Abstract
Digital biomarkers are helping to reshape the understanding of health and disease, which will have an impact in how an individual's relationship to the environment is assessed, how research is conducted, and how treatment effectiveness is determined. In particular, this article highlights key activities by the pharmaceutical industry as they explore the utility and relevance of digital biomarkers across the value chain. Lastly, this paper will discuss how digital biomarkers, in conjunction with digital environmental markers, will pave the way for the creation of healthy spaces that more directly improve patient outcomes.
Keywords: Digital biomarkers, Digital health, Built environment
Introduction
Sensor technology has broad applications in research and healthcare with devices built to monitor blood pressure, skin conductance, mobility, posture, oxygen levels, respiration, sleep, temperature, heart rate, and more [1]. Particularly, many of these devices are being used by the pharmaceutical industry and, as a result, have increased their confidence in the value of digital biomarkers [2] (Table 1).
Table 1.
Examples of commercial and R&D sensors for tracking vital signs and behavior [2]
| Vital sign or behavior | Wearable location (form factor) | Environment location |
|---|---|---|
| Blood pressure | Arm (cuff) | Bed |
| Wrist (watch) Chest (clothing) | Chair | |
| ECG | Chest (phone case, clothing, necklace) | Bed |
| Waist (belt) | Chair | |
| Heart rate | Finger (ring) | Bed |
| Ear (headset, earlobe clip) | Chair | |
| Chest (phone case) | Camera | |
| Nose bridge (glasses) | (face, mirror) | |
| Forehead (hat) | ||
| Pulse oximetry | Finger (ring, glove) | |
| Forehead (mounted sensor) | ||
| Blood glucose | Waist (device) | |
| Eye (contact lens) | ||
| EEG | Head (headset) | |
| Breathing | Chest (device, vest) | Camera (face) |
| Sleep | Wrist (watch) | Bed, mattress |
| Ankle (watch) | Bedside | |
| Head (headset) | Camera | |
| Chest (device) | ||
| Body temperature | Wrist (watch) | Camera |
| Forehead (patch) | ||
| Motion | Wrist (watch) | Camera |
| Ankle (watch) | ||
| Foot (shoe) | ||
| Leg (stocking) | ||
| Waist (belt) | ||
| Chest (necklace) | ||
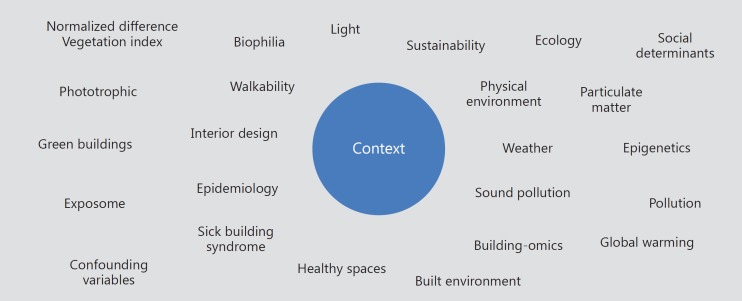
The utilization of such devices will only increase as market forecasts expect the remote monitoring and sensor-based markets to grow rapidly. Such added levels of connectivity will enable a better understanding of human behavior, digital biomarkers, and the role of physical environment. This additional environmental quantification will bring with it additional ter minology from disciplines such as public health, about which pharmaceutical companies are not accustomed to routinely thinking (Fig. 1).
Fig. 1.
Digital biomarker contextual map.
Once sensor implementation challenges – accuracy, privacy, and data integration to name a few – are overcome, a growing body of evidence will highlight the many ways continuous measurement of physiology via biosensors can play a role in managing health and improving access to care [3]. We already see how technology use, via social media, leaves a digital trace, a reflection, of how an individual may be feeling or subtly changing over time, as with sleep or insomnia [4]. Other research shows how such traces correspond to real-world mobility patterns and reflect urban land use [5].
Care delivery has improved as a result of the new and novel data emerging from digital biomarkers, which have enhanced our knowledge of disease progression and helped to identify patient subtypes [6], but overall progress has been minimal to date. Less explored is the context or environment surrounding the capture of digital biomarkers, which can help to strengthen their signal but also provide a glimpse into the everyday life of patient experience.
This discussion will hone in on the particular set of opportunities and incentives that exist in the pharmaceutical industry for digital biomarker research to understand new forms of data, improve research, and measure real-world effectiveness of medications. Additionally, this paper will explore how digital biomarkers can serve as a catalyst for directly modifying the environment to improve health and well-being.
Discussion
The Pharmaceutical Business Model Is Evolving
The pharmaceutical industry is undergoing a business model transformation that is expanding its focus from a product-based company into a more services-oriented model [7]. Competitive forces and the desire to differentiate in the market are primary reasons why service models are being employed. To name a few examples, Verily and Sanofi partnered to create Onduo, a diabetes-based disease management program, while Teva Pharmaceuticals and Roche have taken a more acquisition-based strategy – Teva Pharmaceuticals acquiring Gecko Health Innovations for improved medication adherence and Roche Pharmaceuticals acquiring mySugr to further strengthen its service offering in diabetes.
The hope is that in developing solutions that enhance the patient experience, the combination of drug therapy plus digital health services will improve patient outcomes but also further differentiate the total offering. Regulatory bodies, such as the FDA, have been providing further clarity on new forms of evidence requirements through the 21st Century Cures Act and more recently the creation of their Digital Health Program, as part of the Center for Devices and Radiological Health (CDRH) [8]. These initiatives further enable a more rapid transition by pharma towards new business model exploration.
From a research perspective, the pharmaceutical industry is incentivized to understand and utilize new forms of data and methods to improve its clinical trials. However, when it comes to incorporating novel forms of data that might provide a context to treatments in a study setting, pharmaceutical companies find themselves in a conundrum – include exploratory components and risk a more complex trial, or keep a trial streamlined but potentially miss novel insights by purposely excluding novel data sources or devices. The interplay of these factors has been well documented [9].
From a commercial perspective, pharmaceutical companies face increasing pressure from payers to justify the value of their therapies based on how they perform in the real world. Such real-world evidence represents a way to ensure that medicines are actually benefitting patients, and pharmaceutical companies are paying attention. A recent survey of 15 life sciences companies shows how real-world evidence is a priority, with over 50% of respondents said to be investing in their real-world evidence programs in order to enhance their capability [10].
How Pharmaceutical Companies Are Involved with Digital Biomarkers
In some diseases, digital biomarkers can improve our understanding of the natural history of disease through more continuous measurement of objective health data [11]. Such an approach enhances the often limited clinical encounters patients have with their clinicians, which is particularly relevant in diseases where symptom presence and severity is more variable than initially expected, or in diseases that align to measures captured through current devices, such as mobility in neurodegenerative conditions [12].
Pharmaceutical companies are exploring pilot studies and strategic initiatives across a number of different therapeutic areas, with a range of partners and devices. In some cases pilot studies are meant to test the feasibility of a research process involving wearables, whereas in others the focus is to embark on large, multi-year studies to help shape our knowledge of disease progression through various means, including digital biomarkers.
A representative list of examples is shown below:
Respiratory Conditions. Through its sensor-outfitted inhalers, Propeller Health is able to identify environmental triggers for patients with asthma, chronic obstructive pulmonary disorder, and other respiratory conditions [13]. These efforts have resulted in Asthma Hotspot maps across the US including Louisville, KY. Propeller Health has partnered with numerous health systems and pharmaceutical companies, including GlaxoSmithKline, Boehringer Ingelheim, and AstraZeneca. Boehringer Ingelheim has also partnered with PatientsLikeMe in idiopathic pulmonary fibrosis to test consumer wearables in the real world [14].
Neurodegenerative Conditions. Symptom presence and severity in conditions like Par kinson disease, multiple sclerosis, and Alzheimer disease are benefitting from digital biomarker efforts. Roche built a Parkinson disease app to capture voice-related in formation, Biogen partnered with PatientsLikeMe to understand physical activity measurement in patients with multiple sclerosis, and Neurotrack measured cognitive ability remotely to assess cognition in patients with Alzheimer disease. Formal study results are not available for all, but Parkinson disease results utilizing voice have shown promise in prior efforts [12].
Cardiovascular Disease. Recent efforts to expand our understanding of cardiovascular disease beyond the Framingham Heart Study have started, notably a research grant of USD 75 million for Dr. Calum MacRae from Brigham and Women's Hospital, spearheaded by Verily Life Sciences (Mountain View, CA, USA), AstraZeneca (Cambridge, UK), and the American Heart Association. In part, the study aims to identify the factors that contribute to disease onset and outcomes along the entire patient journey, in a manner that accounts for various data sources including objective sensor information and environmental data.
Diabetes. Diabetes continues to be an area of much innovation for digital therapeutics, in many cases in partnership with pharmaceuetical companies. Omada Health (San Francisco, CA, USA) is a pioneer in bringing evidence-based digital therapeutics to the area of pre-diabetes/diabetes where behavior change approaches leverage coaching plus a host of tools enabling objective data capture. More recently, Merck & Co. and Amazon's Alexa are exploring the creation of diabetes tools, and Sanofi and Verily recently announced Onduo – with funding of USD 500 million dollars to build a next-generation diabetes platform that captures a range of data types and can be applied from research to care.
Virtual Research and Real-World Evidence Benefit from Contextual Data
As digital biomarkers, environmental context, and our need to better quantify disease merge, companies that enable studies to be done remotely without the need of a physical clinical site are proving to be a test bed for pharmaceutical innovation. These virtual research platforms, such as Science37 (Los Angeles, CA, USA), MonARC Bionetworks, Inc. (Palo Alto, CA, USA), Koneksa Health (New York City, NY, USA), Evidation Health (San Mateo, CA, USA), THREAD Research (Tustin, CA, USA), and Medidata Solutions (New York City, NY, USA), all work with pharmaceutical companies in some capacity to help spearhead goals of leveraging novel technology in research studies.
The pharmaceutical industry has come a long way since Pfizer's Overactive Bladder virtual study leveraging “electronic health tools” [15] and the use purely electronic patient-reported outcomes (ePROs). Much more is known about how to recruit patients in clinical trials, and more recently, the term electronic device-reported outcomes (eDROs) has been introduced. eDROs aim to blend objective data from sensor-driven device technologies alongside patient self-report [16]. Environmental or contextual data is not routinely captured alongside ePROs and eDROs. Evidation Health, however, is aiming to better capture real-world contextual information as part of its research platform and showing how its data can be included in real-world assessments of adherence with some success [17], among a number of other use cases.
There are tangible benefits to better capturing contextual information in all of research, but particularly in virtual research as less is known about the situational environment in which the patient may physically be residing. For example, the answers to the following types of questions can not only impact study findings in virtual research, but also the conclusions drawn from assessing the real-world effectiveness of treatments.
Does time of day or light exposure at the time of filling out a PHQ-9 impact the score?
Does ambient sound or music therapy influence outcomes?
How does weather or room temperature affect cognitive status?
How does urban design impact physical activity levels?
Is food availability impacting disease outcomes?
Omada Health has created an evidence-based diabetes prevention program that has been shown to result in weight loss and the lowering of diabetes risk [18]. The program focuses not only on digital health tracking, but also includes access to a coach to help with various lifestyle activities such as nutrition. Food more generally is being recognized as a “beyond the pill” service by pharmaceutical companies, as Merck and Celgene begin to utilize Savor Health's cancer nutrition programs [19]. Sound and music therapy as a digital biomarker is in development, with a potential impact on health measurement, but little evidence has emerged thus far.
Limitations and Barriers to Implementation
While there is much potential in applying these novel technologies and approaches across the pharmaceutical value chain, there are several implementation challenges that must be overcome. Such challenges are not altogether new, as public health researchers have addressed them in the past [20] however, there is some nuance when being applied within a pharmaceutical industry context (Table 2).
Table 2.
How pharmaceutical companies can implement digital biomarkers and account for environmental context
| Barriers to implementation | Considerations |
|---|---|
| 1 Accuracy thresholds and validity | Establish guidelines for how novel digital biomarker technology should be validated; for example, methodologies to compare objective measures to more traditional self-report measures can help increase the uptake of new technologies by researchers and clinicians |
| 2 User adoption and user experience | Excessive and continuous tracking may negatively impact user experience and result in lower engagement; prioritizing the patient experience by measuring only what matters can help reduce the measurement burden |
| 3 Technological platforms | Consumer-facing technology (e.g., smartphones, direct to consumer wearables) may be updated regularly, forcing revalidation studies; new hardware or use of case-specific hardware can help ensure technology consistency over time |
| 4 Regulatory concerns | A more agile regulatory framework can support innovation while ensuring safety; FDA's Digital Health Program is a step in the right direction; however, multiple stakeholders should participate |
| 5 Privacy and security | As person-generated health data continues to grow, the risk of data breaches becomes more significant; alleviating privacy and security concerns by establishing best practices, adhering to privacy guidelines, and ensuring patients are well informed of the risks can help increase comfort of participation |
| 6 Pharmaceutical company organization | Introducing novel technology and concepts (e.g., role of environment) into routine business operations requires top-level senior executive support and clear delineation of where such efforts live within the organization (commercial, R&D, etc.); further, ensuring that innovation efforts are tied to clear business metrics will help to better position internal efforts amongst staff |
Future
Accounting for Environment in Research and Care
Pharmaceutical companies will increasingly look to refine their use of digital biomarkers, either through in-house efforts or in partnership with virtual research platforms. Such efforts will be helped by greater ease of access to government-level data, such as the Environmental Protection Agency, the National Weather Service, and even “noise maps” from the US Bureau of Transportation Statistics [21]. In addition to accessing these types of data sources, new environmental sensing hardware is being launched, such as pollution detecting sensors from Plume Labs (Paris, France).
Treatment facilities are utilizing objective sensor data in a number of clinical settings, such as rehabilitation, with some success [22]. Such models that impact outcomes are highly relevant to pharmaceutical companies that may have a vested interest in those therapeutic areas. In addition, there are now examples of sensors being used in the home environment as part of cancer trials to enable a more real-time assessment of health status [23]. Further, research from the University of California, San Diego, has demonstrated how individuals can carry novel sensors to help quantify city-level environmental variables such as air quality, revealing how exposure levels to particulate matter can vary more than previously thought [24].
Modifying the Built Environment to Improve Health
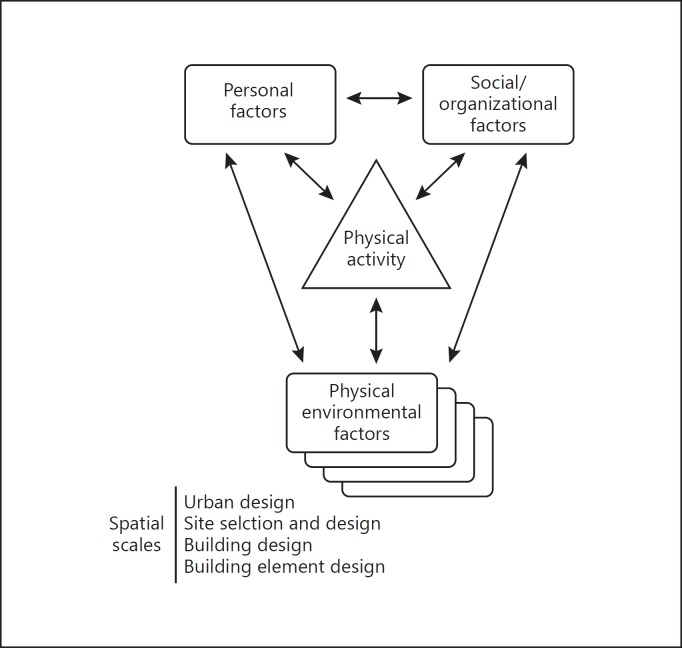
It is well documented that our built environment and urban design impact our health and physical activity [25], and areas like New York City have actively implemented urban design principles to promote health and well-being within the built environment [26]. As such initiatives continue to be implemented, the pharmaceutical industry will be able to leverage these frameworks to implement novel health and disease assessments outside the model of traditional clinical trials. Frameworks around physical activity have been well documented (Fig. 2).
Fig. 2.
A social ecological model of influences on physical activity [25].
Our increasing desire to incorporate context into our definition of health will give rise to digital environmental markers and better elucidate their relationship to digital biomarkers. In this future state, it will become possible to directly modify our built environment in concert or as a result of the device technologies that individuals carry on a day-to-day basis. Physical spaces transform into therapeutic environments where light, sound, pressure, temperature, and other parameters are fine-tuned for optimal health achievement. For example, circadian rhythms can be stabilized by circadian light measures, as is being studied at the Lighting Research Center at the Rensselaer Polytechnic Institute [27].
Stakeholders involved in the business of improving patient outcomes will either compete with new organizations creating “therapeutic environments” or play a key role in their im plementation. The pharmaceutical industry is already moving towards a services-oriented model, and if trends continue, it will likely further extend towards a more experience-driven model that involves helping to shape the environment as part of its overall offering.
The future will have to answer many questions: what is required in order to prescribe therapeutic environments? Which diseases are more likely to benefit from environmental modification? How is the built environment assessed and measured? These questions all need to be addressed thoughtfully, but as health is reframed in the context of the environment, the pharmaceutical industry will similarly need to reframe its thinking about which stakeholders have a seat at the table. Such efforts will continue to include researchers and technologists, but will likely also involve nontraditional stakeholders who are evaluating the role of public health, social determinants of health, and other variables when measuring overall health [28].
Ethics Statement
The author has no ethical conflicts to disclose.
Conflict of Interest Statement
The author provided paid consulting for MonARC Bionetworks, Inc. in 2017.
References
- 1.Li X, Dunn J, Salins D, Zhou G, Zhou W, Schüssler-Fiorenza Rose SM, et al. Digital health: tracking physiomes and activity using wearable biosensors reveals useful health-related information. PLoS Biol. 2017;15:e2001402. doi: 10.1371/journal.pbio.2001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hird N, Ghosh S, Kitano H. Digital health revolution: perfect storm or perfect opportunity for pharmaceutical R&D? Drug Discov Today. 2016;21:900–911. doi: 10.1016/j.drudis.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Chiauzzi E, Rodarte C, Dasmahapatra P. Patient-centered activity monitoring in the self-management of chronic health conditions. BMC Med. 2015;13:77. doi: 10.1186/s12916-015-0319-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain SH, Powers BW, Hawkins JB, Brownstein JS. The digital phenotype. Nat Biotechnol. 2015;33:462–463. doi: 10.1038/nbt.3223. [DOI] [PubMed] [Google Scholar]
- 5.Soliman A, Soltani K, Yin J, Padmanabhan A, Wang S. Social sensing of urban land use based on analysis of Twitter users' mobility patterns. PLos One. 2017;12:e0181657. doi: 10.1371/journal.pone.0181657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quisel T, Lee W-N, Foschini L, Observation time vs. performance in digital phenotyping Proceedings of the 1st Workshop on Digital Biomarkers – Digital Biomarkers 17. Digit Biomark. 2017 DOI 10.1145/3089341.3089347. [Google Scholar]
- 7.Nairain S, Ernst & Young LLP Life Sciences 2025 – Managing Disruptions to Gain Competitive Advantage. 2017 http://www.ey.com/gl/en/industries/life-sciences/ey-vital-signs-managing-disruptions-to-gain-com petitive-advantage (accessed April 5, 2017) [Google Scholar]
- 8.Gottlieb S. FDA Announces New Steps to Empower Consumers and Advance Digital Healthcare. 2017 https://blogs.fda.gov/fdavoice/index.php/2017/07/fda-announces-new-steps-to-empower-consumers-and-advance-digital-healthcare/ (accessed July 29, 2017) [Google Scholar]
- 9.Rosenblatt M. The large pharmaceutical company perspective. N Engl J Med. 2017;376:52–60. doi: 10.1056/NEJMra1510069. [DOI] [PubMed] [Google Scholar]
- 10.Getting Real with Real-World Evidence (RWE) 2017 https://www2.deloitte.com/us/en/pages/life-sciences-and-health-care/articles/real-world-evidence-benchmarking-survey.html (accessed April 2, 2017) [Google Scholar]
- 11.McIninch J, Datta S, DasMahapatra P, Chiauzzi E, Bhalerao R, Spector A, et al. Remote Tracking of Walking Activity in MS Patients in a Real-World Setting. 2015 http://www.neurology.org/content/84/14_Supplement/P3.209. [Google Scholar]
- 12.Kubota KJ, Chen JA, Little MA. Machine learning for large-scale wearable sensor data in Parkinson's disease: concepts, promises, pitfalls, and futures. Mov Disord. 2016;31:1314–1326. doi: 10.1002/mds.26693. [DOI] [PubMed] [Google Scholar]
- 13.Merchant RK, Inamdar R, Quade RC. Effectiveness of population health management using the propeller health asthma platform: a randomized clinical trial. J Allergy Clin Immunol Pract. 2016;4:455–463. doi: 10.1016/j.jaip.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 14.Blaser D, Bhalerao R, Brooks L. Piloting the Use of Consumer Wearable Devices in Idiopathic Pulmonary Fibrosis (IPF) 2015 https://medicinex.stanford.edu/conf/submission/view/520 (accessed June 22, 2017) [Google Scholar]
- 15.Orri M, Lipset CH, Jacobs BP, Costello AJ, Cummings SR. Web-based trial to evaluate the efficacy and safety of tolterodine ER 4mg in participants with overactive bladder: REMOTE trial. Contemp Clin Trials. 2014;38:190–197. doi: 10.1016/j.cct.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Alsumidaie M. An mHealth Perspective on Patient Centricity. 2017 http://www.appliedclinicaltrialsonline.com/mhealth-perspective-patient-centricity (accessed April 6, 2017) [Google Scholar]
- 17.Juusola J, Foschini L, Quisel T. Health Activity Tracking Is Associated with Higher Medication Adherence. SMDM 2015 - 37th Annual Meeting of the Society for Medical Decision Making 2015. 2015 https://smdm.confex.com/smdm/2015mo/webprogram/Paper9247.html (accessed July 28, 2017) [Google Scholar]
- 18.Sepah SC, Jiang L, Peters AL. Long-term outcomes of a web-based diabetes prevention program: 2-year results of a single-arm longitudinal study. J Med Internet Res. 2015;17:e92. doi: 10.2196/jmir.4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bulik BS. Merck, Celgene Go beyond the Pill with Savor's Cancer Nutrition Programs. 2017 http://www.fiercepharma.com/marketing/celgene-merck-go-beyond-pill-nutrition-programs-for-cancer-patients-thanks-to-savor (accessed July 28, 2017) [Google Scholar]
- 20.Chunara R, Wisk LE, Weitzman ER. Denominator issues for personally generated data in population health monitoring. Am J Prev Med. 2017;52:549–553. doi: 10.1016/j.amepre.2016.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dwyer C. How Noisy Is Your Neighborhood? Now There's a Map for That. 2017 http://www.npr.org/sections/thetwo-way/2017/03/23/521227214/how-noisy-is-your-neighborhood-now-theres-a-map-for-that (accessed April 6, 2017) [Google Scholar]
- 22.Wang Q, Markopoulos P, Yu B, Chen W, Timmermans A. Interactive wearable systems for upper body rehabilitation: a systematic review. J Neuroeng Rehabil. 2017;14:20. doi: 10.1186/s12984-017-0229-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patrick K, Wolszon L, Basen-Engquist KM, Demark-Wahnefried W, Prokhorov AV, Barrera S, et al. CYberinfrastructure for COmparative effectiveness REsearch (CYCORE): improving data from cancer clinical trials. Transl Behav Med. 2010;1:83–88. doi: 10.1007/s13142-010-0005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nikzad N, Rosing TŠ, Griswold WG, Verma N, Ziftci C, Bales E, et al. CitiSense. Proceedings of the Conference on Wireless Health – WH 12. Proc Wirel Health. 2012 DOI 10.1145/2448096.2448107. [Google Scholar]
- 25.Zimring C, Joseph A, Nicoll GL, Tsepas S. Influences of building design and site design on physical activity. Am J Prev Med. 2005;28:186–193. doi: 10.1016/j.amepre.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 26.Active Design Guidelines Promoting Physical Activity and Health in Design New York, City of New York. 2010 [Google Scholar]
- 27.Siegel R. “Bluish” Light May Help Alzheimer's Patients Find Bearings. 2014 http://www.npr.org/2014/02/19/279709447/bluish-light-may-help-alzheimers-patients-find-bearings (accessed April 2, 2017) [Google Scholar]
- 28.Barrett MA, Bouley TA. Need for enhanced environmental representation in the implementation of One Health. EcoHealth. 2014;12:212–219. doi: 10.1007/s10393-014-0964-5. [DOI] [PubMed] [Google Scholar]