Abstract
Context
The endocannabinoid (eCB) system partly controls hedonic eating, a major cause of obesity. While some studies suggested an overactivation of the eCB system in obesity, peripheral levels of eCBs across the 24-hour cycle have not been characterized in obese individuals despite the fact that in lean adults, levels of the eCB 2-arachidonoylglycerol (2-AG) vary across the day.
Objective
We sought to examine 24-hour profiles of serum concentrations of 2-AG in healthy obese and nonobese adults, under well-controlled laboratory conditions. We also simultaneously assessed 24-hour profiles of 2-oleoylglycerol (2-OG), leptin, and cortisol in each participant.
Design
With fixed light-dark and sleep-wake cycles, blood sampling was performed over an entire 24-hour period, including identical meals at 0900, 1400, and 1900.
Participants
Twelve obese (8 women, mean body mass index [BMI]: 39.1 kg/m2) and 15 nonobese (6 women; mean BMI: 23.6 kg/m2) healthy adults were studied.
Results
We observed a 24-hour variation of 2-AG levels in obese individuals but, relative to nonobese adults, the amplitude was dampened and the timings of the nadir and peak were delayed by 4 to 5 hours. The profile of 2-OG was similarly misaligned. In contrast, when expressed relative to the 24-hour mean level, the 24-hour rhythm of cortisol and leptin were similar in obese and nonobese participants.
Conclusions
Obesity appears to be associated with a dampening and delay of the 24-hour variation of eCB activity relative to the central circadian signal as well as to the daily leptin rhythm. This misalignment may play a role in the pathophysiology of obesity.
Keywords: endocannabinoids, leptin, cortisol, hedonic eating, circadian rhythms
Excessive food intake relative to energy requirements, often referred to as hedonic eating, is a major contributor to the epidemic of obesity. The endocannabinoid (eCB) system is involved in the regulation of appetite and food intake, particularly of reward mechanisms that govern food intake, or hedonic feeding (1). This system is comprised of the cannabinoid receptors, including the CB1 receptor, the endogenous ligands of these receptors, including 2-arachidonoylglycerol (2-AG) and N-arachidonylethanolamine (AEA or anandamide), and the enzymes responsible for the biosynthesis and degradation of the eCBs (2–6). The eCB receptor ligands 2-AG and AEA can be measured in serum; however, the exact source of these peripherally circulating ligands is unknown (7). The role of the eCB system in the control of feeding, body weight, and peripheral metabolism has been studied in diet-induced or genetically obese animals (8–12) and in humans (13–18). Specifically, CB1 receptor activation is a potent orexigenic signal (19) and antagonism reduces food intake and body weight (20, 21). The CB1 inverse agonist rimonabant was shown to have beneficial metabolic effects beyond those mediated by weight loss, and it was approved in Europe as an appetite-suppressant for humans in 2006 (22–25). The drug was withdrawn in 2008 due to serious psychiatric adverse effects (21). The development of CB1 receptor antagonists or modulators with limited brain penetration is currently an active field of research on the pharmacological treatment of obesity and its complications (26, 27).
The most abundant circulating ligand of the CB1 receptor is 2-AG, with concentrations exceeding those of AEA by 10- to 500-fold (7, 28). Peripheral 2-AG concentrations are thought to reflect release by multiple tissues including adipose tissue, muscle, and brain (7). Elevations in circulating 2-AG levels are associated with increased activation of eCB receptors and thus may promote hedonic eating (7). The hypothesis that overactivation of the eCB system may be a hallmark of human obesity has been examined in multiple studies, with inconsistent results. None of this previous work examined 2-AG levels across the entire 24-hour cycle. Some studies reported elevated basal 2-AG levels in obese compared with lean adults and/or found a positive correlation between measures of adiposity and 2-AG levels (17, 29–31). However, other studies did not detect differences in basal 2-AG levels in obese versus nonobese individuals (32–36). The possibility that palatable food intake may elevate circulating eCB concentrations in obese subjects has been examined in a few studies, also with inconsistent findings (15, 35, 37). In these previous studies, blood samples for measurement of circulating eCB were obtained in the morning. We have, however, recently shown that there is a robust 24-hour rhythm in serum levels of 2-AG in healthy lean adults (38), with a nadir around mid-sleep and an early afternoon peak. Circulating 2-AG concentrations are strikingly dynamic, with an approximately 3-fold increase from nadir to peak concentrations. It is not known whether the robust 24-hour rhythm in levels of 2-AG is altered in obese but otherwise healthy individuals.
Epidemiologic and laboratory studies have shown that disruptions of circadian regulation have adverse metabolic consequences and can result in overeating, weight gain, impaired glucose tolerance, and insulin resistance (39–45). Recent evidence suggests that the timing of caloric intake across the 24-hour day may be a determinant of obesity risk, independently of caloric content (46–51). The timing of food intake is a major synchronizer of circadian clocks in peripheral tissues (52). Abnormal temporal patterns of caloric intake, particularly a preference to eat late in the day (known as a late dietary chronotype), is a potential source of misalignment between the central pacemaker in the hypothalamus and peripheral clocks (53). Surprisingly, the literature documenting the impact of eCB activity on food intake and energy balance has essentially ignored the potential role of the circadian system.
We therefore compared the 24-hour profile of serum 2-AG levels in well-matched healthy obese and nonobese individuals under controlled laboratory conditions to test the hypothesis that the circadian control of circulating concentrations of the major CB1 ligand may be altered in obese adults. To examine circadian alignment, we simultaneously assessed the 24-hour profile of plasma cortisol, widely used as a marker of central circadian timing (54), and the 24-hour profile of plasma leptin, which is partly controlled by the adipocyte circadian clock (55).
Methods
Participants
The protocol was approved by the Institutional Review Board of the University of Chicago. Study procedures took place in the Clinical Research Center. All participants gave written informed consent and were paid for their participation. Subjects were recruited using flyers and public advertisement, and admitted to the University of Chicago Clinical Resource Center after a phone screening to assess eligibility. For the obese group, we recruited healthy men and women from the ages of 18 to 40 years, with a body mass index (BMI; in kg/m2) ≥ 30. In addition to a detailed physical examination and medical history and routine laboratory tests, volunteers underwent an overnight laboratory polysomnography to exclude sleep disorders including obstructive sleep apnea, and a standard 75-g oral glucose tolerance test to exclude diabetes. Inclusion criteria for all participants were habitual sleep duration of 7.5 to 8.5 hours between the hours from 2200 to 0800, no habitual daytime naps, no shift work, and no travel across time zones in the 4 weeks prior to the study. Only nonpregnant women were studied, and the inpatient study was scheduled during the follicular phase of the menstrual cycle. Additional inclusion and exclusion criteria regarding health status were as reported previously (38, 57).
Protocol
During the week preceding the study, participants were instructed to maintain fixed bedtimes designed in accordance with their usual habits, and to not deviate from this schedule by more than 30 minutes. This was verified by continuously monitored wrist activity (Actiwatch; Philips/Respironics). All participants spent 3 consecutive nights with at least 8 to 8.5 hours in bed, in total darkness, in the laboratory. Scheduled time of lights off and on was selected to approximate usual habits and was typically between 2300 and 0730. Throughout the 3-day study, participants were housed in a private room with a window providing natural light. Shades were drawn and lights were dimmed at 1800. Participants had sedentary activities during waking hours. No naps were allowed. Research staff continuously monitored volunteers to ensure compliance. Sleep was recorded via polysomnography on all nights. Following the second night in the laboratory, 24-hour blood sampling at frequent intervals was initiated (38, 57). Concentrations of 2-AG and leptin were assayed at 60-minute intervals while cortisol levels were assayed at 15-minute to 60-minute intervals. None of the data from the obese participants have been previously reported. Data from 12 of the 15 nonobese subjects were included in our previous report (38).
Caloric intake
Caloric intake was strictly controlled throughout the 3-day study. Content of the diet was calculated to meet individual participant’s caloric requirements for sedentary conditions (58). A registered dietitian from the Clinical Resource Center supervised the preparation of all meals. Participants were not allowed to consume any foods or beverages that were not provided by the metabolic kitchen. Meals were served at 0900, 1400, and 1900. The participants were instructed to consume each meal in its entirety within 20 minutes and to remain fasted until presentation of the next meal. During the 24-hour period of blood sampling, participants received 3 identical carbohydrate-rich meals (20% fat, 68% carbohydrate, 12% protein) each meal constituting 33% of total daily caloric need. Thus, calories and macronutrients were evenly distributed across the daytime period.
Sleep recording
Sleep was recorded by polysomnography (Neurofax EEG-1100A, Nihon Kohden, Foothill Ranch, California). The recordings were visually scored in 30-second epochs as wake, rapid eye movement (REM), or non-REM sleep stages N1, N2, and N3 according to standardized criteria (59). Summary variables were calculated as described in our previous studies (38, 57).
Assays
Serum concentrations of 2-AG and its structural analog 2-oleoylglycerol (2-OG) were extracted from serum using Bond Elut C18 solid phase extraction columns (Varian Inc, Lake Forest, CA), as previously described (60). Concentrations of 2-OG were measured as an internal validation of concentrations of 2-AG. The 2 lipids were quantified in the lipid extracts by liquid chromatography-electrospray ionization-mass spectrometry (LC-ESI-MS; Agilent LC-MSD 1100 series) by isotope dilution as described previously (61). Serum cortisol concentrations were measured by an immunochemiluminescent assay (Immulite, Los Angeles, CA). Serum leptin concentrations were measured by radioimmunoassay (Linco Research, St. Charles, MO).
Analysis of individual profiles of 2-AG, 2-OG, cortisol, and leptin levels
Isolated 2-AG values that represented a relative change of more than 100% in comparison with both the preceding and following values and were not concomitant with a similar change in 2-OG level were assumed to represent assay error and were replaced by linear interpolation. A total of 1296 values were measured for both 2-AG and 2-OG. In total, 54 values (4.2%) were interpolated.
To quantify the 24-hour variation of 2-AG, 2-OG, cortisol, and leptin, a best-fit curve was calculated for each individual profile using a robust locally weighted nonlinear regression procedure with a window of 4 hours (62). The peak and the nadir were defined as the maximum and minimum of the regression curve, respectively. The amplitude was defined as half of the difference between the peak and the nadir.
Statistical analysis
All group values are expressed as mean ± standard deviation (SD) for variables that met the assumption of normality while the median and interquartile range (IQR) are reported for variables that were not normally distributed. T-tests were used to compare demographic characteristics, sleep variables, and summary variables extracted from the 24-hour profiles. In each group, the between-subject reproducibility of the timings of the 2-AG peak and nadir was tested using the Rao Spacing Test (63). Correlations were calculated using the Pearson coefficient. For illustrative purposes, the standard error of the mean (SEM) was plotted at each time point to provide a visual estimation of interindividual variability in each group.
Results
In total, 27 individuals were included in the present analyses; 12 obese adults and 15 nonobese. Demographic characteristics are given in Table 1. Sex distribution and age were similar in the 2 groups. None of the participants had obstructive sleep apnea, as evidenced by an apnea-hypopnea index < 5 events per hour derived from in-laboratory polysomnography.
Table 1.
Demographics, Sleep Variables, and Quantitative Characteristics of 2-AG, Leptin, and Cortisol Profiles in Obese and Nonobese Participants
| Demographics | Obese (n = 12) | Nonobese (n = 15) | T-Test |
|---|---|---|---|
| Sex | 8 female, 4 male | 6 female, 9 male | 0.252a |
| Age (yrs) | 27.7 ± 6.9 | 24.5 ± 3.3 | 0.159 |
| BMI (kg/m2) | 39.1 ± 7.2 | 23.6 ± 2.6 | <0.0001 |
| Sleep Characteristics Mean Nights 1–3 | |||
| Sleep period time (min) | 463 ± 16 | 481 ± 23 | 0.028 |
| Sleep efficiency (%) | 87 ± 4.7 | 88.9 ± 4.5 | 0.295 |
| Total sleep time (min) | 420 ± 25 | 452 ± 26 | <0.002 |
| Wake (min) | 53 ± 25 | 32 ± 23 | 0.031 |
| Stages N1 + N2 (% of TST) | 63.3 ± 10.2 | 57.4 ± 7.5 | 0.109 |
| Stage N3 (% of TST) | 12.9 ± 5.4 | 16.7 ± 6.0 | 0.099 |
| REM (% of TST) | 21.9 ± 6.0 | 26.3 ± 3.8 | 0.043 |
| 2-AG | |||
| 24-hour mean (pmol/mL)b | 204 (130, 493) | 260 (152, 468) | 0.664 |
| Peak (pmol/mL)b | 280 (205, 620) | 437 (254, 581) | 0.326 |
| Nadir (pmol/mL)b | 107 (52, 189) | 117 (72, 160) | 0.937 |
| Absolute amplitude (pmol/mL)b | 82 (72, 111) | 154 (79, 220) | 0.133 |
| Relative amplitude (% of mean) | 46 ± 17 | 61 ± 18 | 0.0327 |
| Acrophase (time ± min) | 17h35 ± 215 | 13h20 ± 191 | 0.004 |
| Time of nadir (time ± min) | 07h40 ± 183 | 02h56 ± 163 | 0.0004 |
| Leptin (ng/mL) | |||
| 24 hour mean | 62.9 ± 34.6 | 11.8 ± 11.4 | 0.0003 |
| Amplitude | 15.7 ± 9.0 | 4.2 ± 4.3 | 0.001 |
| Relative amplitude (% of mean) | 26.1 ± 6.7 | 33.7 ± 11.4 | 0.042 |
| Peak | 80.7 ± 44.8 | 16.1 ± 16.4 | 0.0004 |
| Acrophase (time ± min) | 1h35 ± 70 | 48 ± 76 | 0.107 |
| Nadir | 49.4 ± 28.5 | 7.8 ± 8.0 | 0.0003 |
| Time of nadir (time ± min) | 10h30 ± 54 | 10h44 ± 77 | 0.585 |
| Delta time (min) | -535 ± 113 | -596 ± 114 | 0.178 |
| Cortisol (µg/dL) | |||
| 24 hour mean | 7.3 ± 1.3 | 7.2 ± 1.5 | 0.817 |
| Amplitude | 7.2 ± 1.4 | 7.8 ± 1.3 | 0.256 |
| Peak | 16.2 ± 2.7 | 17.2 ± 2.7 | 0.332 |
| Acrophase (time ± min) | 07h35 ± 95 | 08h12 ± 44 | 0.233 |
| Nadir | 1.8 ± 0.8 | 1.6 ± 0.8 | 0.513 |
| Time of nadir (time ± min) | 01h20 ± 82 | 01h10 ± 88 | 0.762 |
| Delta time (min) | 375 ± 140 | 422 ± 104 | 0.345 |
Values reported are mean ± SD for variables that are normally distributed and median ± interquartile range (IQR) for variables that are not normally distributed. T-test assuming unequal variances derived P value.
Abbreviations: 2-AG, 2-arachidonoylglycerol; BMI, body mass index; REM, rapid eye movement; TST, total sleep time.
aChi-square test
bNot normally distributed; median and IQR reported; data are log-transformed for T-test
Sleep
As shown in Table 1, over the 3 study nights, sleep efficiency was similar and near 90% in both groups, typical of good sleep quality. Sleep period time (SPT) was slightly shorter (−18 min; P = 0.028) in the obese group. Obese subjects achieved on average 32 minutes less total sleep time (TST) than nonobese participants (P < 0.002). When sleep stages were expressed as % of TST, obese participants obtained less REM sleep (5% less, P = 0.043) than nonobese subjects.
24-hour profiles of 2-AG (Table 1 and left panels of Figure 1)
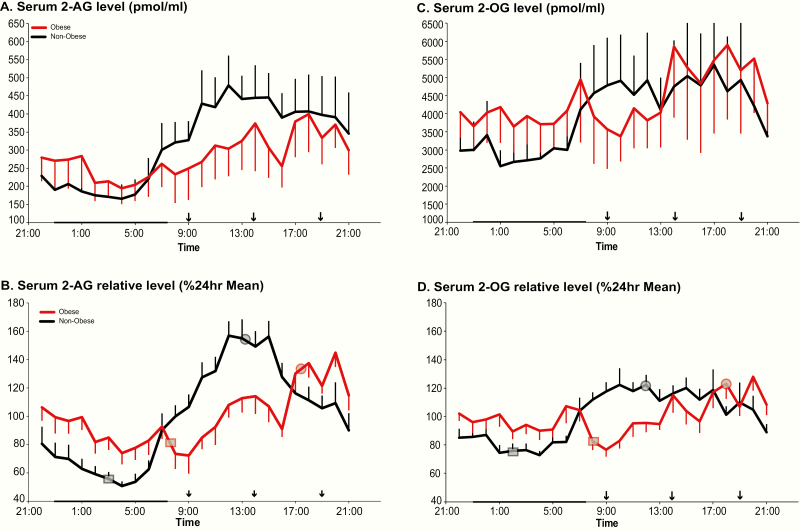
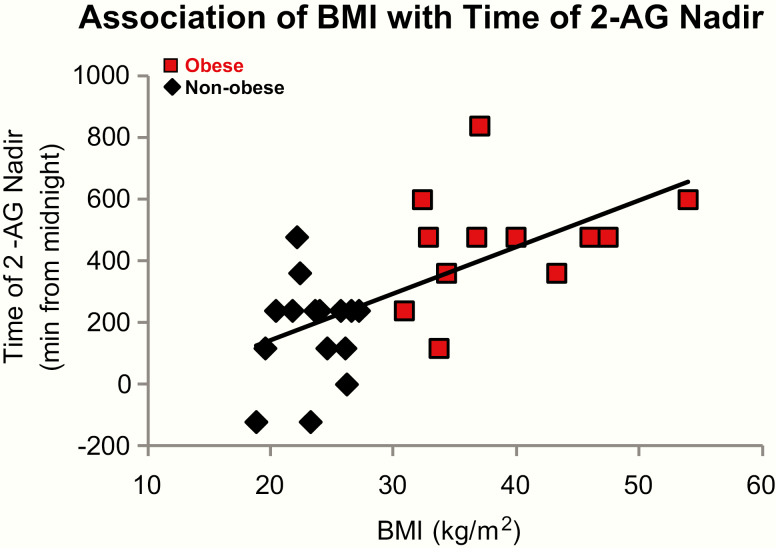
On average, over the entire 24-hour cycle, mean levels of 2-AG were similar in obese versus nonobese participants, with a wide interindividual variability in both groups. In both groups, minimal 2-AG levels were observed during the overnight period when interindividual variability was lower than during the daytime (Fig. 1A). Frequent blood sampling across the daytime revealed a consistent difference between the 2 groups from mid-morning to mid-afternoon, when lower 2-AG levels were observed in the obese subjects (Fig. 1A). In each group, the fitting of nonlinear regression curves identified the existence of a nocturnal nadir and a daytime acrophase. Application of the Rao spacing test showed that the timings of the nadir were significantly clustered either in the middle of the night (nonobese, P < 0.001) or in early morning (obese, P = 0.001). The acrophases occurred consistently around noon in the nonobese participants (nonobese, P < 0.001), but, in comparison, were delayed to the early evening for the obese participants (P < 0.001). To better illustrate the difference in waveshape of the 2-AG profile between groups, each individual profile was expressed as a percentage of its own 24-hour mean (Fig. 1B). In obese compared with nonobese individuals, the 24-hour variation of 2-AG levels was both delayed and dampened (Table 1 and Fig. 1B). We examined area under the curve (AUC) over the sleep period (2300-0700) and during waking (0700-2300). When the AUC was calculated for absolute 2-AG levels, there were no significant differences between the 2 groups during either the sleep period or the wake period. When for each participant, the 2-AG levels were expressed as percentage of the corresponding individual 24-hour mean, we observed that AUC was higher over the sleep period in obese individuals compared with the nonobese (obese: 879% ± 155%, nonobese: 666% ± 156; P = 0.002). Conversely, the AUC during waking hours was lower in the obese versus the nonobese individuals (obese: 1639% ± 168%, nonobese: 1981% ± 128%, P < 0.0001). Interestingly, for all participants considered together, BMI was positively correlated with the timing of the 2-AG nadir, such that the higher the BMI, the later the nadir (Fig. 2); (r = 0.40; P = 0.0004). Notably, this association was not present when we considered either group alone.
Figure 1.
Left panels: Mean 24-hour profiles of serum 2-AG levels in pmol/mL (A) and expressed in % of the individual 24-hour mean (B) in nonobese (black; n = 15) and obese (red; n = 12) participants. Right panels: Mean 24-hour profiles of serum 2-OG levels in pmol/mL (C) and expressed in % of the individual 24-hour mean (D) in nonobese (black; n = 15) and obese (red; n = 12) participants. In B and D, the grey squares on each profile denote the time of the nadir and grey circles the acrophase; normally distributed so shown as mean times for 2-AG and not normally distributed so median times denoted for 2-OG. In all panels, the standard error of the mean (SEM) is represented as a vertical bar at each time point, black vertical arrows denote the identical carbohydrate-rich meals given at 0900, 1400, and 1900 and the black bar represents the approximate time in bed.
Figure 2.
Correlation of BMI (kg/m2) and the timing of the 2-AG nadir was calculated using Pearson coefficient. Considering both groups together, BMI and time of 2-AG nadir were correlated (r2 = 0.40, P = 0.0004), showing that the higher the BMI the more delayed the 2-AG nadir.
24-hour profiles of 2-OG (right panels of Figure 3)
On average, over the entire 24-hour cycle, mean levels of 2-OG, a congener of 2-AG that does not bind eCB receptors, were similar in obese and nonobese participants. Although there was wide interindividual variability in both groups, individual differences in 2-OG mean concentration appeared greater for obese as compared with nonobese individuals, although the difference was not statistically significant.
The temporal variation of 2-OG levels across the 24-hour cycle was parallel to that observed for 2-AG, albeit with blunted amplitude (Fig. 1C). The absolute level of the nadir (median [IQR]; obese: 2061 [1397, 3929] pmol/mL, nonobese: 1667 [1311, 2715] pmol/mL; P = 0.295) and of the peak (median [IQR]; obese: 4186 [2237,7959] pmol/mL, nonobese: 3190 [2677,6353] pmol/mL; P = 0.755) were similar in both groups. In each group, the fitting of nonlinear regression curves identified the existence of a nocturnal nadir and a daytime acrophase. Similar to what we observed for 2-AG, the timing of both the nadir and of the acrophase of 2-OG were delayed in the obese individuals compared with the nonobese individuals; however, in contrast to what was observed for 2-AG, these timing differences between obese and nonobese participants failed to reach significance. To better illustrate the difference in waveshape of the 2-OG profile between groups, each individual profile was expressed as a percentage of its own 24-hour mean. In both groups, the amplitude of the 24-hour variation was lower for 2-OG than 2-AG (nonobese: 42.1 ± 14.1% versus 60.7 ± 17.9%, P < 0.0004; obese: 32.5 ± 17.4% versus 46.0 ± 16.9%, P < 0.02). In obese compared with nonobese individuals, the 24-hour variation of 2-OG levels tended to be blunted (relative amplitude in obese: 32.5 ± 17.4%, relative amplitude in nonobese: 42.1 ± 14.1%; P = 0.138). Further, we observed that the AUC for relative 2-OG levels was significantly higher over the sleep period in obese compared with nonobese individuals (obese: 870% ± 130%, nonobese: 731% ± 112%; P = 0.008). Conversely, the AUC for relative 2-OG levels during waking hours was lower in the obese than in the nonobese individuals (obese: 1529% ± 130%, nonobese: 1669 ± 112%; P = 0.008).
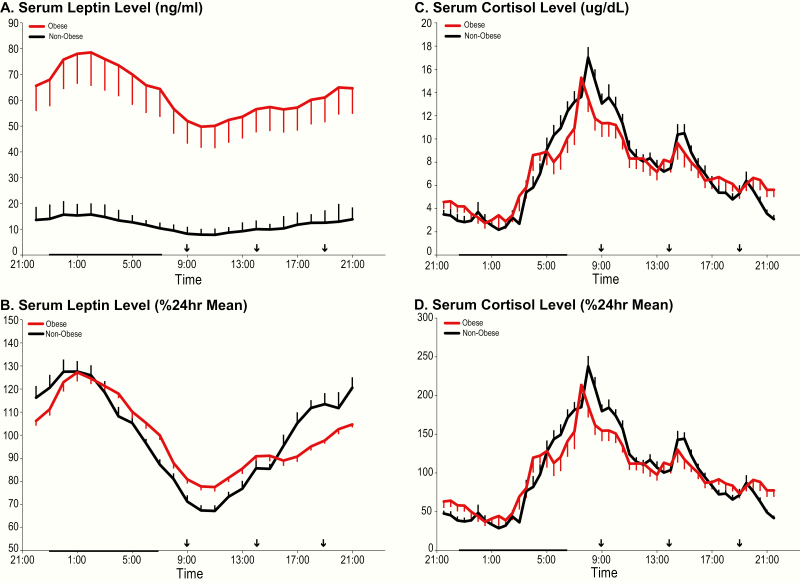
24-hour profiles of leptin and cortisol (Figure 3)
As expected, mean absolute leptin concentrations were much higher in obese than in nonobese participants (Table 1; P = 0.0003) throughout the 24-hour cycle (Fig. 3A). The well-documented diurnal variation of leptin concentrations, with minimum morning levels and a peak at the beginning of the sleep period, was present in both groups. When profiles were expressed as percentage of the individual 24-hour mean (Fig. 3B), the relative amplitude of the rhythm was blunted in obese versus nonobese subjects (Table 1; P = 0.042) and this blunting was due to a higher nadir (obese: 77.1 ± 5.5% versus nonobese: 66.4 ± 8.1%; P = 0.0004) rather than to a lower nocturnal peak. Group differences in timing of the nadir or acrophase were not significant (Table 1).
Figure 3.
Left panels: Mean 24-hour profiles of serum leptin levels in ng/mL (A) and expressed as % of the 24-hour mean (B) in obese (red lines) and nonobese (black lines) participants. Right panels: Mean 24-hour profiles of plasma cortisol in µg/dl (C) and expressed as % of the 24-hour mean (D) in obese (red lines) and nonobese (black lines) participants. In all panels, the standard error of the mean (SEM) is represented as a vertical bar at each time point, the black vertical arrows denote the identical carbohydrate-rich meals given at 0900, 1400, and 1900, and the black bar represents the approximate time in bed.
Mean 24-hour absolute cortisol concentrations and the 24-hour profiles were similar in both groups (Table 1; Fig. 3C). When expressed as a percentage of the individual 24-hour mean, the amplitude was similar in the 2 groups and there were also no differences in the timing of the daily nadir or peak (Fig. 3D).
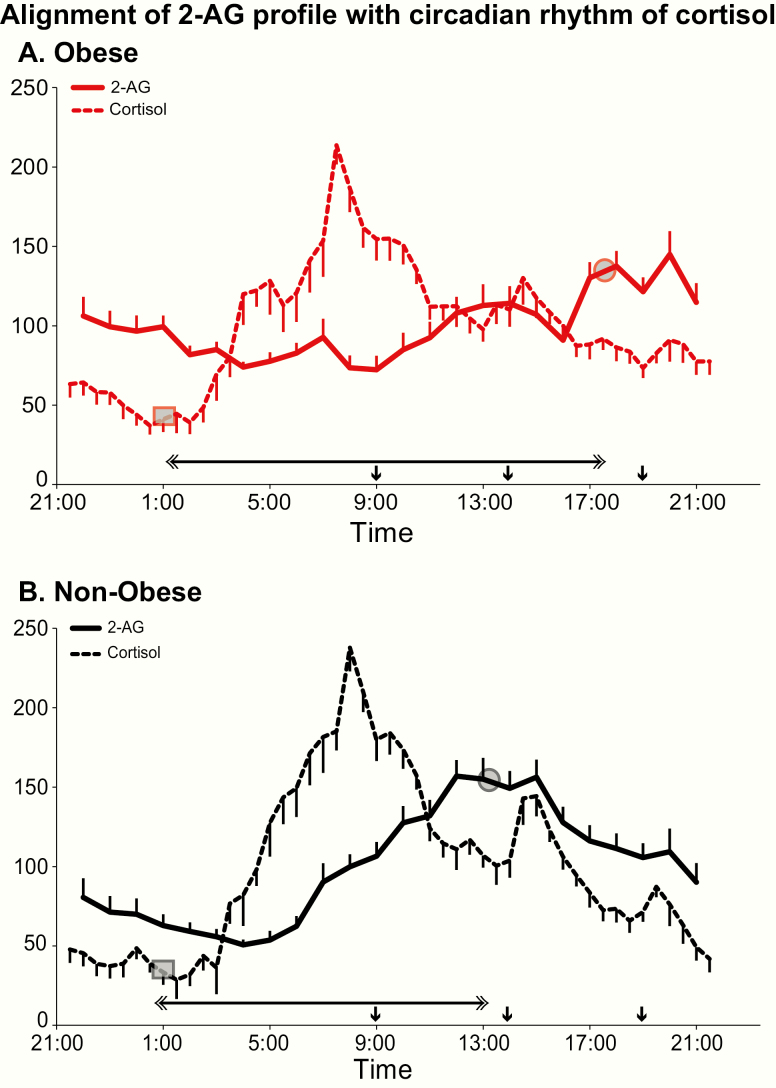
Alignment of the 2-AG profile with cortisol rhythm
Fig. 4 compares the relative 24-hour variation of 2-AG levels with that of cortisol in obese and nonobese participants. Table 2 provides summary measures of the time intervals between phase reference points of the 2-AG and cortisol profile. The timing of the cortisol nadir is considered a more reliable marker of circadian phase than the acrophase, because the latter may be influenced by the time of awakening. In obese individuals, the 2-AG acrophase was delayed by more than 4 hours relative to the cortisol nadir, when compared with nonobese participants (P = 0.009). Similarly, the interval between the cortisol and 2-AG nadirs (P = 0.002), as well as between the cortisol and 2-AG acrophases (P = 0.004), were much longer in the obese versus the nonobese participants, reflecting a significant overall delay of the 2-AG profile relative to the marker of central circadian rhythmicity.
Figure 4.
Alignment of the 24-hour profile of serum 2-AG (solid lines) with the 24-hour centrally controlled circadian rhythm of cortisol (dashed lines) in obese (panel A; red lines) and nonobese (panel B; black lines) participants. For each profile, all data are shown as % of the individual 24-hour mean and the closed circle represents the mean timing of the cortisol nadir and 2-AG acrophase (phase reference point for cortisol and 2-AG) in the corresponding group of participants. The grey squares on each profile denote the time of the nadir and the grey circles the acrophase. The horizontal double-sided arrow denote the time interval separating the phase reference point for 2-AG from the phase reference point for cortisol in obese (top panel) versus nonobese (lower panels) participants. In both panels, the standard error of the mean (SEM) is represented as a vertical bar at each time point and the black vertical arrows denote the identical carbohydrate-rich meals given at 0900, 1400, and 1900.
Table 2.
Misalignment Between 2-AG Profile and the Cortisol and Leptin Profiles
| Obese | Nonobese | P level | |
|---|---|---|---|
| Alignment between 2-AG profile and cortisol profile | |||
| Interval between cortisol and 2-AG acrophases (h:min ± h:min) | 10h00 ± 4h11 | 5h08 ± 3h19 | 0.004 |
| Interval between cortisol and 2-AG nadirs (h:min ± h:min) | 6h20 ± 3h35 | 1h46 ± 3h07 | 0.002 |
| Interval between cortisol nadir and 2-AG acrophase (h:min ± h:min) | 16h15 ± 3h50 | 12h10 ± 3h36 | 0.009 |
| Alignment between 2-AG profile and leptin profile | |||
| Interval between leptin and 2-AG acrophases (h:min ± h:min) | 16h40 ± 2h59 | 11h20 ± 2h14 | < 0.0001 |
| Interval between 2-AG and leptin nadirs (h:min ± h:min) | 2h50 ± 3h15 | 7h48 ± 3h09 | 0.0005 |
| Interval between leptin nadir and 2-AG acrophase (h:min ± h:min) | 7h05 ± 3h29 | 2h36 ± 3h11 | 0.002 |
Values reported are mean ± SD, P value from t-test assuming unequal variances. Abbreviation: 2-AG, 2-arachidonoylglycerol.
Alignment of the 2-AG profile with leptin rhythm
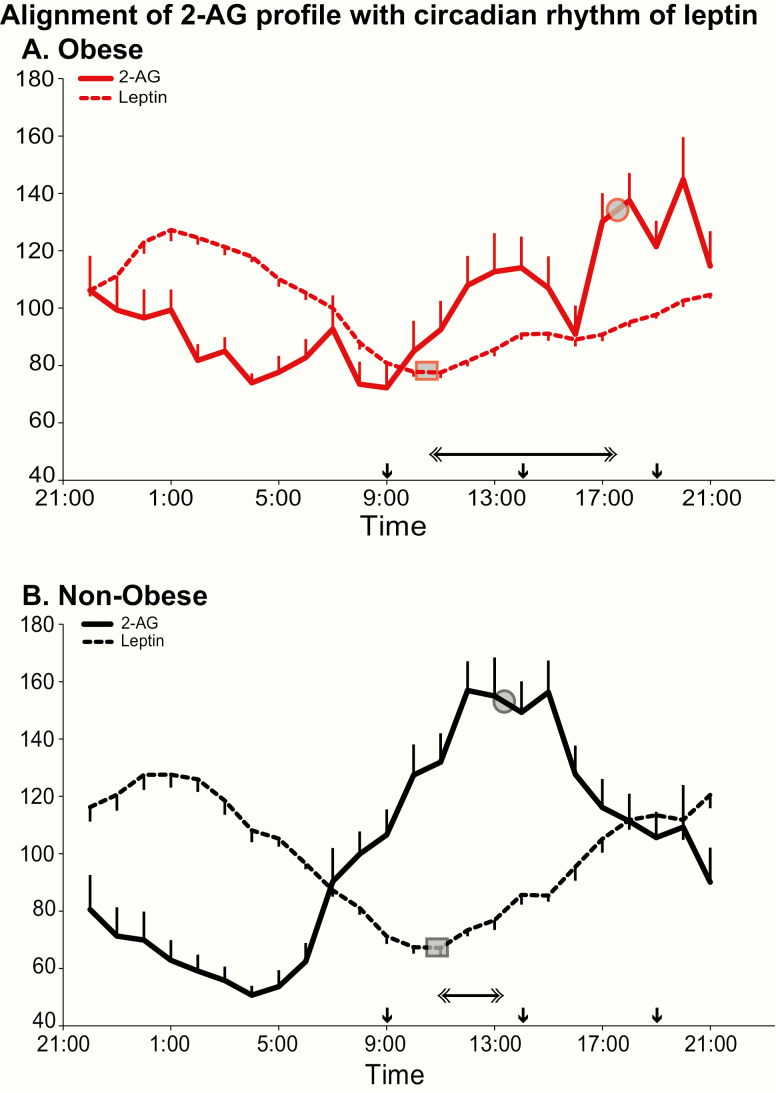
Fig. 5 compares the relative 24-hour variation of 2-AG levels with that of leptin in obese and nonobese participants. Time intervals between phase reference points of the 2 profiles are given in Table 2. In both groups, lower nocturnal levels of 2-AG coincided with higher levels of the adipocyte satiety signal. In nonobese participants, the elevation of 2-AG levels occurred while leptin levels were below their 24-hour mean. In contrast, in obese participants, the rise of 2-AG was extended through the afternoon and early evening, when leptin levels were also rising. This misalignment between the 2-AG profile and the leptin profile is reflected by significant differences between the time intervals separating their respective phase reference points; interval between acrophases (P < 0.0001), interval between nadirs (P = 0.0005), and interval between leptin nadir and 2-AG acrophase (P = 0.002) (Table 2).
Figure 5.
Alignment of the 24-hour profile of serum 2-AG (solid lines) with the 24-hour peripheral rhythm of leptin (dashed lines) in obese (panel A; red lines) and nonobese (panel B; black lines) participants. For each profile, all data are shown as % of the individual 24-hour mean and the closed circle represents the mean timing of the acrophase (phase reference point for 2-AG) or the nadir (phase reference point for leptin) in the corresponding group of participants. The grey squares on each profile denote the time of the nadir and grey circles the acrophase. The horizontal double-sided arrow denote the time interval separating the phase reference point for 2-AG from the phase reference point in obese (top panel) versus nonobese (lower panel) participants. In all panels, the standard error of the mean (SEM) is represented as a vertical bar at each time point and the black vertical arrows denote the identical carbohydrate-rich meals given at 0900, 1400, and 1900.
Discussion
This well-controlled laboratory study delineated the 24-hour profile of 2-AG, the most abundant circulating eCB receptor ligand, in obese but otherwise healthy adults as compared with nonobese subjects with comparable age and sex distributions. The study provides 3 important findings. First, contrary to expectations, levels of 2-AG were not consistently elevated across the 24-hour span in obese participants. Second, the robust 24-hour variation of 2-AG levels previously described in lean participants (38) was also detected in obese subjects, but was dampened and markedly delayed. Parallel findings were observed for the 24-hour profile of 2-OG levels. Third, no differences in the 24-hour profile of cortisol levels were found between obese versus nonobese subjects, and thus the observed delay of the 2-AG profile in obese subjects represents a misalignment relative to central circadian timing. Further, the 2-AG profile in obese participants was misaligned relative to the rhythm of leptin levels.
Previous work has generally assumed that obesity is associated with an overactivation of the eCB system, reflected in higher levels of endogenous ligands of eCB receptors. Findings regarding 2-AG levels were consistent with this hypothesis in some studies (17, 29–31), but not in others (32–36). Data regarding the impact of food intake on circulating eCBs in obesity have also been variable (15, 35, 37). Differences in palatability of the food and degree of visceral adiposity of the participants have been proposed to explain discrepancies (7). Additionally, the majority of previous studies assayed eCBs at one single or a limited set of time points. The present study and our previous work examining the 24-hour profile of AEA (38, 57, 64) indicate that consideration of time of day is imperative to assess eCB activity. In the fasting condition, immediately prior to breakfast, we did not observe significant differences in 2-AG levels between nonobese and obese participants, consistent with previous studies (32–36). We also did not observe consistent postmeal 2-AG excursions in either group whether in the morning, midday, or evening. From 0900 to 1600, 2-AG levels were significantly lower, rather than higher, in the obese subjects despite the fact that they ingested larger meals than nonobese participants since caloric content was tailored to meet individual energy requirements.
The quantification of the wave-shape of each individual profile of 2-AG across the 24-hour cycle revealed that a daily 2-AG rhythm is present in healthy obese individuals but blunted and delayed by 4 to 5 hours when compared with nonobese controls. Analysis of the simultaneous profiles of the congener 2-OG led to similar albeit less robust findings, partly because the amplitude of the 2-OG rhythm was dampened relative to that of 2-AG both in the obese and the nonobese groups. When expressed in percentage of the 24-hour mean, 2-AG levels were higher in the obese group in the overnight period, suggesting that the production of 2-AG was not suppressed during sleep in obese participants to the same extent as in nonobese controls despite the fact that individuals with OSA were excluded and sleep efficiency was similar in both groups. The timing of the nadir was positively correlated with BMI, such that individuals with a higher BMI had a larger delay in the 2-AG nadir. The brisk rise in 2-AG concentrations from the middle-night minimum to lunchtime peak found in nonobese subjects started later in the obese group; thus, from 0900 to 1600, 2-AG concentrations were lower in the obese subjects. In the evening, this difference was reversed, with higher 2-AG levels in the obese subjects. Altogether, these observations suggest that if the drive for hedonic eating is coupled to the rise in circulating 2-AG concentrations across the daytime, hedonically motivated feeding may be stronger later in the day in obese than in lean adults, consistent with data linking obesity, breakfast skipping, and evening eating (46–51).
In contrast, the 24-hour cortisol profile was nearly identical in both groups strongly suggesting that central circadian timing was not affected by excess adiposity in these healthy young participants. Thus, the rhythm of 2-AG concentrations, and presumably of hedonic drive, was clearly misaligned relative to central circadian timing.
The 24-hour profile of leptin, a pivotal marker of both adiposity and satiety, has a well-documented morning nadir and nocturnal acrophase that are largely determined by meal-timing (65), although the rhythm can still be detected when caloric intake is evenly distributed across the 24-hour cycle (66). In the present study, identical meals tailored to meet individual energy requirements were given at the same time in both groups and the timings of the leptin nadir and acrophase were similar in the 2 groups. However, obesity had a major impact on the temporal relationship between leptin and 2-AG profiles that may translate into differences in preferred timing of food intake. Indeed, in nonobese participants, early morning was characterized by changes in concentrations of the 2 signaling molecules that promote appetite (decreasing leptin, increasing 2-AG), while in the evening the concomitance of increasing leptin with decreasing 2-AG is likely to reduce appetite. By comparison, in obese participants, the pre-breakfast period coincided with a lesser decline of leptin and essentially stable 2-AG levels (consistent with reduced stimulation of appetite) while the early evening was associated with a blunted increase in leptin and a sharp rise in 2-AG (both promoting appetite).
The delay of the 2-AG profile, relative to both the central clock and the leptin rhythm, may thus provide a mechanism for the documented, but poorly understood, observation that breakfast skipping and late eating can lead to weight-gain and supports the hypothesis that obese individuals may have a later distribution of total caloric intake across the biological day. Whether the development of this dietary chronotype precedes or follows the delay in the 2-AG rhythm as adiposity increases cannot be addressed by the current data. The delay of the 2-AG profile may have been initiated as a consequence of habitual late eating but could, in turn, contribute to reinforce and perpetuate the dietary behavior. Irrespective of its primary cause, the circadian misalignment of eCB activity relative to the central pacemaker may constitute a mechanism by which obesity risk is fostered. Considering that all participants were maintained on a controlled feeding schedule in which the last meal was at 19:00 for 2 days prior to sampling, it is possible that we may have underestimated the habitual circadian misalignment of 2-AG in our obese participants.
Our study, while well-controlled, has limitations, including the small sample size and cross-sectional design. Sampling at 60-minute intervals may not be sufficient to accurately characterize postprandial 2-AG responses. Our meals had a relatively low fat content and it is possible that they were not palatable enough to elicit an anticipatory or postprandial 2-AG response. We did not quantify visceral adiposity in our participants nor do we have information regarding habitual feeding schedules in these individuals. Both groups included men and women but we were underpowered to examine sex differences. Lastly, our data do not provide insights into the mechanisms causing the profound alterations of the 24-hour profile of 2-AG in obese compared with nonobese individuals.
In conclusion, we observed a dampening and 4- to 5-hour delay of the 24-hour variation of eCB activity relative to the central circadian signal, as well as to the daily leptin rhythm. This misalignment may play a role in the reported preference for a later timing of food intake in obese adults and promote weight gain and other adverse metabolic consequences. These findings warrant further study as they may have important implications for prevention and treatment of obesity.
Acknowledgments
The authors would like to thank Dr. David A. Ehrmann for help with enrolling healthy obese individuals to include in this study. Further, the authors would like to thank Harry Whitmore and Fanny Delebecque for their assistance in data collection.
Financial Support: This study was supported by Grant KL2RR025000 from the National Center for Research Resources; Contract W81XWH-07-2-0071 from the Department of Defense Peer Reviewed Medical Research Program; Grant P50 HD057796 and P01 AG-11412 from the National Institutes of Health, The Research and Education Component of the Advancing a Healthier Wisconsin Endowment at the Medical College of Wisconsin; and a pilot award from the University of Chicago Institute for Translational Medicine supported by Grant UL1 RR024999. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources, the Department of Defense or the National Institutes of Health.
Glossary
Abbreviations
- 2-AG
2-arachidonoylglycerol
- 2-OG
2-oleoylglycerol
- AEA
N-arachidonylethanolamine (anandamide)
- AUC
area under the curve
- BMI
body mass index
- eCB
endocannabinoid
- IQR
interquartile range
- REM
rapid eye movement
- SD
standard deviation
- SEM
standard error of the mean
- SPT
sleep period time
- TST
total sleep time
Additional Information
Disclosure Summary: Authors have nothing to disclose.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Coccurello R, Maccarrone M. Hedonic eating and the “Delicious Circle”: from lipid-derived mediators to brain dopamine and back. Front Neurosci. 2018;12:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gérard CM, Mollereau C, Vassart G, Parmentier M. Molecular cloning of a human cannabinoid receptor which is also expressed in testis. Biochem J. 1991;279(Pt 1):129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Devane WA, Hanus L, Breuer A, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258(5090):1946–1949. [DOI] [PubMed] [Google Scholar]
- 4. Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365(6441):61–65. [DOI] [PubMed] [Google Scholar]
- 5. Mechoulam R, Ben-Shabat S, Hanus L, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50(1):83–90. [DOI] [PubMed] [Google Scholar]
- 6. Sugiura T, Kondo S, Sukagawa A, et al. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215(1):89–97. [DOI] [PubMed] [Google Scholar]
- 7. Hillard CJ. Circulating endocannabinoids: from whence do they come and where are they going? Neuropsychopharmacology. 2018;43(1):155–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jbilo O, Ravinet-Trillou C, Arnone M, et al. The CB1 receptor antagonist rimonabant reverses the diet-induced obesity phenotype through the regulation of lipolysis and energy balance. Faseb J. 2005;19(11):1567–1569. [DOI] [PubMed] [Google Scholar]
- 9. Poirier B, Bidouard JP, Cadrouvele C, et al. The anti-obesity effect of rimonabant is associated with an improved serum lipid profile. Diabetes Obes Metab. 2005;7(1):65–72. [DOI] [PubMed] [Google Scholar]
- 10. Ravinet Trillou C, Arnone M, Delgorge C, et al. Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am J Physiol Regul Integr Comp Physiol. 2003;284(2):R345–R353. [DOI] [PubMed] [Google Scholar]
- 11. Fong TM, Guan XM, Marsh DJ, et al. Antiobesity efficacy of a novel cannabinoid-1 receptor inverse agonist, N-[(1S,2S)-3-(4-chlorophenyl)-2-(3-cyanophenyl)-1-methylpropyl]-2-methyl-2-[[5-(trifluoromethyl)pyridin-2-yl]oxy]propanamide (MK-0364), in rodents. J Pharmacol Exp Ther. 2007;321(3):1013–1022. [DOI] [PubMed] [Google Scholar]
- 12. Chen G, Pang Z. Endocannabinoids and obesity. Vitam Horm. 2013;91:325–368. [DOI] [PubMed] [Google Scholar]
- 13. Witkamp RF. The role of fatty acids and their endocannabinoid-like derivatives in the molecular regulation of appetite. Mol Aspects Med. 2018;64:45–67. [DOI] [PubMed] [Google Scholar]
- 14. Matias I, Gonthier MP, Orlando P, et al. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J Clin Endocrinol Metab. 2006;91(8):3171–3180. [DOI] [PubMed] [Google Scholar]
- 15. Monteleone AM, Di Marzo V, Monteleone P, et al. Responses of peripheral endocannabinoids and endocannabinoid-related compounds to hedonic eating in obesity. Eur J Nutr. 2016;55(4):1799–1805. [DOI] [PubMed] [Google Scholar]
- 16. Monteleone P, Piscitelli F, Scognamiglio P, et al. Hedonic eating is associated with increased peripheral levels of ghrelin and the endocannabinoid 2-arachidonoyl-glycerol in healthy humans: a pilot study. J Clin Endocrinol Metab. 2012;97(6):E917–E924. [DOI] [PubMed] [Google Scholar]
- 17. Gatta-Cherifi B, Matias I, Vallée M, et al. Simultaneous postprandial deregulation of the orexigenic endocannabinoid anandamide and the anorexigenic peptide YY in obesity. Int J Obes. 2012;36(6):880–885. [DOI] [PubMed] [Google Scholar]
- 18. Mennella I, Ferracane R, Zucco F, Fogliano V, Vitaglione P. Food liking enhances the plasma response of 2-Arachidonoylglycerol and of pancreatic polypeptide upon modified sham feeding in humans. J Nutr. 2015;145(9):2169–2175. [DOI] [PubMed] [Google Scholar]
- 19. Williams CM, Rogers PJ, Kirkham TC. Hyperphagia in pre-fed rats following oral delta9-THC. Physiol Behav. 1998;65(2): 343–346. [DOI] [PubMed] [Google Scholar]
- 20. Colombo G, Agabio R, Diaz G, Lobina C, Reali R, Gessa GL. Appetite suppression and weight loss after the cannabinoid antagonist SR 141716. Life Sci. 1998;63(8):PL113–PL117. [DOI] [PubMed] [Google Scholar]
- 21. Sam AH, Salem V, Ghatei MA. Rimonabant: from RIO to Ban. J Obes. 2011;2011:432607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Després JP, Golay A, Sjöström L; Rimonabant in Obesity-Lipids Study Group Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med. 2005;353(20):2121–2134. [DOI] [PubMed] [Google Scholar]
- 23. Scheen AJ, Finer N, Hollander P, Jensen MD, Van Gaal LF; RIO-Diabetes Study Group Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study. Lancet. 2006;368(9548):1660–1672. [DOI] [PubMed] [Google Scholar]
- 24. Van Gaal LF, Scheen AJ, Rissanen AM, Rössner S, Hanotin C, Ziegler O; RIO-Europe Study Group Long-term effect of CB1 blockade with rimonabant on cardiometabolic risk factors: two year results from the RIO-Europe Study. Eur Heart J. 2008;29(14):1761–1771. [DOI] [PubMed] [Google Scholar]
- 25. Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rössner S; RIO-Europe Study Group Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365(9468):1389–1397. [DOI] [PubMed] [Google Scholar]
- 26. Richey JM, Woolcott O. Re-visiting the endocannabinoid system and its therapeutic potential in obesity and associated diseases. Curr Diab Rep. 2017;17(10):99. [DOI] [PubMed] [Google Scholar]
- 27. Amato G, Khan NS, Maitra R. A patent update on cannabinoid receptor 1 antagonists (2015-2018). Expert Opin Ther Pat. 2019;29(4):261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hillard CJ, Weinlander KM, Stuhr KL. Contributions of endocannabinoid signaling to psychiatric disorders in humans: genetic and biochemical evidence. Neuroscience. 2012;204:207–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Engeli S, Böhnke J, Feldpausch M, et al. Activation of the peripheral endocannabinoid system in human obesity. Diabetes. 2005;54(10):2838–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Blüher M, Engeli S, Klöting N, et al. Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes. 2006;55(11):3053–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Côté M, Matias I, Lemieux I, et al. Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. Int J Obes. 2007;31(4):692–699. [DOI] [PubMed] [Google Scholar]
- 32. Matias I, Gatta-Cherifi B, Cota D. Obesity and the endocannabinoid system: circulating endocannabinoids and obesity. Current Obesity Reports. 2012;1(4):229–235. [Google Scholar]
- 33. Sipe JC, Scott TM, Murray S, et al. Biomarkers of endocannabinoid system activation in severe obesity. Plos One. 2010;5(1):e8792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Di Marzo V, Côté M, Matias I, et al. Changes in plasma endocannabinoid levels in viscerally obese men following a 1 year lifestyle modification programme and waist circumference reduction: associations with changes in metabolic risk factors. Diabetologia. 2009;52(2):213–217. [DOI] [PubMed] [Google Scholar]
- 35. Engeli S, Lehmann AC, Kaminski J, et al. Influence of dietary fat intake on the endocannabinoid system in lean and obese subjects. Obesity. 2014;22(5):E70–E76. [DOI] [PubMed] [Google Scholar]
- 36. Carta G, Melis M, Pintus S, et al. Participants with normal weight or with obesity show different relationships of 6-n-Propylthiouracil (PROP) taster status with BMI and plasma endocannabinoids. Sci Rep. 2017;7(1):1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rigamonti AE, Piscitelli F, Aveta T, et al. Anticipatory and consummatory effects of (hedonic) chocolate intake are associated with increased circulating levels of the orexigenic peptide ghrelin and endocannabinoids in obese adults. Food Nutr Res. 2015;59:29678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hanlon EC, Tasali E, Leproult R, et al. Circadian rhythm of circulating levels of the endocannabinoid 2-arachidonoylglycerol. J Clin Endocrinol Metab. 2015;100(1):220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hanlon EC, Van Cauter E. Quantification of sleep behavior and of its impact on the cross-talk between the brain and peripheral metabolism. Proc Natl Acad Sci U S A. 2011;108(Suppl 3):15609–15616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann N Y Acad Sci. 2008;1129:287–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Knutson KL. Sleep duration and cardiometabolic risk: a review of the epidemiologic evidence. Best Pract Res Clin Endocrinol Metab. 2010;24(5):731–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Leproult R, Holmbäck U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014;63(6):1860–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Suwazono Y, Dochi M, Sakata K, et al. A longitudinal study on the effect of shift work on weight gain in male Japanese workers. Obesity. 2008;16(8):1887–1893. [DOI] [PubMed] [Google Scholar]
- 44. Kivimäki M, Batty GD, Hublin C. Shift work as a risk factor for future type 2 diabetes: evidence, mechanisms, implications, and future research directions. Plos Med. 2011;8(12):e1001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lowden A, Moreno C, Holmbäck U, Lennernäs M, Tucker P. Eating and shift work - effects on habits, metabolism and performance. Scand J Work Environ Health. 2010;36(2):150–162. [DOI] [PubMed] [Google Scholar]
- 46. Reid KJ, Baron KG, Zee PC. Meal timing influences daily caloric intake in healthy adults. Nutr Res. 2014;34(11):930–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring). 2011;19(7):1374–1381. [DOI] [PubMed] [Google Scholar]
- 48. Gallant AR, Lundgren J, Drapeau V. The night-eating syndrome and obesity. Obes Rev. 2012;13(6):528–536. [DOI] [PubMed] [Google Scholar]
- 49. Colles SL, Dixon JB, O’Brien PE. Night eating syndrome and nocturnal snacking: association with obesity, binge eating and psychological distress. Int J Obes (Lond). 2007;31(11): 1722–1730. [DOI] [PubMed] [Google Scholar]
- 50. Garaulet M, Madrid JA. Chronobiological aspects of nutrition, metabolic syndrome and obesity. Adv Drug Deliv Rev. 2010;62(9-10):967–978. [DOI] [PubMed] [Google Scholar]
- 51. Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018;27(6):1212–1221.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Arble DM, Sandoval DA. CNS control of glucose metabolism: response to environmental challenges. Front Neurosci. 2013;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity. 2009;17(11):2100–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Oster H, Challet E, Ott V, et al. The functional and clinical significance of the 24-hour rhythm of circulating glucocorticoids. Endocr Rev. 2017;38(1):3–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kettner NM, Mayo SA, Hua J, Lee C, Moore DD, Fu L. Circadian dysfunction induces leptin resistance in mice. Cell Metab. 2015;22(3):448–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Van Cauter EV, Polonsky KS, Blackman JD, et al. Abnormal temporal patterns of glucose tolerance in obesity: relationship to sleep-related growth hormone secretion and circadian cortisol rhythmicity. J Clin Endocrinol Metab. 1994;79(6):1797–1805. [DOI] [PubMed] [Google Scholar]
- 57. Hanlon EC, Tasali E, Leproult R, et al. Sleep restriction enhances the daily rhythm of circulating levels of endocannabinoid 2-arachidonoylglycerol. Sleep. 2016;39(3):653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr. 1985;39(Suppl 1):5–41. [PubMed] [Google Scholar]
- 59. Iber C, Ancoli-Israel S, Chesson A, Quan S.. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, Illinois:American Academy of Sleep Medicine; 2007. [Google Scholar]
- 60. Hill MN, Miller GE, Ho WS, Gorzalka BB, Hillard CJ. Serum endocannabinoid content is altered in females with depressive disorders: a preliminary report. Pharmacopsychiatry. 2008;41(2):48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Patel S, Carrier EJ, Ho WS, et al. The postmortal accumulation of brain N-arachidonylethanolamine (anandamide) is dependent upon fatty acid amide hydrolase activity. J Lipid Res. 2005;46(2):342–349. [DOI] [PubMed] [Google Scholar]
- 62. Cleveland W. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74(368):829–836. [Google Scholar]
- 63. Russell GS, Levitin DJ. An expanded table of probability values for rao’s spacing test. Commun Stat Simul Comput. 1995;24(4):879–888. [Google Scholar]
- 64. Hanlon EC. Impact of circadian rhythmicity and sleep restriction on circulating endocannabinoid (eCB) N-arachidonoylethanolamine (anandamide). Psychoneuroendocrinology. 2020;111:104471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schoeller DA, Cella LK, Sinha MK, Caro JF. Entrainment of the diurnal rhythm of plasma leptin to meal timing. J Clin Invest. 1997;100(7):1882–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Simon C, Gronfier C, Schlienger JL, Brandenberger G. Circadian and ultradian variations of leptin in normal man under continuous enteral nutrition: relationship to sleep and body temperature. J Clin Endocrinol Metab. 1998;83(6):1893–1899. [DOI] [PubMed] [Google Scholar]