Abstract
Cortisol is the main end product of hypothalamic-pituitary-adrenal gland (HPA axis), and melatonin (MT) has a regulating effect on HPA axis, and both are closely related to individual behavior and cognitive function. We aimed to evaluate cortisol and MT roles on children dyslexia in this study.
A total of 72 dyslexic children and 72 controls were recruited in this study. Saliva samples were collected in the morning, afternoon, and night, respectively. The levels of saliva cortisol and MT were measured by enzyme-linked immunosorbent assay method. Differences of cortisol and MT levels between dyslexic and normal children were compared, and the variation trend was also analyzed by dynamic monitoring in 3 time points.
The levels of salivary cortisol and MT in children with dyslexia were all lower than those in normal children whether in the morning (7:30-8:30 am ), at afternoon (15:30-16:30 pm ) or at night (21:30-22:30 pm ) (all P < .001). Compared with normal children, the circadian rhythm variations of salivary cortisol and MT in dyslexic children disappeared and became disordered. The salivary cortisol and MT levels in children with dyslexia were declined throughout the day; and the circadian rhythm was disordered or disappeared.
The results suggest that cortisol and MT levels and their circadian rhythm may affect children dyslexia, but the mechanisms need further exploration.
Keywords: circadian rhythm, cortisol, dyslexia, melatonin, risk factors
1. Introduction
Specific learning disorder is a group of learning ability defects, which refers to the delay in the development of children's learning ability such as reading, spelling and calculation in the early and school age.[1] It is shown that reading accuracy and comprehension, spelling and word ability, basic calculation ability of these children are lower than typically development children of the same age.[2] Clinical classification of specific learning disorder was mainly divided into dyslexia, disorder of written expression and mathematics disorder according to diagnostic and statistical manual of mental disorders, fifth edition (DSM-5).[3] Dyslexia is the most common type, accounting for more than 80%, and the incidence rate in school-age children is about 5% to 15%.[4] The prevalence rate of dyslexia is high, and once children have dyslexia, their behavior, cognition, emotion, social adaptation and other aspects will be affected, seriously hindering children's access to knowledge and improvement of ability.
Dyslexia is related to the development of central nervous system and has a neurobiological basis. Neuroanatomic studies indicated that the heterotopia of neurons in patients with reading disorder lead to the disorder of neuronal sequencing and changes of brain nerve channels, affecting the overall brain function.[5] Some studies have also found that the frontal lobes of the dyslexic patients have been changed; the frontal lobes, angular gyrus, temporo-parietal junction and cerebellum have abnormal activity in multiple brain regions, and the left hemisphere is not activated enough when performing the reading task.[6–8] Meng et al performed functional imaging studies and found that the activated brain regions of Chinese dyslexia children were different from normal children in performing line rhyme task, and that phonological expression of dyslexia children was relatively intact, but the voice operation was abnormal and damaged, which might be due to the reduction or defect of neuronal connections between left superior temporal gyrus and left occipital medial temporal in the process or formation of language or expression.[9] Molecular genetics studies have found that chromosomes 15, 6, 2, 3, and 1 are closely related to dyslexia, and the studies on loci of chromosomes 15 and 6 have been repeated in many independent experiments.[10,11] Different theoretical assumptions are proposed in the cognitive defect theory of dyslexia, which is mainly made up of the defect theory of speech processing,[12] the defect theory of rapid hearing processing,[13] the visual processing theory and the cerebellar theory.[14,15]
Researches of dyslexia mainly focus on brain function, neuroanatomy and neuropsychology, while the research on neuroendocrine is rarely reported. The hypothalamic-pituitary-adrenal gland (HPA axis) is an important part of the human neuroendocrine system. HPA axis plays a key role in maintaining homeostasis and quickly adapting to the environment, and plays an important role in individual emotional regulation, behavior control and cognitive functions. We hypothesize the HPA axis may play important roles in the development of dyslexia. Cortisol is the main end product of the HPA axis, and its secretion level and daily rhythm can directly reflect the activity and function of the HPA axis, which is a key biological marker to study the HPA axis. Previous studies suggested HPA axis was closely related to diseases such as attention deficit hyperactivity disorder (ADHD), and cortisol played an important role in ADHD.[16–18] Other studies have found that dyslexia and ADHD are comorbid, and the co-morbidity rate is up to 18%.[19,20] Therefore, the HPA axis may also have a certain relationship with dyslexia, while there is a lack of research on this topic at present.
Melatonin (MT), a hormone mainly synthesized and secreted by brain pineal gland, is widely distributed in central and peripheral tissues, and has functions of maintaining circadian rhythm, promoting sleep, protecting nerves and regulating endocrine.[21] MT is closely related to cognitive function and emotional regulation and has a regulating effect on HPA axis.[22] MT administration inhibited the development of ADHD phenomena and their related response in an NC/Nga atopic-like mouse model.[23] MT has a certain inhibitory effect on the stress response, and can alleviate the damage caused by acute and chronic stress by regulating the HPA axis. MT can ameliorate cognitive memory by regulation of cyclic adenosine mono phosphate-response element-binding protein expression and the anti-inflammatory response in posttraumatic stress disorder.[24] Due to the important role of MT in cognitive memory, behavior and emotional regulation, regulating the HPA axis, it may also play a role in dyslexia, which lack of studies to confirm.
Therefore, it is of great significance to study the secretion levels of cortisol and MT and their rhythmicity in children with dyslexia, and further explore the correlation between activity and functional status of HPA axis and dyslexia, so as to reveal the possible pathogenesis of dyslexia. In this study, saliva samples were collected from dyslexic and normal children, and cortisol and MT levels were measured to explore the relationships between cortisol, MT secretion levels, their rhythmicity, and dyslexia.
2. Methods
2.1. Subjects
This is a case-control designed study based on previous dyslexia screening in primary school children. Dyslexic children were screened out from the epidemiological survey of school age children in Shantou city of China and diagnosed as dyslexia according to the diagnostic criteria of DSM-5.[3] The 3-staged screening process for the diagnosis of dyslexic children is shown in Figure 1. For patients who meet the inclusion criteria according to DSM-5, general characteristics information was collected after signing the informed consent by the students or their guardians; and then relevant tests such as the dyslexia behavior scale and children's reading ability scale were conducted by specially trained psychiatrists. The healthy children group came from Shantou public primary school, and the non-dyslexic children who volunteered to participate in the study were included. Similarly, the trained psychiatrists conducted the scale of dyslexia behavior and the children's reading ability scale to these children. This survey was approved by the ethics committee of Shantou University Medical College and written informed consents were obtained from participants and guardians prior to the study after detailed explanation of the study to the parents and teachers.
Figure 1.
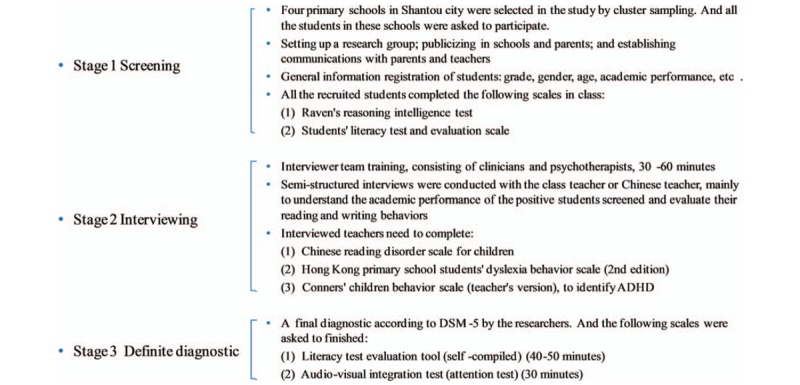
Flowchart of the 3-staged screening process of diagnosis for dyslexic children.
2.2. Saliva sample collection
With the discovery of cortisol level between saliva and serum, the collection and detection of saliva have been more widely used. Salivary cortisol has a good correlation with blood cortisol[25,26] (R = 0.712, P < .001), and can well reflect the level of bioactive free cortisol in blood.[27] The concentration of MT in saliva is also related to the concentration in blood, which can accurately reflect the change of human MT concentration and the change of circadian rhythm.[28,29] Saliva collection is convenient, and has the advantages of being painless, non-invasive and non-stimulating as compared with blood sample collection, which can avoid the instantaneous physiological and biochemical reactions caused by stimulus factors.
Before sample collection, parents and the children were instructed the following notes: do not eat within 1 hour; do not brush teeth and do not drink water within 10 minutes. The collection time is from 07:30 to 8:30, 15:30 to 16:30, and 21:30 to 22:30.
Saliva collection was performed using salivette and the process is as follows: the cotton sliver of the salivette was taken out and put in the sublingual of the students about 3 to 5 minutes, and then put back to the salivette, stored in the refrigerator (0 – 4°C) within 30 minutes. The samples were centrifuged at 3000 rpm for 15 minutes. After the centrifugal saliva flow through a small tube below holes to the bottom of the saliva salivette, about 3 mL (at least 1.5 mL) saliva was collected. The centrifugal saliva was stored at -20°C refrigerator within 4 hours, and for further laboratory analysis. Subjects who were exposed to blood pollution such as bleeding gums were excluded because cortisol levels in the blood are often much higher than salivary cortisol levels.
2.3. Saliva cortisol and MTanalyses
Salivary cortisol and MT concentrations were detected using commercial enzyme-linked immunosorbent assays kits for cortisol (Cat No.: CEA462Ge, Cloud-Clone Corp.) and MT (Cat No.: CEA908Ge, Cloud-Clone Corp.), were purchased from Cloud-Clone Corp. Wuhan (Wuhan, China). Aliquots of each saliva sample were used for the assessment of cortisol and MT based on the instructions of the producer (Cloud Clone, Houston). All measurements in this work were carried out with at least 2 replicates by the same researcher. Intra- and inter- assay coefficients of variation (CV) of salivary cortisol were 4% and 6%, respectively, and levels were expressed as ng/ml. Average intra-and inter-assay CV of MT were less than 2.5%, levels were expressed as pg/mL. In this study, all the saliva samples could be determined for cortisol and MT concentrations, which were above the limit of detection.
2.4. Statistical methods
Categorical data were presented as numbers of samples and percentages (%), while continuous data were presented in the form of mean with standard deviation, or median with inter-quartile range (P25-P75). Normal distribution tests of the data were verified using Kolmogorov-Smirnov and Shapiro-Wilk statistics. For comparing general characteristics, independent sample t test was used for continuous variables, and chi-square test or Fisher exact test for categorical data. Non-normal distribution data were compared between groups by using Mann-Whitney U test. All the statistical analyses and graphing involved use of SPSS23.0 software (SPSS Inc., Chicago, IL), and GraphPad Prism 7 (GraphPad Software, Inc., CA). A 2 side of P < .05 was considered as statistically significant.
3. Results
3.1. General characteristics of the participants
The general information of the research subjects is shown in Table 1. A total of 72 cases were enrolled in the dyslexia group, with 51 (70.8%) boys; and 72 typically development children were recruited as the control group, with 48 (66.7%) boys. The students were aged from 9 to 12 years, with 10.09 ± 1.45 in the dyslexia group, and 10.52 ± 1.24 in the control group. There were no age or sex differences between these 2 groups.
Table 1.
General characteristics of the participants.
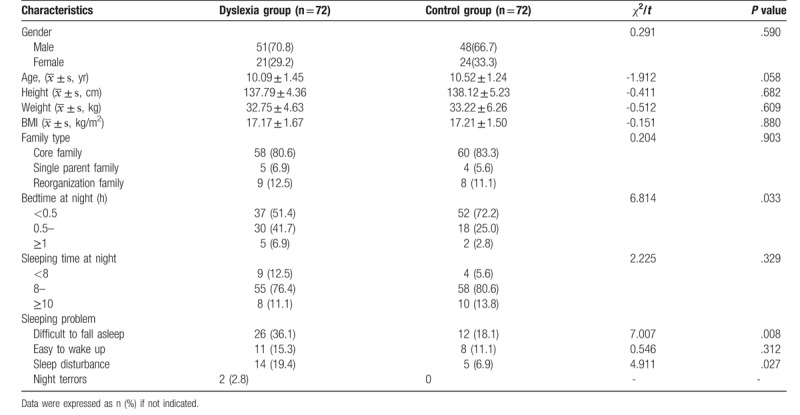
Considering the possible effects of sleeping on hormone secretion, we investigated the sleep conditions of children in the 2 groups, including the difficulty of falling asleep, the amount time of sleep at each night, and possible sleep problems. The results suggested that there was no significant difference in total sleep time between the 2 groups, but dyslexic children took longer time to fall asleep than normal children and had more sleep problems (Table 1).
3.2. Cortisol levels in dyslexia and control groups
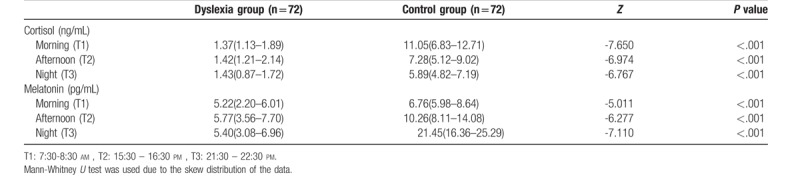
Cortisol levels in the dyslexia group and the control group were shown in Table 2. The differences between dyslexia and control groups at different time points (morning, afternoon and night) were analyzed with the non-parametric tests due to heterogeneity of variance and the skew distribution of the data. Saliva cortisol levels in dyslexic children were all lower in the morning, afternoon and evening than in normal children (all P < .001).
Table 2.
Cortisol and melatonin levels (median, P25-P75) between dyslexia and control groups in the morning, afternoon, and night.
3.3. MT levels in dyslexia and control groups
The results of MT levels in the dyslexia group and the control group were also presented in Table 2. Due to the non-normal distribution of the data, the differences of MT levels between dyslexia and control groups at different time points were analyzed with the nonparametric tests. Regardless of in the morning, at afternoon or at night, the levels of saliva MT in children with dyslexia were lower than those in the normal group (Table 2, all P < .01).
3.4. Circadian rhythm variation of cortisol and MT levels in dyslexia children
Both cortisol and MT have circadian rhythm in normal physiological state. Cortisol is higher in the morning while MT is higher in the evening, so comparing the circadian rhythm of them is of great significance. It can be seen from Figure 2 that there are obvious circadian rhythms variation trend of both cortisol and MT in normal children, with higher in daytime and lower at night for cortisol, and lower in daytime and higher at night for MT. However, the circadian rhythms variation was disappeared in dyslexia children, whether for cortisol or melatonin (Figure 2).
Figure 2.
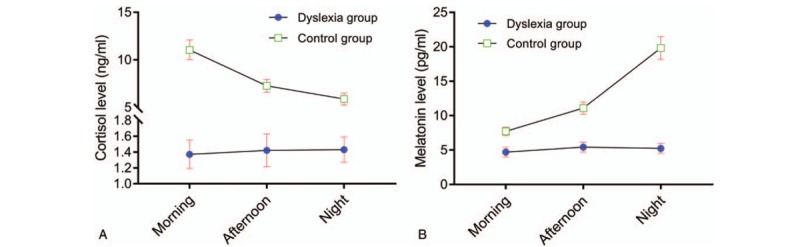
Cortisol and melatonin levels variation trend in the morning, afternoon, and night between dyslexia and control groups. (A) Cortisol level; (B) Melatonin level. Error bars stands for 95% confidence internal (95%CI). CI = confidence internal.
4. Discussion
Dyslexia is the most common type of specific learning disorders, accounting for more than 80%. Dyslexia can have a great impact on children's cognitive function and emotion, which is affected by neuroendocrine factors. The results of this study showed that the saliva cortisol and MT levels of dyslexic children were decreased as compared to typically development children, and the circadian rhythms of them were destroyed. Due to the impaired regulation function on HPA axis, the reduced cortisol and MT and their inordinate rhythm may play a role in promoting the development of dyslexia.
4.1. Cortisol and dyslexia
The cortisol secretion is regulated by HPA axis, and present day high/night low circadian rhythms. Salivary cortisol has been selected as reliable noninvasive biomarkers for HPA axis function in other study previously.[16] Cortisol enters the saliva by passively diffusing through salivary gland somatic cells, therefore the main salivary cortisol is free cortisol with biological activity. Studies have shown that the level of salivary cortisol is well consistent with the level of free cortisol in the blood.[30]
Up to now, there has been scarcely study on salivary cortisol and dyslexia. But in a previous study concerning on children autism, a significant relationship was found between salivary cortisol level and impairments in social interaction and verbal language.[31] The results of this study show that salivary cortisol levels in dyslexic children were lower than those in normal children regardless of morning, afternoon or night, indicating the injury of HPA axis. Significantly higher or lower daily secretion of cortisol than normal value, or the disappeared circadian rhythm may affect individuals’ physical health, behavioral problems, cognitive ability and emotion. Previous studies have shown that children with behavioral problems were associated with baseline cortisol change, and that those with introverted behavior problems had higher baseline cortisol, while those with explicit behavior problems had lower baseline cortisol.[32] Researchers found that children with ADHD showed low response to stress, and their cortisol level was lower than normal children, suggesting that low response of HPA axis is closely related to the core symptoms of ADHD.[33] In addition to reading and writing difficulties, dyslexic children are often accompanied by overt behaviors such as hyperactivity and impulsive behavior, difficulty in paying attention, which may be also associated with low cortisol levels. Dyslexia has been found as comorbid of ADHD,[19,20] cortisol may play an important role in dyslexia, similar as its effects on ADHD.
This study also found that the circadian rhythm (day high and night low) of cortisol in the dyslexic children disappeared, which also revealed from another perspective that the dyslexic children had the disorder of HPA axis activity and function. The rhythmicity of cortisol plays an important role in promoting the normal physiological function of HPA axis. Its rhythm is not only affected by external factors such as sleep and season, but also by stress factors such as mental, psychology or physical status. The pressure faced by dyslexic children is persistent. Their academic achievement is usually low due to learning difficulties, and their parents and teachers do not understand the requirements for learning pressure. Moreover, children with dyslexia often suffer from peer discrimination in the group, which will bring long-term and chronic pressure on them. Psychosocial stress during childhood and adolescence is associated with alterations in the HPA axis, which is implicated in poor health.[34]
Moderate stress exposure may initially cause HPA axis activity increase, but when the pressure continues existing, long-term overloaded work can reduce the flexibility and adjusting role of HPA axis, leading to disorders of the activities. HPA axis disorder may result in reduced cortisol secretion and disappeared rhythm. In turn, changes in cortisol further disrupt the function of the HPA axis, leading to changes in cognition and behavior. Previous studies have also confirmed that neuropsychiatric diseases such as depression, anxiety, stress and hyperactivity disorder are closely related to the dysfunction of the HPA axis,[35,36] the dysfunction of the HPA axis may be also associated with dyslexia.
4.2. MT and dyslexia
MT is a kind of indoline neurohormone secreted by the pineal gland, widely distributed in the central and peripheral tissues. MT is involved in various regulatory processes through autocrine and paracrine effects, such as biorhythm, sleep, antiinflammatory, antioxidant, mood, metabolism and reproduction.[37–40] A large number of studies indicated that MT played nerve protective effects in the pathological process of convulsion brain damage, ischemic hypoxic brain damage, neurodegenerative diseases,[41–43] probably by regulating the neurotransmitter activity or receptor binding with neurotransmitter to exert nerve protective effect. Therefore, MT is considered as a potential way for brain damage interventions. Under normal physiological state, the secretion of MT is mainly affected by the external environment such as light, which has a rhythmical nature of low day and high night. It is generally believed that the level of MT starts to gradually increase around 21:00-22:00 pm after sleeping, reaches the peak at 0:00-3:00 in the morning, and then gradually decreases, and the secretion peak stops at 7:00-9:00 am.[44]
The present study found that the salivary MT in dyslexic children was lower than that of the normal children, and the rhythm of day low/night high disappeared. The decrease of MT level and the disappearance of circadian rhythm may have an impact on functional changes of the HPA axis, thus affecting its cognitive function. Some studies suggest that MT can regulate HPA axis function, and the low level of MT and rhythmic change will reduce the regulating function and further affect the activity of HPA axis and its function.[45,46] Changes in MT secretion rhythm are also seen in other various mental disorders, especially mood disorders.[47,48] Studies showed that the depression level of students with dyslexia was 10% to 15% higher than that of normal students.[49] Students with learning difficulties also report higher academic anxiety, stress, tension, depression, and helplessness. Compared with normal students, students with dyslexia report lower academic achievement, effort involvement, self-efficacy, and sense of belonging, but higher feelings of loneliness and negative emotions.[29,50,51] Prolonged chronic stress will also cause the diurnal rhythm imbalance of MT secretion. The dyslexic children in this study are primary students from grade 3 to 6, their academic achievement has been in a low level all the time, which has not been recognized as dyslexia before accepting the dyslexia screening in our stage I study. They are being in the status of not recognition and understanding for long period, bearing great pressure of study and psychology, which are prone to emotional problems, and further lead to low MT secretion level and circadian rhythm disorder, promote cognitive impairment. As a neuroendocrine hormone, MT is involved in stress response. Detanico et al[52] gave a certain amount of MT treatment to chronic stress mice, which can reduce the elevated level of corticosterone to the normal level and reverse the depressive behavior. By abdominal injection of MT to HPA axis suppression rat induced by corticosterone, the plasma adrenocorticotropic hormone and corticosterone levels are rising, which indicates that exogenous MT can improve the HPA axis suppression state.[53]
4.2.1. Strengths and limitations
Saliva collection has the advantages of convenient, painless, noninvasive and nonstimulating, which can avoid instantaneous physiological and biochemical reactions caused by stimulus factors. Salivary cortisol and MT levels are independent of saliva flow, so measurement of salivary cortisol and MT level is not affected by individual differences in saliva flow. In addition, saliva has the advantage of continuous collection, which is suitable for dynamic monitoring the change of cortisol and MT levels with rhythmical secretion characteristics. Also, there are some limitations existed in this study. Relatively small sample size may limit the deduction from the results. Another limitation of our study is that we were unable to adjust for other saliva biochemical indexes. We have not yet performed intervention and prospective follow up. However, this study firstly evaluated the relationships between cortisol, MT levels and dyslexia, which provide some clues for further researches. In addition, further mechanism explorations are imminently needed based on these findings.
5. Conclusions
The levels of salivary cortisol and MT in children with dyslexia were lower than normal children, and the circadian rhythm of their secretion disappeared, indicating the imbalance of activity and function impairment of HPA axis. HPA axis dysfunction may play an important role in the pathogenesis of dyslexia, but its mechanism has not been defined yet currently and needs to be further studied. Salivary cortisol and MT levels may be used as a reference for early diagnosis of dyslexia and as an indicator of therapeutic effect.
Author contributions
Conceptualization: Kusheng Wu.
Data curation: Yanhong Huang, Meirong He.
Formal analysis: Meirong He, Wenlong Huang.
Investigation: Meirong He, Wenlong Huang.
Methodology: Yanhong Huang, Chongtao Xu, Wenlong Huang.
Resources: Chongtao Xu.
Supervision: Kusheng Wu.
Writing – original draft: Yanhong Huang.
Writing – review & editing: Chongtao Xu, Kusheng Wu.
Kusheng Wu orcid: 0000-0001-5163-2524.
Footnotes
Abbreviations: ADHD = attention deficit hyperactivity disorder, CV = coefficients of variation, DSM-5 = diagnostic and statistical manual of mental disorders, fifth edition, HPA = hypothalamic-pituitary-adrenal gland, MT = melatonin.
How to cite this article: Huang Y, Xu C, He M, Huang W, Wu K. Saliva cortisol, melatonin levels and circadian rhythm alterations in Chinese primary school children with dyslexia. Medicine. 2020;99:6(e19098).
This work was supported by Shantou Science and Technology Project (No.: 170817181930822), the Guangdong Province Office of Education (No.: 2018GXJK044), and the Li Ka Shing Foundation.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Snowling MJ. Dyslexia. Oxford: Blackwell; 2000. [Google Scholar]
- [2].Karande S, Bhosrekar K, Kulkarni M, et al. Health-related quality of life of children with newly diagnosed specific learning disability. J Trop Pediatr 2009;55:160. [DOI] [PubMed] [Google Scholar]
- [3].APA Diagnostic and statistical manual of mental disorders, fifth edition (DSM-5). Washington DC: APA(American Psychiatric Association); 2013. [Google Scholar]
- [4].Song RR, Wu HR. Epidemic study on Chinese dyslexic children. Matern Child Health Care Chin 2008;23:1505–7. [Google Scholar]
- [5].Galaburda AM, Sherman GF, Rosen GD, et al. Developmental dyslexia: four consecutive patients with cortical anomalies. Ann Neurol 2010;18:222–33. [DOI] [PubMed] [Google Scholar]
- [6].Seki A, Koeda T, Sugihara S, et al. A functional magnetic resonance imaging study during sentence reading in Japanese dyslexic children. Brain Dev 2001;23:312–6. [DOI] [PubMed] [Google Scholar]
- [7].Seki A, Okada T, Koeda T, et al. Phonemic manipulation in Japanese: an fMRI study. Cognitive. Brain Res 2004;20:261–72. [DOI] [PubMed] [Google Scholar]
- [8].Shaywitz BA, Shaywitz SE, Pugh KR, et al. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biol Psychiatry 2002;52:101–10. [DOI] [PubMed] [Google Scholar]
- [9].Meng X, You H, Song M, et al. Neural deficits in auditory phonological processing in Chinese children with English reading impairment. Bilingualism Language &. Cognition 2016;19:331–46. [Google Scholar]
- [10].Kong R, Shao S, Wang J, et al. Genetic variant in DIP2A gene is associated with developmental dyslexia in Chinese population. Am J Med Genet B Neuropsychiatr Genet 2016;171:203–8. [DOI] [PubMed] [Google Scholar]
- [11].Nopolahemmi J, Myllyluoma B, Haltia T, et al. A dominant gene for developmental dyslexia on chromosome 3. J Med Genet 2001;38:658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ramus F, Rosen S, Dakin SC, et al. Theories of developmental dyslexia: insights from a multiple case study of dyslexic adults. Brain 2003;126:841–65. [DOI] [PubMed] [Google Scholar]
- [13].Tallal P, Miller S, Fitch RH. Neurobiological basis of speech: a case for the preeminence of temporal processing. Ann N Y Acad Sci 2010;682:27–47. [DOI] [PubMed] [Google Scholar]
- [14].Liu L, Li H, Zhang M, et al. Aberrant topologies and reconfiguration pattern of functional brain network in children with second language reading impairment. Dev Sci 2016;19:657. [DOI] [PubMed] [Google Scholar]
- [15].Nicolson RI, Fawcett AJ, Dean P. Developmental dyslexia: the cerebellar deficit hypothesis. TRENDS NEUROSCI 2001;24:508–11. [DOI] [PubMed] [Google Scholar]
- [16].Angeli E, Korpa T, Johnson EO, et al. Salivary cortisol and alpha-amylase diurnal profiles and stress reactivity in children with attention deficit hyperactivity disorder. Psychoneuroendocrinology 2018;90:174–81. [DOI] [PubMed] [Google Scholar]
- [17].Kamradt JM, Momany AM, Nikolas MA. A meta-analytic review of the association between cortisol reactivity in response to a stressor and attention-deficit hyperactivity disorder. Atten Defic Hyperact Disord 2018;10:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Schloss S, Ruhl I, Muller V, et al. Low hair cortisol concentration and emerging attention-deficit/hyperactivity symptoms in preschool age. Dev Psychobiol 2018;60:722–9. [DOI] [PubMed] [Google Scholar]
- [19].Germano E, Gagliano AP. Comorbidity of ADHD and dyslexia. Dev Neuropsychol 2010;35:475–93. [DOI] [PubMed] [Google Scholar]
- [20].Wiśniewska B, Baranowska W, Wendorff J. The assessment of comorbid disorders in ADHD children and adolescents. Adv Med Sci 2007;52: Suppl 1: 215–7. [PubMed] [Google Scholar]
- [21].Shukla M, Govitrapong P, Boontem P, et al. Mechanisms of melatonin in alleviating Alzheimer's disease. Curr Neuropharmacol 2017;15:1010–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Du X, Pang TY. Is dysregulation of the HPA-Axis a core pathophysiology mediating co-morbid depression in neurodegenerative diseases? Front Psychiatry 2015;6:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Park G, Jung YS, Park MK, et al. Melatonin inhibits attention-deficit/hyperactivity disorder caused by atopic dermatitis-induced psychological stress in an NC/Nga atopic-like mouse model. Sci Rep 2018;8:14981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lee B, Shim I, Lee H, et al. Melatonin ameliorates cognitive memory by regulation of cAMP-response element-binding protein expression and the anti-inflammatory response in a rat model of post-traumatic stress disorder. BMC Neurosci 2018;19:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jung C, Greco S, Nguyen HH, et al. Plasma, salivary and urinary cortisol levels following physiological and stress doses of hydrocortisone in normal volunteers. BMC Endocr Disord 2014;14:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Maas C, Ringwald C, Weber K, et al. Relationship of salivary and plasma cortisol levels in preterm infants: results of a prospective observational study and systematic review of the literature. Neonatology 2014;105:312–8. [DOI] [PubMed] [Google Scholar]
- [27].Bozovic D, Racic M, Ivkovic N. Salivary cortisol levels as a biological marker of stress reaction. Med Arch 2013;67:374–7. [DOI] [PubMed] [Google Scholar]
- [28].Bohak Z, Szabo F, Beckers JF, et al. Monitoring the circadian rhythm of serum and salivary cortisol concentrations in the horse. Domest Anim Endocrinol 2013;45:38–42. [DOI] [PubMed] [Google Scholar]
- [29].Nelson JM, Harwood H. Learning disabilities and anxiety: a meta-analysis. J Learn Disabil 2011;44:3. [DOI] [PubMed] [Google Scholar]
- [30].Estrada-Y-Martin RM, Orlander PR. Salivary cortisol can replace free serum cortisol measurements in patients with septic shock. Chest 2011;140:1216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tordjman S, Anderson GM, Kermarrec S, et al. Altered circadian patterns of salivary cortisol in low-functioning children and adolescents with autism. Psychoneuroendocrinology 2014;50:227–45. [DOI] [PubMed] [Google Scholar]
- [32].Clauss JA, Avery SN, Blackford JU. The nature of individual differences in inhibited temperament and risk for psychiatric disease: a review and meta-analysis. Prog Neurobiol 2015;127-128:23–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].van West D, Claes S, Deboutte D. Differences in hypothalamic-pituitary-adrenal axis functioning among children with ADHD predominantly inattentive and combined types. Eur Child Adolesc Psychiatry 2009;18:543–53. [DOI] [PubMed] [Google Scholar]
- [34].Chiang JJ, Ko A, Bower JE, et al. Stress, Psychological Resources, and HPA and Inflammatory Reactivity During Late Adolescence. Dev Psychopathol 2018. 699–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Holsboer F. Stress, hypercortisolism and corticosteroid receptors in depression: implications for therapy. J Affect Disord 2001;62:77–91. [DOI] [PubMed] [Google Scholar]
- [36].Lupien SJ, McEwen BS, Gunnar MR, et al. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 2009;10:434–45. [DOI] [PubMed] [Google Scholar]
- [37].Besseau L, Benyassi A, Moller M, et al. Melatonin pathway: breaking the ’high-at-night’ rule in trout retina. Exp Eye Res 2006;82:620–7. [DOI] [PubMed] [Google Scholar]
- [38].Bubenik GA. Gastrointestinal melatonin: localization, function, and clinical relevance. Dig Dis Sci 2002;47:2336–48. [DOI] [PubMed] [Google Scholar]
- [39].Thor PJ, Krolczyk G, Gil K, et al. Melatonin and serotonin effects on gastrointestinal motility. J Physiol Pharmacol 2007;58: Suppl 6: 97–103. [PubMed] [Google Scholar]
- [40].Valdes-Tovar M, Escobar C, Solis-Chagoyan H, et al. Constant light suppresses production of Met-enkephalin-containing peptides in cultured splenic macrophages and impairs primary immune response in rats. Chronobiol Int 2015;32:164–77. [DOI] [PubMed] [Google Scholar]
- [41].He P, Ouyang X, Zhou S, et al. A novel melatonin agonist Neu-P11 facilitates memory performance and improves cognitive impairment in a rat model of Alzheimer’ disease. Horm Behav 2013;64:1–7. [DOI] [PubMed] [Google Scholar]
- [42].Maresova D, Riljak V, Mares J. Melatonin modulates hypoxia-induced changes of rat brain excitability. Gen Physiol Biophys 2010;29:67–71. [DOI] [PubMed] [Google Scholar]
- [43].Wilhelm EA, Jesse CR, Bortolatto CF, et al. Correlations between behavioural and oxidative parameters in a rat quinolinic acid model of Huntington's disease: protective effect of melatonin. Eur J Pharmacol 2013;701:65–72. [DOI] [PubMed] [Google Scholar]
- [44].Koch M, Ferreiros N, Geisslinger G, et al. Rhythmic control of endocannabinoids in the rat pineal gland. Chronobiol Int 2015;32:869–74. [DOI] [PubMed] [Google Scholar]
- [45].Rendon NM, Rudolph LM, Sengelaub DR, et al. The agonistic adrenal: melatonin elicits female aggression via regulation of adrenal androgens. Proceedings. Biol Sci 2015. 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zhou J, Zhang J, Luo X, et al. Neu-P11, a novel MT1/MT2 agonist, reverses diabetes by suppressing the hypothalamic-pituitary-adrenal axis in rats. Eur J Pharmacol 2017;812:225–33. [DOI] [PubMed] [Google Scholar]
- [47].Adamczak-Ratajczak A, Kupsz J, Owecki M, et al. Circadian rhythms of melatonin and cortisol in manifest Huntington's disease and in acute cortical ischemic stroke. J Physiol Pharmacol 2017;68:539–46. [PubMed] [Google Scholar]
- [48].Ferri L, Filardi M, Moresco M, et al. Non-24-hour sleep-wake rhythm disorder and melatonin secretion impairment in a patient with pineal cyst. J Clin Sleep Med 2017;13:1355–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sideridis GD. Understanding low achievement and depression in children with learning disabilities: a goal orientation approach. Int Rev Res Ment Retard 2006;31:163–203. [Google Scholar]
- [50].Lackaye TD, Margalit M. Comparisons of achievement, effort, and self-perceptions among students with learning disabilities and their peers from different achievement groups. J Learn Disabil 2006;39:432. [DOI] [PubMed] [Google Scholar]
- [51].Sena JD, Lowe PA, Lee SW. Significant predictors of test anxiety among students with and without learning disabilities. J Learn Disabil 2007;40:360. [DOI] [PubMed] [Google Scholar]
- [52].Detanico BC, Piato AL, Freitas JJ, et al. Antidepressant-like effects of melatonin in the mouse chronic mild stress model. Eur J Pharmacol 2009;607:121–5. [DOI] [PubMed] [Google Scholar]
- [53].Zhong LY, Yang ZH, Li XR, et al. Protective effects of melatonin against the damages of neuroendocrine-immune induced by lipopolysaccharide in diabetic rats. Exp Clin Endocrinol Diabetes 2009;117:463–9. [DOI] [PubMed] [Google Scholar]


