Implications
Red meat is a nutrient dense food providing important amounts of protein, essential amino acids, vitamins, and minerals that are the most common nutrient shortages in the world, including vitamin A, iron, and zinc.
Despite claims by the World Health Organization (WHO) that eating processed meat causes colon cancer and red meat probably causes cancer, the observational data used to support the claims are weak, confounded by multiple unmeasured factors, and not supported by other types of research needed for such a conclusion. Although intervention studies are designed to test the validity of associations found in observational studies, two interventions of low-fat, low-meat diets in volunteers that failed to find a benefit on cancer were not considered in the WHO decision.
It is likely that the association of red-meat consumption with colon cancer is explained either by an inability of epidemiology to detect such a small risk or by combinations of other factors such as greater overweight, less exercise, lower vegetable or dietary fiber intake, and perhaps other habits that differentiate those who eat the most meat from those who eat the least.
Introduction
Red meat is a nutrient dense food that is an important source of complete protein with all essential amino acids, highly bioavailable iron, zinc, selenium, and B vitamins, especially vitamin B12 in the diet. Several of these nutrients are the most common shortfall nutrients in the world that could be alleviated by the consumption of only a few ounces of beef per week (Figure 1; Klurfeld, 2015). Meat has been consumed by humans, sometimes in prodigious amounts, throughout history and is considered by anthropologists as one of the factors that led to evolution of larger brains. In recent decades, many observational studies of people have associated consumption of red or processed meats with a variety of chronic diseases such as multiple types of cancer, various forms of cardiovascular disease, kidney disease, type 2 diabetes, obesity, and total mortality (Boada et al., 2016). Consider if a scientist were claiming that a new drug treated all these illnesses. The overwhelming response would be swift and certain that this was not possible. Yet, critics of meat consumption are firmly convinced that it causes multiple harms despite the softness of data supporting such claims, almost all of which are based on epidemiological associations.
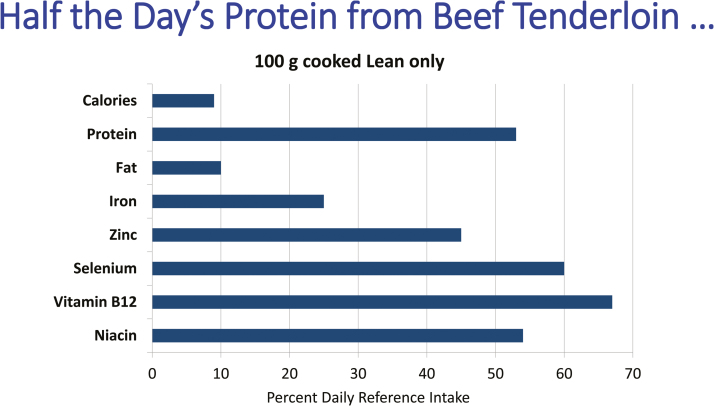
Figure 1.
Approximately 3 ounces of lean beef provides 9% of daily calories in a 2,000 calorie diet and only 10% of fat but more than half the daily needs for protein, selenium, niacin, and vitamin B12, along with a quarter of the iron requirement and almost half the zinc needed. These numbers emphasize the nutrient density of red meat. Reproduced from Klurfeld, 2015.
Epidemiological, or observational, studies provide valuable clues to potential associations between exposure to an agent and development of a disease. Historically, that is how the sources of infectious disease were traced, such as during a cholera epidemic in London in 1854 that John Snow attributed to a contaminated well, or modern outbreaks of food poisoning like E. coli from salad greens. These relatively clear-cut sources of disease can be identified by epidemiological studies because the risk of infection in an exposed individual is several hundred times that of someone not exposed to the same contamination. But, correlation does not equal causation. Many strongly correlated factors may have no relation, such as per capita consumption of margarine in the United States and the divorce rate in the state of Maine, which correlated at a level of 0.99 (the highest possible correlation being 1.00) over a 10-year period (Vigen, 2018).
Meat and cancer—cause and effect or only an association?
In 2015, the World Health Organization’s International Agency for Research on Cancer (IARC) constituted a working group to evaluate the cancer-causing effect of consuming red or processed meat. The 22 members of the working group were all self-nominated; many had spent most of their careers studying the relationship of meat or other dietary factors and cancer. Data used for this exercise were observational studies, animal experiments, and mechanistic studies that might establish a biological link between some component in red or processed meats and development of cancer. This group concluded that animal experiments were not useful in assessing the evidence because these results had no clear effect in either direction of feeding red or processed meat on tumor development. Therefore, the committee relied upon only observational studies, with supporting information from mechanistic studies. However, mechanistic studies are not strong evidence of causality; they simply show that some component in meat could affect noncancer endpoints in studies with animals or cell cultures, and plausibly increase the risk of cancer. In addition, the importance of such mechanistic studies in animals should not outweigh the outcomes from animal studies of cancer development. This means that in experiments in which feeding large amounts of bacon or beef does not increase the risk of cancer in animals that are genetically prone to cancer or given a chemical to induce colon cancer, studies of noncancer, potential biological indicators of cancer risk should count less when the totality of the evidence is assessed. So when evidence of gene damage or oxidative stress in animals is claimed as the supporting mechanisms, but those animals do not get more cancer when fed three times the normal amount of protein combined with a calcium-deficient diet to see an effect, it is illogical to accept the mechanistic studies as confirmation of the epidemiology. In addition, the IARC subgroup who evaluated mechanisms ignored two studies by one of its members in which bacon fed to rats actually significantly suppressed the precancerous indicators (Parnaud et al., 1998; Parnaud et al., 2000).
Although the IARC working group on meat and cancer met in October 2015 and a two-page summary was published immediately after (Bouvard et al., 2015), the full monograph was not published until March 2018 (IARC, 2018). The working group evaluated over 800 epidemiological studies, but only 7 of 14 studies of red meat and 12 of 18 on processed meat found increased risk of colorectal cancer in people eating the most meat. There was insufficient data on meat intake and cancers at other sites in the body for the group to reach a conclusion. IARC stated that the working group was identifying hazard only—not evaluating the risk of getting colon cancer. But, this is circular reasoning because the risk among those eating the most meat determined whether meat consumption was deemed a hazard. Another weakness in the conclusion from IARC is that chance, bias, and confounding could not be ruled out with the same degree of confidence for the relation of red-meat consumption and colon cancer, but these three issues were considered unlikely in the linkage of processed-meat intake and colon cancer. The main reason these differences are not supported is that the same cohort studies provided data on both red and processed meat. Therefore, if chance, bias, and confounding cannot be ruled out for red meat, then those same issues apply to any potential association of processed meat and cancer because the same research data and methods were used from the same subjects.
Nevertheless, the IARC working group concluded that for every 50 grams of processed meat eaten, the relative risk of colon cancer was increased by 18% compared with those who ate the least processed meat. How does this compare with known carcinogens? The increased relative risk of lung cancer from smoking cigarettes is 1000–3000%. The increased relative risk of liver cancer from eating moldy grains contaminated with aflatoxin is about 600%. In fields outside nutrition, the usual threshold for confidence about relative risk is in the range of 200–400%. At the higher end of that range, one can be guardedly confident but “we can hardly ever be confident about estimates of less than 2.0, and when estimates are much below 2.0, we are simply out of business” (Shapiro, 2004); relative risk of 2.0 translates to an increase of 100%. So, an 18% increase equals a relative risk of 1.18, and this score falls substantially below the threshold that epidemiologists in other fields generally accept as worthy of further investigation.
Another indicator of risk is the absolute risk, as opposed to the previously mentioned relative risk. The relative risk is a ratio of the disease rate in the group exposed to the highest amount divided by the rate in the group exposed to the lowest amount but this risk ratio does not reflect the absolute risk of a disease. The lifetime absolute risk of colon cancer in vegetarians is 4.5 out of 100; in people eating 50 grams of processed meat every day for a lifetime, the risk is 5.3 out of 100. These numbers are not statistically distinguishable in epidemiological studies. Today’s standards of evidence generally call for a systematic review of the literature and a quantitative meta-analysis of published studies. Neither of these was performed by IARC. In contrast, other investigators published a meta-analysis shortly after the IARC summary appeared and calculated a 10% increased risk of colorectal cancer (about half the risk concluded by IARC) but found no dose-response, suggesting a lack of specificity in this association (Alexander et al., 2015). This finding means that it did not matter how much meat was eaten by the group with the highest intake across different studies; the highest consumers always had a higher cancer risk, suggesting that other factors not measured affected the risk of colon cancer. In fact, two recent large observational studies comparing vegetarians with meat eaters found no increase of colon cancer risk when adjusting for vegetable intake in one study, or a variety of factors that included socioeconomic status, physical activity, smoking, and alcohol intake (Appleby et al., 2016; Mihrshahi et al., 2017). In other words, people who ate a lot of vegetables and fruit had no increased risk, no matter how much red meat they ate. These results again suggest that there are multiple other lifestyle factors that associate with dietary differences that account for the claimed differences in risk of cancer. A separate recent analysis evaluated health factors associated with meat eating and found that those who ate the most meat weighed more, were less physically active, and had a history of more smoking as well as lower intake of fruits and vegetables (Grosso et al., 2017). Those authors concluded that the differences in health behaviors modify the claimed relationship between diet and chronic disease risk. A take-home message might be that there are healthy and unhealthy lifestyles that contribute to colon cancer and other chronic diseases. Those eating the most red or processed meat may be more likely to ignore other health recommendations and have multiple habits that contribute to the risk of disease.
Observational studies are hypothesis-generating Studies, not proof
One of the weaknesses of most long-term observational studies is the use of a computer-scored food frequency questionnaire to estimate dietary intake. Many food frequency questionnaires ask only about the frequency of consumption, not serving size. Most people who are asked about their habitual diet for the last year are influenced by what they have eaten over the past few weeks. And, there are very few truly objective tests that can be used to validate whether the questionnaire has accurately captured usual dietary intake. Almost all study questionnaires ask about diet at baseline and assume that it has not changed for the duration of follow-up that can last more than 20 years; such an assumption is demonstrably false. Another key issue is whether the food frequency questionnaire is adequate for estimating total calories or protein—both critical for studying the effects of meat consumption on health—and both have tests available that can accurately determine how much has been eaten. The definitive study was done by scientists from the U.S. National Cancer Institute who concluded that the food frequency questionnaire is not able to evaluate the absolute intake of either energy or protein (Schatzkin et al., 2003).
A separate issue that potentially weakens the ability of observational studies to provide solid evidence of a causal relationship between a food and a disease outcome is the large number of comparisons in a study. Outcomes prespecified in an analysis are considered more certain whereas those not specified in advance are considered exploratory and require a higher statistical bar to be considered meaningful. By convention, most scientists use the 5% level of statistical significance as agreement that a group difference is likely reproducible. However, the large numbers of factors in nutrition studies—for example, 125 food items in a typical food frequency questionnaire, 40 nutrients, and 50 disease endpoints or risk factors—yield a total of 406,250 possible outcomes. Five percent of that number means 20,312 could be false positives. If we used a higher barrier to declare significance among endpoints not prespecified, such as 0.5%, the false positives would be reduced by 90% (but would still number more than 2,000 in this example) and most claims about diet and disease would no longer be considered statistically significant and certainly would not represent a treatment effect worthy of further recommendations (Ioannidis, 2018). In fact, there are existing statistical procedures, widely accepted in genetics and other fields that rely on many simultaneous comparisons that control the false discovery rate but this has never been adopted in the nutrition area. High numbers of subjects in a study increase the statistical power but may provide a false sense that an association is meaningful. One example from the epidemiology literature clearly designed to show the limitations of standard statistical approaches in large studies used 10.6 million people equally divided into two cohorts—one for derivation of associations and one for validation of the relationship. The investigators searched 223 of the most common diagnoses for hospitalization in the medical records of the participants and found that 24 were statistically significant in the first cohort based on individuals’ astrological signs. Two of these associations remained statistically significant in the second cohort with relative risks of 1.15 and 1.38, numbers in the same range as the relative risk of processed meat and colon cancer (Austin et al., 2006).
Observational studies of nutrition and health can contribute to the overall assessment of a causal relationship but because an association is weak evidence, they cannot be used by themselves, or as the primary driver, of any conclusions. This was made clear more than 50 years ago by Sir A.B. Hill, who listed nine considerations for conclusions about causality from the scientific literature (Hill, 1965). The factors were strength, consistency, specificity, temporality, biological gradient, plausibility, coherence, experiment, and analogy; the relationship of meat and cancer only fulfils four of these nine, making it relatively weak and uncertain.
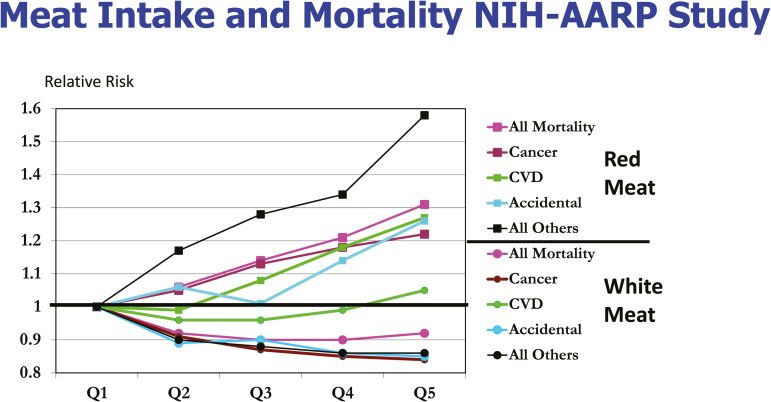
The observational study on meat intake and mortality that critically demonstrated to this author that the association of meat intake with causes of death is not causal was the NIH-AARP Study that followed 500,000 older people and recorded 71,000 deaths (Sinha et al., 2009). If one assumes that the food frequency questionnaire was accurate and that diet did not change during 10 years of follow-up (both of which are generous assumptions), all causes of death were increased in those eating the most red meat and decreased in people eating the most white meat (poultry and fish), except for cardiovascular disease in those eating the most white meat (Figure 2; Klurfeld, 2015). The causes of death were separated into cancer, cardiovascular diseases, accidental, all others, along with total mortality. Relative risk was approximated for both sexes combined from tables for risk in each sex provided in Sinha et al. (2009). The relative risk of dying from cancer among the highest meat eaters was about 1.22 whereas that from accidents was 1.26 and all other causes was 1.58; this latter category included 10 factors including infections, ulcers, chronic obstructive pulmonary disease, liver disease, and kidney disease. The fact that accidents and all other causes of death were increased by red-meat and decreased by white-meat consumption strongly indicates the nonspecific effect of eating these foods. The risk of accidental death was the same as the risk of cancer and risk of a wide-ranging group of 10 causes of death labeled other was significantly higher than that for cancer. These relationships should make it clear that some confounding factors that associate with high red-meat intake not measured, or simply chance, were more likely explanations than a cause and effect from eating red meat (Klurfeld, 2015).
Figure 2.
Relative risk of total mortality among men and women from various causes of death. There were 71,000 deaths among 500,000 participants in the NIH-AARP Cohort Study arranged by quintiles of red or white (poultry and fish) meat intake; Q1 being the lowest and Q5 the highest. Reproduced from Klurfeld, 2015.
Intervention studies with low-meat diets
Most importantly for the IARC report, two major dietary intervention studies that should have contributed to the assessment of the claimed relationship of red meat and cancer were not considered. The first was a study of colon polyps, the precancerous growths that greatly increase the likelihood of developing colon cancer. Almost 1,900 subjects with a recent history of having a polyp removed were divided into a control group that ate their usual diet and a group following a diet characterized by significant decreases in total fat, red, and processed meat along with increases in fruits, vegetables, whole grains, and legumes (Schatzkin et al., 2000). Participants were followed for 3 years and at the end of that time, the recurrence of colon polyps was identical in both diet groups. It is possible that the precancerous stage may not have been the proper time for dietary intervention. The Women’s Health Initiative, therefore, studied a low-fat diet, achieved in large part by reducing red- and processed-meat consumption, among almost 49,000 women (Beresford et al., 2006); about 30,000 followed their normal diets and almost 20,000 were assigned to low-fat diets. After 9 years, the rate of colon cancer was almost identical in the low-fat and control-diet groups. These studies strongly suggest that the observational studies are not supported by dietary intervention studies at either the precancerous or malignant tumor stages of colon cancer.
Diet and disease dead ends
The field of nutrition has a long history of observational studies pointing to a relationship that is not supported by controlled intervention studies that test specific associations. Examples include claims about cancer with the nutrients beta-carotene, vitamins C or E, and selenium. In fact, beta-carotene in high amounts was shown to significantly increase the risk of lung cancer in smokers, in sharp contrast to what observational studies originally suggested. Claims about other outcomes from observational studies that were refuted in clinical trials included those for vitamin D, B vitamins, and multivitamins (Young and Karr, 2011). Most of the claims that high-fat diets led to cancers in various organs including colon, breast, and prostate that drove research for decades have been quietly abandoned.
Even the journal that published the two-page summary of the conclusions from the IARC working group on meat and cancer printed an editorial several months later questioning the validity of the process (Anonymous, 2016). That commentary pointed out the problem of determining reliable findings when data are equivocal and called for internationally agreed-upon methodology for assessing carcinogens.
The conclusion is that there is not good evidence that red- or processed-meat consumption is linked to cancer, but that does not mean eating any amount of meat is compatible with good health. Like any food or nutrient, excess consumption is likely associated with adverse health effects. There are many researchable questions that remain. We do not have valid and reliable data from multiple samples on the chemical changes in charred or smoked meats nor do we know if the amounts of potential carcinogens in such products have an effect in humans. We do know that exposing rodents to 1,000 to 100,000 times the amounts of isolated chemical carcinogens estimated to be in cooked meat leads to cancer, but we have no idea if exposure to much lower levels has any adverse effect. In fact, the concept of hormesis hypothesizes that exposure to low levels of compounds that are harmful at high doses actually leads to a beneficial health effect. In addition, most toxicologists recognize the safety of exposure to small amounts of compounds that are harmful in much greater concentrations even if they do not have a beneficial physiological effect. A similar relation exists for essential nutrients that are needed in small amounts but are toxic in high doses, such as vitamin A or iron; even drinking too much water can lead to fatal consequences. People who eat the most meat are also likely to eat the fewest fruits, vegetables, whole grains, and dietary fiber. Those foods and nutrients are able to modify the gastrointestinal microbiota, the commensal bacteria that plays in increasingly recognized role in human metabolism and maintenance of health, but were not considered by the IARC working group. Recent studies implicate specific strains of bacteria in the development of colon cancer but we are far from conclusive evidence (Dejea et al., 2018). The biggest unresolved question is whether the highest consumption of meat is simply a marker for a set of lifestyle characteristics that increase risk of cancer. These issues are researchable questions that would take a very large group of people who answer questionnaires accurately and would take many years, which translates into a very expensive study. So we are not likely to have a resolution to this conundrum in the foreseeable future unless there is a revolution in our understanding of the causes of colon cancer and our ability to monitor diet and health habits more accurately. In fact, it may not even be worth studying potential risks from single foods and chronic diseases because it diverts attention and resources from focus on the entire diet and associated lifestyle choices that clearly affect long-term health. Coming to a correct conclusion on diet and health does not require absolute proof, but taking a relationship built on weak associations as proof is not helpful to the public or the profession. Science is not possession of the truth but is the systematic, reproducible pursuit of the answers, and we should depend on reliable science for dietary recommendations.
About the Author

David Klurfeld is National Program Leader for Human Nutrition in the Agricultural Research Service of the U.S. Department of Agriculture. His research has focused on the relationship of diet and prevention of chronic diseases such as cancer, heart disease, and gallstones. Among his scientific discoveries are the first demonstration that red-wine consumption resulted in fewer cardiovascular lesions, that the cholesterol-filled cells in human arterial lesions are white blood cells, that reducing calories was more important than reducing fat in the diet for decreasing cancer growth, and a mediator of this last effect was likely insulin-like growth factor-1. He is an Associate Editor of the American Journal for Clinical Nutrition.
Literature Cited
- Alexander D.D., Weed D.L., Miller P.E., and Mohamed M.A.. 2015. Red meat and colorectal cancer: a quantitative update on the state of the epidemiologic science. J. Am. Coll. Nutr. 34:521–543. doi: 10.1080/07315724.2014.992553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous 2016. When is a carcinogen not a carcinogen?Lancet Oncol. 7:295–296. doi: 10.1016/S1470-2045(16)30138-3 [DOI] [Google Scholar]
- Appleby P.N., Crowe F.L., Bradbury K.E., Travis R.C., and Key T.J.. 2016. Mortality in vegetarians and comparable nonvegetarians in the United Kingdom. Am. J. Clin. Nutr. 103:218–230. doi: 10.3945/ajcn.115.119461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin P.C., Mamdani M.M., Juurlink D.N., and Hux J.E.. 2006. Testing multiple statistical hypotheses resulted in spurious associations: a study of astrological signs and health. J. Clin. Epidemiol. 59:964–969. doi: 10.1016/j.jclinepi.2006.01.012 [DOI] [PubMed] [Google Scholar]
- Beresford S.A., Johnson K.C., Ritenbaugh C., Lasser N.L., Snetselaar L.G., Black H.R., Anderson G.L., Assaf A.R., Bassford T., Bowen D., et al. 2006. Low-fat dietary pattern and risk of colorectal cancer: the women’s health initiative randomized controlled dietary modification trial. JAMA. 295:643–654. doi: 10.1001/jama.295.6.643 [DOI] [PubMed] [Google Scholar]
- Boada L.D., Henríquez-Hernández L.A., and Luzardo O.P.. 2016. The impact of red and processed meat consumption on cancer and other health outcomes: epidemiological evidences. Food Chem. Toxicol. 92:236–244. doi: 10.1016/j.fct.2016.04.008 [DOI] [PubMed] [Google Scholar]
- Bouvard V., Loomis D., Guyton K.Z., Grosse Y., Ghissassi F.E., Benbrahim-Tallaa L., Guha N., Mattock H., and Straif K.; International Agency for Research on Cancer Monograph Working Group 2015. Carcinogenicity of consumption of red and processed meat. Lancet. Oncol. 16:1599–1600. doi: 10.1016/S1470-2045(15)00444-1 [DOI] [PubMed] [Google Scholar]
- Dejea C.M., Fathi P., Craig J.M., Boleij A., Taddese R., Geis A.L., Wu X., DeStefano Shields C.E., Hechenbleikner E.M., Huso D.L., et al. 2018. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science. 359:592–597. doi: 10.1126/science.aah3648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosso G., Micek A., Godos J., Pajak A., Sciacca S., Galvano F., and Boffetta P.. 2017. Health risk factors associated with meat, fruit and vegetable consumption in cohort studies: a comprehensive meta-analysis. Plos One. 12:e0183787. doi: 10.1371/journal.pone.0183787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A.B. 1965. The environment and disease: association or causation?Proc. R. Soc. Med. 58:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans 2018. IARC monographs on the evaluation of carcinogenic risks to humans; red meat and processed meat, Vol. 114 Lyon, France: International Agency for Research on Cancer. doi: 10.1016/S1470-2045(15)00444-1 [DOI] [Google Scholar]
- Ioannidis J.P.A. 2018. The proposal to lower P value thresholds to .005. JAMA. 319:1429–1430. doi: 10.1001/jama.2018.15362 [DOI] [PubMed] [Google Scholar]
- Klurfeld D.M. 2015. Research gaps in evaluating the relationship of meat and health. Meat Sci. 109:86–95. doi: 10.1016/j.meatsci.2015.05.022 [DOI] [PubMed] [Google Scholar]
- Mihrshahi S., Ding D., Gale J., Allman-Farinelli M., Banks E., and Bauman A.E.. 2017. Vegetarian diet and all-cause mortality: evidence from a large population-based australian cohort - the 45 and up study. Prev. Med. 97:1–7. doi: 10.1016/j.ypmed.2016.12.044 [DOI] [PubMed] [Google Scholar]
- Parnaud G., Peiffer G., Taché S., and Corpet D.E.. 1998. Effect of meat (beef, chicken, and bacon) on rat colon carcinogenesis. Nutr. Cancer. 32:165–173. doi: 10.1080/01635589809514736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnaud G., Pignatelli B., Peiffer G., Taché S., and Corpet D.E.. 2000. Endogenous N-nitroso compounds, and their precursors, present in bacon, do not initiate or promote aberrant crypt foci in the colon of rats. Nutr. Cancer. 38:74–80. doi: 10.1207/S15327914NC381_11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzkin A., Kipnis V., Carroll R.J., Midthune D., Subar A.F., Bingham S., Schoeller D.A., Troiano R.P., and Freedman L.S.. 2003. A comparison of a food frequency questionnaire with a 24-hour recall for use in an epidemiological cohort study: results from the biomarker-based observing protein and energy nutrition (OPEN) study. Int. J. Epidemiol. 32:1054–1062. doi: 10.1093/ije/dyg264 [DOI] [PubMed] [Google Scholar]
- Schatzkin A., Lanza E., Corle D., Lance P., Iber F., Caan B., Shike M., Weissfeld J., Burt R., Cooper M.R., et al. 2000. Lack of effect of a low-fat, high-fiber diet on the recurrence of colorectal adenomas. Polyp prevention trial study group. N. Engl. J. Med. 342:1149–1155. doi: 10.1056/NEJM200004203421601 [DOI] [PubMed] [Google Scholar]
- Shapiro S. 2004. Looking to the 21st century: have we learned from our mistakes, or are we doomed to compound them?Pharmacoepidemiol. Drug Saf. 13:257–265. doi: 10.1002/pds.903 [DOI] [PubMed] [Google Scholar]
- Sinha R., Cross A.J., Graubard B.I., Leitzmann M.F., and Schatzkin A.. 2009. Meat intake and mortality: a prospective study of over half a million people. Arch. Intern. Med. 169:562–571. doi: 10.1001/archinternmed.2009.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigen T. Spurious correlations [accessed March 22, 2018]. http://www.tylervigen.com/spurious-correlations.
- Young S.S. and Karr A.. 2011. Deming, data and observational studies. A process out of control and needing fixing. Significance. 8:116–120. doi: 10.1111/j.1740-9713.2011.00506.x [DOI] [Google Scholar]