Abstract
Purpose of review
Epstein-Barr virus (EBV) is a known determinant for numerous malignancies and may contribute to autoimmune diseases. The underlining mechanisms behind EBV pathologies is not completely understood. Recently, extracellular vesicles (EVs) released from infected cells have been found to produce profound effects on cellular microenvironments. Therefore, in this review we sought to critically evaluate the roles of EVs in EBV pathogenesis and assess their potential therapeutic and diagnostic utility.
Recent findings
EBV-altered EVs are capable of activating signaling cascades and phenotypic changes in recipient cells through the transfer of viral proteins and RNAs. Moreover, several EV-associated microRNAs have encouraging prognostic or diagnostic potential in EBV-associated cancers.
Summary
Current evidence suggests that EBV-modified EVs affect viral pathogenesis and cancer progression. However, further research is needed to investigate the direct role of both viral and host products on recipient cells and the mechanisms driving viral protein and RNA EV packaging and content modification.
Keywords: Epstein-Barr virus, extracellular vesicles, cancer, exosomes, autoimmune, diagnostic
Introduction:
Epstein-Barr virus (EBV) is a member of the human Herpesviridae family that has established a persistent infection in more than 90% of the world’s population (1). However, in certain susceptible individuals, EBV can lead to the development of a wide range of malignancies including nasopharyngeal carcinoma, gastric adenocarcinoma, Hodgkin lymphoma, Burkitt lymphoma, post-transplant lymphoproliferative disease, NK/T-cell lymphoma, aggressive NK-cell leukemia, lymphomatoid granulomatosis, and diffuse large B cell lymphoma (2, 3). Currently, EBV is estimated to contribute to the development of roughly 200,000 new cancers world-wide each year and account for nearly 2% of all human cancers (4). There is also some evidence that EBV may be an underestimated contributing co-factor to the progression of other malignancies like cervical and breast cancers (5–7). However, more studies are needed considering it is often difficult to establish a causative role of EBV in a particular cancer since only a small percentage of people infected will ever develop malignancies. In addition to cancer, EBV has been linked with autoimmune diseases such as multiple sclerosis and systemic lupus erythematosus (8, 9).
Oncogenic viruses contribute to the development and progression of malignancy by fostering a pro-tumoral microenvironment (10). Extracellular vesicles (EVs) have emerged as key factors driving intercellular communications in tumor microenvironments. Depending on the type of cancer, EVs have been shown to function in cell growth, invasion, metastasis, angiogenesis, drug resistance, and immune cell regulation through the transfer of bioactive molecules (11). EVs contain a diverse repertoire of proteins and RNAs enclosed in a lipid bilayer enriched in sphingolipids and cholesterol (12). Several EV populations are recognized based on their intracellular site of origin and include exosomes and microvesicles (12, 13). Exosomes are small (50–150nm) vesicles that form on the limiting membrane of multi-vesicular bodies, whereas microvesicles bud off from the plasma membrane and are generally larger in size (100–1000nm). The overlap in size and density has made it difficult to get truly pure vesicle populations using methods currently employed in the field for EV isolation. Adding to the complexity is the existence of exosome and microvesicle sub-populations that likely have distinct cargo and functions (**14).
Once released from the cell, EVs can spread locally or enter bodily fluids and be transported to other sites in the body where they can be taken-up by target cells and release their cargo (11, 12). This EV-mediated intercellular molecular exchange can influence recipient cells and contribute to physiological and pathological processes (15–18). The unique properties of EVs has generated tremendous interest for their use as diagnostics, drug delivery vehicles, and therapeutic agents. However, before the full clinical utility of EVs can be achieved, a better understanding of the mechanisms driving biogenesis of EV subpopulations, EV-target cell interactions, and cargo release must be obtained. It is clear from our work and others, that viral infection alters the components and functions of EVs released from infected cells (10, 12, 19). In the case of EBV, it is evident that EVs contribute to pathogenesis of EBV-associated cancers (10).
Viral EV cargo in the context of EBV cancers
EBV has the capability of altering vesicle content and secretion, which can have substantial effects on tissue and tumor microenvironments. EBV packages various viral components, including proteins and RNAs, into EVs (10, 20). The viral manipulation of host secretory pathways has been demonstrated to aid in the evasion of the immune system while promoting cancer progression by modifying the angiogenic, invasive and metastatic potential within the tumor microenvironment (21, 22). The principal oncogene of EBV is latent membrane protein 1 (LMP1) as it is expressed in most EBV-related cancers and is critical for B-cell immortalization and cellular transformation (23). In figure 1, the viral EV cargo secreted by cell type and latency program are depicted and the functions of these products are summarized in table 1.
Figure 1.
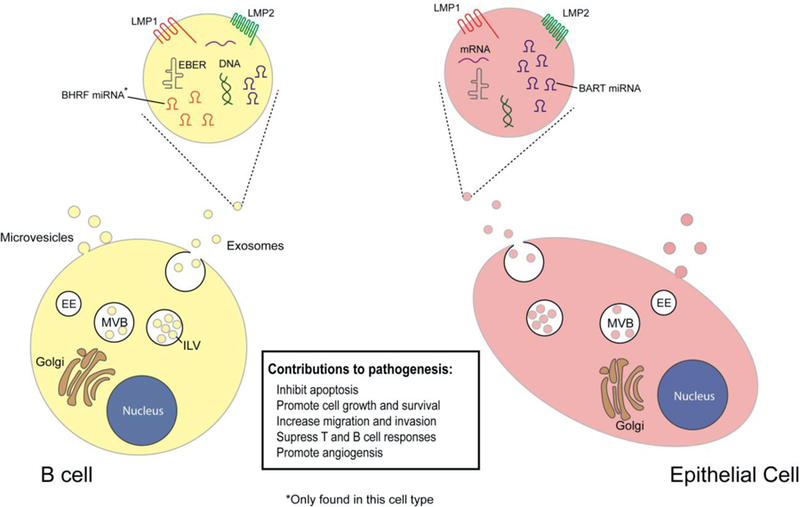
EBV products packaged into extracellular vesicles. Assorted viral components have been found to be incorporated into EVs released from EBV infected cells. There are some differences in components depending on cellular origin and latency type. These EBV-modified EVs perform various functions in recipient cells, such as inhibiting immune responses or promoting cell growth.
Table 1.
Viral EV cargo secreted by cell type
| Viral Product | Cell Lines | Functions | References |
|---|---|---|---|
| LMP1 | BL, GC, LCL, NPC | Enhance cancer growth, metastasis, invasion, and malignancy, immune regulation | Shair (24), Sheu (27), Meckes (15), Aga (29), Dawson (25), Wheelock (31), Wakisaka (33), Tsai (34) |
| LMP2 | BL, LCL, NPC | Promote EMT, enhance cancer progression, immune modulation | Moody (41), Scholle (42), Fukuda (43), Morrison (44), Cen (45) |
| EBER | BL, LCL, NPC | Alter recipient cell function | Ahmed (47), Zeng (48), Lee (49) |
| BHRF1 | BL, HL, LCL, PTLD | Immune regulation | Cai (58), Skinner (59), and Pegtel (16) |
| BART | BL, HL, NPC, GC, NKTL, LCL, DLBCL, PTLD | Immune regulation Cancer progression | Pegtel (16) Meckes (15), Cai (58), Ramayanti (60), Kang (61), Ambrosio (63), Hooykaas (64), Wong (65) |
| mRNA | LCL and NPC | Enhance infection or cancer progression | Canitano (69) |
BL- Burkitt’s lymphoma
HL- Hodgkin’s lymphoma
LCL- Lymphoblastoid Cell Line
PTLD- Post-transplant lymphoproliferative disease
NPC- Nasopharyngeal Carcinoma
GC- Gastric Carcinoma
NKTL- Natural Killer/T-Cell Lymphoma
DLBCL- Diffuse Large B-Cell Lymphoma
Latent membrane protein 1 (LMP1):
LMP1 is a constitutively active CD40 receptor mimic that triggers many pro-oncogenic consequences in cells by recruiting tumor necrosis factor receptor-associated factor (TRAF) proteins and other effector molecules to the C-terminal activating regions (CTAR). This recruitment allows LMP1 to initiate various signaling pathways that inhibit apoptosis and promote cell growth and survival (24–27). LMP1 has been identified in EVs and is capable of inducing similar cellular responses when taken-up by recipient cells (15, *28, 29). Specifically, several studies have demonstrated the ability of LMP1-containing EVs to activate PI3K/AKT and MAPK/ERK pathways in receiver cells as well as inhibit the function of immune cells (21, 22, 29). In fact, LMP1 expression increases levels of EGFR secreted by cells, which likely contributes to the ERK and AKT activating properties of the EVs (15). Along with the cargo modifications, LMP1 has also been found to increase the release of small CD63-containing EVs, perceived as predominately exosomes, from various cell lines and thereby floods tissue environments with virally-modified communicative vehicles (*28).
LMP1 is adept in activating a multitude of pathways that contributes to the progression of cancer (30). One of the recognized characteristics of malignancy progression is the epithelial-mesenchymal transition (EMT), which involves epithelial cells shifting to a more mesenchymal phenotype (31, 32). This phenotypic change is characterized by a cadherin switch, which is when cellular levels of E-cadherin fall while N-cadherin levels increase (31). LMP1 has been found to induce migration and produce a more malignant phenotype in cells by increasing integrin alpha-5 and N-cadherin levels (32). However, whether EV-associated LMP1 produces similar effects in recipient cells has yet to be evaluated. Others have found that LMP1 further promotes malignancy by upregulating hypoxia-inducible factor-1 alpha (HIF1α) and secreting EVs capable of promoting EMT and angiogenesis (29, 33). It is therefore likely that LMP1-modified EVs contribute to EMT and metastatic properties of EBV infected cancer cells.
In addition to enhancing migratory phenotypes, LMP1 supports tumor progression by regulating host immune functions. In a recent study by Tsai et al., LMP1 suppressed host humoral immune responses by blocking differentiation of B cells into antibody secreting cells (ASCs) (34). This study also discovered that LMP1 upregulated the expression of indoleamine 2,3-dioxygenase 1 (IDO1) which is involved in tryptophan production. The tryptophan metabolites inhibit B cell function in the surrounding cells thus further suppressing immunological responses (33). Interestingly, LMP1 was found to regulate programmed cell death protein 1 ligand (PD-L1) in NPC, which is involved in immune suppression, (35) and recently, PD-L1 was detected in exosomes (**36). Therefore, EBV LMP1 may increase packaging of PD-L1 into EVs, conceivably for the modulation of the host immune checkpoints within the tumor microenvironment. This could ultimately contribute to EBV immune evasion strategies and pathogenesis by preventing adequate tumor recognition by immune cells. Immune checkpoint inhibitors are now becoming more widely used for the treatment of certain cancers. Based on current evidence, EBV cancers may therefore be suitable candidates for these therapies.
Since LMP1 can modify EVs, presumably to manipulate the infected microenvironment, gaining a greater understanding of how LMP1 traffics to EVs could foster the generation of novel targeted therapies. Kobayashi et al. determined that farnesylation of the C terminus by ubiquitin C-terminal hydrolase-L1 (UCH-L1) is critical for sorting of LMP1 into EVs (37). Our group later demonstrated that the N terminus and transmembrane region 1 of LMP1 are sufficient for sorting into EVs (*38). Furthermore, a mutant lacking the N terminus and transmembrane domains 1 through 4 failed to be packaged, whereas other mutations resulted in enhanced packaging. These data suggests that there are regulatory mechanisms for LMP1 EV cargo sorting (*38). Alternatively, LMP1 may exists in distinct EV populations with different mechanisms of targeting and biogenesis. For example, LMP1 was detected in fractions consistent with larger microvesicles, as well as the small light and dense EVs described by Kowal and colleagues (**14, *28). In the same study, LMP1 packaging into exosomes requires the tetraspanin protein CD63 (*28, **39). Despite recent advances, the mechanisms controlling LMP1 EV trafficking remain incomplete. Regardless, accumulating evidence supports a role of LMP1-modified EVs as having a prominent involvement in the pathogenesis of EBV-associated cancers.
Latent membrane protein 2 (LMP2):
LMP2 was found by Ikeda and Longnecker to be secreted from cells in exosomes (40). EV-associated LMP2, unlike LMP1, has not been as well studied. Even though LMP2 is critical for maintaining viral latency in EBV infected cells, it is not required for B-cell immortalization (30). Yet, LMP2 has the ability to activate many oncogenic signaling pathways including PI3K/Akt, MAPK/ERK, and NF-kB. LMP2 is required for outgrowth of EBV-infected epithelial cells in vitro (41) and can induce anchorage-independent growth, enhance cell motility and adhesion, and induce EMT (42–44). When cholesterol is depleted in cells, LMP2A cellular levels and EV secretion were both enhanced, but LMP2A was no longer able to be endocytosed (40). LMP2A in the EV fraction is ubiquitinated and not phosphorylated, suggesting that ubiquitination may be important for exosomal loading (40).
Though the effects of LMP2 EVs on recipient cells have not been well studied, LMP1 research suggests that LMP2-modified EVs may both activate pathways and modulate the tumor microenvironment through the promotion of EMT in recipient cells. LMP2 also acts as a B-cell receptor mimic, but the distinct isoforms perform different functions in cells. LMP2a inhibits B-cell activity, while LMP2b activates B-cells (45). This contrasting activity may help maintain EBV latency. Further research is needed to determine if the unique isoforms also effect recipient cells differently. LMP2 promotes malignant progression of NPC therefore it is conceivable that EV-associated LMP2 may exhibit biological effects within the tumor microenvironment (46).
Epstein–Barr virus-encoded RNA (EBER):
In addition to proteins, EBV will package viral RNAs into EVs. Epstein-Barr virus-encoded RNAs (EBERs) are non-coding RNAs that are detected in all EBV infected cells and can be packaged into exosomes with the EBER binding protein La (47). Recently, EBER expression was found capable of distinguishing non-cancerous patients from NPC patients and higher EBER1 levels correlated with increased viability (48). Lee et al. discovered that EBER2 interacts with cellular transcription factor paired box protein 5 (PAX5), which is involved in B cell activation (49). These data suggest that EBV infected cells may use secreted EBERs to alter recipient cell function and could be used as a biomarker for cancer.
Epstein–Barr virus microRNAs:
MicroRNAs (miRNAs) are small noncoding RNAs, typically 20–25 base-pair long, which can act as key regulators of gene expression. The miRNAs can be packaged into EVs and secreted into the extracellular space for cell-to-cell transmission (20, 50). In the case of EBV, miRNAs have been found to play a large role in the pathogenesis of EBV-associated cancers. There are 44 mature EBV miRNAs. Four are mapped to the Bam HI fragment H rightward open reading frame 1 (BHRF1), and forty are mapped to BamHI-A rightward transcript (BART). Different latency stages have unique expression levels of these miRNAs. Nanbo et al. found that cells in type III latency produce the most EVs, when compared with type I latency or EBV-negative (51). Additionally, the cells in type III latency were found to incorporate many viral miRNAs, along with specific host miRNAs (51). EBV encoded miRNAs can regulate viral and host pathways to inhibit apoptosis, promote cell growth, and maintain a persistent infection (52, 53).
EBV miRNAs were found to suppress the release of proinflammatory cytokines, such as IL-12, and repress differentiation of CD4+ T cells. This reduces activation of cytotoxic EBV-specific CD4+ effect T cells (54). EBV infected cells also evade CD8+ T cells by releasing miRNAs that directly target the transported TAP2 and reduce levels of TAP1 and MHC class 1 molecules. The miRNAs are able to decrease levels of EBNA1, which is a target of CD8+ T cells (54). These pathways allow EBV to use EVs to evade the immune system.
Bam HI fragment H rightward open reading frame 1 (BHRF1):
BHRF miRNAs are not typically expressed in nasopharyngeal carcinoma (NPC), unless the cells undergo lytic reactivation, or exhibit a type III latency expression pattern (55). In diffuse large B-cell lymphoma (DLBCL), natural killer/T-cell lymphoma (NKTL), and gastric carcinoma (GC), expression of the BHRF1 miRNA cluster has not been detected (56, 57). However, Cai et al. found that BHRF miRNAs are expressed in cells exhibiting an EBV type III latency program (58). Interleukin 1 (IL-1) receptor 1, which is important for immune regulation, is suppressed by BHRF1–2-5p (59). A study by Pegtel et al. discovered that EVs secreted by EBV-infected B cells containing BHRF1–3 can reduce expression of certain genes, such as the immunostimulatory gene CXCL11, in uninfected recipient cells providing the first evidence of functional miRNA delivery by EVs (16).
BamHI-A rightward transcript (BART):
Ramayanti et al. performed RNA sequencing on plasma RNA from patients with NPC and found that there were higher levels of EBV encoded miRNAs than endogenous miRNA (60). They also discovered that the miRNA profiles differed between patients and that BART13–3p was present in 97% of the samples, suggesting that this BART may be used as a diagnostic marker (60). Additionally, BART miRNAs have been found to be upregulated in GC with BART1 and BART4 being overexpressed (61). High levels of BART miRNAs were detected in DLBCL and NKTL, with the highest expression being BART7, BART22, and BART10 (62). BART miRNAs were found to be expressed at high levels in latently infected epithelial cells and low levels in B cells (58). IL-6 receptor B is targeted by BART6–3p (63), and CREB-binding protein, which is a coactivator in type 1 interferon signaling, is a direct target of BART16 (64). Taken together, these data imply that EBV-modified EVs are important for controlling innate and adaptive antiviral immune responses through miRNA transfer. Since the BART miRNAs can alter various pathways, such as PI3K/AKT and Wnt signaling, or bind to a multitude of tumor suppressor genes (65), it is likely that BART miRNAs in EVs will also target similar pathways in recipient cells.
lncRNA:
Cellular long non-coding RNAs (lncRNA), specifically H19 and H19 antisense, have been found to be packaged into exosomes and released from EBV-positive LCL cells (66). However, little is known about the role these lncRNA play in EBV pathogenesis. H19 was found to have increased levels in retinoblastoma cells and patients with high H19 expression were found to have decreased survival times. Also, knockdown of H19 suppresses cell proliferation and invasion (67). A recent study by Chen et al. additionally found that macrophages in breast cancer, specifically tumor associated (TAMs), secrete EVs containing HIF-1α-stabilizing lncRNA to alter glycolysis in cells within the tumor environment (68). It would be interesting to evaluate whether this occurs in EBV cancers or even what other influences cellular and viral lncRNAs have on the tumor microenvironment.
mRNA:
In addition to non-coding RNAs, EBV also secretes EV-associated mRNA including LMP1, LMP2, Epstein-Barr nuclear antigen 1 (EBNA1) and EBNA2 into the extracellular space (69). More research is required to determine if these mRNA are translated in recipient cells and their downstream effects. It is likely that these mRNAs are be secreted in EVs by infected cells to prime other cells for infection and may enhance cancer progression.
EV diagnostic markers
One of the foremost contributing factors towards improving prognosis and better health outcomes is having the tools and methods allowing for the early identification of diseases. Early diagnosis has been shown to vastly improve patient outcomes in many cancers, as exemplified by those patients diagnosed in stage I/II versus stage III/IV having significantly greater one-year survival rates (70, 71). This is especially true for nasopharyngeal cancers since N3 NPC has been shown to have a high risk of metastasis and low five-year survivability (72). Thus, the development of novel sensitive and specific biomarkers continues to remain a primary focus of various research endeavors. Within the biomarker field of study, EVs are now becoming more universally accepted as a superior source of biomarkers for many diseases, including cancer, and research into EVs as prospective biomarkers has dramatically increased in the past decade.
The benefits of utilizing EVs as biomarkers is that EVs are quite stable, typically minimally invasive to obtain, and have been shown to contain specific cargo relating to the disease and pathology (60, 73). Several recent studies have found the presence or enrichment of certain miRNAs in EVs correlated with particular diseases and even severity or disease stage.
In the case of EBV NPC, plasma EBV DNA and serological IgG assays have been the primary methods for the laboratory detection (74). However, a study by Ramayanti et al. just reported that EV BART13–3P was more sensitive and specific in differentiating EBV NPC from other head and neck cancers (60). As mentioned earlier, EBV BART 1 and 4 were found to be overexpressed in EBV gastric carcinoma. Another study by Tsai et al. found elevated levels of BART 4 as well as BARTs 11, 2, 6, 9, 18 by in situ hybridization of EBV GC surgical specimens (75). Interestingly, BART 9 sequence is homologous to miRNA-200a and miRNA-141, which have been associated with EBV GC EMT phenotype (75). It would be advantageous to examine the levels of these EBV miRNAs in EVs in comparison with the cellular levels since overexpression suggests enhanced secretion. Varying levels of EBV BART miRNAs could potentially not only indicate GC but may also provide additional information on GC phenotypes.
In addition to EBV miRNAs, analyzing host EV miRNA and other non-coding RNAs, may prove worthwhile since cellular expression levels in the context of cancer have been studied extensively. For instance, elevated levels of miRNA-155 as well as the long non-coding RNA PVT1 have both been associated with poor outcomes and survivability in NPC (76, 77). There are ample other host RNA associations in various diseases and analyzing all of the differential expression for each disease type can quickly become complex. Yet, examining EV RNAs could perhaps help narrow down these in order to establish EV profiling assays for each disease. In fact, Taylor et al. established the first multiplexed miRNA EV profiling for ovarian cancer that could not only aid in patient diagnosis but also could be used in screening assays of asymptomatic women (78). Additionally, these multiplex assays potentially increase the prognostic capabilities of the assay which can help better direct patient therapies.
EV therapeutic applications
A key limitation to many types of therapeutics has been effective drug delivery. Recently, there has been a growing interest in nanoparticles as therapy delivery vehicles due to their competent targeting as well as permeability and retention capabilities (79). Nanoparticle delivery systems, including liposomes and mesoporous silica nanoparticles, typically range from 10 to 1000 nm in diameter and can be loaded with chemical therapeutics and miRNAs (79, 80). However, EVs have lately been explored as a possible superior vehicle. Unlike fabricated nanoparticles, EVs have greater stability, are more likely to evade compliment immune responses, and less prone to triggering negative off-target effects (81, 82). Several studies have reported that coupling antigens with EVs increases the immunogenicity and efficacy of potential cancer vaccines (83, 84). This perhaps could be a method for targeting LMP1-induced pathogenic effects in both EBV cancer and autoimmune diseases.
In addition to EV loading methods, the therapeutic application of EVs from specific cell types is being explored for clinical purposes. MSCs, or mesenchymal stem cells, have been researched considerably for potential therapies but recently the EVs from these cells appear more promising because vesicle treatments induce the beneficial effects in target locations without the risk of uninhibited cell proliferation and differentiation (85). A recent study by Yuan et al. found that MSC EV express TNF-related apoptosis-inducing ligand (TRAIL) and could be selectively target and induce apoptosis in vitro (86). TRAIL was previously shown to induce apoptosis in numerous transformed cell types but its utilization has been problematic due to low bioavailability and difficulties with therapeutic delivery (86, 87).
EVs from immature dendritic cells (DC) may also prove to have therapeutic applications. DC-EVs have been found to induce beneficial anti-inflammatory effects that could be utilize in treating autoimmune diseases (88). A study by Kim et al. discovered that DC-EVs exhibited anti-inflammatory effects in vivo and reduced collagen-induced arthritis occurrence as well as severity in mice (89). In addition, DC-EVs are being researched as a possible antitumor therapies and several have made it into clinical trials (90, 91). In a Phase I trial for advanced non-small cell lung cancer, EVs containing MAGE tumor antigens were harvested from patients DCs and readministered (91). The therapy was well tolerated and some patients had positive immune responses and long-term disease stability (91). It may be beneficial to explore both MSC and DC
EVs as therapeutics for EBV associated diseases
Despite promising EV therapeutic uses, there are still obstacles that need be overcome before the widespread clinical application of EV therapies. EV isolation can be arduous and this is further complicated by the inability of current isolation methods to purely separate vesicle types (**14, 85, 92). EVs biogenesis pathways also have yet to be fully appreciated, consequently isolations may contain vesicles from different origins with highly varying cargos (**14, 93). The in vivo consequences of these EVs could pose safety risks so adequate research should be performed prior to the application of these treatments in the clinical setting.
Autoimmune diseases
EBV continues to be implicated in multiple autoimmune diseases, but directly linking infection with disease development can be problematic. Although EBV appears to be a prerequisite for some syndromes, not all who are infected will develop the disease. Regardless, EBV has been connected with several autoimmune diseases but the evidence is strongest for multiple sclerosis (MS), rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) (94–97). Previous research revealed a near 100% EBV-seropositivity in adult and pediatric patients who developed MS (96,95). Recently, over 200 gene loci have been associated with MS risk using genome wide association studies and EBV infection was found to regulate many of these genes, suggesting that EBV infection could be a contributing factor for MS development or pathogenesis (98). RA patients have been found to have higher circulating anti-EBNA1 antibodies (99), elevated blood EBV DNA load (100) and other viral products (i.e. EBERs, LMP1) in synovial fluid (101, 102). Also, SLE patients have elevated circulating EBV DNA, antibodies to EBV EBNAs and viral capsid antigen (VCA) however previously contracting infectious mononucleosis does not lead to an increase chance of developing the disease (103, 104).
One of the accepted means by which viruses are thought to promote the development of autoimmune diseases is through antigenic mimicry. Some viruses have amino acid sequences in their proteins that closely resemble host proteins. This sequence mimicry can lead to immune cells developing antibodies or T cell receptors that can cross-react with host proteins following the viral infection. In the case of EBV, antigenic mimicry has been documented in MS, RA, and SLE (105). Pathogenesis in MS occurs mainly as a result of T cell induced CNS demyelination and chronic inflammation. The MHC class II allele DRB1*1501 has previously been reported as a risk factor for MS but Lang et al. demonstrated the close similarity of the crystal structures of DRB1*1501-MBP (myelin basic protein) peptide and DRB5*1010-EBV peptide presented for T cell recognition (105, 106). For RA and SLE, there are reports of mimicry of EBNA1 peptides with synovial protein and RO/Sm autoantigens respectively (107–109).
It is evident that EVs have many roles in both normal and pathological states. One function of EVs is to serve as a source of antigen presentation complexes that can be taken up by antigen presenting cells (APC) or even possible directly activate T cells (110, 111). EBV has established capabilities in utilizing host EV machinery for the production, processing and release of viral products (15). Interestingly, CD63 is known to have a role in autophagy and trafficking of MHC class molecules for antigen presentation as well as being required for the vesicle secretion of certain viral products (*28, **39, 112). Therefore, it is plausible that EVs displaying the viral mimicry peptides are secreted and could serve as a source for autoimmune clonal expansion of B and T cells. Since EBV LMP1 protein has been shown to increase vesicle secretion, increasing levels of autoimmunogenic EVs may be released with LMP1 expression especially during latency switching (15, *28). As highlighted earlier, LMP1 has been detected in synovial fluid of afflicted joints of RA patients which may also contribute to the release of these mimicry-containing EVs that promote inflammation and pathogenesis (101).
Additionally EVs are capable of stimulating angiogenesis, which can induce damage to cartilage and bone in the joints of RA patients and vasculitis in SLE patients (113). LMP1 is known to increase VEGF expression and LMP1 containing EVs have been reported to promote angiogenesis (15, 114). Increased EBV infected cells (as inferred by elevated DNA levels) in autoimmune patients, may also contribute to increase secretion of pathogenic EVs that are capable of promoting inflammation and immune dysfunction. Altogether the literature supports a pathogenic role for EBV modified EVs in autoimmune diseases.
Conclusions
Viruses have evolved with us and consequently many of the tactics that our bodies have employed to combat infection over the course of time have been met with effective counter adaptive strategies. It can be argued that EBV and other herpesviruses in particular, have been some of the most successful viruses in countering host defenses and exploiting cellular pathways. EVs are essential for the normal function of cells but unfortunately are also exploitable by viruses for the maintenance and spread of infection. Yet, EVs appear to be an opportunity for truly innovative therapeutic interventions that not only can target the viral infection but also the associated pathologies. EVs clearly have a role in the severity of many EBV induce pathologies but EV research may be the means by which we can improve early diagnosis and improve patient outcomes.
Acknowledgements:
We would like to thank Dingani Nkosi, Li Sun, and Monica Abou Harb for helpful discussions and review of this manuscript. This work was supported by a grant from the National Cancer Institute of the National Institutes of Health (R01CA204621) awarded to D.G.M.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest
Allaura S. Cone, Sara B. York, and David G. Meckes Jr. each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.de-The G, Day NE, Geser A, Lavoue MF, Ho JH, Simons MJ, et al. Sero-epidemiology of the Epstein-Barr virus: preliminary analysis of an international study - a review. IARC Sci Publ 1975(11 Pt 2):3–16. [PubMed] [Google Scholar]
- 2.Pattle SB, Farrell PJ. The role of Epstein-Barr virus in cancer. Expert Opin Biol Ther 2006;6(11):1193–205. [DOI] [PubMed] [Google Scholar]
- 3.Ko Y-H. EBV and human cancer. Experimental & molecular medicine 2015;47(1):e130-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen JI, Mocarski ES, Raab-Traub N, Corey L, Nabel GJ. The need and challenges for development of an Epstein-Barr virus vaccine. Vaccine 2013;31, Supplement 2(0):B194–B6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vranic S, Cyprian FS, Akhtar S, Al Moustafa AE. The Role of Epstein-Barr Virus in Cervical Cancer: A Brief Update. Front Oncol 2018;8:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al Moustafa AE, Al-Antary N, Aboulkassim T, Akil N, Batist G, Yasmeen A. Co-prevalence of Epstein-Barr virus and high-risk human papillomaviruses in Syrian women with breast cancer. Hum Vaccin Immunother 2016;12(7):1936–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdallah MOE, Algizouli UK, Suliman MA, Abdulrahman RA, Koko M, Fessahaye G, et al. EBV Associated Breast Cancer Whole Methylome Analysis Reveals Viral and Developmental Enriched Pathways. Front Oncol 2018;8:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan Y, Jakimovski D, Ramanathan M, Weinstock-Guttman B, Zivadinov R. The role of Epstein-Barr virus in multiple sclerosis: from molecular pathophysiology to in vivo imaging. Neural Regen Res 2019;14(3):373–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harley JB, Chen X, Pujato M, Miller D, Maddox A, Forney C, et al. Transcription factors operate across disease loci, with EBNA2 implicated in autoimmunity. Nat Genet 2018;50(5):699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meckes DG. Exosomal Communication Goes Viral. Journal of Virology 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalluri R The biology and function of exosomes in cancer. J Clin Invest 2016;126(4):1208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meckes DG, Raab-Traub N. Microvesicles and Viral Infection. Journal of Virology 2011;85(24):12844–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.N. Hurwitz S, Meckes D Extracellular Vesicle Biogenesis in Cancer2018 11–26 p. [Google Scholar]
- **14.Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proceedings of the National Academy of Sciences 2016;113(8):E968–E77. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes the purification and proteomic analysis of extracellular vesicle subtypes with identification of subtype specific markerss
- 15.Meckes DG, Shair KH, Marquitz AR, Kung CP, Edwards RH, Raab-Traub N. Human tumor virus utilizes exosomes for intercellular communication. Proc Natl Acad Sci U S A 2010;107(47):20370–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, et al. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci U S A 2010;107(14):6328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yogev O, Henderson S, Hayes MJ, Marelli SS, Ofir-Birin Y, Regev-Rudzki N, et al. Herpesviruses shape tumour microenvironment through exosomal transfer of viral microRNAs. PLoS Pathog 2017;13(8):e1006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McNamara RP, Chugh PE, Bailey A, Costantini LM, Ma Z, Bigi R, et al. Extracellular vesicles from Kaposi Sarcoma-associated herpesvirus lymphoma induce long-term endothelial cell reprogramming. PLoS Pathog 2019;15(2):e1007536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raab-Traub N, Dittmer DP. Viral effects on the content and function of extracellular vesicles. Nat Rev Microbiol 2017;15(9):559–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun L, Meckes DG. Methodological Approaches to Study Extracellular Vesicle miRNAs in Epstein–Barr Virus-Associated Cancers International Journal of Molecular Sciences 2018;19:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nanbo A, Kawanishi E, Yoshida R, Yoshiyama H. Exosomes derived from Epstein-Barr virus-infected cells are internalized via caveola-dependent endocytosis and promote phenotypic modulation in target cells. J Virol 2013;87(18):10334–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutzeit C, Nagy N, Gentile M, Lyberg K, Gumz J, Vallhov H, et al. Exosomes derived from Burkitt’s lymphoma cell lines induce proliferation, differentiation, and class-switch recombination in B cells. J Immunol 2014;192(12):5852–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaye KM, Izumi KM, Kieff E. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc Natl Acad Sci U S A 1993;90(19):9150–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shair KH, Schnegg CI, Raab-Traub N. EBV latent membrane protein 1 effects on plakoglobin, cell growth, and migration. Cancer Res 2008;68(17):6997–7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dawson CW, Tramountanis G, Eliopoulos AG, Young LS. Epstein-Barr virus latent membrane protein 1 (LMP1) activates the phosphatidylinositol 3-kinase/Akt pathway to promote cell survival and induce actin filament remodeling. J Biol Chem 2003;278(6):3694–704. [DOI] [PubMed] [Google Scholar]
- 26.Dawson CW, Laverick L, Morris MA, Tramoutanis G, Young LS. Epstein-Barr virus-encoded LMP1 regulates epithelial cell motility and invasion via the ERK-MAPK pathway. J Virol 2008;82(7):3654–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheu LF, Chen A, Wei YH, Ho KC, Cheng JY, Meng CL, et al. Epstein-Barr virus LMP1 modulates the malignant potential of gastric carcinoma cells involving apoptosis. Am J Pathol 1998;152(1):63–74. [PMC free article] [PubMed] [Google Scholar]
- *28.Hurwitz SN, Nkosi D, Conlon MM, York SB, Liu X, Tremblay DC, et al. CD63 Regulates Epstein-Barr Virus LMP1 Exosomal Packaging, Enhancement of Vesicle Production, and Noncanonical NF-κB Signaling. J Virol 2017;91(5). [DOI] [PMC free article] [PubMed] [Google Scholar]; This work assigns a new function for the tetraspanin protien CD63 in controlling LMP1 exosomal trafficking, intracellular signaling and LMP1-mediated enhancement in extracellular vesicle biogenesis.
- 29.Aga M, Bentz GL, Raffa S, Torrisi MR, Kondo S, Wakisaka N, et al. Exosomal HIF1α supports invasive potential of nasopharyngeal carcinoma-associated LMP1-positive exosomes. Oncogene 2014;33(37):4613–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dawson CW, Port RJ, Young LS. The role of the EBV-encoded latent membrane proteins LMP1 and LMP2 in the pathogenesis of nasopharyngeal carcinoma (NPC). Semin Cancer Biol 2012;22(2):144–53. [DOI] [PubMed] [Google Scholar]
- 31.Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. J Cell Sci 2008;121(Pt 6):727–35. [DOI] [PubMed] [Google Scholar]
- 32.Wasil LR, Shair KH. Epstein-Barr virus LMP1 induces focal adhesions and epithelial cell migration through effects on integrin-α5 and N-cadherin. Oncogenesis 2015;4:e171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wakisaka N, Kondo S, Yoshizaki T, Murono S, Furukawa M, Pagano JS. Epstein-Barr virus latent membrane protein 1 induces synthesis of hypoxia-inducible factor 1 alpha. Mol Cell Biol 2004;24(12):5223–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsai CY, Sakakibara S, Yasui T, Minamitani T, Okuzaki D, Kikutani H. Bystander inhibition of humoral immune responses by Epstein-Barr virus LMP1. Int Immunol 2018;30(12):579–90. [DOI] [PubMed] [Google Scholar]
- 35.Fang W, Zhang J, Hong S, Zhan J, Chen N, Qin T, et al. EBV-driven LMP1 and IFN-γ up-regulate PD-L1 in nasopharyngeal carcinoma: Implications for oncotargeted therapy. Oncotarget 2014;5(23):12189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **36.Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018;560(7718):382–6. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study uncovers novel mechism of tumor cell immune evasion through exosomal secretion of PD-L1.
- 37.Kobayashi E, Aga M, Kondo S, Whitehurst C, Yoshizaki T, Pagano JS, et al. C-Terminal Farnesylation of UCH-L1 Plays a Role in Transport of Epstein-Barr Virus Primary Oncoprotein LMP1 to Exosomes. mSphere 2018;3(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- *38.Nkosi D, Howell LA, Cheerathodi MR, Hurwitz SN, Tremblay DC, Liu X, et al. Transmembrane Domains Mediate Intra- and Extracellular Trafficking of Epstein-Barr Virus Latent Membrane Protein 1. J Virol 2018;92(17). [DOI] [PMC free article] [PubMed] [Google Scholar]; The work by Nkosi and colleagues assigns a novel function of the N-terminus and transmembranedomains of LMP1 in extracellular vesicle trafficking.
- **39.Hurwitz SN, Cheerathodi MR, Nkosi D, York SB, Meckes DG. Tetraspanin CD63 Bridges Autophagic and Endosomal Processes To Regulate Exosomal Secretion and Intracellular Signaling of Epstein-Barr Virus LMP1. J Virol 2018;92(5). [DOI] [PMC free article] [PubMed] [Google Scholar]; This report provides a link between endo-lysosomal and autophagy pathways that regulates intercellular signaling by LMP1 and is mediated by CD63.
- 40.Ikeda M, Longnecker R. Cholesterol is critical for Epstein-Barr virus latent membrane protein 2A trafficking and protein stability. Virology 2007;360(2):461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moody CA, Scott RS, Su T, Sixbey JW. Length of Epstein-Barr virus termini as a determinant of epithelial cell clonal emergence. J Virol 2003;77(15):8555–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scholle F, Bendt KM, Raab-Traub N. Epstein-Barr virus LMP2A transforms epithelial cells, inhibits cell differentiation, and activates Akt. J Virol 2000;74(22):10681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fukuda M, Longnecker R. Epstein-Barr virus latent membrane protein 2A mediates transformation through constitutive activation of the Ras/PI3-K/Akt Pathway. J Virol 2007;81(17):9299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morrison JA, Raab-Traub N. Roles of the ITAM and PY motifs of Epstein-Barr virus latent membrane protein 2A in the inhibition of epithelial cell differentiation and activation of {beta}-catenin signaling. J Virol 2005;79(4):2375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cen O, Longnecker R. Latent Membrane Protein 2 (LMP2). Curr Top Microbiol Immunol 2015;391:151–80. [DOI] [PubMed] [Google Scholar]
- 46.Si Y, Deng Z, Lan G, Du H, Wang Y, Si J, et al. The Safety and Immunological Effects of rAd5-EBV-LMP2 Vaccine in Nasopharyngeal Carcinoma Patients: A Phase I Clinical Trial and Two-Year Follow-Up. Chem Pharm Bull (Tokyo) 2016;64(8):1118–23. [DOI] [PubMed] [Google Scholar]
- 47.Ahmed W, Tariq S, Khan G. Tracking EBV-encoded RNAs (EBERs) from the nucleus to the excreted exosomes of B-lymphocytes. Sci Rep 2018;8(1):15438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeng Z, Fan S, Zhang X, Li S, Zhou M, Xiong W, et al. Epstein-Barr virus-encoded small RNA 1 (EBER-1) could predict good prognosis in nasopharyngeal carcinoma. Clin Transl Oncol 2016;18(2):206–11. [DOI] [PubMed] [Google Scholar]
- 49.Lee N, Yario TA, Gao JS, Steitz JA. EBV noncoding RNA EBER2 interacts with host RNA-binding proteins to regulate viral gene expression. Proc Natl Acad Sci U S A 2016;113(12):3221–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee Y, El Andaloussi S, Wood MJ. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet 2012;21(R1):R125–34. [DOI] [PubMed] [Google Scholar]
- 51.Nanbo A, Katano H, Kataoka M, Hoshina S, Sekizuka T, Kuroda M, et al. Infection of Epstein⁻Barr Virus in Type III Latency Modulates Biogenesis of Exosomes and the Expression Profile of Exosomal miRNAs in the Burkitt Lymphoma Mutu Cell Lines. Cancers (Basel) 2018;10(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choy EY, Siu KL, Kok KH, Lung RW, Tsang CM, To KF, et al. An Epstein-Barr virus-encoded microRNA targets PUMA to promote host cell survival. J Exp Med 2008;205(11):2551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cullen BR. Viral and cellular messenger RNA targets of viral microRNAs. Nature 2009;457(7228):421–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tagawa T, Albanese M, Bouvet M, Moosmann A, Mautner J, Heissmeyer V, et al. Epstein-Barr viral miRNAs inhibit antiviral CD4+ T cell responses targeting IL-12 and peptide processing. J Exp Med 2016;213(10):2065–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen SJ, Chen GH, Chen YH, Liu CY, Chang KP, Chang YS, et al. Characterization of Epstein-Barr virus miRNAome in nasopharyngeal carcinoma by deep sequencing. PLoS One 2010;5(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Imig J, Motsch N, Zhu JY, Barth S, Okoniewski M, Reineke T, et al. microRNA profiling in Epstein-Barr virus-associated B-cell lymphoma. Nucleic Acids Res 2011;39(5):1880–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim DN, Seo MK, Choi H, Kim SY, Shin HJ, Yoon AR, et al. Characterization of naturally Epstein-Barr virus-infected gastric carcinoma cell line YCCEL1. J Gen Virol 2013;94(Pt 3):497–506. [DOI] [PubMed] [Google Scholar]
- 58.Cai X, Schäfer A, Lu S, Bilello JP, Desrosiers RC, Edwards R, et al. Epstein-Barr virus microRNAs are evolutionarily conserved and differentially expressed. PLoS Pathog 2006;2(3):e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Skinner CM, Ivanov NS, Barr SA, Chen Y, Skalsky RL. An Epstein-Barr Virus MicroRNA Blocks Interleukin-1 (IL-1) Signaling by Targeting IL-1 Receptor 1. J Virol 2017;91(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramayanti O, Verkuijlen SAWM, Novianti P, Scheepbouwer C, Misovic B, Koppers-Lalic D, et al. Vesicle-bound EBV-BART13–3p miRNA in circulation distinguishes nasopharyngeal from other head and neck cancer and asymptomatic EBV-infections. Int J Cancer 2019;144(10):2555–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kang BW, Choi Y, Kwon OK, Lee SS, Chung HY, Yu W, et al. High level of viral microRNA-BART20–5p expression is associated with worse survival of patients with Epstein-Barr virus-associated gastric cancer. Oncotarget 2017;8(9):14988–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Motsch N, Alles J, Imig J, Zhu J, Barth S, Reineke T, et al. MicroRNA profiling of Epstein-Barr virus-associated NK/T-cell lymphomas by deep sequencing. PLoS One 2012;7(8):e42193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ambrosio MR, Navari M, Di Lisio L, Leon EA, Onnis A, Gazaneo S, et al. The Epstein Barr-encoded BART-6–3p microRNA affects regulation of cell growth and immuno response in Burkitt lymphoma. Infect Agent Cancer 2014;9:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hooykaas MJG, van Gent M, Soppe JA, Kruse E, Boer IGJ, van Leenen D, et al. EBV MicroRNA BART16 Suppresses Type I IFN Signaling. J Immunol 2017;198(10):4062–73. [DOI] [PubMed] [Google Scholar]
- 65.Wong AM, Kong KL, Tsang JW, Kwong DL, Guan XY. Profiling of Epstein-Barr virus-encoded microRNAs in nasopharyngeal carcinoma reveals potential biomarkers and oncomirs. Cancer 2012;118(3):698–710. [DOI] [PubMed] [Google Scholar]
- 66.Gallo A, Vella S, Miele M, Timoneri F, Di Bella M, Bosi S, et al. Global profiling of viral and cellular non-coding RNAs in Epstein-Barr virus-induced lymphoblastoid cell lines and released exosome cargos. Cancer Lett 2017;388:334–43. [DOI] [PubMed] [Google Scholar]
- 67.Li L, Chen W, Wang Y, Tang L, Han M. Long non-coding RNA H19 regulates viability and metastasis, and is upregulated in retinoblastoma. Oncol Lett 2018;15(6):8424–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen F, Chen J, Yang L, Liu J, Zhang X, Zhang Y, et al. Extracellular vesicle-packaged HIF-1alpha-stabilizing lncRNA from tumour-associated macrophages regulates aerobic glycolysis of breast cancer cells. Nat Cell Biol 2019;21(4):498–510. [DOI] [PubMed] [Google Scholar]
- 69.Canitano A, Venturi G, Borghi M, Ammendolia MG, Fais S. Exosomes released in vitro from Epstein-Barr virus (EBV)-infected cells contain EBV-encoded latent phase mRNAs. Cancer Lett 2013;337(2):193–9. [DOI] [PubMed] [Google Scholar]
- 70.Bannister N, Broggio J. Cancer survival by stage at diagnosis for England (experimental statistics): Adults diagnosed 2012, 2013 and 2014 and followed up to 2015 2016. [Google Scholar]
- 71.Neal RD, Tharmanathan P, France B, Din NU, Cotton S, Fallon-Ferguson J, et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer 1122015 p. S92–S107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen J, Liu T, Sun Q, Hu F. Clinical and prognostic analyses of 110 patients with N3 nasopharyngeal carcinoma. Medicine (Baltimore) 2018;97(49):e13483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Momen-Heravi F, Getting SJ, Moschos SA. Extracellular vesicles and their nucleic acids for biomarker discovery. Pharmacol Ther 2018;192:170–87. [DOI] [PubMed] [Google Scholar]
- 74.Fan H, Nicholls J, Chua D, Chan KH, Sham J, Lee S, et al. Laboratory markers of tumor burden in nasopharyngeal carcinoma: a comparison of viral load and serologic tests for Epstein-Barr virus. Int J Cancer 2004;112(6):1036–41. [DOI] [PubMed] [Google Scholar]
- 75.Tsai C-Y, Liu YY, Liu K-H, Hsu J-T, Chen T-C, Chiu C-T, et al. Comprehensive profiling of virus microRNAs of Epstein–Barr virus-associated gastric carcinoma: highlighting the interactions of ebv-Bart9 and host tumor cells. Journal of Gastroenterology and Hepatology 2017;32(1):82–91. [DOI] [PubMed] [Google Scholar]
- 76.Du Z-M, Hu L-F, Wang H-Y, Yan L-X, Zeng Y-X, Shao J-Y, et al. Upregulation of MiR-155 in Nasopharyngeal Carcinoma is Partly Driven by LMP1 and LMP2A and Downregulates a Negative Prognostic Marker JMJD1A. PLOS ONE 2011;6(4):e19137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.He Y, Jing Y, Wei F, Tang Y, Yang L, Luo J, et al. Long non-coding RNA PVT1 predicts poor prognosis and induces radioresistance by regulating DNA repair and cell apoptosis in nasopharyngeal carcinoma. Cell Death & Disease 2018;9(2):235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol 2008;110(1):13–21. [DOI] [PubMed] [Google Scholar]
- 79.Naz S, Wang M, Han Y, Hu B, Teng L, Zhou J, et al. Enzyme-responsive mesoporous silica nanoparticles for tumor cells and mitochondria multistage-targeted drug delivery. International Journal of Nanomedicine 2019;14:2533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jang SC, Gho YS. Could bioengineered exosome-mimetic nanovesicles be an efficient strategy for the delivery of chemotherapeutics? Nanomedicine 2014;9:177+. [DOI] [PubMed] [Google Scholar]
- 81.Kooijmans SA, Vader P, van Dommelen SM, van Solinge WW, Schiffelers RM. Exosome mimetics: a novel class of drug delivery systems. Int J Nanomedicine 72012 p. 1525–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Clayton A, Harris CL, Court J, Mason MD, Morgan BP. Antigen-presenting cell exosomes are protected from complement-mediated lysis by expression of CD55 and CD59. European Journal of Immunology 2003;33(2):522–31. [DOI] [PubMed] [Google Scholar]
- 83.Hartman ZC, Wei J, Glass OK, Guo H, Lei G, Yang XY, et al. Increasing vaccine potency through exosome antigen targeting. Vaccine 2011;29(50):9361–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zeelenberg IS, Ostrowski M, Krumeich S, Bobrie A, Jancic C, Boissonnas A, et al. Targeting tumor antigens to secreted membrane vesicles in vivo induces efficient antitumor immune responses. Cancer Res 2008;68(4):1228–35. [DOI] [PubMed] [Google Scholar]
- 85.Piffoux M, Nicolás-Boluda A, Mulens-Arias V, Richard S, Rahmi G, Gazeau F, et al. Extracellular vesicles for personalized medicine: The input of physically triggered production, loading and theranostic properties. Advanced Drug Delivery Reviews 2019;138:247–58. [DOI] [PubMed] [Google Scholar]
- 86.Yuan Z, Kolluri KK, Gowers KH, Janes SM. TRAIL delivery by MSC-derived extracellular vesicles is an effective anticancer therapy. J Extracell Vesicles 2017;6(1):1265291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 1995;3(6):673–82. [DOI] [PubMed] [Google Scholar]
- 88.Yin W, Ouyang S, Li Y, Xiao B, Yang H. Immature dendritic cell-derived exosomes: a promise subcellular vaccine for autoimmunity. Inflammation 2013;36(1):232–40. [DOI] [PubMed] [Google Scholar]
- 89.Kim SH, Bianco NR, Shufesky WJ, Morelli AE, Robbins PD. Effective treatment of inflammatory disease models with exosomes derived from dendritic cells genetically modified to express IL-4. J Immunol 2007;179(4):2242–9. [DOI] [PubMed] [Google Scholar]
- 90.Besse B, Charrier M, Lapierre V, Dansin E, Lantz O, Planchard D, et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology 2016;5(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med 2005;3(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rider MA, Hurwitz SN, Meckes DG Jr. ExtraPEG: A Polyethylene Glycol-Based Method for Enrichment of Extracellular Vesicles. Scientific Reports 2016;6:23978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kowal J, Tkach M, Théry C. Biogenesis and secretion of exosomes. Current Opinion in Cell Biology 2014;29:116–25. [DOI] [PubMed] [Google Scholar]
- 94.Fujiwara S, Takei M. Epstein-Barr virus and autoimmune diseases. Clinical and Experimental Neuroimmunology 2015;6(S1):38–48. [Google Scholar]
- 95.Afrasiabi A, Parnell GP, Fewings N, Schibeci SD, Basuki MA, Chandramohan R, et al. Evidence from genome wide association studies implicates reduced control of Epstein-Barr virus infection in multiple sclerosis susceptibility. Genome Medicine 2019;11(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pakpoor J, Disanto G, Gerber JE, Dobson R, Meier UC, Giovannoni G, et al. The risk of developing multiple sclerosis in individuals seronegative for Epstein-Barr virus: a meta-analysis: http://dxdoiorg/101177/1352458512449682. 2012. [DOI] [PubMed] [Google Scholar]
- 97.Harley JB, James JA. Epstein-Barr virus infection induces lupus autoimmunity. Bull NYU Hosp Jt Dis 2006;64(1–2):45–50. [PubMed] [Google Scholar]
- 98.Pohl D, Krone B, Rostasy K, Kahler E, Brunner E, Lehnert M, et al. High seroprevalence of Epstein–Barr virus in children with multiple sclerosis 2006. [DOI] [PubMed] [Google Scholar]
- 99.Billings PB, Hoch SO, White PJ, Carson DA, Vaughan JH. Antibodies to the Epstein-Barr virus nuclear antigen and to rheumatoid arthritis nuclear antigen identify the same polypeptide 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Balandraud N, Meynard JB, Auger I, Sovran H, Mugnier B, Reviron D, et al. Epstein-Barr virus load in the peripheral blood of patients with rheumatoid arthritis: Accurate quantification using real-time polymerase chain reaction. Arthritis & Rheumatism 2003;48(5):1223–8. [DOI] [PubMed] [Google Scholar]
- 101.Takei M, Mitamura K, Fujiwara S, Horie T, Ryu J, Osaka S, et al. Detection of Epstein-Barr virus-encoded small RNA 1 and latent membrane protein 1 in synovial lining cells from rheumatoid arthritis patients. International Immunology 1997;9(5):739–43. [DOI] [PubMed] [Google Scholar]
- 102.Masuoka S, Kusunoki N, Takamatsu R, Takahashi H, Tsuchiya K, Kawai S, et al. Epstein-Barr virus infection and variants of Epstein-Barr nuclear antigen-1 in synovial tissues of rheumatoid arthritis. PLoS ONE 2018;13(12):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chougule D, Nadkar M, Rajadhyaksha A, Pandit-Shende P, Surve P, Dawkar N, et al. Association of clinical and serological parameters of systemic lupus erythematosus patients with Epstein-Barr virus antibody profile. Journal of Medical Virology 2018;90(3):559–63. [DOI] [PubMed] [Google Scholar]
- 104.Ulff-Møller CJ, Nielsen NM, Rostgaard K, Hjalgrim H, Frisch M. Epstein–Barr virus-associated infectious mononucleosis and risk of systemic lupus erythematosus. Rheumatology 2010;49(9):1706–12. [DOI] [PubMed] [Google Scholar]
- 105.Lang HLE, Jacobsen H, Ikemizu S, Andersson C, Harlos K, Madsen L, et al. A functional and structural basis for TCR cross-reactivity in multiple sclerosis. Nature Immunology 2002;3(10):940. [DOI] [PubMed] [Google Scholar]
- 106.Warren KG, Catz I, Steinman L. Fine specificity of the antibody response to myelin basic protein in the central nervous system in multiple sclerosis: the minimal B-cell epitope and a model of its features 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fox R, Sportsman R, Rhodes G, Luka J, Pearson G, Vaughan J. Rheumatoid arthritis synovial membrane contains a 62,000-molecular-weight protein that shares an antigenic epitope with the Epstein-Barr virus-encoded associated nuclear antigen 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McClain MT, Heinlen LD, Dennis GJ, Roebuck J, Harley JB, James JA. Early events in lupus humoral autoimmunity suggest initiation through molecular mimicry. Nature Medicine 2005(1):85. [DOI] [PubMed] [Google Scholar]
- 109.Poole BD, Gross T, Maier S, Harley JB, James JA. Lupus-like autoantibody development in rabbits and mice after immunization with EBNA-1 fragments. Journal of Autoimmunity 2008;31(4):362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Clayton A, Court J, Navabi H, Adams M, Mason MD, Hobot JA, et al. Analysis of antigen presenting cell derived exosomes, based on immuno-magnetic isolation and flow cytometry. Journal of Immunological Methods 2001;247(1):163–74. [DOI] [PubMed] [Google Scholar]
- 111.Admyre C, Bohle B, Johansson SM, Focke-Tejkl M, Valenta R, Scheynius A, et al. B cell-derived exosomes can present allergen peptides and activate allergen-specific T cells to proliferate and produce TH2-like cytokines. J Allergy Clin Immunol 2007;120(6):1418–24. [DOI] [PubMed] [Google Scholar]
- 112.Vogt AB, Spindeldreher S, Kropshofer H. Clustering of MHC-peptide complexes prior to their engagement in the immunological synapse: lipid raft and tetraspan microdomains. Immunol Rev 2002;189:136–51. [DOI] [PubMed] [Google Scholar]
- 113.Turpin D, Truchetet ME, Faustin B, Augusto JF, Contin-Bordes C, Brisson A, et al. Role of extracellular vesicles in autoimmune diseases. Autoimmun Rev 2016;15(2):174–83. [DOI] [PubMed] [Google Scholar]
- 114.Xu S, Bai J, Zhuan Z, Li B, Zhang Z, Wu X, et al. EBVLMP1 is involved in vasculogenic mimicry formation via VEGFA/VEGFR1 signaling in nasopharyngeal carcinoma. Oncol Rep 2018;40(1):377–84. [DOI] [PubMed] [Google Scholar]


