Abstract
Aim
The aim was to conduct a systematic review to examine the literature reporting the validity and reliability of wearable physical activity monitoring in individuals with neurological disorders.
Method
A systematic search of the literature was performed using a specific search strategy in PubMed and CINAHL. A search constraint of articles published in English, including human participants, published between January 2008 and March 2017 was applied. Peer-reviewed studies which enrolled adult participants with any neurological disorder were included. For the studies which sought to explore the validity of activity monitors, the outcomes measured using the monitor were compared to a criterion measure of physical activity. The studies' methodological quality was assessed using an adapted version of the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) framework. Data extracted from each study included the following: characteristics of the study participants, study setting, devices used, study protocol/methods, outcomes measured, and the validity/reliability of measurement produced.
Results
Twenty-three studies examining the validity and reliability of 16 different monitors were included. The identified studies comprised participants with a range of different disorders of neurological origin. The available evidence suggests that biaxial or triaxial accelerometer devices positioned around the ankle produce the most accurate step count measurements in patients with neurological disorders. The findings regarding the reliability and validity of activity counts and energy expenditure are largely inconclusive in this population.
Discussion
Ankle-worn biaxial or triaxial accelerometer-type devices provide the most accurate measurement of physical activity. However, further work is required in this field before wearable activity monitoring can be more widely implemented clinically. Standardised activity monitoring protocols are required for implementing these devices in clinical trials and clinical practice, and consensus is required as to the reporting and interpretation of derived variables.
Keywords: Wearable sensor, Activity monitor, Mobility, Motor activity, Physical activity
Introduction
Regular physical activity is essential for health and well-being and has been shown to contribute to the prevention of many illnesses [1, 2, 3, 4], as well as being a vital element in the treatment, rehabilitation, and management of many conditions [5, 6, 7]. Physical activity is defined as “any bodily movement produced by skeletal muscles that results in the expenditure of energy” [8] and can be quantified either in terms of mobility (e.g., number of steps) or energy expenditure. Mobility is central to our quality of life. Mobility limitation is often the first noticeable sign of declining function and is associated with reduced independence and disability [9], longer hospital stays [10], nursing home placement [11], and mortality [9]. Measuring physical activity, and particularly mobility, allows clinicians to understand a patient's functional ability and to decide upon treatment or prognosis. Physical activity and mobility have traditionally been assessed by questionnaires, surveys, and activity diaries. These assessment methods are easy to administer to large groups and can be performed at low cost [12]. However, the subjectivity of these tests means that they have limitations [13]. They may lack the precision needed to detect small changes in physical activity and mobility, and the granularity to characterise daily fluctuations in disease severity. They may also be vulnerable to error caused by manual input either by the patient or the investigator/clinician.
The proliferation of unobtrusive, wearable devices has made it easier to capture objective data relevant to physical activity and mobility. Wearable monitors, which are used to estimate physical activity and mobility, can be broadly classified into one of three types: pedometers, accelerometers, and multisensor systems. Pedometers (e.g., Yamax Digi-Walker) estimate the number of steps taken through mechanical (using a spring-mounted level arm) or digital measurements in only the vertical plane [14]. Accelerometers (e.g., RT3 accelerometer) detect acceleration in one (uniaxial), two (biaxial), or three (triaxial) directions and can determine the quantity and intensity of movement [14]. Multisensor systems (e.g., SenseWear Armband) combine accelerometry with other sensors measuring data such as heart rate, galvanic skin response, or temperature, yielding more data to base physical activity estimations upon.
Using body-worn activity monitors may provide a more robust, objective, and detailed method of assessing physical activity and mobility than traditional assessment methods such as questionnaires and standardised tests. The objective data provided may support the clinical decision-making process, assisting clinicians to better visualise changes in motor function. Furthermore, utilising wearable activity monitors permits continuous patient monitoring by allowing data collection in the patient's own home [15]. These out-of-clinic data may provide a more accurate representation of the patient's ability, as some patients perform better in the clinical environment when under the observation of a clinician [16], while others perform better in the familiar environment of their own home. This approach also has the potential to reduce the burden on both the patient and the clinical site by decreasing the utilisation of valuable resources.
Recent years have witnessed a significant growth in the array of activity monitors with considerable clinical potential. However, despite their potential, they have not yet been widely employed in clinical practice. This may be due to the fact that there is relatively little evidence regarding the accuracy of these activity monitors, and the lack of regulatory approval for many devices. Clinicians may also have concerns related to data privacy and the security of the data produced by these devices. Other cited barriers to their clinical utilisation include the lack of understanding of how to summarise the data gathered to produce meaningful outcome measures that can inform the clinical decision-making process, and also the lack of standards for implementing these devices clinically [17].
A number of reviews have been published which have examined the validity and reliability of using wearable sensors to measure physical activity in chronic lung disease and in stroke patients [14, 18, 19]. The studies included in these reviews were highly heterogeneous in terms of the type of activity monitor used, the activity monitor outcome reported, and the methods used for data collection and analysis. Nonetheless, the evidence presented suggests that multiaxial accelerometer or multisensor devices appear to produce the most valid and reliable data about physical activity in chronic disease populations [14, 18, 19]. A recent systematic review concluded that remote physical activity monitoring is feasible in individuals with neurological diseases, including those with moderate-to-severe disability [20]. Another recent review which evaluated a range of wearable and non-wearable devices for objectively measuring a range of motor symptoms in Parkinson disease (PD) highlighted that while a number of devices can be recommended, further clinimetric testing and clinical validation are required [21]. As yet, no review has broadly summarised the evidence regarding the validity and reliability of wearable activity monitoring in patients with neurological conditions for clinical use. The validity and reliability of activity monitoring in other populations may not translate easily to individuals with neurological diseases, as activity monitoring in individuals with neurological diseases may be complicated by a wide range of neurological impairments such as gait abnormalities [22], weakness [23], spasticity [24], or tremor [25]. For example, it has been demonstrated that gait parameters such as speed and distance can be accurately estimated using a triaxial accelerometer device in healthy adults [26], but the accuracy is not as high in individuals with PD while using the same device [27]. Therefore it is important to explore the validity and reliability of activity monitors in this population before these monitors can be widely implemented in clinical trials and practice.
The aim was to conduct a systematic review to examine the literature reporting on the validity and reliability of activity monitoring in individuals with neurological disorders. This review sought to explore the range of activity monitors that have been evaluated in clinical research studies and to explore the outcome measures of physical activity produced by these monitors. The focus of this review was on the clinical application of activity monitors and was not concerned with studies reporting early developments or validations of algorithms for activity monitoring devices.
Materials and Methods
This review was conducted and is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [28].
Data Sources
The PubMed and CINAHL electronic databases were searched to retrieve relevant articles. These databases were chosen based on the method used in previous literature reviews published in the field [14, 19, 29]. Pilot searches conducted in other electronic databases did not yield applicable results. A search constraint of articles published in the English language, including human participants, between January 2008 and March 2017 was applied. Due to the rapid development of technology in this field, this time frame was selected so as to limit the activity monitors studied to those which are still commercially available.
Search Strategy
The search in PubMed for relevant studies was performed using the free-text and MeSH terms outlined in Appendix 1. The search terms used in the PubMed search were modified for the CINAHL database. The citation lists from all the included studies were also searched, and a search of breadcrumb-related articles was also performed. The search strategy used was developed in consultation with a librarian.
Study Selection
The focus of this review was on an examination of the validity and/or reliability of the range of activity monitors that have been clinically utilised to quantify physical activity and mobility in patients with neurological disorders. Validity and reliability measures are referred to as psychometric properties. Validity refers to how well a test measures what it is purported to measure. Criterion validity was explored in this study, i.e., comparing the activity monitor measurement to a criterion measure of physical activity/mobility. Reliability is the degree to which an assessment tool produces stable and consistent results. The reliability or validity of an assessment tool is indicated by a coefficient, such as the intraclass coefficient or Pearson's correlation coefficient (where values ≥0.90 generally indicate excellent, 0.75–0.90 good, 0.50–0.75 adequate, and <0.50 poor results [30]).
Validation and reliability studies of physical activity monitors are highly heterogeneous. Therefore the inclusion/exclusion criteria outlined in Table 1 were applied for the selection of studies included in this review. Studies performed in laboratory, clinical, or free-living (home/community) environments were included. The abstracts and titles of the studies identified from the search process were assessed and screened by the authors in order to decide whether they were suitable for inclusion [19]. The full-text articles of all potentially relevant studies were then retrieved and assessed for inclusion by the authors based on the defined study inclusion/exclusion criteria.
Table 1.
Study inclusion/exclusion criteria
| Inclusion | Exclusion | |
|---|---|---|
| Study type | Peer-reviewed original papers that evaluated the validity and/or reliability of a wearable activity monitor | Review articles |
| Case reports/intervention studies using an activity monitor as a component of an intervention or to measure the impact of an intervention | ||
| Papers that evaluated the validity of physical activity classification models/algorithms or papers that outlined algorithm development | ||
| Outcomes measured | Measurements which quantify the amount/level of physical activity/mobility, e.g., step counts, distance, intensity of physical activity, walking speed, and energy expenditure | Measurements of postural control Posture classification measurements |
| Population | Adult participants with a neurological condition/disorder, i.e., any disease of the brain, spine, and the nerves that connect them | Healthy volunteers, paediatric participants (<18 years of age), athletic populations |
| Sensor types | Any consumer, research, or medical-grade wearable sensor used to measure physical activity | Smartphone applications, ambient sensors |
| Comparison | Comparison with criterion measure of physical activity/mobility | No comparator |
Data Extraction
Data extracted from each study included the following: characteristics of the study par ticipants, study setting, devices used (make, model, size, weight, and manufacturer), study protocol/methods, outcomes measured, and the validity/reliability of measurement produced. The methodological quality of the validation studies included in this review was assessed using an adapted version of the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) framework [18, 31]. Due to the heterogeneity of the included studies, a meta-analysis was not possible.
Results
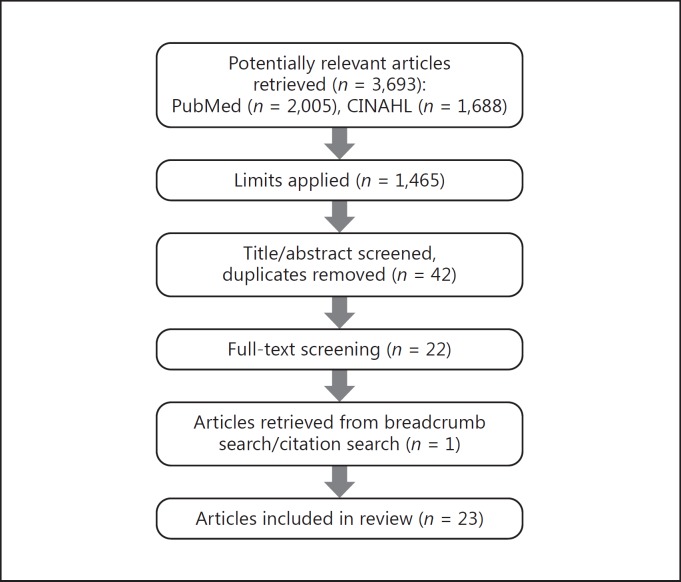
A total of 3,693 potentially relevant articles were retrieved from the literature search performed in the PubMed and CINAHL electronic databases. Figure 1 depicts the study flow throughout the review process. Following a review of the title and abstract and a removal of duplicates, 42 articles remained. The list was subsequently reduced to 22 articles following a review of the full text. One article was retrieved from the citation search/breadcrumb search of the included articles. This yielded a final total of 23 articles for inclusion in this review.
Fig. 1.
Flow chart of the review process.
A detailed description of each study included in this review is presented in Table 2. The methodological quality of each validation study is presented in Appendix 2. Overall the methodological quality of the studies examined was high, with each scoring ≥7. Of the assessed studies, 10 included participants with multiple sclerosis [32, 33, 34, 35, 36, 37, 38, 39, 40, 41], 7 included participants after stroke [32, 33, 42, 43, 44, 45, 46], 4 included participants with PD [27, 33, 47, 48], 3 included participants with a spinal cord injury [32, 49, 50], 2 included participants with cerebral palsy [51, 52], 2 included participants with a traumatic brain injury [32, 42], and participants with Rett syndrome [53] and muscular dystrophy [32] were included in 1 study each. The most frequently used monitors included the StepWatch activity monitor (a biaxial accelerometer) [39, 41, 42, 44, 53], the ActiGraph GT3X (a triaxial accelerometer) [35, 39, 40, 50], the SWA (a multisensor device) [46, 49, 52], and the Digi-Walker pedometer [27, 32, 34, 42]. Table 3 outlines the monitors that were used in the included studies, the outcome measures produced, and the findings reported regarding the validity and reliability of the measurements produced.
Table 2.
Studies included in the systematic review
| Populationand setting | Device | Aim and methods | Endpoints | Findings | |
|---|---|---|---|---|---|
| Bania [51] | 10 participants with spastic diplegic CP (6 male, age: 18. 6±2.7years) were included in the validation study | An activPAL monitor was placed mid-thigh on the frontal aspect of the thigh | To determine the criterion validity and the retest reliability of the activPAL in adolescents and young adults with diplegic CP | Time spent standing | Criterion validityTime standing: R 2 0.97; MD (SD)–0.06 (0.2); 95% LOA–8.6 to 2.6 Time sitting: R 2 0.96; MD (SD) 0.5 (0.5); 95% LOA 1.3 to 20 Step count: R 2 0.99; MD (SD)–13.8 (11.8); 95% LOA 3.3 to 0.8 |
| Time spent sitting | |||||
| Criterion validity study | |||||
| 24 participants with spastic diplegic CP (11 male, age: 18.7±2.9 years) were included in the retest reliability study | The participants were recorded with a video camera for 12 min, during which they were asked to stand up and sit down twice, as well as to complete a 6MWT | Step count (steps/day) | |||
| Criterion: an observer, blinded to the activPAL output, watched the videos and counted the number of steps and the time spent in sitting and standing | Retest reliabilityTime standing: ICC 0.60; MD (SD) −0.31 (1.10); 95% CI–0.081 to 0.19 Time sitting: ICC 0.66; MD (SD) 0.37 (1.28); 95% CI–0.021 to 0.95 Step count: ICC 0.87; MD (SD)–411 (1,301); 95% CI–1,044 to 142 | ||||
| Clinical setting | |||||
| Retest reliability study | |||||
| The participants wore the activPAL monitor for 7 days, and for another 7 days 12 weeks later. They were advised to wear it at all times except during bathing and swimming | |||||
| A daily log was also given to the participants in which to note their activities during the 7-day period | |||||
| Dijkstra et al. [27] | 32 PD patients (17 male, age: 67.3±6.6 years, BMI: 26.7±4.0) | A DynaPort was placed in a belt and positioned between and above the posterior superior iliac spines | To determine the accuracy of an accelerometer system for detecting walking periods and steps in subjects with PD during controlled indoor walking tasks | Gait duration | Gait duration during all tasksAbsolute percentage error DynaPort: 11.1±4.5 |
| Step count | |||||
| Research laboratory | Step counts during all tasksAbsolute percentage error (ICC) DynaPort: 6.9±3.0 (0.98) Left Digi-Walker: 11.1±9.0 (0.87) Right Digi-Walker: 16.3±13.7 (0.75) | ||||
| 8 walking tasks: (1) walking 15 m at the preferred walking speed; (2) walking 15 m slower than preferred; (3) walking 15 m faster than preferred; (4) walking 10 m at one's own pace; (5) walking 5 m at one's own pace; (6) walking 3 m at one's own pace; (7) walking 15 m at the preferred speed while counting backward from 100 to 0 in steps of 5; and (8) walking 15 m at the preferred speed while carrying a tray with two cups filled with water. All tasks were videotaped. The gait characteristics as observed on video were taken as the gold standard | |||||
| A Yamax Digi-Walker (SW-200) was attached to the belt at the left and the right hip | |||||
| Populationand setting | Device | Aim and methods | Endpoints | Findings | |
| Downset al. [53] | 12femaleparticipantswith Rettsyndrome(age:12.9±8.0years) | A StepWatch activity monitor was attached around the right ankle proximal to the lateral malleolus with an elastic and Velcro strap | To assess the accuracy of the StepWatch activity monitor and investigate relationships between daily step counts, gross motor skills, and age | Step count | Step count: MD 0 steps/min; LOA–10 to 10 |
| Agreement did not differ with the level of general (p = 0.389) or complex gross motor skills (p = 0.221) | |||||
| Home environment | Data collection comprised two parts: | ||||
| 1. The participants were videotaped while performing normal walking activities in indoor and outdoor settings for periods of up to 30 min. Gross motor skills were assessed with the Gross Motor Scale for Rett syndrome | |||||
| The participants were less active than their healthy peers (difference 6,086 steps/day;p = 0.001), and physical activity was significantly greater in those who were younger and with greater levels of motor skills | |||||
| 2. The participants wore the StepWatch during 7 consecutive days. Caregivers recorded the time the StepWatch was fitted in the morning and when removed at bedtime | |||||
| Elsworthet al. [32] | 43 adults with neurological conditions (n = 20 stroke,n = 16 MS,n = 5 muscular dystrophy,n = 1 SCI,n = 1 TBI; 26 male, 17 female, age: 54±13 years, BMI: 26±4)a | A Yamax Digi-Walker (SW-200) was positioned midway between the iliac crest and umbilicus over the right leg in line with the midline of the thigh | To assess the accuracy of a pedometer in measuring step counts in neurologically impaired individuals walking at a self-selected walking speed | Step count | All: MD (SD) 27 (11); ICC (95% Cl) 0.73 (0.23 to 0.93);p = 0.003 |
| Stroke: MD (SD) 31 (113); ICC (95% CI) 0.58 (0.20 to 0.81);p = 0.026 | |||||
| The participants were asked to walk along a 16-m walkway in a quiet corridor at their normal speed using walking aids as required for a period of 2 min. An observer manually recorded the participants' step counts using a manual step counter | MS: MD (SD) 23 (81); ICC (95% CI) 0.84 (0.60 to 0.94);p = 0.044 | ||||
| Clinical setting | |||||
| Muscular dystrophy: MD (SD) 7.2 (176); ICC (95% CI) 0.38 (−0.62 to 0.91);p = 0.866 | |||||
| SCI step count difference: 130 steps | |||||
| TBI step count difference: 5 steps | |||||
| Fulk et al. [42] | 30strokeparticipants (15male, age:61.6±10.4years)and 20 TBI participants (19 male, 1female, age:40.3±11.6years) | A Fitbit Ultra and Yamax Digi-Walker (SW-701)were worn on the belt or waistband on the side of the less affected leg | To examine the accuracy of two commercial activity monitors, the Fitbit Ultra and the Nike FuelBand, in identifying stepping activity in people with stroke and TBI and to compare the accuracy of these two activity monitors with that of the StepWatch Activity Monitor and the Yamax Digi-Walker | Step count | All participantsStepWatch: MD (95% CI) 4.7 (1.11 to 8.35); ICC (95% CI) 0.97 (0.92 to 0.99) |
| Fitbit Ultra: MD (95% CI)–9.7 (−0.12 to −19.28); ICC (95% CI) 0.73 (0.56 to 0.83) | |||||
| Researchlaboratory | A Nike FuelBand was worn on the wrist of the less affected arm | ||||
| The participants wore all four activity monitors simultaneously and performed the 2MWT, during which they were videotaped. A researcher counted the steps taken by the participants from the video record | Digi-Walker: MD (95% CI)–28.8 (−12.66 to −43.50); ICC (95% CI) 0.42 (0.14 to 0.63) | ||||
| A StepWatch Activity Monitor was strapped above the lateral malleolus of the less affected leg | |||||
| Nike FuelBand: MD (95% CI)–66.2 (−43.63 to −88.67); ICC (95% CI) 0.20 (−0.076 to 0.46) | |||||
| Haleet al. [33] | 47 participants (17 male, age:63.7±15.5years;MSn = 11, PDn = 7,stroken = 20,controlsn = 9) | An RT3 accelerometer was attached to the waistbelt in a central back position | To investigate the reliability of a triaxial accelerometer to measure physical activity in adults with and without neurologic dysfunction | Activity count | Test-retest reliability activity counts over the two test periodsAll: ICC (95% CI) 0.85 (0.74 to 0.91);p = 0.00; SEM 23% |
| The participants wore the RT3 during waking hours (except while bathing, swimming, or lying in bed) for 7 consecutive days while maintaining their typical weekly schedules. They were instructed to complete a daily activity log. After 7 days a 7-day recall questionnaire was administered. 8 weeks later the procedure was repeated, using the same RT3 unit. The mean daily data for the first 3 days and for 7 days of measuring were calculated | |||||
| Home environment | Activity counts7-day: 124,831±74,373; 3-day: 132,252±92,394;p = 0.03 | ||||
| RT3 data correlation with the 7-day recall questionnaire (R = 0.01, 1%) | |||||
| Hiremathand Ding[49] | 24paraplegicparticipants (19 male, age: 41.4±11.4 years) | An SWA was worn on the right upper arm over the triceps muscle | To evaluate the performance of the SWA and RT3 activity monitors in estimating EE in manual wheelchair users with paraplegia for a variety of physical activities | EE | SWA Absolute percentage error in EE range: 24.4 to 125.8% ICC (LOA) 0.62 (0.49 to 0.73);p<0.05 (for all activities) |
| Researchlaboratory | |||||
| An RT3 was secured around the waist with a belt clip holster | The activity session consisted of resting and three 8-min activity routines: wheelchair propulsion, arm ergometer exercise, and deskwork. The criterion of EE was measured with a K4b2 portable metabolic cart | RT3 Absolute percentage error in EE range: 22.0 to 52.8% ICC (LOA) 0.64 (0.51 to 0.73);p<0.05 (for all activities) | |||
| Population and setting | Device | Aim and methods | Endpoints | Findings | |
| Kayes et al. [34] | 31 participants with MS (10 male, median age: 50 years, range: 34–80) Research laboratory | An Actical accelerometer was mounted onto waistbands and fitted around the participants' waists over the iliac crest of the left hip | To explore the test-retest reliability and validity of the Actical accelerometer in people with MS The participants were scheduled to attend 2 testing sessions, 7 days apart. They completed a series of 6 activities (reading newspaper, washing, vacuuming, stair climbing, 30-s chair stand test, and 6MWT) while wearing the Actical and a Polar heart rate monitor. The Borg RPE was used to measure self-reported activity intensity | Activity count | Test-retest reliabilityICC (95% Cl), bias,p value (95% LOA) Newspaper reading: 0.00 (0.00 to 0.37), 0.4,p = 0.48 (±16) Washing: 0.38 (0.07 to 0.70), 2.7,p = 0.84 (±145) Vacuuming: 0.75 (0.58 to 0.91), 7,p = 0.73 (±247) Stairs: 0.85 (0.76 to 0.95), 96.3,p = 0.26 (±1,065) Chair stand: 0.87 (0.77 to 0.96), 31.8,p = 0.74 (±1,192) 6MWT: 0.90 (0.83 to 0.97),–139,p = 0.33 (±1,330) |
| ValidityEstimate (95% CI),p value Newspaper reading: 0.097 (−0.211 to 0.018),p = 0.10 Washing: 0.02 (-0.002 to 0.042),p = 0.07 Vacuuming: 0.019 (0.007 to 0.030),p = 0.002 Stairs: 0.002 (-0.001 to 0.005),p = 0.16 Chair stand: 0.005 (0.002 to 0.009),p = 0.005 6MWT: 0.004 (0.002 to 0.006),p<0.001 | |||||
| Populationand setting | Device | Aim and methods | Endpoints | Findings | |
| Klassenet al. [43] | 43 participantsafter stroke(30male, age: 65±10.66 years) | A Fitbit One was positioned on each participant's non-paretic side on a waistband and ankle strap (above the lateral malleolus) | To examine the effect of walking speed on the accuracy of an accelerometer-based activity monitor in ambulatory individuals after stroke and to compare the effect of position (waist vs. ankle) on the accuracy of an accelerometer-based activity monitor | Step count | Fitbit anklePercentage error (SD) [95% Cl] 0.3 m/s: 15.8 (22.3) [9.1 to 22.7] 0.4 m/s: 5.5 (10.3) [2.4 to 8.6] 0.5 m/s: 4.5 (6.7) [2.4 to 6.6] 0.6 m/s: 4 (4.9) [2.4 to 5.6] 0.7 m/s: 4.9 (8.2) [2.1 to 7.7] 0.8 m/s: 6.9 (11.7) [2.8 to 11.0] 0.9 m/s: 4.9 (8.3) [1.8 to 8] |
| Research laboratory | |||||
| The participants walked a distance of 15 m for 8 walking trials: 1 trial at a self-selected walking speed and 7 trials from 0.3 to 0.9 m/s in 0.1 m/s increments. Each trial was videorecorded, and two independent viewers counted the actual number of steps from the video recordings of each trial | |||||
| Fitbit waistPercentage error (SD) [95% Cl] 0.3 m/s: 84.6 (30.5) [75.5 to 93.7] 0.4 m/s: 59.1 (40.1) [47.1 to 71.1] 0.5 m/s: 38.3 (33.2) [28.0 to 48.6] 0.6 m/s: 16.6 (17.8) [10.8 to 22.4] 0.7 m/s: 11.8 (17.0) [6.1 to 17.5] 0.8 m/s: 10.1 (13.6) [5.4 to 14.8] 0.9 m/s: 7.7 (8.9) [4.3 to 11.1] | |||||
| Paired ttestFitbit ankle vs. Fitbit waist: 0.3 m/s:p<0.001 0.4 m/s:p<0.001 0.5 m/s:p<0.001 0.6 m/s:p = 0.002 0.7 m/s:p = 0.21 0.8 m/s:p = 0.84 0.9 m/s:p = 0.58 | |||||
| Learmonthet al. [35] | 82 participants with MS (20 male, age: 49.2±9 years) | An ActiGraph GT3X was worn around the waist | To determine the reliability, precision, and clinically important change of accelerometry in participants with MS | Activity count | Activity count: ICC 0.883; 95% Cl 0.815 to 0.926; SEM 28,450; CV 17%; MDC 78,860 |
| Home environment | The participants wore the ActiGraph for 7 days during waking hours exclusive of bathing or swimming. The same procedure was repeated 6 months later | Step count | Step count: ICC 0.907; 95% CI 0.853 to 0.94; SEM 726; CV 16%; MDC 2,011 | ||
| Populationand setting | Device | Aim and methods | Endpoints | Findings | |
| Lord et al. [47] | 12people withPD (4male, age: 70.5±3.3 years)a | The Vitaport activity monitor consists of a portable data recorder attached to a belt worn around the waist, with 5 accelerometers attached to the body: 1 on each leg positioned on the lateral aspect of the mid-thigh, and 3 on the lower third of the sternum | To test the concurrent validity of the Vitaport activity monitor by comparing it to the GAITRite in controls and people with PD, to establish the use of the Vitaport activity monitor during a functional walk test, and to estimate the measurement error of the Vitaport activity monitor under these conditions | Gait speed | Gait speedMD(SD) [95% CI]; ICC Simple walk: −0.06 (0.04) [−0.06 to −0.3]; 0.99 Dual motor: −0.00 (0.09) [−0.06 to 0.5]; 0.91 Dual cognitive: −0.07 (0.05) [−0.10 to −0.04]; 0.97 Multiple task: 0.01 (0.08) [−0.06 to 0.03]; 0.94 |
| Step length | |||||
| Research laboratory | |||||
| Step frequency | |||||
| Four different walking tasks were performed: simple walking, dual motor task, dual cognitive task, and multiple task. Spatial and temporal variables of gait were measured using a GAITRite electronic walkway and the Vitaport activity monitor | |||||
| Step lengthMD(SD) [95% CI]; ICC Simple walk: −0.03 (0.02) [−0.04 to −0.01]; 0.97 Dual motor: 0.00 (0.04) [−0.02 to 0.03]; 0.85 Dual cognitive: 0.03 (0.03) [−0.05 to −0.01]; 0.92 Multiple task: 0.00 (0.02) [−0.01 to 0.02]; 0.93 | |||||
| Step frequencyMD (SD) [95% CI]; ICC Simple walk: 0.15 (0.04) [0.12 to 0.18]; 0.98 Dual motor: 0.14 (0.07) [0.09 to 0.19]; 0.96 Dual cognitive: 0.15 (0.04) [0.12 to 0.18]; 0.98 Multiple task: 0.11 (0.11) [0.03 to 0.18]; 0.92 | |||||
| Motl et al. [36] | 567 participants with MS (93 male, age: 47±10 years) | An ActiGraph 7164 was worn on a belt around the waist above the non-dominant hip | To estimate the reliability of objective measures of physical activity over a period of 6 months in persons with MS | Activity count | Activity counts/daySignificant change in over 6 months;t(474) = 3.92,p = 0.0001,d = 0.18; ICC 0.84 (95% CI 0.81 to 0.87); value significantly different from 0; F (1, 474) = 6.53,p = 0.0001 |
| Home environment | Minutes of MVPA | ||||
| The participants wore the ActiGraph during waking hours, except while bathing, showering, or swimming, for 7 days and then completed a battery of questionnaires that contained the Godin Leisure-Time Exercise Questionnaire (GLTEQ) on the eighth day. The same procedures were completed at baseline and 6 months later at follow-up | |||||
| Minutes of MVPA/daySignificant change over 6 months,t(474) = 5.38,p = 0.0001,d = 0.30; ICC 0.84 (95% CI 0.80 to 0.87); value significantly different from 0; F (1, 474) = 6.09,p = 0.0001 | |||||
| Populationand setting | Device | Aim and methods | Endpoints | Findings | |
| Motl et al. [37] | 51 participantswithMS (8 male, 43female, age:53.1±11.3years) | An actibelt accelerometer was attached to the participants' waist with a special buckle | To determine the accuracy of the actibelt for measuring walking speed during the 6MWT among persons with MS | Walking speed | The actibelt significantly overestimated walking speed (−0.12±0.17 m/s,p<0.0001). The overestimation was more pronounced in participants with moderate (−0.10±0.16 m/s) and severe (−0.26±0.12 m/s) disability. No significant overestimation was seen in those with mild disability (−0.02±0.11) |
| Researchlaboratory | The participants performed a 6MWT in a rectangular, carpeted corridor. The distance traveled (m) was recorded using a measuring wheel and was then converted into actual walking speed (m/s) for comparability with the actibelt output | ||||
| Overall standard error of the estimate: 0.10 m/s (95% Cl 1.10 to 1.50) Percent error rate (SD) [95% Cl] 54 m/min: 4.1 (9.1) [0.9 to 7.3] 80 m/min: 0.2 (0.8) [−0.2 to 0.63] 107 m/min: 0.3 (1.9 [−0.3 to 0.9] | |||||
| Motl et al. [38] | 24 participantswith MS(4male, age: 43.5±12.2 years)a | An ActiGraph 7164 was worn on an elastic belt that was positioned on the participants' right hip | To examine the accuracy of the ActiGraph accelerometer for measuring steps taken during controlled conditions by persons with MS compared with a sample of individuals without MS | Step count | |
| Research laboratory | |||||
| The participants performed three 6MWT on a treadmill, at 54, 80, and 107 m/min. There was a 6-min period of rest between walking periods. The actual number of steps taken was counted by observation using a hand-held tally counter | There was a statistically significant and large main effect for speed [F(2, 92) 59.13,p <0.0001,η2 = 0.17] | ||||
| Mudge andStott[44] | 40 participants with chronic stroke (23 male, 17 female, age: 69.2±12.6 years) | A StepWatch activity monitor was attached to the lateral side of the ankle of the non-paretic leg with a strap or cuff | To assess the test-retest reliability of the StepWatch activity monitor in individuals with chronic stroke | Step count | Total step count: ICC 0.989; CV 10.7%;±95% LOA 37.8% |
| Number of steps at high, medium, and low stepping rates | Number of steps at medium stepping rate: ICC 0.964; CV 17.8%; ±95% LOA 87.1% | ||||
| Home environment | The participants were instructed to wear the monitor for 3 days and for the same 3 days the following week, removing it for sleeping and showering | ||||
| Number of steps at high stepping rate: ICC 0.926; CV 37.6%; ±95% LOA 153% | |||||
| Number of steps at low stepping rate: ICC 0.953; CV 11.1%; ±95% LOA 63.6% | |||||
| Populationand setting | Device | Aim and methods | Endpoints | Findings | |
| Nightingale et al. [50] | 17male manual wheelchair users (age: 36±10 years) | An ActiGraph GT3X and a GENEActiv accelerometer were worn on the right wrist and upper arm | To assess the validity of two commonly used accelerometer devices, at two different anatomical locations, for the prediction of physical activity EE in manual wheelchair users in a controlled laboratory environment | EE | MAPE ActiGraph upper arm: 35.3±30.8% ActiGraph wrist: 33.0±39.5% GENEActiv upper arm: 20.4±14.3% GENEActiv wrist: 21.0±15.1% |
| Community gym | Activity count | ||||
| The participants completed 10 activities: resting, folding clothes, propulsion on a 1% gradient (3-6 and 7 km/h), and propulsion at 4 km/h (with an additional 8% body mass, 2 and 3% gradient) on a motorised wheelchair treadmill. IC was used as a criterion measurement of physical activity EE | Overall percent error of estimate (±95% LOA)ActiGraph upper arm: 15±87% ActiGraph wrist: 14±97% GENEActiv upper arm: 3±49% GENEActiv wrist: 4±50% | ||||
| Pearson CCActiGraph upper arm: 0.68 ActiGraph wrist: 0.82 GENEActiv upper arm: 0.87 GENEActiv wrist: 0.88 | |||||
| Rand et al. [45] | 40 adult communitydwelling participants with stroke (13 male, age: 66.5±9.6 years, BMI: 24.63±3.6) | Two Actical monitors were positioned over the anterior-superior iliac spine on the paretic and the non-paretic side | To assess the reliability of the Actical accelerometer for the paretic and the non-paretic side in people with stroke | EE | Paretic hipActivity count: ICC 0.95 (95% CI 0.92 to 0.97); SEM 18,324; MDC 50,792 EE: ICC 0.95 (95% CI 0.92 to 0.97); SEM 31.38; MDC 86.98 |
| Activity count | |||||
| The participants wore the Actical monitors continuously for 3 days and were instructed to go about their normal lives | |||||
| Home environment | |||||
| Non-paretic hipActivity count: ICC 0.94 (95% CI 0.91 to 0.97); SEM 17,690; MDC 49,035 EE: ICC 0.95 (95% CI 0.90 to 0.96); SEM 32.26; MDC 89.42 | |||||
| Paretic vs. non-paretic hipActivity count: ICC 0.98 (95% CI 0.97 to 0.99); SEM 9,755; MDC 27,039 EE: ICC 0.96 (95% CI 0.93 to 0.98); SEM 27.63; MDC 76.58 | |||||
| Populationand setting | Device | Aim and methods | Endpoints | Findings | |
| Ryanet al. [52] | 18adults(10male, age: 31.9±9.5 years, BMI: 25.3± 4.8) with CPa Clinical setting | An RT3 was worn on the right hip in the mid-axillary line An SWA was positioned over the triceps muscle of the right arm IDEEA sensors were worn on the chest, thighs, and soles of the feet | To evaluate the validity of the SWA, the IDEEA, and the RT3 at estimating EE in adults and children with CP EE data were collected using each monitor during rest and a number of walking activities. IC was used as the criterion measure of EE | EE | MAPE RT3: 17.2% (range 0.4 to 37.9) SWA: 35.5% (range 8.2 to 74.9) IDEEA: 16.3% (range 8.4 to 24.5) LOA RT3:–2.47 to 3.18 kcal/min SWA:–5.38 to 3.35 kcal/min IDEEA:–2.41 to 3.78 kcal/min |
| Sandroff et al. [39] | 63 participants with MS (15 male, age: 50.7±9.2 years) Research laboratory | A StepWatch activity monitor was worn on an elastic strap around the ankle above the right lateral malleolus An ActiGraph GT3X+ was worn on an elastic belt around the waist and above the right hip | To examine the accuracy of the StepWatch and ActiGraph in capturing steps taken at various speeds during over-ground ambulation in people with MS The participants completed three 6MWT: at CWS, at FWS (+0.5 mph of CWS), and at SWS (−0.5 mph of CWS). The actual number of steps taken was counted through direct observation using hand-held tally counters | Step count | ActiGraph step count accuracy: CWS 97.4%; FWS 95.6%; SWS 95.5% StepWatch step count accuracy: CWS 99.8%; FWS 99.9%; SWS 99.9% |
| Sandroff and Motl [40] | 41 participants with MS (5 male, 36 female, age: 47.7±8.8 years) and 41 age-matched healthy controls (5 male, age: 47.7±9.1 years) Research laboratory and home environment | An ActiGraph 7164 and a GT3X accelerometer were worn on an elastic belt around the waist on the non-dominant hip | To compare the activity count outputs from the 7164 and GT3X models of the ActiGraph in persons with MS and healthy controls under free-living and laboratory conditions The participants concurrently wore the accelerometers for 6 days during waking hours, except while swimming, bathing, or showering. They also undertook up to 5 bouts of walking that were each 6 min in duration on a treadmill The 5 possible walking speeds were 54, 67, 80, 94, and 107 m/min | Activity count | Free-living - difference between units12,487 (SD 27,199),p<0.01, ICC 0.983 (95% CI 0.967 to 0.991) Treadmill walking - difference between units54 m/min: 178 (SD 226),p<0.01, ICC 0.869 (95% CI 0.651 to 0.937) 67 m/min: 73 (SD 390),p = 0.09, ICC 0.891 (95% CI 0.831 to 0.930) 80 m/min:–30 (SD 484),p = 0.61, ICC 0.9 (95% CI 0.837 to 0.939) 94 m/min: 42 (SD 664),p = 0.64, ICC (95% CI 0.834 to 0.943) 107 m/min: 70 (SD 893),p = 0.61, ICC (95% CI 0.701 to 0.901) |
| Populationand setting | Device | Aim and methods | Endpoints | Findings | |
| Schmidt et al. [41] | 20 participantsdiagnosed withPD (n = 11) and MS (n = 9) | A StepWatch activity monitor was worn on an elastic strap around the ankle above the lateral malleolus | To explore the validity of the StepWatch Step Activity Monitor (SAM) in assessing stride counts in persons with PD or MS | Number of strides | Pearson CC for MS: 0.99 Pearson CC for PD: 1.0 |
| Clinical setting | The participants walked 15 m over a GaitMat II while wearing the StepWatch activity monitor. The strides counted by the GM were compared with the strides counted by the StepWatch activity monitor | StepWatch mean strides (95% CI): 15.55 (13.43 to 17.67) GaitMat II mean strides (95% CI): 15.85 (13.59 to 18.11) | |||
| Speelman et al. [48] | Part a: 28 participantswithPD(age:65.5±6.6years) | A DynaPort activity monitor was placed in a belt, positioned on the lower back between the posterior superior iliac spines | To evaluate the ability of the DynaPort activity monitor to estimate walking distances in PD | Walking distance | Difference between DynaPort and gold standard: <16% |
| Part b: 23 participantswithPD(age:63.8±9.4years) | Part a: the participants walked at their preferred speed along a marked linear distance in a hallway (ranging between 21 and 27 m) | Step length | In case of a longer walking distance, the LOA were–43 and +41%. The difference between the results and the gold standard did exceed more than 40% | ||
| Community setting | |||||
| Part b: the participants walked along a much longer (max. distance 1,097 m) and more complex“real life” walking trajectory (walking in the hospital corridors, with curves and path deviations). The actually measured walking distance was taken as the gold standard | |||||
| Population and setting | Device | Aim and methods | Endpoints | Findings | |
| Vanroy et al. [46] | 15 stroke patients (9 male, age: 60.4±10.26 years)a | A Yamax Digi-Walker (SW-200) was worn on the anterior side of the hip (on the belt) and the anterolateral side of the knee (patella support strap) on the non-hemiplegic side in stroke patients | To examine the validity and reliability of the SWA and the Digi-Walker in measuring the number of steps and EE in stroke patients and healthy individuals | Step count | Step count Validity (Spearman CC)Treadmill walking: SWA right–0.37 to 0.60; SWA left–0.52 to 0.46; Digi-Walker hip–0.41 to 0.90; Digi-Walker knee 0.30 to 0.69 Normal walking: SWA right–0.13; SWA left–0.23; Digi-Walker hip 0.33; Digi-Walker knee 0.95 Brisk walking: SWA right–0.04; SWA left 0.46; Digi-Walker hip 0.46; Digi-Walker knee 0.98 |
| EE | |||||
| Clinical setting | |||||
| Different activities were performed: treadmill walking, walking up/down a step, cycling, and walking on an even surface. Validity was examined by comparing the number of steps registered by the SWA and the Digi-Walker with that counted with a hand-held tally counter. EE was measured with the SWA and compared to IC. To determine the reliability of the two devices, repeated measurements on the treadmill and bike were compared for the number of steps and EE | |||||
| A SenseWear Armband (Pro2) was worn on both upper arms and positioned on the triceps muscle halfway between the acromion and the olecranon | |||||
| Test-retest reliability (ICC)Treadmill walking 1.5 km/h: SWA right 0.98; SWA left 0.89; Digi-Walker hip 0.88; Digi-Walker knee 0.73 Treadmill walking 3 km/h: SWA right 0.93; SWA left 0.92; Digi-Walker hip 0.96; Digi-Walker knee 0.95 | |||||
| Population and setting | Device | Aim and methods | Endpoints | Findings | |
| <B>EE </B> Validity (Spearman CC)Lying: SWA right 0.56; SWA left 0.49 Standing: SWA right 0.79; SWA left 0.81 Sitting: SWA right 0.78; SWA left 0.85 Treadmill walking: SWA right 0.01 to 0.75; SWA left 0.50 to 0.82 Stepping: SWA right 0.29 to 0.59; SWA left 0.48 to 0.71 Cycling: SWA right 0.54 to 0.71; SWA left 0.00 to 0.52 | |||||
| Test-retest reliability (ICC)Treadmill walking 1.5 km/h: SWA right 0.85; SWA left 0.76 Treadmill walking 3 km/h: SWA right 0.63; SWA left 0.97 Cycling 30 W: SWA right 0.90; SWA left 0.84 Cycling 50 W: SWA right 0.95; SWA left: 0.98 | |||||
| 2MWT, 2-min walk test; 6MWT, 6-min walk test; BMI, body mass index; CC, correlation coefficient; Cl, confidence interval; CV, coefficient of variation; CP, cerebral palsy; CWS, comfortable walking speed; EE, energy expenditure; FWS, fast walking speed; IC, indirect calorimetry; ICC, intra-class correlation coefficients; LOA, limits of agreement; MAPE, mean absolute percentage error; MD, meandifference; MDC, minimal detectable change; MS, multiple sclerosis; MVPA, moderate-to-vigorous-intensity physical activity; PD, Parkinson disease; RPE, rate of perceived exertion; SCI, spinal cord injury; SEM, standard error of measurement; SWS, slow walking speed; TBI, traumatic brain injury.a The study included young healthy participants as well; however, the results are presented only for the population of interest. |
Table 3.
Devices studied and results
| Device | Manufacturer | Device form factor | Reported findings | |||
| population | setting | endpoints | validity/reliability/accuracy | |||
| Triaxial accelerometers | ||||||
| actibelt | Trium | Waistband | MS (37) | Laboratory | Walking speed | Overestimates walking speed significantly in those with moderate (by–0.12±0.17 m/s) and severe (by–0.26±0.12 m/s) disability |
| Actical | Philips Respironics | Clip-on or custom waistband or wristband 1.14× 1.45× 0.43 in | MS (34) | Laboratory | Activity count | Test-retest reliability poor for sedentary and free-living activities, but better for more vigorous or rhythmic activities; validity not established, high variability for all activities |
| (16 g without band) | Stroke (45) | Home | Activity count | Excellent reliability for activity count and EE with Actical worn EE on both the paretic and the non-paretic hip | ||
| ActiGraph GT3X/+ | ActiGraph Corp. | Wristband or waistband 4.6×3.3× 1.5 cm (19 g) | MS (35) | Home | Activity count Step count | Both measures highly reliable across 6 months |
| MS (39) | Laboratory | Step count | ActiGraph worn on the waist is highly accurate (95.6–97.4%) in measuring steps taken under comfortable and fast walking speed; less accurate in measuring steps under slow walking conditions (95.5%), particularly in those with severe disability (87.3%) | |||
| MS (40) | Laboratory and home | Activity count | Activity counts from the ActiGraph 7164 and the GT3X are significantly different under free-living conditions; difference in output due to slow walking speeds | |||
| Manual wheelchair users (50) | Community | EE Activity count | ActiGraph worn on the wrist and upper arm and compared with the GENEActiv device worn on the wrist and upper arm; both ActiGraphs overestimate EE, with the wrist-worn device (percent estimation error: 14%) providing more valid results than that worn on the upper arm (15%) | |||
| DynaPort Activity Monitor | McRoberts | Waistband 6.2×6.2×1.3 cm (55 g) | PD (27) | Laboratory | Step count Gait duration | The DynaPort overestimated gait duration (11.1%) and underestimated the number of steps (6.9%); step count accuracy decreased significantly as the walking distance decreased (10 m, 5.7%; 5 m, 9.6%; 3 m, 18.4%); the DynaPort was less speed dependent and proved to be more appropriate for the PD patients than pedometer methods for walking trajectories of 5 m or more |
| PD (48) | Community | Walking distance Step length | The precision in estimating short walking distances was good (percent error: 16%); however, the precision in estimating long walking distances (percent error: <40%) was less appropriate; the overall moderate precision limits the use of this activity monitor for clinical purposes | |||
| Device | Manufacturer | Device form factor | Reported findings | |||
| population | setting | endpoints | validity/reliability/accuracy | |||
| FitbitUltra | FitbitInc. | Clip 5.5×1.9×1.4 cm (11.34 g) | Stroke and TBI (42) | Laboratory | Step count | The Fitbit Ultra underestimated steps (percent error: 5%); however, it had an acceptable accuracy; it was generally accurate in participants who took more steps, and it may be a less costly alternative to research-based activity monitors for identifying steps taken |
| FitbitOne | FitbitInc. | Clip/wristband 1.9×1×4.8 mm (80 g) | Stroke (43) | Laboratory | Step count | It is more accurate as the walking speed increases and is more accurate when placed at the ankle (percent error range: 4.9–15.8%) versus the waist (7.7–84.6%) |
| GENEActiv | Activinsights | Wristband 4.3×4×1.3 cm (16 g) | Manual wheelchair users (50) | Community | EE Activity coun | The GENEActiv device worn on either the upper arm t (percent error: 3%) or the wrist (4%) provided the most valid prediction of EE |
| Nike FuelBand % | Nike | Wristband | Stroke and TBI (42) | Laboratory | Step count | The Nike FuelBand is not accurate in estimating steps, grossly underestimating steps (33.9%) in this study |
| Device | Manufacturer | Device form factor | Reportedfirn | dings | ||
| population | setting | endpoints | validity/reliability/accuracy | |||
| RT3 accelerometer | Stayhealthy Inc. | Waistband 7.1×5.6×2.8 cm (65.2 g) | MS, PD, stroke (33) | Home | Activity count | Good test-retest reliability in measuring free-living activity; the daily data collected in the first 3 days were significantly different from those collected over 7 days; a 7-day monitoring period provides the most reliable measurement of physical activity |
| SCI (49) | Laboratory | EE | Overestimated EE (percent estimation error range: 22–52.8%); however, EE estimations with the RT3 were closer to the criterion EE than those with the SWA | |||
| CP (52) | Clinic | EE | The LOA revealed that the RT3 provided the best agreement with the indirect calorimeter in estimating EE compared to the SWA and IDEEA; however, the RT3 could significantly overestimate or underestimate individual estimates of EE (LOA–67.2 to 86.3% of the mean EE), with smaller errors for over-ground walking compared to treadmill walking | |||
| Biaxial accelerometers | ||||||
| StepWatch Activity Monitor | Modus Health | Ankle band 7×5×2 cm (38 g) | MS (39) | Laboratory | Step count | Accurately measures step counts at slow (99%), comfortable (99.8%), and fast (99.6%) walking speeds |
| PD and MS (43) | Clinic | Number of strides | Accurately counts the number of strides in both MS (Pearson CC 0.99) and PD (Pearson CC 1.0) patients | |||
| Stroke and TBI (42) | Laboratory | Step count | Accurately counts steps, with only marginal overestimation (percent error: 2.4%) in this population | |||
| Stroke (44) | Home | Step count | The total step count has excellent test-retest reliability when used for 3 days in individuals with stroke; monitoring for less than a 3-day period is not recommended due to high variability | |||
| Rett syndrome (27) | Home | Step count | Accurately counts steps (mean difference: 0 steps/min); agreement did not differ with the level of general or complex gross motor skills | |||
| Device | Manufacturer | Device form factor | Reported findings | |||
| population | setting | endpoints | validity/reliability/accuracy | |||
| Uniaxial accelerometers | ||||||
| ActiGraph 7164 | ActiGraph Corp. | Clip-on or waist/wristband 5.1×4.1×1.5 cm (45.5 g) | MS (36) | Home | Activity coun Minutes of MVPA | t Highly reliable over a 6-month period in an MS population |
| MS (38) | Home | Step count | Accurately measures steps during moderate (percent error: 0.2%) and fast (0.3%) walking in persons with MS; however, there is a small degree of underestimation of step counts during slower walking (4.1%) | |||
| MS (40) | Laboratory and home | Activity coun | t Activity counts from the ActiGraph 7164 and the GT3X are significantly different under free-living conditions; difference in output due to slow walking speeds | |||
| ActivPAL | PAL Technologies Ltd. | Adheres directly to skin using PALStickies (hydrogel/waterproof attachment pad) 3.5×5.3×7 cm (15 g) | CP (51) | Clinic | Time spent standing Time spent sitting Time spent lying Step counts | Validity was high (r2≥0.96); the limits of group agreement were relatively narrow, but the LOA for individuals were narrow only for the number of steps (>5.5%); the relative reliability was high for the number of steps and moderate for the time spent sitting and lying and the time spent standing; the ActivPAL is sufficiently accurate and reliable to be used for research purposes, but less so for measuring physical activity and sedentary behaviour in an individual |
| Multisensors | ||||||
| SWA | BodyMedia, Inc. | Biaxial accelerometer, heat flux sensor, skin temperature sensor, near-body ambient temperature sensor, and galvanic skin response sensor; armband around the right upper arm 8.5×5.3×2 cm (79 g including armband) | Stroke (46) | Clinic | Step count EE | There was a poor validity of the SWA in measuring steps and EE during a range of activities and walking tasks; there was good-to-excellent test-retest reliability in measuring steps and EE |
| SCI (49) | Laboratory | EE | Significantly overestimated EE (percent error estimation range: 24.4–125.8%) in this population | |||
| CP (52) | Clinicl | EE | Overestimated EE in adults with CP, with smaller errors for over-ground walking compared to treadmill walking | |||
| IDEEA | MiniSun, LLC | Intelligent Device for Energy Expenditure and Activity (IDEEA) 5 biaxial accelerometers collect data and transmit it through thin, flexible wires to a recorder; the accelerometer is placed on the chest, thighs, and soles of the feet Recorder: 7×5.4×1.7 cm (59 g) Sensor: 1.8×1.5×0.3 cm (2 g) | CP (52) | Clinic | EE | The mean absolute percentage error was smallest for the IDEEA (range: 8.4–24.5%) when compared to the RT3 and the SWA |
| Device | Manufacturer | Device form factor | Reported findings | |||
| population | setting | endpoints | validity/reliability/accuracy | |||
| VitaportActivityMonitor | TEMEC Instruments Inc. | Five accelerometers attached to the body connected to a portable battery-powered activity monitor (Vitaport) by cables which run under the clothes; the Vitaport is attached to a belt worn around the waist, with 1 accelerometer on each and 3 placed on the lower third of the sternum 9×4.5×1.5 cm (1,360 g) | PD (47) | Laboratory | Gait speed Step length Step frequency | Excellent validity (ICC(2, 2) = 0.92–0.99,p<0.0001) for the use of the Vitaport Activity Monitor to measurespatiotemporalgait characteristics during a functional walking test for PD |
| Spring-mounted lever arm pedometer | ||||||
| Digi-Walker SW-701/SW-200 | Yamax Corporation | Clip to waistband or belt 5×3.8×1.4 cm (21 g) | Stroke and TBI (42) | Laboratory | Step count | Moderate accuracy, tending to underestimate steps in this study (percent error: 14.7%) |
| Stroke, MS, SCI, ABI, muscular dystrophy (32) | Clinic | Step count | Undercounts steps (percent error: 24–35%) in a neurological population; however, this is not strongly related to walking speed | |||
| Stroke (46) | Clinic | Step count | Reliably counts steps; wearing it on the knee is a valid option for measuring steps, except during high-intensity walking; the device is more valid as walking speed increases | |||
| PD (34) | Laboratory | Step count | Underestimates step counts (percent error: left, 11.1%; right, 16.3%) and is less accurate for short trajectories and as the walking pace decreases |
ABI, acquired brain injury; CC, correlation coefficient; CP, cerebral palsy; EE, energy expenditure; ICC, intra-class correlation coefficient; LOA, limits of agreement; MS, multiple sclerosis; MVPA, moderate-to-vigorous-intensity physical activity; PD, Parkinson disease; SCI, spinal cord injury; TBI, traumatic brain injury.
Discussion
This review identified 23 studies examining the validity and reliability of 16 different monitors (9 triaxial accelerometer-type monitors, 1 biaxial accelerometer-type monitor, 2 uniaxial accelerometer-type monitors, 3 multisensor devices, and 1 spring-mounted lever arm pedometer-type monitor) in individuals with neurological disorders. The studies included in this review were highly heterogeneous in terms of their study design, the participants included, the activity monitors used, the placement of the monitor on the body, the outcomes measured, and the algorithms used to calculate the measurement outcome. Therefore it was difficult to directly compare the findings. Nevertheless an attempt was made to summarise the key findings of the papers.
Step counts are the most frequently reported outcome measure of physical activity in individuals with neurological disorders, and the evidence suggests that ankle-worn biaxial or triaxial accelerometer-type devices provide the most accurate measurement. There is conflicting evidence regarding the validity and reliability of wearable activity monitors in measuring activity counts, while accelerometer-type devices appear to be more appropriate in estimating energy expenditure than multisensor devices, which are more frequently used. The sections below describe these findings in greater detail.
Ankle-Worn Devices Provide the Most Accurate Measurement of Step Counts
Step counts are the most frequently reported outcome measure of physical activity in individuals with neurological disorders. The evidence suggests that for accurate and reliable measurements of step counts, a number of factors need to be considered. Firstly the validity and reliability of wearable activity monitors in measuring step counts appears to be dependent on the type of device that is used. Spring-mounted lever arm pedometers appear to underestimate step counts in participants with a range of neurological disorders, particularly at slower walking speeds, and are also less accurate for short walking trajectories [27, 32, 42]. Similarly uniaxial accelerometers appear to underestimate steps in slower walking conditions [38, 51]. Using multiaxial accelerometers appears to be a less speed-dependent method of counting steps, producing more accurate measurements [27, 35, 39, 42, 44, 53]. The validity and reliability of wearable activity monitors in measuring step counts also appears to be dependent upon the position on the body in which the device is placed. Activity monitors positioned on the ankle appear to be more accurate than wrist-mounted and waist-mounted devices in counting steps, particularly during slow walking conditions [39, 42, 43]. This may be because larger accelerations occur at the ankle during walking due to the distance from the pivot point of the hip. Many device manufacturers recommend that monitoring devices are best positioned at the waist for usability reasons. However, wearing a device around the waist in the centre of the back is considered uncomfortable by many, especially when sitting and driving [33], making long-term adherence in the home environment a challenge to achieve. Therefore, for accurate step count measurements in individuals with neurological disorders, it is recommended, based on the evidence, to use biaxial or triaxial accelerometer-type devices, positioning the device around the ankle.
Validity and Reliability of Accelerometer Activity Counts
A number of studies have reported accelerometer activity counts as a measure of physical activity [33, 34, 35, 36, 40, 45, 50]; however, the evidence is conflicting regarding the validity and reliability of this measure in patients with neurological disorders. Some authors report good reliability in measuring activity counts during free-living activities from an activity monitor positioned at the waist [33, 36, 45]. However, others found that an accelerometer positioned around the waist was not reliable at measuring activity counts during sedentary and free-living activities [34]. Two studies reported on the validity of activity count outputs from accelerometers, and conflicting findings were also reported [34, 50].
Multisensor Devices Are Inaccurate in Estimating Energy Expenditure
Four studies included in this review reported on the accuracy of using a wearable device in estimating energy expenditure in this population. Multisensor devices such as the SWA are most frequently used to estimate energy expenditure, but this device was shown to be inaccurate in estimating energy expenditure in this population [27, 49, 52]. Similarly, the multisensor Intelligent Device for Energy Expenditure and Activity (IDEEA) was shown to be inaccurate in estimating energy expenditure [52]. Accelerometer devices may be more appropriate for estimating energy expenditure. The Actical triaxial accelerometer was shown to have excellent day-to-day reliability in estimating energy expenditure during free-living physical activities in individuals with stroke living in the community [45].
Limitations
There are a number of limitations to this review that need to be considered. The majority of the studies identified in this review include a small sample of participants. In addition the majority of the studies were conducted in a controlled laboratory or clinical setting, and much more work is required to establish the accuracy of measurements in free-living environments. Despite the best efforts of the authors, it is possible that some studies were not identified in the literature search, or were excluded given the study selection criteria and the electronic databases that were searched to retrieve studies. However, given the systematic approach that was adopted, this review can be accepted as an accurate reflection of the existing evidence exploring activity monitoring in individuals with neurological disorders. Nonetheless this is a rapidly expanding and evolving field of research; therefore the findings of this review should be substantiated as new evidence emerges and new studies are published.
Recommendations for Clinical Use
Activity monitors selected for clinical use should be confined to those for which there is a body of evidence outlining the validity and the reliability of the measurements produced. In addition the psychometric properties of the monitor selected should be established in the clinical cohort of interest, as the validity and reliability of a monitor in one cohort does not necessarily infer the same in another. Furthermore consideration should be given to the stage and severity of a disorder to ensure the monitor selection is appropriate.
Besides the validity and the reliability of the measurements produced, other factors need to be considered when selecting a device. One should also consider the cost of obtaining the device (hardware and software) and its attachments (e.g., belts or adhesives). Other factors which also warrant attention include the aesthetic appearance of the device, the comfort and wearability of the device, the user experience, the obtrusiveness of the device, the device's durability, and the privacy and discretion afforded with its use. Taking these human factors into consideration when selecting a device will help minimise the impact of potential user acceptance issues that may arise.
Further work is required in this field before wearable activity monitoring can be more widely implemented. Standardised activity monitoring protocols need to be developed for implementing these devices in clinical trials and clinical practice, and consensus is required as to the reporting and interpretation of derived variables.
Conclusions
Recent technological advances have led to the development of a wide range of devices capable of measuring physical activity and mobility. Wearable sensors have immense potential in clinical trials and clinical practice; however, as yet they have not been widely adopted. This review of the literature attempted to summarise the evidence exploring the validity and reliability of body-worn monitors that measure physical activity and mobility in patients with neurological disorders. The variety of methods used in the included studies limits the ability to draw definitive conclusions. Nonetheless, the evidence appears to suggest that multiaxial accelerometer devices – in particular the StepWatch activity monitor and the ActiGraph GT3X – positioned around the ankle most accurately measure step counts in patients with neurological disorders, and are acceptable in slow walking conditions. The findings regarding the reliability and validity of activity counts and energy expenditure are largely inconclusive in this population.
Appendix 1
Search Terms
Free-text and MeSH terms used in the PubMed database:
accelerometry [MeSH] OR accelerometer OR accelerometer and gyroscope OR pedometer OR physical activity trackers OR activity trackers OR activity monitor OR activity tracker OR fitness tracker OR physical activity tracker OR physical activity monitor OR step counter OR wearable technology OR wearable sensor OR wearable device OR wearable OR sensor OR inertial sensor OR inertial measurement unit OR IMU
AND
physical fitness [MeSH] OR motion [MeSH] OR energy metabolism [MeSH] OR motor activity [MeSH] OR steps
AND
validity OR validation OR validation study OR reliability OR reliability study OR accuracy OR comparison OR comparison study
Appendix 2
Quality Assessment of Method Comparison Validation Studies
The following criteria were used for assessing the methodologic quality of method comparison studies as either “yes,” “no,” or “unclear”:
Ethics Statement
The authors have no ethical conflicts to disclose.
Conflict of Interest Statement
I.C. and L.W. work for Novartis. O.M.G. is on secondment with Novartis.
Funding Sources
This publication has emanated from research conducted with the financial support of Science Foundation Ireland (SFI) under Grant No. 15/IFA/3009.
References
- 1.Thompson P, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, et al. American Heart Association Council on Clinical Cardiology Subcommittee on Exercise, Rehabilitation, and Prevention; American Heart Association Council on Nutrition, Physical Activity, and Metabolism Subcommittee on Physical Activity: Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity) Circulation. 2003;107:3109–3116. doi: 10.1161/01.CIR.0000075572.40158.77. 10.1161/01.CIR.0000075572.40158.77.12821592. [DOI] [PubMed] [Google Scholar]
- 2.Hu G, Lakka TA, Kilpeläinen TO, Tuomilehto J. Epidemiological studies of exercise in diabetes prevention. Appl Physiol Nutr Metab. 2007;32:583–595. doi: 10.1139/H07-030. 10.1139/H07-030.17510700. [DOI] [PubMed] [Google Scholar]
- 3.Lee IM. Physical activity and cancer prevention - data from epidemiologic studies. Med Sci Sports Exerc. 2003;35:1823–1827. doi: 10.1249/01.MSS.0000093620.27893.23. 10.1249/01.MSS.0000093620.27893.23.14600545. [DOI] [PubMed] [Google Scholar]
- 4.Howe TE, Shea B, Dawson LJ, Downie F, Murray A, Ross C, et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2011;7:CD000333. doi: 10.1002/14651858.CD000333.pub2. 10.1002/14651858.CD000333.pub2.21735380. [DOI] [PubMed] [Google Scholar]
- 5.Poirier P, Després JP. Exercise in weight management of obesity. Cardiol Clin. 2001;19:459–470. doi: 10.1016/s0733-8651(05)70229-0. 10.1016/S0733-8651(05)70229-0.11570117. [DOI] [PubMed] [Google Scholar]
- 6.Hernández-Molina G, Reichenbach S, Zhang B, Lavalley M, Felson DT. Effect of therapeutic exercise for hip osteoarthritis pain: results of a meta-analysis. Arthritis Rheum. 2008;59:1221–1228. doi: 10.1002/art.24010. 10.1002/art.24010.18759315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobs PL, Nash MS. Exercise recommendations for individuals with spinal cord injury. Sports Med. 2004;34:727–751. doi: 10.2165/00007256-200434110-00003. 10.2165/00007256-200434110-00003.15456347. [DOI] [PubMed] [Google Scholar]
- 8.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126–131. 3920711. [PMC free article] [PubMed] [Google Scholar]
- 9.Hirvensalo M, Rantanen T, Heikkinen E. Mobility difficulties and physical activity as predictors of mortality and loss of independence in the community-living older population. J Am Geriatr Soc. 2000;48:493–498. doi: 10.1111/j.1532-5415.2000.tb04994.x. 10.1111/j.1532-5415.2000.tb04994.x.10811541. [DOI] [PubMed] [Google Scholar]
- 10.Bo M, Fonte G, Pivaro F, Bonetto M, Comi C, Giorgis V, et al. Prevalence of and factors associated with prolonged length of stay in older hospitalized medical patients. Geriatr Gerontol Int. 2016;16:314–321. doi: 10.1111/ggi.12471. 10.1111/ggi.12471.25752922. [DOI] [PubMed] [Google Scholar]
- 11.Miller EA, Weissert WG. Predicting elderly people's risk for nursing home placement, hospitalization, functional impairment, and mortality: a synthesis. Med Care Res Rev. 2000;57:259–297. doi: 10.1177/107755870005700301. 10.1177/107755870005700301.10981186. [DOI] [PubMed] [Google Scholar]
- 12.Bandmann E. Physical activity questionnaires: a critical review of methods used in validity and reproducibility studies; thesis. Stockholm. 2008 [Google Scholar]
- 13.Jørstad-Stein E, Hauer K, Becker C, Bonnefoy M, Nakash R, Skelton D, et al. Suitability of physical activity questionnaires for older adults in fall-prevention trials: a systematic review. J Aging Phys Act. 2005;13:461–481. doi: 10.1123/japa.13.4.461. 10981186. [DOI] [PubMed] [Google Scholar]
- 14.Van Remoortel H, Giavedoni S, Raste Y, Burtin C, Louvaris Z, Gimeno-Santos E, et al. Validity of activity monitors in health and chronic disease: a systematic review. Int J Behav Nutr Phys Act. 2012;9:84. doi: 10.1186/1479-5868-9-84. 10.1186/1479-5868-9-84.22776399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel S, Park H, Bonato P, Chan L, Rodgers M. A review of wearable sensors and systems with application in rehabilitation. J Neuroeng Rehabil. 2012;9:21. doi: 10.1186/1743-0003-9-21. 10.1186/1743-0003-9-21.22520559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robles-García V, Corral-Bergantiños Y, Espinosa N, Jácome MA, García-Sancho C, Cudeiro J, et al. Spatiotemporal gait patterns during overt and covert evaluation in patients with Parkinson's disease and healthy subjects: is there a Hawthorne effect? J Appl Biomech. 2015;31:189–194. doi: 10.1123/jab.2013-0319. 10.1123/jab.2013-0319.25536440. [DOI] [PubMed] [Google Scholar]
- 17.Byrom B, Rowe DA. Measuring free-living physical activity in COPD patients: deriving methodology standards for clinical trials through a review of research studies. Contemp Clin Trials. 2016;47:172–184. doi: 10.1016/j.cct.2016.01.006. 10.1016/j.cct.2016.01.006.26806669. [DOI] [PubMed] [Google Scholar]
- 18.Dhillon SS, Sima CA, Kirkham AR, Syed N, Camp PG. Physical activity measurement accuracy in individuals with chronic lung disease: a systematic review with meta-analysis of method comparison studies. Arch Phys Med Rehabil. 2015;96:2079.e10–2088.e10. doi: 10.1016/j.apmr.2015.05.015. 10.1016/j.apmr.2015.05.015.26049088. [DOI] [PubMed] [Google Scholar]
- 19.Gebruers N, Vanroy C, Truijen S, Engelborghs S, De Deyn PP. Monitoring of physical activity after stroke: a systematic review of accelerometry-based measures. Arch Phys Med Rehabil. 2010;91:288–297. doi: 10.1016/j.apmr.2009.10.025. 10.1016/j.apmr.2009.10.025.20159136. [DOI] [PubMed] [Google Scholar]
- 20.Block VAJ, Pitsch E, Tahir P, Cree BAC, Allen DD, Gelfand JM. Remote physical activity monitoring in neurological disease: a systematic review. PLoS One. 2016;11:e0154335. doi: 10.1371/journal.pone.0154335. 10.1371/journal.pone.0154335.27124611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Godinho C, Domingos J, Cunha G, Santos AT, Fernandes RM, Abreu D, et al. A systematic review of the characteristics and validity of monitoring technologies to assess Parkinson's disease. J Neuroeng Rehabil. 2016;13:24. doi: 10.1186/s12984-016-0136-7. 10.1186/s12984-016-0136-7.26969628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gehlsen G, Beekman K, Assmann N, Winant D, Seidle M, Carter A. Gait characteristics in multiple sclerosis: progressive changes and effects of exercise on parameters. Arch Phys Med Rehabil. 1986;67:536–539. 3741079. [PubMed] [Google Scholar]
- 23.Moreno Catalá M, Woitalla D, Arampatzis A. Central factors explain muscle weakness in young fallers with Parkinson's disease. Neurorehabil Neural Repair. 2013;27:753–759. doi: 10.1177/1545968313491011. 10.1177/1545968313491011.23774123. [DOI] [PubMed] [Google Scholar]
- 24.Thibaut A, Chatelle C, Ziegler E, Bruno MA, Laureys S, Gosseries O. Spasticity after stroke: physiology, assessment and treatment. Brain Inj. 2013;27:1093–1105. doi: 10.3109/02699052.2013.804202. 10.3109/02699052.2013.804202.23885710. [DOI] [PubMed] [Google Scholar]
- 25.Jankovic J. Parkinson's disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–376. doi: 10.1136/jnnp.2007.131045. 10.1136/jnnp.2007.131045.18344392. [DOI] [PubMed] [Google Scholar]
- 26.Zijlstra W, Hof AL. Assessment of spatio-temporal gait parameters from trunk accelerations during human walking. Gait Posture. 2003;18:1–10. doi: 10.1016/s0966-6362(02)00190-x. 10.1016/S0966-6362(02)00190-X.14654202. [DOI] [PubMed] [Google Scholar]
- 27.Dijkstra B, Zijlstra W, Scherder E, Kamsma Y. Detection of walking periods and number of steps in older adults and patients with Parkinson's disease: accuracy of a pedometer and an accelerometry-based method. Age Ageing. 2008;37:436–441. doi: 10.1093/ageing/afn097. 10.1093/ageing/afn097.18487266. [DOI] [PubMed] [Google Scholar]
- 28.Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement [reprinted from Ann Intern Med] Phys Ther. 2009;89:873–880. 19723669. [PubMed] [Google Scholar]
- 29.Taraldsen K, Chastin SFM, Riphagen II, Vereijken B, Helbostad JL. Physical activity monitoring by use of accelerometer-based body-worn sensors in older adults: a systematic literature review of current knowledge and applications. Maturitas. 2012;71:13–19. doi: 10.1016/j.maturitas.2011.11.003. 10.1016/j.maturitas.2011.11.003.22134002. [DOI] [PubMed] [Google Scholar]
- 30.Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15:155–163. doi: 10.1016/j.jcm.2016.02.012. 10.1016/j.jcm.2016.02.012.27330520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whiting P, Rutjes AWS, Reitsma JB, Bossuyt PMM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:25. doi: 10.1186/1471-2288-3-25. 10.1186/1471-2288-3-25.14606960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elsworth C, Dawes H, Winward C, Howells K, Collett J, Dennis A, et al. Pedometer step counts in individuals with neurological conditions. Clin Rehabil. 2009;23:171–175. doi: 10.1177/0269215508098895. 10.1177/0269215508098895.19164404. [DOI] [PubMed] [Google Scholar]
- 33.Hale LA, Pal J, Becker I. Measuring free-living physical activity in adults with and without neurologic dysfunction with a triaxial accelerometer. Arch Phys Med Rehabil. 2008;89:1765–1771. doi: 10.1016/j.apmr.2008.02.027. 10.1016/j.apmr.2008.02.027.18760161. [DOI] [PubMed] [Google Scholar]
- 34.Kayes NM, Schluter PJ, McPherson KM, Leete M, Mawston G, Taylor D. Exploring Actical accelerometers as an objective measure of physical activity in people with multiple sclerosis. Arch Phys Med Rehabil. 2009;90:594–601. doi: 10.1016/j.apmr.2008.10.012. 10.1016/j.apmr.2008.10.012.19345774. [DOI] [PubMed] [Google Scholar]
- 35.Learmonth YC, Dlugonski DD, Pilutti LA, Sandroff BM, Motl RW. The reliability, precision and clinically meaningful change of walking assessments in multiple sclerosis. Mult Scler. 2013;19:1784–1791. doi: 10.1177/1352458513483890. 10.1177/1352458513483890.23587605. [DOI] [PubMed] [Google Scholar]
- 36.Motl RW, McAuley E, Klaren R. Reliability of physical-activity measures over six months in adults with multiple sclerosis: implications for designing behavioral interventions. Behav Med. 2014;40:29–33. doi: 10.1080/08964289.2013.821966. 10.1080/08964289.2013.821966.24512363. [DOI] [PubMed] [Google Scholar]
- 37.Motl RW, Weikert M, Suh Y, Sosnoff JJ, Pula J, Soaz C, et al. Accuracy of the actibelt® accelerometer for measuring walking speed in a controlled environment among persons with multiple sclerosis. Gait Posture. 2012;35:192–196. doi: 10.1016/j.gaitpost.2011.09.005. 10.1016/j.gaitpost.2011.09.005.21945386. [DOI] [PubMed] [Google Scholar]
- 38.Motl RW, Snook EM, Agiovlasitis S. Does an accelerometer accurately measure steps taken under controlled conditions in adults with mild multiple sclerosis? Disabil Health J. 2011;4:52–57. doi: 10.1016/j.dhjo.2010.02.003. 10.1016/j.dhjo.2010.02.003.21168808. [DOI] [PubMed] [Google Scholar]
- 39.Sandroff BM, Motl RW, Pilutti LA, Learmonth YC, Ensari I, Dlugonski D, et al. Accuracy of StepWatchTM and ActiGraphTM accelerometers for measuring steps taken among persons with multiple sclerosis. PLoS One. 2014;9:e93511. doi: 10.1371/journal.pone.0093511. 10.1371/journal.pone.0093511.24714028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sandroff BM, Motl RW. Comparison of ActiGraph activity monitors in persons with multiple sclerosis and controls. Disabil Rehabil. 2013;35:725–731. doi: 10.3109/09638288.2012.707745. 10.3109/09638288.2012.707745.23557239. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt AL, Pennypacker ML, Thrush AH, Leiper CI, Craik RL. Validity of the StepWatch Step Activity Monitor: preliminary findings for use in persons with Parkinson disease and multiple sclerosis. J Geriatr Phys Ther. 2011;34:41–45. doi: 10.1519/JPT.0b013e31820aa921. 10.1519/JPT.0b013e31820aa921.21937891. [DOI] [PubMed] [Google Scholar]
- 42.Fulk GD, Combs SA, Danks KA, Nirider CD, Raja B, Reisman DS. Accuracy of 2 activity monitors in detecting steps in people with stroke and traumatic brain injury. Phys Ther. 2014;94:222–229. doi: 10.2522/ptj.20120525. 10.2522/ptj.20120525.24052577. [DOI] [PubMed] [Google Scholar]
- 43.Klassen TD, Simpson LA, Lim SB, Louie DR, Parappilly B, Sakakibara BM, et al. “Stepping up ” activity poststroke: ankle-positioned accelerometer can accurately record steps during slow walking. Phys Ther. 2016;96:355–360. doi: 10.2522/ptj.20140611. 10.2522/ptj.20140611.26251478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mudge S, Stott NS. Test-retest reliability of the StepWatch Activity Monitor outputs in individuals with chronic stroke. Clin Rehabil. 2008;22:871–877. doi: 10.1177/0269215508092822. 10.1177/0269215508092822.18955419. [DOI] [PubMed] [Google Scholar]
- 45.Rand D, Eng J, Tang P, Jeng J, Hung C. How active are people with stroke? Use of accelerometers to assess physical activity. Stroke. 2009;40:163–168. doi: 10.1161/STROKEAHA.108.523621. 10.1161/STROKEAHA.108.523621.18948606. [DOI] [PubMed] [Google Scholar]
- 46.Vanroy C, Vissers D, Cras P, Beyne S, Feys H, Vanlandewijck Y, et al. Physical activity monitoring in stroke: SenseWear Pro2 Activity accelerometer versus Yamax Digi-Walker SW-200 pedometer. Disabil Rehabil. 2014;36:1695–1703. doi: 10.3109/09638288.2013.859307. 10.3109/09638288.2013.859307.24279597. [DOI] [PubMed] [Google Scholar]
- 47.Lord S, Rochester L, Baker K, Nieuwboer A. Concurrent validity of accelerometry to measure gait in Parkinsons disease. Gait Posture. 2008;27:357–359. doi: 10.1016/j.gaitpost.2007.04.001. 10.1016/j.gaitpost.2007.04.001.17604630. [DOI] [PubMed] [Google Scholar]
- 48.Speelman AD, van Nimwegen M, Borm GF, Bloem BR, Munneke M. Monitoring of walking in Parkinson's disease: validation of an ambulatory activity monitor. Parkinsonism Relat Disord. 2011;17:402–404. doi: 10.1016/j.parkreldis.2011.02.006. 10.1016/j.parkreldis.2011.02.006.21367643. [DOI] [PubMed] [Google Scholar]
- 49.Hiremath SV, Ding D. Evaluation of activity monitors in manual wheelchair users with paraplegia. J Spinal Cord Med. 2011;34:110–117. doi: 10.1179/107902610X12911165975142. 10.1179/107902610X12911165975142.21528634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nightingale TE, Walhin JP, Thompson D, Bilzon JL. Influence of accelerometer type and placement on physical activity energy expenditure prediction in manual wheelchair users. PLoS One. 2015;10:e0126086. doi: 10.1371/journal.pone.0126086. 10.1371/journal.pone.0126086.25955304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bania T. Measuring physical activity in young people with cerebral palsy: validity and reliability of the ActivPALTM monitor. Physiother Res Int. 2014;19:186–192. doi: 10.1002/pri.1584. 10.1002/pri.1584.24634324. [DOI] [PubMed] [Google Scholar]
- 52.Ryan JM, Walsh M, Gormley J. A comparison of three accelerometry-based devices for estimating energy expenditure in adults and children with cerebral palsy. J Neuroeng Rehabil. 2014;11:116. doi: 10.1186/1743-0003-11-116. 10.1186/1743-0003-11-116.25097005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Downs J, Leonard H, Hill K. Initial assessment of the StepWatch Activity MonitorTM to measure walking activity in Rett syndrome. Disabil Rehabil. 2012;34:1010–1015. doi: 10.3109/09638288.2011.630773. 10.3109/09638288.2011.630773.22107440. [DOI] [PubMed] [Google Scholar]