Abstract
Water–salt stress and nutrient limitation may affect leaf economic spectrum of halophytes and confuse our understanding on plant physiological principles in a changing world. In this study, three halophytic plant communities of Phragmites australis, Suaeda salsa, and Tamarix chinensis, were selected in two sites (sites 1 and 2) on the west coast of Bohai Sea. The net photosynthetic rate (Pn), transpiration rate (Tr), stomatal conductance (Gs), leaf vapor pressure deficit (VPDleaf) and their influencing factors were studied to test the possible carbon assimilation strategies of the halophytes. P. australis had higher Pn, Tr, and Gs than S. salsa and T. chinensis in both sites. Similar trends were found for leaf P and photosynthetic N and P efficiency (PNUE and PPUE, respectively) in one or both sites. By contrast, the leaf dry mass per area (LMA) increased in the order of P. australis < S. salsa < T. chinensis in both sites. For identical species in different sites, Pn, leaf P, and PNUE were lower but Tr, VPDleaf, leaf N, leaf N:P, and PPUE were higher in site 1 than in site 2 for one or more halophytes. Although soil physicochemical properties in different sites explained several variations among the halophytes, two-way ANOVA indicated that the species can explain most of the leaf traits compared with the site. LMA also had significant nonlinear relationships with Pn, Tr, Gs, and VPDleaf. PNUE and PPUE showed positive correlation with Pn in both sites, but they decreased in the power-law function with increasing LMA. Overall, the redundancy analysis showed that the gas exchange capacity of the halophytic plant communities was significantly affected by PPUE (60.0% of explanation), PNUE (57.1%), LMA (35.0%), leaf P (22.0%), and soil N (15.8%).
Introduction
Photosynthesis is one of the most important physiological processes in plants [1]. The photosynthetic products, i.e., carbon hydrates, are the basic material and energy source for all living beings [2]. Leaf N and P concentrations are crucial parameters for gas exchange activities [3–5]. Over the past decades, ecologists have become increasingly concerned with quantifying the correlations between photosynthetic characters and leaf nutrients. These ecologists have discovered that foliar N and/or P are positively correlated with the photosynthetic rate (Pn) in regional and global scales [6–10].
The physiological responses of plants to N availability are well-documented [11–13]. For example, the correlations between photosynthetic capacity and leaf N concentration are always shown positively for many plants in terrestrial ecosystems [6–10, 14], because most of the leaf N is invested in photosynthetic apparatus [15, 16]. Although the relationships between photosynthetic capacity and leaf P concentration are not familiar with photosynthesis-leaf N [17, 18], leaf P is correlated with photosynthetic capacity positively under P-limited conditions [4, 9, 19]. However, those correlations are poor under P-rich conditions [20, 21]. Other leaf traits, such as leaf texture and structure, can also affect leaf photosynthesis under many conditions. For example, high leaf dry mass per area (LMA) in a barren environment indicates a large carbohydrate allocation pattern in leaves, thereby leading to a nutrient dilution effect in the photosynthetic apparatus and reducing leaves’ carbon assimilation capacity [22]. The LMA is also strongly correlated with leaf P than with leaf N in P-limited ecosystems [23].
The large-scale quantification for leaf trait relationships has considerably improved our understanding of the leaf economic spectrum, but this spectrum tends to be regulated by soil nutrient supply [24]. Photosynthesis is an environmentally sensitive activity in the changing world [25]. Thus, nutrient deficiency in soils might trigger a series of adjustments, which will eventually lead to fluctuations in the leaf economic spectrum. At present, the world is undergoing a series of climate changes, such as the increase in atmospheric CO2, global warming, and N deposition. These phenomena are mainly caused by anthropogenic activities and predicted to increase in the near future [26]. Under these climate change conditions, the primary production of plants is predicted to depend on P but not N availability [27], because the P-limited phenomenon may be more prevalent than we think in the changing world [28]. Although various useful information on P-limited ecosystems such as tropical forests, rivers, and lakes in many areas [9, 29, 30] has been documented, evidence regarding the halophytic plant communities of coastal wetlands is limited.
In general, N is the predominant limiting nutrient of plants in coastal wetland [31]. However, in recent decades, atmospheric N deposition has become widespread in coastal areas due to the increasing population density [32]. The atmospheric P deposition is almost negligible [33], but it results in a high anthropogenic N deposition and high deposited N:P ratios in those coastal regions. The amounts of fertilizer inputs also have increased exponentially since the 1950s, which indicates that coastal waters receive more N than P fertilizers through surface runoff and sea-going rivers [32, 34]. Therefore, the imbalance in N and P inputs leads to a shift from N-limited to P-limited environments in coastal wetlands. This nutrient limitation shift may affect the relationships between leaf traits and ultimately change the leaf economic spectrum of plant. The leaves of halophytes are different from those of other terrestrial plants. Halophytes often have large photosynthetic cells and chloroplast ultrastructures due to their long-term adaptation to salt stress, thereby possibly affecting their leaf economic spectrum and confusing our understanding of plant physiological principles under climate changes.
In this study, three typical halophytic plant communities of Phragmites australis, Suaeda salsa, and Tamarix chinensis were selected in two sites (sites 1 and 2) on the west coast of Bohai Sea. The objectives of this study are as follows: (1) to document the gas exchange characteristics and their correlations with other leaf traits of halophytes in the coastal wetlands; (2) to test the influencing factors of gas exchange capacity of halophytes in the coastal wetlands in nutrient limitation conditions; and (3) to discuss the possible strategies adopted by halophytes in photosynthetic carbon assimilation under the nutrient limitation conditions.
Materials and methods
Ethics statement
Our experiment was performed in the Yellow River Delta Nature Reserve and the Beidagang Wetland Nature Reserve, China. This study was approved by the Yellow River Delta Nature Reserve Management Bureau of Shandong and Beidagang Wetland Nature Reserve Management Center of Tianjin. We confirmed that our study has no harm to the environment, and the field studies did not involve endangered or protected species.
Site description
This study was carried out in two typical coastal wetlands on the west coast of Bohai Sea. Site 1 is located at the Yellow River Delta Nature Reserve (37°35′–38°12′N, 118°33′–119°20′E) of Dongying, China. Dongying is an agricultural high-tech industry demonstration area in Shandong Province. The total area of Yellow River Delta Nature Reserve is 1530 km2, with a supratidal area of 827 km2, intertidal area of 382.5 km2, and shallow sea area (<3 m in low-tide) of 320.5 km2. In this study, the halophytic plant communities were selected from coastal wetlands of the supratidal area in site 1. This region has a typical continental monsoon climate. The mean annual temperature, evaporation, and precipitation are 12.3°C, 1926.1 mm, and 542.3 mm, respectively. The frost-free period is 199 days, and the rainy season starts in June and ends in August. Intrazonal tidal soil and salty soil are distributed in this region, and the dominant species are P. australis, T. chinensis, and S. salsa [35]. Site 2 is located on the Beidagang Wetland Nature Reserve (38°36′–38°57′N, 117°11′–117°37′E) of Tianjin, China. In contrast to the agricultural city of Dongying, Tianjin is an important industrial city of China. The total area of Beidagang Wetland Nature Reserve is 348.87 km2, with a core area of 115.72 km2, buffer area of 91.96 km2, and experimental area of 141.19 km2. Beidagang Wetland Nature Reserve is the largest wetland nature reserve of Tianjin, and the types of wetlands include coastal wetlands, rivers, swamps, ditches, and other artificial wetlands. The halophytic plant communities were selected from coastal wetlands of the experimental area in site 2. The climate of this region is dominated by a northern subtropical monsoon. The mean annual temperature, evaporation, and precipitation are 11.3°C, 1500 mm, and 600 mm, respectively. The rainy season extends from June to September [36]. The soil types of Tianjin coastal wetlands are meadow solonchak and salinized meadow soil, and the dominant species are herbaceous plants, that is, S. salsa and P. australis and shrubs, such as T. chinensis.
Photosynthesis measurements
In the wet season of 2015, the three typical halophyte plant communities of P. australis, S. salsa and T. chinensis were chosen at each site. Six quadrats (1 m × 1 m for P. australis and S. salsa communities and 3 m × 3 m for T. chinensis community) were randomly set in each community. In each community, five individuals were selected for photosynthesis measurement. In this research, a portable leaf chamber and open system infrared gas analyzer (CI-340; CID, Inc., USA) were used to measure the photosynthetic characters, including the net photosynthetic rate (Pn), transpiration rate (Tr), stomatal conductance (Gs), and leaf vapor pressure deficit (VPDleaf). Similar and uniform sunlit days were selected to minimize the sources of diurnal heterogeneity. The gas analyzer was calibrated daily and checked periodically throughout the measurement period. Measurement was performed from mid-morning to late morning (08:30–10:30, depending on local weather). Leaves with no signs of injury or disease for each individual were measured at ambient CO2 concentrations (approximately 370 μmol·mol−1) and under natural light conditions. The photosynthetic photon flux density ranged from 1000 μmol·m−2·s−1 to 1200 μmol·m−2·s−1, which proved to be saturated for photosynthesis rate. One leaf chamber (CI-301LC-2, CID, Inc., USA) was used for P. australis measurement. Another leaf chamber (CI-301LC-5, CID, Inc., USA) was used for S. salsa and T. chinensis measurement. The air flow rate was 0.3 L·min-1, and the ambient temperature ranged from 26°C to 28°C.
Field sampling
After photosynthesis measurement, the same leaves were removed from the branches of each individual for leaf area measurement. For all halophytes, multiple leaves were collected, and the leaf area was measured by a laser area meter (CI-202, CID, Inc., USA). Then, the leaves were dried in an oven for 65°C for at least 48 h and weighed. LMA was calculated as leaf dry mass divided by leaf area. Finally, all dried leaves were ground into powder for N and P measurements. Five soil samples were also collected randomly at the depth of 0–10 cm in each quadrat. Then, the samples of the same layers were mixed as one sample, placed inside polyethylene bags, and brought to the laboratory. A total of 36 soil samples were collected from both the two sites. All soil samples were air dried and ground into powder for chemical analysis.
Measurements for plant and soil nutrients
The total N concentrations of plant and soil samples was determined using an elemental analyzer (Vario EL III, Elementar, Germany), whereas the total P concentrations of the plant and soil samples were measured by inductively coupled plasma optical emission spectrometry (Varian, Inc., USA).
Photosynthetic nutrient use efficiency calculation
Photosynthetic data and leaf N or P were used to calculate the photosynthetic N (PNUE) and P use efficiency (PPUE), as defined by Field and Mooney [14]. PNUE was calculated as the ratio between photosynthetic rate per mass (Pn/LMA) and leaf N concentration, and PPUE was computed as the ratio between photosynthetic rate per mass and leaf P concentration.
Soil physicochemical property measurements
Soil pH was measured by a potentiometric method with a soil–water ratio of 1:2.5 (IQ-150 Spectrum Technologies, Inc., Germany). The soil water content was measured by a soil moisture measuring instrument (HD2, IMKO, Germany). The soil salt concentration was determined using the weighing method with a soil–water ratio of 1:5. To avoid experimental errors, we repeated all measurements thrice.
Data analysis
All data used in this study were the arithmetical averages of multiple repetitions (S1 Dataset). Then, one-way ANOVA was used to test the soil physicochemical properties (soil N, soil P, salinity, SWC, and pH), gas exchange parameters (Pn, Tr, Gs, and VPDleaf), leaf chemical and structural traits (leaf N, leaf P, leaf N:P, and LMA), and photosynthetic nutrient use efficiency (PNUE and PPUE) of the three halophytic plant communities at the same site. In multiple comparison tests, the Games–Howell method was used when variances were assumed to be heterogeneous using the Levene’s test. Tukey’s method was applied when variances were homogeneous. For identical species between the two sites, the differences were tested by t-test. We also used the regression approach to two-way ANOVA as implemented in a general linear model using type Ш sums of squares. The model included species, site, and their interaction (species × site). The significance of the effects was tested using F-ratios between the mean squares of effects and residuals. All statistical analyses were conducted in IBM SPSS Statistics 22.0 for Windows (SPSS Inc., Chicago, IL, USA).
A redundancy analysis (RDA) and Monte Carlo test were performed using Canoco 4.5 software to evaluate the relationships between gas exchange characteristics and their influencing factors of all halophytic plant communities, including P. australis, S. salsa, and T. chinensis in sites 1 and 2. A standardized major axis regression was used to describe the pairwise relationships of PNUE-Pn and PPUE-Pn. The allometric equation parameters were calculated using (S) MATR version 2.0 [37]. The heterogeneity of the regression slopes was tested using a method introduced by Warton and Weber [38]. When the homogeneity of the slope occurred, the differences in the y-intercept of the regression slopes were also tested by WALD test. In the present study, the carbon assimilation strategies of the halophytes in different sites were explained by slopes and y-intercepts.
Results
Gas exchange characteristics of halophytes
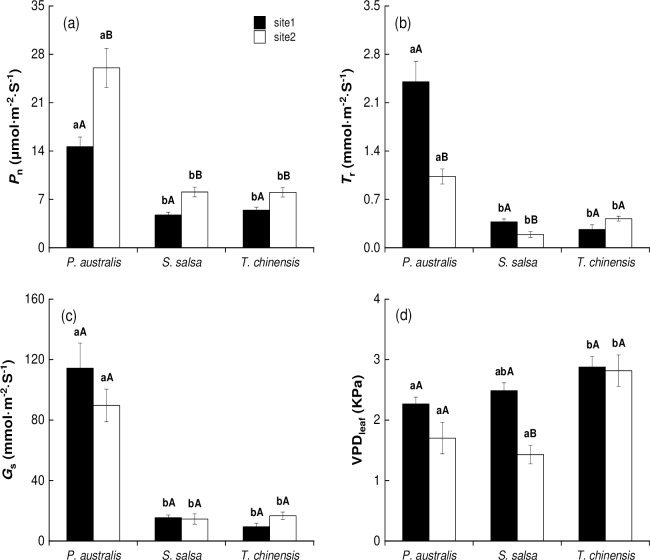
P. australis had higher Pn, Tr, and Gs than S. salsa and T. chinensis (Fig 1A, 1B and 1C; P < 0.05). However, the differences between S. salsa and T. chinensis were not significant (P > 0.05). T. chinensis had higher VPDleaf than P. australis and S. salsa in site 2 (Fig 1D, P < 0.05), but the differences between P. australis and S. salsa were not significant (Fig 1D, P > 0.05).
Fig 1. Gas exchange characters (mean ± SE) of halophytic plant communities.
(a) Net photosynthetic rate (Pn), (b) transpiration rate (Tr), (c) stomatal conductance (Gs), and (d) leaf vapor pressure deficit (VPDleaf). The different lower letters indicate significant differences (P<0.05) between the different species in the same site; the different upper letters indicate significant differences (P<0.05) between the same species in different sites.
For identical species in different sites, P. australis in site 1 had lower Pn but higher Tr than P. australis in site 2 (Fig 1A and 1B, P < 0.05). S. salsa and T. chinensis in site 1 had lower Pn than those in site 2. However, the Tr and VPDleaf of S. salsa were higher in site1 than in site 2 (Fig 1A, 1B and 1D; P < 0.05). The Gs of the three species had no significant differences between the two sites (Fig 1C, P > 0.05).
LMA and leaf nutrient conditions of halophytes
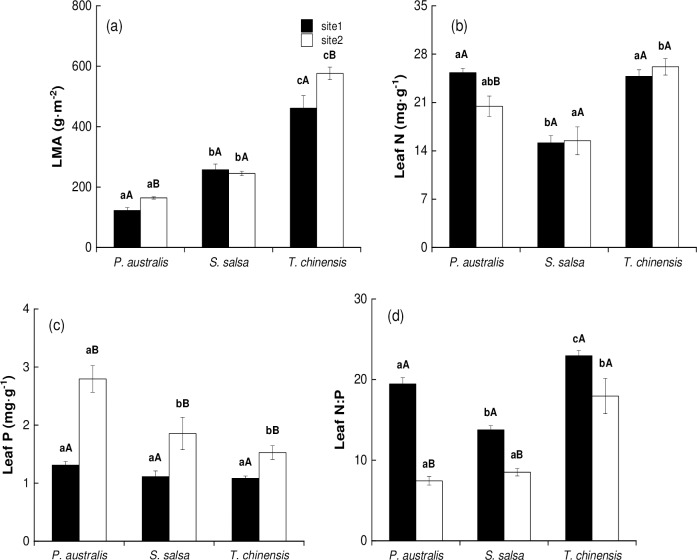
For different species in site 1, the LMA was highest for T. chinensis, followed by S. salsa and P. australis (Fig 2A, P < 0.05). Leaf N was lowest for S. salsa but did not differ between P. australis and T. chinensis (Fig 2B, P > 0.05). The difference for leaf P among the three halophytic plant communities in site 1 was not significant (Fig 2C, P > 0.05). However, leaf N:P was highest for T. chinensis, followed by P. australis and then S. salsa (Fig 2D, P < 0.05).
Fig 2. Leaf mass per area (LMA) and leaf nutrient conditions (mean ± SE) of halophytic plant communities.
(a) LMA, (b) leaf N, (c) leaf P, and (d) leaf N:P. The different lower letters indicate significant differences (P<0.05) between the different species in the same site; the different upper letters indicate significant differences (P<0.05) between the same species in different sites.
For different species in site 2, the LMA was highest for T. chinensis, followed by S. salsa and P. australis (Fig 2A, P < 0.05). T. chinensis had higher leaf N than S. salsa (Fig 2B, P < 0.05), but the differences between P. australis and the two other communities were not significant (P > 0.05). Leaf P was highest for S. salsa, followed by P. australis and T. chinensis (Fig 2C, P < 0.05). Leaf N:P was highest for T. chinensis (Fig 2D, P < 0.05), followed by S. salsa and P. australis, but the difference between these two species was not significant (P > 0.05).
For identical species in different sites, P. australis in site 1 had lower LMA and leaf P, but higher leaf N and N:P than in site 2. S. salsa in site 1 had lower leaf P and higher N:P than in site 2. However, the differences for LMA and leaf N between the two sites were not significant. T. chinensis in site 1 had lower LMA and leaf P than in site 2 (Fig 2A and 2C, P < 0.05). However, the differences for leaf N and leaf N:P between the two study sites were not significant (Fig 2B and 2D, P > 0.05).
Relationships between LMA and gas exchange characteristics
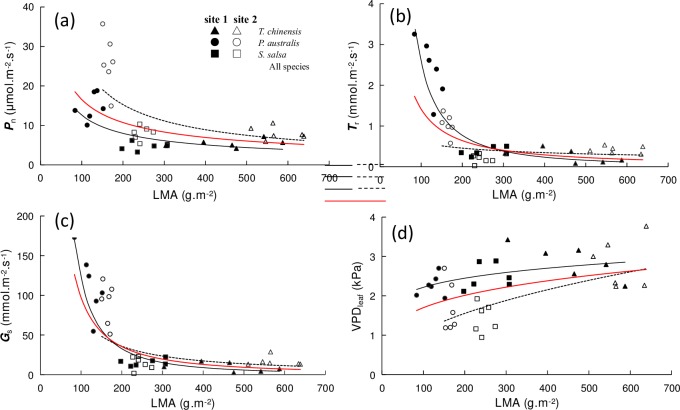
Leaf Pn showed a significant decrease in power law with increasing LMA for species in site 1 (Fig 3A, P = 0.001, R2 = 0.489) and site 2 (Fig 3A, P = 0.001, R2 = 0.481), respectively. A similar trend was found for all species pooled together (Fig 3A, P < 0.001, R2 = 0.321). Tr showed a significant decrease in power law with increasing LMA for the species in site 1 (Fig 3B, P < 0.001, R2 = 0.792) and for all the species pooled together (Fig 3B, P < 0.001, R2 = 0.375). However, the changes for Tr with increasing LMA for species in site 2 were not significant (Fig 3B, P = 0.358, R2 = 0.053).
Fig 3. Relationships between LMA and gas exchange characteristics of halophytic plant communities.
(a) LMA and Pn (equation for site 1: y = 255.59x−0.654, R2 = 0.489, P = 0.001; equation for site 2: y = 935.77x−0.777, R2 = 0.481, P = 0.001; equation for all species: y = 295.94x−0.626, R2 = 0.321, P < 0.001); (b) LMA and Tr (equation for site 1: y = 7037x−1.721, R2 = 0.792, P < 0.001; equation for site 2: y = 3.8727x−0.403, R2 = 0.053, P = 0.358; equation for all species: y = 268.35x−1.139, R2 = 0.375, P < 0.001); (c) LMA and Gs (equation for site 1: y = 729930x−1.89, R2 = 0.804, P < 0.001; equation for site 2: y = 9216.9x−1.048, R2 = 0.270, P = 0.027; equation for all species: y = 80893x−1.46, R2 = 0.511, P < 0.001); (d) LMA and VPDleaf (equation for site 1: y = 1.1491x0.1426, R2 = 0.272, P = 0.026; equation for site 2: y = 0.1273x0.4721, R2 = 0.397, P = 0.005; equation for all species: y = 0.5527x0.2437, R2 = 0.160, P = 0.015).
Gs showed a significant decrease in power law with increasing LMA for the species in sites 1 (Fig 3C, P < 0.001, R2 = 0.804) and 2 (Fig 3C, P = 0.027, R2 = 0.270) for all the species pooled together (Fig 3C, P < 0.001, R2 = 0.511). VPDleaf showed a significant increase in power law with increasing LMA for the species in sites 1 (Fig 3D, P = 0.026, R2 = 0.272) and 2 (Fig 3D, P = 0.005, R2 = 0.397). A similar trend was found for all the species pooled together (Fig 3D, P = 0.015, R2 = 0.160).
Relationships between leaf nutrient conditions and Pn
The correlations found between leaf N and Pn were not significant (Fig 4A, P > 0.05). However, a significant linear relationship was found between Leaf P and Pn for the species in site 2 (Fig 4B, P < 0.001, R2 = 0.589). A similar trend was found for all the species pooled together (Fig 4B, P < 0.001, R2 = 0.542).
Fig 4. Relationships between leaf nutrient conditions and Pn of halophytic plant communities.
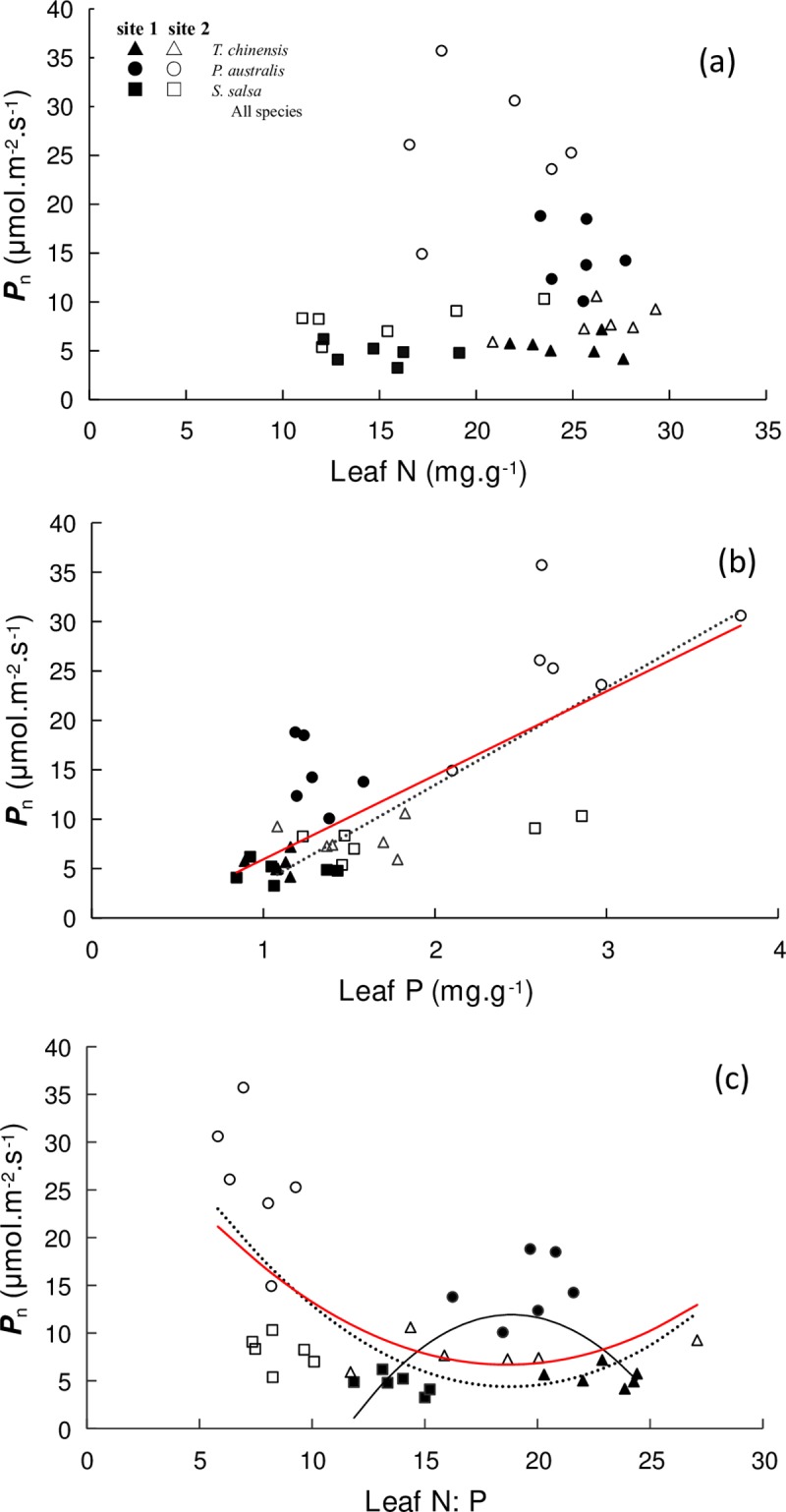
(a) Leaf N and Pn (equations for sites 1 and 2 and all species: P > 0.05). (b) Leaf P and Pn (equation for site 1: P = 0.081; equation for site 2: y = 9.8482x − 6.2357, R2 = 0.589, P < 0.001; equation for all species: y = 8.5006x − 2.5626, R2 = 0.542, P < 0.001). (c) Leaf N:P and Pn (equation for site 1: y = −0.2213x2 + 8.3418x − 66.676, R2 = 0.377, P = 0.029; equation for site 2: y = 0.1112x2 − 4.1707x + 43.505, R2 = 0.372, P = 0.031; equation for all species: y = 0.0879x2 − 3.277x + 37.24, R2 = 0.306, P = 0.002).
Leaf N:P showed a nonlinear relationship for the species in sites 1 (Fig 4C, P = 0.029, R2 = 0.377) and 2 (Fig 4C, P = 0.031, R2 = 0.372). For the species in site 2, Pn decreased until N:P increased to approximately 20 mg‧g−1 and then increased along with leaf N:P. However, the opposite trend was found for the species in site 1. For all species pooled together, the quadratic regression also suggested that Pn decreased until N:P increased to approximately 20 mg‧g−1 and then increased along with leaf N:P (Fig 4C, P = 0.002, R2 = 0.305).
PNUE and PPUE of halophytes
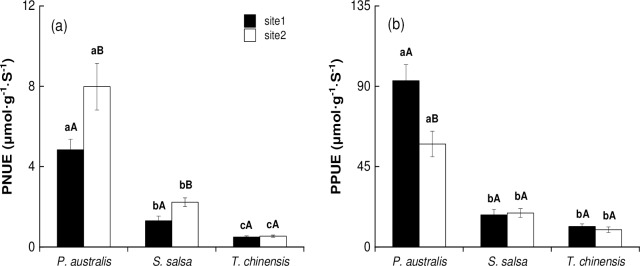
For different species at the same site, PNUE and PPUE were highest for P. australis, followed by S. salsa and T. chinensis (Fig 5A and 5B, P < 0.05). For identical species in different sites, P. australis in site 1 had lower PNUE but higher PPUE than site 2 (Fig 5A and 5B, P < 0.05). For S. salsa, PNUE was lower in site 1 than in site 2 (Fig 5A, P < 0.05). However, the differences for PPUE between the two study sites were not significant (Fig 5B, P > 0.05). For T. chinensis, PNUE and PPUE had no significant differences between the two sites (Fig 5A and 5B, P > 0.05).
Fig 5. Photosynthetic N and P use efficiency (PNUE and PPUE; mean ± SE) of halophytic plant communities.
(a) PNUE; (b) PPUE. The different lower letters indicate significant differences (P<0.05) between the different species in the same site; the different upper letters indicate significant differences (P<0.05) between the same species in different sites.
Relationships between photosynthetic nutrient use efficiency and Pn
PNUE was positively correlated with Pn in sites 1 (Fig 6A, P < 0.001, R2 = 0.838) and 2 (Fig 6A, P < 0.001, R2 = 0.904). The slopes of PNUE-Pn did not significantly differ between the two regression lines (P > 0.05), with a common slope of 0.539 (95% CI = [0.439, 0.661]). However, the y-intercept of PNUE-Pn in site 2 was higher than that in site1 (P = 0.017). PPUE was positively correlated with Pn in sites 1 (Fig 6B, P < 0.001, R2 = 0.925) and 2 (Fig 6B, P < 0.001, R2 = 0.870). The slopes of PPUE-Pn did not differ between the two regression lines (P > 0.05), and the average was 0.631 (95% CI = [0.526, 0.762]). The y-intercept of PPUE-Pn in site 2 was also higher than that in site 1 (P < 0.001).
Fig 6. Relationships between photosynthetic nutrient use efficiency and Pn of halophytic plant communities.
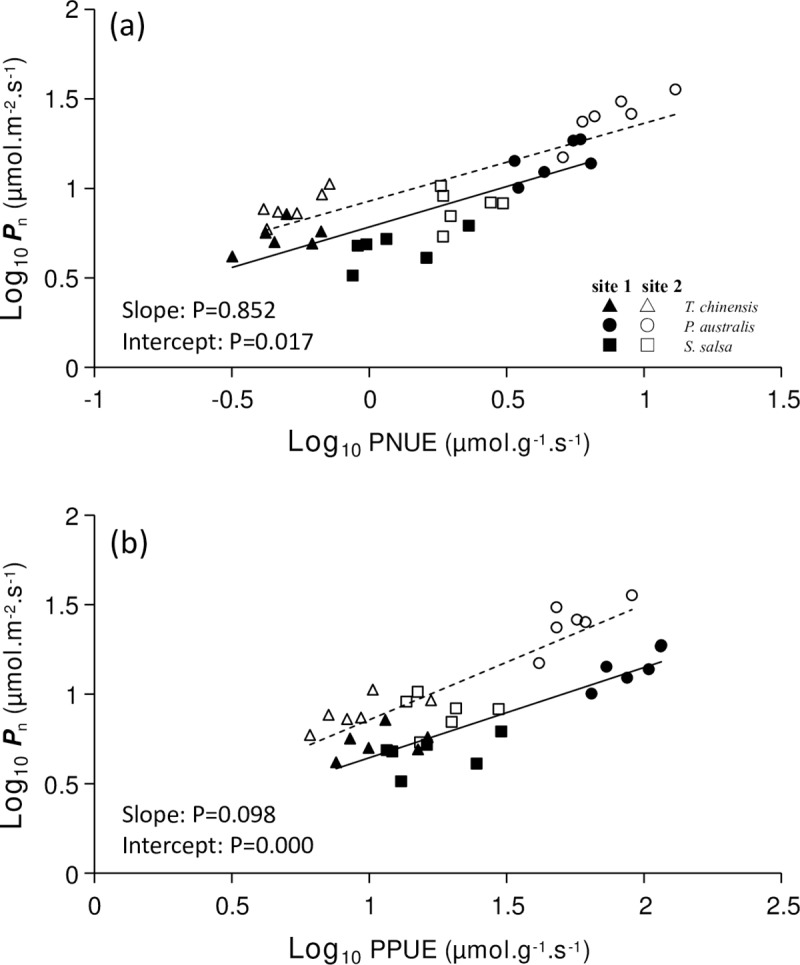
(a) PNUE and Pn (equation for site 1: y = 0.4522x+0.7842, R2 = 0.679, P < 0.001; equation for site 2: y = 0.4345x + 0.9289, R2 = 0.676, P < 0.001; with data log10-transformed); (b) PPUE and Pn (equation for site 1: y = 0.5042x + 0.1405, R2 = 0.812, P < 0.001; equation for site 2: y = 0.6439x + 0.2127, R2 = 0.752, P < 0.001; with data log10-transformed).
Relationships between LMA and photosynthetic nutrient use efficiency
PNUE showed a significant increase in power law when LMA decreased for the species in sites 1 (Fig 7A, P < 0.001, R2 = 0.908) and 2 (Fig 7A, P < 0.001, R2 = 0.923) and for all the species pooled together (Fig 7A, P < 0.001, R2 = 0.805). PPUE showed a significant increase in power law when LMA decreased for species in sites 1 (Fig 7B, P < 0.001, R2 = 0.849) and 2 (Fig 7B, P < 0.001, R2 = 0.812) and for all species pooled together in the two study sites (Fig 7B, P < 0.001, R2 = 0.832).
Fig 7. Relationships between LMA and photosynthetic nutrient use efficiency of halophytic plant communities.
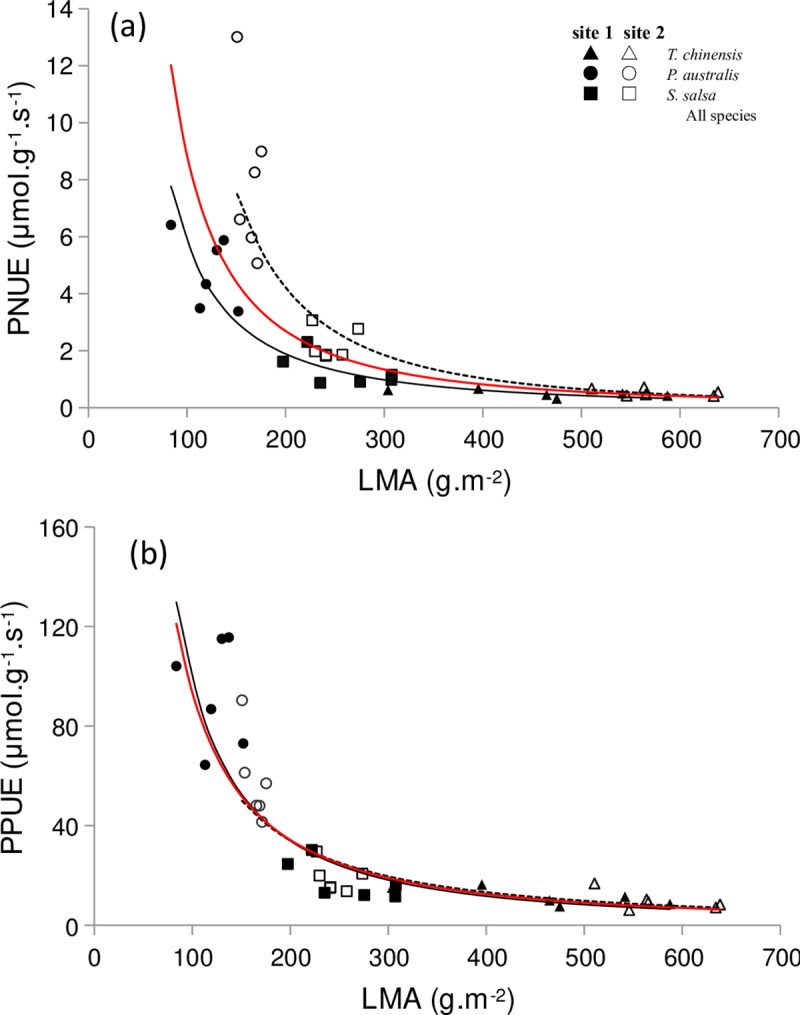
(a) LMA and PNUE (equation for site 1: y = 10339x−1.625, R2 = 0.909, P < 0.001; equation for site 2: y = 204171x−2.036, R2 = 0.923, P < 0.001; equation for all species: y = 24214x−1.719, R2 = 0.805, P < 0.001); (b) LMA and PPUE (equation for site 1: y = 118828x−1.54, R2 = 0.849, P < 0.001; equation for site 2: y = 45843x−1.36, R2 = 0.812, P < 0.001; equation for all species: y = 74475x−1.451, R2 = 0.832, P < 0.001).
Soil physicochemical properties in different sites
For different communities in site 1, T. chinensis had higher soil N and SWC but lower pH than P. australis and S. salsa (Table 1, P < 0.05). Soil salinity was higher in the community of S. salsa than in the communities of P. australis and T. chinensis (Table 1, P < 0.05). For soil P, the differences among the three communities were not significant (Table 1, P > 0.05).
Table 1. Soil physicochemical properties of halophytic plant communities in two sites.
| Study sites | Community species | Soil N (mg.kg-1) | Soil P (mg.kg-1) | Salinity (‰) | SWC (%) | pH |
|---|---|---|---|---|---|---|
| Site 1 | P. australis | 0.63±0.06aA | 0.52±0.07aA | 9.95±2.02aA | 37.02±1.13aA | 8.47±0.09aA |
| S.salsa | 0.71±0.14aA | 0.56±0.04aA | 17.40±3.97bA | 36.82±0.56aA | 8.39±0.23aA | |
| T. chinensis | 0.93±0.24bA | 0.50±0.07aA | 11.70±2.49aA | 40.95±2.63bA | 8.12±0.11bA | |
| Site 2 | P. australis | 0.45±0.05aB | 0.56±0.03aA | 23.12±2.07aB | 30.57±5.11aB | 8.14±0.09aB |
| S.salsa | 0.59±0.10bA | 0.61±0.04bA | 27.40±5.28abB | 24.68±8.57aB | 8.21±0.08aA | |
| T. chinensis | 0.59±0.09bB | 0.53±0.03aA | 29.05±5.23bB | 26.97±4.36aB | 8.26±0.18aB |
The different lower letters indicate significant differences (P<0.05) between the different species in the same site; the different upper letters indicate significant differences (P<0.05) between the same species in different sites.
For different communities in site 2, P. australis had lower soil N and salinity than S. salsa and T. chinensis (Table 1, P < 0.05). Soil P was highest in the community of S. salsa, followed by the communities of P. australis and T. chinensis, which did not differ from each other. The differences for SWC and pH among the three communities were not significant (Table 1, P > 0.05).
For identical community in different sites, P. australis in site 1 had higher soil N, SWC, and pH but lower salinity than those in site 2 (Table 1, P < 0.05). Soil P did not differ between the P. australis communities in sites 1 and 2 (P > 0.05). For S. salsa communities, soil N, soil P and pH were common in the two sites. However, that community in site 1 had lower salinity and higher SWC than that in site 2 (Table 1, P < 0.05). T. chinensis had higher soil N and SWC but lower salinity and pH in site 1 than in site 2 (Table 1, P < 0.05), but the difference for soil P between the two sites was not significant (P > 0.05).
Effects of site, species, and their interaction on leaf traits
The results of two-way ANOVA with the effects of site, species, and their interactions on leaf functional traits showed that site had no effect on Gs and leaf N but had a significant effect on other functional traits (Table 2, P < 0.001). Species had a significant effect on all leaf photosynthetic functional traits (Table 2, P < 0.001). Except for leaf P and leaf N:P, all F-ratios for species were larger than those for site, thereby indicating a high explanation for species. In this analysis, all leaf functional traits (except for Gs) were also influenced by the interactions between site and species. However, the F-ratios for site × species were lower than those for site and species separately, thereby suggesting a slightly weak explanation for their interactions (Table 2, P < 0.01).
Table 2. Results of a two-way analysis of variance to test the effects of site, species, and their interactions on the leaf traits.
| Leaf traits | Site | Species | Site × Species |
|---|---|---|---|
| Pn (μmol.m-2.s-1) | 26.263** | 66.477** | 6.304** |
| Tr (mmol.m-2.s-1) | 18.026** | 73.154** | 17.952** |
| Gs (mmol.m-2.s-1) | 0.803NS | 73.996** | 1.982NS |
| VPDleaf (kPa) | 12.837** | 13.937** | 3.398* |
| LMA (g.m-2) | 7.798** | 168.395** | 4.617* |
| Leaf N (mg.g-1) | 0.999NS | 33.467** | 3.334* |
| Leaf P (mg.g-1) | 44.882** | 11.501** | 5.424* |
| Leaf N:P ratio | 75.192** | 42.808** | 7.213** |
| PNUE (μmol.g-1.s-1) | 9.700** | 66.457** | 4.409* |
| PPUE (μmol.g-1.s-1) | 8.727** | 99.950** | 8.232** |
F-ratios are shown in the table
**indicates significant at 0.01 level
* indicates significant at 0.05 level, NS indicates no significant effect
RDA for gas exchange characters and their influencing factors
The RDA results showed that the explanatory of the four gas exchange characteristics for all halophytic plant communities in two sites were 71.6% and 2.3% (Fig 8), that is, the two axes explained 73.9% variations of the gas exchange capacity. Most of the information on the gas exchange characteristics for halophytic plant communities and their influencing factors could be reflected by the first two axes.
Fig 8. Redundancy analysis (RDA) of relationships between gas exchange characteristics and the influencing factors of halophytic plant communities.
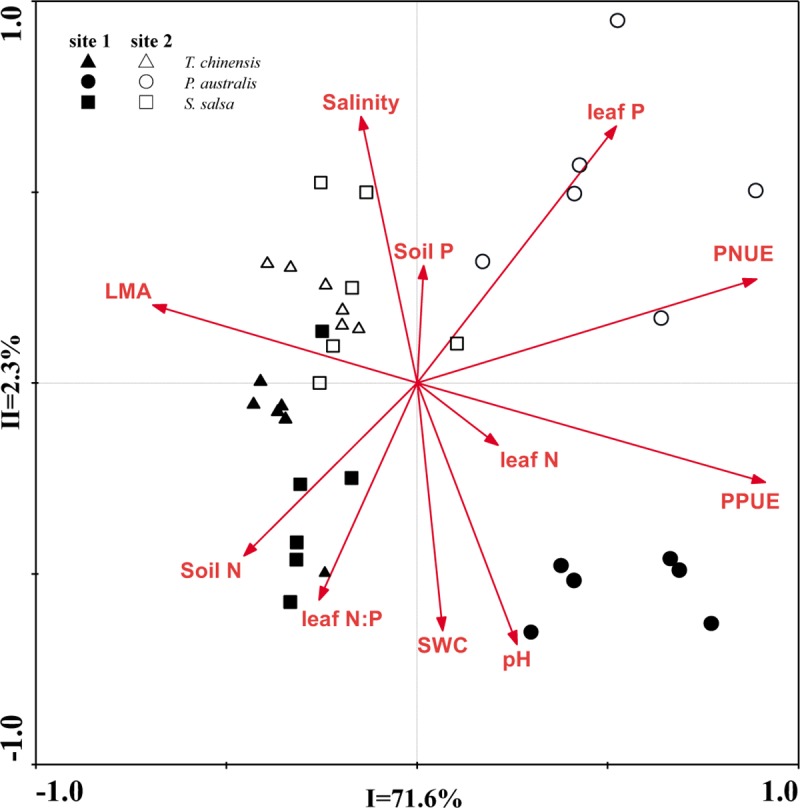
The Monte-Carlo test results showed that the important values (F) of the influencing factors decreased in the order of PPUE > PNUE > LMA > leaf P > soil N > pH > leaf N:P > leaf N > salinity > SWC > soil P (Table 3). The effects for PPUE (Table 3, P = 0.002), PNUE (P = 0.002), LMA (P = 0.002), leaf P (P = 0.002), and soil N (P = 0.02) were significant, and their explanations were 60.0%, 57.1%, 35.0%, 22.0%, and 15.8%, respectively (Table 3). In contrast with these factors, the effects for pH, leaf N:P, leaf N, salinity, SWC, and soil were not significant P (Table 3, P > 0.05). PPUE, PNUE, LMA, leaf P, and soil N were the main driving factors of gas exchange capacity for all communities in the two sites.
Table 3. Importance ranking and significance of influencing factors in their explanation on the gas exchange capacity of halophytic plant communities.
| Influencing factors | Importance ranking | Explanation (%) | F | P |
|---|---|---|---|---|
| PPUE | 1 | 60.0 | 51.095 | 0.002 |
| PNUE | 2 | 57.1 | 45.222 | 0.002 |
| LMA | 3 | 35.0 | 18.295 | 0.002 |
| Leaf P | 4 | 22.0 | 9.383 | 0.002 |
| Soil N | 5 | 15.8 | 6.395 | 0.02 |
| pH | 6 | 7.1 | 2.587 | 0.08 |
| Leaf N:P | 7 | 6.9 | 2.527 | 0.082 |
| Leaf N | 8 | 4.1 | 1.471 | 0.23 |
| Salinity | 9 | 3.9 | 1.378 | 0.24 |
| SWC | 10 | 2.4 | 0.824 | 0.338 |
| Soil P | 11 | 0.7 | 0.247 | 0.732 |
Significant results are shown in bold (P < 0.05).
N:P thresholds for halophytes in different sites
For the species in site 1, the N:P thresholds of P. australis and T. chinensis were higher than 16 (Fig 9A), which indicated the P limitation for the two species in this habitat. However, all the N:P thresholds of S. salsa were lower than 16, and 50% of the thresholds were lower than 14 (Fig 9A), thereby suggesting N limitation or ambiguous limitation for S. salsa in site 1.
Fig 9. Relationships between N and P of plants and soils.
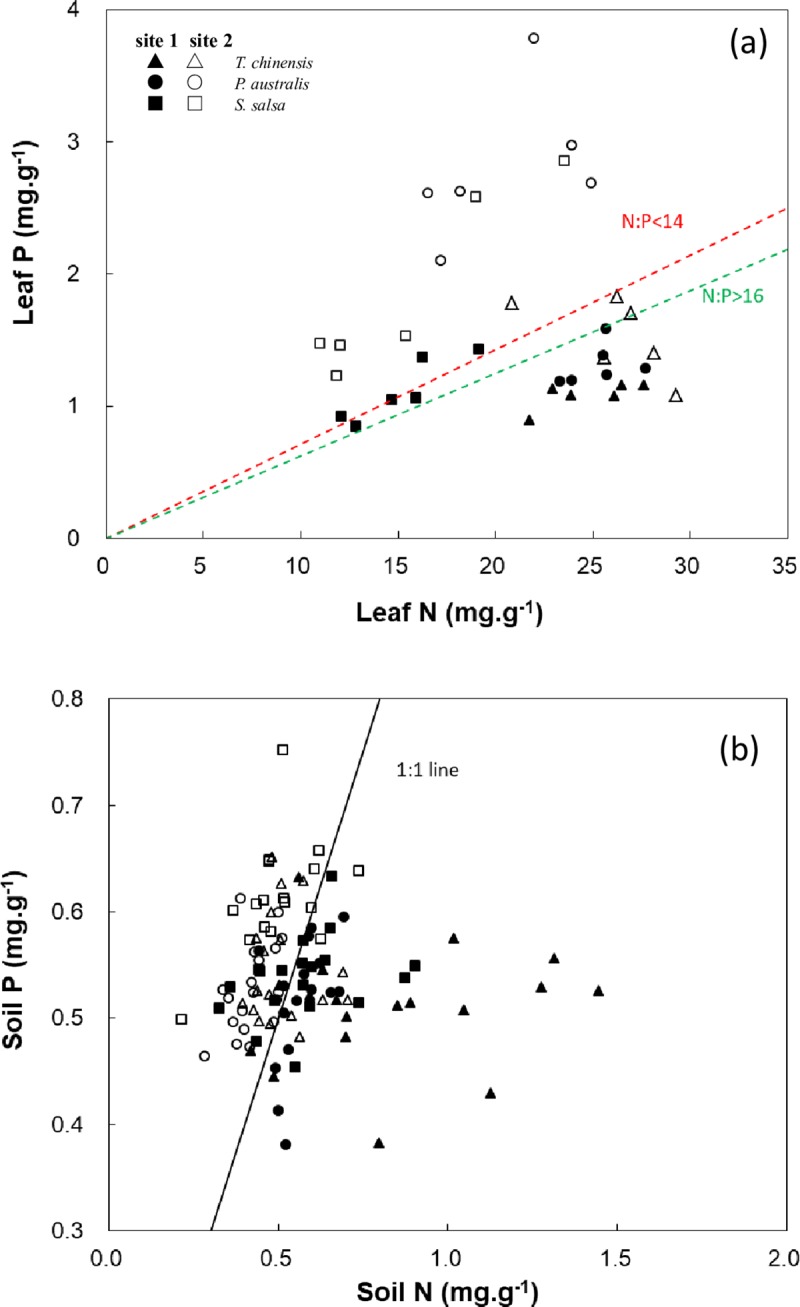
(a) N versus P of plants; (b) N versus P of soils.
For species in site 2, The N:P thresholds of P. australis and S. salsa were lower than 14 (Fig 9A), which indicated a N limitation for the two species in this habitat. For T. chinensis in site 2, 50% of the thresholds were higher than 16 (Fig 9A), thereby suggesting that T. chinensis was grown under P-limited conditions in site 2. However, a sample with a N:P of <16 can either show N limitation or ambiguous limitation for T. chinensis in site 2.
For soil samples in the communities of site 1, 75.93% of the N:P thresholds were higher than 1 (Fig 9B), which indicated that a large N input occurred in these habitats. However, for all the soil samples in the communities of site 2, only 12.96% of the N:P thresholds were higher than 1 (Fig 9B), which suggested that the soil was rich in P in these habitats.
Discussion
Gas exchange capacity and leaf nutrient conditions
Photosynthesis is an important indicator for the estimation of plant productivity. However, the photosynthetic rate is not enough to elucidate the gas exchange capacity of a plant. In many studies, the leaf functional traits (i.e., Pn, Tr, Gs and VPDleaf) and leaf structural traits (i.e., LMA, leaf N and P) are generally used to evaluate the carbon assimilation patterns of different ecosystems [39–41]. The current study’s results indicated that P. australis had higher Pn, Tr and Gs than S. salsa and T. chinensis (Fig 1) in both sites, which may have been affected by the genera and the environmental conditions. However, the gas exchange characteristics are intrinsic, and environmental determinants remain unclear in previous studies [4]. No direct evidence showed positive connections between soil properties and photosynthetic rate in each site. For example, P. australis had lower soil N and SWC but higher Pn, Tr, and Gs than T. chinensis in site 1 (Table 1; Fig 1). This result indicated that those redundant nutrients and water are generally used to synthesize compounds for stress resistance in the saline–alkaline habitat. For different sites, the communities in site 1 had higher soil N and SWC and lower salinity than those in site 2 (Table 1). However, Pn was lower in site 1 than in site 2 (Fig 1). The photosynthetic capacity was determined by the surroundings and influenced by genetic factors. To some extent, the photosynthetic capacity and water use efficiency of the plants are determined by their intrinsic factors under specific environmental conditions [42]. In this research, the F-ratios of species are higher than the site, and their interaction also confirmed the inference in Table 2.
Leaf N and P are two important intrinsic factors related to photosynthesis. These factors are relatively constant in the given environmental conditions and reflect highly adaptable strategies for plants in carbon fixation [17, 41]. Therefore, exploring the relationships between gas exchange characters and leaf traits are crucial in understanding the morphogenesis and evolution of halophytes. The photosynthetic rate of plants has significant positive correlations with N and P concentrations in leaves [6–10]. In the present study, Pn did not have a significant correlation with leaf N in both sites, which indicated that the Pn might not have changed with increasing soil N. However, the Monte-Carlo test in RDA showed that soil N had a significant effect on the gas exchange capacity of halophytes (Table 3, P = 0.02). This result may be correlated with N absorption and utilization strategies. The photosynthetic rate of two herbaceous did not significantly differ upon the application of nitrogen fertilizer [16]. The electron transport of chloroplast thylakoid membrane and the other sections involved in the photoreaction is also unaffected by nitrogen nutrition [43]. By contrast, leaf P had a positive correlation with Pn in site 2. However, that relationship was weak in site 1 (Fig 4B). The differences in leaf P-Pn between the two sites may be related to their different nutrient limitation conditions. The difference for soil P between the two sites was not significant, but soil N was significantly higher in site 1 than in site 2 (Table 1), which indicated a high N:P ratio in the soil of site 1 (Fig 9). As suggested in previous studies [44], the high input ratios of N and P result in P limitation in site 1. Meanwhile, P is easily decoupled from C and N cycles under P-limited conditions [32, 45]; this finding can explain the phenomena in our research.
Gas exchange capacity and LMA
The photosynthetic rate is tightly connected with LMA in many terrestrial ecosystems [6, 10, 40]. In our research, the gas exchange characteristics of the two study sites showed no evident changes with leaf nutrient conditions. However, the two study sites were significantly affected by LMA in an exponential manner (Fig 3A, 3B, 3C and 3D). In most situations, wetland plants are generally expressed through physiological, morphological, and genetic changes in response to waterlogging stress [46, 47]. All species tend to maintain their gas exchange characteristics by optimizing LMA [40], which might be a possible strategy for halophytes to adapt to an environment with short-term flooding. In the present study, LMA was highest for T. chinensis (461.31 and 576.34 g‧m−2 in sites 1 and 2, respectively), followed by S. salsa (257.49 and 244.97 g‧m−2, respectively) and P. australis (122.56 and 164.15 g‧m−2, respectively). As a woody plant, the LMA of T. chinensis is two- to threefold higher than that of annual and perennial herbs (S. salsa and P. australis), which proves that the LMA of trees and shrubs is larger than herbs [10]. In summary, the morphological structure of the plant leaves in coastal wetlands differs from those in other ecosystems. The leaves of halophytes are generally small in size, mostly narrow or coniferous, scaly, and fleshy due to flooding and salinity stress [47]. These characteristics result in considerable changes in LMA. The large variation of LMA among halophytes may be the main reason of the anomalous fluctuations in their gas exchange characteristics. RDA result supported our deduction and indicated that LMA has negative and significant effect on the gas exchange capacity of halophytes. By definition, the larger the LMA is, the higher proportion of the biomass allocated to the leaves per unit area will be [40, 41]. Thus, high LMA has a dilution effect on the nutrient content of per leaf area, which reduces their gas exchange capacity of halophytes. Here, the explanation of LMA was 35% (Table 3), which was lower than those of PPUE and PNUE but higher than those of other influencing factors. In this scenario, LMA was tightly coupled with PNUE and PPUE in both sites (Fig 7), thereby suggesting that photosynthetic nutrient use efficiency determined the gas exchange capacity of halophytes.
Gas exchange capacity and photosynthetic nutrient use efficiency
Photosynthesis is related to the nutrients in leaves and leaf structure and to the photosynthetic nutrient use efficiency [41, 48, 49]. The PNUE and PPUE of halophytic plant communities in the two sites showed the same trend as Pn, that is, P. australis > S. salsa > T. chinensis (Fig 5A and 5B). In many studies, PPUE and PNUE proved to be the important indicators of plant response to soil nutrient status [48, 49]. For example, plants tend to enhance their nutrient use efficiency when soil nutrients are scarce. However, low nutrient use efficiency indicates that plant communities in this habitat are not restricted by such nutrients [50–52]. In the present study, in site 2, soil N was low, and the leaf N:P ratios of P. australis and S. salsa were smaller than 14 (Fig 9A), which indicated that those herbs were limited by N in this site. The high PNUE of P. australis and S. salsa in site 2 was an adaptive strategy for plant in N-limitation habitat. As previously discussed, the increased soil N and P inputs in site 1 may lead to a shift from N-limited to P-limited situations, which were often found in the estuarine and coastal wetlands. Here, the leaf N:P ratios of P. australis and T. chinensis in site 1 were larger than 16, which also provided additional evidence for this scenario.
The curves of PNUE-Pn and PPUE-Pn fitted significantly in sites 1 and 2 (Fig 6A and 6B), respectively, which indicated that the photosynthetic nutrient use efficiency was more useful than nutrient concentrations in affecting plant photosynthesis. The shift in the slope and intercept of the fitting curves between leaf traits represents certain biological significance [9, 49]. In the present study, the slopes of PNUE-Pn and PNUE-Pn were not significant different between the two sites (Fig 6A and 6B), which indicated that the nutrient utilization strategy of the two sites was indiscriminate. By contrast, the y-intercepts of PNUE-Pn and PNUE-Pn were significantly higher in site 2 than in site 1 (Fig 6A and 6B), which indicated that the plant in site 1 had higher PNUE and PPUE than those in site 2 at a given amount of carbon assimilation. Hence, the plants in site 1 tend to allocate minimal N and P to photosynthetic apparatus in keeping similar carbon assimilation. RDA result suggested that PPUE and PNUE were the first two influencing factors in depicting the gas exchange capacity of halophytes in both sites (Fig 8, Table 3). Thus, halophytes may promote their photosynthetic nutrient use efficiency and decrease the assimilated allocation per leaf area to cope with the nutrient limitation changes in coastal wetlands.
Conclusions
The photosynthetic capacity of halophytes was not primarily controlled by nutrient concentration in leaves. It depended on photosynthetic nutrient use efficiency. PNUE and PPUE showed a significant decrease in power law with increasing LMA for all the species in both sites. Thus, halophytes may promote their photosynthetic nutrient use efficiency and decrease the assimilated allocation per leaf area to cope with the nutrient limitation changes in coastal wetlands. RDA showed that the gas exchange capacity of the halophytic plant communities was significantly affected by PPUE (60.0% of explanation), PNUE (57.1%), LMA (35.0%), leaf P (22.0%), and soil N (15.8%). The plant productivity of the terrestrial plants depended more on the availability of nutrients than their concentrations under climate change conditions, thereby helping elucidate the leaf economic spectrum and physiological principles of halophytes in coastal wetlands.
Supporting information
(XLSX)
Acknowledgments
We thank Yi Zheng, Chong You, Minghua Wang and Chengliang Bi for their work in the field and in the laboratory. We are very grateful to the staff of the Yellow River Delta Ecology Research Station of Coastal Wetland for their field support.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work is financially supported by National Natural Science Foundation of China (41303057), and Tianjin Research Program of Application Foundation and Advanced Technology (14JCYBJC23000). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Xu ZZ, Jiang YL, Zhou GS. Response and adaptation of photosynthesis, respiration, and antioxidant systems to elevated CO2 with environmental stress in plants. Front Plant Sci, 2015; 6:701 10.3389/fpls.2015.00701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsuji Y, Yamazaki M, Suzuki I, Shiraiwa Y. Quantitative analysis of carbon flow into photosynthetic products functioning as carbon storage in the marine coccolithophore, Emiliania huxleyi. Mar Biotechnol. 2015; 17: 428–440. 10.1007/s10126-015-9632-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.César HH, Alejandro EC, Jose LS, Josep P, Rodrigo V, Jose RRL. High Vcmax, Jmax and photosynthetic rates of Sonoran Desert species: Using nitrogen and specific leaf area traits as predictors in biochemical models. J Arid Environ. 2018; 156:1–8. [Google Scholar]
- 4.Bahar NHA, Gauthier PPG, O'Sullivan OS, Brereton T, Evans JR, Atkin OK. Phosphorus deficiency alters scaling relationships between leaf gas exchange and associated traits in a wide range of contrasting Eucalyptus species. Funct Plant Biol. 2018; 45: 813–826. [DOI] [PubMed] [Google Scholar]
- 5.Crous KY, O’Sullivan OS, Zaragoza-Castells J, Bloomfield KJ, Negrini ACA, Meir P, et al. Nitrogen and phosphorus availabilities interact to modulate leaf trait scaling relationships across six plant functional types in a controlled-environment study. New Phytol. 2017; 215: 992–1008. 10.1111/nph.14591 [DOI] [PubMed] [Google Scholar]
- 6.He JS, Wang ZH, Wang XP, Schmid B, Zuo WY, Zhou M, et al. A test of the generality of leaf trait relationships on the Tibetan Plateau. New Phytol. 2006; 170: 835–848. 10.1111/j.1469-8137.2006.01704.x [DOI] [PubMed] [Google Scholar]
- 7.Leishman MR, Haslehurst T, Ares A, Baruch Z. Leaf trait relationships of native and invasive plants: community- and global-scale comparisons. New Phytol. 2007; 176: 635–643. 10.1111/j.1469-8137.2007.02189.x [DOI] [PubMed] [Google Scholar]
- 8.Reich PB, Ellsworth DS, Walters MB, Vose JM, Gresham C, Volin JC, et al. Generality of leaf trait relationships: A test across six biomes. Ecology. 1999; 80: 1955–1969. [Google Scholar]
- 9.Liu FD, Yang WJ, Wang ZS, Xu Z, Liu H, Zhang M, et al. Plant size effects on the relationships among specific leaf area, leaf nutrient content, and photosynthetic capacity in tropical woody species. Acta Oecol. 2010; 2: 149–159. [Google Scholar]
- 10.Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, et al. The worldwide leaf economics spectrum. Nature. 2004; 428: 821–827. 10.1038/nature02403 [DOI] [PubMed] [Google Scholar]
- 11.Egli P, Schmid B. Relationships between leaf nitrogen and limitations of photosynthesis in canopies of Solidago altissima. Acta Oecol. 1999; 20: 559–570. [Google Scholar]
- 12.Shangguan ZP, Shao MA, Dyckmans J. Nitrogen nutrition and water stress effects on leaf photosynthetic gas exchange and water use efficiency in winter wheat. Environ. Exp. Bot. 2000; 44: 141–149. 10.1016/s0098-8472(00)00064-2 [DOI] [PubMed] [Google Scholar]
- 13.DaMatta FM, Loos RA, Silva EA, Loureiro ME. Limitations to photosynthesis in Coffea canephoraas a result of nitrogen and water availability. J Plant Physiol. 2002; 159: 975–981. [Google Scholar]
- 14.Field C, Mooney HA. P hotosynthesis—nitrogen relationship in wild plants In: On the Economy of Plant Form & Function (ed. Givnish TJ). Cambridge, Cambridge University Press; 1986. [Google Scholar]
- 15.Hikosaka K, Osone Y. A paradox of leaf-trait convergence: why is leaf nitrogen concentration higher in species with higher photosynthetic capacity? J Plant Res. 2009; 122: 245–251. 10.1007/s10265-009-0222-z [DOI] [PubMed] [Google Scholar]
- 16.Zhong C, Jian SF, Huang J, Jin QY, Cao XC. Trade-off of within-leaf nitrogen allocation between photosynthetic nitrogen-use efficiency and water deficit stress acclimation in rice (Oryza sativa L.). Plant Physiol Bioch. 2019; 135: 41–50. [DOI] [PubMed] [Google Scholar]
- 17.Cordell S, Goldstein G, Meinzer FC, Vitousek PM. Regulation of leaf life-span and nutrient-use efficiency of Metrosideros polymorpha trees at two extremes of a long chronosequence in Hawaii. Oecologia. 2001; 127: 198–206. 10.1007/s004420000588 [DOI] [PubMed] [Google Scholar]
- 18.Whitehead D, Boelman NT, Turnbull MH, Griffin KL, Tissue DT, Barbour MM, et al. Photosynthesis and reflectance indices for rainforest species in ecosystems undergoing progression and retrogression along a soil fertility chronosequence in New Zealand. Oecologia. 2005; 144: 233–244. 10.1007/s00442-005-0068-6 [DOI] [PubMed] [Google Scholar]
- 19.Yu Q, Wu H, He N, Lü X, Wang Z, Elser JJ, et al. Testing the growth rate hypothesis in vascular plants with above- and below-ground biomass. PLoS One. 2012; 7: e32162 10.1371/journal.pone.0032162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reich PB, Schoettle AW. Role of phosphorus and nitrogen in photosynthetic and whole plant carbon gain and nutrient use efficiency in eastern white pine. Oecologia. 1988; 77: 25–33. 10.1007/BF00380920 [DOI] [PubMed] [Google Scholar]
- 21.Wright IJ, Reich PB, Westoby M. Strategy shifts in leaf physiology, structure and nutrient content between species of high- and low-rainfall and high- and low-nutrient habitats. Funct Ecol. 2001; 15: 423–434. [Google Scholar]
- 22.Wright IJ, Reich PB, Cornelissen JHC, Falster DS, Garnier E, Hikosaka K, et al. Assessing the generality of global leaf trait relationships. New Phytol. 2005; 166: 485–496. 10.1111/j.1469-8137.2005.01349.x [DOI] [PubMed] [Google Scholar]
- 23.Kirschbaum MUF, Tompkins D. Photosynthetic responses to phosphorus nutrition in Eucalyptus grandis seedlings. Funct Plant Biol. 1990; 17: 527–535. [Google Scholar]
- 24.Niinemets Ü, Kull K. Leaf structure vs. nutrient relationships vary with soil conditions in temperate shrubs and trees. Acta Oecol. 2003; 24: 209–219. [Google Scholar]
- 25.Centritto M, Loreto F. Photosynthesis in a changing world: photosynthesis and abiotic stresses. Agric Ecosyst Environ. 2005; 106: 115–117. [Google Scholar]
- 26.Solomon S, Climate change the physical science basis, American Geophysical Union. 2007; 9: 123–124. [Google Scholar]
- 27.Sheffield J, Wood EF, Roderick ML. Little change in global drought over the past 60 years. Nature. 2012, 491: 435–438. 10.1038/nature11575 [DOI] [PubMed] [Google Scholar]
- 28.Yuan ZY, Chen HYH. Decoupling of nitrogen and phosphorus in terrestrial plants associated with global changes. Nat Clim Chang. 2015; 5: 465. [Google Scholar]
- 29.Elser JJ, Bracken MES, Cleland EE, Gruner DS, Harpole WS, Hillebrand H, et al. Global analysis of nitrogen and phosphorus limitation of primary producer in freshwater, marine and terrestrial ecosystems. Ecol Lett. 2007; 10: 1135–1142. 10.1111/j.1461-0248.2007.01113.x [DOI] [PubMed] [Google Scholar]
- 30.Elser JJ, Andersen T, Baron JS, Bergström AK, Jansson M, Kyle M, et al. Shifts in lake N:P stoichiometry and nutrient limitation driven by atmospheric nitrogen deposition. Science. 2009; 326: 835–837. 10.1126/science.1176199 [DOI] [PubMed] [Google Scholar]
- 31.Verhoeven JTA, Laanbroek HJ, Rains MC, Whigham DF. Effects of increased summer flooding on nitrogen dynamics in impounded mangroves. J Environ Manage. 2014; 139: 217–226. 10.1016/j.jenvman.2014.02.035 [DOI] [PubMed] [Google Scholar]
- 32.Peñuelas J, Poulter B, Sardans J, Ciais P, van der Velde M, Bopp L, et al. Human-induced nitrogen-phosphorus imbalances alter natural and managed ecosystems across the globe. Nature Commun. 2013; 4: 2934. [DOI] [PubMed] [Google Scholar]
- 33.Mahowald N, Jickells TD, Baker AR, Artaxo P, Benitez-Nelson CR, Bergametti G, et al. The global distribution of atmospheric phosphorus sources, concentrations and deposition rates, and anthropogenic impacts. Global Biogeochem Cy. 2008; 22: GB4026. [Google Scholar]
- 34.Peñuelas J, Sardans J, Rivas-Ubach A, Janssens IA. The human-induced imbalance between C, N and P in Earth’s life system. Global Change Biol. 2012; 18: 3–6. [Google Scholar]
- 35.Mou XJ, Sun ZG, Wang LL, Dong HF. Characteristics of nitrogen accumulation and allocation of Suaeda salsa in different growth conditions of intertidal zone in yellow river estuary. Wetland Science. 2010; 8: 57–66. [Google Scholar]
- 36.Xie Z, Xu L, Duan X, Xu X. Analysis of boundary adjustments and land use policy change–A case study of Tianjin Palaeocoast and Wetland National Natural Reserve, China. Ocean Coast Manage. 2012; 56: 56–63. [Google Scholar]
- 37.Falster DS, Warton DI, Wright IJ. (S)MATR: standardized major axis tests and routines. Version 2.0. 2006; Available from: http://www.bio.mq.edu.au/ecology/SMATR2.0 [Google Scholar]
- 38.Warton DI, Weber NC. Common slope tests for bivariate errors-in-variables models. Biometrical J. 2002; 44: 161–174. [Google Scholar]
- 39.Geng Y, Ma WH, Wang L, Baumann F, Kühn P, Scholten T, et al. Linking above‐ and belowground traits to soil and climate variables: an integrated database on China's grassland species. Ecology. 2017; 98: 1471–1471. 10.1002/ecy.1780 [DOI] [PubMed] [Google Scholar]
- 40.Osnas JLD, Katabuchi M, Kitajima K, Wright SJ, Reich PB, Van Bael SA, et al. Divergent drivers of leaf trait variation within species, among species, and among functional groups. P Natl Acad Sci USA. 2018; 115, 5480–5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kikuzawa K. Leaf phenology as an optimal strategy for carbon gain in plants. Can J Bot. 1995; 73: 158–163. [Google Scholar]
- 42.Santos CMD, Endres L, Ferreira VM, Silva JV, Rolim EV, Wanderley HCL. Photosynthetic capacity and water use efficiency in Ricinus communis (L.) under drought stress in semi-humid and semi-arid areas. An Acad Bras Cienc. 2017; 89: 3015–3029. 10.1590/0001-3765201720160729 [DOI] [PubMed] [Google Scholar]
- 43.Evans J, Crisovan E, Barry K, Daum C, Jenkins J, Kunde Ramamoorthy G, et al. Diversity and population structure of northern switchgrass as revealed through exome capture sequencing. Plant J. 2015; 84: 800–815. 10.1111/tpj.13041 [DOI] [PubMed] [Google Scholar]
- 44.Vitousek PM, Porder S, Houlton BZ, Chadwick OA. Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen-phosphorus interactions. Ecol Appl. 2010; 20: 5–15. 10.1890/08-0127.1 [DOI] [PubMed] [Google Scholar]
- 45.Sardans J, Rivas-Ubach A, Peñuelas J. The C:N:P stoichiometry of organisms and ecosystems in a changing world: A review and perspectives. Perspect Plant Ecol Evol Syst. 2012; 14: 33–47. [Google Scholar]
- 46.Romanello GA, Chuchra-Zbytniuk KL, Vandermer JL, Touchette BW. Morphological adjustments promote drought avoidance in the wetland plant Acorus americanus. Aquat Bot. 2008; 89: 390–396. [Google Scholar]
- 47.Voesenek LACJ, Colmer TD, Pierik R, Millenaar FF, Peeters AJM. How plants cope with complete submergence. New Phytol. 2006; 170: 213–226. 10.1111/j.1469-8137.2006.01692.x [DOI] [PubMed] [Google Scholar]
- 48.Li JY, Guo QX, Zhang JX, Korpelainen H, Li CY. Effects of nitrogen and phosphorus supply on growth and physiological traits of two Larix species. Environ Exp Bot. 2016; 130: 206–215. [Google Scholar]
- 49.Liu FD, Yang WJ, Zhang M, Liu YH, Zheng JW, Wang WJ, et al. Does strategy of resource acquisition in tropical woody species vary with life form, leaf texture and canopy gradient? Eur J Forest Res. 2010; 129: 1093–1108. [Google Scholar]
- 50.Aerts R, Chapin FS. The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. Adv Ecol Res. 1999; 30: 1–67. [Google Scholar]
- 51.Aerts R, de Caluwe H, Beltman B. Is the relation between nutrient supply and biodiversity co-determined by the type of nutrient limitation? Oikos. 2003; 101: 489–498. [Google Scholar]
- 52.Fynn RWS, Morris CD, Kirkman KP. Plant strategies and trait trade-offs influence trends in competitive ability along gradients of soil fertility and disturbance. J Ecol. 2005; 93: 384–394. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.