Abstract
Ready to Eat (RTE) cooked meat products are among the most consumed RTE food subcategories in the EU. They are also associated with the highest number of listeriosis cases per year. Contamination with Listeria monocytogenes may arise from post‐processing and its growth is often supported by the pH and water activity of the product. L. monocytogenes may grow during refrigeration and reach unacceptable levels at the time of consumption, posing a public health risk. The aim of this study was to conduct a Quantitative Microbiological Risk Assessment (QMRA) of L. monocytogenes in a traditional Italian RTE cooked meat product. Data for the risk assessment included prevalence and concentration of the microorganism, temperature‐time conditions during transport and storage, information on the growth of the microorganism and its potential for disease (dose–response). These data were obtained from laboratory analysis of product samples (n = 50), a consumer survey (n = 160), recordings of temperatures of domestic refrigerators (n = 60) and were complemented with information from the literature. The data were described with appropriate probability distributions and introduced into a previously described growth model of L. monocytogenes. Based on the above components, a probabilistic model was created to evaluate the growth of L. monocytogenes at each stage of the product pathway (retail storage, transportation and domestic storage) using Monte Carlo simulations. The model design for this pathogen/food product combination, alongside with the findings of the study are included in a separate publication (manuscript under preparation). The results may help risk managers to apply appropriate control measures to minimise the public health risk. The project contributed to further education of the fellow, especially in the use of QMRA risk analysis tools and laid the foundations for future collaborations between the fellow's home institution, the University of Crete, Greece and the University of Perugia, Italy.
Keywords: Listeria monocytogenes, risk assessment, RTE meat, head cheese, QMRA
1. Introduction
Listeria monocytogenes remains a significant public health concern, despite not being among the most commonly reported causes of food‐borne illness (Buchanan et al., 2017). Listeriosis may present a mild infection in healthy people, but it can be very serious among susceptible populations, characterised by high mortality and hospitalisation rates. In addition, the number of detected outbreaks is usually low, since most invasive listeriosis cases appear as sporadic infections (EFSA, 2018). Consequently, there is great uncertainty about the true burden of listeriosis and under‐reporting has been estimated at around a factor of two in the UK and North America (EFSA BIOHAZ Panel, 2018).
L. monocytogenes is a bacterium that is common in the environment and can be found in agricultural and food production environments, where once established, it tends to persist through the creation of biofilms (Gray et al., 2018). Possible entry routes of the bacterium in the final product are both raw material contamination and cross‐contamination during food processing. Ready to Eat (RTE) foods have been shown to be one of the most important vehicles responsible for human infections by several studies (Cenci‐Goga et al., 2018; Kurpas et al., 2018). RTE foods typically associated with human listeriosis, include ‘meat and meat products’ (Cenci‐Goga et al., 2008), ‘fish and fish products’ and ‘milk and milk products’, as well as foods of plant origin and frozen foods. In the 2018 EFSA scientific opinion on listeriosis (EFSA BIOHAZ Panel, 2018), which considers foods of animal origin, it was reported that cooked meat and heat‐treated sausages were the RTE food subcategories with most consumed servings per person and per year in the European Union (EU)/European Economic Area (EEA). Simultaneously, cooked meat products were associated with the largest number of listeriosis cases per year (more than 850). Depending on the formulation and storage conditions, almost all RTE foods may support growth of L. monocytogenes and therefore have the potential to cause disease, especially when consumed by the susceptible population (Cenci‐Goga et al., 2012, 2015)
L. monocytogenes prevalence and contamination data of various RTE food categories (meat, milk, fish and their products) marketed in the EU is important for estimating the public health risk for listeriosis. Nevertheless, in the 2018 EFSA opinion, it was reported that 41% of listeriosis outbreaks are linked to foods not considered in the Opinion, thus highlighting the need for more QMRA studies to generate data on both meat and plant derived RTE foods (EFSA BIOHAZ Panel, 2018).
Up to date, neither contamination levels nor a risk assessment study for the Italian Head Cheese are available in literature. The traditionally cooked deli meat product, named “Coppa di Testa”, is produced seasonally by several small and large processing establishments in Italy, using local pork (or hog) meat. It has been identified as a product that can support the growth of L. monocytogenes once contaminated with the bacterium, therefore posing a potential risk for public health (Bardasi et al., 2010). Different variations of the head cheese are also manufactured in several parts of the world, including the EU and the USA. The product has been previously linked to outbreaks of invasive listeriosis in the USA (2011) and more recently in an outbreak that occurred in Italy between May 2015 and March 2016 (CDC, 2011; Duranti et al., 2018). In addition, detection of L. monocytogenes during routine testing has resulted in recent Italian head cheese recalls.1 , 2
2. Description of work programme
2.1. Aims
The overall objective of the work programme was to apply ‘risk assessment’ methodology in order to estimate the public health risk from L. monocytogenes following consumption of ‘Coppa Di Testa’ head cheese. As a scientific process, risk assessment determines the relationship between exposure to a given hazard under a defined set of conditions and the likelihood of an adverse health effect or disease (McLauchlin et al., 2004; Koutsoumanis and Aspridou, 2016). In this work, we applied a Quantitative Microbial Risk Assessment (QMRA) model, which was run on experimental and literature derived data. More specifically, the aim was to:
Collect and analyse information on the prevalence of L. monocytogenes in product samples, time/temperature storage conditions, consumption data; identify where critical data and/or knowledge is lacking;
Characterise the nature and size of the microbial food safety risk due to L. monocytogenes in RTE Coppa Di Testa products.
In addition, through this assessment we aimed to identify factors that contribute most significantly to the risk and suggest potential management strategies to reduce the food safety risk due to L. monocytogenes. The main output of the work programme will be the preparation of a manuscript describing the application of a QMRA model for L. monocytogenes in RTE Coppa Di Testa, in order to disseminate the results of the project to the wider scientific community. The methodology and the steps applied for the QMRA as well as other activities in which the fellow has been involved are briefly described below.
2.2. Activities/Methods
2.2.1. Production process, sample analysis and consumer survey
In order to better understand the possible entry points for L. monocytogenes, the production of ‘Coppa di Testa’ was carefully recorded during visits of the fellow and other lab members to various production facilities in the area of Umbria, Italy. Coppa di testa is a traditional cooked pork salami seasonally produced by several small and large processing establishments using local pork (or hog) meat. Briefly, it is made by deboned head meat with the addition of tongue and rind. Salt and spices are added and the product is placed in moulds, portioned and vacuum‐packaged before distributed to retail stores.
Also, as mentioned above, available literature data on Coppa di Testa are very limited. Therefore, to acquire data on L. monocytogenes prevalence required for the stochastic model described below, we sought to collect and directly analyse a number of samples in the laboratory, complementing this analysis with data from local authorities if possible. About 50 random products were purchased from different retail shops either vacuum packed or sliced. Analysis was carried out at the hosting institution and involved measurements on intrinsic factors of the product such as pH and water activity as well as microbiological analysis according to the methods previously described (Cenci‐Goga et al., 2012). The ISO 11290 method (ISO, 1996) was used to isolate Listeria monocytogenes.
In addition, information on Coppa di Testa domestic storage and consumption habits of consumers was derived after conducting a consumer survey. A relevant questionnaire was prepared, in which consumers were asked to complete information on (i) their personal characteristics (age, gender, susceptible group or not); (ii) the size (portions) and frequency (per week) of the product consumption; as well as (iii) the time required for product transport to home and (iv) the time of domestic storage before consumption for vacuum‐packed and sliced products. The anonymous consumer survey was distributed not only via web‐based social network platforms such as Facebook, but also in printed form via short consumer interviews during visits to local facilities, i.e. a house for the elderly, supermarkets, medical practices. In total, our survey resulted in more than 160 responses among the Italian population. Responses coming from consumers of other countries were not included. Nevertheless, thanks to the assistance of Dr. Giorgiana Catunescu, also participating in the EU‐FORA programme, the questionnaire was made available to a number of Romanian consumers, yielding about 100 responses. Since a product that is similar to Coppa di Testa is produced and consumed in Romania, the survey data may be useful in a future study. Among the 162 Italian participants (39% men and 61% women), 20% of men and 10% of women were > 65 years of age. Briefly, the results of the survey show that the majority (82%) of the consumers do not consume Coppa di Testa on a regular basis. However, about 15% of the participants, consume the product once per week and 3% consume it 2–3 times per week. The majority prefers to consume it mostly during dinner (50%) and lunch (42%) and less frequently during breakfast (8%) with the average slice number/person/week estimated at 3.7 slices.
2.2.2. Application of stochastic mathematical modelling for QMRA – model development
The fellow was involved in the development and application of a stochastic model, as an important tool for quantitative microbial risk analysis. The overall risk assessment was based on four separate stages (in accordance with Codex Alimentarius) namely (i) hazard identification, (ii) hazard characterisation, (iii) exposure assessment and (iv) risk characterisation. More specifically:
Collection of literature data on listeriosis and the behaviour of L. monocytogenes in meat derived RTE foods, especially in Coppa di Testa head cheese (hazard identification);
Presentation of the potential for L. monocytogenes to cause illness in human populations based on literature (hazard characterisation/dose–response);
Determination of the exposure to L. monocytogenes from consumption of head cheese: collection and analysis of data on prevalence and on consumption habits of a sample group among the population (exposure assessment);
Application of an appropriate mathematical model, integrating exposure assessment and hazard characterisation, in order to estimate the public health risk from consumption of head cheese contaminated with L. monocytogenes (risk characterisation).
The product pathway and the risk assessment process is diagrammed in Figure 1.
Figure 1.
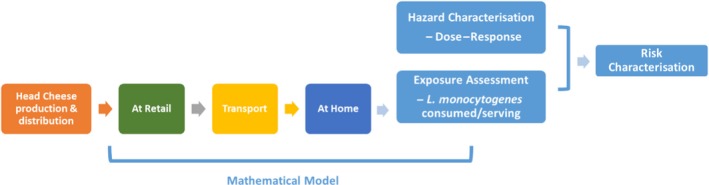
The product pathway and risk assessment process
Initially, the literature was scanned and publications relevant to ‘L. monocytogenes and RTE meat products’ were retrieved through searches using the PubMed3 database, Google Scholar,4 Scopus5 and Web of Science6. Information was also retrieved from websites belonging to relevant organisations and authorities (e.g. WHO, EFSA, FDA). For hazard characterisation, a dose–response curve was necessary and describes the possibility of illness following consumption of a certain number of pathogenic bacterial cells. Based on several previous QMRA studies, (Giovannini et al., 2007; Ross et al., 2009; Mataragas et al., 2010; Bassett et al., 2012), the general form of the exponential dose–response model by FAO/WHO was selected for this work (FAO/WHO, 2004).
For the exposure assessment, we needed to describe how often and at what levels, consumers in the population consume the hazard in the food of interest (Lammerding and Fazil, 2000). The important output from the exposure assessment was the ‘number of L. monocytogenes per serving of contaminated Coppa di Testa’ was and this was estimated using information about the frequency of contamination (prevalence) and the final contamination levels at the point of consumption. The prevalence was estimated by microbial analysis of samples, whereas predictive microbiology was applied to calculate the final contamination levels from initial contamination levels (at the point of retail or production) as well as growth of L. monocytogenes based on product formulation, times and temperatures of distribution and storage prior to consumption. For this purpose, the fellow used data derived from expert opinion (producers and retailers), product labels, as well as temperature data obtained after conducting a study on 60 domestic refrigerators. In addition, information on consumption (size and the number of servings) as well as product storage habits was obtained by the anonymous consumer survey described above. Consumption size and frequency data from the survey were fitted into distributions using @Risk and applied to the model calculations. A schematic overview of the influence diagram for the exposure assessment is presented in Figure 2.
Figure 2.
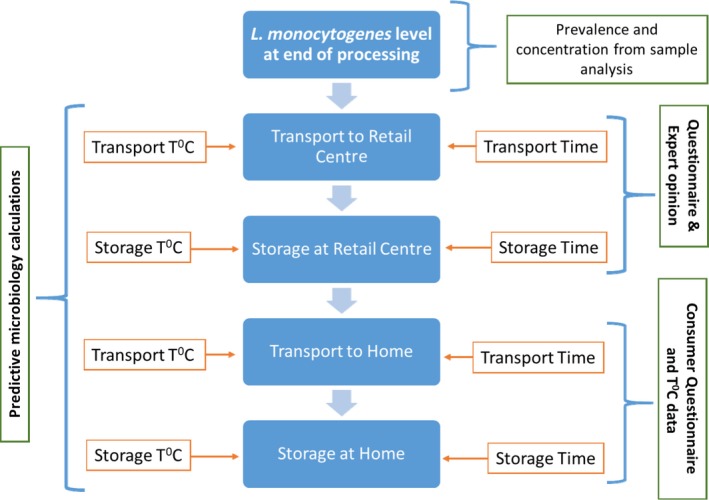
Influence Diagram of the overall structure of the exposure assessment part of the model. The various stages between production and consumption are discretely modelled and the output of each stage is influenced by model inputs as depicted by the arrow points
Finally, in order to perform a QMRA, the fellow worked on developing a stochastic model and integrated the steps of hazard characterisation (dose–response relationship) and exposure assessment, leading to the risk characterisation output. The model implemented Monte Carlo simulations and was designed using the @Risk simulation software (@Risk 7.6 for Excel, Palisade, Ithaca, USA), as an add‐in to Microsoft Excel. The mathematical model describes the possibility of post‐production contamination of Coppa di Testa with L. monocytogenes and the effect of temperature and time, during transport and storage, on the growth of the bacterium. The model structure incorporates data from our laboratory sample analysis and from the literature, information derived from questionnaires, previous risk assessments and on consultation with experts. Integrated with the dose–response relationship for L. monocytogenes, the model calculates the likelihood of public health adverse effects following consumption of the product. A manuscript currently under preparation includes a comprehensive description of the QMRA study, details on the model structure and mathematical calculations and the results of the study. It is expected to be submitted for publication in a peer‐reviewed journal in the coming period.
2.2.3. Other activities during the EU‐FORA fellowship
During the fellowship, the fellow had the opportunity to participate to various scientific activities. These included:
Presentation of the Risk assessment methodology for microbial risks in foods (lecture to undergraduate students at the University of Perugia)
Participation to the annual European College of Veterinary Public Health (ECVPH) conference, organised in Perugia (October 2018)
A one‐day trip accompanying university students to inspection visits to a large meat‐canning factory.
Visits to the local slaughterhouse accompanied by a tutorial on animal welfare measures, microbial contamination risks and inspection during the process.
Visits to processed meat production plants and observation of the production procedures for Coppa di Testa and other processed meats. Conducted interviews of producer managers regarding the process and safety measures against contamination.
Dissemination of the survey questionnaires to the public through visits to supermarkets, a house for the elderly etc.
Preparation of an abstract/poster for the National conference AIVI (National Congress of the Italian Association of Veterinary Food Hygienists), Bari, Italy September 11–13 (upcoming event).
Preparation of two research manuscripts: a review manuscript on L. monocytogenes (title not available yet) and a second manuscript with results from the main subject of this work programme (Title: Quantitative risk assessment of Listeria monocytogenes in ready‐to‐eat head cheese in Italy). These manuscripts are currently under preparation.
Preparation of a joint application for funding of a 3‐year research project between the institution of the fellow (University of Crete, Greece) and the hosting institution (University of Perugia, Italy), aiming to create future collaboration opportunities between the two organisations. The proposal has been submitted (March 2019) to the Hellenic Foundation for Research and Innovation (HFRI) and at the time of writing, is under review.
Finally, the fellow attended seminars at the Department of Veterinary Medicine and participated in outdoor activities organised by the hosting laboratory.
3. Conclusions
The main focus of the work programme was the development and application of a quantitative microbial risk assessment model in order to estimate the public health risk for listeriosis following consumption of Italian head cheese. The programme has enabled the fellow to gain expertise in risk assessments related to microbiological hazards. Through this process, the fellow learned how to design a risk assessment pathway and to build a QMRA model by combining information, experimental data and mathematical equations while taking into account all input parameters (i.e. temperature, time, microbial population) that affect the output. One important aspect of this was the familiarisation of the fellow with relevant online risk assessment platforms and especially the @Risk software package, often used as a powerful risk analysis tool. Both the fellow and the supervisor agree that the EU‐FORA programme was a valuable opportunity to discuss opinions, methodologies and science, and an important step to building a professional network that will serve as a basis for future collaboration in risk assessment studies.
Abbreviations
- EEA
European Economic Area
- EU‐FORA
European Food Risk Assessment Fellowship Programme
- FAO
Food and Agriculture Organization of the United Nations
- FDA
Food and Drug Administration
- WHO
World Health Organization
- RTE
Ready to eat
Suggested citation: Department of Veterinary Medicine, University of Perugia, Perugia, Italy , Hadjicharalambous C, Grispoldi L and Goga BC, 2019. Quantitative risk assessment of Listeria monocytogenes in a traditional RTE product. EFSA Journal 2019;17(S2):e170906, 9 pp. 10.2903/j.efsa.2019.e170906
Acknowledgements: This report is funded by EFSA as part of the EU‐FORA programme.
Approved: 31 July 2019
Notes
References
- Bardasi L, Bonilauri P, Rugna G, Galletti G, Fedrizzi G, Santandrea G, Gandolfi P, Vecchi G and Merialdi G, 2010. Growth of naturally occurring Listeria innocua in Coppa di Testa. Italian Journal of Food Safety, 1, 35–39. [Google Scholar]
- Bassett J, Nauta M, Lindqvist R and Zwietering M, 2012. Tools for Microbiological risk assessment. ILSI Europe. ILSI Europe Report Series, In. [Google Scholar]
- Buchanan Robert L, Gorris Leon GM, Hayman Melinda M, Jackson Timothy C and Whiting Richard C, 2017. A review of Listeria monocytogenes : An update on outbreaks, virulence, dose‐response, ecology, and risk assessments. Food Control, 75, 1–13. [Google Scholar]
- CDC (Centers for Disease Control and Prevention), 2011. Outbreak of invasive listeriosis associated with the consumption of hog head cheese–Louisiana, 2010. MMWR. Morbidity and Mortality Weekly Report, 60, 401–405. [PubMed] [Google Scholar]
- Cenci‐Goga BT, Ranucci D, Miraglia D and Cioffi A, 2008. Use of starter cultures of dairy origin in the production of Salame nostrano, an Italian dry‐cured sausage. Meat Science, 78, 381–390. [DOI] [PubMed] [Google Scholar]
- Cenci‐Goga BT, Rossitto PV, Sechi P, Parmegiani S, Cambiotti V and Cullor JS, 2012. Effect of selected dairy starter cultures on microbiological, chemical and sensory characteristics of swine and venison (Dama dama) nitrite‐free dry‐cured sausages. Meat Science, 90, 599–606. [DOI] [PubMed] [Google Scholar]
- Cenci‐Goga BT, Karama M, Sechi P, Iulietto MF, Novelli S, Selvaggini R and Mattei S, 2015. Growth Inhibition of Selected Microorganisms by an Association of Dairy Starter Cultures and Probiotics. Italian Journal of Animal Science, 14, 3745. [Google Scholar]
- Cenci‐Goga BT, Karama M, Sechi P, Iulietto MF, Grispoldi L, Selvaggini R, Ceccarelli M and Barbera S, 2018. Fate of selected pathogens in spiked <<SALAME NOSTRANO>> produced without added nitrates following the application of NONIT technology. Meat Science, 139, 247–254. [DOI] [PubMed] [Google Scholar]
- Duranti A, Sabbatucci M, Blasi G, Acciari VA, Ancora M, Bella A, Busani L, Centorame P, Camma C, Conti F, De Medici D, Di Domenico M, Di Marzio V, Filippini G, Fiore A, Fisichella S, Gattuso A, Gianfranceschi M, Graziani C, Guidi F, Marcacci M, Marfoglia C, Neri D, Orsini M, Ottaviani D, Petruzzelli A, Pezzotti P, Rizzo C, Ruolo A, Scavia G, Scuota S, Tagliavento G, Tibaldi A, Tonucci F, Torresi M, Migliorati G and Pomilio F, 2018. A severe outbreak of listeriosis in central Italy with a rare pulsotype associated with processed pork products. Journal of Medical Microbiology, 67, 1351–1360. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2018. Outcome of the public consultation on the draft Scientific Opinion on Listeria monocytogenes contamination of ready‐to‐eat foods and the risk for human health in the EU. EFSA Supporting Publications 2018:en‐1352, 67 pp. https://www.efsa.europa.eu/en/supporting/pub/en-1352 [DOI] [PMC free article] [PubMed]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), Ricci A, Allende A, Bolton D, Chemaly M, Davies R, Escámez Fernández PS, Girones R, Herman L, Koutsoumanis K, Nørrung B, Robertson L, Ru G, Sanaa M, Simmons M, Skandamis P, Snary E, Speybroeck N, Ter Kuile B, Threlfall J, Wahlström H, Takkinen J, Wagner M, Arcella D, Da Silva Felicio MT, Georgiadis M, Messens W and Lindqvist R, 2018. Scientific Opinion on the Listeria monocytogenes contamination of ready‐to‐eat foods and the risk for human health in the EU. EFSA Journal 2018;16(1):5134, 173 pp. 10.2903/j.efsa.2018.5134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO/WHO (Food and Agriculture Organization of the United Nations and World Health Organization), 2004. Risk assessment of Listeria monocytogenes in ready‐to‐eat foods. TECHNICAL REPORT. Microbiological risk assessment series 5. Food and Agriculture Organization of the United Nations, Viale delle Terme di Caracalla, 00100 Rome, Italy. Available online: http://www.fao.org/es/esn/food/risk_mra_listeria_report_en
- Giovannini A, Migliorati G, Prencipe V, Calderone D, Zuccolo C and Cozzolino P, 2007. Risk assessment for listeriosis in consumers of Parma and San Daniele hams. Food Control, 18, 789–799. [Google Scholar]
- Gray JA, Chandry PS, Kaur M, Kocharunchitt C, Bowman JP and Fox EM, 2018. Novel Biocontrol Methods for Listeria monocytogenes Biofilms in Food Production Facilities. Frontiers in Microbiology, 9, 605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISO , 1996. ISO 11290‐1:1996: Microbiology of food and animal feeding stuffs ‐ horizontal method for the detection and enumeration of Listeria monocytogenes ‐ Part 1: detection method. [Google Scholar]
- Koutsoumanis Konstantinos P and Aspridou Z, 2016. Moving towards a risk‐based food safety management. Current Opinion in Food Science, 12, 36–41. [Google Scholar]
- Kurpas M, Wieczorek K and Osek J, 2018. Ready‐to‐eat meat products as a source of Listeria monocytogenes . Journal of Veterinary Research, 62, 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammerding AM and Fazil A, 2000. Hazard identification and exposure assessment for microbial food safety risk assessment. International Journal of Food Microbiology, 58, 147–157. [DOI] [PubMed] [Google Scholar]
- Mataragas M, Zwietering MH, Skandamis PN and Drosinos EH, 2010. Quantitative microbiological risk assessment as a tool to obtain useful information for risk managers–specific application to Listeria monocytogenes and ready‐to‐eat meat products. International Journal of Food Microbiology, 141(Suppl 1), S170–S179. [DOI] [PubMed] [Google Scholar]
- McLauchlin J, Mitchell RT, Smerdon WJ and Jewell K, 2004. Listeria monocytogenes and listeriosis: a review of hazard characterisation for use in microbiological risk assessment of foods. International Journal of Food Microbiology, 92, 15–33. [DOI] [PubMed] [Google Scholar]
- Ross T, Rasmussen S, Fazil A, Paoli G and Sumner J, 2009. Quantitative risk assessment of Listeria monocytogenes in ready‐to‐eat meats in Australia. International Journal of Food Microbiology, 131, 128–137. [DOI] [PubMed] [Google Scholar]


