Abstract
Arbuscular mycorrhizal fungi (AMF) are important mutualistic microbes in soil, which have capacity to form mutualistic associations with most land plants. Arbuscular mycorrhizal fungi play an important role in plant invasions and their interactions with invasive plants have received increasing attention. However, the chemical mechanisms underlying the interactions of AMF and invasive plants are still poorly understood. In this study we aim to test whether root secondary chemicals are related to enhanced AMF colonization and rapid growth in an invasive tree. We conducted a common garden experiment in China with Chinese tallow tree (Triadica sebifera) to examine the relationships among AMF colonization and secondary metabolites in roots of plants from introduced (USA) and native (China) populations. We found that AMF colonization rate was higher in introduced populations compared to native populations. Roots of plants from introduced populations had lower levels of phenolics and tannins, but higher levels of flavonoids than those of plants from native populations. Flavonoids were positively correlated with AMF colonization, and this relationship was especially strong for introduced populations. Besides, AMF colonization was positively correlated with plant biomass suggesting that higher root flavonoids and AMF colonization may impact plant performance. This suggests that higher root flavonoids in plants from introduced populations may promote AMF spore germination and/or attract hyphae to their roots, which may subsequently increase plant growth. Overall, our results support a scenario in which invasive plants enhance their AMF association and invasion success via genetic changes in their root flavonoid metabolism. These findings advance our understanding of the mechanisms underlying plant invasion success and the evolutionary interactions between plants and AMF. Understanding such mechanisms of invasive plant success is critical for predicting and managing plant invasions in addition to providing important insights into the chemical mechanism of AMF–plant interactions.
Keywords: Biomass, Chinese tallow tree, flavonoids, invasive population, secondary metabolism
In this study we found that the arbuscular mycorrhizal fungi (AMF) colonization rate was higher in introduced populations compared to native populations. Roots of plants from introduced populations had higher levels of flavonoids and the concentration of flavonoids in roots was positively correlated with AMF colonization, especially strong for introduced populations. Furthermore, AMF colonization was positively correlated with plant biomass. These findings suggest that higher root flavonoids in plants from introduced populations may promote AMF spore germination and/or attract hyphae to their roots, which may subsequently increase plant growth.
Introduction
Plant invasions can damage the ecological environment (Tanveer et al. 2018) by reducing the diversity or abundance of native plant and animal communities (Moroń et al. 2009; Stefanowicz et al. 2017). In order to clarify the mechanisms underlying plant invasions, there have been an increasing number of studies on the role of below-ground biota, such as the effects of soil microbes on invasive plants (Rout and Callaway 2012; Li et al. 2017; Verbeek and Kotanen 2019). Arbuscular mycorrhizal fungi (AMF), an important group of symbiotic microbes (van Kleunen et al. 2018), have been found to play a role in plant invasions (Richardson et al. 2010; van Kleunen et al. 2018; Yong et al. 2018). However, it is not known why invasive plants or introduced populations of invasive plants often have higher rates of mycorrhizal colonization than native plants or native populations of invasive plants, respectively.
Arbuscular mycorrhizal fungi build symbiotic relationships with >80 % of terrestrial plants, including many invasive plants (Reinhart and Callaway 2006; Zdenka et al. 2013; Horn et al. 2017; Mello and Balestrini 2018). They typically benefit their host plants by promoting soil nutrient mobilization and absorption (Rillig 2010; Datta and Kulkarni 2014; Bunn et al. 2015; Kim et al. 2015; Jiang et al. 2018; Zhang et al. 2019). At present, an increasing number of studies have demonstrated that AMF can have an important role in plant invasion success (Dawkins and Esiobu 2017; Zhang et al. 2018). The enhanced mutualisms hypothesis indicates that invasive species can alter the AMF community and receive greater benefits from them compared to co-occurring native plants, which may facilitate their invasion (Reinhart and Callaway 2006) by increasing survival, growth rate and/or competitiveness (Sun and He 2010; Lekberg et al. 2013; Zhang et al. 2017). One study found that plant invasions can increase the diversity of AMF by comparing uninvaded and invaded sites in Hawaii (Gomes et al. 2018). Another study found that the invasive plants, Ambrosia artemisiifolia and Bidens pilosa, have higher AMF colonization rates than the native plant Setaria viridis when they are planted together (Zhang et al. 2018). Furthermore, the invasive Eurasian forbs knapweed (Centaurea stoebe), leafy spurge (Euphorbia esula) and Canada goldenrod (Solidago canadensis) each benefit in competition with native plants when mycorrhizae are present (Sun and He 2010; Lekberg et al. 2013). The invasive Chinese tallow tree (Triadica sebifera) has also been found to gain more benefits from mycorrhizal associations than co-occurring native trees in a range of soil fertilities (Nijjer et al. 2004, 2008; Paudel et al. 2014). Hence, it can be inferred that the AMF may play an important role in the establishment and spread of invasive plants (Sielaff et al. 2019). Although we have a wealth of evidence showing that invasive plants and introduced populations of invasive plants often benefit more from AMF associations than native species or native populations, respectively, we know little about what drives these higher rates of mycorrhizal colonization that underlie these high colonization rates and benefits.
Indeed, some studies have explored the chemical mechanisms that drive differences in mycorrhizal colonization rates for plants in general. For instance, a study on the root exudates from tomato found an unknown active factor, which is a methanol-soluble compound but not the strigolactone analog GR24, stimulates AMF growth and branching (Sun et al. 2012). A study on legumes showed that flavonoids play an important role in signalling, establishment and regulation of mycorrhizal endosymbiosis (Singla and Garg 2017), as well as playing a role in anti-herbivore defence (Xiao et al. 2019) and antioxidant activity (Ma et al. 2017). This finding is supported by an RNAi silencing study, which found that mycorrhizal colonization is affected by flavonoids and polyamines in soybeans (Salloum et al. 2018). In addition, research on the effect of the essential oil from aromatic lavender (Lavandula stoechas) on two mycorrhizal species indicated that it was beneficial for the colonization of Septoglomus deserticola and Rhizophagus intraradices (Hassiotis and Orfanoudakis 2018). Other studies have demonstrated that some flavonoids can induce AMF spore germination and hyphal branching, potentially increasing the colonization rate on plant roots (Akiyama et al. 2005; Liu et al. 2014). For example, the flavonoid apigenin is able to enhance hyphal branching and root colonization at 0.5 μM concentration (Scervino et al. 2006). A study on melon found that flavonoids are involved in the regulation of AMF infection in its roots (Akiyama et al. 2002). However, there are no investigations of the regulation of AMF by secondary chemicals in invasive plants or the potential role of differences in secondary chemicals, especially root flavonoids, of introduced vs. native populations of invasive plants on their AMF associations.
Chinese tallow tree (T. sebifera) is a deciduous tree that is originally from China (Pattison and Mack 2008). It is introduced to USA in the late 18th century where it has become invasive (Pile et al. 2017). Previous studies showed that plants from introduced populations have higher AMF colonization (Yang et al. 2013, 2015c), more rapid growth and greater competitiveness (Huang et al. 2012b; Siemann et al. 2017) and higher foliar flavonoids than native populations (Wang et al. 2012). In this study, we investigated whether higher flavonoids in roots of T. sebifera plants from introduced populations are correlated with higher AMF colonization rates, which contribute to their rapid growth.
Materials and Methods
Seeds collection
We collected seeds of T. sebifera from 12 populations in southern China (native populations) and 10 populations across the south-eastern USA (invasive populations) in November 2015 (Table 1). These populations included the likely source and recipient populations from the two major North American introduction events (Dewalt et al. 2011). We hand-collected seeds from 5 to 10 trees in every population. We removed the waxy layer around these seeds by soaking them in water with laundry detergent (Huang et al. 2012a), then we rinsed them and put them in the refrigerator (4 °C) in wet sand. After 30 days, we planted them in sterile garden soil.
Table 1.
The geographical coordinates of Triadica sebifera populations from the native (China, 12 populations) and introduced (USA, 10 populations) ranges in this study.
| ID | Site of seed collection | Latitude | Longitude |
|---|---|---|---|
| Native populations (in China) | |||
| CH-DW | Dawu, Hubei | 31°28′N | 114°16′E |
| CH-GL | Guilin, Guangxi | 25°04′N | 110°18′E |
| CH-HeS | Hengyang, Hunan | 27°14′N | 112°46′E |
| CH-HF | Hefei, Anhui | 31°50′N | 117°09′E |
| CH-HuS | Huangshan, Anhui | 30°00′N | 117°59′E |
| CH-LiA | Lin’an, Zhejiang | 30°47′N | 120°03′E |
| CH-ML | Miluo, Hunan | 28°53′N | 113°12′E |
| CH-NJ | Nanjing, Fujian | 24°42′N | 117°3′3E |
| CH-WX | Wuxi, Jiangsu | 31°36′N | 120°14′E |
| CH-YS | Yangshan, Guangdong | 24°35′N | 112°41′E |
| CH-ZS | Zhangshu, Jiangxi | 28°02′N | 115°25′E |
| CH-YT | Yingtan, Jiangxi | 28°19′N | 117°03′E |
| Introduced populations (in USA) | |||
| US-AL-1 | Tillman’s Corner, AL | 30°35′N | 88°09′W |
| US-FL-4 | Callahan, FL | 30°35′N | 81°47′W |
| US-GA-1 | Hutchinson Island, GA | 32°06′N | 81°06′W |
| US-GA-2 | Sapelo Island, GA | 31°23′N | 81°15′W |
| US-LA-1 | Lake Charles, LA | 30°14′N | 93°09′W |
| US-LA-5 | Pumpkin Center, LA | 30°28′N | 90°32′W |
| US-SC-1 | Limehouse, SC | 32°09′N | 81°06′W |
| US-TX-2 | La Marque, TX | 29°22′N | 95°02′W |
| US-TX-4 | Lynchburg, TX | 29°47′N | 95°02′W |
| US-TX-5 | Port Arthur, TX | 29°53′N | 94°02′W |
Soil
We mixed together (1:1 volume) commercial river sand and soil which were collected from a field at Henan University (Kaifeng, Henan province, China) in which maize had been grown in the previous season. We filled 198 plastic pots (height: 15 cm, upper diameter: 19 cm, bottom diameter: 12 cm) each with 1.5 kg of this soil mixture.
Experimental design
To test the relationship between plant growth and AMF colonization, we carried out a common garden experiment from June to October in 2016 at Henan University. In July, when seedlings had reached the four-leaf stage, nine seedlings of each population individually were transplanted into the pots with the sand/soil mix. These seedlings were protected with nylon mesh (40 openings per inch) from herbivory and placed in an open-sided greenhouse, and then they were watered daily.
After 105 days, these seedlings were clipped at ground level before we collected soil samples to measure hyphal density. Then, we carefully washed the roots from the soil and took a subsample of fine roots to assess AMF colonization rate. Following that, we dried (40 °C for 48 h) and then weighed above-ground and below-ground biomass, separately.
AMF colonization
We cleared fresh fine roots with 10 % KOH for 60 min at 90 °C, acidified them with 2 % HCl for 5 min and then stained them for 30 min at 90 °C with 0.05 % trypan blue following published protocols (Nijjer et al. 2008). We mounted 30 1-cm fine root segments from each plant onto slides. Arbuscular mycorrhizal fungi colonization rate was estimated by using the gridline intersect method with 300 intersection points per plant (Brundrett et al. 1995).
Hyphal density
We estimated hyphal length in soil following a modification of published methods (Hanssen et al. 1974; Johansen et al. 1992). We blended 2 g soil and 50 mL of Deionized water for 30 s at high speed (10 000 rpm). We transferred each soil solution into a 500-mL beaker with 200 mL of Deionized water and mixed it with a magnetic stirrer (900 rpm). We let the solution rest for 30 s, then took 5 mL of this solution from a depth of 1 cm from the top and filtered it through a Millipore filters (0.45 μm) three times per soil sample (three separate filters). We covered the filters for 5 min with 0.05 % trypan blue. We dried them and recorded the presence of hyphae at 25 intersections of a 1-mm grid at ×200 magnification. We calculated hyphal density by the formula: Hyphal density (m g−1 dry soil) = 0.14399 × number of crossings (Jakobsen et al. 1992; Gao et al. 2016).
Total soluble sugars
We ground dried roots with a ball mill (Hengao HMM-400A, Tianjin Hengao Technology Development Co., Ltd). We extracted total soluble sugars from 100 mg root samples using 95 % ethanol and determined the concentrations of soluble sugar with the colourimetry of sulfuric acid-anthrone method (Yemm and Willis 1954).
Secondary metabolites
We measured the concentration of total phenolics by the Folin-Ciocaileu colourimetric method (Stankovic et al. 2011) and the concentration of total flavonoids by the modified method of aluminium nitrate colourimetric (Medini et al. 2014; Li et al. 2019). We measured total tannin with the vanillin-hydrochloric acid method (Price et al. 1978).
Data analysis
We used analysis of variance (ANOVA) to test the dependence of AMF colonization, hyphal density, total soluble sugars and secondary compounds on population origin (fixed factor) and population nested in origin as a random factor (proc mixed, SAS 9.4). We used ANOVA to test the dependence of AMF colonization on root flavonoids, population origin and their interaction as fixed terms and population nested in origin as a random factor. We used Pearson correlations to examine the relationships among AMF colonization and plant mass (the total mass of above-ground and below-ground biomass), the root concentrations of polyphenols, tannins, soluble sugars and flavonoids (proc corr, SAS 9.4). Because native and introduced population plants differed significantly in AMF colonization and root flavonoids and visual inspection indicated different relationships between root flavonoids and AMF colonization for introduced vs. native populations, we performed separate Pearson correlations for them as well.
Results
AMF of native and introduced populations
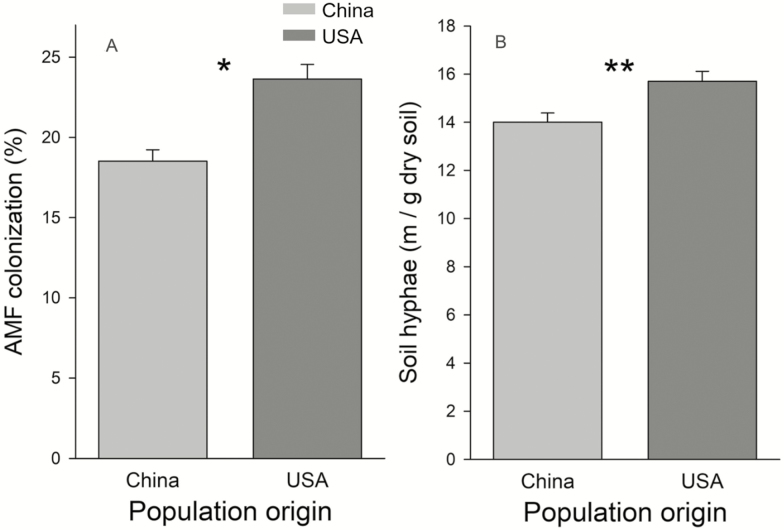
Plants from introduced populations had higher levels of AMF colonization on their roots than native populations (Fig. 1A; F1, 20 = 5.18, P = 0.0341) and a greater density of hyphae in soil associated with their roots (Fig. 1B; F1, 20 = 9.18, P = 0.0060). Populations varied in their AMF colonization (Z = 2.62, P = 0.0044) but not density of hyphae in soil (Z = 0.61, P = 0.5431).
Figure 1.
(A) AMF colonization on roots and (B) fungal hyphae in soils associated with T. sebifera plants from native (China) or introduced (USA) populations. Difference of native (China) and introduced (USA): *P < 0.05; **P < 0.01.
Concentrations of metabolite in the roots of native and introduced populations
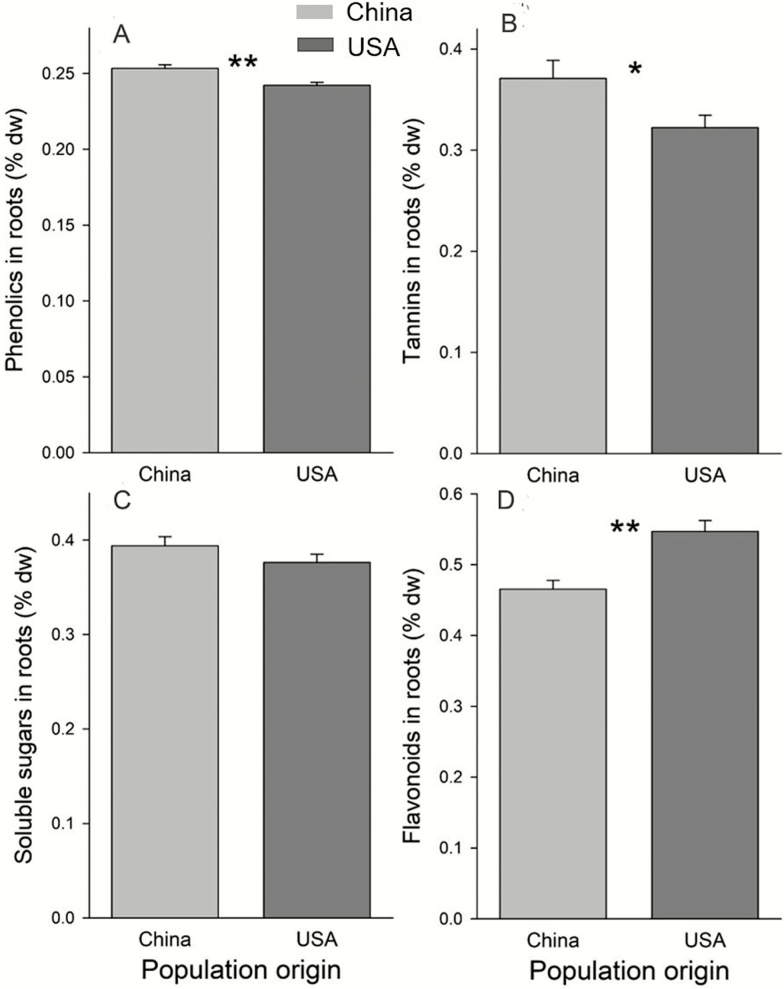
Compared to plants from native populations, those from introduced populations had lower root concentrations of phenolics (Fig. 2A; F1, 15 = 11.08, P = 0.0046) and tannins (Fig. 2B; F1, 15 = 5.03, P = 0.0405), comparable concentrations of soluble sugars (Fig. 2C; F1, 15 = 1.87, P = 0.1919) and higher concentrations of flavonoids (Fig. 2D; F1, 15 = 10.81, P = 0.0050). None of these chemical concentrations varied with population (all P > 0.10).
Figure 2.
Concentrations of (A) phenolics, (B) tannins, (C) soluble sugars and (D) flavonoids in roots of T. sebifera plants from native (China) or introduced (USA) populations. Difference of naive (China) and introduced (USA): *P < 0.05; **P < 0.01.
The relationship between flavonoids and AMF
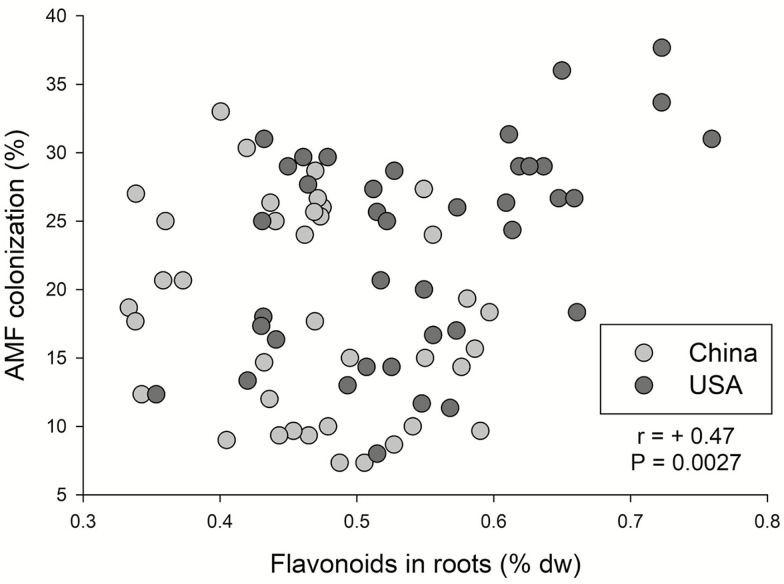
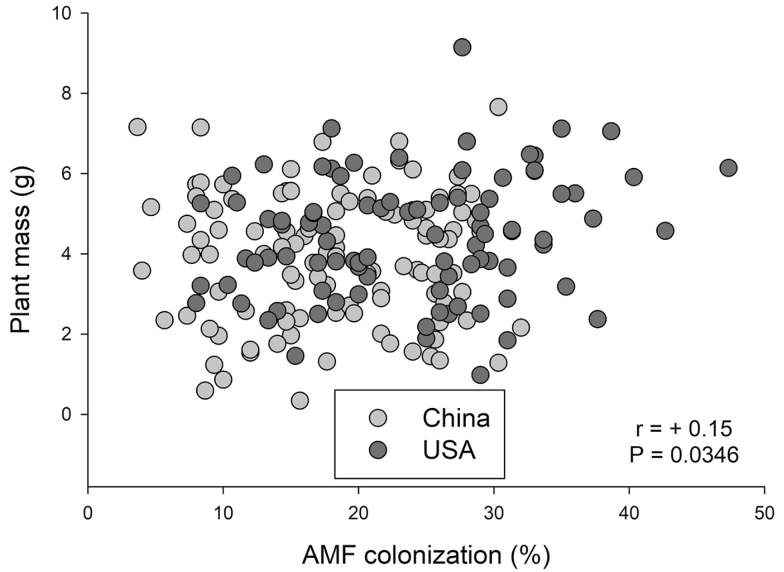
AMF colonization was significantly positively correlated with the concentration of root flavonoids (Fig. 3; r = +0.47, P = 0.0027) but was not related to concentrations of phenolics (P = 0.5017), tannins (P = 0.7394) or soluble sugars (P = 0.7252). When introduced and native populations were examined separately, the correlation between root flavonoids and AMF colonization was significant for introduced populations (r = +0.48, P = 0.0027) but not native populations (P = 0.17). Plant mass and AMF colonization rate were positively correlated (Fig. 4; r = +0.15, P = 0.0346).
Figure 3.
The relationship between root flavonoids and AMF colonization. The r and P-values are from a Pearson correlation with all plants.
Figure 4.
The relationship between AMF colonization and plant mass. The r and P-values are from a Pearson correlation with all plants.
Discussion
An increasing number of studies support an important role for symbiotic micro-organisms in plant invasions (Dawson and Schrama 2016; van Kleunen et al. 2018), however, we know little about how invasive plants enhance their interaction with AMF. Yet, knowing the mechanisms that regulate these symbiotic relationships would help to understand, predict and manage plant invasions. In this study, we found that introduced populations of T. sebifera enhanced their symbiotic relationship with AMF apparently by having higher levels of root flavonoids. That in turn may increase their growth ability and competitiveness in the introduced range. To the best of our knowledge, this study is the first to report such a linkage between root secondary chemicals, AMF and invasive plant growth by comparing the differences between plants from the introduced and native populations.
Variation of AMF relationships between population origins
Previous studies have reported that T. sebifera plants from the introduced range have higher AMF colonization rates than those from the native range, which is consistent with our results (Yang et al. 2013, 2015c). As was the case in these other studies, we also found that AMF colonization and plant growth were positively related. Although we did not examine other benefits of AMF colonization, they have been associated with higher levels of tolerance to soil salinity and water stress (Yang et al. 2015a, b), which may help to explain the high tolerance of T. sebifera to these stressful conditions in the introduced range (Howard 2012; Paudel and Battaglia 2013, 2015).
Our results indicated that T. sebifera plants are able to increase AMF associations and benefit their success as has been shown for other invasive plants. For example, the invasive plants, Agropyron cristatum, C. stoebe and E. esula may increase the abundance and diversity of AMF communities and accelerate their invasions (Reinhart et al. 2017). In addition, activated carbon applications to invasive populations of S. canadensis limit their selective enhancement of beneficial AMF (Yuan et al. 2014) and support a role for root secondary chemicals in this enhancement. This is in agreement with our finding that flavonoids enhance AMF colonization. Although soil hyphae likely include many of fungal types other than AMF, our finding that hyphae were abundant in soil associated with plants from introduced populations supports a role for root exudates in shaping the soil microbial community (Siegrid et al. 2007).
Flavonoids were positively correlated with AMF colonization
Previous studies have shown that secondary metabolites in plants play a role in regulating AMF symbiosis (Kohki et al. 2005; Kikuchi et al. 2007; Barto et al. 2010), such as flavones, phenolics and saponins which are three general types of secondary metabolites in many plants (Scervino et al. 2007), that can accumulate in soil (Zhang et al. 2011). Phenolics may reduce AMF colonization (Piotrowski et al. 2008), but flavones (e.g. quercetin and luteolin) may enhance AMF symbiosis (Siegrid et al. 2007). In this study, we found that introduced populations of T. sebifera had lower levels of root phenolics and tannins but higher flavonoids indicating that introduced populations may have genetic traits that enhance AMF colonization, which is consistent with the finding that flavonoids increasing the rhizobium nodules in legumes (Liu et al. 2017). Additionally, AMF could regulate the nodulation of rhizobium (Jin et al. 2018; Girardin et al. 2019). The positive correlation we found between flavonoids and AMF supports a higher overall level of root flavonoids as driving higher AMF colonization. But the distinct relationships of flavonoids and AMF colonization for introduced vs. native populations suggest that the flavonoid chemical composition may also vary between population origins and contribute to variation in AMF colonization. However, it is unclear which specific flavonoid chemicals have the main regulatory effect on AMF colonization for T. sebifera or other invasive plants.
Conclusions
Many studies have examined the secondary compounds in introduced vs. native populations of invasive plants but these have largely focused on anti-herbivore defences. Here we found evidence that T. sebifera plants may produce more root flavonoids or different types of root flavonoids which increases their AMF associations and in turn their rapid growth. Because there are many types of flavonoids, future studies should examine the variation in individual flavonoids and test their effects on spore germination and hyphal growth of AMF in the introduced range. Because our study did not experimentally manipulate root chemicals this would strengthen the inferences regarding their effects on AMF colonization. In addition, it is critical to understand how differences in secondary chemicals in T. sebifera and native plants in the introduced range may influence the plant invasive success, especially with mycorrhizal associations being more beneficial to T. sebifera than native tree species with which it co-occurs (Nijjer et al. 2004). Indeed, understanding the traits of introduced plants that make them likely to have enhanced benefits from mycorrhizae in the introduced range compared to native plants with which they compete is critical for predicting and managing plant invasions.
Supporting Information
The following additional information is available in the online version of this article—
Supplement. Supporting information for DATA.
Supplement S2. Supporting information for data analysis CODE.
Data
The complete data for the analyses are also available as Supporting Information.
Sources of Funding
This work was supported by the National Natural Science Foundation of China (31600300, 31971558, and 31770414) and the National Key Research and Development Program (YFC20171200100).
Contributions by the Authors
J.D. designed this experiment and revised the manuscript; Y.P. and B.T. carried out the experiment; E.S. did the data analysis; B.T. drafted the manuscript; J.D. and E.S. edited the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
There is no any conflict of interest in this study.
Literature Cited
- Akiyama K, Matsuoka H, Hayashi H. 2002. Isolation and identification of a phosphate deficiency-induced\r, c\r, -glycosyl flavonoid that stimulates arbuscular mycorrhiza formation in melon roots. Molecular Plant-Microbe Interactions 15:334–340. [DOI] [PubMed] [Google Scholar]
- Akiyama K, Matsuzaki K, Hayashi H. 2005. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435:824–827. [DOI] [PubMed] [Google Scholar]
- Barto K, Friese C, Cipollini D. 2010. Arbuscular mycorrhizal fungi protect a native plant from allelopathic effects of an invader. Journal of Chemical Ecology 36:351–360. [DOI] [PubMed] [Google Scholar]
- Brundrett M, Melville L, Peterson L. 1995. Practical methods in mycorrhiza research. The New Phytologist 131:289. [Google Scholar]
- Bunn RA, Ramsey PW, Lekberg Y. 2015. Do native and invasive plants differ in their interactions with arbuscular mycorrhizal fungi? A meta-analysis. Journal of Ecology 103:1547–1556. [Google Scholar]
- Datta P, Kulkarni M. 2014. Influence of two “AM” fungi in improvement of mineral profile in Arachis hypogaea L. under salinity stress. Legume Research 37:321–328. [Google Scholar]
- Dawkins K, Esiobu N. 2017. Arbuscular and ectomycorrhizal fungi associated with the invasive Brazilian pepper tree (Schinus terebinthifolius) and two native plants in South Florida. Frontiers in Microbiology 8:665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson W, Schrama M. 2016. Identifying the role of soil microbes in plant invasions. Journal of Ecology 104:1211–1218. [Google Scholar]
- DeWalt SJ, Siemann E, Rogers WE. 2011. Geographic distribution of genetic variation among native and introduced populations of Chinese tallow tree, Triadica sebifera (Euphorbiaceae). American Journal of Botany 98:1128–1138. [DOI] [PubMed] [Google Scholar]
- Gao C, Kim YC, Zheng Y, Yang W, Chen L, Ji NN, Wan SQ, Guo LD. 2016. Increased precipitation, rather than warming, exerts a strong influence on arbuscular mycorrhizal fungal community in a semiarid steppe ecosystem. Botany 94:1–11. [Google Scholar]
- Girardin A, Wang T, Ding Y, Keller J, Buendia L, Gaston M, Ribeyre C, Gasciolli V, Auriac MC, Vernié T, Bendahmane A, Ried MK, Parniske M, Morel P, Vandenbussche M, Schorderet M, Reinhardt D, Delaux PM, Bono JJ, Lefebvre B. 2019. LCO receptors involved in arbuscular mycorrhiza are functional for Rhizobia perception in legumes. Current Biology 29:4249–4259.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes SIF, Merckx VSFT, Hynson NA. 2018. Biological invasions increase the richness of arbuscular mycorrhizal fungi from a Hawaiian subtropical ecosystem. Biological Invasions 20:2421–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanssen JF, Thingstad TF, Goksøyr J. 1974. Evaluation of hyphal lengths and fungal biomass in soil by a membrane filter technique. Oikos 25:102–107. [Google Scholar]
- Hassiotis CN, Orfanoudakis M. 2018. The impact of Lavandula stoechas L. degradation on arbuscular mycorrhizal fungi, in a Mediterranean ecosystem. Applied Soil Ecology 126:182–188. [Google Scholar]
- Horn S, Hempel S, Verbruggen E, Rillig MC, Caruso T. 2017. Linking the community structure of arbuscular mycorrhizal fungi and plants: a story of interdependence? The ISME Journal 11:1400–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard JJ. 2012. Hurricane Katrina impact on a leveed bottomland hardwood forest in Louisiana. American Midland Naturalist 168:56–69. [Google Scholar]
- Huang W, Carrillo J, Ding J, Siemann E. 2012a. Invader partitions ecological and evolutionary responses to above- and belowground herbivory. Ecology 93:2343–2352. [DOI] [PubMed] [Google Scholar]
- Huang W, Carrillo J, Ding J, Siemann E. 2012b. Interactive effects of herbivory and competition intensity determine invasive plant performance. Oecologia 170:373–382. [DOI] [PubMed] [Google Scholar]
- Jakobsen I, Abbott LK, Robosen AD. 1992. External hyphae of vesicular–arbuscular mycorrhizal fungi associated with Trifolium subterraneum L. 1. Spread of hyphae and phosphorus inflow into roots. The New Phytologist 120:371–380. [Google Scholar]
- Jiang Y, Xie Q, Wang W, Yang J, Zhang X, Yu N, Zhou Y, Wang E. 2018. Medicago AP2-domain transcription factor WRI5a is a master regulator of lipid biosynthesis and transfer during mycorrhizal symbiosis. Molecular Plant 11:1344–1359. [DOI] [PubMed] [Google Scholar]
- Jin Y, Chen Z, Yang J, Mysore KS, Wen J, Huang J, Yu N, Wang E. 2018. IPD3 and IPD3L function redundantly in rhizobial and mycorrhizal symbioses. Frontiers in Plant Science 9:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen A, Jakobsen I, Jensen ES. 1992. External hyphae of vesicular–arbuscular mycorrhizal fungi associated with Trifolium subterraneum L. The New Phytologist 124:61–68. [Google Scholar]
- Kikuchi K, Matsushita N, Suzuki K, Hogetsu T. 2007. Flavonoids induce germination of basidiospores of the ectomycorrhizal fungus Suillus bovinus. Mycorrhiza 17:563–570. [DOI] [PubMed] [Google Scholar]
- Kim YC, Gao C, Zheng Y, He XH, Yang W, Chen L, Wan SQ, Guo LD. 2015. Arbuscular mycorrhizal fungal community response to warming and nitrogen addition in a semiarid steppe ecosystem. Mycorrhiza 25:267–276. [DOI] [PubMed] [Google Scholar]
- Kohki A, Ken-Ichi M, Hideo H. 2005. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435:824–827. [DOI] [PubMed] [Google Scholar]
- Lekberg Y, Gibbons SM, Rosendahl S, Ramsey PW. 2013. Severe plant invasions can increase mycorrhizal fungal abundance and diversity. The ISME Journal 7:1424–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Fan R, Guo S, Wang P, Zhu X, Fan Y, Chen Y, He K, Kumar A, Shi J, Wang Y, Li L, Hu Z, Song CP. 2019. The Arabidopsis MYB transcription factor, MYB111 modulates salt responses by regulating flavonoid biosynthesis. Environmental and Experimental Botany 166:103807. [Google Scholar]
- Li YP, Feng YL, Kang ZL, Zheng YL, Zhang JL, Chen YJ. 2017. Changes in soil microbial communities due to biological invasions can reduce allelopathic effects. Journal of Applied Ecology 54:1281–1290. [Google Scholar]
- Liu YC, Qin XM, Xiao JX, Tang L, Wei CZ, Wei JJ, Zheng Y. 2017. Intercropping influences component and content change of flavonoids in root exudates and nodulation of faba bean. Journal of Plant Interactions 12:187–192. [Google Scholar]
- Liu HL, Tan Y, Nell M, Zitter-Eglseer K, Wawscrah C, Kopp B, Wang SM, Novak J. 2014. Arbuscular mycorrhizal fungal colonization of Glycyrrhiza glabra roots enhances plant biomass, phosphorus uptake and concentration of root secondary metabolites. Journal of Arid Land 6:186–194. [Google Scholar]
- Ma Y, Wu Y, Yang J, Liang H, Shang F. 2017. Flavonoids content and antioxidant activity of ethanol extracts of Osmanthus fragrans flowers. Bangladesh Journal Botany 46:907–915. [Google Scholar]
- Medini F, Fellah H, Ksouri R, Abdelly C. 2014. Total phenolic, flavonoid and tannin contents and antioxidant and antimicrobial activities of organic extracts of shoots of the plant Limonium delicatulum. Journal of Taibah University for Science 8:216–224. [Google Scholar]
- Mello A, Balestrini R. 2018. Recent insights on biological and ecological aspects of ectomycorrhizal fungi and their interactions. Frontiers in Microbiology 9:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroń D, Lenda M, Skórka P, Szentgyörgyi H, Settele J, Woyciechowski M. 2009. Wild pollinator communities are negatively affected by invasion of alien goldenrods in grassland landscapes. Biological Conservation 142:1322–1332. [Google Scholar]
- Nijjer S, Rogers WE, Lee CTA, Siemann E. 2008. The effects of soil biota and fertilization on the success of Sapium sebiferum. Applied Soil Ecology 38:1–11. [Google Scholar]
- Nijjer S, Rogers WE, Siemann E. 2004. The effect of mycorrhizal inoculum on the growth of five native tree species and the invasive Chinese tallow tree (Sapium sebiferum). In: 3rd Big Thicket Science Conference on Biodiversity and Ecology of the West Gulf Coastal Plain Landscape, Beaumont, TX, 357–368. [Google Scholar]
- Pattison RR, Mack RN. 2008. Potential distribution of the invasive tree Triadica sebifera (Euphorbiaceae) in the United States: evaluating CLIMEX predictions with field trials. Global Change Biology 14:813–826. [Google Scholar]
- Paudel S, Baer SG, Battaglia LL. 2014. Arbuscular mycorrhizal fungi (AMF) and success of Triadica sebifera invasion in coastal transition ecosystems along the northern Gulf of Mexico. Plant and Soil 378:337–349. [Google Scholar]
- Paudel S, Battaglia LL. 2013. Germination responses of the invasive Triadica sebifera and two co-occurring native woody species to elevated salinity across a gulf coast transition ecosystem. Wetlands 33:527–535. [Google Scholar]
- Paudel S, Battaglia LL. 2015. The role of light, soil and human factors on the probability of occurrence of an invasive and three native plant species in coastal transitions of coastal Mississippi, USA. Journal Plant Ecology 8:491–500. [Google Scholar]
- Pile LS, Wang GG, Stovall JP, Siemann E, Wheeler GS, Gabler CA. 2017. Mechanisms of Chinese tallow (Triadica sebifera) invasion and their management implications - a review. Forest Ecology and Management 404:1–13. [Google Scholar]
- Piotrowski JS, Morford SL, Rillig MC. 2008. Inhibition of colonization by a native arbuscular mycorrhizal fungal community via Populus trichocarpa litter, litter extract, and soluble phenolic compounds. Soil Biology & Biochemistry 40:709–717. [Google Scholar]
- Price ML, Van Scoyoc S, Butler LG. 1978. A critical evaluation of the vanillin reaction as an assay for tannin in sorghum grain. Journal Agricultural and Food Chemistry 26:1214–1218. [Google Scholar]
- Reinhart KO, Callaway RM. 2006. Soil biota and invasive plants. The New Phytologist 170:445–457. [DOI] [PubMed] [Google Scholar]
- Reinhart KO, Lekberg Y, Klironomos J, Maherali H. 2017. Does responsiveness to arbuscular mycorrhizal fungi depend on plant invasive status? Ecology and Evolution 7:6482–6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson DM, Allsopp N, D’Antonio CM, Milton SJ, Rejmánek M. 2010. Plant invasions–the role of mutualisms. Biological Reviews of the Cambridge Philosophical Society 75:65–93. [DOI] [PubMed] [Google Scholar]
- Rillig M. 2010. Arbuscular mycorrhizae and terrestrial ecosystem processes. Ecology Letters 7:740–754. [Google Scholar]
- Rout ME, Callaway RM. 2012. Interactions between exotic invasive plants and soil microbes in the rhizosphere suggest that ‘everything is not everywhere’. Annals of Botany 110:213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloum MS, Menduni MF, Benavides MP, Larrauri M, Luna CM, Silvente S. 2018. Polyamines and flavonoids: key compounds in mycorrhizal colonization of improved and unimproved soybean genotypes. Symbiosis 76:265–275. [Google Scholar]
- Scervino JM, Ponce MA, Erra-Bassells R, Bompadre MJ, Vierheilig H, Ocampo JA, Godeas A. 2006. Glycosidation of apigenin results in a loss of its activity on different growth parameters of arbuscular mycorrhizal fungi from the genus Glomus and Gigaspora. Soil Biology & Biochemistry 38:2919–2922. [Google Scholar]
- Scervino JM, Ponce MA, Erra-Bassells R, Bompadre J, Vierheilig H, Ocampo JA, Godeas A. 2007. The effect of flavones and flavonols on colonization of tomato plants by arbuscular mycorrhizal fungi of the genera Gigaspora and Glomus. Canadian Journal of Microbiology 53:702–709. [DOI] [PubMed] [Google Scholar]
- Siegrid S, Venasius L, Ingrid L, Peter S, Thanasan K, Jean-Patrick T, Horst V. 2007. Flavonoids and strigolactones in root exudates as signals in symbiotic and pathogenic plant-fungus interactions. Molecules 12:1290–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sielaff AC, Polley HW, Fuentes-Ramirez A, Hofmockel K, Wilsey BJ. 2019. Mycorrhizal colonization and its relationship with plant performance differs between exotic and native grassland plant species. Biology Invasions 21:1981–1991. [Google Scholar]
- Siemann E, DeWalt SJ, Zou JW, Rogers WE. 2017. An experimental test of the EICA hypothesis in multiple ranges: invasive populations outperform those from the native range independent of insect herbivore suppression. AoB Plants 9:plw087; doi: 10.1093/aobpla/plw087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla P, Garg N. 2017. Plant flavonoids: key players in signaling, establishment, and regulation of rhizobial and mycorrhizal endosymbioses. In: Varma A, Prasad R, Tuteja N, eds. Mycorrhiza - function, diversity, state of the art. Cham, Switzerland: Springer International Publishing, 133–176. [Google Scholar]
- Stankovic MS, Niciforovic N, Topuzovic M, Solujic S. 2011. Total phenolic content, flavonoid concentrations and antioxidant activity, of the whole plant and plant parts extracts from Teucrium montanum L. Var. Montanum, F. Supinum (L.) Reichenb. Biotechnology and Biotechnological Equipment 25:2222–2227. [Google Scholar]
- Stefanowicz AM, Stanek M, Nobis M, Zubek S. 2017. Few effects of invasive plants Reynoutria japonica, Rudbeckia laciniata and Solidago gigantea on soil physical and chemical properties. The Science of the Total Environment 574:938–946. [DOI] [PubMed] [Google Scholar]
- Sun ZK, He WM. 2010. Evidence for enhanced mutualism hypothesis: Solidago canadensis plants from regular soils perform better. PLoS One 5:e15418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Wang J, Zhu L, Liao D, Gu M, Ren L, Kapulnik Y, Xu G. 2012. An active factor from tomato root exudates plays an important role in efficient establishment of mycorrhizal symbiosis. PLoS One 7:e43385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanveer A, Ali HH, Manalil S, Raza A, Chauhan BS. 2018. Eco-biology and management of alligator weed Alternanthera philoxeroides) (Mart.) Griseb: a review. Wetlands 38:1067–1079. [Google Scholar]
- van Kleunen M, Bossdorf O, Dawson W. 2018. The ecology and evolution of alien plants. Annual Review of Ecology, Evolution, and Systematics 49:25–47. [Google Scholar]
- Verbeek JD, Kotanen PM. 2019. Soil-mediated impacts of an invasive thistle inhibit the recruitment of certain native plants. Oecologia 190:619–628. [DOI] [PubMed] [Google Scholar]
- Wang Y, Siemann E, Wheeler GS, Zhu L, Gu X, Ding J. 2012. Genetic variation in anti‐herbivore chemical defences in an invasive plant. Journal of Ecology 100:894–904. [Google Scholar]
- Xiao L, Carrillo J, Siemann E, Ding J. 2019. Herbivore-specific induction of indirect and direct defensive responses in leaves and roots. AoB Plants 11:plz003; doi: 10.1093/aobpla/plz003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Carrillo J, Jin H, Shang L, Hovick SM, Nijjer S. 2013. Plant–soil biota interactions of an invasive species in its native and introduced ranges: implications for invasion success. Soil Biology Biochemistry 65:78–85. [Google Scholar]
- Yang Q, Li B, Siemann E. 2015a. Positive and negative biotic interactions and invasive Triadica sebifera tolerance to salinity: a cross-continent comparative study. Oikos 124:216–224. [Google Scholar]
- Yang Q, Li B, Siemann E. 2015b. The effects of fertilization on plant-soil interactions and salinity tolerance of invasive Triadica sebifera. Plant and Soil 394:99–107. [Google Scholar]
- Yang Q, Wei SJ, Shang L, Carrillo J, Gabler CA, Nijjer S, Li B, Siemann E. 2015c. Mycorrhizal associations of an invasive tree are enhanced by both genetic and environmental mechanisms. Ecography 38:1112–1118. [Google Scholar]
- Yemm EW, Willis AJ. 1954. The estimation of carbohydrates in plant extracts by anthrone. The Biochemical Journal 57:508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong Z, Xia L, Gao Y, Liu H, Gao YB, van der Heijden MGA, Ren AZ. 2018. Plant endophytes and arbuscular mycorrhizal fungi alter plant competition. Functional Ecology 32:1168–1179. [Google Scholar]
- Yuan Y, Tang J, Leng D, Hu S, Yong JW, Chen X. 2014. An invasive plant promotes its arbuscular mycorrhizal symbioses and competitiveness through its secondary metabolites: indirect evidence from activated carbon. PLoS One 9:e97163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zdenka B, Lucy G, Bruce TJA, Michael B, Caulfield JC, Christine W, Pickett JA, David J. 2013. Underground signals carried through common mycelial networks warn neighbouring plants of aphid attack. Ecology Letters 16:835–843. [DOI] [PubMed] [Google Scholar]
- Zhang FJ, Li Q, Chen FX, Xu HY, Inderjit, Wan FH. 2017. Arbuscular mycorrhizal fungi facilitate growth and competitive ability of an exotic species Flaveria bidentis. Soil Biology & Biochemistry 115:275–284. [Google Scholar]
- Zhang F, Li Q, Yerger EH, Chen X, Shi Q, Wan F. 2018. AM fungi facilitate the competitive growth of two invasive plant species, Ambrosia artemisiifolia and Bidens pilosa. Mycorrhiza 28:703–715. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang P, Xue K, Hao YB, Wang YF, Cui XY. 2019. Trait complementarity between fine roots of Stipa purpurea and their associated arbuscular mycorrhizal fungi along a precipitation gradient in Tibetan alpine steppe. Journal of Mountain Science 16:542–547. [Google Scholar]
- Zhang S, Zhu W, Wang B, Tang J, Chen X. 2011. Secondary metabolites from the invasive Solidago canadensis L. accumulation in soil and contribution to inhibition of soil pathogen Pythium ultimum. Applied Soil Ecology 48:280–286. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.