Abstract
Background
The motor subscale of the Movement Disorder Society-Unified Parkinson's Disease Rating Scale (MDS-UPDRS-III) has limited applicability for the assessment of motor fluctuations in the home setting.
Methods
To assess whether a self-administered, tablet-based application can reliably quantify differences in motor performance using two-target finger tapping and forearm pronation-supination tasks in the ON (maximal dopaminergic medication efficacy) and OFF (reemergence of parkinsonian deficits) medication states, we recruited 11 Parkinson disease (PD) patients (age, 60.6 ± 9.0 years; disease duration, 12.8 ± 4.1 years) and 11 healthy age-matched controls (age, 62.5 ± 10.5 years). The total number of taps, tap interval, tap duration, and tap accuracy were algorithmically calculated by the application, using the more affected side in patients and the dominant hand in healthy controls.
Results
Compared to the OFF state, PD patients showed a higher number of taps (84.2 ± 20.3 vs. 54.9 ± 26.9 taps; p = 0.0036) and a shorter tap interval (375.3 ± 97.2 vs. 708.2 ± 412.8 ms; p = 0.0146) but poorer tap accuracy (2,008.4 ± 995.7 vs. 1,111.8 ± 901.3 pixels; p = 0.0055) for the two-target task in the ON state, unaffected by the magnitude of coexistent dyskinesia. Overall, test-retest reliability was high (r >0.75) and the discriminatory ability between OFF and ON states was good (0.60 ≤ AUC ≤ 0.82). The correlations between tapping data and MDS-UPDRS-III scores were only moderate (−0.55 to 0.55).
Conclusions
A self-administered, tablet-based application can reliably distinguish between OFF and ON states in fluctuating PD patients and may be sensitive to additional motor phenomena, such as accuracy, not captured by the MDS-UPDRS-III.
Keywords: App-based digital biomarkers, Motor symptoms, Objective monitoring of motor symptoms, Parkinson disease-related motor symptoms
Introduction
A growing body of evidence suggests that biometric monitoring using technology-based objective measures will become a valuable supplement to routine neurological evaluations [1, 2, 3, 4, 5, 6]. Technology-based objective measures can reduce rater bias and interrater variability and increase sensitivity, elucidating important subclinical changes [4, 7, 8]. As of November 2015, there were at least 73 different devices (22 wearable, 38 nonwearable, and 13 hybrid) developed to assess Parkinson disease (PD) [9], and, as of October 2016, at least 47 assessed limb bradykinesia specifically [10]. Among the wearable devices, only Kinesia [11], a hybrid device, and 6 other wearable devices have been used to assess PD by groups other than the developers [12, 13, 14, 15, 16, 17]. Besides a lack of validation, additional reasons that may contribute to the limited use of these technologies include skepticism about the usefulness of data, a perception of a high burden required for adoption, unsustained adherence, a discrepancy between research and clinical utility, the unclear value for informing management decisions, and a lack of compatibility between systems, which affect data integration and analytics [4, 18].
Finger tapping data have been successfully used to evaluate motor function in PD [19, 20, 21]. Individuals with PD often have motor fluctuations in response to commonly used medications for the condition. These fluctuations, termed “ON” and “OFF” states, are closely monitored in clinical care and PD clinical trials. We sought to assess whether a tablet-based application (iMotor; Apptomics Inc., Wellesley Hills, MA, USA) can reliably detect and quantify differences in motor performance in PD-associated OFF (reemergence of parkinsonian deficits) and ON (maximal dopaminergic medication efficacy) medication states using a standard finger tapping test.
Methods
Study Population and Design
We prospectively enrolled consecutive PD patients undergoing assessment eligibility for deep-brain stimulation from December 2015 to March 2016. Inclusion criteria were the diagnosis of idiopathic PD according to UK Brain Bank criteria [22]; Hoehn and Yahr stage I–III in the ON state [23]; and an age of 18–75 years. Excluded were any patients with history or clinical features suggestive of an atypical parkinsonian syndrome and any comorbid central nervous system disorder which could contribute to motor impairment. Healthy spouses and caregivers of PD patients were enrolled and matched for age as healthy controls. The University of Cincinnati institutional review board approved the study, and all participants gave written informed consent.
OFF/ON Assessment
The participants provided demographic information and their medical history in a structured interview on the day of their OFF/ON assessment for deep-brain stimulation eligibility. Motor subscale scores on the Movement Disorder Society version of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS-III) and iMotor tapping data were collected during both medication states. Clinical assessment, scale administration, and iMotor testing were performed in the practically defined OFF state, i.e., after withholding all dopaminergic medications for ≥12 h, and again in the ON state, i.e., after the usual dopaminergic regimen had become clinically most effective. The maximum dopaminergic medication effectiveness was determined by the combined judgment of the patient and a movement disorder specialist through a semi-structured interview and documented by a reduction in the MDS-UPDRS-III score within 60 min after medication administration.
Tablet App Testing
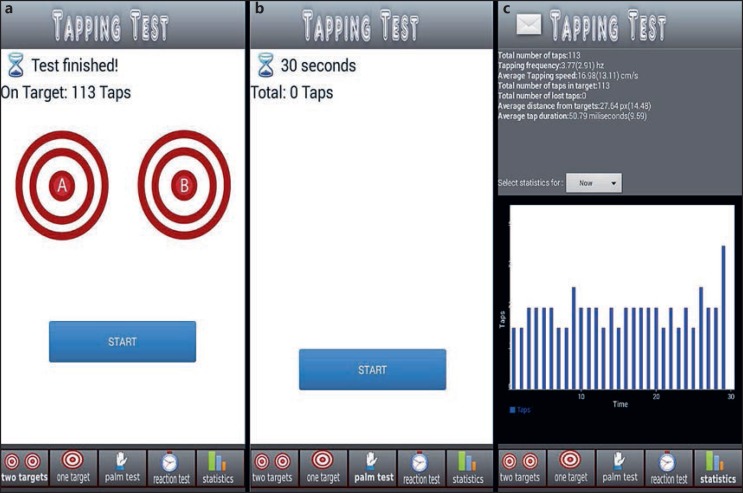
Site personnel trained the participants to follow the screens of the iMotor tablet app (Fig. 1) to perform the following tests during their OFF and ON states:
Fig. 1.
Screenshots of iMotor-based tapping tests. a Two-target test: the participants alternatingly tapped with the index finger, as fast and as accurately as possible, the centers of two concentric circles on the tablet screen. b Pronation-supination test: the participants alternatingly tapped the palmar and dorsal surfaces of their hand as fast as possible on the tablet screen. c Sample patient score report, available immediately to the patient.
Two-target finger tapping test: the participants were prompted to alternatingly tap the centers of two concentric circles with the index finger, as fast and accurately as possible; double and mis-taps due to tremor, dyskinesia, or touch screen errors were not recorded by the application in order to minimize errors in tapping data collection
Pronation-supination test: the participants were prompted to alternatingly tap the palmar and dorsal surfaces of their hand on the tablet screen as fast as possible
The total number of taps, tap interval (time [ms] between two consecutive finger/hand screen taps), tap duration (time [ms] the index finger/hand touches the screen per tap), and tap accuracy (tap distance [pixels] from the center of the target) were recorded.
The duration of each test was 30 s. User fatigue was assessed retrospectively using the patient score report (Fig. 1c). The total duration of the patient interface with the application was, on average, 5 min. The patients performed each test twice with their more affected hand (as assessed by the investigator), the controls with their dominant hand. All tests were performed with the tablet resting flat on a table in front of the patient. The tablet was in portrait orientation during the two-target test and in landscape orientation during the pronation-supination test.
Blinded Video Analysis
Videos of the patients at rest and while performing the MDS-UPDRS-III finger tapping and pronation-supination tasks, taken in the OFF and ON states, were randomized for later blinded rating. Senior Movement Disorder Fellows (F.R.-P., J.V., and L.L.) blindly rated the dyskinesia severity during each activity (0 = absent; 1 = mild/no interference with activity; 2 = severe/interferes with activity). The dyskinesia distribution was categorized into: (1) any; (2) axial; (3) appendicular in the active limb; and (4) appendicular in the nonactive limb. The dyskinesia severity ratings were then compared with the tapping accuracy scores.
Statistical Analysis
It was estimated that a sample size of 11 was needed to detect between-group differences in motor performance with 80% power and a 1% level of significance, adjusted due to multiple tests, assuming at least a 20% mean difference in each test with a 15% standard deviation (SD), estimated based on a previous study using tapping tests that compared PD patients with controls [21], using a paired t test. Data are reported as means ± SD, where applicable. Categorical variables were compared using Fisher's exact test, while quantitative variables were compared using the t test between controls and PD cases. Test-retest reliability and MDS-UPDRS-III score correlations were measured using a concordance correlation coefficient (r). Tapping data were averaged across multiple readings and compared between the OFF and the ON state. Between the OFF and the ON state, all data were compared using paired t tests. Ordinal survey satisfaction data were compared using the Wilcoxon rank-sum test between controls and PD cases. The presence of dyskinesia was compared between the OFF and the ON state using McNemar's test in each condition. Tapping accuracy was compared according to dyskinesia status and for different types using the Wilcoxon rank-sum test. Paired t test results were summarized using mean changes along with 95% confidence intervals and p values. Further, logistic regression models were performed to evaluate the individual discriminatory performance of each test in differentiating between PD-ON and PD-OFF after adjusting for clustering effects. The results were summarized using AUC (area under the receiver operating characteristic curve) and CC (correct classification) accuracy. p values <0.05 were considered significant results. All statistical analyses were carried out using SAS 9.3 and STATA 13.
Results
Patients
The cohort included 11 PD patients (73% men; 60.6 ± 9.0 years old) and 11 controls (46% men; 62.5 ± 10.5 years old) (Table 1). The patients' mean MDS-UPDRS-III score was 48.6 ± 8.2 in the OFF state and 23.0 ± 6.1 in the ON state (p < 0.0001).
Table 1.
Demographics
| Controls (n = 11) | PD patients (n = 11) | p value | |
|---|---|---|---|
| Age, years | 62±11 | 61±9 | 0.65 |
| Male | 5 (46) | 8 (73) | 0.19 |
| Education, years | 16±3 | 17±2 | 0.6 |
| White | 10 (91) | 10 (91) | 1 |
| Disease duration, years | - | 13±4 | - |
| MDS-UPDRS-III OFF score | - | 49±8 | - |
| MDS-UPDRS-IIION score | - | 23±6 | - |
| Dyskinesia (OFF state) | - | 0 | - |
| Dyskinesia (ON state) | - | 10 (91) | - |
| Dyskinesia interfered with UPDRS ratings (ON state) | - | 2 (18) | - |
| LEDD, mg | 2,199±992; 1,200–3,920 | - |
Data are reported asn (%) or mean± SD; range. PD, Parkinson disease; MDS-UPDRS, Movement Disorder Society-Unified Parkinson's Disease Rating Scale; LEDD, levodopa equivalent daily dose.
Tablet App Results
Two-Target Finger Tapping Test
Compared to the OFF state, there were more total taps (84.2 ± 20.3 vs. 54.9 ± 26.9 taps; p = 0.0036) and shorter tap intervals in the ON state (375.3 ± 97.2 vs. 708.2 ± 412.8 ms), but with a lower tapping accuracy (2,008.4 ± 995.7 vs. 1,111.8 ± 901.3 pixels; p = 0.0055) (Table 2). The controls tended to tap more accurately and had more total taps than the PD patients in the ON state (p = 0.1828 and p = 0.1517, respectively).
Table 2.
iMotor testing and reliability
| Controls | PD-OFF |
PD-ON |
pvaluea | pvalueb | pvaluec | AUC | CC, % | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mean± SD | reliability | UPDRS correlation | mean± SD | reliability | UPDRS correlation | |||||||
| Two-target test | ||||||||||||
| Total taps | 101±32 | 55±27 | 0.96 | −0.55 | 84±20 | 0.85 | −0.53 | 0.002 | 0.15 | 0.004 | 0.82 | 73 |
| Tap interval, ms | 324±93 | 708±413 | 0.92 | 0.51 | 375±97 | 0.75 | 0.41 | 0.01 | 0.22 | 0.01 | 0.80 | 77 |
| Tap duration, ms | 113±120 | 74±65 | 0.88 | 0.35 | 108±158 | 0.95 | −0.40 | 0.36 | 0.94 | 0.55 | 0.60 | 46 |
| Tap accuracy, pixels | 1,515±646 | 1,112±901 | 0.77 | −0.51 | 2,008±996 | 0.90 | −0.46 | 0.24 | 0.18 | 0.006 | 0.78 | 73 |
| Pronation-supination test | ||||||||||||
| Total taps | 84±21 | 55±21 | 0.60 | −0.31 | 78±20 | 0.83 | −0.55 | 0.006 | 0.54 | 0.003 | 0.77 | 68 |
| Tap interval, ms | 377±92 | 650±387 | 0.81 | 0.27 | 409±112 | 0.76 | 0.42 | 0.04 | 0.50 | 0.03 | 0.75 | 64 |
| Tap duration, ms | 4±2 | 3±1 | 0.06 | 0.10 | 3±2 | 0.88 | 0.20 | 0.26 | 0.89 | 0.46 | 0.45 | 50 |
Data are reported as the mean± SD unless specified otherwise. Data were collected for most affected side and dominant hand forp and controls, respectively. PD-OFF/ON, Parkinson disease patients in the OFF state (reemergence of parkinsonian deficits)/ON state (maximal dopaminergic medication efficacy); UPDRS, Movement Disorder Society-Unified Parkinson's Disease Rating Scale; AUC, area under the curve for PD-OFF vs. PD-ON; CC, correct classification.
Unpaired/Satterthwaitet test between controls and PD-OFF.
Unpaired/Satterthwaitet test between controls and PD-ON.
Pairedt test between PD-OFF and PD-ON.
Pronation-Supination Test
Compared to the OFF state, there were more total taps (78.1 ± 20.2 vs. 54.9 ± 20.8 taps; p = 0.0027) and shorter tap intervals in the ON state (408.6 ± 112.2 vs. 649.9 ± 386.5 ms; p = 0.0315). Accuracy was not measured during the pronation-supination test.
Tablet App Performance
With the exception of tap duration, the other iMotor-measured tasks distinguished PD-OFF from PD-ON (AUC = 0.75–0.82; CC = 64–77%) (Table 2). Test-retest reliability was high in both the ON state (r = 0.85 ± 0.07; r range, 0.75–0.95) and the OFF state (r = 0.71 ± 0.31; r range, 0.06–0.96). Two-target and pronation-supination tapping test data correlated only moderately with MDS-UPDRS-III scores (−0.55 to 0.51).
Dyskinesia and Accuracy
Dyskinesia severity did not affect tapping accuracy (Table 3). Tapping accuracy tended to be lower in patients who were dyskinetic at rest (p = 0.1709), but this trend did not persist during the two-target tapping and pronation-supination tasks.
Table 3.
Tapping accuracy categorized by the presence of dyskinesia according to blinded video ratings
| Dyskinesia type | No dyskinesia | Dyskinesia | pvalue | κ | |
|---|---|---|---|---|---|
| At rest | any | 1,668±884 | 2,293±713 | 0.17 | 0.32 |
| limb | 1,701±1,126 | 2,378±762 | 0.17 | 0.63 | |
| axial | 1,824±1,187 | 2,230±778 | 0.41 | 0.88 | |
| Finger tapping | any | 2,691±1,664 | 1,857±867 | 0.41 | 0.31 |
| axial | 2,663±1,178 | 1,763±876 | 0.18 | 0.27 | |
| active limb | 2,043±1,089 | 1,915±890 | 1 | 0.27 | |
| nonactive limb | 2,642±1,130 | 1,646±770 | 0.16 | 0.26 | |
| Pronation-supination | any | 2,453±1,498 | 1,842±812 | 0.61 | 0.39 |
| axial | 2,218±1,310 | 1,888±865 | 0.78 | 0.74 | |
| active limb | 2,072±1,073 | 1,723±712 | 0.91 | 0.24 | |
| nonactive limb | 2,243±1,241 | 1,727±612 | 0.65 | 0.02 | |
Data reported are pixels away from the center of the target (less is more accurate). Patients were categorized as having dyskinesia vs. no dyskinesia by blinded video analysis. Dyskinesias were further defined as: any; axial; active limb (extremity associated with the task); and nonactive limb (extremities not associated with the task).κvalues represent slight (0.01–0.20), fair (0.21–0.40), moderate (0.41–0.60), substantial (0.61–0.80), and almost perfect agreement (0.81–0.99) [28].
Discussion
The tablet-based application captured an increase in the total number of taps and a corresponding reduction in the tap interval in the ON state compared to the OFF state, reliably distinguishing these two states. Notably, motor accuracy, a feature not readily measurable with clinical scales, lowered with an increased tapping frequency in the ON state. This reduction in motor accuracy was not affected by (or due to) any coexistent dyskinesia. Finally, the results were consistent between the two tapping tests, and repeatable within each test for both medication states.
An increased tapping speed may require sacrificing accuracy, or speed and accuracy may be modulated by separate mechanisms. The lower tapping accuracy in the ON (hyperdopaminergic) state may be due to the hypothesis of Vaillancourt et al. [24]: a dopamine overdose effect in the ventral and anterior striatum could negatively affect the cognitive challenge of tapping both quickly and accurately. In fact, multiple cognitive domains may be adversely affected by dopamine flooding [25]. The blinded video analysis suggested dyskinesia did not affect accuracy, regardless of the presence of dyskinesia in the upper or lower extremity. Voluntary movement during the tapping tests may have overridden dyskinetic movement. Tremor could have influenced tapping accuracy, but it is unlikely to fully account for the observed reduction, since PD patients in the OFF state tended to be more accurate than controls.
It is important to note that tapping tasks are widely used by clinicians as methods of assessing bradykinesia, a core element of PD. However, total taps and tap accuracy are not properly captured by the MDS-UPDRS-III, which focuses on rhythm, interruptions, slowing, and decrements in amplitude. These differences may have contributed to what represents only a moderate correlation with MDS-UPDRS-III scores. As subjective scales are limited in accuracy and reliability [1], a moderate correlation suggests a tablet-based application may be capable of detecting differences in motor function beyond those probed by the MDS-UPDRS-III. In fact, the test-retest reliability for both medication states was higher than that reported for the MDS-UPDRS-III (0.60–0.71) [26]. Thus, a “perfect” correlation of any technology-based objective measure with MDS-UPDRS-III scores may not be achievable or even desirable if objective measures capture motor data more accurately and reliably.
PD-specific motor scales also have limitations in logistic deployment, reliability, and spatial resolution [1, 2, 27]. These shortcomings may lower the signal-to-noise ratio in the measurement of relevant endpoints in clinical trials, lowering the ability of finding statistically significant differences between interventions of interest. On the other hand, technology-based objective measures can detect functional abnormalities without reliance on skilled administrators, and the results can be comparable across centers, understood by patients, and readily integrated into medical records. Importantly, technology-based objective measures can be easily deployed in the home setting, capture different time points in the dopaminergic cycle, cover a larger spectrum of motor behaviors, and aid in decision-making and response monitoring. Within this context, assessment of motor fluctuation with technology-based objective measures, both in clinical trials and in daily monitoring, offers potential improvement in PD and other movement disorders.
Some limitations need to be acknowledged with regard to the study design and the tablet-based application. The small sample size does not allow robust generalizations. However, there was sufficient power for the relatively large effects between the OFF and the ON medication state to be significant. Also, dopaminergic states were considered binary rather than continuous, as is common in clinical trials. Recording motor function at multiple levels of dopaminergic stimulation would have been optimal, but it is methodologically more complex and time-consuming. Additionally, upon conducting our analysis, the variation observed in some measures was higher than the predicted SD of 15%. However, robust statistical significance was still met for the primary outcome measures, and, therefore, enrollment was stopped. Further, the tablet-based application relied on tapping tests as a proxy for motor function, largely by virtue of its widespread use as part of the MDS-UPDRS-III. It may not be the task best reflective of global movement, which was not characterized by complementary measures. Finally, there could have been touch screen errors that were not observed by the study personnel monitoring the test and that were not recorded by the application.
Conclusions
A tablet-based application reliably captured differences in motor performance between the OFF and the ON state of PD patients, identifying a tradeoff between velocity and accuracy for tapping. The moderate correlation between iMotor and MDS-UPDRS scores suggests that tablet-based applications may be more sensitive and accurate at measuring performance than clinical scales, and that they could serve as useful adjuncts in clinical trials and home-based assessments.
Statement of Ethics
The authors confirm that all subjects gave written, informed consent. The study protocol was approved by the University of Cincinnati Institutional Review Board (#2014-0626).
Disclosure Statement
B.D.W. is supported by the NIH (T32GM063483-14). G.M. is the founder and CEO of Apptomics, Inc. A.K.D. is supported by the NIH as a co-investigator (1R01HL125016-01) and as a collaborator (R21 AI118228). He has also been serving as a statistician in 4 CPRIT grants (PP110156, PP140211, PP150031, and PP130083), Coldwell (co-investigator), and MSA Coalition (collaborator) and as a principal investigator in a TTUHSC ELP mini seed grant. He is a director of biostatistics and epidemiology consulting laboratory at the TTUHSC ELP.
S.P. is a full-time employee at TEVA Pharmaceuticals and co-chair of the Task Force on Technology for the International Parkinson and Movement Disorder Society. S.K., J.R.L.C., and E.S. have nothing to disclose. A.P.D. has served as a consultant for Merz Pharma, US WorldMeds, and Auspex Pharmaceuticals and has received honoraria from UCB.
F.R.-P., J.V., L.L., I.T., and A.S. have nothing to disclose. A.J.E. is the chair of the Task Force on Technology for the International Parkinson and Movement Disorder Society, is supported by the NIH, and has received grant support from Cleveland Medical Devices Inc./Great Lakes NeuroTechnologies, the Davis Phinney Foundation, and the Michael J. Fox Foundation; personal compensation as a consultant/scientific advisory board member for Solvay, Abbott, Chelsea Therapeutics, TEVA, Impax, Merz, Lundbeck, and Eli Lilly; honoraria from TEVA, UCB, the American Academy of Neurology, and the Movement Disorder Society; and publishing royalties from Lippincott Williams & Wilkins, Cambridge University Press, and Springer. He has no financial interests in, nor has received compensation from, iMotor or Apptomics, Inc.
Funding Sources
The study was unfunded. Apptomics, Inc. provided two tablets, the iMotor application, and technical support. With the exception of G.M. and S.P., none of the investigators have a financial interest in iMotor or Apptomics, Inc.
Author Contributions
1. Research project: (A) conception; (B) organization; (C) execution
2. Statistical analysis: (A) design; (B) execution; (C) review and critique
3. Manuscript preparation: (A) writing of the first draft; (B) review and critique
B.D.W.: 1B, 1C, 2C, and 3A
G.M.: 1B, 1C, 2C, and 3B
A.K.D.: 1C, 2A, 2B, 2C, and 3B
S.P., S.L., J.R.L.C., E.S., A.P.D., F.R.-P., J.V., L.L., I.T., and A.S.: 1C and 3B
A.J.E.: 1A, 1B, 1C, 2C, and 3B
References
- 1.Heldman DA, Espay AJ, LeWitt PA, Giuffrida JP. Clinician versus machine: reliability and responsiveness of motor endpoints in Parkinson's disease. Parkinsonism Relat Disord. 2014;20:590–595. doi: 10.1016/j.parkreldis.2014.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heldman DA, Giuffrida JP, Chen R, Payne M, Mazzella F, Duker AP, et al. The modified bradykinesia rating scale for Parkinson's disease: reliability and comparison with kinematic measures. Mov Disord. 2011;26:1859–1863. doi: 10.1002/mds.23740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lieber B, Taylor BE, Appelboom G, McKhann G, Connolly ES., Jr Motion sensors to assess and monitor medical and surgical management of Parkinson disease. World Neurosurg. 2015;84:561–566. doi: 10.1016/j.wneu.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 4.Espay AJ, Bonato P, Nahab FB, Maetzler W, Dean JM, Klucken J, et al. Technology in Parkinson's disease: challenges and opportunities. Mov Disord. 2016;31:1272–1282. doi: 10.1002/mds.26642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arora S, Venkataraman V, Zhan A, Donohue S, Biglan KM, Dorsey ER, et al. Detecting and monitoring the symptoms of Parkinson's disease using smartphones: a pilot study. Parkinsonism Relat Disord. 2015;21:650–653. doi: 10.1016/j.parkreldis.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 6.Dorsey ER, Papapetropoulos S, Xiong M, Kieburtz K. The first frontier: digital biomarkers for neurodegenerative disorders. Digital Biomarkers. 2017;1:6–13. doi: 10.1159/000477383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horak FB, Mancini M. Objective biomarkers of balance and gait for Parkinson's disease using body-worn sensors. Mov Disord. 2013;28:1544–1551. doi: 10.1002/mds.25684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maetzler W, Domingos J, Srulijes K, Ferreira JJ, Bloem BR. Quantitative wearable sensors for objective assessment of Parkinson's disease. Mov Disord. 2013;28:1628–1637. doi: 10.1002/mds.25628. [DOI] [PubMed] [Google Scholar]
- 9.Godinho C, Domingos J, Cunha G, Santos AT, Fernandes RM, Abreu D, et al. A systematic review of the characteristics and validity of monitoring technologies to assess Parkinson's disease. J Neuroeng Rehabil. 2016;13:24. doi: 10.1186/s12984-016-0136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasan H, Athauda DS, Foltynie T, Noyce AJ. Technologies assessing limb bradykinesia in Parkinson's disease. J Parkinsons Dis. 2017;7:65–77. doi: 10.3233/JPD-160878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giuffrida JP, Riley DE, Maddux BN, Heldman DA. Clinically deployable Kinesia technology for automated tremor assessment. Mov Disord. 2009;24:723–730. doi: 10.1002/mds.22445. [DOI] [PubMed] [Google Scholar]
- 12.Mancini M, King L, Salarian A, Holmstrom L, McNames J, Horak FB. Mobility lab to assess balance and gait with synchronized body-worn sensors. J Bioeng Biomed Sci. 2011;(suppl 1):007. doi: 10.4172/2155-9538.S1-007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aminian K, Robert P, Buchser EE, Rutschmann B, Hayoz D, Depairon M. Physical activity monitoring based on accelerometry: validation and comparison with video observation. Med Biol Eng Comput. 1999;37:304–308. doi: 10.1007/BF02513304. [DOI] [PubMed] [Google Scholar]
- 14.Speelman AD, van Nimwegen M, Borm GF, Bloem BR, Munneke M. Monitoring of walking in Parkinson's disease: validation of an ambulatory activity monitor. Parkinsonism Relat Disord. 2011;17:402–404. doi: 10.1016/j.parkreldis.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Hale LA, Pal J, Becker I. Measuring free-living physical activity in adults with and without neurologic dysfunction with a triaxial accelerometer. Arch Phys Med Rehabil. 2008;89:1765–1771. doi: 10.1016/j.apmr.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 16.Van Hees VT, Slootmaker SM, De Groot G, Van Mechelen W, Van Lummel., RC Reproducibility of a triaxial seismic accelerometer (DynaPort) Med Sci Sports Exerc. 2009;41:810–817. doi: 10.1249/MSS.0b013e31818ff636. [DOI] [PubMed] [Google Scholar]
- 17.Godfrey A, Lara J, Munro CA, Wiuff C, Chowdhury SA, Del Din S, et al. Instrumented assessment of test battery for physical capability using an accelerometer: a feasibility study. Physiol Meas. 2015;36:N71–N83. doi: 10.1088/0967-3334/36/5/N71. [DOI] [PubMed] [Google Scholar]
- 18.Maetzler W, Klucken J, Horne M. A clinical view on the development of technology-based tools in managing Parkinson's disease. Mov Disord. 2016;31:1263–1271. doi: 10.1002/mds.26673. [DOI] [PubMed] [Google Scholar]
- 19.Djurić-Jovičić M, Petrović I, Ječmenica-Lukić M, Radovanović S, Dragašević-Mišković N, Belić M, et al. Finger tapping analysis in patients with Parkinson's disease and atypical parkinsonism. J Clin Neurosci. 2016;30:49–55. doi: 10.1016/j.jocn.2015.10.053. [DOI] [PubMed] [Google Scholar]
- 20.Giancardo L, Sánchez-Ferro A, Arroyo-Gallego T, Butterworth I, Mendoza CS, Montero P, et al. Computer keyboard interaction as an indicator of early Parkinson's disease. Sci Rep. 2016;6:34468. doi: 10.1038/srep34468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitsi G, Mendoza EU, Wissel BD, Barbopoulou E, Dwivedi AK, Tsoulos I, et al. Biometric digital health technology for measuring motor function in Parkinson's disease: results from a feasibility and patient satisfaction study. Front Neurol. 2017;8:273. doi: 10.3389/fneur.2017.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 1988;51:745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 24.Vaillancourt DE, Schonfeld D, Kwak Y, Bohnen NI, Seidler R. Dopamine overdose hypothesis: evidence and clinical implications. Mov Disord. 2013;28:1920–1929. doi: 10.1002/mds.25687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanna-Pladdy B, Pahwa R, Lyons KE. Paradoxical effect of dopamine medication on cognition in Parkinson's disease: relationship to side of motor onset. J Int Neuropsychol Soc. 2015;21:259–270. doi: 10.1017/S1355617715000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez-Martin P, Rodriguez-Blazquez C, Alvarez-Sanchez M, Arakaki T, Bergareche-Yarza A, Chade A, et al. Expanded and independent validation of the Movement Disorder Society-Unified Parkinson's Disease Rating Scale (MDS-UPDRS) J Neurol. 2013;260:228–236. doi: 10.1007/s00415-012-6624-1. [DOI] [PubMed] [Google Scholar]
- 27.Espay AJ, Beaton DE, Morgante F, Gunraj CA, Lang AE, Chen R. Impairments of speed and amplitude of movement in Parkinson's disease: a pilot study. Mov Disord. 2009;24:1001–1008. doi: 10.1002/mds.22480. [DOI] [PubMed] [Google Scholar]
- 28.Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37:360–363. [PubMed] [Google Scholar]