theory of stone formation which proposes that the plaque initially forms in the basement membrane of the thin loops of Henle [8,10]. However, others have suggested that the blood vessels are the origin [19], or that crystal-induced kidney cell injury is the cause [20,12]. In any case, once these mineral plaques spread throughout the interstitial tissue and break through the papillary surface epithelium, they become exposed to the urine, and with its different pH and composition, the mineral phase of CaOx grows on the plaque, resulting in stone formation [21,3,6,8,7,9,10,13,15,22].
Despite years of scientific research into the mechanisms of plaque formation and stone growth, the pathogenesis of RP is difficult to unravel due to the complexity of the renal and urinary environments where these two minerals are formed. [7,23,24,19,25]. The wide variety of molecules present in urine can heavily influence the thermodynamics and kinetics of processes that control crystal formation [25–28,9] or crystal aggregation [29]. While there are many hypotheses regarding the roles of the various macromolecules with respect to crystal nucleation [30,27,31–33,25,26,34], they are often described as ‘inhibitory’ or ‘stimulatory’, which falls short of providing a satisfactory explanation if the reaction were to proceed by a non-classical crystallization process, which is now known to be the case for many biominerals that form via an amorphous precursor phase [35–39].
Several of the RP and stone morphologies resemble those of calcium-based mineral products formed by an amorphous precursor [7,10,16,12], and particularly those seen by our group when using the polymer additives [40,41,27,6,9,10,42,16,12,43–45]. For example, when charged polypeptides are added to the crystallization solution to mimic the highly acidic proteins commonly associated with biominerals, they sequester ions or ion clusters (which may be in the form of prenucleation clusters (PNCs) and/or a preexisting liquid condensed phase (LCP) [46,47,45]), while simultaneously suppressing classical nucleation of crystalline phase, to induce liquid–liquid phase separation of colloids of hydrated, ion-enriched amorphous nano-droplets [27,45,41,48]. These so-called polymer-induced liquid-precursor (PILP) droplets are so highly hydrated that this amorphous precursor has fluidic character, at least in the early stages [27,44,48,49,46]. This allows the droplets to coalesce into smooth coatings and films prior to crystallization, which, upon pseudomorphic transformation, produces non-equilibrium morphologies and textures in the mineral products which are unlike the faceted crystals produced by the classical crystallization mechanism [40,27,45,44,41,42,50,49,51]. From a materials science perspective, such morphologies and textures provide ‘mineralogical signatures’ that can help identify the mechanism(s) by which the crystals were formed.
Our hypothesis that RP and stones might be initiated via a PILP-like process is based on the fact that the renal and urinary environment contains many different acidic macromolecular species, and given the non-specificity of the PILP process, these charged (and often intrinsically disordered) proteins could conceivably induce or promote this non-classical crystallization pathway under moderate supersaturation conditions [42]. Many acidic proteins are found in stone matrix [25], and these proteins could form a PILP-like phase and become entrapped during solidification. Non-classical mineralization processes have been demonstrated for a variety of anionic as well as cationic polymers [52,53], and in our model system of collagen mineralization, osteopontin (OPN) has been found to be highly potent [54]. OPN is a multifunctional protein found in bone, but is also prevalent in both RP and stones [13–15, 31], and thus is believed to play a significant role in stone formation. However, though OPN is a well-known ‘inhibitor’ of crystal nucleation [34,55–58], our group has shown that it can induce the PILP process because the inhibitory action is what enables the polymer to concentrate ions while suppressing nucleation, thereby producing the amorphous precursor [59,54,42].
Our goal here is to develop a model system of the two stages of idiopathic composite stone formation, where in the first stage we examine the mineralization of renal tissue with CaP, and in the second stage, examine how this ‘biomimetic Randall’s plaque’ then influences the overgrowth of calcium oxalate into a ‘biomimetic stone’, both in-vitro and in-vivo with a rat model. The second stage studies will be reported on separately, as part of a series. This RP model is particularly valuable when considering the renal and urinary environments, both of which have variable conditions as well as many macromolecular species. We can examine how different variables influence such processes, individually or in controlled combinations, with the ultimate goal of developing strategies to prevent stones, or treatments and therapeutics to ameliorate RP and thus stone formation.
Previously, we described the mineralization of MatriStem® (decellularized porcine bladder) with CaP to create ‘biomimetic RP’ [59]. For the set of experiments presented herein, we have been able to use actual kidney tissues, kindly provided by Dr. Edward Ross, Dr. Brad Willenberg (College of Medicine, University of Central Florida), and Dr. Christopher Batich (Department of Materials Science and Engineering, University of Florida), who have developed a protocol for the decellularization of porcine kidneys [60]. These kidney tissues provide an enhanced matrix for examining ‘biomimetic’ RP because RP is thought to initiate in the basement membranes, and these tissues possess numerous tubules and thus a correspondingly high density of basement membranes from within each of these tubules. However, while this is an even closer mimic to the native structures found in the kidney, one must bear in mind that the tissues are decellularized, and thus appear somewhat different without the renal epithelial cells lining the tubules.
Materials and Methods:
Porcine kidneys were decellularized according to our collaborators’ previously published protocol [60]. For mineralization experimentation, the tissues were cut into approximately 10 mm × 5 mm sections, and were mineralized using a 4.5 mM calcium and 2.1 mM phosphate solution in tris buffer containing 0.9% (w/v) NaCl and 0.02% (w/v) sodium azide at a pH of 7.4, which is based on a recipe that favors the apatite phase of CaP, and which we have used in our prior CaP PILP experiments. For inducing the PILP mineralization process, 50 μg/ml of poly-L aspartic-acid (pAsp·sodium salt, MW = 27 kDa, polydispersity index of 1.00–1.20, Alamanda Polymers) or OPN, purified from bovine milk [61] (Lacprodan OPN-10, provided by Arla Foods Ingredients Group P/S, Denmark) was added to the solution, while the conventional crystallization reaction contained no polymer. The samples were removed after the course of 2–4 days for the OPN conditions and 7–14 days for the conventional and polyAsp conditions (because our prior studies have shown that OPN has a faster mineralization time [54]), rinsed thoroughly in dI-water, and lyophilized. These scaffolds, along with a non-mineralized specimen, were first scanned with Cu-Kα X-ray radiation from a PANalytical X’Pert Pro Powder Diffractometer at 45 kV and 40 mA, using a step size of 0.01°mrad s−1 over a 2Ɵ range of 10–70°. They were then mounted on aluminum Scanning electron microscopy (SEM) stubs and coated with carbon for microanalysis using a JEOL 6400 SEM or and FEI XL-40 FEG SEM, both of which are equipped with an energy dispersive spectrometer (EDS) for x ray microanalysis. For further analysis using transmission electron microscopy (TEM), the samples were removed from the solution, rinsed, and immediately immersed in 4% paraformaldehyde in phosphate buffer (pH 7.4) and fixed for a minimum of 3 days. After fixing, the samples were rinsed in phosphate buffer, dehydrated via graded ethanol solutions, and embedded in LR white hard resin (Electron Microscopy Sciences). Thin sections (0.05 μm) were cut, stained with 2% uranyl acetate, and analyzed with a Hitachi 7600 TEM. For TEM selected area electron diffraction (SAED) analysis, a JEOL 200CX and a JEOL 2010F were used. All chemicals were purchased from Fisher unless otherwise noted.
Results:
Representative SEM micrographs of the kidney tissues before mineralization and after mineralization under the three conditions are seen in Figure 1. Compared to the Matristem® of our prior study, this tissue has a large amount of tubules characteristic of the kidney structure. Best depicted in the unmineralized group, the tubular areas have a smooth morphology which is indicative of the basement membrane (Fig 1a), which is in contrast to the fibrillar morphology associated with the interstitium (Fig 1b, Fig. S1a). After mineralization via the conventional process, superficial HA spherulites nucleated on the surface of both tissue types (Fig. 1c–d). With the poly-Asp and OPN additives, there was no external spherulitic crust (Fig. 1e–h), but inside the tissue there was a large amount of mineral, mainly associated with collagen fibrils in the interstitium (Fig. 1f,h, and Fig. S1b). OPN appeared to produce a higher mineral content, as judging by the larger Ca/P peaks (from mineral) relative to C/O peaks (from organic matrix) in the EDS spectra (insets). On the basement membrane side, with polyAsp, small specks of mineral were present on, or embedded within, the basement membrane (Fig. 1e inset). This is more readily seen in the backscattered SEM images which give z-contrast enhancement for Ca & P (Fig. S1c–d). Such specks could not be distinguished in the OPN sample because of the overall higher mineral content of the underlying mineralized collagen (Fig. 1g), which also appeared to have disrupted the basement membranes. X-ray diffraction (XRD) data indicates that the mineral phase was apatitic for all three of the mineralization conditions (Fig. S2).
Fig. 1.
SEM micrographs of decellularized porcine kidney tissue prior to mineralization (a-b), after mineralization via the conventional process (c-d), with polyAsp additive (e-f), and with OPN additive (g-h). (a) Cross-sections of unmineralized tissue show the kidney’s highly tubular structure with varying diameters of the tubules, and the insert shows the smooth, tightly-bound morphology of the tubular walls, consistent with the structure of basement membrane. (b) The interstitial regions exhibit a fibrous texture consistent with extracellular matrix (ECM). EDS spectra (inset) show no CaP. (c-d) After conventional mineralization, the tissue has become encrusted with spherulitic mineral. Towards the interior of the tissue, however, only a few spherulites are present (c, mid to lower left regions). At higher magnification, one can see that the spherulites are comprised of HAp crystallites with a platy morphology (c, insert). (d) Spherulites in the interstitial areas appear to have randomly nucleated on the surface of the collagen fibrils. An EDS spectrum of this region shows small Ca and P peaks relative to the C and O peaks of organic matrix (inset). (e-f) Tissue mineralized with polyAsp additive did not produce spherulites, yet mineral is present, as seen in the EDS spectra (insets). Mineral is not visibly present on the tubules, but at higher magnification (e, top insert), the small bright spots show higher Z contrast indicative of CaP. Higher mag. images in supplement Figure S1c,d, using backscatter mode to enhance z-contrast, show mineral specks that often appear to be embedded under the membrane. (f) The presence of mineral in the interstitial areas is also not apparent, but EDS shows a large amount of Ca and P (inset). Although EDS cannot be used in a quantitative fashion with such rough surfaces, such large Ca and P peaks (relative to the C and O peaks from organic matrix) generally indicate that the scaffold is well mineralized, and with no obvious evidence of crystals on the surface, once can conclude that much of the mineral must be within the fibrils. Some regions contained highly aligned fibrils, which also became well mineralized (supplement Fig. S1a,b). (g-h) Tissue mineralized with OPN additive showed tubular structures that were no longer smooth. As indicated by EDS (g, inset), these regions are highly mineralized. The thin membranes appear to have become disrupted by the excessive mineral, with thickened, rigid fibrils creating a rough and lumpy appearance, even in regions where the membrane is still present. Given the micron penetration depth of EDS, small bright specks (if present) cannot be distinguished from the highly mineralized background of underlying interstitial collagen. (h) There are no obvious HAp crystals in the interstitial areas, yet the EDS shows a large amount of Ca and P, indicating that the mineral is intrafibrillar. Scale bars are as follows: (a) 50 μm, insert 20 μm; (b) 10 μm; (c) 200 μm, insert 20 μm; (d) 50 μm; (e) 100 μm, insert 20 μm; (f) 20 μm; (g) 50 μm, insert 20 μm; (h) 20 μm.
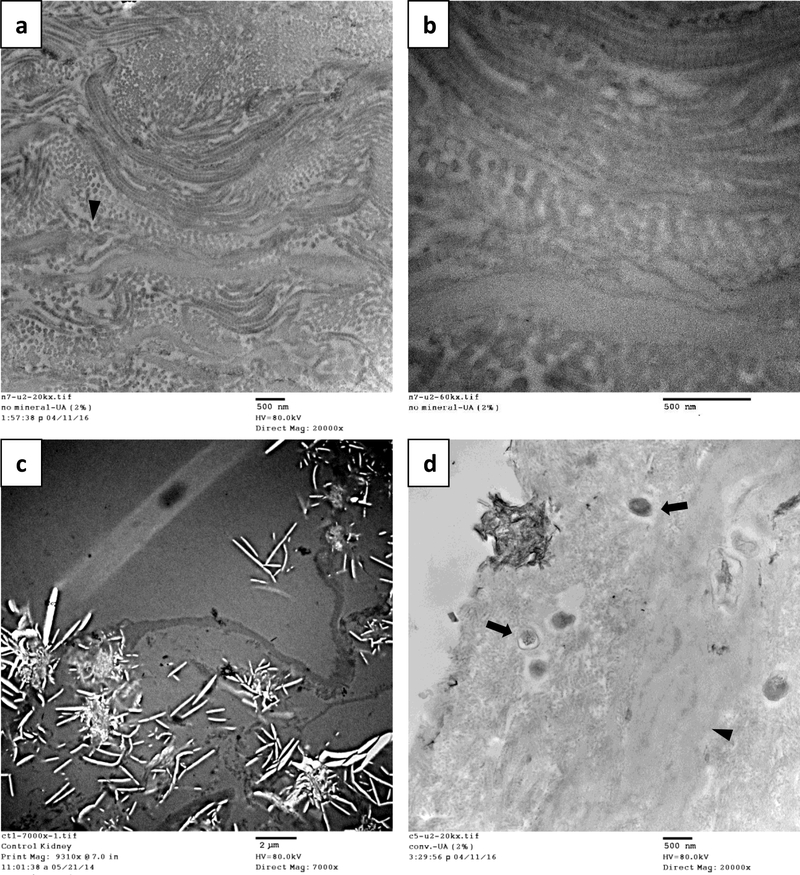
Figure 2 shows the ultrastructure of the decellularized kidney tissues as seen by TEM. In this region, which appears to be mostly interstitial tissue, collagen fibrils can be seen to form as stacked layers in the cross-section and longitudinal directions (Fig. 2a–b). Parallel layers of collagen were also seen in some SEM images (supplement Fig. S1a,b). At higher magnification (Fig. 2b), the characteristic banding pattern of the collagen can be seen, so the large and non-banded fibrous structures are likely elastic fibers [62]. Kidney tissues mineralized by the conventional process (no polymer additive) showed a large amount of crystal “ghosts” in the surface regions where large platelets had pulled out during microtoming (Fig. 2c). In the interior of the tissue, non-collagenous areas are present alongside fibrous areas, with only sparse evidence of mineral. However, some small dark spheres are present which appear to be amorphous mineral that hasn’t yet crystallized, as did the dark crystal cluster seen at the surface (Fig. 2d and Fig. S3d–f).
Fig. 2.
TEM of decellularized kidney tissues before mineralization with CaP (a-b) and after conventional mineralization with CaP (c-d), stained with 2% UA. (a) In unmineralized tissues, bundled collagen fibrils are present and seem to form layers in the cross-section and longitudinal directions. (b) In the longitudinal direction at higher magnifications, the characteristic banding pattern of type-I collagen can be distinguished. These regions (most likely the interstitium) are interrupted by occasional non-collagenous regions (arrowheads), which may be elastic fibers. (c) With conventional mineralization, a large amount of crystal “ghosts” of the spherulitic platelets are found at the surface regions. Large rigid crystals often get pulled out during microtoming, leaving behind pores that are bright in transmission. (d) Non-collagenous areas are again present in the interior (arrowheads), alongside fibrous areas, and there is relatively little infiltration of mineral phase in the tissue region. However, one crystal cluster is seen at the surface, and there are also some spherical structures with moderate contrast that may be early stage amorphous mineral (arrows). They do not yet contain the dark streaks seen for crystals (as in the surface cluster), and selected area electron diffraction (SAED) taken of these types of dark structures found no Bragg spots indicative of crystallinity (Fig. S4f, insert). Micrograph scale bars: (a) 500 nm, (b) 500 nm, (c) 2 μm, and (d) 500 nm.
Figure 3 shows TEM micrographs depicting various stages of mineralization in the presence of the two polymeric additives. In the polyAsp system, in regions with a small amount of mineral (Fig. 3a), the crystal alignment and texture resembles a sheaf-of-wheat structure. Although not apparent at this early stage, the mineral is associated with collagen. This becomes more evident when comparing to the image in Figure 3c, where there is sufficient mineral along the length of the fibrils to more readily identify them as mineralized collagen. In some areas the crystals appear to be projecting out of the fibril (Fig. 3a,c), becoming extrafibrillar crystals, unlike those seen with the OPN additive, which shows mostly aligned crystallites (Fig. 3b,d). Figures 3d,f show the late mineralization stage, where it appears that mineral starts off with a fine texture, but then becomes course and less organized as the crystals escape the confines of the fibrils. This is readily seen for some fibrils in supplement Figure S4, which includes SAED of crystal organization inside and outside individual fibrils.
Fig. 3.
TEM comparing tissues mineralized with CaP via the PILP process using polyAsp additive on the left (a,c,e) and the OPN additive on the right (b,d,f), depicting various stages of mineralization (from early at the top, to late at the bottom). All except (e) have been stained with 2% UA. (a) With polyAsp, dark regions of mineralized collagen are present alongside areas of what appear to be to non-collagenous regions, which could correspond to other components of the interstitium or to the basement membrane. The alignment and texture of the crystals resemble a sheaf-of-wheat structure, and although the crystals are not fully aligned, we believe the mineral is initially intrafibrillar. The reason for this becomes more apparent when comparing to the images in (c), where the fibrillar structures can be more readily identified in longitudinal sections where there is sufficient mineral present. Though the crystals show partial alignment, in some areas, they appear to be projecting from the fibrils as extrafibrillar crystals (a, arrowheads). (c) In the more heavily mineralized region, there are areas of less mineralized fibrils present alongside dark, highly mineralized fibrils, suggesting the mineral has spread throughout this collagenous region but without filling in other components in the tissue. (e & f) In the late mineralization stage, a dense array of crystals has formed with needle-like and/or plate-like morphologies typical of HAp. The finer texture of the crystals at the top is typical of intrafibrillar crystals, while the larger and more distinct crystals at the bottom of both images appear to be extrafibrillar, having grown beyond the constraints of the collagen fibrils. To note, the collagen banding pattern cannot be detected in these images because it becomes masked upon infiltration of mineral. Scale bars are as follows: (a) 500 nm; (b) 500 nm; (c) 500 nm; (d) 500 nm; (e) 100 nm; (f) 500 nm.
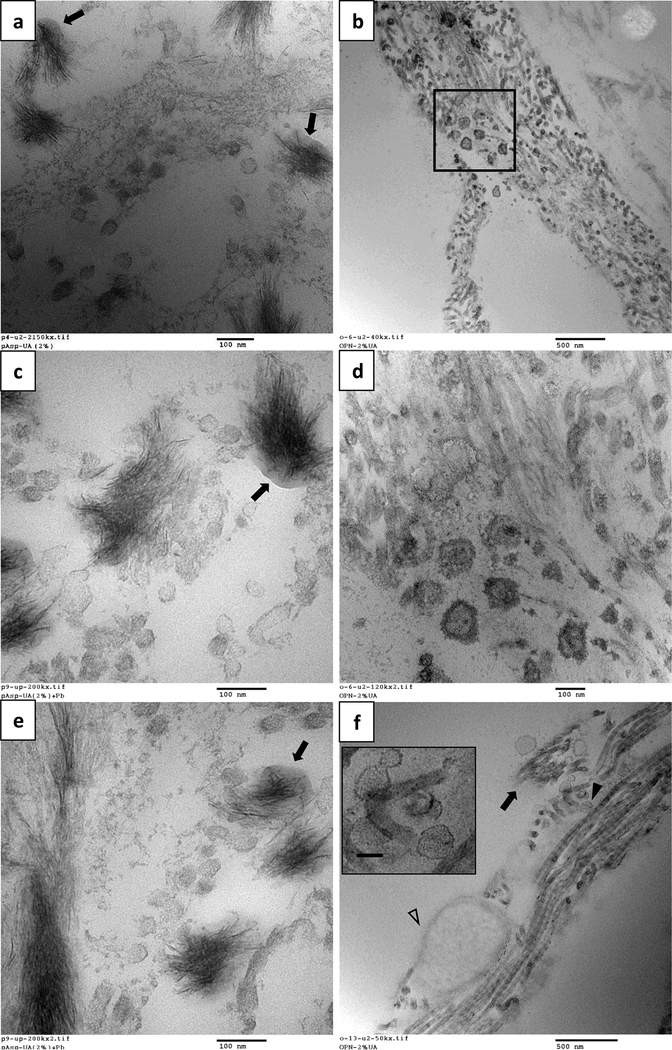
Although the polyAsp and OPN additive lead to an overall mineralization pattern that is similar, some intriguing differences were observed. For the polyAsp mineralized tissues, an irregularly shaped, amorphous substance can be seen around the mineral bundles (Fig. 4a,c,e). Given its fluidic appearance, we speculate that these globs are PILP phase that has accumulated on the collagen. This was only seen with polyAsp additive, and not OPN. On the other hand, with the OPN additive and not the polyAsp additive, interesting spherical entities were observed (Fig. 4. b,d,f). The first type, seen in Figure 4b&d, shows dark rounded structures with a diameter greater than 100 nm, larger than the collagen fibrils in this tissue (typically around 50 – 70 nm in diameter). At higher magnification (Fig. 4d), one can see a concentric double-layer pattern. Figure 4f shows a different type of spherical structure that was found in a region of collagen fibrils that were in the early stages of mineralization (as judging by the enhanced contrast of the collagen banding pattern). Although these are also around 100 nm, one can see on close examination that these have an outer rim and multiple concentric layers.
Fig. 4.
Differing nanoscale features observed for polyAsp additive on the left (a ,c ,e) versus OPN additive on the right (b, d, f). All TEM sections were stained with 2% UA (c and e were also stained with lead citrate). (a,c,e) With polyAsp additive, an amorphous substance of moderate contrast can be seen intermingled with the bundles of crystallites (arrows). Given that it has a fluidic appearance, it is thought to be PILP phase that has accumulated on the collagen fibrils. The thin crystals growing from this phase seem fairly well aligned, but also exhibit some splay in orientation, analogous to that seen in Figure 3a. Many spherical structures are also present, and with diameters less than 100 nm, they are likely cross-sections of collagen fibrils in the early stages of infiltration of the amorphous CaP precursor. (b,d,f) With OPN additive, there are many small dark spherical structures, often found closer to the non-fibrous regions. Even the fibrous structures do not appear to be type-I collagen because they lack of the distinct banding pattern (compare to the fibrils seen in (f)). These may be basement membrane components or elastic fibers. (d) At a higher magnification of the boxed area in (b), the spherical structures, approximately 100 nm, have a concentric double layer. Although they could be collagen fibrils in cross section, with both intra- and extrafibrillar mineral, they appear considerably larger than the majority of the fibrils present in these sections (typically around 70 nm in diameter). They could alternatively be mineral accumulation in and on matrix vesicles. (f) In this region, the collagen fibrils have strong contrast and appear to be mineralizing, but the mineral has not yet hidden the collagen d-period banding and may still be amorphous given that it lacks distinct dark streaks typically seen for crystallites. There are areas along the length of the fibrils that show diminishing z-contrast and banding (arrow). Spherical structures (arrowhead) are also present, and though these vary in diameter, most are around 100 nm. These spherules do not resemble those in (d), and a few, when examined closely (f, insert), have multiple concentric layers which more closely resemble early-stage spherules in Randall’s plaques. The large oval structure (open arrowhead) appears similar to images in the literature of the cross section of elastic fibers which have an amorphous elastin component interior [62]. Scale bars are as follows: (a) 100 nm; (b) 500 nm; (c) 100 nm; (d) 100 nm; (e) 100 nm; (f) 500 nm.
Discussion:
The decellularized porcine kidneys have a large amount of tubules, and thus basement membranes surrounding them (composed primarily of laminin, type IV collagen and heparan sulfate proteoglycan [63]), as well as interstitial tissue between the tubules (composed of primarily collagen type I and elastin). Having both types of tissues present in these samples allowed for the study of mineral deposition in both the regions where native RP is found. Although the cells have been removed from the kidneys, and thus the transport mechanisms of ions into the tissues is no longer the same as in-vivo conditions, we hypothesized that, given the very different compositions and structure of these two tissue types, they might show uniquely different features under the same mineralization reaction conditions; and indeed, this has been found to be the case.
In general, the PILP-mineralized tissues were very different from the conventionally mineralized tissue, while the differences between using the polyAsp and OPN additive were more subtle. OPN achieves a much higher degree of mineral in a relatively short time compared to polyAsp, which is in agreement with our prior studies [59,54,64,65]. With both polymers, the mineral was able to infiltrate further into the interior of the tissue as compared to the conventional mineralization, which we have argued is due to the liquid-like characteristics of the amorphous precursor [41,59]. One interesting finding is that although the collagen mineralization seems to start off intrafibrillar for both the polyAsp and OPN reactions, in the case of polyAsp, crystals seem to grow beyond the constraints of the fibrils to become extra-fibrillar shortly thereafter. Given that only the polyAsp system showed globs of a nondescript phase co-located with the collagen fibrils where mineral was emerging (Fig. 4a,c,e) perhaps the polyAsp PILP phase accumulates into larger drops or globs that provide an ion enriched phase for continued growth of crystals outward from the fibrils. The OPN PILP droplets may stay nanoscopic as they attach to the collagen along its length (due to collagen binding domains in OPN [66–68]), thereby enabling a faster and more uniform infiltration of the precursor nanodroplets. This could explain our prior SEM observations that OPN leads to more uniformity of mineralization than polyAsp [69], where the latter exhibits nodules or bulges along the length of the fibrils in the early stages. Whether this is relevant or not to plaque formation is unclear, but it does provide insight into some of the more specific differences between using a protein versus a protein mimic in our model system.
Another difference between the additives was seen in the TEM evidence of CaP spherule type deposits that were not found in the early stage ‘plaques’ in the polyAsp system, but were prevalent with OPN system. As seen in Figure 4d, the ~100 nm spherules have some concentric laminations somewhat resembling the early-stage Randall’s plaque spherules found in the basement membrane [9,10]. Although these circular structures could just be cross-sections of mineralizing fibrils (which do become thicker upon mineralization), there did not appear to be collagen fibrils in this region, as judging by the lack of banding in the higher magnification image of Fig. 4d. Thus, we believe these structures may be mineral accumulation in matrix vesicles, which are similar in size [12], and would be expected to form only a double concentric layer from interior and exterior mineral, as was the case here. The other type of spherules seen in Figure 4f have a more distinct border and, when examined closely, exhibit faint multiple concentric layers that more closely resemble early-stage spherules in Randall’s plaques. However, the layers are rather faint in contrast relative to literature examples of plaque spherules, which are stained with uranyl acetate and lead citrate [9,10]. This could be due to a variety of factors: our samples may have been captured in the very early stages of formation before the PILP phase had densified into amorphous calcium phosphate and excluded the impurities (which leads to concentric bands [70]); or perhaps the relatively low concentration of UA stain we used didn’t highlight the organic matter sufficiently to see the protein layers. As mentioned, the polyAsp additive showed no evidence of such spherules. This could relate to its lacking the more specific binding domains that are found on OPN. In addition to its cell binding ligand, OPN is known to bind to various organics in the extracellular matrix [66], and it has a putative mineral binding domain as well [71,72]. However, concentrically-layered mineral granules are found in many organisms [73], so this effect is not likely to be limited to only OPN protein.
Another observation in our system is that it appears that the mineral favors collagen mineralization initially, as opposed to forming spherules. In native plaque formation, the tubule fluids are separated from the interstitium by the basement membrane and their attached cells, thus it is likely that mineral will deposit there first before reaching the interstitial collagen; however, in our system, both tissue types are exposed to the mineralization solution simultaneously. This further supports the premise that collagen matrix can stimulate mineralization under PILP-like conditions, while non-PILP conditions lead to a more indiscriminate ‘crashing out’ of HAp deposits on the surface, as occurs on any heterogeneous nucleating surface under sufficient supersaturation. In the interstitial areas, mineral in both of the PILP-mineralized substrates are needle- and plate-like, and appear closely associated with collagen (i.e., they are smaller and well aligned), as seen in native RP [12]. In fact, the TEM images of the late mineralization stages shown in Figures 3d,f look quite similar to TEM images of native interstitial RP [59].
In comparing to the literature, ex vivo studies have found that when RP spreads to the interstitium, much of the mineral appears to be inside the collagen fibrils [6,12]. There is even evidence that shows early stage mineral starting within collagen fibrils and/or on matrix vesicles present in the interstitium [74,12]. Collagen is generally a poor nucleator of hydroxyapatite (HAp), which requires relatively high supersaturations and, even then, in the conventional crystallization process, HAp crystals nucleate predominantly as extrafibrillar spherulites on the surface of the scaffold. However, our group and others have shown that charged macromolecular additives can promote intrafibrillar mineralization of type-I collagen, and although the mechanism of infiltration remains controversial [44,41,48,53,75,76,52], one thing that is clear is that the mineral precursor phase likes to accumulate inside of collagen fibrils. In our model systems, we have seen that polymer-stabilized solutions remain clear and free of precipitates (of a size that scatters light); however, when a collagen matrix is added, the mineral precursor nanodroplets are sequestered directly into the fibrils, which then become well mineralized before crystals grow on the surface [59,54,42]. Thus, it is possible that the collagen in the interstitial plaque helps to stimulate and/or spread the mineral formation by providing a nanoporous scaffold throughout which the precursor phase can accumulate and spread. This suggests that ‘inhibitory’ proteins that are presumably designed to keep the mineral phase from nucleating, may instead result in the formation of an amorphous phase, which, in the presence of a collagen matrix, ends up promoting mineralization.
This discussion about OPN leading to a non-classical mineralization of interstitial tissue must lead one to wonder, why would OPN be secreted if it leads to the spread of mineralized tissue? One might speculate that OPN, being a protein that is found in multiple locations and functions throughout the body, could simply be secreted from the more general foreign body response, such as by macrophages (or other cells) in response to undesired mineral. This is a somewhat non-satisfying argument because it is hard to imagine that such an advanced organ like the kidney would be stuck using an evolutionarily ineffective protein that causes an undesirable effect. But perhaps this effect is not undesirable. Considering that high levels of calcium can damage cells, a means of sequestering and isolating the ‘toxic’ metal ions in the form of a non-toxic mineral presumably lies at the evolutionary foundation of all biologically controlled mineralizations. So perhaps sequestering away the ions to the interior of collagen fibrils may provide some protective mechanism to the organ as a whole. One could imagine that such a natural phenomenon evolved similarly to collagen mineralization becoming the basis for bone formation, the latter of which indeed plays an important metabolic role in calcium storage and mobilization. In the case of bone, the HAp crystals within collagen are exceedingly small and metastable, which provides a means for mobilization by osteoclast cells upon enzymatic removal of the protective collagen. In contrast, conventional HAp is not readily resorbed by the body because of its larger size and higher degree of crystallinity [77]. Thus, the same may be true in kidney tissue; although not benefiting from osteoclast remodeling, the macrophages (of related lineage) could potentially assist with gradual removal of such mineral plaques. Indeed, it has been shown that macrophages can phagocytose CaOx stone fragments [78], so it is not unreasonable to predict that this cellular mechanism could be available for removal of RPs as well, as long as they are kept in a quantity or form that is ‘digestible’. Given that RP deposits are common even in non-stone formers [79,80], RP in and of itself may not necessarily be the culprit of idiopathic stone formation, but rather an excessive buildup or a faulty removal mechanism of RP may be responsible [81,82,78].
In addition to the intrafibrillar mineral in native RP, as discussed above, mineral spherulites similar to those produced during the conventional crystallization here have also been seen in native RP, just underneath or at the surface of the papillary epithelium where the plaque has broken across [16,12]. This extrafibrillar crystal crust may have occurred because the mineral was not confined by the collagen where the matrix terminates, or when the collagen had already been completely filled with mineral, at which point extrafibrillar crystals emerge [59]. This was seen in the late stage mineral in our system, where the crystals continued to grow beyond the fibrils and became much larger and less organized (Fig. 3d,f and Fig. S4 with SAED patterns). Of course extrafibrillar mineral could simply be caused by the classical crystallization mechanism. However, in considering the argument made above regarding mineralized collagen providing an apt storage mechanism for high ion loads, if the conventional type HAp spherulites were to form in the tissue instead, then larger and less soluble crystals would be much more difficult for any cell type to remove.
When using in-vitro model systems, one must consider the weaknesses of the model. Cells can play an important role in mineralization processes by directing ion transport, which is substantially different in this beaker reaction. Another concern is that while this substrate does have the native kidney architecture, decellularization is a harsh process, and many other components, such as lipids and glycosaminoglycans (GAGs), may have been removed or altered during the process, and these could certainly affect mineralization. Nevertheless, we believe this model system is helping to identify how mineral precursors interact with different tissue matrices, which can then be combined with various mixtures of other species present in the urinary environment, thus providing a means for studying the mechanism(s) of CaP growth, and ultimately CaOx overgrowth, the focus of our Stage 2 study (to be presented in a follow-up paper).
Conclusions:
Our preliminary studies with the decellularized tissues seem promising in that they are leading to mineralogical features that mimic those seen in RP. Overall, when the mineralization pathway follows the non-classical pathway of the PILP process, the mineralized substrates resemble native RP more so than the conventional reaction, supporting our hypothesis that some of the acidic urinary macromolecules could play an influential role in inducing a non-classical or PILP-type process. The conventional mineralization led to mineral crusts that resemble other features of RP, thus suggesting that both classical and non-classical mechanisms could be at play in plaque formation. Based on the differences between these types of mineral, we propose a new hypothesis regarding the role of ‘inhibitory’ proteins by suggesting that they could provide a protective means for dealing with excessively high ion loads in the kidney tissue by storing the mineral, either as an amorphous and thus more soluble phase, or as tiny metastable crystallites within collagen, both of which could be more readily removed by cells than the conventional HAp spherulites. In conclusion, as our ‘biomimetic plaque’ is now being used to grow ‘biomimetic’ stones with similar morphological features as those found in native idiopathic CaOx-CaP composite stones (Stage 2 paper), we believe that this approach will continue to provide a valuable model system for studying the pathogenesis of this complex disease in the presence of other urinary species.
Supplementary Material
Acknowledgements:
Research reported in this publication was supported by the National Institute of Diabetes And Digestive And Kidney Diseases of the National Institutes of Health under Award Number R01DK092311. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Drs. Brad Willenberg, Edward Ross (College of Medicine, University of Central Florida), and Christopher Batich (Department of Materials Science and Engineering, University of Florida) for providing the decellularized porcine kidney tissues. We would also like to thank Drs. Sharon Matthews and Jill Verlander for their training and expertise in tissue sample preparation, microtomy, and microscopy done at the College of Medicine Electron Microscopy Core Facility at the University of Florida.
Funding: There were no external sources of funding beyond the NIH grant acknowledged above.
Footnotes
Conflict of Interest: All authors declare that they have no conflict of interest in this work.
Ethical approval: This article does not contain any studies with human participants performed by any of the authors. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed (UF IACUC Protocol # 201607895).
References:
- 1.Miller NL, Gillen DL, Williams JC, Evan AP, Bledsoe SB, Coe FL, Worcester EM, Matlaga BR, Munch LC, Lingeman JE (2009) A formal test of the hypothesis that idiopathic calcium oxalate stones grow on Randall’s plaque. BJU Int 103 (7):966–971. doi: 10.1111/j.1464-410X.2008.08193.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodgers AL, De Klerk DP (1986) Crystalluria and urolithiasis in a relatively stone-free population. Scan Electron Microsc (Pt 3):1157–1167 [PubMed] [Google Scholar]
- 3.Coe FL, Evan AP, Worcester EM, Lingeman JE (2010) Three pathways for human kidney stone formation. Urol Res 38:3:147–160. doi:DOI 10.1007/soo240-010-0271-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evan A, Worcester E, Coe F, Williams J Jr., Lingeman J (2015) Mechanisms of human kidney stone formation. Urolithiasis 43: Suppl 1:S19–S32. doi:DOI 10.1007/s00240-014-0701-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Randall A (1937) The origin and growth of renal calculi. Annals of Surgery 105 (6):1009–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evan A, Lingeman J, Coe FL, Worcester E (2006) Randall’s plaque: Pathogenesis and role in calcium oxalate nephrolithiasis. Kidney Int 69 (8):1313–1318 [DOI] [PubMed] [Google Scholar]
- 7.Evan AP, Coe FL, Lingeman JE, Shao Y, Sommer AJ, Bledsoe SB, Anderson JC, Worcester EM (2007) Mechanism of Formation of Human Calcium Oxalate Renal Stones on Randall’s Plaque. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology 290 (10):1315–1323. doi: 10.1002/ar.20580 [DOI] [PubMed] [Google Scholar]
- 8.Evan AP (2010) Physiopathology and etiology of stone formation in the kidney and the urinary tract. Pediatric Nephrology 25:831–841. doi:DOI 10.1007/s00467-009-1116-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evan AP, Coe FL, Rittling SR, Bledsoe SM, Shao Y, Lingeman JE, Worcester EM (2005) Apatite plaque particles in inner medulla of kidneys of calcium oxalate stone formers: Osteopontin localization. Kidney Int 68 (1):145–154 [DOI] [PubMed] [Google Scholar]
- 10.Evan AP, Lingeman JE, Coe FL, Parks JH, Bledsoe SB, Shao YZ, Sommer AJ, Paterson RF, Kuo RL, Grynpas M (2003) Randall’s plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. Journal of Clinical Investigation 111 (5):607–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bushinsky DA (2003) Nephrolithiasis: site of the initial solid phase. The Journal of Clinical Investigation 111 (5):602–605. doi: 10.1172/JCI18016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan SR, Rodriguez DE, Gower LB, Monga M (2012) Association of Randall Plaque With Collagen Fibers and Membrane Vesicles. The Journal of Urology 187 (March):1094–1100. doi:DOI: 10.1016/j.juro.2011.10.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bagga HS, Chi T, Miller J, Stoller ML (2013) New insights into the pathogenesis of renal calculi. Urologic Clinics of North America 40 (1):1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matlaga BR, Williams JC Jr, Kim SC, Kuo RL, Evan AP, Bledsoe SB, Coe FL, Worcester EM, Munch LC, Lingeman JE (2006) Endoscopic evidence of calculus attachment to Randall’s plaque. The Journal of urology 175 (5):1720–1724. doi: 10.1016/S0022-5347(05)01017-7 [DOI] [PubMed] [Google Scholar]
- 15.Sayer JA, Carr G, Simmons NL (2004) Nephrocalcinosis: molecular insights into calcium precipitation within the kidney. Clinical science 106 (6):549–561 [DOI] [PubMed] [Google Scholar]
- 16.Khan SR (1997) Calcium Phosphate/Calcium Oxalate Crystal Association in Urinary Stones: Implications for Heterogeneous Nucleation of Calcium Oxalate. The Journal of Urology 157 (1):376–383 [PubMed] [Google Scholar]
- 17.Bazin D, Daudon M (2012) Pathological calcifications and selected examples at the medicine-solid-state physics interface. J Phys D: Appl Phys 45 (38):383001 [Google Scholar]
- 18.Khan SR, Finlayson B, Hackett R (1984) Renal papillary changes in patient with calcium oxalate lithiasis. Urology 23 (2):194–199 [DOI] [PubMed] [Google Scholar]
- 19.Stoller ML, Meng MV, Abrahams HM, Kane JP (2004) The primary stone event: a new hypothesis involving a vascular etiology. The Journal of urology 171 (5):1920–1924 [DOI] [PubMed] [Google Scholar]
- 20.Khan S (2006) Renal tubular damage/dysfunction: key to the formation of kidney stones. Urol Res 34(2):86–91. doi: 10.1007/s00240-005-0016-2 [DOI] [PubMed] [Google Scholar]
- 21.Coe FL, Evan AP, Lingeman JE, Worcester EM (2010) Plaque and deposits in nine human stone diseases. Urol Res on-line first. doi:DOI 10.1007/s00240-010-0296-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tiselius H-G (2011) A hypothesis of calcium stone formation: an interpretation of stone research during the past decades. Urol Res 39:4:231–243. doi:DOI: 10.1007/s00240-010-0349-3 [DOI] [PubMed] [Google Scholar]
- 23.Hug S, Grohe B, Jalkanen J, Chan B, Galarreta B, Vincent K, Lagugne-Labarthet F, Lajoie G, Goldberg HA, Karttunen M, Hunter GK (2012) Mechanism of inhibition of calcium oxalate crystal growth by an osteopontin phosphopeptide. Soft Matter 8 (4):1226–1233. doi: 10.1039/C1SM06232H [DOI] [Google Scholar]
- 24.Stoller ML, Low RK, Shami GS, Mccormick VD, Kerschma RL (1996) High resolution radiography of cadaveric kidneys: Unraveling the mystery of randall’s plaque formation. The Journal of Urology 156:1263–1266. doi:022-5347/96/1564-1263$03.00/ [DOI] [PubMed] [Google Scholar]
- 25.Thurgood LA, Cook AF, Sørensen ES, Ryall RL (2010) Face-specific incorporation of osteopontin into urinary and inorganic calcium oxalate monohydrate and dihydrate crystals. Urol Res 38:357–376. doi:DOI 10.1007/s00240-010-0300-7 [DOI] [PubMed] [Google Scholar]
- 26.Canales BK, Anderson L, Higgins L, Slaton J, Roberts KP, Liu NT, Monga M (2008) Comprehensive proteomic analysis of human calcium oxalate monohydrate kidney stone matrix. Journal of Endourology 22 (6):1161–1167. doi: 10.1089/end.2007.0440 [DOI] [PubMed] [Google Scholar]
- 27.Gower LB, Amos FF, Khan SR (2010) Mineralogical Signatures of Stone Formation Mechanisms. Urol Res 38:4:281–292. doi: 10.1007/s00240-010-0288-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan SR, Atmani F, Glenton P, Hou ZC, Talham DR, Khurshid M (1996) Lipids and membranes in the organic matrix of urinary calcific crystals and stones. Calcif Tissue Int 59 (5):357–365 [DOI] [PubMed] [Google Scholar]
- 29.Sheng XX, Jung TS, Wesson JA, Ward MD (2005) Adhesion at calcium oxalate crystal surfaces and the effect of urinary constituents. Proc Natl Acad Sci U S A 102 (2):267–272. doi: 10.1073/pnas.0406835101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christmas KG, Gower LB, Khan SR, El-Shall H (2002) Aggregation and Dispersion Characteristics of Calcium Oxalate Monohydrate: Effect of Urinary Species. J Colloid Interface Sci 256:168–174 [DOI] [PubMed] [Google Scholar]
- 31.Khan SR, Kok DJ (2004) Modulators of urinary stone formation. Frontiers in Bioscience 9 (May):1450–1482 [DOI] [PubMed] [Google Scholar]
- 32.Kolbach AM, Afzal O, Halligan B, Sorokina E, Kleinman JG, Wesson JA (2012) Relative deficiency of acidic isoforms of osteopontin from stone former urine. Urol Res 40 (5):447–454. doi: 10.1007/s00240-012-0459-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lan M, Lucy L, Andrew PE, Andre JS, John CL, Xue-Ru W (2007) Renal calcinosis and stone formation in mice lacking osteopontin, Tamm-Horsfall protein, or both. American Journal of Physiology - Renal Physiology 293 (6):F1935–F1943. doi: 10.1152/ajprenal.00383.2007 [DOI] [PubMed] [Google Scholar]
- 34.Gericke A, Qin C, Spevak L, Fujimoto Y, Butler WT, Sorensen ES, Boskey AL (2005) Importance of phosphorylation for osteopontin regulation of biomineralization. Calcif Tissue Int 77 (1):45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Yoreo JJ, Gilbert PUPA, Sommerdijk NAJM, Penn RL, Whitelam, Joester D, Zhang H, Rimer JD, Navrotsky A, Banfield JF, Wallace AF, Michel FM, Meldrum FC, Cölfen H, Dove PM (2015) Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science 349 (6247). doi: 10.1126/science.aaa6760 [DOI] [PubMed] [Google Scholar]
- 36.Karthika S, Radhakrishnan TK, Kalaichelvi P (2016) A Review of Classical and Nonclassical Nucleation Theories. Crystal Growth & Design 16 (11):6663–6681. doi: 10.1021/acs.cgd.6b00794 [DOI] [Google Scholar]
- 37.Lee J, Yang J, Kwon SG, Hyeon T (2016) Nonclassical nucleation and growth of inorganic nanoparticles. Nature Reviews Materials:16034. doi: 10.1038/natrevmats.2016.34 [DOI] [Google Scholar]
- 38.Rodriguez-Navarro C, Ruiz-Agudo E, Harris J, Wolf SE (2016) Nonclassical crystallization in vivo et in vitro (II): Nanogranular features in biomimetic minerals disclose a general colloid-mediated crystal growth mechanism. J Struct Biol. doi: 10.1016/j.jsb.2016.09.005 [DOI] [PubMed] [Google Scholar]
- 39.Wolf SE, Gower LB (2017) Challenges and Perspectives of the Polymer-Induced Liquid-Precursor Process: the Pathway from Liquid-Condensed Mineral Precursors to Mesocrystalline Products In: Driessche AESv, Kellermeier M, Benning LG, Gebauer D (eds) New perspectives on mineral nucleation and growth: From solution precursors to solid materials. Springer International Publishing, Switzerland, pp 43–75. doi:DOI: 10.1007/978-3-319-45669-0_3 [DOI] [Google Scholar]
- 40.Amos FF, Dai L, Kumar R, Khan SR, Gower LB (2009) Mechanism of formation of concentrically laminated spherules: implication to Randall’s plaque and stone formation. Urol Res 37:1:11–17. doi:doi: 10.1007/s00240-008-0169-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olszta MJ, Cheng X, Jee SS, Kumar R, Kim Y-Y, Kaufman MJ, Douglas EP, Gower LB (2007) Bone structure and formation: A new perspective. Materials Science & Engineering R-Reports 58 (3–5):77–116. doi: 10.1016/j.mser.2007.05.001 [DOI] [Google Scholar]
- 42.Gower LB (2008) Biomimetic Model Systems for Investigating the Amorphous Precursor Pathway and Its Role in Biomineralization. Chemical Reviews 108 (11):4551–4627. doi:doi: 10.1021/cr800443h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amos FF, Olszta MJ, Khan SR, Gower LB (2006) Relevance of a Polymer-Induced Liquid-Precursor (PILP) Mineralization Process to Normal and Pathological Biomineralization In: Königsberger E, Königsberger L (eds) Biomineralization- Medical Aspects of Solubility, vol Chapter 4. John Wiley & Sons, Ltd, West Sussex, England, pp 125–217 [Google Scholar]
- 44.Jee S-S, Thula TT, Gower LB (2010) Development of bone-like composites via the polymer-induced liquid-precursor (PILP) process. Part 1: Influence of polymer molecular weight. Acta Biomaterialia 6(9):3676–3686. doi:doi: DOI: 10.1016/j.actbio.2010.03.036 [DOI] [PubMed] [Google Scholar]
- 45.Gower LB, Odom DJ (2000) Deposition of calcium carbonate films by a polymer-induced liquid-precursor (PILP) process. J Crystal Growth 210:4:719–734 [Google Scholar]
- 46.Wolf SLP, Caballero L, Melo F, Cölfen H (2016. online) Gel-Like Calcium Carbonate Precursors Observed by in-situ AFM. Langmuir online. doi:DOI: 10.1021/acs.langmuir.6b03974 [DOI] [PubMed] [Google Scholar]
- 47.Bewernitz MA, Gebauer D, Long J, Cölfen H, Gower LB (2012) A metastable liquid precursor phase of calcium carbonate and its interactions with polyaspartate. Faraday Discussions 159:291–312. doi: 10.1039/c2fd20080e [DOI] [Google Scholar]
- 48.Olszta MJ, Odom DJ, Douglas EP, Gower LB (2003) A new paradigm for biomineral formation: Mineralization via an amorphous liquid-phase precursor. Connective Tissue Research 44:326–334. doi: 10.1080/03008200390181852 [DOI] [PubMed] [Google Scholar]
- 49.Kim Y-Y, Hetherington NBJ, Noel EH, Kroeger R, Charnock JM, Christenson HK, Meldrum FC (2011) Capillarity Creates Single-Crystal Calcite Nanowires from Amorphous Calcium Carbonate. Angew Chem-Int Edit 50 (52):12572–12577. doi: 10.1002/anie.201104407 [DOI] [PubMed] [Google Scholar]
- 50.Kim Y-Y, Douglas EP, Gower LB (2007) Patterning inorganic (CaCO3) thin films via a polymer-induced liquid-precursor process. Langmuir 23 (9):4862–4870. doi: 10.1021/la0619751 [DOI] [PubMed] [Google Scholar]
- 51.Kim YY, Kulak AN, Li YT, Batten T, Kuball M, Armes SP, Meldrum FC (2009) Substrate-directed formation of calcium carbonate fibres. J Mater Chem 19 (3):387–398. doi: 10.1039/b813101e [DOI] [Google Scholar]
- 52.Niu L-n, Jee SE, Jiao K, Tonggu L, Li M, Wang L, Yang Y-d, Bian J-h, Breschi L, Jang SS, Chen J-h, Pashley DH, Tay FR (2017) Collagen intrafibrillar mineralization as a result of the balance between osmotic equilibrium and electroneutrality. Nat Mater 16 (3):370–378. doi:10.1038/nmat478910.1038/nmat4789http://www.nature.com/nmat/journal/v16/n3/abs/nmat4789.html#supplementary-informationhttp://www.nature.com/nmat/journal/v16/n3/abs/nmat4789.html#supplementary-information [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nudelman F, Pieterse K, George A, Bomans PHH, Friedrich H, Brylka LJ, Hilbers PAJ, de With G, Sommerdijk NAJM (2010) The role of collagen in bone apatite formation in the presence of hydroxyapatite nucleation inhibitors. Nature Materials 9 (12):1004–1009. doi:DOI: 10.1038/NMAT2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodriguez DE, Thula-Mata T, Toro EJ, Yeh Y-W, Holt C, Holliday LS, Gower LB (2014) Multifunctional role of osteopontin in directing intrafibrillar mineralization of collagen and activation of osteoclasts. Acta Biomaterialia 10:1 (1):494–507. doi:10.1016/j.actbio.2013.10.01010.1016/j.actbio.2013.10.010http://dx.doi.org/10.1016/j.actbio.2013.10.010http://dx.doi.org/10.1016/j.actbio.2013.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wesson JA, Johnson RJ, Mazzali M, Beshensky AM, Stietz S, Giachelli C, Liaw L, Alpers CE, Couser WG, Kleinman JG, Hughes J (2003) Osteopontin is a critical inhibitor of calcium oxalate crystal formation and retention in renal tubules. J Am Soc Nephrol 14 (1):139–147. doi: 10.1097/01.asn.0000040593.93815.9d [DOI] [PubMed] [Google Scholar]
- 56.Kleinman JG, Beshensky A, Worcester EM, Brown D (1995) Expression of osteopontin, a urinary inhibitor of stone mineral crystal growth, in rat kidney. Kidney Int 47 (6):1585–1596. doi: 10.1038/ki.1995.222 [DOI] [PubMed] [Google Scholar]
- 57.Shiraga H, Min W, Vandusen WJ, Clayman MD, Miner D, Terrell CH, Sherbotie JR, Foreman JW, Przysiecki C, Neilson EG, Hoyer JR (1992) Inhibition of calcium-oxalate crystal-growth in vitro by uropontin - another member of the aspartic acid-rich protein superfamily. Proc Natl Acad Sci, USA 89 (1):426–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Worcester EM, Beshensky AM (1995) Osteopontin inhibits nucleation of calcium oxalate crystals. Ann NY Acad Sci 760:375–377 [DOI] [PubMed] [Google Scholar]
- 59.Chidambaram A, Rodriguez D, Khan S, Gower L (2015) Biomimetic Randall’s plaque as an in vitro model system for studying the role of acidic biopolymers in idiopathic stone formation. Urolithiasis 43:1 (1 Supplement (January)):77–92. doi: 10.1007/s00240-014-0704-x PMC: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4285617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ross EA, Williams MJ, Hamazaki T, Terada N, Clapp WL, Adin C, Ellison GW, Jorgensen M, Batich CD (2009) Embryonic Stem Cells Proliferate and Differentiate when Seeded into Kidney Scaffolds. J Am Soc Nephrol 20:2338–2347. doi:doi: 10.1681/ASN.2008111196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sørensen ES, Ostersen S, Chatterton D, Holst HH, Albertsen K (2007) Process for isolation of osteopontin from milk. Google Patents,
- 62.Ross R (1973) The elastic fiber- A review. The Journal of Histochemistry and Cytochemistry 21:3:199–208 [DOI] [PubMed] [Google Scholar]
- 63.Badylak SF, Freytes DO, Gilbert TW (2009) Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomaterialia 5 (1):1–13. doi: 10.1016/j.actbio.2008.09.013 [DOI] [PubMed] [Google Scholar]
- 64.Saxena NS (2017) Optimization of the Polymer-Induced Liquid-Precursor Process for Dentin Remineralization Doctoral, University of Florida, Gainesville, FL [Google Scholar]
- 65.Saxena N, Mizels J, Rodriguez VGD, Wingender ALB, Gower L (2017. (anticipated)) Comparative study of osteopontin versus polyaspartate for collagen mineralization. Acta Biomaterialia (anticipated) [Google Scholar]
- 66.Scatena M, Liaw L, Giachelli CM (2007) Osteopontin. A Multifunctional Molecule Regulating Chronic Inflammation and Vascular Disease. Arteriosclerosis, Thrombosis, and Vascular Biology 27 (11):2302–2309. doi: 10.1161/atvbaha.107.144824 [DOI] [PubMed] [Google Scholar]
- 67.Kaartinen MT, Pirhonen A, Linnala-Kankkunen A, Mäenpää PH (1997) Transglutaminase-catalyzed cross-linking of osteopontin is inhibited by osteocalcin. J Biol Chem 272:36 (Sep.):22736–22741. [DOI] [PubMed] [Google Scholar]
- 68.Martin SM, Schwartz JL, Giachelli CM, Ratner BD (2004) Enhancing the biological activity of immobilized osteopontin using a type - 1 collagen affinity coating. Journal of Biomedical Materials Research Part A 70A (1):10–19. doi:doi: 10.1002/jbm.a.30052 [DOI] [PubMed] [Google Scholar]
- 69.Thula TT, Svedlund F, Rodriguez DE, Podschun J, Pendi L, Gower LB (2011) Mimicking the Nanostructure of Bone: Comparison of Polymeric Process-Directing Agents. Polymers 3:1 (1):10–35. doi:doi: 10.3390/polym3010010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dai L, Cheng X, Gower LB (2008) Transition Bars during Transformation of an Amorphous Calcium Carbonate Precursor. Chem Mat 20 (22):6917–6928. doi: 10.1021/cm800760p [DOI] [Google Scholar]
- 71.McKee MD, Nancl A, Khan SR (1995) Ultrastructural immunodetection of osteopontin and osteocalcin as major matrix components of renal calculi. Journal of Bone and Mineral Research 10(12):1913–1929. doi: 10.1002/jbmr.5650101211 [DOI] [PubMed] [Google Scholar]
- 72.Hunter GK, O’Young J, Grohe B, Karttunen M, Goldberg HA (2010) The flexible polyelectrolyte hypothesis of protein-biomineral interaction. Langmuir 26:24:18639–18646. doi:DOI: 10.1021/la100401r [DOI] [PubMed] [Google Scholar]
- 73.Ryall RL (2008) The future of stone research: rummagings in the attic, Randall’s plaque, nanobacteria, and lessons from phylogeny. Urol Res DOI 10.1007/s00240-007-0131-3. doi:DOI 10.1007/s00240-007-0131-3 [DOI] [PubMed] [Google Scholar]
- 74.Golub EE (2011) Biomineralization and matrix vesicles in biology and pathology. Seminars in immunopathology 33 (5):409–417. doi: 10.1007/s00281-010-0230-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Deshpande AS, Beniash E (2008) Bioinspired Synthesis of Mineralized Collagen Fibrils. Cryst Growth Des 8 (8):3084–3090. doi:doi: 10.1021/cg800252f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Price PA, Toroian D, Lim JE (2009) Mineralization by Inhibitor Exclusion- The calcification of collagen with fetuin. J Biol Chem 284 (25):17092–17101. doi: 10.1074/jbc.M109.007013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tang R, Wang L, Nancollas GH (2004) Size-effects in the dissolution of hydroxyapatite: an understanding of biological demineralization. J Mater Chem 14:2341–2346. doi:DOI: 10.1039/b401097c [DOI] [Google Scholar]
- 78.Kusmartsev S, Dominguez-Gutierrez PR, Canales BK, Bird VG, Vieweg J, Khan SR (2016) Calcium Oxalate Stone Fragment and Crystal Phagocytosis by Human Macrophages. The Journal of Urology 195 (4, Part 1):1143–1151. doi: 10.1016/j.juro.2015.11.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Haggitt RC, Pitcock JA (1971) Renal medullary calcifications: a light and electron microscopic study. J Urol 106:3:342–347 [DOI] [PubMed] [Google Scholar]
- 80.Low RK, Stoller ML (1997) Endoscopic mapping of renal papillae for Randall’s plaques in patients with urinary stone disease. J Urol 158:2062–2064 [DOI] [PubMed] [Google Scholar]
- 81.Taguchi T, Ikoma T, Tanaka J (2002) An improved method to prepare hyaluronic acid and type II collagen composite matrices. Journal of Biomedical Materials Research 61 (2):330–336 [DOI] [PubMed] [Google Scholar]
- 82.Okada A, Yasui T, Fujii Y, Niimi K, Hamamoto S, Hirose M, Kojima Y, Itoh Y, Tozawa K, Hayashi Y, Kohri K (2010) Renal macrophage migration and crystal phagocytosis via inflammatory-related gene expression during kidney stone formation and elimination in mice: Detection by association analysis of stone-related gene expression and microstructural observation. Journal of Bone and Mineral Research 25 (12):2701–2711. doi: 10.1002/jbmr.158 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.