Abstract
Physical properties of clusters, i.e. systems composed of a ‘small’ number of particles, are qualitatively different from those of infinite systems. The general approach to the problem of clustering is suggested. Clusters, as they are seen in the graphs theory, are discussed. Various physical mechanisms of clustering are reviewed. Dimensional properties of clusters are addressed. The dimensionality of clusters governs to a great extent their properties. Weakly and strongly coupled clusters are discussed. Hydrodynamic and capillary interactions giving rise to clusters formation are surveyed. Levitating droplet clusters, turbulent clusters and droplet clusters responsible for the breath-figures self-assembly are considered. Entropy factors influencing clustering are considered. Clustering in biological systems results in non-equilibrium multi-scale assembly, where at each scale, self-driven components come together by consuming energy in order to form the hierarchical structure.
This article is part of the theme issue ‘Bioinspired materials and surfaces for green science and technology (part 3)’.
Keywords: cluster, self-organization, dimension, capillary interactions, hydrodynamic interactions, biological clusters
1. Introduction: what are ‘clusters’ and ‘clustering’?
The term ‘cluster’ denotes a collection of similar, although not necessarily identical, things that occur together. The word originates from the Middle English clustre, Old English clyster (a clot or a bunch of grapes), thus in the Wycliffite Bible: ‘Thi tetes shul ben as the clustris of a vyne’ (‘May your breasts be like clusters of grapes’, Song of Songs 7:8, translation of Hebrew  ) [1].
) [1].
Although the phenomenon of clustering is ubiquitous in natural and artificial systems, it is difficult to exactly define ‘cluster’ and ‘clustering’, which is the common problem for very general terms. Scientists speak about the cluster model of nuclei [2], clusters of atoms and molecules [3], clusters of nanoparticles [4], clusters of genes [5] and clusters of galaxies [6]. In astronomy, researchers speak about superclusters of galaxies, which are the largest clearly defined in the Universe [7]. Therefore, clustering occurs on all of the possible spatial scales, as well as in the temporal domains [8,9].
In computer science, clusters are defined as groups of objects that have maximum similarity with other objects within the group, and minimum similarity with objects in other groups [8,9]. The clusters may be classified as physical and informational (or data) ones [10], as depicted in figure 1. However, this classification is conditional, given that clusters of genes [5] are both physical objects and data carriers. Our review is about physical clusters.
Figure 1.

Classification of clusters and clustering processes. (Online version in colour.)
In physics and chemistry, clusters refer to small (between three and several millions) conglomerations of atoms or molecules, or nanoparticles, which are intermediate in size and properties between a molecule and a bulk solid. The current interests in clusters and the emergence of the so-called cluster science was stimulated by the development of the nanoscience and nanotechnology [11–14]. Clusters provide a bridge between properties of isolated molecules and bulk matter, particularly those properties, such as the phase transitions, which have no counterpart in individual objects. Moreover, phase transition is a property of the infinite system and it is impossible for clusters.
In a more general sense, physical clusters may be classified as those dominated by fundamental physical forces, such as gravitational forces (clusters of galaxies [6,7]), electromagnetic or nuclear forces (clusters of nuclei and clusters of atoms and molecules [2,3]) and clusters formed by intermediate phases (gaseous or liquid). This kind of cluster is represented by recently discovered levitating droplet clusters bonded by aerodynamic interactions occurring in water vapour flow [15–20], droplet clusters formed by the breath-figures self-assembly, in which water droplets interact via evaporated polymer solutions [21–25] and capillary clusters formed by floating micro- and nanoparticles and bubbles [25–32]. The mixed situation, when clusters of particles interact via media and in parallel via fundamental (say electrostatic) interactions, was reported [33–37]. Clustering in biological systems was broadly discussed [38–40].
It is noteworthy that the droplet and capillary clusters reported in [11–28] are two-dimensional (2D) ones. The subclass of 2D clusters are the chain-like clusters in which particles or droplets form polymer-like structures due to a structural phase transition [15,37,38,41–43]. The distinction between 3D (bulk), 2D (plane) and 1D (linear or chain) clusters is important, due to the fact that the fundamental thermodynamic properties of 3D, 2D and 1D systems differ very strongly [44,45]. Thus, for example, phase transitions are impossible in 1D systems under certain assumptions [44]. Moreover, the Ising model suggests that ‘true’ phase transitions are impossible in finite systems, in particular they are impossible in clusters, whatever their dimensions are. The aforementioned temporal clustering represents a kind of 1D clustering [8,9].
2. Dimensional properties of clusters
Mathematically, clusters are related to networks and graphs. Consider a cluster built of particles, such as those depicted in figure 2. The particles may interact one with another, but they do not necessarily interact. The cluster shown in figure 2 may be seen as a graph, where particles correspond to vertices, and edges, in turn, correspond to interactions. The clustering coefficient C may be introduced [46]
| 2.1 |
Figure 2.

Cluster built of interacting particles seen as the network. Particles shown as circles correspond to vertices; edges, shown as lines, correspond to physical interactions between the particles. (Online version in colour.)
The clustering coefficient is a number between zero and one, which serves as a measure of the relative frequency of triangles, and it quantifies the network transitivity (the probability that two particles interacting with a given particle, also interact with one another). The clustering coefficient is equal to zero, when there is no clustering (zero triangles appear on the graph). In other words, a clustering coefficient is a measure of the degree to which vertices in a graph (and particles in the cluster) tend to cluster together and it is used to quantify aggregation in granular media [47].
Since the 1990s, a number of important discoveries have been made about scaling behaviour and topology of real-life networks, including experimental properties such as small-world networks and the scale-free behaviour [48–50]. The small-world concept implies that despite their large size, in most networks, there is a relatively short path between any two nodes. The scale-free behaviour implies a power-law relation between the number of nodes and the number of neighbours, which is somewhat similar to the statistical Benford law [51]. These concepts from the network topology and graphs theory turn out to be instructive for physical characterization of clusters. For example, the small-world effect and scale-free behaviour were reported for packing problems related to aggregation of granular media [52].
3. Clusters dominated by fundamental physical interactions: strongly and weakly coupled systems
The reductionist approach concentrating on individual properties of atoms and molecules is opposite to the approach focusing on the properties of condensed phases comprising a large number of particles (atoms or molecules). The realm between these limits remained untouched until the late 1970s. Clusters are aggregates of particles intermediate in size between individual particles and aggregates large enough to be called the bulk matter. Consider the cluster built of N particles or molecules with the constant number density n = N/V. Suppose the attractive potential between two particles to be of the general form , where α = const and m is an integer.
The total interaction energy W of one particle with all other molecules of the cluster is given by
| 3.1 |
where d is the diameter of the particles and L is the size of the system. Note that the clustering coefficient C = 1 is this case because each particle interacts with every other particle. If d/L ≪ 1 and m > 3, large distance contributions to the interaction will disappear [53]. This makes possible the existence of intensive thermodynamic properties of condensed phases, which are independent of the size of the system L. Such systems are called weakly coupled, due to the fact that most of the energy of a particle comes from interactions between nearby neighbours.
However, for d/L ≪ 1, m < 3, the contributions from more distant particles will dominate over those of nearby ones [53]. In such cases (which are called strongly coupled systems), the size of the system matters, as occurs for gravitational forces where n = 1 and where distant planets, stars and galaxies are still strongly interacting with one another. Note that the boundary value m = 3 arises from the 3D nature of the space. This marginal class of systems whose interaction decays with an exponent equal to their dimensionality D (in other words, D = m) will be discussed below in the context of microfluidic droplet clusters.
The size of clusters is often of the same order of the diameters of the particles, namely d/L ≅ 1, and it follows from equation (3.1) that the size of the system must be taken into account for any value of m. Therefore, we conclude that the properties of clusters are never intensive and depend on the size of a cluster; this makes the study of cluster systems extremely challenging. In particular, the extensive nature of properties of clusters manifests itself in the well-known problem of the Tolman length [54], estimating the extent by which the surface tension γ of a small liquid drop (in other words, a cluster of water molecules) deviates from its planar value.
When clusters are governed by fundamental interactions and zero screening is assumed, the particles form the fully connected graph [46], and the clustering coefficient C introduced by equation (1.1) equals to unity, i.e. C = 1.
4. Clusters of particles interacting via intermediate media
(a). Capillary clusters
This very different kind of cluster is represented by the systems of macroscopic bodies interacting via liquid or gaseous phase. Consider the group of floating polymer bodies shown in figure 3. These bodies tend to form a cluster driven by the capillary forces illustrated by figure 4 (φ1 and φ2 are interfacial angles (defined as the meniscus slope angles) at the contact lines separating particles and liquid). Two different situations should be distinguished, as depicted in figure 4. In the first (shown in figure 4a,c,e), the particles (in our case, water droplets) are freely floating. The forces acting in this case were called the flotation forces by Kralchevsky et al. [26–29]. The attraction appears because the liquid meniscus changes the gravitational potential energy of two particles which decreases as they approach each other. Hence, the origin of this force is the particle weight (including the Archimedes force) [26–29]. Thus, it is well expectable that this force is negligible for micro-scaled floating objects, which are characterized by dimensions much smaller than the capillary length (where Δρ is the contrast in the densities of the floating body and liquid [55]). Kralchevsky et al. [26–29] stated that the flotation force disappears for spherical particles with radius smaller than 10 µm.
Figure 3.

Cluster built from floating polyethylene particles is shown. The scale bar is 3 mm. (Online version in colour.)
Figure 4.
Lateral capillary forces acting between solid particles. φ1 and φ2 are the interfacial angles (the meniscus slope angle) at the contact lines separating particles and liquid. (a,c,e) flotation forces; (b,d,f) immersion forces.
A force of capillary attraction also appears when the particles (instead of freely floating) are partially immersed in a liquid layer on a substrate, as shown in figure 4b,d,f. In this case, the deformation of the liquid surface is related to the wetting properties of the particle surface, namely the position of the triple line and the magnitude of contact angle, rather than to gravity. These forces were called the immersion forces by Kralchevsky et al. [26–29]. It should be emphasized that the immersion force can be significant even when R ≅ 10 nm [26–29].
The immersion forces may be either attractive (figure 4b) or repulsive (figure 4d). This is governed by the signs of the meniscus slope angles φ1 and φ2. As shown in [21–24], the capillary force is attractive when: sin φ1 sin φ2 > 0, and it will be repulsive, when: sin φ1 sin φ2 < 0. When the capillary forces are attractive, they give rise to the self-assembly or clustering of the floating objects. The theory developed by Kralchevsky et al. predicts the following asymptotic expression for the lateral immersion force acting between two particles (droplets) of radii R, separated by a centre-to-centre distance L
| 4.1 |
where K1(x) is the modified Bessel function of the first order and q is the parameter with the dimensions of m−1 introduced in [26–29], and R is the radius of floating particles. It is seen from equation (4.1) that the immersion force increases with the increase in the radius of floating objects. This prediction waits for experimental verification. It was already demonstrated by Bragg & Nye [30] and Lomer [31] that the capillary interaction of bubbles promotes the assemblage of bubbles, representing the crystal structure of real metals. It was also suggested that capillary forces are responsible for the breath-figures self-assembly discussed in [21–25]. However, the precise mechanism of breath-figures clustering remains obscure. Limaye et al. [56] suggested one more mechanism of attraction of floating bodies, namely their hydrodynamic attraction.
Capillary interaction is not the only kind of physical interaction acting between particles placed at the liquid/vapour interface. It was demonstrated by Pieranski that the repulsive electrostatic interactions between floating particles may be no less important than capillary ones [33]. The potential U(L) arising from the electrostatic interaction of floating particles is described by the function
| 4.2 |
where a1 and a2 are the pre-factors that determine the order of magnitude of the screened Coulomb, diffuse double layer and the dipole–dipole interaction, respectively; kB is the Boltzmann constant, is the inverse Debye screening length and T is the absolute temperature [33,57]. At large enough particle separations (), the dipolar contribution dominates the interaction. A wide range of experiments confirms the dipolar nature of the interactions, showing that the interparticle potential decays as L−3 [37]. Thus, clustering and self-assembly of floating particles result from both capillary and electrostatic interactions. Clustering in this case arises from media (liquid)-inspired and electrostatic forces. When capillary clusters are addressed, the effect of screening is pronounced. In other words, the particles do not feel the presence of distant ones, thus the graph representing the cluster is not fully connected and the clustering coefficient C < 1. Regrettably, there are no reports devoted to the calculation of the clustering coefficient for clusters built of objects interacting via gaseous or liquid media. This challenging task should be addressed in the future research.
(b). Two-dimensional and three-dimensional droplet clusters arising from hydrodynamic interactions
Forces acting between floating particles discussed in the previous section are essentially static. Another kind of cluster appears when droplets flowing inside a microchannel interact hydrodynamically [58–60]. Consider the continuous two-phase flow of water droplets dispersed in oil driven in a channel at low Reynolds number of . Note that the 2D flow enables the exact solution of equations of hydrodynamics [58,59]. It was demonstrated that the motion of the droplets induces dipolar flow fields, which result in hydrodynamic interactions between droplets (see §2). The 2D microfluidic droplet ensemble belongs to the marginal class of systems whose interaction decays with an exponent equal to their dimensionality, thus we have in this case the strongly coupled system, exposed to long-range effects [58,59]. However, it was shown that summation of the long-range 2D hydrodynamic forces over droplet clusters converges in contrast with the divergence of long-range hydrodynamic forces in the 3D situation [58,59]. The collective phenomena such as phonons emerge in chain-like clusters of water droplets dispersed in oil [58,59].
Hydrodynamic interactions occur also under sedimentation of colloidal particles. However, under 3D sedimentation experiments, the strong coupling gives rise to long-range correlations in the form of swirls that depend on the geometry of the boundaries [61]. The main difficulty with strongly coupled systems is their non-additivity, the inability to break them into weakly interacting subsystems [58–61]. Again, the dimensionality of clusters plays a crucial role in constituting their physical properties.
(c). The droplet cluster: water droplets interacting via the gaseous phase
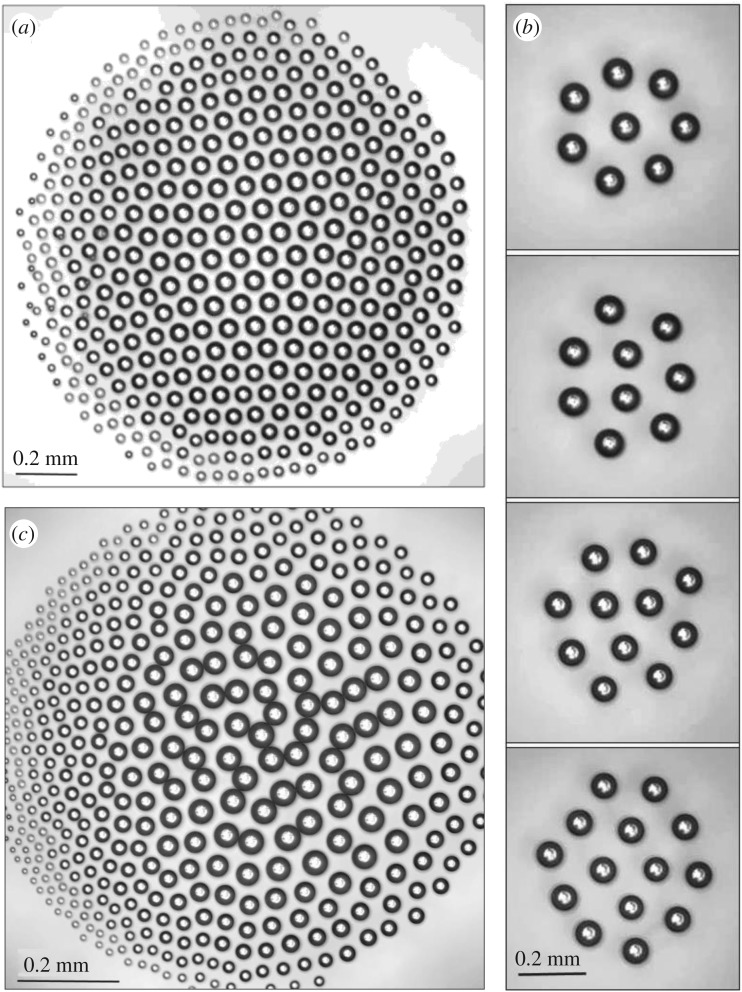
Ordered, self-assembled 2D clusters, depicted in figure 5, formed by droplets interacting via the gaseous phase were revealed and reported in [15–20]. It was demonstrated that the self-assembled monolayer of hexagonally ordered microdroplets (10–200 µm diameter) is condensed over a locally heated surface of water levitating at heights of the same order as the droplet diameter. The microdroplets do not coalesce with the substrate water layer due to complex aerodynamic forces between them in the ascending vapour flow. Typical water temperatures are 60–95°C, although the phenomenon has been observed also at lower temperatures of about 25°C [20]. Such clusters emerge over locally heated spots of a liquid surface [15–20]. The weight of droplets is equilibrated by the drag force of the ascending vapour jet rising over the heated spot [15,17]. Due to the attraction to the centre of the heated area combined with aerodynamic repulsion between the droplets, the clusters form structures that are quite diverse and different from densest packing of hard spheres [17].
Figure 5.
The configurations of levitating droplet clusters are depicted: (a) the hexagonal 2D lattice of the typical ‘large’ clusters is shown; (b) clusters built of 8–14 droplets are depicted [17]; (c) the cluster comprising chain structures is presented [19].
Dynamic ordering of clusters may be quantified with the Voronoi entropy [17]. It was suggested that not only aerodynamic but also electrostatic interactions are responsible for the ordering [16]. However, experiments performed with the use of the external electric field demonstrated the crucial role of the aerodynamic interactions in the ordering of the droplet clusters. Anyway, both fundamental (electrostatic) and media-driven interactions are inherent for droplet clusters [16]. The aerodynamic interactions are short-range, whereas electrostatic interactions are long-range ones, thus the resulting clustering coefficient for droplet cluster supplied by equation (2.1) equals to unity. It is recognized that the clustering coefficient, introduced by equation (2.1), ignores the strength of interactions between the objects forming the cluster. Thus, a more reasonable physical (weighed) coefficient considering the nature of these interactions quantifying the phenomenon of clustering should be introduced, which is elaborated now by our group and which is out of the scope of the present review.
(d). Turbulent droplets clustering
One more example of droplets clustering is supplied by aggregation of water droplets in cumulus clouds [62–65]. In contrast with the aforementioned 2D clusters, the aggregation of droplets in clouds is a 3D effect, which does not result in the formation of ordered structures. The phenomenon takes place at the high Reynolds numbers ; thus, the effects due to the turbulence became important. This turbulent clustering is also known as the ‘preferential concentration’ effect. Essential processes that govern the evolution of the entire system take place at the scale of the particle size, which is smaller than the dissipation scale [62]. The clusters of inertial particles or droplets are formed and disappear permanently even in the case of the so-called homogeneous turbulence. Consideration of collisions between droplets followed by their coalescence is important for the understanding of the cloud clusters [62].
(e). Self-assembled breath figures
One more exemplification of 2D droplets-built clusters is supplied by the so-called breath-figures patterns. The breath figures consisting of regular hexagonal clusters of microdroplets are formed when water vapour contacts a cold solid surface, such as glass, when one breathes on it. The breath figures were studied already by J. Aiken and by Lord Rayleigh [66,67]; however, the mechanism of the interaction between the droplets leading to their clustering and non-coalescence is still not well understood. Most researchers tend to agree that non-coalescence is due to the Marangoni convection and variations in the temperature and humidity next to condensing microdroplets [23,24]. It was suggested that the capillary interactions discussed in §4a are responsible for the ordering observed under the breath-figures self-assembly [23,24].
Breath figures are used for the formation of regular honeycomb arrangements of micropores on polymer surfaces, depicted in figure 6. These micropores are formed by rapid evaporation of polymer solutions in humid atmosphere [21–25]. Rapid evaporation of the solvent cools the solution resulting in intensive condensation of water droplets at the surface. The droplets then sink into the solution, eventually forming a honeycomb pattern (figure 6). The correlational analysis of the SEM images indicated hierarchical short-range (ca 5 µm) and large-scale (ca 50 µm) ordering of the honeycomb structures. The quantification of ordering in droplet clusters and breath-figures patterns may be achieved by a diversity of mathematical techniques including calculation of the Voronoi entropy, Fourier analysis of the images, calculation of the Minkowski functionals and the correlational analysis [68].
Figure 6.
Typical micro-porous polymer (polystyrene) pattern arising from the breath-figures self-assembly process. Clusters of water droplets condensed on the rapidly evaporated surface of polymer solution give rise to the ordered micro-porous topography. The scale bar is 20 µm.
(f). Formation of clusters and entropy considerations
Until now, we considered only energy contributions to the formation of clusters of interacting objects. Obviously, entropy considerations are well expected to be important for the understanding of clusters formation. Somewhat surprisingly entropy considerations are essential for the analysis of clustering on all of possible scales: from subatomic to astronomic ones [69,70]. We restrict ourselves to the very important and ubiquitous entropy-driven ‘hydrophobic effect’. The hydrophobic effect is the observed tendency of non-polar particles to aggregate in an aqueous solution and exclude water molecules [71,72]. The hydrophobic interaction originates from the disruption of hydrogen bonds between molecules of liquid water by the non-polar solute [71]. By aggregating together, non-polar molecules reduce the surface area exposed to water and minimize this disruptive effect. This reduction in the surface is entropically favourable [71,72]. This entropic effect leads to the clustering of hydrophobic colloidal particles [71]. Hydrophobic interactions, like other entropic effects, increase in strength with increasing temperature [71]. Thus, the formation of clusters may be stipulated by the entropic factors as well as by the energetic ones. Again, there is no research devoted to the calculation of the clustering coefficient for the clusters, driven by the hydrophobic effect.
(g). Clustering in biological systems
The phenomenon of clustering in living matter is ubiquitous and is not well understood due to its inherent complexity [38–40,73]. It can be viewed as a non-equilibrium hierarchical assembly, where at each scale, self-driven components come together by consuming energy in order to form increasingly complex structures [73]. The examples include bacterial turbulence [74,75] and fish schooling in the ocean [76]. It was shown that there exists a trade-off between grouping (clustering) and the optimizing of the energetic efficiency of individual locomotion for swimming fishes [76].
The complexity of biological clusters arises from the fact that they are built from active (self-propelling) matter (bacteria, nematodes, fishes, etc.). Even the pair interactions between self-propelled swimmers become complicated, due to the fact that the pair dynamics depends on the Reynolds number and time [73]. Moreover, the scaling arguments and the estimation of the relevant dimensionless numbers (such as Re) become subtle. Even if the individual swimmer is small (say microscopic), when a cluster of such swimmers is considered, the relevant length scale used to calculate Re may be that of the collective rather than the individual swimmer [73]. It is noteworthy that swimming bacteria sometimes demonstrate the turbulent dynamics in a system which is nominally in the regime of Stokes flow (Re ≪ 1) [77]. Establishment of the nature of coupling (strong/weak) as well as the calculation of the clustering coefficient for the clusters built of living entities becomes an extremely challenging task.
5. Conclusion
An interest in clusters composed of the small number of physical entities was strengthened in the context of nanoscience [78,79]. In this article, we suggested a general approach to the problems of clusters and clustering. The classification of clusters distinguishing between physical, information (data) and biological clusters can be proposed. Clusters of any kind are treated mathematically as graphs or networks, with the clustering coefficient defined as a measure of the degree to which vertices in a graph tend to cluster together [46]. Physical clusters are aggregates of objects, such as particles, droplets or bubbles, intermediate in size between individual entities and aggregates large enough to be called the bulk matter.
The stability of physical clusters is controlled either by fundamental physical interactions or interactions via the gaseous or liquid media. The energy of attraction may be represented by the potential . The physical properties of the cluster are dependent, to a large extent, on the interrelation between the exponent, m, and the dimension of the cluster, D. When m> D, the clusters are weakly coupled, and the particles are mainly influenced by their nearest neighbours. On the other hand, when m<D, the contributions from more distant particles become significant and dominate over neighbouring particles. The intermediate case, m = D, is common for 2D droplet clusters driven by hydrodynamic interactions, such as disk-shaped water droplets dispersed in oil [58–60].
It was shown that summation of the long-range 2D hydrodynamic forces over droplet clusters converges in contrast with the divergence of long-range hydrodynamic forces in the 3D situation [58,59]. However, the physical properties of clusters, composed of the small number of particles, are never intensive, and they depend on the size of a cluster, which makes the study of cluster systems extremely challenging. Generally, the properties of systems containing infinitely many particles are qualitatively different from those of finite systems. In particular, phase transitions cannot occur in any finite system; they are solely a property of infinite systems, in particular there is no phase transition in any finite-temperature system in one dimension [44,80].
The entropy considerations are important for the understanding of properties of clusters as well as energetic ones. The entropy factors to a great extent govern aggregation of hydrophobic particles, dispersed in water [69,71,72]. The properties, structures and ordering of droplet clusters levitating on the hot water/vapour interface and turbulent droplet clusters are addressed [15–20,62–65,81]. The phenomenon of clustering occurs on all of the possible spatial scales, as well as in the broad variety of temporal domains.
Data accessibility
This article has no additional data.
Authors' contributions
All authors equally contributed to the paper.
Competing interests
We declare we have no competing interests.
Funding
The authors gratefully acknowledge the Russian Science Foundation (project 19-19-00076) for the financial support of this work.
References
- 1.Oxford English Dictionary. online. See https://www.oed.com/view/Entry/34883) (accessed 1 September 2019).
- 2.Saito S. 1969. Interaction between clusters and Pauli principle. Prog. Theor. Phys. 41, 705–722. ( 10.1143/PTP.41.705) [DOI] [Google Scholar]
- 3.Haberland H. 1994. Clusters of atoms and molecules. Berlin, Germany: Springer. [Google Scholar]
- 4.Johnston RL. 2003. Evolving better nanoparticles: genetic algorithms for optimising cluster geometries. Dalton Trans. 22, 4193–4207. ( 10.1039/b305686d) [DOI] [Google Scholar]
- 5.Yi G, Sze SH, Thon MR. 2007. Identifying clusters of functionally related genes in genomes. Bioinformatics 23, 1053–1060. ( 10.1093/bioinformatics/btl673) [DOI] [PubMed] [Google Scholar]
- 6.Press WH, Schechter P. 1974. Formation of galaxies and clusters of galaxies by self-similar gravitational condensation. Astrophys. J. 187, 425–438. ( 10.1086/152650) [DOI] [Google Scholar]
- 7.Fabian AC. 1992. Clusters and superclusters of galaxies. NATO ASI series, V. 366. Cambridge, UK: Springer. [Google Scholar]
- 8.Liao TW. 2005. Clustering of time series data—a survey. Pattern Recognit. 38 1857–1874. ( 10.1016/j.patcog.2005.01.025) [DOI] [Google Scholar]
- 9.Aghabozorgi S, Shirkhorshidi AS, Wah TY. 2015. Time-series clustering—a decade review. Information Systems 53, 16–38. ( 10.1016/j.is.2015.04.007) [DOI] [Google Scholar]
- 10.Kraskov A, Stögbauer H, Andrzejak RG, Grassberger P. 2005. Hierarchical clustering using mutual information. Europhys. Lett. 70, 278–284. ( 10.1209/epl/i2004-10483-y) [DOI] [Google Scholar]
- 11.Cleveland CL, Landman U. 1992. Dynamics of cluster-surface collisions. Science 257, 355–361. ( 10.1126/science.257.5068.355) [DOI] [PubMed] [Google Scholar]
- 12.Brauman JI. 1996. Clusters. Science 271, 889 ( 10.1126/science.271.5251.889) [DOI] [Google Scholar]
- 13.Liu K, Cruzan JD, Saykally RJ. 1996. Water clusters. Science 271, 929–933. ( 10.1126/science.271.5251.929) [DOI] [PubMed] [Google Scholar]
- 14.Alivisatos AP. 1996. Semiconductor clusters, nanocrystals, and quantum dots. Science 271, 933–937. ( 10.1126/science.271.5251.933) [DOI] [Google Scholar]
- 15.Fedorets AA. 2004. Droplet cluster. JETP Lett. 79, 372–374. ( 10.1134/1.1772434) [DOI] [Google Scholar]
- 16.Fedorets AA, Dombrovsky LA, Bormashenko E, Nosonovsky M. 2019. On relative contribution of electrostatic and aerodynamic effects to dynamics of a levitating droplet cluster. Int. J. Heat Mass Transf. 133, 712–717. ( 10.1016/j.ijheatmasstransfer.2018.12.160) [DOI] [Google Scholar]
- 17.Fedorets AA, Frenkel M, Bormashenko E, Nosonovsky M. 2017. Small levitating ordered droplet clusters: Stability, symmetry, and Voronoi entropy. J. Phys. Chem. Lett. 8, 5599–5602. ( 10.1021/acs.jpclett.7b02657) [DOI] [PubMed] [Google Scholar]
- 18.Dombrovsky LA, Fedorets AA, Medvedev DN. 2016. The use of infrared irradiation to stabilize levitating clusters of water droplets. Infrared Phys. Technol. 75 124–132. ( 10.1016/j.infrared.2015.12.020) [DOI] [Google Scholar]
- 19.Fedorets AA, Bormashenko Ed, Dombrovsky LA, Nosonovsky M. 2019. Droplet clusters: nature-inspired biological reactors and aerosols. Phil. Trans. R. Soc. A 377, 20190121 ( 10.1098/rsta.2019.0121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fedorets AA, Dombrovsky LA, Ryumin PI. 2017. Expanding the temperature range for generation of droplet clusters over the locally heated water surface. Int. J. Heat Mass Transf. 113, 1054–1058. ( 10.1016/j.ijheatmasstransfer.2017.06.015) [DOI] [Google Scholar]
- 21.François B, Pitois O, François J. 1995. Polymer films with a self-organized honeycomb morphology. Adv. Mater. 7, 1041–1044. ( 10.1002/adma.19950071217) [DOI] [Google Scholar]
- 22.Bunz UHF. 2006. Breath figures as a dynamic templating method for polymers and nanomaterials. Adv. Mater. 18, 973–989. ( 10.1002/adma.200501131) [DOI] [Google Scholar]
- 23.Munoz-Bonilla A, Fernández-García M, Rodriguez-Hernandez J. 2014. Towards hierarchically ordered functional porous polymeric surfaces prepared by the breath figures approach. Prog. Polym. Sci. 39, 510–554. ( 10.1016/j.progpolymsci.2013.08.006) [DOI] [Google Scholar]
- 24.Bormashenko E. 2017. Breath-figure self-assembly, a versatile method of manufacturing membranes and porous structures: physical, chemical and technological aspects. Membranes 7, 45 ( 10.3390/membranes7030045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang A, Bai H, Li L. 2015. Breath figure: a nature-inspired preparation method for ordered porous films. Chem. Rev. 115, 9801–9868. ( 10.1021/acs.chemrev.5b00069) [DOI] [PubMed] [Google Scholar]
- 26.Kralchevsky PA, Danov KD, Denkov ND.. 2003. Chemical physics of colloid systems and interfaces, chapter 5. In Surface and colloidal chemistry (ed. Birdi KS.). Boca Raton, FL: CRC Press. [Google Scholar]
- 27.Kralchevsky PA, Nagayama K. 1994. Capillary forces between colloidal particles. Langmuir 10, 23–36. ( 10.1021/la00013a004) [DOI] [Google Scholar]
- 28.Kralchevsky PA, Nagayama K. 2000. Capillary interactions between particles bound to interfaces, liquid films and biomembranes. Adv. Colloid Interface Sci. 85, 145–192. ( 10.1016/S0001-8686(99)00016-0) [DOI] [PubMed] [Google Scholar]
- 29.Kralchevsky PA, Paunov VN, Ivanov IB, Nagayama K. 1992. Capillary meniscus interactions between colloidal particles attached to a liquid-fluid interface. J. Colloid Interface Sci. 151, 79–94. ( 10.1016/0021-9797(92)90239-I) [DOI] [Google Scholar]
- 30.Bragg L, Nye JFA. 1947. Dynamical model of a crystal structure. Proc. R. Soc. Lond. A 190, 474–481. ( 10.1098/rspa.1947.0089) [DOI] [Google Scholar]
- 31.Lomer WM. 1949. The forces between floating bubbles and a quantitative study of the Bragg ‘Bubble Model’ of a crystal. Math. Proc. Cambridge Phil. Soc. 45, 660–673. ( 10.1017/S0305004100025342) [DOI] [Google Scholar]
- 32.Multanen V, Pogreb R, Bormashenko Ye, Shulzinger E, Whyman G, Frenkel M, Bormashenko Ed. 2016. Under-liquid self-assembly of submerged buoyant polymer particles. Langmuir 32, 5714–5720. ( 10.1021/acs.langmuir.6b00636) [DOI] [PubMed] [Google Scholar]
- 33.Pieranski P. 1980. Two-dimensional interfacial colloidal crystals. Phys. Rev. Lett. 45, 569–573. ( 10.1103/PhysRevLett.45.569) [DOI] [Google Scholar]
- 34.Shavlov AV, Dzhumandzhi VA. 2016. Metastable states and coalescence of charged water drops inside clouds and fog. J. Aerosol Sci. 91, 54–61. ( 10.1016/j.jaerosci.2015.10.001) [DOI] [Google Scholar]
- 35.Fedorets AA, Dombrovsky LA, Gabyshev DN, Bormashenko E, Nosonovsky M.. 2020. An effect of external electric field on dynamics of levitating water droplets. Int. J. Therm. Sci. (under review). [Google Scholar]
- 36.Masschaele R, Park BJ, Furst EM, Fransaer J, Vermant J. 2010. Finite ion-size effects dominate the interaction between charged colloidal particles at an oil-water interface. Phys. Rev. Lett. 105, 048303 ( 10.1103/PhysRevLett.105.048303) [DOI] [PubMed] [Google Scholar]
- 37.Singh P, Joseph DD, Aubry N. 2010. Dispersion and attraction of particles floating on fluid–liquid surfaces. Soft Matter 6, 4310–4325. ( 10.1039/C000495M) [DOI] [Google Scholar]
- 38.Bowen JD, Stolzenbach KD, Chisholm SW. 1993. Simulating bacterial clustering around phytoplankton cells in a turbulent ocean. Limnol. Oceanogr. 38, 36–51. ( 10.4319/lo.1993.38.1.0036) [DOI] [Google Scholar]
- 39.Corsettia A, Lavermicocca P, Morea M, Baruzzi F, Tostic N, Gobbetti M. 2001. Phenotypic and molecular identification and clustering of lactic acid bacteria and yeasts from wheat (species Triticum durum and Triticum aestivum) sourdoughs of Southern Italy. Int. J. Food Microbiol. 64, 95–104. ( 10.1016/S0168-1605(00)00447-5) [DOI] [PubMed] [Google Scholar]
- 40.Ballerini M, et al. 2008. Interaction ruling animal collective behavior depends on topological rather than metric distance: evidence from a field study. Proc. Natl Acad. Sci. USA 105, 1232–1237. ( 10.1073/pnas.0711437105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McMullen A, Holmes-Cerfon M, Sciortino F, Grosberg AY, Brujic J. 2018. Freely jointed polymers made of droplets. Phys. Rev. Lett. 121, 138002 ( 10.1103/PhysRevLett.121.138002) [DOI] [PubMed] [Google Scholar]
- 42.Muševič I, Škarabot M, Tkalec U, Ravnik M, Žumer S. 2006. Two-dimensional nematic colloidal crystals self-assembled by topological defects. Science 313, 954–958. ( 10.1126/science.1129660) [DOI] [PubMed] [Google Scholar]
- 43.Fedorets Al, Frenkel M, Legchenkova I, Shcherbakov D, Dombrovsky L, Nosonovsky M, Bormashenko E. 2019. Self-arranged levitating droplet clusters: a reversible transition from hexagonal to chain structure. Langmuir 35, 15 330–15 334. ( 10.1021/acs.langmuir.9b03135) [DOI] [PubMed] [Google Scholar]
- 44.Landau LD, Lifshitz E. 2013. Course of theoretical physics, V. 5, statistical physics, 3rd edn Oxford, UK: Butterworth-Heinemann. [Google Scholar]
- 45.Kosterlitz JM, Thouless DJ. 1973. Ordering, metastability and phase transitions in two-dimensional systems. J. Phys. C: Solid State Phys. 6, 1181 ( 10.1088/0022-3719/6/7/010) [DOI] [PubMed] [Google Scholar]
- 46.Girvan M, Newman MEJ. 2002. Community structure in social and biological networks. Proc. Natl Acad. Sci. USA 99, 7821–7826. ( 10.1073/pnas.122653799) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arévalo R, Zuriguel I, Maza D. 2010. Topology of the force network in the jamming transition of an isotropically compressed granular packing. Phys. Rev. E 81, 041302 ( 10.1103/PhysRevE.81.041302) [DOI] [PubMed] [Google Scholar]
- 48.Watts DJ, Strogatz SH. 1998. Collective dynamics of small-world networks. Nature 393, 440–442. ( 10.1515/9781400841356.301) [DOI] [PubMed] [Google Scholar]
- 49.Barabási A-L, Albert R. 1999. Emergence of scaling in random networks. Science 286, 509–512. ( 10.1126/science.286.5439.509) [DOI] [PubMed] [Google Scholar]
- 50.Albert R, Barabási A-L. 2002. Statistical mechanics of complex networks. Rev. Modern Phys. 74, 47–97. ( 10.1103/RevModPhys.74.47) [DOI] [Google Scholar]
- 51.Whyman G, Ohtori N, Shulzinger E, Bormashenko Ed. 2016. Revisiting the Benford law: when the Benford-like distribution of leading digits in sets of numerical data is expectable? Physica A: Stat. Mech. Appl. 461, 595–601. ( 10.1016/j.physa.2016.06.054) [DOI] [Google Scholar]
- 52.Andrade JS Jr, Herrmann HJ, Andrade RF, de Silva LR.. 2005. Apollonian networks: simultaneously scale-free, small world, euclidean, space filling, and with matching graphs. Phys. Rev. Lett. 94, 018702 ( 10.1103/PhysRevLett.94.018702) [DOI] [PubMed] [Google Scholar]
- 53.Israelichvili J. 2011. Intermolecular and surface forces, 3rd edn Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 54.Schmelzer JWP, Abyzov AS, Baidakov VG. 2019. Entropy and the Tolman parameter in nucleation theory. Entropy 21, 670 ( 10.3390/e21070670) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Gennes PG, Brochard-Wyart F, Quéré D. 2003. Capillarity and wetting phenomena. Berlin, Germany: Springer. [Google Scholar]
- 56.Limaye AV, Narhe RD, Dhote AM, Ogale SB. 1996. Evidence for convective effects in breath figure formation on volatile fluid surfaces. Phys. Rev. Lett. 76, 3762–3765 ( 10.1103/PhysRevLett.76.3762). [DOI] [PubMed] [Google Scholar]
- 57.Hurd AJ. 1985. The electrostatic interaction between interfacial colloidal particles. J. Phys. A: Math. Gen. 18, L1055–L1060. ( 10.1088/0305-4470/18/16/011). [DOI] [Google Scholar]
- 58.Beatus Ts, Shani I, Bar-Ziv RH, Tlusty Ts. 2017. Two-dimensional flow of driven particles: a microfluidic pathway to the non-equilibrium frontier. Chem. Soc. Rev. 46, 5620–5646. ( 10.1039/C7CS00374A) [DOI] [PubMed] [Google Scholar]
- 59.Beatus Ts, Bar-Ziv RH, Tlusty Ts. 2012. The physics of 2D microfluidic droplet ensembles . Phys. Rep. 516, 103–145. ( 10.1016/j.physrep.2012.02.003) [DOI] [PubMed] [Google Scholar]
- 60.Raj MD, Gnanasekaran A, Rengaswamy R. 2019. On the role of hydrodynamic interactions in the engineered-assembly of droplet ensembles. Soft Matter 15, 7863–7875. ( 10.1039/c9sm01528k) [DOI] [PubMed] [Google Scholar]
- 61.Segrè PN, Herbolzheimer E, Chaikin PM. 1997. Long-range correlations in sedimentation. Phys. Rev. Lett. 79, 2574–2577. ( 10.1103/PhysRevLett.79.2574) [DOI] [Google Scholar]
- 62.Shaw RA. 2003. Particle-turbulence interactions in atmospheric clouds. Annu. Rev. Fluid Mech. 35, 183–227. ( 10.1146/annurev.fluid.35.101101.161125) [DOI] [Google Scholar]
- 63.Dombrovsky LA, Zaichik LI. 2010. An effect of turbulent clustering on scattering of microwave radiation by small particles in the atmosphere. J. Quant. Spectr. Radiat. Transf. 111, 234–242. ( 10.1016/j.jqsrt.2009.09.003) [DOI] [Google Scholar]
- 64.Matsuda K, Onishi R, Hirahata M, Kurose K, Takahashi K, Komori S. 2014. Influence of microscale turbulent droplet clustering on radar cloud observations. J. Atmos. Sci. 71, 3569–3582. ( 10.1175/JAS-D-13-0368.1) [DOI] [Google Scholar]
- 65.Ariki T, Yoshida K, Matsuda K, Yoshimatsu K. 2018. Scale-similar clustering of heavy particles in the inertial range of turbulence. Phys. Rev. E 97, 033109 ( 10.1103/PhysRevE.97.033109) [DOI] [PubMed] [Google Scholar]
- 66.Aitken J. 1895. Breath figures. Proc. R. Soc. Edinb. 20, 94–97. ( 10.1017/S0370164600048434) [DOI] [Google Scholar]
- 67.Rayleigh L. 1911. Breath figures. Nature 86, 416–417. ( 10.1038/086416d0) [DOI] [Google Scholar]
- 68.Bormashenko E, Frenkel M, Vilk A, Legchenkova I, Fedorets AA, Aktaev NE, Dombrovsky LA, Nosonovsky M. 2018. Characterization of self-assembled 2D patterns with Voronoi entropy. Entropy 20, 956 ( 10.3390/e20120956) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shi Z, Ford JV, Wei S, Castleman AW Jr. 1993. Water clusters: contributions of binding energy and entropy to stability. J. Chem. Phys. 99, 8009 ( 10.1063/1.465678). [DOI] [Google Scholar]
- 70.Lloyd-Davies EJ, Ponman TJ, Cannon DB. 2000. The entropy and energy of intergalactic gas in galaxy clusters. Monthly Notic. R. Astron. Soc. 315, 689–702. ( 10.1046/j.1365-8711.2000.03380.x) [DOI] [Google Scholar]
- 71.Chandler D. 2005. Interfaces and the driving force of hydrophobic assembly. Nature 437, 640–647. ( 10.1038/nature04162) [DOI] [PubMed] [Google Scholar]
- 72.Breslow R. 1991. Hydrophobic effects on simple organic reactions in water. Acc. Chem. Res. 24, 159–164. ( 10.1021/ar00006a001) [DOI] [Google Scholar]
- 73.Klotsa D. 2019. As above, so below, and also in between: mesoscale active matter in fluids. Soft Matter 15, 8946–8950. ( 10.1039/C9SM01019J) [DOI] [PubMed] [Google Scholar]
- 74.Wensink HH, Dunkel J, Heidenreich S, Drescher K, Goldstein RE, Löwen H, Yeomans JM. 2012. Meso-scale turbulence in living fluids. Proc. Natl Acad. Sci. USA 109, 14 308–14 313. ( 10.1073/pnas.1202032109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dunkel J, Heidenreich S, Drescher K, Wensink HH, Bär M, Goldstein RE. 2013. Fluid dynamics of bacterial turbulence. Phys. Rev. Lett. 110, 228102 ( 10.1103/PhysRevLett.110.228102) [DOI] [PubMed] [Google Scholar]
- 76.Noda T, Fujioka K, Fukuda H, Mitamura H, Ichikawa K, Arai N. 2016. The influence of body size on the intermittent locomotion of a pelagic schooling fish. Proc. R. Soc. B 283, 290–291. ( 10.1098/rspb.2015.3019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cisneros LH, Cortez R, Dombrowski C, Goldstein RE, Kessler JO. 2007. Fluid dynamics of self-propelled microorganisms, from individuals to concentrated populations. Exp. Fluids 43, 737–753. ( 10.1007/978-3-642-11633-9_10) [DOI] [Google Scholar]
- 78.Schmid G, Bäunle M, Geerkens M, Heim I, Osemann C, Sawitowski T. 1999. Current and future applications of nanoclusters. Chem. Soc. Rev. 28 179–185. ( 10.1039/A801153B) [DOI] [Google Scholar]
- 79.Schmid G, Fenske D. 2010. Metal clusters and nanoparticles. Phil. Trans. R. Soc. A 368, 1207–1210. ( 10.1098/rsta.2009.0281) [DOI] [PubMed] [Google Scholar]
- 80.Kadanoff LP. 2009. More is the same; phase transitions and mean field theories. J. Stat. Phys. 137, 777–797 ( 10.1007/s10955-009-9814-1) [DOI] [Google Scholar]
- 81.Fedorets AA, Dombrovsky LA, Ryumin PI. 2017. Expanding the temperature range for generation of droplet clusters over the locally heated water surface. Int. J. Heat Mass Transf. 113, 1054–1058. ( 10.1016/j.ijheatmasstransfer.2017.06.015) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.