Abstract
Risk ranking is a versatile tool used to prioritise activities performed by public health regulatory bodies. It also allows efficient communication between all stakeholders in the process of risk analysis. However, risk ranking methods are still not optimal. Because of the different approaches employed in the risk assessment of microbiological agents and chemicals, it is difficult to rank them together using the same metrics. In our work, we first discuss differences and commonalities between chemical and microbiological risk assessment to provide a starting point for consideration of a common risk ranking platform. In the second part, we perform risk ranking of contaminants and regulated chemicals using the recently developed Risk Thermometer tool. In this approach, chemicals are not ranked solely on the basis of the margin of exposure between a reference value and the exposure, but also by considering the severity of the critical health effects used. The results show that ranking using both methods provides different results from the use of either method alone. Overall, specific chemical groups (i.e. heavy metals, pesticides, etc.) do not generally rank higher or lower, but individual compounds are scattered in the rankings from low to high. Risk ranking methods demand further development to gain wide acceptability and recognition.
Keywords: risk ranking, chemical hazards, microbiological hazards, risk thermometer
1. Introduction
Public health institutions need to carefully optimise their workload and directions of activities owing to limited resources. In this context, risk ranking can help to set priorities. Ideally, ranking by risk assessors should be risk‐ and science‐based to provide risk managers with clear input and exclude subjectivity. It should also provide a clear scientific input to risk communication.
Recently, Van der Fels‐Klerx et al. (2018) reviewed risk ranking methods. Methods have been classified as quantitative, semi‐quantitative and qualitative. Quantitative risk ranking is preferable because semi‐quantitative and qualitative methods use different practices and metrics, and the results may not fully correlate with quantitative risk assessments. For chemical hazards, risk ratio, scoring, flow charts and risk matrices have been considered for ranking. Cost of illness (CoI), health‐adjusted life years (HALY) and expert judgements were most commonly applied to microbiological hazards. There is a clear difference in preferred methods used for chemical and microbiological risk ranking because chemical and microbiological risk assessments (CRA and MRA) are based on different principles. This may prevent chemical and microbiological risks from being effectively ranked using the same platform and metrics. Specifically, chemical risk is assessed if the estimated exposure is below the established health‐based guidance values (HBGV), while for microbiological risk, the probability or risk of disease (or similar) associated with the estimated exposure is assessed.
The use of a risk ratio and its variants has been the most commonly applied approach for risk assessment of chemicals, including ranking. By such ratios, the HBGVs, i.e. the tolerable daily intakes (TDI) for food contaminants and the acceptable daily intakes (ADI) for food additives, are compared with corresponding estimates of chemical exposure. The TDI and ADI are usually set by dividing a reference point (RP), derived from experimental data, by an overall adjustment factor (AF) of 100 to account for differences between animals and humans as well as differences in susceptibility within the human population. The (risk) ratio between the RP and the exposure is known as the margin of exposure (MOE). For compounds that are both genotoxic and carcinogenic, an MOE higher that 10,000 is generally regarded as of low concern (EFSA, 2005). For non‐genotoxic compounds, an MOE of > 100 would correspond to an exposure below the HBGV (provided that a default overall AF of 100 is applied in the establishment of the HBGV). The drawback with this type of method is that an extra safety margin specifically for the nature/severity of the critical effect is only applied in the case of genotoxic cancer risk. To address this issue, a tool called Risk Thermometer was developed by the Swedish National Food Agency (NFA; Sand et al., 2015). This method uses an extra AF that accounts for the severity of the critical health effect in a systematic manner, resulting in a modified risk ratio denoted as the severity‐adjusted margin of exposure (SAMOE).
This work has been performed within in the framework of the ‘Risk ranking of chemical and microbiological hazards in food’ project. Partners in the project are the NFA and the Finnish Food Safety Authority (EVIRA), and the project is supported by the European Food Safety Authority (EFSA).
2. Description of work programme
2.1. Aims
2.1.1.
Objective 1
Analysis of CRA and MRA methods and principles for ranking risks in each area. To identify differences and commonalities in methods, concepts and data requirements for CRA and MRA as a starting point for exploring the possibility of developing a common platform for risk ranking of microbiological and chemical risks.
Objective 2
Application of the Risk Thermometer tool to rank chemicals for which EFSA has made risk assessments, and for which the required data are available on toxicity and exposure at the European level. The purpose of this study is to investigate how a large group of chemicals (e.g. regulated products such as pesticides and food additives and other substances like heavy metals, dioxins, diverse halogenated molecules and mycotoxins) become distributed between the five risk grades of the Risk Thermometer scale.
2.2. Activities/methods
2.2.1.
Differences and commonalities between CRA and MRA
Literature searches were performed to identify and summarise important aspects of risk assessment and risk ranking methods for chemical and microbiological hazards. This included differences and similarities in terms of approaches (e.g. bottom–up, top–down, extrapolation methods), concepts (e.g. acute or chronic exposure), assumptions (including the use of assessment factors) and the necessary data requirements. Literature where simultaneous ranking of microbiological and chemical risks had been attempted was also identified along with the approaches used for those studies.
Risk ranking of chemicals using the Risk Thermometer tool
A case study was performed by applying the Risk Thermometer to chemicals for which EFSA has made risk assessments providing both toxicity and exposure data in the European Union (EU). A detailed description of the Risk Thermometer can be found in Sand et al. (2015). The required input parameters for this analysis are described below. Data on these parameters were mainly extracted from EFSA scientific opinions and reports.
Reference point (RP): RPs in terms of a benchmark dose (BMD), a no‐observed‐adverse‐effect level (NOAEL) or the lowest‐observed‐adverse‐effect level (LOAEL) were used. In cases where the BMD method had been applied, both the BMD and BMD lower level limit (BMDL) are preferably needed, and also the BMD upper level limit (BMDU), if available. The BMD was regarded as the geometrical mean of the BMDL and BMDU in cases when the BMDL and BMDU were provided, but not the BMD. If data on BMDL and BMDU were not available, a default 0.2 log uncertainty was applied to the BMD in both directions as a qualitative measure of uncertainty. When only the BMDL was available, a default 0.4 log uncertainty was applied as a qualitative measure of uncertainty.
Adjustment factors (AFs): Default AFs to account for intraspecies variability (3.16 for toxicokinetics and 3.16 for toxicodynamics) and interspecies variability (3.98 for toxicokinetics and 2.51 for toxicodynamics) were generally used when the RP was based on experimental data. Whenever available, chemical‐specific AFs were applied according to the specific EFSA risk assessments used as a basis.
The severity factor (SF): The severity of the critical effect was classified according to a health effect classification scheme developed by the NFA (Sand et al., 2015). Based on this classification, the value‐based SF was set by experts at NFA: the SF can adopt values of 1 (100), 3.16 (100.5), 10 (101), 31.6 (101.5) or 100 (102). A factor of 1 is given to ‘mild’ critical effects, like changes in non‐specific biomarkers, while a factor of 100 is ascribed to cancer or severe developmental effects, for example.
Exposure (E): The mean and 95th percentile (if available) of exposure for European adults were considered. Upper‐, middle‐ and lower‐boundary results, which describe the impact of how concentration values below the limit of detection/quantification are treated, were used as part of the uncertainty analysis. The middle‐boundary result was used as a point estimate, while the lower‐ and upper‐boundaries were allowed to describe uncertainty. The geometrical mean of the lower and upper boundary results was used as a point estimate in cases when the middle‐boundary result was not provided.
The Risk Thermometer is based on a severity‐adjusted margin of exposure (SAMOE) approach. The SAMOE depends on the parameters described above in bullets 1–4, such that SAMOE = RP⁄(AF × SF × E). Risk ranking is performed based on the SAMOE value, which is presented as a point estimate with a 90% confidence interval describing uncertainty. At the level of the Risk Thermometer, the SAMOE is classified into one of five risk classes corresponding to different levels of population health concerns (Sand et al., 2015).
As a comparative starting point in these analyses, a more traditional risk ratio was calculated: the ratio between the HBGV and the mean exposure. This analysis was limited to chemicals with established HBGVs.
A user interface for the Risk Thermometer tool has been developed in Matlab (MathWorks, Inc., USA). Results have been further evaluated using Excel® (Microsoft Corp. USA).
3. Conclusions
3.1. Differences and commonalities between CRA and MRA
This work is still in draft form and the main conclusions are not yet formulated since the analysis is ongoing. The information presented here is therefore to be considered provisional.
Risk assessment in combination with risk management and risk communication constitute the core of risk analysis. Risk assessment is science‐based, with a clear separation between hazard and risk in both MRA and CRA. CRA and MRA follow the same basic four‐step procedure of hazard identification, hazard characterisation, exposure assessment and risk characterisation.
Acute vs lifetime hazards
Since chemical and microbiological hazards substantially differ in their characteristics, persistence, survivability and adverse health effects, differences have emerged between CRA and MRA. For chemicals, the potential risks associated with low‐level exposure over a long time period are usually of concern. In contrast, with supporting clinical data and case studies, it is easier to diagnose microbiological hazards causing an acute illness and to establish a link to the food chain. Microbes also do not build up in the body as many chemicals do (e.g. cadmium, dioxins). Exposure to specific microbes leads to a certain degree of immunity, decreasing or preventing the risk of re‐infection.
Exposure assessment
Microbes can multiply in foods depending on food handling, storage and processing. Exposure assessment in MRA, therefore, requires a multistep analysis to provide an estimate of microbial contamination at the consumption level. However, some toxins secreted by microorganisms behave similarly to chemicals that are stable upon storage and treatment.
Threshold vs non‐threshold
With the notable exception of genotoxic carcinogens considered to have a non‐threshold dose–response at low doses, most chemicals are generally considered to follow threshold dose–response models. Microbial dose–response modelling in MRA generally uses non‐threshold models that may include host, pathogen and epidemiologic parameters.
Risk assessment vs safety assessment
CRA and MRA differ in what determines their fitness for purpose. When exposure assessment in CRA is below the HBGV, or the MOE > 10,000 in the case of genotoxic compounds, the risk of adverse effects is considered to be of low concern. The approach in MRA provides the probability of illness as a consequence of exposure. Overall, safety assessment in CRA and risk assessment in MRA are based on problems related to the consequences of exposure, which are mainly chronic and acute, respectively.
Variability and uncertainties
There is an array of variability and uncertainty sources in CRA and MRA. In CRA, variability within the human population and uncertainties in route‐to‐route extrapolation, duration of exposure in experimental animal studies, the dose–response curve, the nature and severity of the effects, extrapolation from animal species to humans, and concentrations of chemicals in commodities below the limit of detection or quantification are the main issues. In MRA, the main sources of variability include differences within the human population as well as the genetic variability of microbial strains. Additional sources of uncertainty include events along and within the food chain that predictive microbiology models are often used to describe. In both CRA and MRA, consumption data depend on dietary survey methods. In MRA, separate treatment of variability and uncertainty has become a common practice through the development of two‐dimensional models and stochastic modelling. In CRA, uncertainty and variability are described at the level of exposure, whereas HBGVs are fixed upon application of uncertainty and variability factors (even though uncertainty may be addressed at the level of the RP derivation).
Exposure sources
CRA calculates a cumulative risk for a specific compound found in different foods, since the occurrence of chemicals is generally not limited to a single food. In contrast, MRA can be for one food and one pathogen, one food and several pathogens, or many foods or a group of similar foods and one pathogen, or many foods and many pathogens. Pathogen survival depends on physical and processing parameters for the food across the farm‐to‐fork supply chain, requiring a separate calculation for each specific food. However, cumulative risks to several foods can also be calculated in an MRA.
3.2. Risk ranking of chemicals using the Risk Thermometer tool
Based on EFSA reports, we extracted data on toxicity and exposure for > 40 food chemical contaminants and > 25 regulated substances (pesticides, sweeteners, food colour additives).
Chemicals were first ranked in line with a traditional risk ratio method by dividing HBGVs by the corresponding (mean) exposure estimated for adults in the EU (Figure 1). Heavy metals (nickel, cadmium) rank the highest in risk (considering that the risk ratio indirectly relates to response/effect and risk), while aluminium, copper, organic and inorganic mercury rank lower. From all EFSA‐evaluated mycotoxins with HBGVs, the T‐2 + HT‐2 group ranks the highest. Dithiocarbamates rank the highest from all pesticides considered, which may be because they are commonly used phytopharmaceutical substances. Dioxin, like polychlorinated biphenyls (PCBs) and nitrates, also rank high, next to nickel and cadmium. Glyphosate, despite recently drawing widespread public attention, ranks very low among all pesticides. Food colour additives (E 102, E 104, E 124) likewise rank much lower than the heavy metals.
Figure 1.
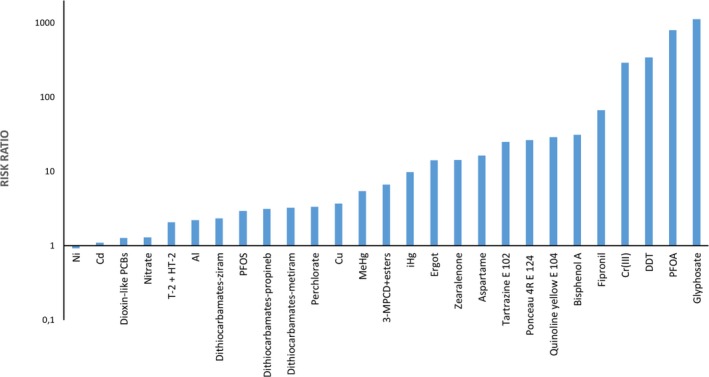
Risk ranking of selected contaminants and regulated substances using the risk ratio, HBGV:exposure. Mean European exposure for adults was applied in the calculations
In the next step, risk ranking of selected chemicals was performed using the Risk Thermometer (Figure 2). As described earlier, this tool uses an extra assessment factor (the SF) that depends on the critical health effect used for each chemical. Based on their SAMOE value, chemicals are classified into five classes.
Figure 2.
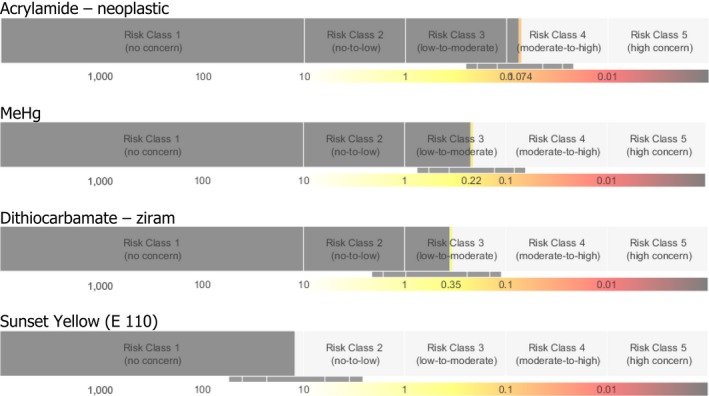
Risk ranking of selected contaminants and regulated substances using the Risk Thermometer. The grey bars describe the estimate (point estimate and 90% confidence interval) of the severity‐adjusted margin of exposure (SAMOE) to the compound that places it in a particular Risk Class that is associated with a described level of health concern (Sand et al., 2015). Calculations are based on the European mean exposure
The results in Figure 2 show that ranking of chemicals by the Risk Thermometer differs from the ranking obtained by the traditional risk ratio method. The genotoxic compound acrylamide ranks higher than methylmercury and the dithiocarbamate pesticide ziram. Since neurodevelopmental disorders and less‐severe organ‐specific changes are the critical effects of methylmercury and ziram, respectively, they are ranked differently compared with the results obtained by the risk ratio method. Glyphosate and the food colour Sunset Yellow (E 110) rank significantly lower, which is also observed in with ranking based on risk ratio.
Ranking of chemicals using a traditional risk ratio method has several drawbacks. For example, some non‐genotoxic compounds do not have a TDI/ADI for diverse reasons. Also, genotoxic compounds do not have HBGVs; safety is assessed by MOE. Upon consideration of an additional uncertainty factor of 100 (that accounts for the process of carcinogenicity), genotoxic compounds with MOEs > 10,000 are regarded as safe or to be of low concern (EFSA 2005). These two groups of compounds cannot be ranked with other compounds by the classical risk ratio ranking approach. The approach used in the Risk Thermometer allows calculation of SAMOE for all compounds, with or without set HBGVs, including genotoxic compounds. Besides accounting for the severity of effect, uncertainty is also addressed by the SAMOE/Risk Thermometer in contrast with traditional methods. The Risk Thermometer can therefore be regarded as an upgrade of risk ratio methods, providing more realistic results for a decision‐making process.
Abbreviations
- ADI
allowed daily intake
- BMD
benchmark dose level
- BMDL
benchmark dose lower level limit
- BMDU
benchmark dose upper level limit
- CoI
cost of illness
- CRA
chemical risk assessment
- EVIRA
Finnish Food Safety Authority
- HALY
health‐adjusted life years
- HBGV
health‐based guidance values
- LOAEL
lowest‐observed‐adverse‐effect level
- MOE
margin of exposure
- MRA
microbiological risk assessment
- NFA
Swedish National Food Agency
- NOAEL
no‐observed‐adverse‐effect level
- PCB
polychlorinated biphenyl
- RP
reference point
- SAMOE
severity‐adjusted margin of exposure
- SF
severity factor
- TDI
tolerable daily intake
Suggested citation: Swedish National Food Agency (NFA), Uppsala, Sweden , Langerholc T, Lindqvist R and Sand S, 2018. Risk ranking of chemical and microbiological hazards in food. EFSA Journal 2018;16(S1):e160813, 9 pp. 10.2903/j.efsa.2018.e160813
Acknowledgements: This report is funded by EFSA as part of the EU‐FORA programme.
Approved: 6 July 2018
References
- EFSA (European Food Safety Authority), 2005. Opinion of the Scientific Committee on a request from EFSA related to A Harmonised Approach for Risk Assessment of Substances Which are both Genotoxic and Carcinogenic. EFSA Journal 2005;3(10):282, 31 pp. 10.2903/j.efsa.2005.282 [DOI] [Google Scholar]
- Sand S, Bjerselius R, Busk L, Eneroth H, Färnstrand JS and Lindqvist R, 2015. The Risk Thermometer ‐ a tool for risk comparison. Swedish National Food Agency, 2015, 59 pp. Available online: https://www.livsmedelsverket.se/globalassets/rapporter/2015/the-risk-thermometer.pdf
- Van der Fels‐Klerx HJ, Van Asselt ED, Raley M, Poulsen M, Korsgaard H, Bredsdorff L, Nauta M, D'agostino M, Coles D, Marvin HJP, Frewer LJ, 2018. Critical review of methods for risk ranking of food‐related hazards, based on risks for human health. Critical Reviews in Food Science and Nutrition, 58, 178–193. 10.1080/10408398.2016.1141165 [DOI] [PubMed] [Google Scholar]


