Abstract
Endocrine disruptors (EDs) are exogenous compounds that interfere with the hormone system, affecting human health and environment. Specific legislative obligations have been introduced in the European Union (EU) to gradually eliminate EDs in water, industrial chemicals and pesticides. However, identification of EDs is the first and essential step towards regulation and appropriate risk management. Scientific criteria and guidance for ED assessment have recently been established for pesticides in the EU. In this project, the ED properties of the non‐pesticide chemical Bisphenol AF (BPAF), analogue and potential substitute of Bisphenol A were evaluated by the application of the EU criteria and guidance in the frame of human health risk assessment. A data dossier was built by a systematic literature review (WOS, Scopus, Pubmed, Embase), title/abstract screening (RAYYAN) and full‐text examination. All relevant information was extracted and systematically reported, and reliability and relevance of data were assessed (SciRAP). Data were synthesised into lines of evidence for (i) endocrine activity, (ii) adversity and (iii) general toxicity, and weight of evidence evaluation was applied. The initial analysis of the evidence showed potential endocrine adverse effects and endocrine activity, meeting the ED criteria and leading the assessment to the mode of action (MoA) analysis. The biological plausibility of the link between the adverse effects and the endocrine activity was investigated based on current scientific knowledge. Empirical support for dose–response and temporal concordance was evaluated, and the key events were assessed in terms of essentiality, consistency, analogy and specificity. Finally, an overall conclusion of the ED properties of BPAF was drawn. The EU criteria and guidance for EDs assessment were successfully applied to BPAF demonstrating its endocrine activity and adversity based on weight of evidence methodology and MoA analysis. The Fellow greatly increased her knowledge and hands‐on experience on ED assessment in the EU regulatory context contributing to implement transparency and structure in health risk assessment.
Keywords: bisphenol AF, endocrine disruptor, risk assessment
1. Introduction
The hormone system has an essential role in the regulation of many physiological functions such as body development, growth, reproduction, metabolism, immunity, inflammation and behaviour (Chrousos, 2007). Endocrine disruptors (EDs) are exogenous compounds that interfere with any aspect of endogenous hormone system, including hormones production, release, transport, metabolism, binding, action or elimination, negatively affecting human health (Lee, 2018; Pouzaud et al., 2018). They represent a special and challenging form of toxicity as their effects depend on both the level and timing of exposure, being especially critical in developmental stages (WHO/UNEP, 2012).
EDs are highly heterogeneous chemicals – including pesticides, fungicides, plastics, plasticisers and heavy metals – with diverse applications at industrial, agricultural, pharmaceutical and cosmetic level, which result in contaminant residues in food and other consumer products leading to human exposure to ED mixtures (Schug et al., 2016).
Scientific understanding of the health impacts of ED substances has been growing in recent years and progressively raised awareness of ED‐related concerns (Gore et al., 2015). The European Commission initiated in 1999 a strategy to develop a legislative framework on EDs pursuing the harmonisation of hazard‐based criteria for EDs identification. The European Commission's Endocrine Disrupters Expert Advisory group described the three elements required to identify an ED, in line with the World Health Organization definition (WHO, 2002). Accordingly, an ED substance has to show (i) endocrine adverse health effects in individuals and/or their offspring, (ii) endocrine activity through an endocrine mode of action (MoA) and (iii) a plausible and clear‐established link between the adverse effects and the endocrine MoA (Munn and Goumenou, 2013). However, to demonstrate that a given substance is an ED represents a huge challenge due to the complex and critical roles of the endocrine system in maintaining the homeostasis of all biological processes, as well as the multiple pathways and mechanisms involved (Beausoleil et al., 2018). Scientific criteria to identify substances with ED properties have been recently implemented in plant protection products (PPP) regulation (European Union, 2009), and biocidal products (BP) regulation (European Union, 2012) applying from June 2018 and November 2018, respectively. The European Commission entrusted the European Food Safety Authority (EFSA) and the European Chemicals Agency (ECHA) to develop a guidance document for the implementation of ED criteria pursuant to the PPP and BP regulations. The recent published ECHA/EFSA guidance intends to reduce subjectivity and conflicting procedures for determining ED properties by guiding applicants and assessors of the competent regulatory authorities, contributing to the harmonisation between industry, authorities and academia with regard to ED toxicity assessment (ECHA/EFSA, 2018).
2. Description of work programme
The work programme was based on the application of the EU criteria and ECHA/EFSA guidance for ED identification to a non‐pesticide compound in the frame of health risk assessment. Although the ED criteria cover all ED effects, the guidance mainly addresses EATS (estrogen, androgen, thyroid, steroidogenesis) modalities due to their relatively good mechanistic understanding and the availability of standardised in vivo and in vitro test guidelines with broad scientific agreement.
2.1. Aims
The aim of the project was (i) to identify strengths and specific challenges in the process described in the ECHA/EFSA guidance for ED assessment, and (ii) to explore its application on a non‐pesticide model substance, Bisphenol AF (BPAF), by evaluating the ED properties for human health according to the EU scientific criteria.
2.2. Activities and methods
2.2.1. Selection of the model substance
Selecting the model substance was the very first and extremely important step of the project. The ideal model substance should have been extensively investigated with enough scientific data to allow the whole ED evaluation; however, due to personnel and time resources, data amount should also be handling and not extremely large to be completely analysed by the fellow during the EU‐FORA programme.
Under the European regulation of chemicals REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals), substances having ED properties have to be identified as Substances of Very High Concern (SVHC) as first step towards regulation and appropriate risk management. The use of SVHC is controlled by temporary authorisations conditioning its uses and strongly encouraging its substitution (European Union, 2006; Beausoleil et al., 2018). Although REACH regulation does not provide any specific ED criteria, the European Commission last communication on EDs claimed the development of a horizontal approach for ED identification across EU legislation built on the pesticides criteria (EC Communication, 2018).
With the objective of applying the ED scientific criteria on a REACH chemical, information sources were consulted including the SVHC list (https://echa.europa.eu/candidate-list-table), SIN (Substitute It Now) list (https://chemsec.org/sin-list) and REACH chemicals regulation (European Union, 2006) collecting data on several compounds about their registration status (candidate list, authorisation list, restriction list, CoRAP list, full registration, intermediate registration, notification of new substance), production amounts (tonnes/year), hazard category classification and reason for inclusion in the SVHC or SIN list when it applies. At this point, compound search was framed on phthalates and bisphenols after analysing systematic reviews (Rochester and Bolden, 2016; Skledar and Masic, 2016; NTP 2017) that highlighted the wide use of these substances despite the lack of comprehensive knowledge on their ED properties. In order to increase the value of the work and provide useful information to both the scientific community and competent authorities, we informed the Swedish Chemicals Agency (KemI) about the ongoing project asking their interest and opinion with regard to the regulatory relevance of our final substance candidates: Bisphenol AF, Bisphenol B, Bisphenol F and Bisphenol S. Finally, according to our preliminary data search and the KemI suggestion, BPAF (Figure 1) was selected as the model substance for the ED assessment. The cooperation with KemI is an example of successful communication between academia and national authorities increasing work efficiency and ensuring regulatory relevance by sharing knowledge and expertise in risk assessment. The project workflow for the further steps is shown in Figure 2.
Figure 1.
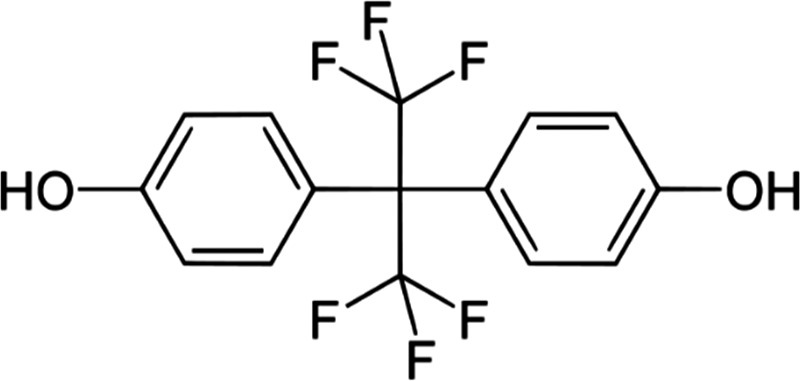
Bisphenol AF (BPAF) chemical structure. IUPAC name: 4‐[1,1,1,3,3,3‐hexafluoro‐2‐(4‐hydroxyphenyl)propan‐2‐yl]phenol; CAS number: 1478‐61‐1
Figure 2.
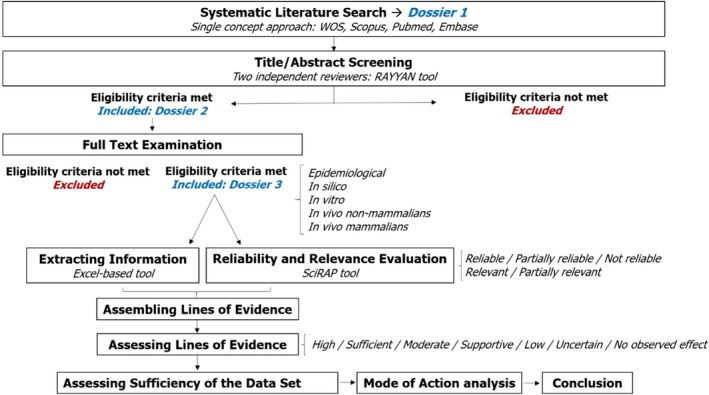
Workflow for Bisphenol AF assessment based on the ECHA/EFSA guidance
2.2.2. Systematic literature search
Data for assessing potential ED properties of BPAF was gathered by a structured literature review based on the principles of systematic review methodology in the electronic databases WOS, Pubmed, Scopus and Embase. Information experts from Karolinska Institutet library service were consulted at this point to guarantee that the methodology that fits best the search purpose was applied. A single concept approach by using several search terms (CAS number, IUPAC name and chemical name synonyms) was applied as a first step, according to the ECHA/EFSA guidance. Since the number of hits retrieved by the single concept search was not excessively large, additional search refinement was not required for further screening steps. The individual studies from all databases were transferred into the electronic reference management software EndNote (http://kib.ki.se) where reference duplicates were removed to obtain the preliminary dossier (dossier 1).
2.2.3. Screening and selection of the studies
Two steps were applied for the studies selection from the preliminary dossier: (i) screening by title and abstract, and (ii) full‐text examination (Figure 2). Title and abstract screening was independently performed by two reviewers at different institutions and countries (RIVM – National Institute for Public Health and the Environment, The Netherlands; KI – Karolinska Institutitet, Sweden) by RAYYAN tool (https://rayyan.qcri.org/). The included and excluded studies were critically identified after defining the problem formulation (scope, scientific needs/objectives and feasibility), the PECO (population, exposure, comparator and outcome) statements and the eligibility (inclusion/exclusion) criteria, according the EFSA systematic review methodology (EFSA 2010).
Studies meeting the eligibility criteria were kept for next screening step. Studies clearly not relevant to the problem formulation or meeting the exclusion criteria were excluded. When exclusion could not be made based on the title/abstract, studies were kept for subsequent full‐text examination performed by the fellow. Studies in conflict between the two reviewers were resolved by discussion and the final included studies formed the title and abstract dossier (dossier 2). It should be noted that cooperation between institutions (RIVM and KI) was essential at this step, providing the fellow experience in networking and external scientific collaboration.
A deep examination at full‐text level was then performed by the fellow for the screened studies, where those considered that met the eligibility criteria were included into the full‐text dossier (dossier 3) and preliminary classified into epidemiological, in silico, in vitro, in vivo mammalians and in vivo non‐mammalians.
2.2.4. Extracting and reporting the information
All the information from dossier 3, including relevant endocrine‐related parameters, as well as general toxicity endpoints was extracted and systematically reported for both positive and negative results. The supplementary Excel‐based tool from the ECHA/EFSA guidance document (Appendix E – Excel template for reporting the available information relevant for ED assessment) was used to collect all data describing each parameter in one single row as recommended in the guidance. Once all the parameters were entered, a data matrix was applied into the Excel tool to clearer reorganise the information and data clustering.
2.2.5. Evaluating reliability and relevance
The relevance (appropriateness of the data for the intended purpose of the assessment) and reliability (inherent quality of the test method and level of reporting) of each individual study included in dossier 3 was assessed by the online web tool Science in Risk Assessment and Policy – SciRAP (http://www.scirap.org). SciRAP provides predefined criteria and a colour coding tool aimed to promote structure and transparency in the evaluation of ecotoxicity and toxicity (in vitro and in vivo) studies for hazard and risk assessment of chemicals. When a study contained both in vitro and in vivo information, two parallel SciRAP evaluations were performed.
Relevance evaluation allows the classification into three categories (relevant, partially relevant, not relevant) based on five criteria: substance identity, concentrations, test system/animal model, administration route and studied endpoint. However, according to the systematic review methodology, studies were efficiently assessed for relevance against inclusion criteria in two steps: (i) screening of titles and abstracts for relevance to the study question and (ii) full‐text examination for the eligibility of studies (EFSA 2010). Therefore, assessment of relevance at this stage was considered as a confirmation and only two categories (relevant and partially relevant) were included since the not relevant studies were excluded at the initial steps of the protocol.
Reliability consisted of two blocks evaluating 23 or 30 criteria for reporting quality, and 15 or 18 criteria for methodological quality, for in vitro and in vivo studies, respectively. The output for each assessed study was provided as an Excel file containing a colour profile (qualitative evaluation) and a ranking score (quantitative evaluation) used to rate the studies as (i) reliable, (ii) partially reliable and (iii) not reliable. Five pure in silico and four epidemiological studies, not applicable for SciRAP tool, were manually evaluated by similar SciRAP‐based criteria.
2.2.6. Assembling lines of evidence
The extracted parameters along with the study quality assessment scores were assembled into lines of evidence for (a) endocrine activity, (b) adversity and (c) general toxicity. Each group was subdivided into categories based on the nature of the data as shown in Table 1.
Table 1.
Lines of evidence classification in groups and subgroups
| Lines of evidence classification | |||
|---|---|---|---|
| Groups | Endocrine activity | Adversity | General toxicity |
| Subgroups | In silico | In vivo: EATS‐mediated | Cellular toxicity |
| In vitro mechanistic | In vivo: EATS‐sensitive but not diagnostic | Target organ toxicity | |
| In vivo mechanistic | Epidemiological | Systemic toxicity | |
2.2.7. Assessing lines of evidence
Each individual line of evidence was assessed considering the quantity and quality of both the studies and the included parameters, as well as their coherence and/or conflicting information. In this way, a ranking composed of seven categories was defined based on the examples from the guidance and a weight of evidence document from the European Commission Scientific Committee on Health, Environmental and Emerging Risks (SCHEER 2018). Ranking categories were designed to categorised each assessed line of evidence after summarising the information included in each, and weight of evidence was described as follows: (1) high: several studies in different species/strains indicating clear and coherent evidence in the absence of conflicts; (2) sufficient: more than two studies in different species/strains indicating clear and coherent evidence; (3) moderate: two or more studies not necessarily in different species/strains showing coherent evidence; (4) supportive: one or more studies showing clear trend or indication of evidence but not enough available data; (5) low: one or more studies showing slight trend or indication of evidence not clearly demonstrated with not enough available data; (6) uncertain: one or more studies showing conflicting and not coherent results hindering evidence assessment; (7) no observed effect: one or more studies showing no effects observed.
After assessing each individual line of evidence, a similar approach was applied to the subgroup evaluation and the assessment of the integrated lines of evidence for each group: endocrine activity, adversity and general toxicity (Table 1).
2.2.8. Assessment of sufficiency of the data set
According to the ECHA/EFSA guidance, the sufficiency of the collected data set – lines of evidence – should be assessed with regard to EATS‐mediated adversity and EATS‐related endocrine activity, in order to identify the specific next‐step scenario that should be followed to proceed with the assessment. Six different scenarios are described in the guidance, represented as a decision tree, which evaluates: (i) if endocrine activity and adversity have been sufficiently investigated, (ii) if endocrine activity and adversity have been observed.
With this aim, the amount and type of available information was evaluated for adversity and endocrine activity. The final dossier generated in the present work included studies covering all the levels established in the OECD conceptual framework (CF) for the testing and assessment of ED chemicals (OECD 2012). Level 1 (existing data and non‐test information) was filled by in silico studies while epidemiological studies were considered as supportive data. In vitro studies performed in several cell lines from mice, rats, monkeys and mainly humans were classified as level 2 – in vitro assays providing mechanistic data. Additional mechanistic data were obtained from ToxCast studies and yeast bioassays. OECD CF level 3 (in vivo assays providing data about selected endocrine mechanism and pathways), level 4 (in vivo assays providing data on adverse effects on endocrine relevant endpoints) and level 5 (in vitro assays providing more comprehensive data on adverse effects on endocrine relevant endpoints over more extensive parts of the life cycle of the organism) were represented by in vivo studies in fish (medaka and mainly zebrafish), amphibian and in vivo mammalian studies in mice and rats.
Overall, it was concluded that the available information showed potential endocrine‐related adverse effects and endocrine activity; therefore, ED criteria were met and a MoA analysis was required according to the ECHA/EFSA guidance.
2.2.9. Mode of action analysis
As the guidance describes, a MoA consists of a sequence of measurable events at molecular, cellular and individual levels that link the molecular initiating event (MIE), to the adverse outcome (AO) through intermediate key events (KEs). MoA analysis consists of two steps: (1) postulate a MoA and (2) evaluate the MoA by the establishment of a biologically plausible link between endocrine activity and adverse effect.
To postulate the MoA, the adverse effects that showed the highest weight of evidence were initially selected as AOs. The information in the lines of evidence that was considered biologically connected to the AOs was organised into biological levels and a preliminary hypothesis was drawn defining the events chain from the molecular/cellular level to the individual/population AO (Figure 3).
Figure 3.
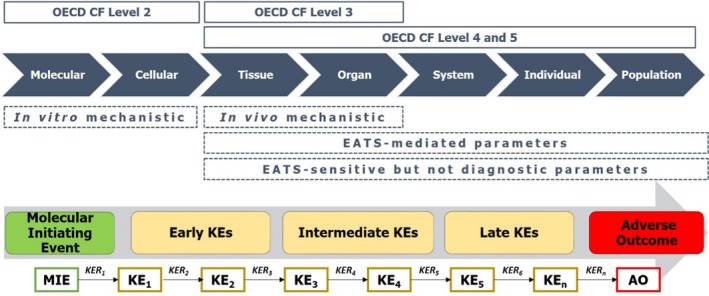
Scheme of the MoA postulation based on the ECHA/EFSA guidance for EDs assessment
To evaluate the MoA, the biological plausibility of the link between each adverse effect and the endocrine activity was investigated based on current scientific knowledge by applying weight of evidence approach. Each KE in the postulated MoA was identified, briefly described and evaluated based on the supporting evidence from the available literature. Biological plausibility of the KE relationships (KERs) was assessed based on the broader knowledge of biology, this means the current understanding of physiology, endocrinology and toxicology. As recommended in the guidance, biological plausibility of KERs was weighted as: (i) strong: if is there is extensive understanding of the KER based on extensive previous documentation and broad acceptance, (ii) moderate: if the KER is plausible based on analogy with accepted biological relationships, but scientific understanding is not completely established and (iii) weak: if the structural or functional relationship between the KEs is not understood. At this step, empirical support for dose–response/incidence concordance (earlier KEs are observed at below or similar doses than later KEs and AO) and temporal concordance (earlier KEs are observed in studies of similar or shorter duration than later KEs and AO) was evaluated as strong, moderate or weak. The individual KEs in the MoA were assessed with regard to (i) essentiality: the sequence of events in the postulated MoA is reversible if dosing is stopped or a KE prevented; (ii) consistency: repeatability of the KE in different studies/species/strains/systems; (iii) analogy: the postulated KEs also occur for other substances for which the same MoA has already been established; and (iv) specificity: the MoA for the adverse effect is endocrine related and not an indirect result of other non‐endocrine‐mediated toxicity. Finally, the overall conclusion of the ED properties of BPAF was reported based on the weight of evidence that supported the postulated MoA.
2.2.10. EU‐FORA Fellowship additional activities
In addition to the work at the Unit of Biochemical Toxicology at the Institute of Environmental Medicine (IMM), Karolinska Institutet, the Fellow attended the four EU‐FORA modules organised by EFSA (Italy), AGES (Austria), BfR (Germany) and EFET (Greece) where a wide training in risk assessment provided her extremely useful knowledge and practice. Moreover, additional activities positively contributed the work development and results dissemination, as well as her training and learning:
Course on Health Risk Assessment of Reproductive Toxicity and Endocrine Disruptors, Karolinska Institutet (IMM), Sweden.
Lecture at the Doctoral Programme Seminar – Environmental Factors and Health (EFH), Karolinska Institutet (IMM), Sweden.
Lecture at the Master of Food Quality and Safety, University of Valencia (UV), Spain.
Study visit to the Swedish Chemicals Agency (KemI), Sweden.
Open lecture attendance; Toxicology: should we really take the risk to communicate? – Lucia de Luca. Karolinska Institutet (IMM), Sweden.
Webinar on BioSecurity – International Hellenic University, Greece.
Poster presentation at the XXIII Spanish Congress of Toxicology (AETOX – Spanish Association of Toxicology), Spain.
Poster presentation at the 55th Congress of the European Society of Toxicology (EUROTOX – Federation of European Toxicologists & European Societies of Toxicology), Finland.
3. Conclusions
The ECHA/EFSA guidance for ED assessment was successfully applied to evaluate the ED properties for human health of BPAF, a potential substitute for BPA, as an illustration of its application on a non‐pesticide model substance. According to the EU criteria and guidance, BPAF showed endocrine activity and adversity based on systematic review approach, weight of evidence methodology and MoA analysis. The Fellow gained extensive knowledge and hands‐on experience on EDs assessment in the EU regulatory context as part of chemical risk assessment, and as an essential step towards regulation and appropriate risk management. This included the application of methodologies to increase transparency and structure in health risk assessment, such as systematic review, weight of evidence, and AO Pathways (AOPs). Moreover, a remarkable collaboration network was developed within the project demonstrating successful scientific communication and cooperation between different research groups and institutions (RIVM), as well as between academia and national authorities (KemI).
Abbreviations
- AO
Adverse Outcome
- AOP
Adverse Outcome Pathway
- BP
biocidal products
- BPA
Bisphenol A
- BPAF
Bisphenol AF
- EATS
Estrogen, Androgen, Thyroid, Steroidogenesis
- ECHA
European Chemicals Agency
- ED
Endocrine Disruptor
- KemI
Swedish Chemicals Agency
- KEs
Key Events
- KERs
Key Events Relationship
- MIE
Molecular Initiating Event
- MoA
Mode of Action
- OECD CF
Organisation for Economic Co‐operation and Development Conceptual Framework
- PPP
Plant protection products
- REACH
Registration, Evaluation, Authorisation and Restriction of Chemicals
- RIVM
National Institute for Public Health and the Environment, The Netherlands
- SciRAP
Science in Risk Assessment and Policy
- SIN
Substitute It Now
- SVHC
Substances of Very High Concern
Suggested citation: Karolinska Institutet, Institute of Environmental Medicine, Sweden , Escrivá L, Hanberg A, Zilliacus J and Beronius A, 2019. Assessment of the endocrine disrupting properties of Bisphenol AF according to the EU criteria and ECHA/EFSA guidance. EFSA Journal 2019;17(S2):e170914, 10 pp. 10.2903/j.efsa.2019.e170914
Acknowledgements: This report is funded by EFSA as part of the EU‐FORA programme.
Approved: 31 July 2019
References
- Beausoleil C, Emond C, Cravedi JP, Antignac JP, Applanat M, Appenzeller BR, Beaudouin R, Belzunces LP, Canivenc‐Lavier MC, Chevalier N, Chevrier C, Elefant E, Eustache F, Habert R, Kolf‐Clauw M, Le Magueresse‐Battistoni B, Mhaouty‐Kodja S, Minier C, Multigner L, Schroeder H, Thonneau P, Viguié C, Pouzaud F, Ormsby JN, Rousselle C, Verines‐Jouin L, Pasquier E and Michel C, 2018. Regulatory identification of BPA as an endocrine disruptor: context and methodology. Molecular and Cellular Endocrinology, 475, 4–9. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, 2007. Organization and integration of the endocrine system: the arousal and sleep perspective. Sleep Medicine Clinics, 2, 125–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EC Communication , 2018. 734 final. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of The Regions. Towards a Comprehensive European Union Framework on Endocrine Disruptors. Available online: http://ec.europa.eu/transparency/regdoc/rep/1/2018/EN/COM-2018-734-F1-EN-MAIN-PART-1.PDF
- ECHA/EFSA (European Chemicals Agency/European Food Safety Authority), 2018. Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and (EC) No 1107/2009. EFSA Journal 2018;16(6):5311, 135 pp. 10.2903/j.efsa.2018.5311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2010. Application of systematic review methodology to food and feed safety assessments to support decision making. EFSA Journal 2010;8(6):1637, 90 pp. 10.2903/j.efsa.2010.1637 [DOI] [Google Scholar]
- European Union , 2006. Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC.
- European Union , 2009. Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC.
- European Union , 2012. Regulation (EU) No 528/2012 of the European Parliament and of the Council of 22 May 2012 concerning the making available on the market and use of biocidal products.
- Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J and Zoeller RT, 2015. EDC‐2: The Endocrine Society's Second Scientific Statement on Endocrine‐Disrupting Chemicals. Endocrine Reviews, 36, E1–E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, 2018. Evidence of the possible harm of endocrine‐disrupting chemicals in humans: ongoing debates and key issues. Endocrinology and Metabolism, 33, 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munn S and Goumenou M, 2013. Key scientific issues relevant to the identification and characterisation of endocrine disrupting substances‐ Report of the Endocrine Disrupters Expert Advisory Group. Toxicology Letters, 221, S170. [Google Scholar]
- NTP (National Toxicology Program), 2017. NTP Research Report on Biological Activity of Bisphenol A (BPA) Structural Analogues and Functional Alternatives. [PubMed]
- OECD Series on Testing and Assessment, 2012. OECD Conceptual Framework for Testing and Assessment of Endocrine Disrupters (as revised in 2012).
- Pouzaud F, Thierry‐Mieg M, Burga K, Vérines‐Jouin L, Fiore K, Beausoleil C, Michel C, Rousselle C and Pasquier E, 2018. Concerns related to ED‐mediated effects of Bisphenol A and their regulatory consideration. Molecular and Cellular Endocrinology, 475, 92–106. [DOI] [PubMed] [Google Scholar]
- Rochester JR, Bolden AL, 2016. Bisphenol S and F: a systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environmental Health Perspectives, 123, 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHEER , 2018. Scientific Committee on Health, Environmental and Emerging Risks SCHEER. Memorandum on weight of evidence and uncertainties. Revision 2018.
- Schug TT, Johnson AF, Birnbaum LS, Colborn T, Guillette LJ Jr, Crews DP, Collins T, Soto AM, Vom Saal FS, McLachlan JA, Sonnenschein C and Heindel JJ, 2016. Minireview: endocrine disruptors: past lessons and future directions. Molecular Endocrinology, 30, 833–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skledar D and Masic P, 2016. Bisphenol A and its analogs: do their metabolites have endocrine activity? Environmental Toxicology and Pharmacology, 47, 182–199. [DOI] [PubMed] [Google Scholar]
- WHO , 2002. International Programme on Chemical Safety In: Damstra T, Barlow S, Bergman A, Kavlock R, Van Der Kraak G. Global Assessment of the State of the Science of Endocrine Disruptors. Available online: http://www.who.int/ipcs/publications/new_issues/endocrine_disruptors/en/ [Google Scholar]
- WHO/UNEP , 2012. State of the science of endocrine disrupting chemicals – 2012. An assessment of the state of the science of endocrine disruptors prepared by a group of experts for the United Nations Environment Programme and World Health Organization.


