Abstract
Objective
Fasting plasma glucose (FPG), 2-hour plasma glucose (2hPG) from a 75-g oral glucose tolerance test (OGTT) and glycated hemoglobin (HbA1c) can lead to different results when diagnosing prediabetes and diabetes. The Hemoglobin Glycation Index (HGI) quantifies the interindividual variation in glycation resulting in discrepancies between FPG and HbA1c. We used data from the Vitamin D and Type 2 Diabetes (D2d) study to calculate HGI, to identify HGI-associated variables, and to determine how HGI affects prediabetes and diabetes diagnosis.
Measurements
A linear regression equation [HbA1c (%) = 0.0164 × FPG (mg/dL) + 4.2] was derived using the screening cohort (n = 6829) and applied to calculate predicted HbA1c. This was subtracted from the observed HbA1c to determine HGI in the baseline cohort with 2hPG data (n = 3945). Baseline variables plus prediabetes and diabetes diagnosis by FPG, HbA1c, and 2hPG were compared among low, moderate, and high HGI subgroups.
Results
The proportion of women and Black/African American individuals increased from low to high HGI subgroups. Mean FPG decreased and mean HbA1c increased from low to high HGI subgroups, consistent with the HGI calculation; however, mean 2hPG was not significantly different among HGI subgroups.
Conclusions
High HGI was associated with Black race and female sex as reported previously. The observation that 2hPG was not different across HGI subgroups suggests that variation in postprandial glucose is not a significant source of population variation in HGI. Exclusive use of HbA1c for diagnosis will classify more Black individuals and women as having prediabetes compared with using FPG or 2hPG.
Keywords: hemoglobin glycation, prediabetes, diagnosis, type 2, oral glucose tolerance test, observational study
Fasting plasma glucose (FPG), 2-hour plasma glucose (2hPG) from a 75-g oral glucose tolerance test (OGTT), and glycated hemoglobin A1c (HbA1c) are each used clinically for the diagnosis and management of prediabetes and diabetes. While FPG and 2hPG are specific measures of blood glucose concentration, HbA1c reflects more than just glycemia. Research has shown that diabetes classification based on these 3 tests can differ markedly in human populations (1, 2). One major cause of differences in the diagnostic specificity of each test is interindividual variation in the quantitative relationship between HbA1c and plasma glucose concentration (3, 4). For example, Blacks/African Americans tend to have higher HbA1c levels than whites with similar blood glucose concentrations (3, 5, 6). It has been suggested that interindividual differences in red blood cell life span or other biological factors can influence HbA1c levels independent of the effect of plasma glucose concentration (7). Variation in the quantitative relationship between HbA1c and blood glucose has important clinical implications for how HbA1c, FPG, and 2hPG should be used in the diagnosis of prediabetes and diabetes and for prediction of diabetes complications (8).
The Hemoglobin Glycation Index (HGI) is a biomarker of population variation in HbA1c due to factors other than blood glucose concentration (9). HGI quantifies the magnitude and direction of interindividual variation in HbA1c based on the difference between an observed (measured) HbA1c and a predicted HbA1c. The predicted HbA1c is calculated by inserting an individual’s FPG into a linear regression equation that describes the quantitative relationship between FPG and HbA1c in a study population (10–12). Because of how HGI is calculated, people with a low HGI phenotype have higher FPG than people with a high HGI phenotype and similar HbA1c. Likewise, people with a high HGI phenotype have higher HbA1c than people with a low HGI phenotype and similar FPG. Thus, one might predict that disproportionately more people with low HGI would be classified as having prediabetes or diabetes based on FPG; while disproportionately more people with high HGI would be classified as having prediabetes or diabetes based on HbA1c. It is less clear whether variation in postprandial glucose is associated with HGI, or whether the proportion of diagnoses of prediabetes or diabetes based on 2hPG differs among HGI subgroups (13).
It has been previously reported that patients with type 1 or type 2 diabetes and high HGI calculated using either FPG or mean blood glucose (MBG, from patient meters or 1-day profile sets collected before and after meals) were at greater risk for microvascular complication hypoglycemia and cardiovascular disease (10, 11, 14–17). Moreover, higher HGI in people without diabetes has been associated with increased coronary artery calcification (18), carotid atherosclerosis (19), hepatic steatosis (20), kidney dysfunction (21), and inflammation (12, 19, 22). Other investigators have suggested that because HGI is strongly associated with HbA1c, it cannot be an independent predictor of microvascular complications (23). The degree to which HGI represents a more discriminating biomarker of risk for complications compared with HbA1c alone remains to be established.
The Vitamin D and Type 2 Diabetes (D2d) study is a large prospective clinical trial designed to investigate the effect of vitamin D supplementation on prevention of diabetes in people with prediabetes (24). This analysis used D2d study data (individuals with complete screening and baseline visit data) to calculate HGI, to identify demographic and clinical variables associated with HGI, and to determine how HGI is associated with prediabetes and diabetes diagnosis. These analyses will lay the groundwork for future longitudinal analyses of prospective diabetes outcomes over time in participants with low and high HGI.
Materials and Methods
Overview of the D2d study
D2d is a US-based clinical trial conducted at 22 sites to evaluate oral administration of vitamin D compared with placebo for prevention of diabetes in people at high risk for diabetes. The experimental design of D2d has been published (24). The study was approved and monitored by an independent data and safety monitoring board and the institutional review board of each collaborating site.
Study population
Target participants were adults at high risk for diabetes (24). At the screening visit, glycemic criteria for prediabetes were preliminarily evaluated by measuring FPG and HbA1c either in a local laboratory or at the D2d central laboratory. If participants had FPG and HbA1c values that suggested they might qualify for the study (cutoffs varied slightly between centers but generally required FPG and HbA1c to be in the prediabetes range), participants were invited to proceed to a baseline visit on the same day or on another day. At the baseline visit, a 75-g OGTT was performed, and fasting FPG, 2hPG, and HbA1c were measured at the D2d central laboratory to determine final eligibility. Participants ultimately randomized had to meet at least 2 criteria for prediabetes as established by the American Diabetes Association (ADA) in 2010 (25): FPG: 100 to 125 mg/dL (5.6–6.9 mmol/L); 2hPG: 140 to 199 mg/dL (7.8–11.0 mmol/L) in a 75-g OGTT; and/or HbA1c: 5.7% to 6.4% (39–46 mmol/mol). Other entry criteria included age ≥ 30 years (≥ 25 years for American Indians, Alaska Natives, Native Hawaiians, or other Pacific Islanders) and body mass index (BMI) of 24 to 42 kg/m2 (22.5 to 42 kg/m2 for Asians). For this current analysis, data from the whole screening cohort were used to derive a linear regression equation of HbA1c versus FPG and to derive HGI tertile cutpoints. HbA1c and FPG from the baseline visit were then entered into the regression equation to calculate HGI and to divide participants in the baseline cohort into HGI subgroups based on the screening HGI tertile cutpoints.
Laboratory methods
At the baseline visit, blood samples for glucose analysis were collected in sodium fluoride tubes, refrigerated, and centrifuged within 45 minutes of collection. Plasma from these samples was stored at −70°C and shipped frozen to the D2d central laboratory. These samples were analyzed for glucose using a hexokinase method (Roche Glucose HK Gen.3 on the Cobas Integra 400 or Cobas c311 analyzer, Roche Diagnostics, Indianapolis, IN), standardized against isotope dilution mass spectrometry (ID/MS). Blood samples for HbA1c analysis were collected in EDTA tubes and stored refrigerated until they were shipped refrigerated. HbA1c was measured within 7 days of collection using an ion-exchange high-performance liquid chromatography (HPLC) method (Tosoh G8, Tosoh Bioscience, South San Francisco, CA). This method is certified by the National Glycohemoglobin Standardization Program (NGSP), and the D2d Central Laboratory is certified by NGSP as a Level I Laboratory with documented traceability to the Diabetes Control and Complications Trial Reference Method (26). At the beginning of the study, approximately half of the screening samples were analyzed at each local site by a variety of methods for glucose and HbA1c.
Calculation of hemoglobin glycation index (HGI)
A linear regression equation of HbA1c versus FPG was derived using all data from the screening cohort with the exception of 2 outliers for FPG and HbA1c that were not clinically plausible (n = 6829). Due to the structure of the D2d study, the larger screening cohort was used to derive the regression equation because the initial screening process limited the range of FPG and HbA1c values in the subsequent baseline cohort. HGI was then calculated as the difference between the observed (measured) HbA1c and a predicted HbA1c derived by inserting a date-matched blood glucose measurement into the linear regression equation (see below) and applied to the subset of participants in the baseline cohort with complete values for FPG, HbA1c, and 2hPG (n = 3945).
Data analyses
The baseline cohort was divided into low, moderate, and high HGI subgroups based on screening cohort HGI tertile cutpoints. Available baseline variables that have been shown to be important in previous HGI studies (age, BMI, sex, race, and ethnicity) were compared among the 3 HGI subgroups. Race was defined as Black/African American or non-African American; ethnicity was classified as Hispanic/Latino or non-Hispanic/Latino. Analysis of variance (ANOVA) for continuous variables (age, BMI) and chi-square analysis for categorical variables (sex, race, ethnicity) were used to determine differences among HGI groups. Comparisons of glycemia by FPG, HbA1c, and 2hPG were made among the 3 HGI subgroups via ANOVA. Statistical significance was determined using two-tailed tests with a significance level of P < 0.05. All analyses were conducted using R version 3.4.2.
Results
Characteristics of screening and baseline cohort
Characteristics of the screening and baseline cohort are presented in Table 1 and the groups were noted to be similar overall.
Table 1.
Characteristics of the Screening and Baseline Cohort
| Variable | Screening N = 6829 | Baseline N = 3945 | Low HGI N = 1495 | Moderate HGI N = 1419 | High HGI N = 1031 | P Valuea |
|---|---|---|---|---|---|---|
| HGI, mean (SD) | 0.00 | -0.06 | -0.35 | -0.01 | 0.30 | <0.001 |
| (0.38) | (0.29) | (0.17) | (0.08) | (0.13) | ||
| Age (y), mean (SD) | 59.4 | 59.4 | 59.4 | 59.6 | 59.3 | 0.62 |
| (10.2) | (10.2) | (10.4) | (10.2) | (9.8) | ||
| Sex, n (%) | <0.001 | |||||
| Female | 3371 | 1799 | 596 | 668 | 535 | |
| (49.4) | (45.6) | (39.9) | (47.1) | (51.9) | ||
| Male | 3458 | 2146 | 899 | 751 | 496 | |
| (50.6) | (54.4) | (60.1) | (52.9) | (48.1) | ||
| Race, n (%) | <0.001 | |||||
| Black/African American | 1746 | 1008 | 190 | 347 | 471 | |
| (25.6) | (25.6) | (12.7) | (24.5) | (45.7) | ||
| Non-African American | 5083 | 2937 | 1305 | 1072 | 560 | |
| (74.4) | (74.4) | (87.3) | (75.5) | (54.4) | ||
| Ethnicity, n (%) | 0.06 | |||||
| Hispanic | 744 | 382 | 124 | 154 | 104 | |
| (10.9) | (9.7) | (8.3) | (10.9) | (10.1) | ||
| Non-Hispanic | 6085 | 3563 | 1371 | 1265 | 927 | |
| (89.1) | (90.3) | (91.7) | (89.1) | (89.9) | ||
| BMI (kg/m 2 ), mean (SD) | 31.9 | 31.9 | 31.9 | 31.7 | 32.0 | 0.18 |
| (4.5) | (4.5) | (4.4) | (4.5) | (4.6) | ||
| FPG (mg/dL), mean (SD) | 103.6 | 106.7 | 111.7 | 105.5 | 101.1 | <0.001 |
| (14.6) | (10.7) | (10.9) | (9.2) | (9.1) | ||
| HbA1c (%), mean (SD) | 5.9 | 5.9 | 5.7 | 5.9 | 6.1 | <0.001 |
| (0.5) | (0.4) | (0.2) | (0.2) | (0.2) | ||
| 2hPG (mg/dL), mean (SD) | N/A | 137.9 | 137.6 | 137.6 | 138.7 | 0.80 |
| (44.9) | (45.7) | (44.7) | (44.1) |
The Baseline cohort was divided into Low, Moderate, and High subgroups.
Abbreviations: 2hPG, 2-hour plasma glucose; ANOVA, analysis of variance; BMI, body mass index; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin A1c; HGI, Hemoglobin Glycation Index; SD, standard deviation.
aANOVA for continuous variables and chi-square analysis for categorical variables were used to determine differences among low, moderate, and high HGI groups
HGI cutpoints
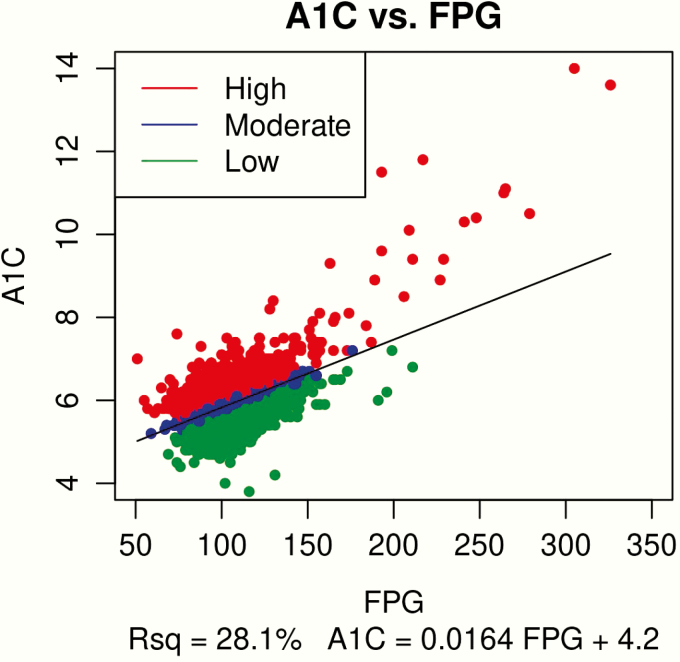
Based on data from the screening visit, a linear regression equation [HbA1c (%) = 0.0164 × FPG (mg/dL) + 4.2] was established and then applied to calculate predicted HbA1c and HGI for each participant in the baseline cohort (Fig. 1). Cutpoints for assignment to either the low (≤ −0.153), moderate (> −0.153 to 0.130), or high (> 0.130) HGI subgroup were established based on HGI tertiles established using the screening data and then applied to the baseline cohort.
Figure 1.
Diagram of Linear Regression Equation Based on the Entire Screening Cohort (N = 6830). Red indicates high HGI, blue indicates moderate HGI, and green indicates low HGI.
Association between HGI cutpoints and characteristics of baseline cohort
The proportions of women and Blacks/African Americans increased across subgroups of HGI from low to high HGI (P < 0.001). Age, ethnicity, and BMI were not different among HGI subgroups (P = 0.62, P = 0.06, and P = 0.18, respectively). Mean FPG decreased and HbA1c increased going from low to high HGI subgroups, consistent with how HGI is calculated. Mean 2hPG was not significantly different among HGI subgroups (P = 0.80).
Association between HGI cutpoints and diagnosis of prediabetes and diabetes
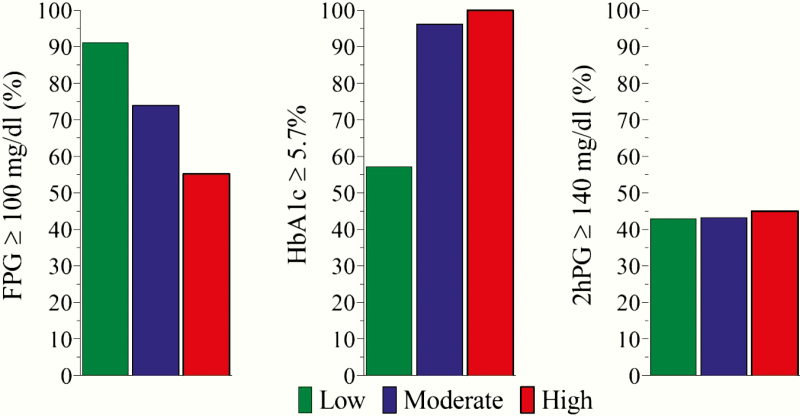
Over 80% of low HGI participants had FPG in the prediabetes range compared with just over 50% of high HGI participants (P < 0.001) (Table 2). In contrast, over 90% of high HGI participants had HbA1c in the prediabetes range compared with less than 60% of low HGI participants (P < 0.001). There was no difference in the prevalence of people with 2hPG in the prediabetes range among HGI subgroups (P = 0.29). Fig. 2 uses the values from Table 2 to show that the combined diagnoses of prediabetes and diabetes differed among HGI subgroups based on FPG (≥ 100 mg/dL) and HbA1c (≥ 5.7% / 39 mmol/mol) but not on the results of the 2hPG level (≥ 140 mg/dL).
Table 2.
Prediabetes and Diabetes Diagnosis by HGI Subgroup
| Total N = 3945 | Low HGI N = 1495 | Moderate HGI N = 1419 | High HGI N = 1031 | P Value | |
|---|---|---|---|---|---|
| FPG, n (%) | <0.001 | ||||
| Normal | 965 (24.5) | 133 (8.9) | 370 (26.1) | 462 (44.8) | |
| Prediabetes | 2819 (71.5) | 1242 (83.1) | 1015 (71.5) | 562 (54.5) | |
| Diabetes | 161 (4.0) | 120 (8.0) | 34 (2.4) | 7 (0.7) | |
| HbA1c, n (%) | <0.001 | ||||
| Normal | 698 (17.7) | 641 (42.9) | 56 (3.9) | 1 (0.1) | |
| Prediabetes | 3197 (81.0) | 852 (57.0) | 1362 (96.0) | 983 (95.3) | |
| Diabetes | 50 (1.3) | 2 (0.1) | 1 (0.1) | 47 (4.6) | |
| 2hPG, n (%) | 0.29 | ||||
| Normal | 2226 (56.4) | 853 (57.1) | 806 (56.8) | 567 (55.0) | |
| Prediabetes | 1360 (34.5) | 497 (33.2) | 482 (34.0) | 381 (37.0) | |
| Diabetes | 359 (9.1) | 145 (9.7) | 131 (9.2) | 83 (8.0) |
Abbreviations: 2hPG, 2-hour plasma glucose; BMI, body mass index; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin A1c; HGI, Hemoglobin Glycation Index.
Figure 2.
Prevalence of combined diagnoses of prediabetes and diabetes based on FPG, HbA1c, and 2hPG by HGI subgroups.
Discussion
HGI is the residual derived using a blood glucose versus HbA1c linear regression equation from a reference population; whereas, the glycosylation (glycation) gap is a similar residual derived using fructosamine versus HbA1c. Dr. Hempe and colleagues first proposed the HGI in 2002 (14) and the glycosylation gap was proposed by Cohen et al in 2003 (15). In the last 2 years there have been at least 20 reports looking at relationships between HGI and chronic disease in both nondiabetic and diabetic study populations. People with high HGI exhibit similar traits whether the glucose component of the regression equation is estimated using FPG, MBG, or fructosamine (15, 27, 28) and regardless of whether MBG was calculated using patient glucose meter data (14, 16), continuous glucose monitoring (17), or 7-point glucose profile sets (9). Thus, not sampling blood glucose in the postprandial period does not appear to affect HGI. The observation of phenotypic consistency, regardless of how blood glucose status is measured, strongly suggests that FPG, MBG, and fructosamine all similarly reflect the most important aspects of person-to-person variation in blood glucose that affect the quantitative relationship between blood glucose and HbA1c measured by HGI. If using MBG, fructosamine, and FPG to calculate HGI all give similar results, then FPG will be the metric of choice because it is simpler, less expensive, and more widely used, especially in developing countries.
Our screening and baseline data from the D2d study identified differences among HGI subgroups in race and sex, some of which have been reported in previous studies. Our analysis showed no difference in BMI among HGI subgroups. This observation is similar to what has been reported in previous analyses of HGI and BMI in people with diabetes (10, 29–33). In contrast, multiple studies have reported that BMI is higher in people without diabetes with high HGI (18, 19, 22). Whether low or high HGI predicts who will develop type 2 diabetes is a question we plan to examine using longitudinal data that recently became available in the D2d study.
Our analysis found that the high HGI subgroup included disproportionately more Blacks/African Americans and women. Evidence of racial variation in HGI confirms multiple reports which have shown that Blacks/African-Americans tend to have higher HbA1c compared with whites with similar blood glucose levels in both nondiabetic and diabetic study populations (3, 5, 6, 22, 34, 35). The high HGI subgroup in the D2d study also contained disproportionately more women as previously observed in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial (10). Evidence of a relationship between sex and HGI has not been consistent across studies, however, and thus whether or not HGI is associated with sex remains equivocal. The etiology of race or sex differences in HGI could be related to heritable differences in erythrocyte lifespan (36), glucose transport (37), or the fact that both women and people of more recent African descent tend to have lower than average hemoglobin levels (6, 38, 39).
As predicted, the low HGI subgroup had disproportionately more individuals diagnosed with prediabetes or diabetes based on FPG alone and the high HGI subgroup had disproportionately more individuals diagnosed with prediabetes or diabetes based on HbA1c alone (Table 2 and Fig. 2). We acknowledge that these findings were the result of how HGI is calculated. However, the third metric of glycemic status, 2hPG, was not significantly different among HGI subgroups. Other investigators (40, 41) have hypothesized that biological variation in HGI might be an analytical artifact caused by person-to-person variation in postprandial glucose excursions that are not detected when FPG is used to calculate HGI. This hypothesis is supported by the results of a study in people without diabetes, with prediabetes, and with treatment-naïve diabetes, conducted by Ahn et al (12) who reported higher 2hPG levels in high-HGI study participants. In contrast, our results agree with those of Marini et al (19), who reported no difference in 2hPG between HGI subgroups in a study population without diabetes or with prediabetes, potentially indicating minimal contribution of postprandial glucose excursions to the biological variation in HGI. This would also make sense, since people without diabetes spend very little time in the postprandial state.
If 2hPG is not significantly different among HGI subgroups, we hypothesize that HGI may be a useful biomarker whenever there is a discrepancy between HbA1c and FPG. In people with a low HGI phenotype who should have lower HbA1c levels than their high HGI counterparts, an elevated HbA1c in the prediabetes or diabetes range may be demonstrating postprandial glucose intolerance/insulin insufficiency and would require an OGTT to further determine blood glucose control. Alternatively, in people with a high HGI phenotype who should have lower FPG levels than their low HGI counterparts, an elevated FPG in the prediabetes or diabetes range may be demonstrating hyperglucagonemia, (42) for example, and an OGTT could help further characterize any additional postprandial glucose intolerance. In addition, use of an OGTT for diabetes screening may be preferred to prevent overdiagnosis based on HbA1c alone in those with high HGI or overdiagnosis based on FPG in those with low HGI. However, the value of HGI in predicting the future incidence of diabetes in those with prediabetes cannot be determined from this cross-sectional analysis and requires longitudinal follow-up. With the diabetes outcomes now reported in the D2d study, we will have additional information to determine which HGI subgroup is more likely to progress to diabetes. Additional studies are needed to investigate the value of HGI in clinical practice to help determine the most appropriate screening method for prediabetes and diabetes in certain populations.
Our study has several strengths, including a large sample size, extensive phenotyping of a large number of people at risk for diabetes, and extensive evaluation of glycemic status (including FPG, HbA1c, and 2hPG). Our approach was consistent with several other studies using HGI. Our study did have some limitations. A small subset of the screening tests used in the HGI regression equation were analyzed at different local laboratories introducing possible interlaboratory variation; however, about half of the screening labs were analyzed in the central lab and all local labs had NGSP certification for HbA1c. In addition, we used the screening population to derive the equation and then applied it to the baseline population. Although the baseline cohort had less glycemic variability because participants had to pass the screening glycemic criteria to attend a baseline visit, the demographic characteristics of the 2 populations were similar. Because fructosamine was not measured in this study, the glycation gap could not be calculated and compared with results using HGI. Limitations of HGI as a clinical tool include the lack of a single regression equation that can be used to calculate HGI for every population.
Future work could examine data from continuous glucose monitoring during the 3 months prior to the HbA1c measurement as a direct measure of mean glycemia, although this method would be difficult to implement in large clinical trials, such as the D2d study. Moreover, similar interindividual variation was seen between HbA1c and mean glucose from continuous glucose monitoring data in the development of the Glucose Management Indicator (GMI), an estimated HbA1c level derived from a regression equation of mean glucose and measured HbA1c in diabetes patients (42).
In conclusion, evidence of clinically significant interindividual variation in the relationship between blood glucose and HbA1c complicates the use of any single test for the diagnosis of prediabetes and diabetes. HGI classification may be affected by race and sex. Moreover, blood glucose control as measured by FPG and HbA1c may be influenced by HGI classification, but 2hPG may help better characterize blood glucose control in certain populations. Our results suggest that normative values and longitudinal follow-up using HGI should be further evaluated, since HGI may serve as an additional variable when deciding which tests to use in which patients for diagnosing prediabetes and diabetes.
Acknowledgments
The authors thank the D2d investigators, staff, and trial participants for their outstanding dedication and commitment to the study. Dr. Pittas was supported in part by generous donations to the Tupper Research Fund at Tufts Medical Center.
Financial Support: The planning phase of D2d was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) through a multicenter clinical study implementation planning grant (U34) to Tufts Medical Center in Boston, MA (U34DK091958; principal investigator A.G.P.). Planning was also supported in part by the Intramural Research Program of the NIDDK. The conduct of D2d is primarily supported by NIDDK and the Office of Dietary Supplements of the National Institutes of Health through the multicenter clinical study cooperative agreement (U01DK098245; principal investigator A.G.P.) to Tufts Medical Center where the D2d Coordinating Center is based. The U01 grant mechanism establishes the NIDDK project scientist (M.A.S.) as a member of the D2d Research Group. The study also received secondary funding from the American Diabetes Association (1-14-D2d-01). The D2d investigators and the NIDDK project scientist had a role in the design and conduct of the study; interpretation of the data and data collection; management, analysis, review and approval of the manuscript; and the decision to submit the manuscript for publication.
Clinical Trial Information: ClinicalTrials.gov identifier NCT01942694.
Author Contributions: D.S.H., N.R., A.G.P., C.W.L., J.M.H., C.V.D. contributed to the design, acquisition and interpretation of data, draft, revisions, and final approval of the manuscript. All other authors contributed to the acquisition and interpretation of data, critical review and edits of the drafts, and final approval of the manuscript.
Glossary
Abbreviations
- 2hPG
2-hour plasma glucose
- ANOVA
analysis of variance
- BMI
body mass index
- D2d
Vitamin D and Type 2 Diabetes
- FPG
fasting plasma glucose
- HbA1c
glycated hemoglobin A1c
- HGI
Hemoglobin Glycation Index
- MBG
mean blood glucose
- NGSP
National Glycohemoglobin Standardization Program
- OGTT
oral glucose tolerance test
Contributor Information
D2d Research Group:
Anastassios G Pittas, Irwin Brodsky, Lisa Ceglia, Chhavi Chadha, Ranee Chatterjee, Bess Dawson-Hughes, Cyrus Desouza, Rowena Dolor, John Foreyt, Adline Ghazi, Daniel S Hsia, Karen C Johnson, Sangeeta R Kashyap, Sun Kim, Erin S LeBlanc, Michael R Lewis, Emilia Liao, Saul Malozowski, Lisa M Neff, Patrick O’Neil, Jean Park, Anne Peters, Lawrence S Phillips, Richard Pratley, Philip Raskin, Neda Rasouli, David Robbins, Clifford Rosen, Vanita R Aroda, Patricia Sheehan, Myrlene A Staten, and William C Knowler
Additional Information
Disclosure statement: The authors have nothing to disclose.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
D2d Research Group collaborators
Steering Committee
Anastassios G. Pittas, MD MS, Tufts Medical Center, Boston, MA (Chair)
Irwin Brodsky, MD, Maine Medical Center Research Institute, Scarborough, ME
Lisa Ceglia, MD MS, Tufts Medical Center, Boston, MA
Chhavi Chadha, MD, HealthPartners Research Foundation, Minneapolis, MN
Ranee Chatterjee, MD, Duke University Medical Center, Durham, NC
Bess Dawson-Hughes, MD, Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University, Boston, MA
Cyrus Desouza, MBBS, Omaha VA Medical Center, University of Nebraska Medical Center, Omaha, NE
Rowena Dolor, MD MHS, Duke University Medical Center, Durham, NC
John Foreyt, PhD, Baylor College of Medicine, Houston, TX
Adline Ghazi, MD, MedStar Good Samaritan Hospital, Baltimore, MD
Daniel S. Hsia, MD, Pennington Biomedical Research Center, Baton Rouge, LA
Karen C. Johnson, MD MPH, University of Tennessee Health Science Center, Memphis, TN
Sangeeta R. Kashyap, MD, Cleveland Clinic, Cleveland, OH
Sun Kim, MD, Stanford University Medical Center, Stanford, CA
Erin S. LeBlanc, MD MPH, Kaiser Permanente Center for Health Research NW, Portland, OR
Michael R. Lewis, MD MBA, University of Vermont–Central Laboratory, Burlington, VT
Emilia Liao, MD, Northwell Health Lenox Hill Hospital, New York, NY
Saul Malozowski, MD PhD, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD
Lisa M. Neff, MD, Northwestern University, Chicago, IL
Patrick O’Neil, PhD, Medical University of South Carolina, Charleston, SC
Jean Park, MD, MedStar Health Research Institute, Hyattsville, MD
Anne Peters, MD, Keck School of Medicine of the University of Southern California, Los Angeles, CA
Lawrence S. Phillips, MD, Atlanta VA Medical Center, Decatur, GA and Emory University School of Medicine, Atlanta, GA
Richard Pratley, MD, AdventHealth Translational Research Institute for Metabolism and Diabetes, Orlando, FL
Philip Raskin, MD, University of Texas Southwestern Medical Center, Dallas, TX
Neda Rasouli, MD, University of Colorado, School of Medicine and VA Eastern Colorado Health Care System, Aurora, CO
David Robbins, MD, University of Kansas Medical Center, Kansas City, KS
Clifford Rosen, MD, Maine Medical Center Research Institute, Scarborough, ME
Past Steering Committee members
Vanita R. Aroda, MD, Brigham and Women’s Hospital, Boston, MA
Patricia Sheehan, RN MPH MS, Spaulding Rehabilitation Network, Charlestown, MA
Myrlene A. Staten, MD, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD
Advisor
William C. Knowler, MD DrPH, National Institute of Diabetes and Digestive and Kidney Diseases, Phoenix, AZ
References
- 1. Menke A, Rust KF, Savage PJ, Cowie CC. Hemoglobin A1c, fasting plasma glucose, and 2-hour plasma glucose distributions in U.S. population subgroups: NHANES 2005-2010. Ann Epidemiol. 2014;24(2):83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Selvin E, Crainiceanu CM, Brancati FL, Coresh J. Short-term variability in measures of glycemia and implications for the classification of diabetes. Arch Intern Med. 2007;167(14):1545–1551. [DOI] [PubMed] [Google Scholar]
- 3. Bergenstal RM, Gal RL, Connor CG, et al. ; T1D Exchange Racial Differences Study Group Racial differences in the relationship of glucose concentrations and hemoglobin A1c levels. Ann Intern Med. 2017;167(2):95–102. [DOI] [PubMed] [Google Scholar]
- 4. Bao X, Wan M, Gu Y, et al. Red cell distribution width is associated with hemoglobin A1C elevation, but not glucose elevation. J Diabetes Complications. 2017;31(10):1544–1548. [DOI] [PubMed] [Google Scholar]
- 5. Selvin E, Sacks DB. Variability in the relationship of hemoglobin A1c and average glucose concentrations: how much does race matter? Ann Intern Med. 2017;167(2):131–132. [DOI] [PubMed] [Google Scholar]
- 6. Kamps JL, Hempe JM, Chalew SA. Racial disparity in A1C independent of mean blood glucose in children with type 1 diabetes. Diabetes Care. 2010;33(5):1025–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cohen RM, Smith EP. Frequency of HbA1c discordance in estimating blood glucose control. Curr Opin Clin Nutr Metab Care. 2008;11(4):512–517. [DOI] [PubMed] [Google Scholar]
- 8. Sacks DB. A1C versus glucose testing: a comparison. Diabetes Care. 2011;34(2):518–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McCarter RJ, Hempe JM, Gomez R, Chalew SA. Biological variation in HbA1c predicts risk of retinopathy and nephropathy in type 1 diabetes. Diabetes Care. 2004;27(6):1259–1264. [DOI] [PubMed] [Google Scholar]
- 10. Hempe JM, Liu S, Myers L, McCarter RJ, Buse JB, Fonseca V. The hemoglobin glycation index identifies subpopulations with harms or benefits from intensive treatment in the ACCORD trial. Diabetes Care. 2015;38(6):1067–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Steen SC, Schrieks IC, Hoekstra JB, et al. ; AleCardio study group The haemoglobin glycation index as predictor of diabetes-related complications in the AleCardio trial. Eur J Prev Cardiol. 2017;24(8):858–866. [DOI] [PubMed] [Google Scholar]
- 12. Ahn CH, Min SH, Lee DH, et al. Hemoglobin glycation index is associated with cardiovascular diseases in people with impaired glucose metabolism. J Clin Endocrinol Metab. 2017;102(8):2905–2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Monnier L, Colette C. Contributions of fasting and postprandial glucose to hemoglobin A1c. Endocr Pract. 2006;12 Suppl 1:42–46. [DOI] [PubMed] [Google Scholar]
- 14. Hempe JM, Gomez R, McCarter RJ Jr, Chalew SA. High and low hemoglobin glycation phenotypes in type 1 diabetes: a challenge for interpretation of glycemic control. J Diabetes Complications. 2002;16(5):313–320. [DOI] [PubMed] [Google Scholar]
- 15. Cohen RM, Holmes YR, Chenier TC, Joiner CH. Discordance between HbA1c and fructosamine: evidence for a glycosylation gap and its relation to diabetic nephropathy. Diabetes Care. 2003;26(1):163–167. [DOI] [PubMed] [Google Scholar]
- 16. Soros AA, Chalew SA, McCarter RJ, Shepard R, Hempe JM. Hemoglobin glycation index: a robust measure of hemoglobin A1c bias in pediatric type 1 diabetes patients. Pediatr Diabetes. 2010;11(7):455–461. [DOI] [PubMed] [Google Scholar]
- 17. Wilson DM, Xing D, Cheng J, et al. ; Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group Persistence of individual variations in glycated hemoglobin: analysis of data from the Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Randomized Trial. Diabetes Care. 2011;34(6):1315–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rhee EJ, Cho JH, Kwon H, et al. Association between coronary artery calcification and the hemoglobin glycation index: the Kangbuk Samsung Health Study. J Clin Endocrinol Metab. 2017;102(12):4634–4641. [DOI] [PubMed] [Google Scholar]
- 19. Marini MA, Fiorentino TV, Succurro E, et al. Association between hemoglobin glycation index with insulin resistance and carotid atherosclerosis in non-diabetic individuals. Plos One. 2017;12(4):e0175547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fiorentino TV, Marini MA, Succurro E, et al. Association between hemoglobin glycation index and hepatic steatosis in non-diabetic individuals. Diabetes Res Clin Pract. 2017;134:53–61. [DOI] [PubMed] [Google Scholar]
- 21. Fiorentino TV, Marini MA, Succurro E, et al. Elevated hemoglobin glycation index identify non-diabetic individuals at increased risk of kidney dysfunction. Oncotarget. 2017;8(45):79576–79586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu S, Hempe JM, McCarter RJ, Li S, Fonseca VA. Association between inflammation and biological variation in hemoglobin A1c in U.S. Nondiabetic Adults. J Clin Endocrinol Metab. 2015;100(6):2364–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lachin JM, Genuth S, Nathan DM, Rutledge BN. The hemoglobin glycation index is not an independent predictor of the risk of microvascular complications in the Diabetes Control and Complications Trial. Diabetes. 2007;56(7):1913–1921. [DOI] [PubMed] [Google Scholar]
- 24. Pittas AG, Dawson-Hughes B, Sheehan PR, et al. ; D2d Research Group Rationale and design of the Vitamin D and Type 2 Diabetes (D2d) study: a diabetes prevention trial. Diabetes Care. 2014;37(12):3227–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Feasibility of centralized measurements of glycated hemoglobin in the Diabetes Control and Complications Trial: a multicenter study. The DCCT Research Group. Clin Chem. 1987;33(12):2267–2271. [PubMed] [Google Scholar]
- 27. Nayak AU, Singh BM, Dunmore SJ. Potential clinical error arising from use of HbA1c in diabetes: effects of the glycation gap. Endocr Rev. 2019;40(4):988–999. [DOI] [PubMed] [Google Scholar]
- 28. Nayak AU, Nevill AM, Bassett P, Singh BM. Association of glycation gap with mortality and vascular complications in diabetes. Diabetes Care. 2013;36(10):3247–3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kim MK, Jeong JS, Kwon HS, Baek KH, Song KH. Concordance the hemoglobin glycation index with glycation gap using glycated albumin in patients with type 2 diabetes. J Diabetes Complications. 2017;31(7):1127–1131. [DOI] [PubMed] [Google Scholar]
- 30. van Steen SC, Woodward M, Chalmers J, et al. ; ADVANCE Collaborative Group Haemoglobin glycation index and risk for diabetes-related complications in the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial. Diabetologia. 2018;61(4):780–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang JS, Hung YJ, Lu YC, et al. Difference between observed and predicted glycated hemoglobin at baseline and treatment response to vildagliptin-based dual oral therapy in patients with type 2 diabetes. Diabetes Res Clin Pract. 2018;138: 119–127. [DOI] [PubMed] [Google Scholar]
- 32. Chen YW, Wang JS, Sheu WH, et al. Hemoglobin glycation index as a useful predictor of therapeutic responses to dipeptidyl peptidase-4 inhibitors in patients with type 2 diabetes. Plos One. 2017;12(2):e0171753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cheng PC, Hsu SR, Cheng YC, Liao PM. The hemoglobin glycation index correlates with efficacy of metformin therapy in individuals newly diagnosed with type 2 diabetes mellitus. Int J Clin Exp Med. 2017;10:3742–3746 [Google Scholar]
- 34. Herman WH, Cohen RM. Racial and ethnic differences in the relationship between HbA1c and blood glucose: implications for the diagnosis of diabetes. J Clin Endocrinol Metab. 2012;97(4):1067–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ziemer DC, Kolm P, Weintraub WS, et al. Glucose-independent, black-white differences in hemoglobin A1c levels: a cross-sectional analysis of 2 studies. Ann Intern Med. 2010;152(12):770–777. [DOI] [PubMed] [Google Scholar]
- 36. Cohen RM, Franco RS, Khera PK, et al. Red cell life span heterogeneity in hematologically normal people is sufficient to alter HbA1c. Blood. 2008;112(10):4284–4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Khera PK, Joiner CH, Carruthers A, et al. Evidence for interindividual heterogeneity in the glucose gradient across the human red blood cell membrane and its relationship to hemoglobin glycation. Diabetes. 2008;57(9):2445–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gould BJ, Davie SJ, Yudkin JS. Investigation of the mechanism underlying the variability of glycated haemoglobin in non-diabetic subjects not related to glycaemia. Clin Chim Acta. 1997;260(1):49–64. [DOI] [PubMed] [Google Scholar]
- 39. Beutler E, West C. Hematologic differences between African-Americans and whites: the roles of iron deficiency and alpha-thalassemia on hemoglobin levels and mean corpuscular volume. Blood. 2005;106(2):740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Basu S, Raghavan S, Wexler DJ, Berkowitz SA. Characteristics associated with decreased or increased mortality risk from glycemic therapy among patients with type 2 diabetes and high cardiovascular risk: machine learning analysis of the ACCORD Trial. Diabetes Care. 2018;41(3):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Riddle MC, Gerstein HC Comment on Hempe et al. The hemoglobin glycation index identifies subpopulations with harms or benefits from intensive treatment in the ACCORD trial. Diabetes Care 2015;38:1067-1074. Diabetes Care. 2015;38(10):e170–e171. [DOI] [PubMed] [Google Scholar]
- 42. Bergenstal RM, Beck RW, Close KL, et al. Glucose Management Indicator (GMI): a new term for estimating a1c from continuous glucose monitoring. Diabetes Care. 2018;41(11): 2275–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]