Abstract
We examined the mineralogical, chemical and isotopic compositions of secondary fluid inclusions in olivine-rich rocks from two active serpentinization systems: the Von Damm hydrothermal field (Mid-Cayman Rise) and the Zambales ophiolite (Philippines). Peridotite, troctolite and gabbroic rocks in these systems contain abundant CH4-rich secondary inclusions in olivine, with less abundant inclusions in plagioclase and clinopyroxene. Olivine-hosted secondary inclusions are chiefly composed of CH4 and minor H2, in addition to secondary minerals including serpentine, brucite, magnetite and carbonates. Secondary inclusions in plagioclase are dominated by CH4 with variable amounts of H2 and H2O, while those in clinopyroxene contain only CH4. We determined hydrocarbon abundances and stable carbon isotope compositions by crushing whole rocks and analysing the released volatiles using isotope ratio monitoring—gas chromatography mass spectrometry. Bulk rock gas analyses yielded appreciable quantities of CH4 and C2H6 in samples from Cayman (4–313 nmol g−1 CH4 and 0.02–0.99 nmol g−1 C2H6), with lesser amounts in samples from Zambales (2–37 nmol g−1 CH4 and 0.004–0.082 nmol g−1 C2H6). Mafic and ultramafic rocks at Cayman exhibit δ13CCH4 values of −16.7‰ to −4.4‰ and δ13CC2H6 values of −20.3‰ to +0.7‰. Ultramafic rocks from Zambales exhibit δ13CCH4 values of −12.4‰ to −2.8‰ and δ13CC2H6 values of −1.2‰ to −0.9‰. Similarities in the carbon isotopic compositions of CH4 and C2H6 in plutonic rocks, Von Damm hydrothermal fluids, and Zambales gas seeps suggest that leaching of fluid inclusions may provide a significant contribution of abiotic hydrocarbons to deep-sea vent fluids and ophiolite-hosted gas seeps. Isotopic compositions of CH4 and C2H6 from a variety of hydrothermal fields hosted in olivine-rich rocks that are similar to those in Von Damm vent fluids further support the idea that a significant portion of abiotic hydrocarbons in ultramafic-influenced vent fluids is derived from fluid inclusions.
This article is part of a discussion meeting issue ‘Serpentinite in the Earth system’.
Keywords: abiotic organic synthesis, methane, fluid inclusions, serpentinization, hydrothermal systems
1. Introduction
Identifying sources and sinks for methane (CH4) has remained one of the most important problems in hydrothermal research due to methane's numerous roles in the cycling of carbon, the evolution of microbial life and the limits of microbial life in extreme geochemical environments. Oxidation of abiotic CH4 can represent a major source of metabolic energy for microbial organisms in diverse geological environments where mafic and ultramafic rock occur, including mid-ocean ridge and off-axis environments, passive margins, subduction zones, orogenic belts and ophiolites [1–5]. As serpentinization systems have existed throughout most of Earth's history, it is likely that such systems represent some of the most ancient microbial habitats on Earth (e.g. [6,7]). Indeed, the synthesis of abiotic hydrocarbons in serpentinization systems plays a central role in current hypotheses for the origin and early evolution of life on Earth (e.g. [8,9]) and possibly elsewhere in the solar system (e.g. [10,11]).
Seawater-derived hot-spring fluids at oceanic spreading centres are highly enriched in dissolved CH4 relative to seawater, where CH4(aq) concentrations are typically below 2 nmol kg−1 [12]. In general, fluids that interact with ultramafic rocks contain aqueous CH4 concentrations of the order of 2–3 mmolal [13–16], while those that interact with mafic rocks typically contain less than 1 mmolal CH4(aq) (e.g. [13]). Methane is also a common feature of continental ultramafic-influenced cold seep fluids, with some releasing reduced gases that contain up to 93% CH4 by volume [17–19]. Previous studies have demonstrated that fluid inclusions are common in gabbroic rocks and are believed to represent a major reservoir of abiotic CH4 in the lower oceanic crust [20–23]. Although it has long been postulated that such fluid inclusions may contribute to the inventory of CH4 in ridge-crest hydrothermal fluids [21,24,25], more recent studies have provided compelling evidence for a direct link based on the chemical and isotopic composition of vent fluid CH4 [16,26].
The purpose of this study was to characterize the chemical and isotopic compositions of low molecular weight hydrocarbons in fluid inclusions hosted in olivine-rich rocks, in order to facilitate a comparison to hydrocarbons venting from submarine hydrothermal systems and continental serpentinite-hosted gas seeps. To this end, we measured the relative abundances and carbon isotopic compositions of low molecular weight hydrocarbons in fluid inclusions hosted in mafic and ultramafic rocks from the Mount Dent oceanic core complex at the Mid-Cayman Rise (figure 1) and in ultramafic rocks from the Zambales ophiolite, Philippines (figure 2). The results allow constraints to be placed on the source of hydrocarbons in gas seeps at the Zambales ophiolite and in vent fluids emanating from the Von Damm hydrothermal field at Mount Dent, which have implications for the origin of abiotic CH4 in other submarine and subaerial vent systems.
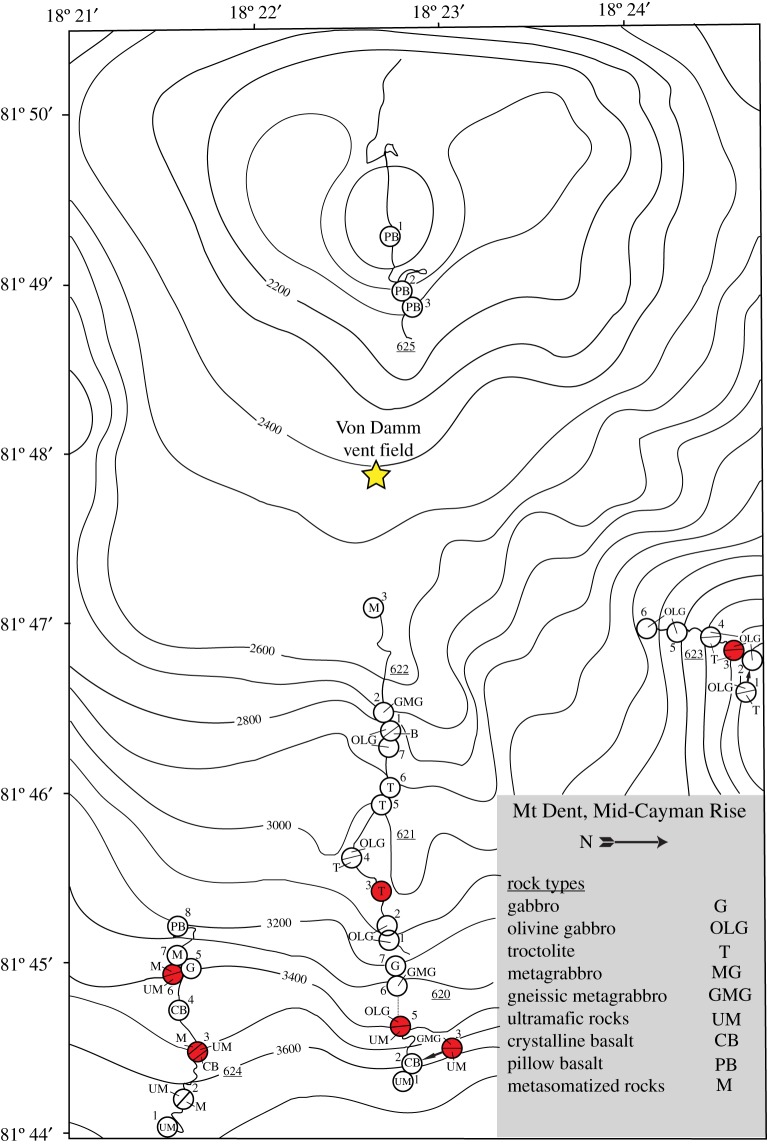
Figure 1.
Bathymetric map of the eastern flank of the Mount Dent oceanic core complex on the Mid-Cayman Rise showing the locations of dive tracks and stations from the Cayman Trough Project. The distribution of rock types is shown for each numbered station. Samples used in this study are displayed as red circles. The approximate location of the Von Damm hydrothermal field (yellow star) is shown for comparison. Figure modified from Stroup & Fox [27]. (Online version in colour.)
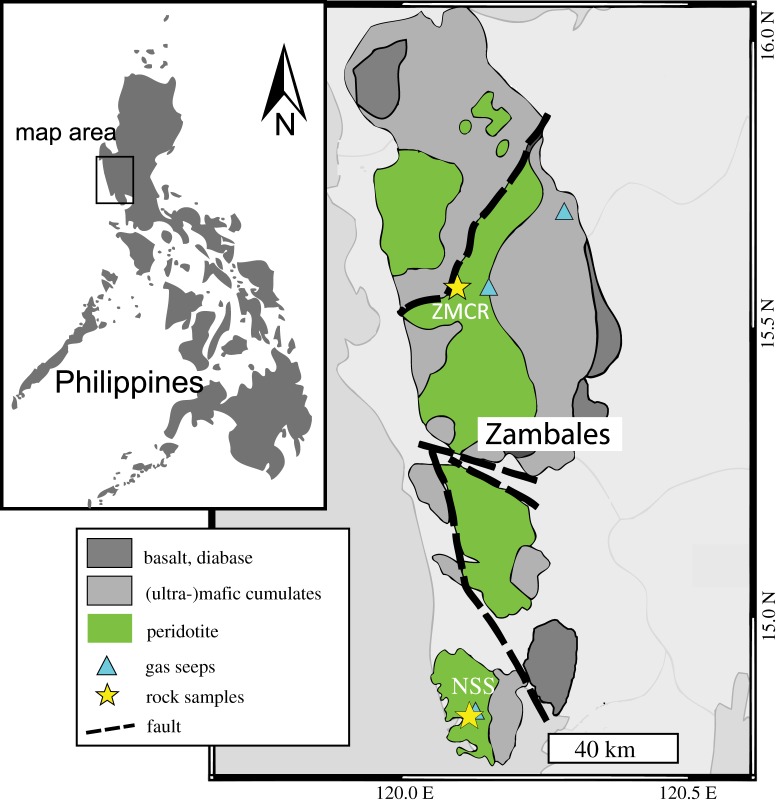
Figure 2.
Map of the Zambales Ophiolite Complex in the Philippines displaying sampling locations and gas seeps. Figure modified from Dimalanta et al. [28] with gas seep GPS locations from Vacquand et al. [19]. (Online version in colour.)
2. Material and methods
(a). Sample materials
Seven olivine-rich gabbroic and mantle rocks collected from the eastern flank of the Mount Dent oceanic core complex at the Mid-Cayman Rise (figure 1) using the deep submergence vehicle Alvin in 1976 [27,29] were acquired from the Seafloor Samples Laboratory at Woods Hole Oceanographic Institution. The approximately 110 km long Mid-Cayman Rise is an ultraslow spreading centre (15–17 mm yr−1) located between the Caribbean and the North American plates and is one of the deepest spreading centres worldwide [30]. The Mt Dent oceanic core complex, which rises approximately 3 km from the axial rift valley near the central portion of the Mid-Cayman Rise, has been exhumed by long-lived detachment faulting and hosts the Von Damm hydrothermal field [16,31–33]. Hydrothermal fluids venting at Von Damm are Mg- and SO4-poor, and enriched in dissolved SiO2, H2, H2S, CH4 and C2H6, which is consistent with hydrothermal alteration of mafic (gabbro, diabase, basalt) and ultramafic (peridotite) rocks present at Mt Dent [27,34]. The rocks from the Mid-Cayman Rise examined here include one gneissic metagabbro, two olivine gabbros, two troctolites, one serpentinized spinel harzburgite and one serpentinized spinel lherzolite (table 1). Thin section petrography indicates that the harzburgite and lherzolite samples are 80% and 90% serpentinized, respectively. Olivine in the samples is primarily altered to serpentine and magnetite, while orthopyroxene is altered to serpentine, chlorite and minor talc. Cross-cutting veins composed of serpentine and magnetite suggest the occurrence of multiple serpentinization events. More detailed descriptions of the mineralogical and chemical compositions of each sample are presented in previous studies [27,35,36].
Table 1.
Volatile and mineral contents of secondary inclusions determined from in situ Raman analyses. Normalized modes of primary minerals determined from visual estimates in thin section. Ant, antigorite; Brc, brucite; Cal, calcite; Cpx, clinopyroxene; Ctl, chrysotile; Dol, dolomite; Lz, lizardite; Mag, magnetite; Mg-Cal, high-Mg calcite; Mgs, magnesite; Oliv, olivine; Pg, paragonite; Plag, plagioclase; Sht, shortite; Tr-Act, tremolite-actinolite.
| composition of secondary inclusions in mineral hosts | normalized primary mode (vol.%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| location | lithology | sample | olivine | plagioclase | clinopyroxene | hornblende | Plag | Oliv | Cpx | Opx | LOI (wt.%) |
| Cayman | gneissic metagabbro | ALV 620-3C | CH4, H2, Brc, Lz, Tlc | CH4, H2O, Pg | CH4, Mg-Cal, Tr-Act | CH4, Lz, Ant, Cal | — | — | — | — | 1.497 |
| olivine gabbro | ALV 620-5-1 | CH4, H2, Brc, Ctl, Lz, Ant, Mag, Dol | CH4, H2, H2O, Pg, Cal, Sht? | CH4, Tr-Act | — | 75 | 5 | 20 | 0 | 1.695 | |
| ALV 624-5-1 | CH4, H2, Brc, Ctl, Lz, Mag, Mg-Cal | CH4, H2O, Cal | CH4 | — | 50 | 35 | 15 | 0 | 1.394 | ||
| troctolite | ALV 621-3-1 | CH4, H2, Brc, Ctl, Lz, Mag, Cal | CH4, H2, H2O, Cal | — | — | 80 | 20 | 0 | 0 | 3.464 | |
| ALV 623-3-2 | CH4, H2, Brc, Ctl, Lz, Ant, Mag, Cal, Dol, Mgs | CH4, H2, H2O, Pg, Cal | CH4, Tr-Act | — | 90 | 9 | 1 | 0 | 0.985 | ||
| peridotite | ALV 624-3-3 | CH4, H2, Brc, Ctl, Lz, Ant, Mag | — | — | — | 0 | 85 | 1 | 14 | 10.40 | |
| ALV 624-6-2 | CH4, H2, Brc, Ctl | — | — | — | 0 | 90 | 1 | 9 | 11.82 | ||
| Zambales | dunite | ZMCR-02 | CH4, H2, Brc, Ctl, Lz, Ant | — | — | — | 0 | 99 | 1 | 0 | 13.49 |
| ZMCR-03 | H2, Brc, Ctl, Lz | — | — | — | 0 | 99 | 1 | 0 | 13.72 | ||
| peridotite | NSS-02 | CH4, H2, Brc, Lz, Ctl | — | — | — | 0 | 88 | 2 | 10 | 3.657 | |
| NSS-04 | CH4, H2, Brc, Lz, Ctl, Ant, Dol | — | — | — | 0 | 90 | 1 | 9 | 3.358 | ||
Additional ultramafic rocks were collected from the Zambales ophiolite in the Philippines (figure 2), approximately 5 km from reported CH4-rich gas seeps [17,19,37,38]. The Zambales ophiolite complex is part of a series of suprasubduction zone ophiolites at the western seaboard of the Philippine archipelago [39]. Its layered sequence consists of pillow lavas, sheeted dikes, gabbros, layered cumulates, transition zone dunites and residual peridotites, suggesting that it originated in magmatically robust oceanic crust, likely at a fast-spreading mid-ocean ridge [39]. Two partially serpentinized dunites were sampled at the Coto Mine, a chromite mine located in the Zambales Mineral Chromite Reservation in the Coto Block (N15°34′21.0′′, E120°05′47.9′′). The dunites are approximately 70% altered to a secondary mineral assemblage primarily composed of serpentine, brucite and trace quantities of magnetite and Ni-sulfides. Two serpentinized harzburgites were recovered from Nagsasa Cove in the San Antonio Massif, Philippines (N14°49′41.1′′, E120°7′3.2′′). These samples underwent approximately 20% serpentinization, resulting in the formation of serpentine, minor brucite and traces of micrometre-sized magnetite.
(b). Analytical methods
Thin sections of rock samples were examined with a petrographic microscope in transmitted and reflected light to document the types, sizes and distribution of fluid inclusions and their host mineralogy. A Horiba Jobin-Yvon LabRAM HR confocal Raman spectrometer with a 473 nm laser, 600 mm−1 grating, 100 µm confocal pinhole and 100 µm slit width was used to identify the volatile contents of individual inclusions and the mineralogy of inclusion walls. Quantitative CH4 measurements were acquired using an 1800 mm−1 grating for improved spectral resolution. Spectra represent the average of at least three 30–120 s acquisitions typically conducted in the 150–2300 cm−1 and 2750–4400 cm−1 Raman shift regions. Estimates of CH4 gas densities and pressures in fluid inclusions of select samples were determined from the measured Raman shift of the C–H symmetric stretching band relative to the band position of pure methane at 21°C and atmospheric pressure. Methane gas densities were calculated using the calibration of Zhang et al. [40], and CH4 pressures were calculated using the calibration of Lu et al. [41].
Confocal Raman analysis requires a minimum fluid inclusion size of approximately 1 µm that depends on the inclusion depth and opacity of the host mineral. Detection limits for Raman spectroscopy are instrument-specific and depend on measurement conditions, which can vary from sample to sample and even within individual mineral grains. For instance, while it is possible to detect CO2(g) with a density of 0.001–0.002 g cm−3 in large (greater than 10 µm) fluid inclusions near the mineral surface [42–44], higher vapour densities are needed to detect CO2(g) in smaller inclusions or those buried deep in the mineral host. Wopenka & Pasteris [42] have reported detection limits for CH4(g) (0.0002 g cm−3) and H2(g) (0.0001 g cm−3) that are an order of magnitude lower than that for CO2(g). Although detection limits reported in the literature apply only to analytical conditions at the time of measurement, they are representative of typical sensitivity levels for the Raman spectroscopic techniques used in the present study.
The chemical and carbon isotopic compositions of volatile species contained within fluid inclusions were analysed by isotope ratio monitoring–gas chromatography mass spectrometry (irm-GCMS) following extraction of gases from fluid inclusions by mechanical crushing of whole-rock samples (figure 3). Unlike other extraction techniques, such as thermal decrepitation, crushing liberates gases only from inclusions and minimizes thermogenic formation of gases or thermal decomposition that can alter gas compositions and abundances [45–47]. In particular, thermal decrepitation methods have been shown to generate significant quantities of CO2, CO and CH4 by pyrolysis of high molecular weight organic material [45,48,49].
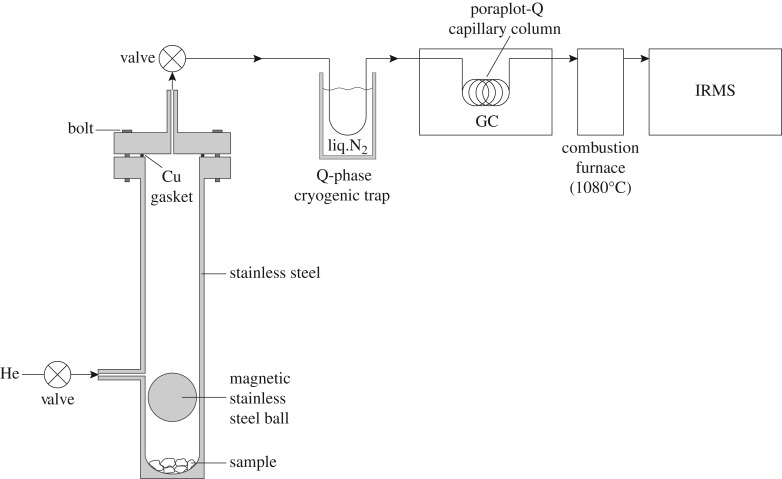
Figure 3.
Schematic diagram of the stainless steel crushing device in line with an isotope ratio monitoring–gas chromatography mass spectrometry (irm-GCMS) system.
Whole-rock samples were cut into centimetre-sized pieces using a diamond-tipped blade, partially crushed using a tungsten-carbide jaw crusher, and then dry sieved to collect the 1–5 mm size fraction. Because olivine grains were partially altered in all samples, it was not feasible to separate them from secondary mineral products and collect pure olivine separates. Therefore, only bulk rocks were analysed in this study. Gases were extracted from 1 to 5 mm whole-rock chips by placing a 1–4 g aliquot of each sample in a gas-tight, magnetically operated, stainless steel crushing device under an atmosphere of helium (figure 3). The crushing device was heated to 70°C during all steps of the analysis to reduce adsorption of gases onto mineral surfaces and facilitate their release into the carrier gas. After purging with helium to remove air, the crushing chamber was isolated from the He flow path using a valve, and a stainless steel ball inside the crushing device was manually raised and lowered 800 times using a magnet to pulverize the sample. After crushing, the chamber was sparged with He and the released volatiles were focused on a cryogenic trap immersed in liquid N2. The cryogenic trap was interfaced directly to the irm-GCMS system for compositional and carbon isotopic analysis.
Crushing rocks in steel crushers is known to generate small amounts of gases, including H2, CO, CH4 and C2+ hydrocarbons by frictional heating during steel-to-steel contact [50–54]. Blank levels are reduced by the presence of sample material which can act as a cushion during contact between metal surfaces [53]. Blank experiments performed during this study revealed that moving the stainless steel ball up and down inside an empty crushing device continuously generated measurable quantities of CH4 and C2H6. The level of hydrocarbon contamination was minimized by using a large sample size (1–4 g) to produce higher levels of sample-derived hydrocarbons and to cushion contact between the stainless-steel ball and inner walls of the chamber during crushing. Prior to analysing replicate aliquots of the same sample, the previously crushed aliquot was removed from the crushing device. Material that had adhered to the stainless-steel ball and the inside walls of the crushing device from a previous aliquot of the same sample was not removed in order to minimize steel-to-steel contact during subsequent crushing. Between analyses of different rock samples, the crushing device was scrubbed with a plastic brush to remove all rock powder, sonicated in water for 10 min and then dried in air at 60°C.
To quantify the blank, gases released during the crushing of two different blank materials were measured: (i) three previously pulverized samples (ALV 621-3-1, 623-3-2, 624-3-3) that were combusted in a muffle furnace at 1000°C in air for 24 h and (ii) 3 mm diameter combusted Pyrex beads. The purpose of combusting the samples was to remove carbonate and organic carbon contaminants. A 2 g fraction of each blank material was crushed and analysed using the same procedure as described for the samples. Crushing of combusted Pyrex beads yielded CH4 and C2H6 quantities of no more than 0.10 nmol g−1 and 0.0017 nmol g−1, respectively (electronic supplementary material, table S1). This amount of CH4 represents 0.02% of the CH4 yield from the most CH4-rich sample (ALV 620-3C) and 4% of the CH4 yield from the least CH4-rich sample (ZMCR-03). Ethane background levels represent 0.2% of the yield from the most C2H6-rich sample (ALV 620-3C) and 43% of the yield from the least C2H6-rich sample (ZMCR-02). For samples analysed for the carbon isotope composition of C2H6, the C2H6 blank represents 4% of the yield from the least C2H6-rich sample (ALV 623-3-2). The previously pulverized and combusted rock samples yielded C2H6 blank levels appreciably lower than those from the Pyrex beads and CH4 blank levels comparable to those from the Pyrex beads, except for sample ALV 621-3-1 which released higher amounts of CH4 (electronic supplementary material, table S1).
The abundance and carbon isotopic composition of CH4 and C2H6 were determined using an irm-GCMS system consisting of an Agilent 6890 gas chromatograph coupled to a Thermo-Finnigan Delta Plus XL mass spectrometer through a Finnigan Gas Chromatograph Combustion Interface III held at 1080°C with a constant oxygen trickle. A Poraplot-Q capillary column was used to separate hydrocarbon compounds in the gas chromatograph. Stable carbon isotope values are reported in standard δ-notation relative to the Vienna Pee Dee Belemnite as follows:
| 2.1 |
where Rspl and Rstd are the carbon isotope ratios (13C/12C) of the sample and standard, respectively. Average carbon isotope values and hydrocarbon abundances for each sample were obtained from analyses of two to five replicates of the bulk sample material.
Helium abundances and isotopic compositions were analysed by crushing in vacuum at the Isotope Geochemistry Facility at Woods Hole Oceanographic Institution. Whole-rock chips were dry sieved to collect the 1–2 mm size fraction. The chips were then sonicated in distilled water followed by acetone, and dried in a laminar flow hood under air. Approximately 0.3 g of sample was loaded into an ultra-high vacuum crusher [55] and crushed 20 times using a magnetic stainless steel piston. All He measurements were performed using a branch tube magnetic sector mass spectrometer, details of which are described in Kurz et al. [56]. Measured 3He/4He ratios are normalized to the atmospheric 3He/4He ratio, Ra (1.4 × 10−6).
3. Results
(a). Distribution of secondary fluid inclusions
Secondary fluid inclusions in the samples occur as trails that extend along healed microfractures in minerals (figure 4) and across grain boundaries between adjacent minerals. Several generations of inclusion trails can be distinguished from cross-cutting relationships. Olivine contains the highest density of secondary fluid inclusions in all rock types. Olivine-hosted secondary fluid inclusions are particularly abundant in metagabbro, olivine gabbro and troctolite, with significantly fewer observed in peridotite and dunite. Individual inclusions range from less than 1 to 40 µm in diameter, with most inclusions being 1–15 µm in diameter. Plagioclase-hosted secondary fluid inclusions are typically smaller in size with diameters of 1–10 µm, but can range up to 25 µm in diameter, and are much less abundant than those in olivine. Inclusion trails within plagioclase are common in gabbro and troctolite, but rare in the gneissic metagabbro sample (ALV 620-3C) which exhibits strong alteration of plagioclase. By contrast, this sample contains abundant secondary inclusions in hornblende. Isolated inclusions of probable primary origin were detected in plagioclase but not in olivine. Few fluid inclusions are observed in clinopyroxene in any of the lithologies.
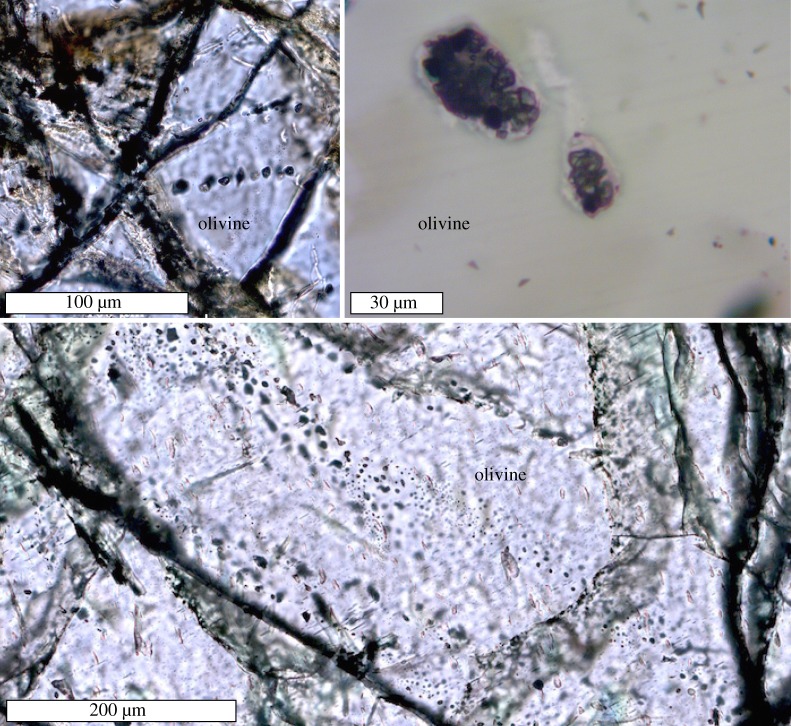
Figure 4.
Representative thin section photomicrographs showing trails of CH4-rich secondary fluid inclusions cross cutting olivine in troctolite from the Mid-Cayman Rise (Sample ALV 623-3-2; plane-polarized light). (Online version in colour.)
(b). Composition of secondary fluid inclusions
Olivine-hosted secondary fluid inclusions in all Cayman samples are dominated by CH4, with minor H2 (table 1; electronic supplementary material, figure S1). Carbon dioxide gas was not detected by Raman spectroscopy in any secondary inclusion in olivine. Preliminary data from sample ALV 624-3-3 indicate estimated CH4 vapour densities of 0.16–0.29 g cm−3 and pressures of 20–54 MPa. Two-dimensional confocal Raman mapping shows abundant alteration minerals that typically occur as spatially discrete phases lining the inside walls and filling much of the interior of fluid inclusions (figure 5). Brucite and serpentine minerals, including chrysotile, lizardite and antigorite, are observed in olivine-hosted inclusions in all Cayman samples examined in this study. Magnetite and carbonate minerals, including calcite, high-Mg calcite, dolomite and magnesite, occur in olivine-hosted inclusions in most Cayman samples. No magnetite was detected in samples ALV 620-3C and 624-6-2, and no carbonates were detected in samples ALV 620-3C, 624-3-3 and 624-6-2. Free H2O was not detected by Raman spectroscopy, either as a liquid or vapour phase, in any olivine-hosted secondary inclusion.
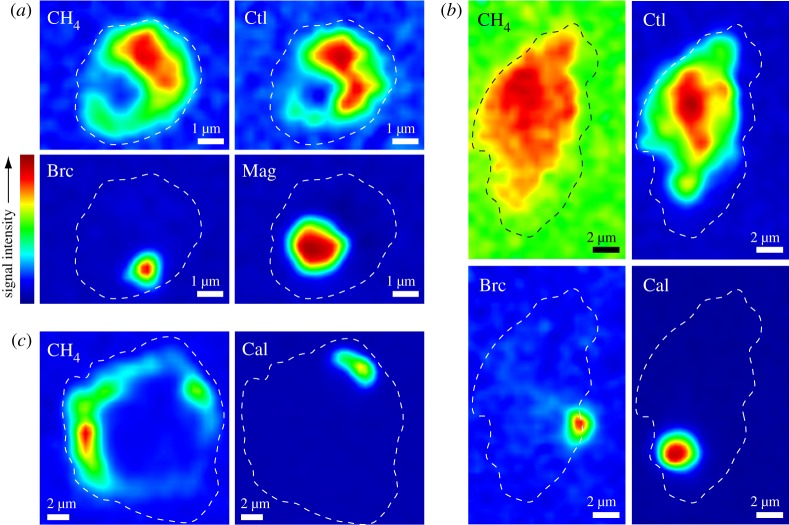
Figure 5.
False-colour hyperspectral Raman maps showing the locations of mineral and gaseous species in secondary fluid inclusions hosted in olivine (a,b) and plagioclase (c) in Mid-Cayman Rise samples ALV 624-5-1 (a,c) and ALV 621-3-1 (b). Colours represent relative intensities of the characteristic Raman band for a particular phase, with warmer colours indicating higher signal intensities. Dashed lines represent the approximate outlines of the fluid inclusions. Brc, brucite; Cal, calcite; Ctl, chrysotile; Mag, magnetite. (Online version in colour.)
Secondary fluid inclusions in plagioclase are vapour- and liquid-rich, hosting gaseous CH4 with variable amounts of liquid H2O (figure 5, table 1 and electronic supplementary material, figure S2). Secondary inclusions in samples ALV 620-5-1, 621-3-1 and 623-3-3 also contain minor H2. Calcite and paragonite are common daughter minerals in plagioclase-hosted secondary inclusions. Shortite [Na2Ca2(CO3)3], a sodium–calcium carbonate, was identified in Raman spectra of one plagioclase-hosted secondary inclusion in sample ALV 620-5-1.
Raman spectroscopy reveals that secondary fluid inclusions in clinopyroxene are vapour-dominated, with CH4 as the sole volatile component (table 1 and electronic supplementary material, figure S3). High-Mg calcite was identified in the gneissic metagabbro sample ALV 620-3C. Tremolite-actinolite was detected in all samples, except ALV 624-5-1, on the basis of Raman band positions at 3677 cm−1 and 3662 cm−1 (electronic supplementary material, figure S3), with additional bands at lower wavenumbers (3645 cm−1, 3626 cm−1) occurring in some samples.
Olivine-hosted secondary fluid inclusions in ultramafic rocks from the Zambales ophiolite contain variable amounts of gaseous CH4 and H2 (table 1). Raman measurements reveal that H2 is the dominant volatile component within olivine-hosted secondary inclusions in samples ZMCR-02 and ZMCR-03. Minor CH4 was additionally detected in ZMCR-02, but was not detected in ZMCR-03. Samples NSS-02 and NSS-04, in contrast, host more abundant CH4 with minor H2. Raman data from olivine-hosted inclusions in NSS-04 yield estimated CH4 vapour densities of less than or equal to 0.09 g cm−3 and pressures of up to 11 MPa. Olivine in all Zambales samples hosts brucite and serpentine minerals, including chrysotile, lizardite and antigorite. Dolomite was also detected in some olivine-hosted secondary inclusions in sample NSS-04.
(c). Hydrocarbon abundances and isotopes
(i). Hydrocarbon measurements
Chemical and carbon isotopic analyses of extracted hydrocarbon gases from whole-rock samples are displayed in table 2. Values reported for the abundance of individual hydrocarbons represent minimum values as it is not possible to break open all inclusions through crushing. No correlations were observed between carbon isotopic compositions and hydrocarbon abundances or CH4/C2H6 ratios. The measured δ13C values of CH4 were identical within analytical error (±0.5‰) for replicate analyses of the same sample. However, C2H6 was distinctly more 13C-depleted in the first aliquot analysed for all samples, except for the most C2H6-rich sample, ALV 620-3C, which showed consistent δ13CC2H6 values for replicate analyses. The remaining samples exhibited a trend toward more 13C-enriched C2H6 with analysis of successive aliquots of sample material (electronic supplementary material, figure S4). Moving the stainless steel ball up and down inside an empty crushing device yielded δ13C values for CH4 (−36‰) and C2H6 (−29‰) that were significantly more negative than sample measurements, suggesting that the 13C-depleted C2H6 observed in the first sample aliquots reflects contamination from the crushing device. Analysis of the first sample aliquot was conducted in a clean crushing device free of any rock powder, while analyses of subsequent aliquots were performed in a crushing device lined with previously pulverized sample material. A similar trend in CH4 isotopic composition was not observed, likely because CH4 concentrations are higher than those of C2H6.
Table 2.
Average hydrocarbon abundances and carbon isotopic compositions in whole-rock samples from the Mid-Cayman Rise and the Zambales ophiolite. Here n, number of analyses; n.a., not analysed.
| CH4 | C2H6 | CH4/C2H6 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| location | lithology | sample | Avg (nmol g−1) | 2σ | n | Avg δ13C (‰) | 2σ | n | Avg (nmol g−1) | 2σ | Avg δ13C (‰) | 2σ | n | Avg | 2σ |
| Cayman | gneissic metagabbro | ALV 620-3C | 313 | 23 | 6 | −14.0 | 0.4 | 3 | 0.99 | 0.09 | −12.5 | 0.4 | 4 | 315 | 28 |
| olivine gabbro | ALV 620-5-1 | 254 | 32 | 5 | −4.4 | 0.3 | 3 | 0.68 | 0.05 | +0.7 | 0.3 | 2 | 355 | 60 | |
| ALV 624-5-1 | 93 | 5 | 5 | −7.2 | 0.3 | 2 | 0.13† | −13.9† | 676† | ||||||
| troctolite | ALV 621-3-1 | 78 | 9 | 7 | −7.9 | 0.2 | 2 | 0.084 | 0.014 | −10.6 | 0.1 | 2 | 924 | 9 | |
| ALV 623-3-2 | 72 | 7 | 10 | −16.7 | 0.3 | 3 | 0.047† | −20.3† | 1372† | ||||||
| peridotite | ALV 624-3-3 | 4.3 | 1.1 | 2 | −15.8 | 0.3 | 2 | 0.015 | 0.000 | n.a. | 2 | 350 | 10 | ||
| ALV 624-6-2 | 9.9 | 1.3 | 3 | −11.6 | 0.6 | 3 | 0.020 | 0.003 | n.a. | 3 | 488 | 11 | |||
| Zambales | dunite | ZMCR-02 | 2.0 | 0.3 | 2 | −10.9 | 0.0 | 2 | 0.013 | 0.004 | n.a. | 2 | 163 | 33 | |
| ZMCR-03 | 1.7 | 0.2 | 5 | −12.4 | 1.5 | 5 | 0.004† | n.a. | 431† | ||||||
| peridotite | NSS-02 | 23 | 2 | 5 | −3.0 | 0.1 | 5 | 0.075 | 0.013 | −0.9 | 0.2 | 3 | 327 | 56 | |
| NSS-04 | 37 | 7 | 6 | −2.8 | 0.0 | 2 | 0.082 | 0.010 | −1.2 | 0.4 | 3 | 382 | 1 | ||
†δ13C values of C2H6 and CH4/C2H6 ratios represent minimum values due to contamination detected from the steel crusher. See text for details.
The δ13CC2H6 values for most samples from the Mid-Cayman Rise and Zambales ophiolite appeared to level off to within analytical error (±0.5‰) after analysis of the second or third aliquot of sample material (electronic supplementary material, figure S4). Average carbon isotope compositions of C2H6 in these samples were thus obtained from two to four successive analyses whose δ13CC2H6 values are identical within analytical error (±0.5‰). However, the C2H6 isotopic compositions of two samples (ALV 624-5-1 and ALV 623-3-2) did not level off. Visual inspection revealed that these samples did not adhere well to the stainless steel surfaces of the crushing device during pulverization, likely resulting in higher levels of frictional heating and background hydrocarbon production during crushing. Therefore, C2H6 isotope compositions of samples ALV 624-5-1 and ALV 623-3-2 may be more affected by hydrocarbon contamination from the crushing device, and the measured δ13CC2H6 values likely reflect minimum values. Samples ALV 624-5-1 and ALV 623-3-2 are characterized by very low degrees of alteration relative to the other samples. We speculate that the greater abundance of hydrous alteration minerals, including serpentine, talc and tremolite, in the more altered samples produces a ‘stickier’ rock powder that can adhere more effectively to the stainless steel walls of the crushing device, thus helping to minimize the production of background hydrocarbons by frictional heating.
(ii). Hydrocarbon compositions of Cayman and Zambales samples
The greatest absolute abundance of hydrocarbons in olivine-rich mafic and ultramafic rocks from the Mid-Cayman Rise is observed in sample ALV 620-3C, with average CH4 and C2H6 contents of 313 nmol g−1 and 0.99 nmol g−1, respectively, while the lowest quantity of hydrocarbons is observed in sample ALV 624-3-3, with average CH4 and C2H6 contents of 4.3 nmol g−1 and 0.015 nmol g−1, respectively (table 2). Most Cayman samples have CH4/C2H6 ratios between 315 and 924. However, ALV 623-3-2 has a much higher CH4/C2H6 ratio of 1372 relative to the other samples.
Methane in all Cayman samples shows a wide range of δ13C values from −16.7‰ to −4.4‰ (table 2). Similarly, δ13C values for C2H6 in most Cayman samples span a broad range from −20.3‰ to −10.6‰, except in sample ALV 620-5-1, which shows extremely 13C-enriched C2H6 with a δ13CC2H6 value of +0.7‰. Sample ALV 620-5-1 exhibits a trend toward more 13C-enriched C2H6 with increasing number of crushes of the same sample aliquot, tentatively suggesting that there are distinct populations of fluid inclusions in the sample. However, no trend was evident in the CH4 isotopic composition or CH4/C2H6 ratio with cumulative number of crushes. Serpentinized peridotite samples from Cayman contained insufficient C2H6 for stable isotope measurements.
Serpentinized dunite and peridotite from the Zambales ophiolite also show a wide range of hydrocarbon abundances and carbon isotopic compositions (table 2). Samples NSS-02 and NSS-04 have higher average CH4 contents (23–37 nmol g−1) and C2H6 contents (0.075–0.082 nmol g−1) relative to the more extensively serpentinized samples ZMCR-02 and ZMCR-03 (1.7–2.0 nmol g−1 CH4 and 0.004–0.013 nmol g−1 C2H6). All samples show a relatively narrow range in average CH4/C2H6 ratios from 163 to 431. Samples ZMCR-02 and ZMCR-03 exhibit δ13CCH4 values of −12.4‰ to −10.9‰. They contained insufficient C2H6 for stable isotope measurements. By contrast, samples NSS-02 and NSS-04 exhibit more 13C-enriched CH4, with δ13CCH4 values of −3.0‰ to −2.8‰. C2H6 in the NSS samples is slightly 13C-enriched relative to CH4 and shows average δ13CC2H6 values of −1.2‰ to −0.9‰.
(d). Helium contents and isotopes
4He contents of Mid-Cayman Rise samples exhibit a wide range from 4.7 × 10−7 to 9.0 × 10−11 cm3 STP g−1 (table 3). Samples of serpentinized peridotite contain the lowest abundance of He, with 4He contents that are one to two orders of magnitude lower than those in plutonic rock samples. With the exception of the highest He abundance in the gneissic metagabbro sample (ALV 620-3C), measured 4He contents are 0.4–5 orders of magnitude lower than those reported for basaltic glasses from the Mid-Cayman Rise [57] (figure 6). Helium isotope compositions of most Cayman rocks fall within a relatively narrow range of 7.02–8.32 Ra, and are consistent with 3He/4He ratios documented in basaltic glasses from the Mid-Cayman Rise and other ocean ridges [57–59] (figure 6). Average 3He/4He ratios of 6.58 and 9.91 Ra for samples ALV 624-3-3 and 624-6-2, respectively, fall just outside this range, but have larger uncertainties owing to low He abundances in the samples.
Table 3.
Helium in whole-rock samples from the Mid-Cayman Rise and the Zambales ophiolite. All measurements were performed by crushing in vacuum.
| 3He/4He | |||||||
|---|---|---|---|---|---|---|---|
| location | lithology | sample | crush 4He (cc STP/g) | crush 4He (nmol g−1) | (R/Ra) | 2σ | CH4/3He |
| Cayman | gneissic metagabbro | ALV 620-3C | 4.68 × 10−7 | 2.09 × 10−2 | 8.32 | 0.16 | 1.28 × 109 |
| olivine gabbro | ALV 620-5-1 | 2.26 × 10−9 | 1.01 × 10−4 | 7.02 | 0.24 | 2.56 × 1011 | |
| ALV 624-5-1 | 7.70 × 10−9 | 3.44 × 10−4 | 7.25 | 0.17 | 2.67 × 1010 | ||
| troctolite | ALV 621-3-1 | 6.46 × 10−9 | 2.88 × 10−4 | 7.53 | 0.19 | 2.57 × 1010 | |
| ALV 623-3-2 | 3.53 × 10−8 | 1.57 × 10−3 | 7.66 | 0.15 | 4.27 × 109 | ||
| peridotite | ALV 624-3-3 | 8.97 × 10−11 | 4.01 × 10−6 | 9.91 | 1.23 | 7.70 × 1010 | |
| ALV 624-6-2 | 2.47 × 10−10 | 1.10 × 10−5 | 6.58 | 0.59 | 9.76 × 1010 | ||
| Zambales | dunite | ZMCR-02 | 1.33 × 10−9 | 5.92 × 10−5 | 3.00 | 0.16 | 8.06 × 109 |
| peridotite | NSS-02 | 9.12 × 10−11 | 4.07 × 10−6 | 5.57 | 0.92 | 7.23 × 1011 | |
| NSS-04 | 1.15 × 10−10 | 5.11 × 10−6 | 4.00 | 1.01 | 1.30 × 1012 |
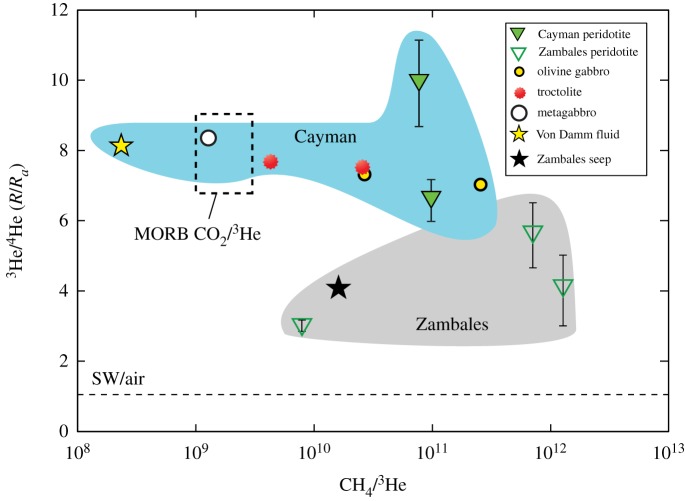
Figure 6.
Plot of 3He/4He (R/Ra) versus CH4/3He for whole-rock samples from the Mid-Cayman Rise and Zambales ophiolite. Except for the gneissic metagabbro sample, all samples exhibit CH4/3He ratios that are significantly higher than the average ratio for mid-ocean ridge basalt (MORB). High CH4/3He ratios may reflect inefficient helium extraction by crushing during the helium analyses or addition of He to the vent fluid from a source other than fluid inclusions. See text for discussion. (Online version in colour.)
Similar to serpentinized peridotite samples from the Mid-Cayman Rise, samples from the Zambales ophiolite have very low He abundances, with 4He contents of 9.1 × 10−11 to 1.3 × 10−9 cm3 STP g−1 (table 3). Average 3He/4He ratios range from 3.00 to 5.57 Ra and are lower than 3He/4He ratios in most of the Cayman samples, despite the larger uncertainties in the measured values.
4. Discussion
(a). Formation and compositional evolution of carbon-rich fluid inclusions
The present study provides new constraints on the H2, CH4, C2H6 and alteration mineral contents of fluid inclusions in partially serpentinized peridotite and gabbroic rocks. These inclusions form when carbon-bearing aqueous fluids percolating in the lower lithosphere are trapped in primary minerals during healing of microfractures below the brittle–ductile transition temperature of the host mineral (less than 600–800°C for olivine; [60]). In mid-ocean ridge settings, trapped fluids likely represent seawater-derived fluids enriched in magmatic volatiles that circulated at depth close to the ridge axis, with CO2 being derived from seawater, magmatic outgassing or both. Trapped water is likely seawater-derived since exsolution of volatiles from mid-ocean ridge basalt (MORB) magma produces chiefly CO2 [61]. Owing to the strong temperature dependence of primary and secondary mineral stability during fluid–rock alteration, cooling of the trapped fluid and host mineral during exhumation creates a large thermodynamic drive for the aqueous alteration of the primary host mineral [23]. Olivine becomes thermodynamically unstable in the presence of water at temperatures below approximately 400°C and will react with water to form serpentine minerals, brucite, magnetite and H2(g) under closed-system conditions, according to the generalized serpentinization reaction [23,62–64]:
| 4.1 |
The occurrence of reaction 4.1 at the scale of individual fluid inclusions that represent closed-system micro-reactors is indicated by the ubiquitous presence of serpentine, brucite, magnetite and H2 within olivine-hosted fluid inclusions examined in this study (table 1; electronic supplementary material, figure S1).
The accumulation of H2 during serpentinization has profound implications for carbon speciation in fluid inclusions due to the strong thermodynamic drive for the reduction of CO2 to CH4 and lesser quantities of C2+ alkanes via the general reaction
| 4.2 |
at temperatures approximately below 350°C [65]. In addition to the formation of hydrocarbons, CO2 may also be consumed by the precipitation of carbonate minerals that are observed in some samples. Phase equilibria constraints indicate that carbonates can coexist in chemical equilibrium with CH4 under a wide range of reducing conditions [66]. However, because the generation of H2 in fluid inclusions depends on the oxidation of ferrous iron to ferric iron, the uptake of ferrous iron into the FeCO3 component of carbonate minerals is expected to limit the amount of H2 generated by serpentinization [67], thereby reducing the thermodynamic drive for hydrocarbon production [68]. The occurrence of Ca-bearing carbonates in olivine-hosted fluid inclusions in several samples suggests that circulating fluids contained dissolved Ca that was derived from seawater or from chemical interaction with Ca-bearing minerals in the surrounding rock.
The absence of free H2O detected in secondary inclusions in olivine points to a complete or near-complete extent of serpentinization, resulting in the complete uptake of trapped H2O into the hydrous minerals serpentine and brucite [23]. Furthermore, the lack of detectable CO2 gas in olivine-hosted fluid inclusions suggests that all CO2 was reduced to hydrocarbons or precipitated as carbonate minerals. Our observations contrast with previous findings of CH4 + CO2 ± H2O-bearing fluids in olivine-hosted inclusions in Southwest Indian Ridge (SWIR) gabbroic rocks [20,21], whose compositions suggest disequilibrium between trapped volatiles and the olivine host [23].
Unlike olivine, plagioclase is Fe-poor and equilibration with trapped fluid during cooling may yield limited amounts of H2 from ferrous iron oxidation. Accordingly, the presence of H2 and CH4 in plagioclase-hosted inclusions in some Cayman samples (table 1; electronic supplementary material, figure S2) suggests that requisite H2 for CO2 reduction to CH4 was derived from a source external to the fluid inclusion or generated in situ by radiolysis due to the decay of radiogenic elements in plagioclase. Although H2 was not detected in any clinopyroxene-hosted secondary fluid inclusion, H2 may have been present at a level below the detection limit of Raman spectroscopy, or may have been completely consumed by CO2 reduction to CH4 (table 1; electronic supplementary material, figure S3). Generation of H2 within clinopyroxene-hosted inclusions may occur by oxidation of ferrous iron initially present in clinopyroxene to ferric iron in tremolite-actinolite; however, the amount of H2 that can be generated by this mechanism is limited due to the low ferric iron contents generally observed in tremolite-actinolite [69]. The possibility also exists that either H2 or CH4 in clinopyroxene-hosted inclusions may have been externally derived.
(i). Sources of helium and carbon in secondary fluid inclusions
At the Mid-Cayman Rise, 3He/4He ratios in lower crustal plutonic and mantle rock samples vary from 6 to 10 Ra (figure 6) and are consistent with a mantle source for helium. 4He abundances in Cayman samples are approximately 1–5 orders of magnitude lower than those in MORB from fast-spreading ocean ridges [70] and may reflect the magma-poor source region of the ultraslow-spreading Mid-Cayman Rise or loss of 4He during rock alteration. At Zambales, 3He/4He ratios of 3.0–5.6 Ra in olivine-rich rocks examined in this study are significantly lower than those typically in the mantle as exemplified by MORB (7–9 Ra). These low 3He/4He ratios likely reflect the addition of radiogenic 4He from U-Th decay in the ultramafic protolith [17,19].
The isotopic composition of CH4 trapped within fluid inclusions in olivine-rich rocks from the Mid-Cayman Rise and Zambales ophiolite is characterized by a large range of δ13C values that varies from −16.7 to −2.8‰. The more positive end of this range directly overlaps with the isotopic composition of mantle-derived CO2 (figure 7), consistent with CH4 formation from a mantle carbon source. Samples that contain CH4 that is more depleted in 13C than mantle-derived CO2 are consistent with isotopic fractionation during CH4 formation. The extent of isotopic fractionation during CO2 reduction to CH4 is dependent on several factors, including formation temperature, partitioning of carbon among different species, such as CO2, CH4, C2+ alkanes and carbonate minerals, and the presence of an open or closed chemical system. Partial reduction of CO2 to hydrocarbons under hydrothermal conditions generates CH4 and C2+ alkanes that are depleted in 13C relative to the initial CO2 source [83–86]. For example, equilibrium 13C/12C fractionation factors (1000lnα) between dissolved CO2 and CH4 are −30 and −18 at 200°C and 300°C, respectively [84]. Generation of CH4 with mantle δ13C values (−10‰ to −4‰) from magmatic CO2 thus suggests the near-quantitative reduction of CO2 to CH4 in a closed system, consistent with the lack of detectable CO2 in fluid inclusions. Assuming the fluid inclusion remains a closed system, CH4 with a δ13C value that is significantly more negative than −10‰ points to the incomplete reduction of magmatic CO2 to CH4.
Figure 7.
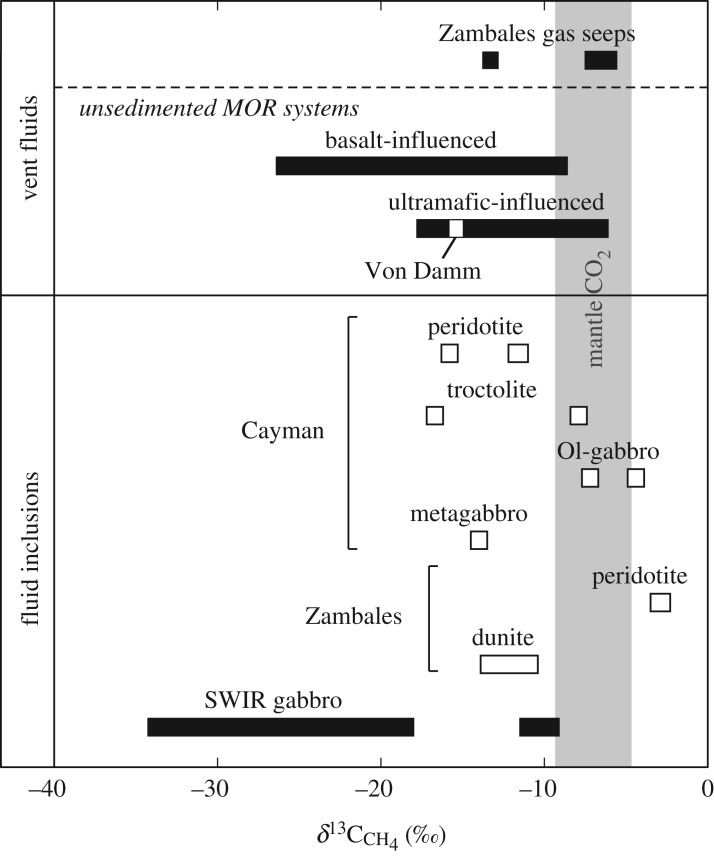
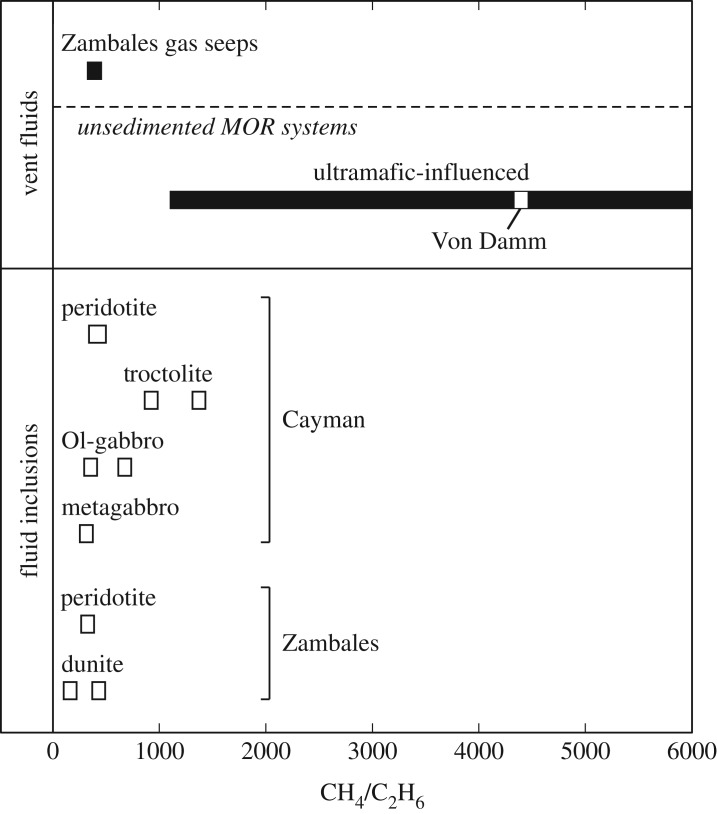
Ranges in carbon isotopic compositions of CH4 from whole-rock samples in this study, Zambales gas seeps, mid-ocean ridge (MOR) vent fluids and Southwest Indian Ridge (SWIR) gabbros. δ13CCH4 values in mafic and ultramafic rocks from Cayman encompass the δ13C value of dissolved CH4 in Von Damm endmember fluids (−15.4‰), suggesting that CH4 budgets at Von Damm may be influenced by interaction with olivine-rich rocks in the subsurface. δ13C values of CH4 issuing from Zambales gas seeps at Los Fuegos Eternos (−7.0 ± 0.4‰) and Nagsasa (−5.6‰) lie in between the ranges of δ13CCH4 in serpentinized dunite and peridotite from the ophiolite, suggesting that CH4 gas in Zambales may be sourced from these two rock types. See text for discussion. Data are from [13,14,16,17,19,21,22,71–82].
Previous studies have examined the chemical and isotopic compositions of CH4-rich fluid inclusions in gabbroic rocks from the ultraslow-spreading SWIR and Mid-Cayman Rise [20–22]. Comparison of the concentrations of CH4 extracted from fluid inclusions in this study (1.7–313 nmol g−1) with concentrations of CH4 released from SWIR gabbros during thermal decrepitation (120–2900 nmol g−1) [21,22] indicates generally higher values in the SWIR samples with some degree of overlap. The majority of SWIR samples are characterized by δ13CCH4 from −34.2‰ to −18.0‰, with two samples exhibiting more positive δ13C values of −11.5‰ and −9.1‰ [20–22]. Isotopic analysis of bulk carbon in the Mid-Cayman Rise samples by elemental analysis combined with isotope ratio mass spectrometry (EA-IRMS) indicates values that vary from −26.7‰ to −23.6‰ [22]. With the exception of the two most positive values, δ13C values for CH4 in SWIR gabbros and for bulk carbon in Mid-Cayman Rise gabbros are significantly more negative than δ13CCH4 values of −16.7‰ to −2.8‰ measured in plutonic rocks during this study (figure 7).
Isotopic analyses of CH4 in SWIR gabbros were performed by step-heating of the samples to 450–500°C and greater than 900–1000°C in the absence of O2 to decrepitate fluid inclusions and release trapped gases; EA-IRMS used for bulk carbon analysis of the Mid-Cayman Rise gabbros involved combusting samples at 1060°C [21,22]. In the case of the SWIR analyses, heating at these temperatures in the absence of O2 can generate CH4 by pyrolysis of organic carbon material, which can represent a variable but significant portion of the total carbon content of fresh and altered igneous rocks. For example, studies of basaltic glass and mantle xenoliths demonstrate the occurrence of condensed carbonaceous material as films and discrete particles along crack surfaces, grain boundaries and fluid inclusion walls, with bulk δ13C values of −29‰ to −22‰ [87–93]. Gabbroic and ultramafic rocks in altered oceanic crust contain variable but significant quantities of organic carbon, up to 7000 ppm, with a similar range in carbon isotopic compositions [94–97]. Thus, it is highly likely that heating of the SWIR samples to decrepitate fluid inclusions resulted in the pyrolysis of higher molecular weight organic compounds and the generation of 13C-depleted CH4. Accordingly, the carbon isotopic compositions of CH4 reported for SWIR gabbros [21,22] likely include a contribution of pyrolytic CH4, resulting in δ13CCH4 values that are more negative than those of CH4 released from fluid inclusions in this study.
(b). Implications for the origin of methane in hydrothermal fluids and gas seeps
(i). Ridge-crest hydrothermal fluids
The existence of abundant CH4-bearing fluid inclusions in rocks that compose a major fraction of the oceanic lithosphere has potential implications for the chemical composition of fluids venting from axial hot springs. Convective circulation of hot and highly reactive aqueous fluids in the crust may release volatiles trapped in fluid inclusions through dissolution of their mineral hosts during hydrothermal alteration and weathering. Fracturing of the olivine host likely represents an additional mechanism to release volatiles from fluid inclusions. The contribution of fluid inclusions to the chemical inventory of ridge-crest hydrothermal fluids can be assessed by examining the composition of vent fluids at the Von Damm hydrothermal field situated on the southern flank of the Mt Dent oceanic core complex at the Mid-Cayman Rise [31,32], in close proximity to the sampling locations of rocks analysed during this study (figure 1). Hydrothermal activity at the Von Damm vent field involves the venting of near neutral pH(25°C) fluids at temperatures up to 226°C [16]. Endmember vent fluids are highly reducing, with dissolved H2 concentrations of 18.2 mmol kg−1, and contain substantial quantities of dissolved CH4 (2.81 mmol kg−1), C2H6 (639 nmol kg−1) and C3H8 (56 nmol kg−1) [16]. Based on the observation that CH4 in the Von Damm vent fluids contained radiocarbon (14C) at the limit of detection, while dissolved ΣCO2 contained five times more 14C, McDermott et al. [16] concluded that carbon in CH4 was not derived from dissolved CO2 in convecting seawater-derived hydrothermal fluids. Additional evidence to support this assertion was provided by the concentration and stable carbon isotope composition of vent fluid ΣCO2, which was identical to that in the seawater source fluid, indicating that there had been no ΣCO2 addition or consumption during hydrothermal circulation. Collectively, these observations led McDermott et al. [16] to postulate that CH4 and possibly other low molecular weight hydrocarbons in the Von Damm vent fluids were leached from fluid inclusions in deep-seated reaction zones during hydrothermal circulation.
Leaching of CH4 and other hydrocarbons from fluid inclusions represents a fundamentally different process than paradigms for CH4 formation that involve the reduction of dissolved CO2 during convection of seawater-derived fluids in the crust. Stable isotope, radiocarbon and mass balance constraints at the Von Damm hydrothermal field indicate that abiotic CH4 formation does not occur in actively circulating fluids beneath Mt Dent [16]. This suggests that insufficient time is available to surmount the large kinetic barriers inhibiting CO2 reduction to CH4 under hydrothermal conditions [64,67,98–101]. Estimated crustal residence times of less than 3–10 years for high-temperature (greater than 200°C) ridge-crest hydrothermal fluids [102,103] and hundreds to 10 000 years for lower temperature ridge flank hydrothermal fluids [104–106] suggest that even longer reaction times may be necessary to generate significant quantities of abiotic CH4 from CO2 during active fluid convection. However, longer reaction times can be achieved by trapping volatiles within fluid inclusions in lower crustal plutonic and mantle rocks. Constraints from thermochronology indicate that rocks exhumed along oceanic detachment faults take 0.3–0.7 Ma to cool from approximately 850°C to 200°C [107–111]. Exhumation by detachment faulting involves horizontal motion (off-axis, at a rate that is roughly half of the spreading rate; [112]) in addition to approximately 5–10 km of vertical motion [113]. Detachment faults are known to steepen with depth at the ridge axis (e.g. [114,115]), which means that a significant portion of the cooling time of an inclusion-bearing rock can be spent moving mostly upwards in the near-axis domain. This may provide sufficient time for the reduction of CO2 to CH4 to occur in fluid inclusions while the host rock remains close to the spreading axis. For instance, that could be the case at the near-axis Logatchev hydrothermal site on the Mid-Atlantic Ridge. By contrast, host rocks can move farther off-axis in less magma-rich regions of spreading centres where more extension is accommodated through slip on detachment faults, as may be the case for the off-axis Von Damm and Lost City sites. In both cases, carbon-rich fluid inclusions may persist in the subsurface of oceanic core complexes for hundreds of thousands of years before extraction by circulating seawater-derived fluids, which may allow sufficient time for the reduction of dissolved CO2 to CH4 and other low molecular weight hydrocarbons.
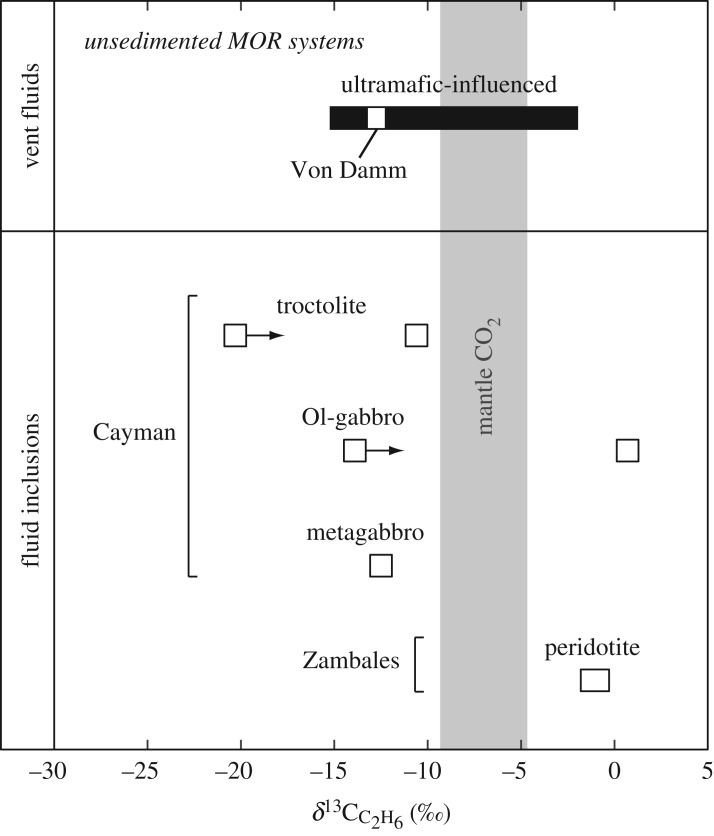
Aqueous CH4 in the Von Damm vent fluids has a δ13C value of −15.4‰, which falls within the range of δ13CCH4 values measured in mafic and ultramafic rocks from the Mt Dent oceanic core complex of −16.7‰ to −4.4‰ (figure 7). Similarly, the range of δ13CC2H6 values in the Cayman rock samples (−20.3‰ to −10.6‰), with the exception of ALV 620-5-1 (+0.7‰), encompasses the δ13C value of −12.7‰ for aqueous C2H6 in the Von Damm vent fluid (figure 8). Moreover, 3He/4He ratios of 6.58–9.91 Ra in Cayman rocks all show mantle-like values within analytical error (figure 6) and are consistent with the average 3He/4He ratio (8.14 ± 0.13 Ra) in Von Damm hydrothermal fluids [16]. The similarities in the carbon and He isotopic compositions between Von Damm fluids and Cayman rocks are consistent with a model in which hydrocarbons and helium in Von Damm fluids may be derived by leaching of volatiles trapped within subsurface olivine-rich rocks.
Figure 8.
Ranges in carbon isotopic compositions of C2H6 from whole-rock samples in this study and ultramafic-influenced MOR vent fluids. Symbols with arrows denote minimum values due to C2H6 contamination detected from the crushing device (see text for details). The δ13C value of dissolved C2H6 in Von Damm fluids (−12.7‰) is similar to δ13CC2H6 values for most Cayman samples, with the exception of ALV 620-5-1. See text for discussion. Data are from [14,16,71,72,81,82].
While hydrocarbon isotopic compositions in vent fluid and fluid inclusions show a high degree of overlap, the molar CH4/C2H6 ratios of 315–1372 measured in Cayman rocks are significantly lower than the Von Damm endmember fluid ratio of 4397 [16] (figure 9). Differences in CH4/C2H6 ratios between the rocks and fluids may reflect the decomposition of C2+ hydrocarbons during transport in the Von Damm hydrothermal fluids at elevated temperatures. Laboratory hydrothermal experiments have demonstrated relatively rapid oxidative degradation of C2+ hydrocarbons in the presence of Fe-sulfide and oxide minerals that buffer the redox state to conditions analogous to ridge-crest hydrothermal systems [116]. Experimentally determined half-lives of C2H6 by oxidative decomposition at 300–350°C are on the order of a few months [116], and are shorter than residence times of less than 3–10 years estimated for actively convecting high-temperature fluids in submarine hydrothermal systems [102,103].
Figure 9.
Range in CH4/C2H6 ratios from whole-rock samples in this study, Zambales gas seeps and ultramafic-influenced MOR vent fluids. CH4/C2H6 ratios in Zambales gas seeps are similar to measured ratios in serpentinized dunite and peridotite from the ophiolite. By contrast, CH4/C2H6 ratios in Von Damm vent fluids are more than three times higher than those in Cayman rocks. See text for discussion. Data are from [13,14,16,17,81].
Rocks from the Mid-Cayman Rise, except for sample ALV 620-3C, exhibit CH4/3He ratios significantly higher than the Von Damm vent fluid ratio of 2.34 × 108, indicating the addition of carbon or loss of helium in the rocks (figure 6). Several factors may account for such higher ratios, in particular the different methodologies used to analyse hydrocarbon and helium contents. Hydrocarbon abundances were measured by crushing whole-rock samples 800 times each, whereas He contents were measured by crushing whole-rock samples only 20 times each, likely resulting in more incomplete rupturing of fluid inclusions. Furthermore, vacuum crushing and melting experiments demonstrate that the majority (79–95%) of helium in oceanic peridotites and mylonites is contained within mineral matrices as opposed to fluid and melt inclusions [117]. Because mineral matrices may be more rugged than fluid inclusions, vacuum crushing of partially serpentinized Cayman peridotites may have released a smaller fraction of He relative to inclusion-hosted CH4, resulting in elevated CH4/3He ratios. Another process that may lower CH4/3He ratios in vent fluids at Von Damm is the addition of mantle He from sources other than fluid inclusions, such as the crystallization of igneous rocks at depth. Since He in fluid inclusions is also mantle-derived, there would be no difference in the isotopic composition of He in rock samples and vent fluids.
Whole-rock hydrocarbon abundances in Cayman rock samples can be used to estimate the minimum water/rock mass ratios needed to account for the concentrations of dissolved CH4 (2.81 mmol kg−1) and C2H6 (639 nmol kg−1) observed in endmember fluids at the Von Damm hydrothermal field [16]. Water/rock mass ratios were obtained by dividing the concentration of these species in the rock by their concentration in the endmember vent fluid. For these calculations, it was assumed that hydrocarbons are quantitatively leached during hydrothermal alteration by convecting fluids. Because the measured hydrocarbon abundances in Cayman rocks represent minimum values due to incomplete crushing of fluid inclusions, calculated water/rock ratios likely underestimate actual values. The CH4 abundances in Cayman samples yield water/rock mass ratios of 0.002–0.11, while ratios calculated from C2H6 contents are approximately an order of magnitude higher and vary from 0.02 to 1.6 (table 4). Higher values calculated for C2H6 may reflect oxidative degradation of C2H6 in the fluids following extraction from the rock.
Table 4.
Calculated water–rock (W/R) mass ratios needed to account for the concentrations of dissolved CH4 and C2H6 in Von Damm hydrothermal fluids.
| W/R | |||
|---|---|---|---|
| rock type | sample | CH4 | C2H6 |
| gneissic metagabbro | ALV 620 3C | 0.11 | 1.56 |
| olivine gabbro | ALV 620 5-1 | 0.09 | 1.07 |
| ALV 624 5-1 | 0.03 | 0.21 | |
| troctolite | ALV 621 3-1 | 0.03 | 0.13 |
| ALV 623 3-2 | 0.03 | 0.07 | |
| peridotite | ALV 624 3-3 | 0.002 | 0.02 |
| ALV 624 6-2 | 0.004 | 0.03 | |
Water/rock mass ratios for Von Damm vent fluids can also be estimated using the abundance of inorganic trace elements. Based on the concentrations of Li and Rb in Von Damm vent fluids and Cayman rocks, water/rock mass ratios vary from 0.2 to 0.8 [34], consistent with the rock-dominated conditions indicated by hydrocarbon abundances in this study. The broader range of values calculated from hydrocarbon abundances and the extension to substantially lower values may be attributed to alteration of the rock samples, which may have released trapped volatiles from fluid inclusions prior to their analysis. Peridotite samples, in particular, are more than 80–90% serpentinized according to visual estimates in thin section, suggesting that only a small fraction of secondary fluid inclusions have remained intact since their formation.
To examine the potential of fluid inclusions as a source of hydrocarbons in the Von Damm vent site, we estimate the CH4 flux that can be supplied by the downward migration of cracks that would enable convecting fluids to access inclusion-bearing rocks in the oceanic lithosphere. Seismic data by Harding et al. [33] suggest that hydrothermal circulation at Von Damm could be fueled by a cracking front mining heat from hot rocks beneath the Mt Dent oceanic core complex (e.g. [118]). Based on the flux of fluid estimated to vent at Von Damm (approx. 500 kg s−1; [119]) and the concentration of dissolved CH4 in endmember vent fluids (2.81 mmol l−1 [16]), release of CH4 from fluid inclusion-bearing rocks would have to supply approximately 1 mol CH4 s−1 to the endmember fluid. Assuming the crust contains approximately 1 mol CH4 m−3 rock (corresponding to the most CH4-rich rock sample measured in this study), such a CH4 flux could be achieved by a cracking front that propagates downward at approximately 10 cm yr−1, allowing the instantaneous release of CH4 from the fluid inclusions to the hydrothermal fluid over a horizontal area of approximately 10 km2. This area is consistent with the size of the seismically inferred heat source beneath Von Damm [33]. Furthermore, such a migration rate for the cracking front is within the bounds of rates estimated from models of thermal cracking in the oceanic lithosphere [118]. In addition, if we consider that hydrothermal circulation beneath Van Damm is occurring over the entire area of the Mt Dent oceanic core complex (of the order of 100 km2), then the cracking front would only need to propagate downward at approximately 1 cm yr−1. Propagation at this rate would roughly match the slip rate of the detachment fault that hosts the Von Damm field and allow the cracking front to remain stationary as the detachment fault exhumes deeper CH4-bearing rocks that continuously replenish the supply of CH4 available for leaching.
Dissolved hydrocarbons in vent fluids from unsedimented, ultramafic-influenced hydrothermal sites from a broad range of seafloor locations show remarkably similar concentrations and isotopic compositions to those in Von Damm vent fluids, despite the widely different subsurface temperatures, alteration histories and inorganic fluid chemistries of the hydrothermal sites (figures 7 and 8). For example, dissolved CH4 exhibits a relatively narrow range in concentration from 0.5 to 3.5 mM, while dissolved C2H6 exhibits concentrations of 0.1–5.7 µM, with the majority of values falling between 0.6 and 1 µM for fluids from the Lost City, Rainbow, Logatchev, and even the mafic-hosted Lucky Strike hydrothermal fields [13,14,16,80,81,120]. δ13C values vary from −17.8‰ to −6.1‰ in CH4, and from −15.2‰ to −2.0‰ in C2H6 [13,14,16,80,81]. In addition, recent analyses of clumped methane isotopologues reveal similar apparent equilibrium temperatures of for a variety of ultramafic- and basalt-influenced seafloor hydrothermal systems [26]. In contrast to hydrocarbon abundances and isotopic compositions, measured vent fluid temperatures and pH(25°C) vary substantially from 96°C to 370°C and from 3.3 to 10.2, respectively. The similarity in hydrocarbon abundances and isotopic compositions at these vent fields with those observed at Von Damm suggests that leaching of volatiles from fluid inclusions during convective circulation of seawater-derived hydrothermal fluids may be a ubiquitous process.
Examination of figure 7 reveals that the range of δ13CCH4 values observed for CH4 trapped in olivine-rich rocks from the Mid-Cayman Rise also shows a high degree of overlap with δ13CCH4 values measured for dissolved CH4 in basalt-influenced hydrothermal systems. This similarity suggests that fluids hosted in mafic substrates may additionally be mining CH4 from fluid inclusions in olivine-rich gabbroic rocks of oceanic layer 3 [20–23]. Variations of CH4(aq) concentrations measured in mafic-hosted hydrothermal systems may therefore reflect the abundance of olivine-rich gabbro interacting with convecting hydrothermal fluids along their flow path [22].
(ii). Zambales gas seeps
Abundant CH4-rich fluid inclusions in olivine-rich rocks at the Zambales ophiolite represent a significant reservoir of hydrocarbons that may contribute to active gas seeps. Reduced gases venting from fractured, partially serpentinized ultramafic rock in the Zambales ophiolite contain up to 58.5 vol.% H2 and up to 55 vol.% CH4 [17,19,37,38]. Seep gases also contain elevated amounts of He (up to 6.9 ppm), C2H6 (up to 0.15 vol.%) and other C2+ hydrocarbons [17]. Estimated serpentinization temperatures for the Zambales ophiolite range from 350°C in the geological past to less than 100°C for present-day serpentinization [121,122].
Comparison of the relative abundances and isotopic compositions of volatiles in seep gases with those measured in olivine-rich rocks from the Zambales ophiolite reveals remarkable similarities. In particular, CH4 gas collected from the Los Fuegos Eternos and Nagsasa seeps has average δ13CCH4 values of −7.0‰ and −5.6‰, respectively [17,19], which fall within the range of −12.4‰ to −2.8‰ measured for CH4 hosted in fluid inclusions in serpentinized dunite and peridotite from the ophiolite (figure 7). Moreover, the range of CH4/C2H6 ratios measured in serpentinized dunite and peridotite from Zambales during this study (163–431) overlaps with the range of CH4/C2H6 ratios in Zambales gas seeps (325–457) [17] (figure 9). Average 3He/4He ratios of 3.0–5.6 Ra in serpentinized dunite and peridotite (table 3) also overlap with 3He/4He ratios of 4.03 ± 0.05 to 0.49 Ra at Los Fuegos Eternos and 4.35 Ra at Nagsasa gas seeps [17,19]. Similarly, the average CH4/3He ratio in Zambales gas seeps (1.6 × 1010 ± 0.2 × 1010) [17] lies between measured CH4/3He ratios in serpentinized samples (8.06 × 109−1.30 × 1012) (figure 6). Overall, the abundance and isotopic compositions of hydrocarbons and He in ultramafic rocks presented here are remarkably similar to those of gases emanating from the Zambales ophiolite, suggesting that mining of fluid inclusion-hosted volatiles during alteration or fracturing of rocks may be a significant source of hydrocarbons at Zambales and possibly other continental gas seeps. For instance, available geochemical and radiometric constraints for the origin of CH4 venting at Chimaera (Turkey), a serpentinite-hosted gas seep active for more than two millennia [123], are consistent with a fluid inclusion source. Abiotic CH4 is interpreted to have formed at 120–140°C, which is significantly higher than the current temperature in the source rock [124], suggesting that CH4 must have formed in the geological past when temperatures were higher. This interpretation is in keeping with radiocarbon constraints [123] and recent mass balance estimates indicating fluid inclusion abundances are sufficiently high to account for the source of abiotic CH4 at Chimaera [23].
5. Conclusion
Lower crustal and upper mantle rocks at the Mid-Cayman Rise and the Zambales ophiolite contain appreciable quantities of CH4 and C2H6 hosted in secondary fluid inclusions in olivine ± plagioclase ± clinopyroxene. Whole-rock CH4 and C2H6 carbon isotopic compositions at the Mid-Cayman Rise are similar to the range observed in fluids venting at the Von Damm hydrothermal field and other ultramafic-influenced hydrothermal sites on the seafloor. Likewise, hydrocarbon and noble gas compositions in fluid inclusions in rocks from the Zambales ophiolite are strikingly similar to those of nearby gas seeps. These findings suggest that leaching of fluid inclusions by dissolution or fracturing of the rock may provide a significant contribution of hydrocarbons to hydrothermal fluids at Von Damm, gas seeps at Zambales, and possibly other ultramafic-influenced submarine and subaerial vent sites. It is also possible that CH4 in basalt-hosted vent fluids may be derived from leaching of fluid inclusions in gabbroic rocks. While convecting seawater-derived fluids transport dissolved hydrocarbons to the seafloor, current evidence does not support their direct involvement in hydrocarbon production. Instead, CH4 and C2+ alkanes appear to be formed in isolation from percolating fluids, such as in fluid inclusions where CO2 reduction can take place over geologic time scales. It is proposed that CO2 and H2O become trapped in primary minerals as carbon-rich aqueous fluids circulate within cooling gabbroic and mantle rocks [23]. As temperatures decrease, entrapped CO2 and H2O react with their host minerals and undergo chemical re-equilibration. Reaction of olivine with H2O below approximately 400°C is expected to generate H2, thereby driving the reduction of CO2 and resulting in the formation of secondary fluid inclusions enriched in CH4, C2H6 and potentially other low molecular weight hydrocarbons. Subsequent extraction and transport of hydrocarbons to vent sites is mediated by convecting hydrothermal fluids. These findings have profound implications for models that describe the flux of carbon between the lithosphere and oceans as well as its impact on microbial vent communities in seafloor and subseafloor environments that use reduced carbon species as a source of carbon and metabolic energy.
Supplementary Material
Acknowledgements
We thank Mark Kurz and Joshua Curtice for performing the helium isotopic measurements. We thank Karmina Aquino and Emmanuel Codillo for collecting samples from the Zambales ophiolite, and Karmina Aquino for performing some of the Raman analyses on the Zambales samples. This work benefited from fruitful discussions with Jean-Arthur Olive. Constructive reviews by Jeff Alt, associate editor Andrew McCaig and an anonymous reviewer greatly improved the manuscript.
Data accessibility
The datasets supporting this article are included in tables and figures within this manuscript.
Authors' contributions
F.K. and J.S.S. conceived this study. All authors contributed to the conception and design of the method for extracting volatile carbon species from rock-hosted fluid inclusions. N.G.G. and S.P.S. collected the data, and all authors were involved in the interpretation of the acquired dataset. N.G.G. wrote the manuscript, with significant contributions to drafting and revision made by all authors. The final version of this manuscript has been approved by all authors.
Competing interests
We declare we have no competing interests.
Funding
This study was supported by the National Science Foundation through grant no. OCE-1634032 to F.K. and J.S.S. Additional financial support was provided by Louise Von Damm.
References
- 1.Mottl MJ, Komor SC, Fryer P, Moyer CL. 2003. Deep-slab fluids fuel extremophilic Archaea on a Mariana forearc serpentinite mud volcano: Ocean Drilling Program Leg 195. Geochem. Geophys. Geosyst. 4, 9009 ( 10.1029/2003GC000588) [DOI] [Google Scholar]
- 2.Schrenk MO, Kelley DS, Bolton SA, Baross JA. 2004. Low archaeal diversity linked to subseafloor geochemical processes at the Lost City Hydrothermal Field, Mid-Atlantic Ridge. Environ. Microbiol. 6, 1086–1095. ( 10.1111/j.1462-2920.2004.00650.x) [DOI] [PubMed] [Google Scholar]
- 3.Schrenk MO, Brazelton WJ, Lang SQ. 2013. Serpentinization, carbon, and deep life. Rev. Mineral. Geochem. 75, 575–606. ( 10.2138/rmg.2013.75.18) [DOI] [Google Scholar]
- 4.Curtis AC, Wheat CG, Fryer P, Moyer CL. 2013. Mariana forearc serpentinite mud volcanoes harbor novel communities of extremophilic Archaea. Geomicrobiol. J. 30, 430–441. ( 10.1080/01490451.2012.705226) [DOI] [Google Scholar]
- 5.Quéméneur M, et al. 2014. Spatial distribution of microbial communities in the shallow submarine alkaline hydrothermal field of the Prony Bay, New Caledonia. Environ. Microbiol. Rep. 6, 665–674. ( 10.1111/1758-2229.12184) [DOI] [PubMed] [Google Scholar]
- 6.Sleep NH, Bird DK, Pope EC. 2011. Serpentinite and the dawn of life. Phil. Trans. R. Soc. B 366, 2857–2869. ( 10.1098/rstb.2011.0129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takai K, Nakamura K, Suzuki K, Inagaki F, Nealson KH, Kumagai H. 2006. Ultramafics-Hydrothermalism-Hydrogenesis-HyperSLiME (UltraH3) linkage: a key insight into early microbial ecosystem in the Archean deep-sea hydrothermal systems. Paleontol. Res. 10, 269–282. ( 10.2517/prpsj.10.269) [DOI] [Google Scholar]
- 8.Martin W, Baross J, Kelley D, Russell MJ. 2008. Hydrothermal vents and the origin of life. Nat. Rev. Microbiol. 6, 805–814. ( 10.1038/nrmicro1991) [DOI] [PubMed] [Google Scholar]
- 9.Lane N, Allen JF, Martin W. 2010. How did LUCA make a living? Chemiosmosis in the origin of life. Bioessays 32, 271–280. ( 10.1002/bies.200900131) [DOI] [PubMed] [Google Scholar]
- 10.Deamer D, Damer B. 2017. Can life begin on Enceladus? A perspective from hydrothermal chemistry. Astrobiology 17, 834–839. ( 10.1089/ast.2016.1610) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waite JH, et al. 2017. Cassini finds molecular hydrogen in the Enceladus plume: evidence for hydrothermal processes. Science 356, 155–159. ( 10.1126/science.aai8703) [DOI] [PubMed] [Google Scholar]
- 12.Charlou JL, Donval JP. 1993. Hydrothermal methane venting between 12°N and 26°N along the Mid-Atlantic Ridge. J. Geophys. Res. 98, 9625–9642. ( 10.1029/92JB02047) [DOI] [Google Scholar]
- 13.Charlou JL, Donval JP, Fouquet Y, Jean-Baptiste P, Holm N. 2002. Geochemistry of high H2 and CH4 vent fluids issuing from ultramafic rocks at the Rainbow hydrothermal field (36°14′N, MAR). Chem. Geol. 191, 345–359. ( 10.1016/S0009-2541(02)00134-1) [DOI] [Google Scholar]
- 14.Proskurowski G, Lilley MD, Seewald JS, Früh-Green GL, Olson EJ, Lupton JE, Sylva SP, Kelley DS. 2008. Abiogenic hydrocarbon production at Lost City Hydrothermal Field. Science 319, 604–607. ( 10.1126/science.1151194) [DOI] [PubMed] [Google Scholar]
- 15.Seyfried WE, Pester NJ, Tutolo BM, Ding K. 2015. The Lost City hydrothermal system: Constraints imposed by vent fluid chemistry and reaction path models on subseafloor heat and mass transfer processes. Geochim. Cosmochim. Acta 163, 59–79. ( 10.1016/j.gca.2015.04.040) [DOI] [Google Scholar]
- 16.McDermott JM, Seewald JS, German CR, Sylva SP. 2015. Pathways for abiotic organic synthesis at submarine hydrothermal fields. Proc. Natl Acad. Sci. USA 112, 7668–7672. ( 10.1073/pnas.1506295112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abrajano TA, Sturchio NC, Bohlke JK, Lyon GL, Poreda RJ, Stevens CM. 1988. Methane-hydrogen gas seeps, Zambales Ophiolite, Philippines: deep or shallow origin? Chem. Geol. 71, 211–222. ( 10.1016/0009-2541(88)90116-7) [DOI] [Google Scholar]
- 18.Hosgormez H, Etiope G, Yalçin MN. 2008. New evidence for a mixed inorganic and organic origin of the Olympic Chimaera fire (Turkey): a large onshore seepage of abiogenic gas. Geofluids 8, 263–273. ( 10.1111/j.1468-8123.2008.00226.x) [DOI] [Google Scholar]
- 19.Vacquand C, Deville E, Beaumont V, Guyot F, Sissmann O, Pillot D, Arcilla C, Prinzhofer A. 2018. Reduced gas seepages in ophiolitic complexes: evidences for multiple origins of the H2-CH4-N2 gas mixtures. Geochim. Cosmochim. Acta 223, 437–461. ( 10.1016/j.gca.2017.12.018) [DOI] [Google Scholar]
- 20.Kelley DS. 1996. Methane-rich fluids in the oceanic crust. J. Geophys. Res. Earth 101, 2943–2962. ( 10.1029/95jb02252) [DOI] [Google Scholar]
- 21.Kelley DS, Früh-Green GL. 1999. Abiogenic methane in deep-seated mid-ocean ridge environments: insights from stable isotope analyses. J. Geophys. Res. 104, 10 439–10 460. ( 10.1029/1999JB900058) [DOI] [Google Scholar]
- 22.Kelley DS, Früh-Green GL. 2001. Volatile lines of descent in submarine plutonic environments: insights from stable isotope and fluid inclusion analyses. Geochim. Cosmochim. Acta 65, 3325–3346. ( 10.1016/S0016-7037(01)00667-6) [DOI] [Google Scholar]
- 23.Klein F, Grozeva NG, Seewald JS. 2019. Abiotic methane synthesis and serpentinization in olivine-hosted fluid inclusions. Proc. Natl Acad. Sci. USA 116, 17 666–17 672. ( 10.1073/pnas.1907871116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelley DS, Baross JA, Delaney JR. 2002. Volcanoes, fluids, and life at mid-ocean ridge spreading centers. Annu. Rev. Earth Planet. Sci. 30, 385–491. ( 10.1146/annurev.earth.30.091201.141331) [DOI] [Google Scholar]
- 25.McCollom TM, Seewald JS. 2007. Abiotic synthesis of organic compounds in deep-sea hydrothermal environments. Chem. Rev. 107, 382–401. ( 10.1021/cr0503660) [DOI] [PubMed] [Google Scholar]
- 26.Wang DT, Reeves EP, McDermott JM, Seewald JS, Ono S. 2018. Clumped isotopologue constraints on the origin of methane at seafloor hot springs. Geochim. Cosmochim. Acta 223, 141–158. ( 10.1016/j.gca.2017.11.030) [DOI] [Google Scholar]
- 27.Stroup JB, Fox PJ. 1981. Geologic investigations in the Cayman Trough: evidence for thin oceanic crust along the Mid-Cayman Rise. J. Geol. 89, 395–420. ( 10.1086/628605) [DOI] [Google Scholar]
- 28.Dimalanta CB, Salapare RC, Faustino-Eslava DV, Ramos NT, Queaño KL, Yumul GP, Yang TF. 2015. Post-emplacement history of the Zambales Ophiolite Complex: insights from petrography, geochronology and geochemistry of Neogene clastic rocks. J. Asian Earth Sci. 104, 215–227. ( 10.1016/j.jseaes.2014.07.021) [DOI] [Google Scholar]
- 29.Ballard R, et al. 1979. Geological and geophysical investigation of the Midcayman Rise spreading center: initial results and observations. In Deep drilling results in the Atlantic Ocean: ocean crust (eds Talwani M, Harrison CG, Hayes DE), pp. 66–93. Washington, DC: American Geophysical Union. [Google Scholar]
- 30.Hayman NW, Grindlay NR, Perfit MR, Mann P, Leroy S, De Lépinay BM.. 2011. Oceanic core complex development at the ultraslow spreading Mid-Cayman Spreading Center. Geochem. Geophys. Geosyst. 12, Q0AG02 ( 10.1029/2010GC003240) [DOI] [Google Scholar]
- 31.German CR, et al. 2010. Diverse styles of submarine venting on the ultraslow spreading Mid-Cayman Rise. Proc. Natl Acad. Sci. USA 107, 14 020–14 025. ( 10.1073/pnas.1009205107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Connelly DP, et al. 2012. Hydrothermal vent fields and chemosynthetic biota on the world's deepest seafloor spreading centre. Nat. Commun. 3, 620 ( 10.1038/ncomms1636) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harding JL, Van Avendonk HJA, Hayman NW, Grevemeyer I, Peirce C, Dannowski A.. 2017. Magmatic-tectonic conditions for hydrothermal venting on an ultraslow-spread oceanic core complex. Geology 45, 839–842. ( 10.1130/G39045.1) [DOI] [Google Scholar]
- 34.McDermott JM. 2015. Geochemistry of deep-sea hydrothermal vent fluids from the Mid-Cayman Rise, Caribbean Sea. PhD thesis, Massachusetts Institute of Technology, USA. [Google Scholar]
- 35.Malcolm FL. 1979. Petrography, mineral chemistry and microstructures of gabbros from the Mid-Cayman Rise spreading center. MS thesis, State University of New York at Albany, USA. [Google Scholar]
- 36.Elthon D. 1987. Petrology of gabbroic rocks from the Mid-Cayman rise spreading center. J. Geophys. Res. 92, 658–682. ( 10.1029/JB092iB01p00658) [DOI] [Google Scholar]
- 37.Thayer TP. 1966. Serpentinization considered as a constant-volume metasomatic process. Am. Mineral. 51, 685–710. [Google Scholar]
- 38.Abrajano TA, Sturchio NC, Kennedy BM, Lyon GL, Muehlenbachs K, Bohlke JK. 1990. Geochemistry of reduced gas related to serpentinization of the Zambales ophiolite, Philippines. Appl. Geochem. 5, 625–630. ( 10.1016/0883-2927(90)90060-I) [DOI] [Google Scholar]
- 39.Yumul GP, et al. In press Slab rollback and microcontinent subduction in the evolution of the Zambales Ophiolite Complex (Philippines): a review. Geosci. Front. ( 10.1016/j.gsf.2018.12.008) [DOI] [Google Scholar]
- 40.Zhang J, Qiao S, Lu W, Hu Q, Chen S, Liu Y. 2016. An equation for determining methane densities in fluid inclusions with Raman shifts. J. Geochem. Explor. 171, 20–28. ( 10.1016/j.gexplo.2015.12.003) [DOI] [Google Scholar]
- 41.Lu W, Chou IM, Burruss RC, Song Y. 2007. A unified equation for calculating methane vapor pressures in the CH4-H2O system with measured Raman shifts. Geochim. Cosmochim. Acta 71, 3969–3978. ( 10.1016/j.gca.2007.06.004) [DOI] [Google Scholar]
- 42.Wopenka B, Pasteris JD. 1987. Raman intensities and detection limits of geochemically relevant gas mixtures for a laser Raman microprobe. Anal. Chem. 59, 2165–2170. ( 10.1021/ac00144a034) [DOI] [Google Scholar]
- 43.Rosso KM, Bodnar RJ. 1995. Microthermometric and Raman spectroscopic detection limits of CO2 in fluid inclusions and the Raman spectroscopic characterization of CO2. Geochim. Cosmochim. Acta 59, 3961–3975. ( 10.1016/0016-7037(95)94441-H) [DOI] [Google Scholar]
- 44.Lamadrid HM. 2016. Geochemistry of fluid-rock processes. PhD thesis, Virginia Polytechnic Institute and State University, USA. [Google Scholar]
- 45.Piperov NB, Penchev NP. 1973. A study on gas inclusions in minerals. Analysis of the gases from micro-inclusions in allanite. Geochim. Cosmochim. Acta 37, 2075–2097. ( 10.1016/0016-7037(73)90009-4) [DOI] [Google Scholar]
- 46.Roedder E. 1984. Fluid inclusions. Washington, DC: Mineralogical Society of America. [Google Scholar]
- 47.Salvi S, Williams-Jones AE. 2003. Bulk analysis of volatiles in fluid inclusions. Mineral. Assoc. Canada Short Course Ser. 32, 247–278. [Google Scholar]
- 48.Norman DI, Sawkins FJ. 1987. Analysis of volatiles in fluid inclusions by mass spectrometry. Chem. Geol. 61, 1–10. ( 10.1016/0009-2541(87)90020-9) [DOI] [Google Scholar]
- 49.Norman DI, Harrison RW, Andres CB. 1991. Geology and geochemical analysis of mineralizing fluids at the St. Cloud and U.S. Treasury Mines, Chloride Mining District, New Mexico. J. Geochem. Explor. 42, 61–89. ( 10.1016/0375-6742(91)90060-8) [DOI] [Google Scholar]
- 50.Abell PI, Draffan CH, Eglinton G, Hayes JM, Maxwell JR, Pillinger CT. 1970. Organic analysis of the returned Apollo 11 lunar sample. Proc. Apollo 11 Lunar Sci. Conf. 2, 1757–1773. [DOI] [PubMed] [Google Scholar]
- 51.Andrawes F, Holzer G, Roedder E, Gibson EKJ, Oro J. 1984. Gas chromatographic analysis of volatiles in fluid and gas inclusions. J. Chromatogr. 302, 181–193. ( 10.1016/S0021-9673(01)89010-5) [DOI] [PubMed] [Google Scholar]
- 52.Andrawes FF, Gibson EKJ. 1979. Release and analysis of gases from geological samples. Am. Mineral. 64, 453–463. [Google Scholar]
- 53.Welhan JA. 1988. Methane and hydrogen in mid-ocean-ridge basalt glasses: Analysis by vacuum crushing. Can. J. Earth Sci. 25, 38–48. ( 10.1139/e88-004) [DOI] [Google Scholar]
- 54.Roedder E. 1972. Composition of fluid inclusions. U.S. Geol. Surv. Prof. Pap. 440, 1–164. ( 10.3133/pp440JJ) [DOI] [Google Scholar]
- 55.Kurz MD, Gurney JJ, Jenkins WJ, Lott DE. 1987. Helium isotopic variability within single diamonds from the Orapa kimberlite pipe. Earth Planet. Sci. Lett. 86, 57–68. ( 10.1016/0012-821X(87)90188-9) [DOI] [Google Scholar]
- 56.Kurz MD, Curtice J, Lott DE, Solow A. 2004. Rapid helium isotopic variability in Mauna Kea shield lavas from the Hawaiian Scientific Drilling Project. Geochem. Geophys. Geosyst. 5, Q04G14 ( 10.1029/2002GC000439) [DOI] [Google Scholar]
- 57.Kurz MD, Jenkins WJ. 1981. The distribution of helium in oceanic basalt glasses. Earth Planet. Sci. Lett. 53, 41–54. ( 10.1016/0012-821X(81)90024-8) [DOI] [Google Scholar]
- 58.Allègre CJ, Staudacher T, Sarda P, Kurz MD. 1983. Constraints on evolution of Earth's mantle from rare gas systematics. Nature 303, 762–766. ( 10.1038/303762a0) [DOI] [Google Scholar]
- 59.Hiyagon H, Ozima M, Marty B, Zashu S, Sakai H. 1992. Noble gases in submarine glasses from mid-oceanic ridges and Loihi seamount: constraints on the early history of the Earth. Geochim. Cosmochim. Acta 56, 1301–1316. ( 10.1016/0016-7037(92)90063-O) [DOI] [Google Scholar]
- 60.Harper D. 1985. Tectonics of slow spreading mid-ocean ridges and consequences of a variable depth to the brittle/ductile transition. Tectonics 4, 395–409. ( 10.1029/TC004i004p00395) [DOI] [Google Scholar]
- 61.Jones MR, et al. 2019. New constraints on mantle carbon from Mid-Atlantic Ridge popping rocks. Earth Planet. Sci. Lett. 511, 67–75. ( 10.1016/j.epsl.2019.01.019) [DOI] [Google Scholar]
- 62.Allen DE, Seyfried WEJ. 2003. Compositional controls on vent fluids from ultramafic-hosted hydrothermal systems at mid-ocean ridges: An experimental study at 400°C, 500 bars. Geochim. Cosmochim. Acta 67, 1531–1542. ( 10.1016/S0016-7037(02)01173-0) [DOI] [Google Scholar]
- 63.Klein F, Bach W, McCollom TM. 2013. Compositional controls on hydrogen generation during serpentinization of ultramafic rocks. Lithos 178, 55–69. ( 10.1016/j.lithos.2013.03.008) [DOI] [Google Scholar]
- 64.McCollom TM, Klein F, Robbins M, Moskowitz B, Berquó TS, Jöns N, Bach W, Templeton A. 2016. Temperature trends for reaction rates, hydrogen generation, and partitioning of iron during experimental serpentinization of olivine. Geochim. Cosmochim. Acta 181, 175–200. ( 10.1016/j.gca.2016.03.002) [DOI] [Google Scholar]
- 65.Shock EL. 1990. Geochemical constraints on the origin of organic compounds in hydrothermal systems. Orig. Life Evol. Biosph. 20, 331–367. ( 10.1007/BF01808115) [DOI] [PubMed] [Google Scholar]
- 66.Früh-Green GL, Connolly JAD, Plas A, Kelley DS, Grobety B. 2004. Serpentinization of oceanic peridotites: implications for geochemical cycles and biological activity. In The subseafloor biosphere at mid-ocean ridges (eds Wilcock WSD, Delong EF, Kelley DS, Baross JA, Craig Cary S), pp. 119–136. Washington, DC: American Geophysical Union. [Google Scholar]
- 67.Klein F, McCollom TM. 2013. From serpentinization to carbonation: new insights from a CO2 injection experiment. Earth Planet. Sci. Lett. 379, 137–145. ( 10.1016/j.epsl.2013.08.017) [DOI] [Google Scholar]
- 68.Hentscher M. 2012. Thermodynamic investigations of microbial metabolism and abiotic organic synthesis in seafloor hydrothermal systems. PhD thesis, University of Bremen. [Google Scholar]
- 69.Evans BW, Yang H. 1998. Fe-Mg order-disorder in tremolite-actinolite-ferro-actinolite at ambient and high temperature. Am. Mineral. 83, 458–475. ( 10.2138/am-1998-5-606) [DOI] [Google Scholar]
- 70.Kurz MD, Moreira M, Curtice J, Lott DE, Mahoney JJ, Sinton JM. 2005. Correlated helium, neon, and melt production on the super-fast spreading East Pacific Rise near 17°S. Earth Planet. Sci. Lett. 232, 125–142. ( 10.1016/j.epsl.2005.01.005) [DOI] [Google Scholar]
- 71.Des Marais DJ, Moore JG.. 1984. Carbon and its isotopes in mid-oceanic basaltic glasses. Earth Planet. Sci. Lett. 69, 43–57. ( 10.1016/0012-821X(84)90073-6) [DOI] [Google Scholar]
- 72.Sakai H, Des Marais DJ, Ueda A, Moore JG.. 1984. Concentrations and isotope ratios of carbon, nitrogen and sulfur in ocean-floor basalts. Geochim. Cosmochim. Acta 48, 2433–2441. ( 10.1016/0016-7037(84)90295-3) [DOI] [PubMed] [Google Scholar]
- 73.Charlou JL, Donval JP, Douville E, Jean-Baptiste P, Radford-Knoery J, Fouquet Y, Dapoigny A, Stievenard M. 2000. Compared geochemical signatures and the evolution of Menez Gwen (35°50′N) and Lucky Strike (37°17′N) hydrothermal fluids, south of the Azores Triple Junction on the Mid-Atlantic Ridge. Chem. Geol. 171, 49–75. ( 10.1016/S0009-2541(00)00244-8) [DOI] [Google Scholar]
- 74.Gamo T, et al. 2001. Chemical characteristics of newly discovered black smoker fluids and associated hydrothermal plumes at the Rodriguez Triple Junction, Central Indian Ridge. Earth Planet. Sci. Lett. 193, 371–379. ( 10.1016/S0012-821X(01)00511-8) [DOI] [Google Scholar]
- 75.Takai K, Gamo T, Tsunogai U, Nakayama N, Hirayama H, Nealson KH, Horikoshi K. 2004. Geochemical and microbiological evidence for a hydrogen-based, hyperthermophilic subsurface lithoautotrophic microbial ecosystem (HyperSLiME) beneath an active deep-sea hydrothermal field. Extremophiles 8, 269–282. ( 10.1007/s00792-004-0386-3) [DOI] [PubMed] [Google Scholar]
- 76.Charlou JL, Fouquet Y, Donval JP, Auzende JM, Jean-Baptiste P, Stievenard M, Michel S. 1996. Mineral and gas chemistry of hydrothermal fluids on an ultrafast spreading ridge: East Pacific Rise, 17° to 19°S (Naudur cruise, 1993) phase separation processes controlled by volcanic and tectonic activity. J. Geophys. Res. 101, 15 899–15 919. ( 10.1029/96JB00880) [DOI] [Google Scholar]
- 77.Merlivat L, Pineau F, Javoy M. 1987. Hydrothermal vent waters at 13°N on the East Pacific Rise: isotopic composition and gas concentration. Earth Planet. Sci. Lett. 84, 100–108. ( 10.1016/0012-821X(87)90180-4) [DOI] [Google Scholar]
- 78.Evans WC, White LD, Rapp JB. 1988. Geochemistry of some gases in hydrothermal fluids from the southern Juan de Fuca Ridge. J. Geophys. Res. 93, 15 305–15 313. ( 10.1029/JB093iB12p15305) [DOI] [Google Scholar]
- 79.Welhan JA, Craig H. 1983. Methane, hydrogen and helium in hydrothermal fluids at 21°N on the East Pacific Rise. In Hydrothermal processes at seafloor spreading centers (eds Rona PA, Boström K, Laubier L, Smith KL), pp. 391–409. Boston, MA: Springer. [Google Scholar]
- 80.Kelley DS, et al. 2005. A serpentinite-hosted ecosystem: the Lost City hydrothermal field. Science 307, 1428–1434. ( 10.1126/science.1102556) [DOI] [PubMed] [Google Scholar]
- 81.Charlou JL, Donval JP, Konn C, Ondréas H, Fouquet Y, Jean-Baptiste P, Fourré E. 2010. High production and fluxes of H2 and CH4 and evidence of abiotic hydrocarbon synthesis by serpentinization in ultramafic-hosted hydrothermal systems on the Mid-Atlantic Ridge. In Diversity of hydrothermal systems on slow spreading ocean ridges (eds Rona PA, Devey CW, Dyment J, Murton BJ), pp. 265–296. Washington, DC: American Geophysical Union. [Google Scholar]
- 82.Blank JG, Delaney JR, Des Marais DJ.. 1993. The concentration and isotopic composition of carbon in basaltic glasses from the Juan de Fuca Ridge, Pacific Ocean. Geochim. Cosmochim. Acta 57, 875–887. ( 10.1016/0016-7037(93)90175-V) [DOI] [PubMed] [Google Scholar]
- 83.McCollom TM, Seewald JS. 2006. Carbon isotope composition of organic compounds produced by abiotic synthesis under hydrothermal conditions. Earth Planet. Sci. Lett. 243, 74–84. ( 10.1016/j.epsl.2006.01.027) [DOI] [Google Scholar]
- 84.Horita J, Berndt ME. 1999. Abiogenic methane formation and isotopic fractionation under hydrothermal conditions. Science 2, 1055–1057. ( 10.1126/science.285.5430.1055) [DOI] [PubMed] [Google Scholar]
- 85.Sherwood Lollar B, Lacrampe-Couloume G, Voglesonger K, Onstott TC, Pratt LM, Slater GF. 2008. Isotopic signatures of CH4 and higher hydrocarbon gases from Precambrian Shield sites: a model for abiogenic polymerization of hydrocarbons. Geochim. Cosmochim. Acta 72, 4778–4795. ( 10.1016/j.gca.2008.07.004) [DOI] [Google Scholar]
- 86.Etiope G, Sherwood Lollar B. 2013. Abiotic methane production on earth. Rev. Geophys. 51, 276–299. ( 10.1002/rog.20011) [DOI] [Google Scholar]
- 87.Pineau F, Mathez EA. 1990. Carbon isotopes in xenoliths from the Hualalai Volcano, Hawaii, and the generation of isotopic variability. Geochim. Cosmochim. Acta 54, 217–227. ( 10.1016/0016-7037(90)90209-4) [DOI] [Google Scholar]
- 88.Tingle TN, Hochella MF, Becker CH, Malhotra R. 1990. Organic compounds on crack surfaces in olivine from San Carlos, Arizona and Hualalai Volcano, Hawaii. Geochim. Cosmochim. Acta 54, 477–485. ( 10.1016/0016-7037(90)90337-K) [DOI] [Google Scholar]
- 89.Tingle TN, Mathez EA, Hochella MF Jr.. 1991. Carbonaceous matter in peridotites and basalts studied by XPS, SALI, and LEED. Geochim. Cosmochim. Acta 55, 1345–1352. ( 10.1016/0016-7037(91)90312-S) [DOI] [Google Scholar]
- 90.Mathez EA. 1987. Carbonaceous matter in mantle xenoliths: composition and relevance to the isotopes. Geochim. Cosmochim. Acta 51, 2339–2347. ( 10.1016/0016-7037(87)90288-2) [DOI] [Google Scholar]
- 91.Sugisaki R, Mimura K. 1994. Mantle hydrocarbons: abiotic or biotic? Geochim. Cosmochim. Acta 58, 2527–2542. ( 10.1016/0016-7037(94)90029-9) [DOI] [PubMed] [Google Scholar]
- 92.Mathez EA, Delaney JR. 1981. The nature and distribution of carbon in submarine basalts and peridotite nodules. Earth Planet. Sci. Lett. 56, 217–232. ( 10.1016/0012-821X(81)90129-1) [DOI] [Google Scholar]
- 93.Deines P. 2002. The carbon isotope geochemistry of mantle xenoliths. Earth Sci. Rev. 58, 247–278. ( 10.1016/S0012-8252(02)00064-8) [DOI] [Google Scholar]
- 94.Delacour A, Früh-Green GL, Bernasconi SM, Schaeffer P, Kelley DS. 2008. Carbon geochemistry of serpentinites in the Lost City Hydrothermal System (30°N, MAR). Geochim. Cosmochim. Acta 72, 3681–3702. ( 10.1016/j.gca.2008.04.039) [DOI] [Google Scholar]
- 95.Shilobreeva S, Martinez I, Busigny V, Agrinier P, Laverne C. 2011. Insights into C and H storage in the altered oceanic crust: Results from ODP/IODP Hole 1256D. Geochim. Cosmochim. Acta 75, 2237–2255. ( 10.1016/j.gca.2010.11.027) [DOI] [Google Scholar]
- 96.Schwarzenbach EM, Früh-Green GL, Bernasconi SM, Alt JC, Plas A. 2013. Serpentinization and carbon sequestration: a study of two ancient peridotite-hosted hydrothermal systems. Chem. Geol. 351, 115–133. ( 10.1016/j.chemgeo.2013.05.016) [DOI] [Google Scholar]
- 97.Klein F, Humphris SE, Guo W, Schubotz F, Schwarzenbach EM, Orsi WD. 2015. Fluid mixing and the deep biosphere of a fossil Lost City-type hydrothermal system at the Iberia Margin. Proc. Natl Acad. Sci. USA 112, 12 036–12 041. ( 10.1073/pnas.1504674112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McCollom TM, Seewald JS. 2001. A reassessment of the potential for reduction of dissolved CO2 to hydrocarbons during serpentinization of olivine. Geochim. Cosmochim. Acta 65, 3769–3778. ( 10.1016/S0016-7037(01)00655-X) [DOI] [Google Scholar]
- 99.McCollom TM, Seewald JS. 2003. Experimental constraints on the hydrothermal reactivity of organic acids and acid anions: I. Formic acid and formate. Geochim. Cosmochim. Acta 67, 3625–3644. ( 10.1016/S0016-7037(03)00136-4) [DOI] [Google Scholar]
- 100.Seewald JS, Zolotov MY, McCollom T. 2006. Experimental investigation of single carbon compounds under hydrothermal conditions. Geochim. Cosmochim. Acta 70, 446–460. ( 10.1016/j.gca.2005.09.002) [DOI] [Google Scholar]
- 101.Grozeva NG, Klein F, Seewald JS, Sylva SP. 2017. Experimental study of carbonate formation in oceanic peridotite. Geochim. Cosmochim. Acta 199, 264–286. ( 10.1016/j.gca.2016.10.052) [DOI] [Google Scholar]
- 102.Kadko D, Butterfield DA. 1998. The relationship of hydrothermal fluid composition and crustal residence time to maturity of vent fields on the Juan de Fuca Ridge. Geochim. Cosmochim. Acta 62, 1521–1533. ( 10.1016/S0016-7037(98)00088-X) [DOI] [Google Scholar]
- 103.Kadko D, Moore W. 1988. Radiochemical constraints on the crustal residence time of submarine hydrothermal fluids: Endeavor Ridge. Geochim. Cosmochim. Acta 52, 659–668. ( 10.1016/0016-7037(88)90328-6) [DOI] [Google Scholar]
- 104.Elderfield H, Wheat CG, Mottl MJ, Monnin C, Spiro B. 1999. Fluid and geochemical transport through oceanic crust: a transect across the eastern flank of the Juan de Fuca Ridge. Earth Planet. Sci. Lett. 172, 151–165. ( 10.1016/S0012-821X(99)00191-0) [DOI] [Google Scholar]
- 105.Walker BD, McCarthy MD, Fisher AT, Guilderson TP. 2008. Dissolved inorganic carbon isotopic composition of low-temperature axial and ridge-flank hydrothermal fluids of the Juan de Fuca Ridge. Mar. Chem. 108, 123–136. ( 10.1016/j.marchem.2007.11.002) [DOI] [Google Scholar]
- 106.Wheat CG, Fisher AT. 2008. Massive, low-temperature hydrothermal flow from a basaltic outcrop on 23 Ma seafloor of the Cocos Plate: chemical constraints and implications. Geochem., Geophys. Geosyst. 9, Q12O14 ( 10.1029/2008GC002136) [DOI] [Google Scholar]
- 107.Grimes CB, John BE, Cheadle MJ, Wooden JL. 2008. Protracted construction of gabbroic crust at a slow spreading ridge: constraints from 206Pb/238U zircon ages from Atlantis Massif and IODP Hole U1309D (30°N, MAR). Geochem. Geophys. Geosyst. 9, Q08012 ( 10.1029/2008GC002063) [DOI] [Google Scholar]
- 108.Baines AG, Cheadle MJ, John BE, Grimes CB, Schwartz JJ, Wooden JL. 2009. SHRIMP Pb/U zircon ages constrain gabbroic crustal accretion at Atlantis Bank on the ultraslow-spreading Southwest Indian Ridge. Earth Planet. Sci. Lett. 287, 540–550. ( 10.1016/j.epsl.2009.09.002) [DOI] [Google Scholar]
- 109.John BE, Foster DA, Murphy JM, Cheadle MJ, Baines AG, Fanning CM, Copeland P. 2004. Determining the cooling history of in situ lower oceanic crust-Atlantis Bank, SW Indian Ridge. Earth Planet. Sci. Lett. 222, 145–160. ( 10.1016/j.epsl.2004.02.014) [DOI] [Google Scholar]
- 110.Schwartz JJ, John BE, Cheadle MJ, Reiners PW, Baines AG. 2009. Cooling history of Atlantis Bank oceanic core complex: evidence for hydrothermal activity 2.6 Ma off axis. Geochem. Geophys. Geosyst. 10, Q08020 ( 10.1029/2009GC002466) [DOI] [Google Scholar]
- 111.Grimes CB, Cheadle MJ, John BE, Reiners PW, Wooden JL. 2011. Cooling rates and the depth of detachment faulting at oceanic core complexes: evidence from zircon Pb/U and (U-Th)/He ages. Geochem. Geophys. Geosyst. 12, Q0AG01 ( 10.1029/2010GC003391) [DOI] [Google Scholar]
- 112.Buck WR, Lavier LL, Poliakov ANB. 2005. Modes of faulting at mid-ocean ridges. Nature 434, 719–723. ( 10.1038/nature03358) [DOI] [PubMed] [Google Scholar]
- 113.Schoolmeesters N, Cheadle MJ, John BE, Reiners PW, Gee J, Grimes CB. 2012. The cooling history and the depth of detachment faulting at the Atlantis Massif oceanic core complex. Geochem. Geophys. Geosyst. 13, Q0AG12 ( 10.1029/2012GC004314) [DOI] [Google Scholar]
- 114.DeMartin BJ, Sohn RA, Canales JP, Humphris SE. 2007. Kinematics and geometry of active detachment faulting beneath the Trans-Atlantic geotraverse (TAG) hydrothermal field on the Mid-Atlantic Ridge. Geology 35, 711–714. ( 10.1130/G23718A.1) [DOI] [Google Scholar]
- 115.Parnell-Turner R, Sohn RA, Peirce C, Reston TJ, MacLeod CJ, Searle RC, Simão NM. 2017. Oceanic detachment faults generate compression in extension. Geology 45, 923–926. ( 10.1130/G39232.1) [DOI] [Google Scholar]
- 116.Seewald JS. 2001. Aqueous geochemistry of low molecular weight hydrocarbons at elevated temperatures and pressures: constraints from mineral buffered laboratory experiments. Geochim. Cosmochim. Acta 65, 1641–1664. ( 10.1016/S0016-7037(01)00544-0) [DOI] [Google Scholar]
- 117.Kurz MD, Warren JM, Curtice J. 2009. Mantle deformation and noble gases: helium and neon in oceanic mylonites. Chem. Geol. 266, 10–18. ( 10.1016/j.chemgeo.2008.12.018) [DOI] [Google Scholar]
- 118.Olive JA, Crone TJ. 2018. Smoke without fire: How long can thermal cracking sustain hydrothermal circulation in the absence of magmatic heat? J. Geophys. Res. Solid Earth 123, 4561–4581, ( 10.1029/2017JB014900) [DOI] [Google Scholar]
- 119.Hodgkinson MRS, Webber AP, Roberts S, Mills RA, Connelly DP, Murton BJ. 2015. Talc-dominated seafloor deposits reveal a new class of hydrothermal system. Nat. Commun. 6, 10150 ( 10.1038/ncomms10150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Schmidt K, Koschinsky A, Garbe-Schönberg D, de Carvalho LM, Seifert R. 2007. Geochemistry of hydrothermal fluids from the ultramafic-hosted Logatchev hydrothermal field, 15°N on the Mid-Atlantic Ridge: temporal and spatial investigation. Chem. Geol. 242, 1–21. ( 10.1016/j.chemgeo.2007.01.023) [DOI] [Google Scholar]
- 121.Abrajano TA. 1984. Igneous petrology and low temperature geochemistry of the Acoje massif, Zambales Ophiolite, Philippines: mineralogical, isotopic, and mixed-volatile equilibria. PhD thesis, Washington University in St. Louis, USA. [Google Scholar]
- 122.Sturchio NC, Abrajano TA, Murowchick JB, Muehlenbachs K. 1989. Serpentinization of the Acoje massif, Zambales ophiolite, Philippines: hydrogen and oxygen isotope geochemistry. Tectonophysics 168, 101–107. ( 10.1016/0040-1951(89)90370-3) [DOI] [Google Scholar]
- 123.Etiope G, Schoell M. 2014. Abiotic gas: atypical, but not rare. Elements 10, 291–296. ( 10.2113/gselements.10.4.291) [DOI] [Google Scholar]
- 124.Young ED, et al. 2017. The relative abundances of resolved 12CH2D2 and 13CH3D and mechanisms controlling isotopic bond ordering in abiotic and biotic methane gases. Geochim. Cosmochim. Acta 203, 235–264. ( 10.1016/J.GCA.2016.12.041) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article are included in tables and figures within this manuscript.