Abstract
EFSA regards the household as a stage in the food chain that is important for the final number of food‐borne infections. The fate of a pathogen in the private kitchen largely depends on consumer hygiene during preparation of food and on its proper cooking, especially in the case of meat. Unfortunately, detailed information on the microbiological survival in meat products after heating in the consumer kitchen is lacking. The aim of the study was to improve the estimation of the inactivating effect on pathogens by heating meat or a meat product by the consumer in the kitchen. On that account, artificially contaminated meat and meat products were cooked according to several degrees of doneness and simulating real world conditions, and bacterial survival was measured. Heat camera pictures and button temperature loggers inserted into the food matrix served to record time and the temperature of heating. Temperature, time and the microbial survival ratio observed served to inform a mathematical model able to explain the thermal inactivation of meat or a meat product in home settings. The results of the study would help to improve microbiological comparative exposure assessments of pathogens in food, as an attribution tool and as a supportive tool for risk‐based sampling in monitoring and surveillance.
Keywords: QMRA, Exposure assessment, D/z model, home preparation, meat, heating, inactivation
1. Introduction
Quantitative microbiological risk assessment (QMRA) is a methodology that combines information, data and mathematical models to evaluate food‐, direct contact‐ and environmental‐related microbiological public health risks (Evers et al., 2008). In the case of food, QMRAs rely on mathematical models to describe the sequential steps of a pathogen along the food chain, embracing the food animals on the farm, transport, slaughter, processing, retail and preparation by the consumer in the households. The exposure of a population to a microbiological hazard is a key step of QMRA. The estimated exposure of consumers to a pathogen, coupled with a dose–response relationship, enables an approximation to be made of the number of human cases attributable to the pathogen occurring in the population of interest (Chardon and Evers, 2017). The assessment of exposure to microbiological agents requires, as a basic input, data such as the number of portions of products eaten by the consumers (food consumption data), the prevalence of contaminated portions and the concentration of the microorganism in the portion. Nevertheless, the fate of a pathogen in food and, in turn, the level of human exposure, is associated with consumer behaviour in the kitchen. The European Food Safety Authority (EFSA) estimates that the largest proportion of food‐borne outbreaks that occur in the European Union originate in the household, with meat and meat products the type of food most frequently implicated (EFSA, 2016). Notably, information on the extent of microbiological survival in meat products after heating in the consumer's kitchen is lacking. Many studies have simulated the heat inactivation of bacterial cultures grown in liquid media (De Jesús and Whiting, 2003; Gabriel, 2012; Smelt and Brul, 2014; Adhikari et al., 2016; Haberbeck et al., 2017). The results of these studies have been used to predict the thermal inactivation of the bacteria present in meat. Correct estimation of this inactivating effect in a real‐world situation (a consumer preparing food in the kitchen) would serve to decrease the uncertainty in exposure assessment calculations. The hypothesis behind our study is that the bacterial survival in meat after heating is higher than in microbiological culture media. Therefore, we performed experiments to quantify the degree of thermal inactivation of bacteria in meat and meat preparations. By recording the time and the temperature to which food was exposed, we wanted to ascertain whether the resulting bacterial inactivation could be explained by a D/z model (Smelt and Brul, 2014) with, only when necessary, the inclusion of additional explanatory variables.
2. Description of work programme
The work programme was carried out at the Centre for Zoonoses and Environmental Microbiology (Z&O) at RIVM, the Netherlands, which has extensive and long experience of performing QMRAs for pathogens in food, water and the environment. The work programme was integrated into a wider project on microbiological comparative exposure assessment of pathogens in food, as an attribution tool and as a supportive tool for risk‐based sampling in monitoring and surveillance. The activities proposed were aimed at improving the estimation of the inactivating effect on pathogens by the heating of meat or a meat product by the consumer in the kitchen.
2.1. Aims
The activities of the work programme were aimed at:
obtaining survival ratios for the domestic preparation of meat or meat products to then be used in a QMRA;
developing and characterising a thermal inactivation model explaining bacterial heat survival in meat prepared at home.
2.2. Activities/methods
The work programme used the results of some realistic experiments using meat and meat preparations artificially contaminated with Escherichia coli, which served as model bacterium, while monitoring the temperature with a heat camera and button data loggers inserted in the food matrix. Beefsteaks, plain beef and mixed (50% beef and 50% pork) meat preparations (meatball, hamburger and meat crumble) were spiked with a known amount of E. coli and then fried simulating home conditions until meeting various levels of readiness preferred by consumers. The temperature of the food during frying was measured and recorded by taking pictures with a heat camera (Figure 1) and, in the case of hamburger and meatballs, also by button loggers embedded in the food matrix (Figure 2). The extent of survival together with the recorded temperature and duration of frying served to build a D/z model explaining the survival observed in the different food types based of the frying style applied.
Figure 1.
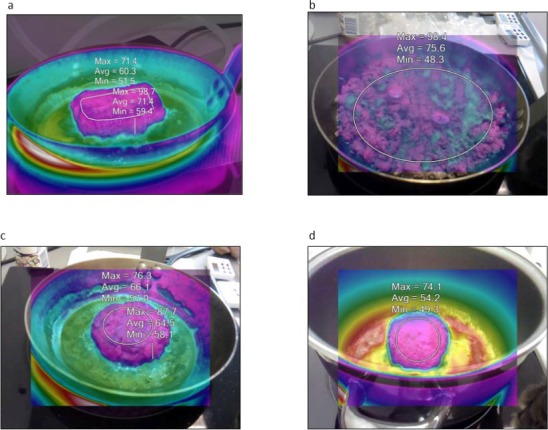
Infrared pictures with temperature measurements of the beefsteak, crumbs, hamburger and meatball (Figure 1a–d)
Figure 2.
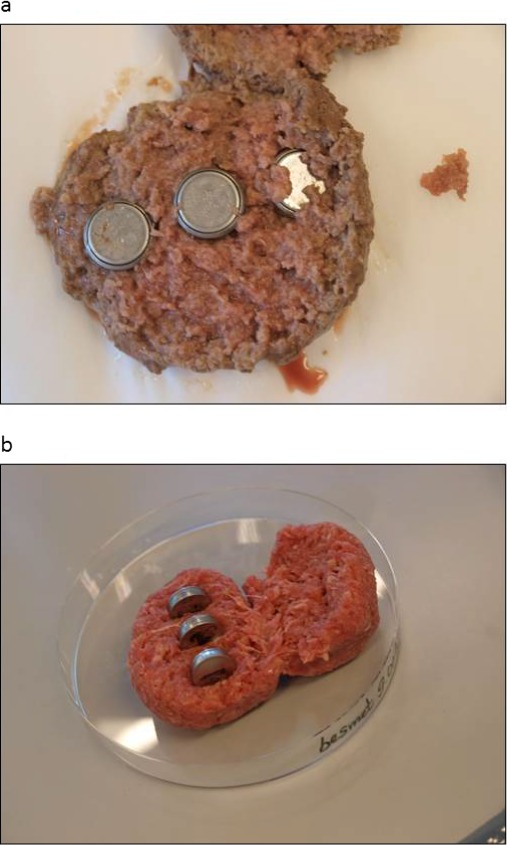
Temperature log buttons inserted in the medium fried pork–beef hamburger and meatball (Figure 2a and b)
The first activity of the programme was a thorough statistical analysis of the inactivation resulting from the heating. We used a Bayesian approach to interpret the results of plate counts or of a combination of plate counts and presence/absence after enrichment.
In the second step of the programme, we took advantage of the inactivation observed in the experiments and the matrix of time–temperature–location (on or in the meat) data to estimate the parameter values of the heating (D/z) model. The result of this step was the generation of a set of Dref and Z values compatible with the survival ratio observed in the experiments. This activity is still ongoing (at the time of writing) and is expected to be finished by the end of May 2018.
The final step of the work programme foresees an in‐depth study of the current available scientific articles on the bacterial survival after heating in laboratory liquid media or in or on meat. The ultimate aim is to develop a theory and possibly an explicit mathematical model which can explain and describe the inactivation results obtained by these studies. Again, this task is expected to be accomplished in June 2018.
The main of output of the work programme will be the preparation of a manuscript describing a model explaining the thermal inactivation of bacteria occurring during meat preparation at home. In order to reach the widest range of possible readers and give the results of the work programme the greatest visibility, the manuscript will be submitted to a journal dealing with microbial food safety rather than one dealing with mathematical modelling and risk analysis.
3. Conclusions
The experiments performed, of which the analyses have yet to be finalised, revealed that the residual bacteria found after frying the beefsteak will almost all originate from the side. This result has its explanation in the higher bacterial contamination present on the side and the lower temperature values this part of the beefsteak experiences during heating when compared to the top and bottom surfaces. The number of microorganisms found in the hamburgers after frying declined with the length of the thermal treatment. The composition of the hamburger, either beef or a blend of beef and pork, seems to have no influence on the number of bacteria surviving the heating process. Similarly, in the case of meatballs, no difference in the number of microorganisms was found between meatballs made of plain beef or a blend of beef and pork when the same level of readiness was compared. Interestingly, variability of survival was observed, probably connected to the shape of the meat balls which results in an uneven temperature distribution during the frying process.
The work programme proposed made the fellow acquainted with the best‐suited statistical methods to describe the uncertainty associated with microbiological data. This process was also coupled with learning how to use @RISK, one of the software packages often used as a risk analysis tool. In addition, the fellow learned how to combine temperature and time data to explain thermal inactivation of microorganisms by means of a D/z model. Overall, the proposed work programme was in line with the background and expertise of the fellow. Furthermore, he joined and actively participated in the meetings and seminars organised by the Z&O Centre throughout the year. No issue has hampered or slowed the implementation of the agreed work programme, given the constant and effective guidance offered by the supervisor and his collaborators. The programme enables the fellow to return to his home institution with the expertise to build QMRAs able to answer relevant microbiological risk questions. Both the fellow and the supervisor agree that the EU‐FORA programme provides a valuable opportunity to exchange opinions and methodologies on relevant public health issues and to build a professional and personal network that will serve as the basis for future cooperation.
Abbreviations
- RIVM
Rijksinstituut voor Volksgezondheid en Milieu
- QMRA
quantitative microbiological risk assessment
- Z&O
Centre for Zoonoses and Environmental Microbiology
Suggested citation: National Institute for Public Health and the Environment (RIVM), the Netherlands , Pesciaroli M, Chardon JE and Evers EG, 2018. Modelling of inactivation through heating for quantitative microbiological risk assessment (QMRA). EFSA Journal 2018;16(S1):e16089, 8 pp. 10.2903/j.efsa.2018.e16089
Acknowledgements: This report is funded by EFSA as part of the EU‐FORA programme.
Approved: 6 July 2018
References
- Adhikari A, Bary A, Cogger C, James C, Ünlü G and Killinger K, 2016. Thermal and starvation stress response of Escherichia coli O157:H7 isolates selected from agricultural environments. Journal of Food Protection, 79, 1673–1679. [DOI] [PubMed] [Google Scholar]
- Chardon JE and Evers EG, 2017. Improved swift Quantitative Microbiological Risk Assessment (sQMRA) methodology. Food Control, 73, 1285–1297. [Google Scholar]
- De Jesús AJ and Whiting RC, 2003. Thermal inactivation, growth, and survival studies of Listeria monocytogenes strains belonging to three distinct genotypic lineages. Journal of Food Protection, 66, 1611–1617. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2016. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2015. EFSA Journal 2016;14(12):4634, 231 pp. 10.2903/j.efsa.2016.4634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers EG, Klerx HJVDF, Nauta MJ, Schijven JF, Havelaar AH, 2008. Campylobacter source attribution by exposure assessment. International Journal of Risk Assessment and Management, 8, 174. [Google Scholar]
- Gabriel AA, 2012. Influences of heating temperature, pH, and soluble solids on the decimal reduction times of acid‐adapted and non‐adapted Escherichia coli O157:H7 (HCIPH 96055) in a defined liquid heating medium. International Journal of Food Microbiology, 160, 50–57. [DOI] [PubMed] [Google Scholar]
- Haberbeck LU, Wang X, Michiels C, Devlieghere F, Uyttendaele M and Geeraerd AH, 2017. Cross‐protection between controlled acid‐adaptation and thermal inactivation for 48 Escherichia coli strains. International Journal of Food Microbiology, 241, 206–214. [DOI] [PubMed] [Google Scholar]
- Smelt JPPM and Brul S, 2014. Thermal Inactivation of Microorganisms. Critical Reviews in Food Science and Nutrition, 54, 1371–1385. [DOI] [PubMed] [Google Scholar]


