Abstract
Background:
It has been estimated that a substantial portion of chronic and noncommunicable diseases can be caused or exacerbated by exposure to environmental chemicals. Multiple lines of evidence indicate that early life exposure to environmental chemicals at relatively low concentrations could have lasting effects on individual and population health. Although the potential adverse effects of environmental chemicals are known to the scientific community, regulatory agencies, and the public, little is known about the mechanistic basis by which these chemicals can induce long-term or transgenerational effects. To address this question, epigenetic mechanisms have emerged as the potential link between genetic and environmental factors of health and disease.
Objectives:
We present an overview of epigenetic regulation and a summary of reported evidence of environmental toxicants as epigenetic disruptors. We also discuss the advantages and challenges of using epigenetic biomarkers as an indicator of toxicant exposure, using measures that can be taken to improve risk assessment, and our perspectives on the future role of epigenetics in toxicology.
Discussion:
Until recently, efforts to apply epigenomic data in toxicology and risk assessment were restricted by an incomplete understanding of epigenomic variability across tissue types and populations. This is poised to change with the development of new tools and concerted efforts by researchers across disciplines that have led to a better understanding of epigenetic mechanisms and comprehensive maps of epigenomic variation. With the foundations now in place, we foresee that unprecedented advancements will take place in the field in the coming years. https://doi.org/10.1289/EHP6104
Introduction
Exposure to environmental chemicals in the air, water, soil, food, and consumer products, whether intentional or not, is an everyday occurrence (ACOG Committee Opinion No. 575 2013). Because global chemical production and usage are projected to increase in the coming decades (OECD 2012), there is a growing recognition that, although many comforts of modern life are due in part to chemicals that are on the market today, their sound management is critical for the preservation of human health and the environment (Massy and Jacobs 2013).
A recent assessment by an expert panel convened by the World Health Organization estimated that as many as 20% of cancers, 31% of cardiovascular diseases, 42% of stroke cases, and 35% of chronic obstructive pulmonary disease cases are attributable to environmental factors (Prüss-Ustün et al. 2016). Chronic illnesses that may be caused or exacerbated by environmental chemicals include asthma, obesity, Alzheimer’s disease, diabetes, infertility, and ovarian dysgenesis syndrome (reviewed by Bijlsma and Cohen 2016). Taken together, it has been estimated that the health and socioeconomic costs associated with environmental chemical exposure are likely to exceed 10% of the global gross domestic product, amounting to approximately $6.23 trillion (Grandjean and Bellanger 2017).
These reports indicate that there are major inadequacies in current risk assessment and hazard management practices. First, toxicity studies are generally conducted with individual chemicals with the objective of providing an estimated point of departure (Goodson et al. 2015). Such assessments, although undoubtedly useful, are ineffective at predicting the hazardous effects of chemicals that exhibit nonmonotonic dose responses and low dose relationships (reviewed by Vandenberg et al. 2012) and fail to account for mixture effects (reviewed by Bijlsma and Cohen 2016). An additional challenge is that a subgroup of these chemicals, endocrine-disrupting chemicals (EDCs), can mimic or alter endogenous endocrine processes (Alofe et al. 2019). Therefore, it is often challenging to disentangle the effects of environmental hazards (such as EDCs) from those of inherent differences within and between populations (Nadal et al. 2005). Indeed, multiple reports have shown that the effects of an exposure could differ in individuals depending on the individuals’ age or sex (ACOG Committee Opinion No. 575 2013). The burden of environmental hazards is reported to be inequitably distributed according to socioeconomic status, disproportionately affecting vulnerable populations (Brown 1995). Moreover, efforts to pinpoint associations between environmental chemicals and health effects in longitudinal studies are often complicated by the fact that chemical environments change relatively rapidly over time (LaKind et al. 2016).
To date, several major classes of hazardous or potentially hazardous environmental chemicals have been identified. These include toxic elements (e.g., arsenic, cadmium, lead, mercury); naturally occurring animal, plant, and food allergens; pesticides, persistent organic pollutants (e.g., perfluorooctanoic acid, polychlorinated biphenyls); volatile organic compounds (e.g., benzene, formaldehyde, gasoline); plastic components (e.g., bisphenol A, phthalates); and air pollutants (e.g., asbestos, radon, tobacco smoke) (reviewed by Bijlsma and Cohen 2016; Sears and Genuis 2012). Biochemical effects commonly reported to be triggered by environmental toxicants include oxidative stress, endocrine disruption, genotoxicity, enzyme inhibition, epigenetic changes, and dysbiosis (reviewed by Herceg et al. 2018; Sears and Genuis 2012). Evidently, there is a need for a concerted effort across all levels to understand and mitigate the impact of toxic exposures on human health and the environment.
A compelling puzzle in the field is the observation that a subgroup of environmental toxicants can induce persistent phenotypic effects after transient exposures, generating adverse effects that can be manifested years or even generations later (Gore et al. 2011; Skinner et al. 2008; Uzumcu et al. 2004; Vandenberg 2013). Although the mechanisms by which these long-lasting or transgenerational adverse effects occur have yet to be fully elucidated, one explanation for this phenomenon is that environmental toxicants could perturb epigenetic mechanisms (Verma 2015). Described as the interface between the environment and the genome, epigenetic marks are responsive to environmental stimuli and transmissible and are, therefore, promising mechanisms by which the effects of environmental toxicants are perpetuated (Herceg and Vaissière 2011).
In this commentary, we discuss the impact that environmental chemicals can have on epigenetic regulation, the impacts of chemical-induced epigenetic changes on health outcomes, current risk assessment strategies for detecting epigenetic modifiers, and the unique challenges of and strategies for incorporating epigenomic analyses in toxicology.
Epigenetics and Its Growing Role in Environmental Epidemiology
Epigenetic mechanisms refer collectively to mitotically or meiotically heritable alterations in gene expression (and associated phenotypic traits) that do not arise from changes in DNA nucleotide sequences (reviewed by Bird 2002; Feil and Fraga 2012). The definition of epigenetics encompasses chromatin modifications that directly influence chromatin conformation (i.e., DNA methylation and histone posttranslational modifications), as well as the activity of noncoding RNAs (ncRNAs), in view of accumulating evidence that ncRNAs can influence chromatin conformation and induce heritable effects (Ashe et al. 2012; Braconi et al. 2010; Buckley et al. 2012; Burton et al. 2011; Esteller 2011; Fabbri et al. 2007; Garzon et al. 2009; Lei et al. 2016) (Figure 1). Although these three modes of epigenetic regulation converge on a common cellular process (i.e., regulating gene expression), the mechanisms may act with different degrees of dynamic effects, strengths, and reversibility (Bintu et al. 2016). Moreover, although they are often studied independently, it is becoming increasingly clear that there is extensive cross-talk between DNA methylation, histone modifications, and ncRNAs (Hanly et al. 2018; Rose and Klose 2014; Vaissière et al. 2008).
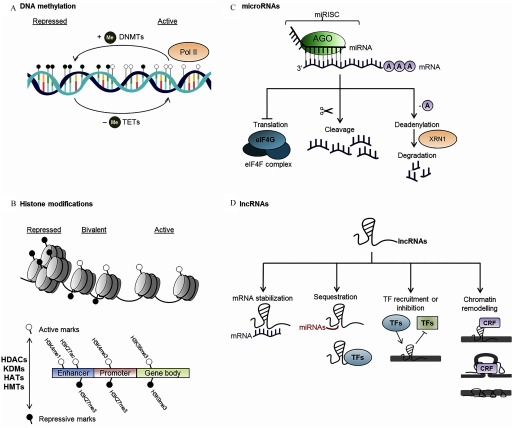
Figure 1.
Diagrammatical summary of epigenetic modes on action. (A) The deposition of methyl groups is mediated by the DNA methyltransferases (DNMTs), and their removal is mediated by the ten-eleven translocation (TET) family proteins (reviewed by Greenberg and Bourc’his 2019; Wu and Zhang 2010). Increased deposition of methyl groups promotes the condensation of chromatin, whereas reduced DNA methylation is associated with increased accessibility to transcription machinery [represented by RNA polymerase II (Pol II)]. DNA hypermethylation generally leads to the silencing of gene expression when it occurs at gene promoters (reviewed by Greenberg and Bourc’his 2019; Wu and Zhang 2010). (B) Histone modifications are deposited by multiple classes of enzymes [histone acetyltransferases (HATs), histone methyltransferases (HMTs), histone deacetylases (HDACs), and histone lysine demethylases (KDMs)] (reviewed by Chen et al. 2017). Examples of histone modifications illustrated here are histone H3 lysine 27 trimethylation (H3K27me3) and H3 lysine 9 trimethylation (H3K9me3), which are usually associated with repression of gene transcription, and H3 lysine 4 monomethylation (H3K4me1), H3 lysine 4 trimethylation (H3K4me3), H3 lysine 36 trimethylation (H3K36me3), and H3 lysine 27 acetylation (H3K27ac) marks that are typically associated with activation of transcription. Simultaneous deposition of opposing histone marks is associated with poised or bivalent chromatin, which is in a transitional state poised to be resolved into active or repressed states (reviewed by Chen et al. 2017). (C) microRNAs (miRNAs) bound to the Argonaute (AGO) protein make up the miRNA-induced silencing complex (miRISC). miRNAs direct the miRISC to target mRNAs base-pairing partially to complementary binding sites, which will be cleaved by catalytically active AGO proteins (O’Brien et al. 2018). Alternatively, AGO proteins can recruit additional protein partners, initiating the process of deadenylation, decapping and 5ʹ-to-3ʹ mRNA degradation by 5ʹ-to-3ʹ exoribonuclease 1 (XRN1) (reviewed by Jonas and Izaurralde 2015). There has also been evidence that miRNAs inhibit translation by inhibiting the eukaryotic initiation factor 4F (eIF4F) complex, although this process has yet to be fully elucidated (reviewed by Jonas and Izaurralde 2015). 5ʹ polyA tails are denoted by circles labeled A. (D) Long noncoding RNAs (lncRNAs) may influence gene expression by increasing or decreasing target mRNA stability, by acting as decoys to miRNA and transcription factors (TFs), thus sequestering them from their cognate promoters, or they may recruit or inhibit TF binding to their target sites on the chromatin (reviewed by Angrand et al. 2015). lncRNAs may also recruit chromatin remodeling factors (CRFs) such as the polycomb repressive complexes or cohesin proteins that recruit histone modifier complexes or initiate long-range chromatin looping (reviewed by Angrand et al. 2015). The Xist lncRNA triggers stable repression of the presumptive inactive X-chromosome by physically coating the X-chromosome (reviewed by Lee and Bartolomei 2013).
Epigenetic mechanisms regulate transcriptional activity across the genome and can thereby determine temporal and spatial gene expression patterns (Baylin and Jones 2011; Jaenisch and Bird 2003). The heritability of epigenetic marks allows for faithful maintenance of cell identity (Lee et al. 2014), whereas their reversibility allows for developmental plasticity, enabling the manifestation of different phenotypes in response to developmental cues and environmentally induced changes from a single unchanged genotype (Low and Gluckman 2018). Although some degree of stochastic epigenetic alterations, or epigenetic drift, can be observed in aging or as asymptomatic occurrences (Bjornsson et al. 2008; Feil and Fraga 2012; Fraga 2009; Wong et al. 2010), epigenetic aberrations have been described as important drivers of neurological diseases and cancer (Herceg et al. 2018; Zoghbi and Beaudet 2016). Therefore, external stimuli that can cause shifts away from baseline rates of age-related epigenetic drift have been described as elements that cause “environmental deflection” (Kochmanski et al. 2017).
Moreover, epigenetics can provide key mechanistic evidence for the developmental origins of health and disease (DOHaD) hypothesis (Goyal et al. 2019). The DOHaD concept was proposed as a means to explain epidemiological evidence linking early life exposure to environmental factors with the onset of adverse health effects later in life (Barouki et al. 2018; Barrett 2017; Bianco-Miotto et al. 2017). DOHaD proposes that early developmental stages are windows of vulnerability to psycho-social or chemical stressors. Hence, relatively small alterations at this stage of life could have impacts on risk of diseases across the life course into adulthood. This is in line with the notion that profound epigenetic reprograming, characterized by a sequential genome-wide erasure and de novo lineage-specific establishment of epigenetic marks, underlies pluripotency and lineage commitment, which occur early in embryonic development (Lee et al. 2014). Thus, perturbations during this window of development could enable the propagation of aberrant epigenetic patterns and associated gene activity states at doses of exposure that would otherwise be biologically inconsequential (Anway et al. 2005; Breton et al. 2017).
Environmental Toxicants as Disruptors of Epigenetic Regulation
Following the frequent inclusion of epigenetics as a parameter in environmental epidemiology studies, numerous reports have been made in recent years associating alterations in DNA methylation with various environmental factors, including biological agents, dietary habits, and air pollution (Ambatipudi et al. 2016; Barouki et al. 2018; de FC Lichtenfels et al. 2018; Degli Esposti et al. 2017; Fasanelli et al. 2019; Feil and Fraga 2012; Hattori and Ushijima 2016; Herceg et al. 2018; Martin and Fry 2018; Perrier et al. 2019; Woo et al. 2018). Similarly, many reports indicate that microRNA (miRNA) profiles are responsive to various environmental exposures, including air pollution [R Chen et al. (2018); Espín-Pérez et al. (2018), both epidemiological studies], nanoparticles [Brzóska et al. (2019), utilizing human liver cells], endocrine disruptors, such as bisphenol A (BPA) [Chou et al. (2017), using human endometrial cells, and Martínez-Ibarra et al. (2019), using human blood samples], and dichlorodiphenyltrichloroethane (DDT) [Krauskopf et al. (2017), using human blood]. Long noncoding RNAs (lncRNAs), although still a relatively new area of research, have been reported to be associated with levels of phthalates in first trimester urine of pregnant women (LaRocca et al. 2014), benzene in seven exposed individuals (Bai et al. 2014b), and occupational exposure to cadmium (Zhou et al. 2015) and to be responsive to developmental exposure to BPA in mice (Kumamoto and Oshio 2013) and to certain heavy metals in in vitro (Tani et al. 2014; Zhou et al. 2015) and in vivo experimental systems (Zhou et al. 2015). Alterations to histone marks have been reported in response to various substances including arsenic in mice (Cronican et al. 2013) and in cell lines (Jo et al. 2009; Li et al. 2002; Zhou et al. 2009), BPA [Senyildiz et al. (2017), using human neuroblastoma cells], phthalates [Sonkar et al. (2016), in mesenchymal stem cells], and dichlorodiphenyltrichloroethane [Ben Maamar et al. (2018), in male rats descended from exposed gestating female rats].
Table 1 provides a non-exhaustive summary of environmental chemicals with demonstrated epigenome-altering properties. Here, we discuss three salient examples, namely, benzene, vinclozolin, and BPA, in greater detail.
Table 1.
Summary of evidence of environmental chemicals and their effect on epigenetic regulation.
Note: Animal studies include findings in both vertebrate and invertebrate models. Plus signs (+) indicate that there is evidence that the compound(s) in question exhibits epigenetic-modulating properties in the systems specified. —, Not applicable; As, arsenic; BDE 47, tetrabromodiphenyl ether; Bi, bismuth; BPA, bisphenol A; Cd, cadmium; Cr, chromium; DBP, dibutyl phthalate; DEHP, di-2-ethylhexyl phthalate; EDC, endocrine-disrupting chemical; Fe, iron; Li, lithium; Ni, nickel; Pb, lead; p,pʹ-DDE, p,pʹ-dichlorodiphenoxydichloroethylene; S, sulfur; Sb, antimony; V, vanadium.
Benzene—Epigenome Perturbations at Low-Level Exposures
Benzene, a common aromatic compound in crude oils, is a human carcinogen (IARC 2018) and may be one of the most potent epigenetic modifiers in the environment. Airborne, occupational exposure to benzene has been consistently associated with hypomethylation in repetitive elements in peripheral blood DNA obtained from occupationally exposed individuals (Bollati et al. 2007; Fustinoni et al. 2012; Seow et al. 2012) and is associated with lower levels of -methylguanine-DNA methyltransferase (MGMT) promoter methylation in whole blood DNA (Li et al. 2017). Of note, significant differential methylation was reported between a group of individuals with a median personal benzene exposure of (Bollati et al. 2007) and in individuals exposed to a median 8-h time-weighted average benzene exposure of (Li et al. 2017), both of which are below the National Institute of Occupational Safety and Health’s recommended exposure limit for benzene () (NIOSH 2019). Similarly, individuals exposed to benzene at median concentrations of had significantly higher global levels of H3 lysine 4 trimethylation (H3K4me3) marks compared with a control group (J Li et al. 2018). Perturbations to miRNA (Bai et al. 2014a; Yang Liu et al. 2016) and lncRNA (Bai et al. 2014b) expression have likewise been detected in pooled plasma (Yang Liu et al. 2016) and peripheral blood mononuclear cell (Bai et al. 2014a, 2014b) samples of benzene-exposed individuals. Although it has been reported that environmental exposures to benzene also affect the transcriptome in blood mononuclear cells (McHale et al. 2009, 2011), the direct contribution of benzene-induced epigenetic alterations to the transcriptome remains to be established.
Benzene and some of its metabolites have also been shown to induce global hypomethylation in in vitro (Coulter et al. 2013; Hu et al. 2014; Ji et al. 2010) and in vivo (Philbrook and Winn 2015) experimental systems, recapitulating the effects observed in exposed humans. Cells subjected to multiple rounds of exposure to hydroquinone, a benzene metabolite, over a period of 5 weeks exhibited a gradual increase in the levels of H3K4me3, an active histone mark (Mancini et al. 2017). In mice, exposure to benzene was associated with lower levels of peripheral blood cells and hematopoietic progenitor cells in the bone marrow and alterations to the miRNAs mmu-miR-34a-5p; mmu-miR-342-3p; mmu-miR-100-5p, mmu-miR-181a-5p, and mmu-miR-196b-5p, many of which are dysregulated in hematological malignancies (Wei et al. 2015).
Vinclozolin—Transgenerational Impacts on the Epigenome
Vinclozolin is a dicarboximide fungicide used on fruits, vegetables, ornamental plants, and turf grass and is a prominent example of an environmental toxicant capable of triggering adverse health effects long after the initial exposure (Uzumcu et al. 2004). Mice exposed to vinclozolin in utero displayed significantly higher numbers of apoptotic germ cells at puberty [postnatal day (PND) 20], an effect that became more pronounced in adulthood (PND60) (Uzumcu et al. 2004). Moreover, maternal exposure to vinclozolin negatively affected fertility in male mice in the descendent F1 generation and epigenetic alterations in more than 1,000 targets (primarily hypomethylation events) compared with controls (Lee and Oh 2012). Guerrero-Bosagna et al. (2012) further observed that differential DNA methylation patterns could be observed up to the F3 generation of vinclozolin-lineage mice (Guerrero-Bosagna et al. 2012). Interestingly, administration of vinclozolin to pregnant mice led to higher levels of DNA methylation in the promoters of two paternally imprinted genes [imprinted maternally expressed transcript (H19) and maternally expressed 3 (Gtl2) also known as Meg3] and lower levels of DNA methylation in regions regulating three maternally imprinted genes [small nuclear ribonucleoprotein polypeptide N (Snrpn), paternally-expressed Gene 1 (Peg1) also known as Mest, and paternally expressed 3 (Peg3)] (Stouder and Paoloni-Giacobino 2010). In Sprague-Dawley rats, alterations in more than 200 small ncRNAs (Schuster et al. 2016) and 99 histone retention sites (Ben Maamar et al. 2018) were observed in the sperm from the F3 generation of vinclozolin-lineage rats compared with the control group. The F3 generation of vinclozolin-lineage male rats had higher incidence of prostate histopathology and abnormalities and displayed altered gene expression, ncRNA expression, and DNA methylation patterns compared with the untreated controls (Klukovich et al. 2019).
Because the abovementioned studies suggest that vinclozolin can induce transgenerational epigenetic effects, it stands to reason that differential epigenetic marks observed in the F3 generation should also be present in the F1 and F2 generations of the vinclozolin-lineage rats or mice. Intriguingly, however, analyses of F1 and F3 vinclozolin-lineage rats revealed that although there were a total of 290 differentially methylated regions (DMRs) () in the F1 generation (control vs. vinclozolin-lineage animals) and a total of 916 total DMRs in the F3 generation, there was no overlap between the F1 and F3 DMRs (Beck et al. 2017). This indicates that the epigenetic alterations accumulated in vinclozolin-lineage rats or mice are not directly inherited by subsequent generations. Instead, exposure could result in alterations to developmental programing and epigenetic mechanisms in early generations that are transmitted to subsequent generations, amplifying differences in epigenetic patterns between control and exposure-lineage mice.
One caveat to these findings, however, is that the transgenerational epigenetic effects of vinclozolin were reported in studies using intraperitoneal administration of vinclozolin at body weight per day (Ben Maamar et al. 2018; Lee and Oh 2012; Nilsson et al. 2018; Stouder and Paoloni-Giacobino 2010; Uzumcu et al. 2004). This is orders of magnitude above the calculated chronic population adjusted dose of (U.S. EPA 2000) or the estimated occupational exposure in production workers () (Zober et al. 1995). To our knowledge, there is currently no evidence that vinclozolin induces transgenerational effects when administered at lower doses and via dermal and/or oral routes, which are arguably closer to the likely routes of vinclozolin exposure in humans.
Bisphenol A—a Stealthy Hazard
No summary on environmental toxicants could be complete without the mention of BPA. BPA is now a household name, and it is not uncommon to see “BPA-free” statements on plastic products or printed material. Despite efforts to limit the usage of BPA, BPA production is predicted to increase in the coming decades (reviewed by Almeida et al. 2018). BPA is a known endocrine disruptor, exhibiting agonistic effects on both estrogen receptors alpha and beta (), and antagonistic effects on the androgen receptor (Delfosse et al. 2014). A wide range of health effects have been associated with BPA exposure, including obesity, reduced reproductive capacity, and metabolic disease (Manikkam et al. 2013). Traces of BPA were detected in 92.6% of sampled participants from the National Health and Nutrition Examination Survey (NHANES) (Calafat et al. 2008), suggesting that exposure to BPA is nearly ubiquitous.
BPA exposure has been consistently associated with hypomethylation in repetitive elements in humans (Faulk et al. 2015; Miao et al. 2014; Zheng et al. 2017). In a cross-sectional human study, multivariate analysis indicated that workplace BPA exposure was associated with significantly lower long interspersed nuclear element 1 (LINE-1) methylation levels relative to an unexposed control group, and that urine BPA levels were significantly inversely associated with LINE-1 methylation in sperm (Miao et al. 2014). In corroboration with this, a similar study reported that sperm collected from BPA-exposed men exhibited 19.6% higher levels of global 5-hydroxymethylcytosine, an intermediate of the demethylating process by ten-eleven translocation (TET) enzymes, compared with men without occupational exposure to BPA (Zheng et al. 2017). Interestingly, an epigenome-wide study on human fetal liver tissue reported that although exposure was generally associated with global hypomethylation, increasing BPA levels were positively correlated with hypermethylation of CpG islands, suggesting that the effects of BPA on DNA methylation and gene expression are genomic region-dependent (Faulk et al. 2015). In utero exposure to BPA was also found to induce persistent epigenetic effects. Using data from a longitudinal birth cohort, Goodrich et al. (2016) reported that blood leukocyte DNA methylation in participating peri-adolescents was positively correlated with increasing BPA levels both in archived maternal urinary samples collected during the third trimester of pregnancy and in the urinary samples obtained from the peri-adolescents themselves. Although this positive correlation was observed at all analyzed loci, namely, the LINE-1 repetitive elements, and imprinted genes H19, IGF2, and HSD11B2, statistical significance was observed only when associating maternal urinary BPA levels with IGF2 hypermethylation (Goodrich et al. 2016). However, these findings are not completely concordant with the previously described results in human subjects whereby BPA exposure was associated with decreased LINE-1 methylation. Nevertheless, taken together, these reports suggest that environmentally relevant concentrations of BPA can affect the epigenome.
The effect of BPA on the epigenome, including persistent and early life effects, has been demonstrated in numerous experimental models (Table 1). For example, prenatal exposure to BPA resulted in dysregulated expression of the lncRNAs Xist and its antisense Tsix in the mouse brain, suggesting that in utero BPA exposure during the critical period of brain development may affect neurodevelopment through the deregulation of epigenetic mechanisms involved in X-chromosome inactivation (Kumamoto and Oshio 2013). Epigenetic alterations were detected in mice exposed to environmentally relevant doses (as low as ) of BPA during development (Anderson et al. 2012). Similarly, rats exposed to transient, environmentally relevant doses of BPA exhibited greater prostate gland susceptibility to adult-onset precancerous lesions and persistent alterations in DNA methylation patterns (Ho et al. 2006).
However, it is worth noting that studies on BPA have not been immune to flaws in design and execution. An expert opinion on the design and execution of the Consortium Linking Academic and Regulatory Insights on BPA Toxicity (CLARITY-BPA) study has highlighted that the use of gavage as a route of exposure, without a nongavaged negative control, could complicate the interpretation of the results and lead to erroneous conclusions (vom Saal 2019). This argument was previously made by Vandenberg et al. (2014) in the context of EDCs (Vandenberg et al. 2014), indicating that further review and reconsideration of supposedly established methods may be in order.
Discussion
Epigenetic Marks in Risk Assessment
As described in the previous section, stable epigenetic patterns can be observed in response to a wide range of environmental toxicants. Thus, we hold the opinion that there are clear benefits to using epigenetic marks as biomarkers of exposure, for reasons which will be discussed in this section. For instance, DNA methylation events are particularly attractive as biomarkers because of their relative robustness in different storage conditions (Ghantous et al. 2014; Thirlwell et al. 2010; Wong et al. 2008).
Moreover, the properties of DNA methylation biomarkers as both stable and responsive to environmental cues make them attractive candidates as markers of exposure and predictive markers of disease (Ladd-Acosta 2015). DNA methylation-based predictors of health and lifestyle factors such as smoking habits (Ambatipudi et al. 2016; Bojesen et al. 2017; McCartney et al. 2018; Philibert et al. 2012, 2016), alcohol consumption (McCartney et al. 2018), and body mass index (McCartney et al. 2018) have been described and have been proposed as molecular predictors of disease and morbidity or as an alternative to self-reported measurements. Because epigenetic marks can persist even after cessation of the exposure, using epigenetic marks as biomarkers of exposure could be particularly useful in situations where the exposures tested have short half-lives in vivo (Ladd-Acosta 2015).
As biomarkers of disease, epigenetic disease signatures, or episignatures, have been described in cancers (Crujeiras et al. 2019), neurological diseases (Bend et al. 2019; De Jager et al. 2014; Henderson-Smith et al. 2019), and heart diseases (Roetker et al. 2018) and may be applied as molecular predictors of disease or indicators of disease progression. Epigenetics-based diagnostic tests are already commercially available; examples include the DNA methylation-based Epi proColon® 2.0 CE (Epigenomics AG) for colorectal cancer (Lamb and Dhillon 2017) and the miRNA-based RosettaGX Reveal™ (Rosetta Genomics) for thyroid malignancies (Lithwick-Yanai et al. 2017).
Episignatures as biomarkers have, in theory, the potential of capturing information on current tissue states, predisposition to disease, and the cumulative effects of environmental stressors and exposures. However, although carcinogenicity testing is an important component of testing for regulatory approval, the role of toxico-epigenomics in the risk assessment process has yet to be established (Herceg et al. 2013; Ray et al. 2014).
Challenges of Applying Epigenetics in Toxicology
A major impediment to the application of epigenomic analysis in toxicology is the fact that consensus normal epigenomes have yet to be fully defined for all tissue and cell types (Stueve et al. 2016; Tonge and Gant 2016). Given the large epigenetic variability within and between tissue types, age groups, and populations, reference epigenomes are particularly critical (Marczylo et al. 2016; Marsit 2015) because comparing epigenetic patterns across heterogeneous sample groups without suitable adjustment will, in our experience, substantially reduce statistical power.
These challenges could be overcome in the near future with the introduction of correction methods for cell-composition effects (Breeze et al. 2016; Houseman et al. 2016; McGregor et al. 2016; Zou et al. 2014) and the generation of cell-type-specific epigenomic maps by large-scale initiatives of the International Human Epigenome Consortium (Bujold et al. 2016), namely, Encyclopedia of DNA Elements (ENCODE) (Djebali et al. 2012; ENCODE Project Consortium 2012), the National Institutes of Health Roadmap Epigenomics Program (Roadmap Epigenomics Consortium et al. 2015), the BLUEPRINT projects (Fernández et al. 2016), and the 4D Nucleome Project (Dekker et al. 2017). The Toxicant Exposures and Responses by Genomic and Epigenomic Regulators of Transcription (TaRGET) program provides an invaluable resource of high-quality transcriptomic, epigenomic, and chromatin accessibility data from experimental in vivo systems that not only enables an improved understanding of the potential impacts of environmental exposures on the epigenome but also provides a means to better assess the utility of surrogate tissues (Wang et al. 2018). However, although these resources have brought unprecedented progress to the field, gaps remain in the representation of different human populations and phenotypes that need to be addressed.
Another challenge that especially affects the testing of EDCs is the fact that the health outcomes associated with exposure and epigenetic alterations can occur years or generations after the initial exposure. As discussed in previous sections, epigenetic impacts are often amplified when exposure occurs in early life (Barouki et al. 2018)—a vulnerable period that is not generally amenable to human testing. A further complication is the fact that epigenetic mechanisms act in concert to have a net functional effect on chromatin accessibility and gene regulation (Kelly et al. 2010). Therefore, a single end point measure is often insufficient to gain a thorough understanding of the epigenotoxicity of a compound.
A potential solution for studying environmental chemicals (such as EDCs) with unknown or persistent effects is to conduct real-time observations of epigenetic states. In recent years, applications of reporter systems have enabled real-time observation of DNA methylation, enhancing our understanding of the dynamics of epigenetic states in development. For instance, in vitro reporters (Bintu et al. 2016; Stelzer et al. 2015), fluorescent probes specific for DNA methylation (Kumar et al. 2018), the MethylRO mouse (Ueda et al. 2014), and the mCherry-MBD transgenic zebrafish line (Zhang et al. 2017) enable users to observe spatial and temporal changes to epigenetic states. An application of fluorescence lifetime imaging-based Förster resonance energy transfer (FLIM-FRET) has demonstrated the interactions between epigenetic marks and the and the disruption of these interactions by the antiestrogen tamoxifen (Liu et al. 2019). High-throughput applications of this approach could enable the screening of other endocrine regulators in response to a wide array of environmental chemicals. Other live-cell methods include bimolecular anchor detector (BiAD) sensors, which have been applied for the detection of DNA methylation and H3K9me3 levels in living cells (Lungu et al. 2017). This system gives a fluorescence readout dependent on co-occurrent binding of epigenetic mark-specific detector proteins and site-specific anchor proteins, which are targeted to sites of interest by zinc-finger, transcription activator-like (TAL)-effector or clustered regularly interspaced short palindromic repeats (CRISPR)-dCas9 systems (Lungu et al. 2017).
The third challenge is the limitations of current epigenomic assays. Current assays can be broadly divided into three categories: measurements of global epigenetic effects, locus-specific assays, and epigenome-wide methods. Commonly used measurements of global epigenetic effects include techniques such as high-performance liquid chromatography (Roberts et al. 2018), mass spectrometry (Chen et al. 2013; Molden and Garcia 2014; Yuan et al. 2014), dot blots (Jia et al. 2017), enzyme-linked immunosorbent assay (reviewed by Andersen et al. 2015), luminometric methylation assays (Karimi et al. 2006), and bisulfite pyrosequencing of repetitive elements (Yang et al. 2004). Cell-based reporter assays for global methylation have also been described (Baba et al. 2019; Yoshida et al. 2016). Although these techniques have undoubtedly produced valuable findings, potentially epigenotoxic compounds may induce locus-specific effects that are too subtle to be detected by these methods. Moreover, whereas DNA methylation levels in repetitive elements are often used as surrogates for global DNA methylation, these readings do not always correlate well with true global DNA methylation (BLUEPRINT Consortium 2016).
Techniques that enable locus-specific assays include bisulfite pyrosequencing, amplicon bisulfite sequencing, and cell-based reporter assays (Han et al. 2013; Stelzer et al. 2015). In vivo models include intracisternal A-particle (IAP) mouse models, based on IAP retrotransposon reporter-type systems (Blewitt and Whitelaw 2013). IAP mouse models display a range of coat color and tail morphology changes that reflect epigenetic states in response to test materials administered to the mice. One limitation of these models is that changes in tail kinks and coat color may be subtle and could be subject to observer bias. Moreover, a limitation that affects all locus-specific assays is that a priori knowledge of the gene to be analyzed is required, making such assays less useful in screening for new epigenetic modifiers with unknown targets.
Epigenome-wide methods, as the name suggests, provides high resolution and coverage for the epigenetic mark analyzed. Requiring no a priori knowledge of potential targets, epigenome-wide methods enable the discovery of new biomarkers and target regions. Examples include DNA methylation arrays, reduced-representation bisulfite sequencing (RRBS) (Meissner et al. 2005), histone chromatin immunoprecipitation sequencing (ChIP-seq) (Schmidl et al. 2015), and whole-genome bisulfite sequencing (Urich et al. 2015). These methods, although highly informative, are to our knowledge not currently amenable to miniaturization or cost effective for screening large libraries of compounds at a time. Nevertheless, this could change in the near future as more resources are dedicated to this field of study and the costs of sequencing and analysis are reduced.
Evidently, none of the discussed methods would be universally applicable. In a review by Rasoulpour et al. (2011), the authors suggested that the ideal toxicology screen should be scalable to medium or high throughput, be in vitro based, be cost effective, have robust end points, and have good false-positive/negative rates (Rasoulpour et al. 2011). Although none of the methods discussed match such an ideal screening method (Figure 2), future technological advances could make this a reality.
Figure 2.
Comparison of major techniques for epigenetics analyses on subjective scales for scalability and direct biological relevance. Axes are in arbitrary scales, with scalability denoting how amenable a given technique is to be implemented in a high-throughput screening setting. Direct biological relevance denotes how information-rich the results of a given technique are. Note: ATAC-seq, assay for transposase-accessible chromatin using sequencing; ChIP, chromatin immunoprecipitation sequencing; ELISA, enzyme-linked immunosorbent assay; HPLC, high-performance liquid chromatography; RRBS, reduced-representation bisulfite sequencing; WGBS, whole-genome bisulfite sequencing.
One particularly exciting avenue of development is the rapid advance of genome editing technology. The relative efficiency and adaptability of CRISPR/Cas9/Cas12a (Campa et al. 2019; Ding et al. 2019) techniques could soon give rise to multi-end point, high-throughput, in vitro reporter assays for epigenetic modifiers. In our view, the first challenge to reach this goal is to identify robust and biologically relevant end points for which reporter assays could be generated. Future assays could reflect higher-order chromatin structures in biologically important regions of the chromatin, as opposed to being locus-specific. One of the most exciting new developments in the field has been the development and refinement of techniques that profile chromatin accessibility, whether by sequencing (Buenrostro et al. 2015; Corces et al. 2018) or super-resolution imaging (Bintu et al. 2018). As discussed previously, examining a single aspect of epigenetic regulation may often be insufficient, because epigenetic mechanisms typically work in concert. By assessing higher-order chromatin states, these techniques take into account the summation of these mechanisms. Although these methods are currently not as easily implemented in a high-throughput setting, further research in this field is certainly warranted.
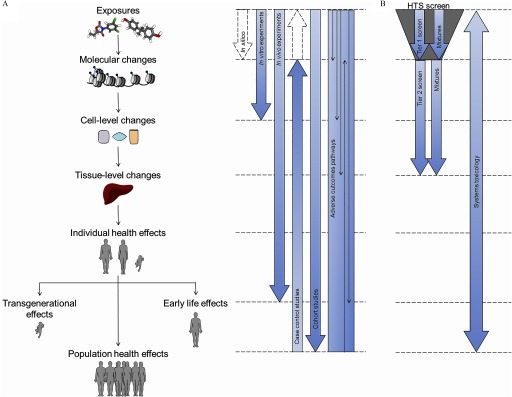
Finally, it may also be argued that in vitro screening measures would be limited in identifying biologically relevant epigenetic toxicants. However, we hold the opinion that the implementation of screening programs is still useful because they could lead to the identification of highly epigenotoxic compounds, thus halting their use or introduction into the environment until further analyses are carried out. Moreover, it is worth noting that each class of method—whether in vitro, in vivo, in silico, or based on populations—will have a different capacity to provide different levels of evidence, as well as different strengths and weaknesses (Figure 3A).
Figure 3.
An integrative method for investigating the impact of environmental chemicals on the epigenome and a proposed approach for toxico-epigenomic screening. (A) Comparison of in silico, in vitro, in vivo, population-based, and integrative methods in fully understanding the potential effects of environmental chemicals on the epigenome. Dotted arrows indicate situations where evidence can be inferred but not directly proved by the described methods. (B) An illustration of a proposed approach for toxicoepigenomic screening. A high-throughput screen (HTS) using in vitro and in silico methods can be conducted using single compounds and mixtures. Hits identified from the Tier 1 screen can be characterized more extensively using relevant in vitro and in vivo experiments. Finally, a systems toxicology approach could be used to integrate all data, including human data, to generate a complete profile of epigenotoxicology.
Even assuming that all the technical challenges can be overcome, interpreting epigenetic results from the perspective of classical toxicology could still be complicated. For instance, if a change in epigenetic state is identified, is it truly an adverse event, or is it an early adaptive response? Is there a no-effect threshold when it comes to epigenetic modification?
Strategies for Toxico-Epigenomic Screening
In addition to technical improvements, screening can be improved by reassessing current strategies for evaluating environmental toxicants as epigenetic modifiers. In this section, we discuss potential strategies for conducting epigenomic screening in the face of current technical limitations.
Applying drug discovery pipelines for identifying epigenetic modifiers.
Perspectives from the drug discovery pipeline could prove useful for developing similar approaches for toxicological screening. The burgeoning fields of computer-aided drug design and molecular docking have been successfully implemented for drug discovery processes (Macalino et al. 2015). By using homology models of the DNMTs or, in theory, any of the epigenetic enzymes, it is possible to perform virtual screens of thousands of compounds at relatively little cost. As reviewed by Lu et al. (2018), this approach has already led to the discovery of the DNMT inhibitors RG108, NSC14778, nanaomycin A, SET7 inhibitor DC_S100, and EZH2 inhibitor DCE_254, among others (Lu et al. 2018). This approach could also be applied in toxicology to screen for potential epigenetic disruptors. However, although in silico approaches offer invaluable opportunities for streamlining target discovery processes, this does not preclude the usual standards of in vivo and in vitro testing. It does, however, enable the prioritization of potential targets.
On a related point, another approach for identifying lead chemicals is to pinpoint molecular features that enable the identification of epigenetic perturbagens. To date, common features have been identified in a subset of epigenetic modifiers, for instance phenoxyacetic acid moieties in fibrates, and the presence of halogenated polycyclic features in DDT, polychlorinated biphenyls, and chlordane (Kobets et al. 2019). This approach has been challenging because nongenotoxic epigenetic agents typically lack easily identifiable DNA-reactive chemical features. Moreover, as far as we know, the features identified in fibrates and polychlorinated compounds are not direct evidence of epigenetic-modifying ability. However, we expect that improvements in computational technology, artificial intelligence, and our understanding of the chemical processes underlying epigenetic modifications could bring new advances in this area of study.
Advances in high-content screening or high-content analysis (HCS/HCA) can also be adapted for toxico-epigenomics. HCS/HCA has been applied in drug discovery and has been driven by advances in automated microscopy and image analysis (Esner et al. 2018). An example of this is the recently described microscopic imaging of the epigenetic landscape (MIEL) system (Farhy et al. 2019). In this approach, the authors applied HCS approaches for multiparametric staining and imaging of multiple epigenetic marks. They demonstrated that the MIEL machine-learning algorithm could accurately rank and classify epigenetically active drugs, proving that the imaging of epigenetic marks and chromatin states can be added to the battery of possible HCA phenotypic screens (Farhy et al. 2019).
Emulating the endocrine disruptor screening program.
One overarching approach may be to emulate and extend the Endocrine Disruptor Screening Program (EDSP), which was implemented by the U.S. Environmental Protection Agency (EPA) in response to mounting concerns over EDCs (Fenner-Crisp et al. 2000). The EDSP screens for chemicals that could perturb estrogen, androgen, and thyroid systems in humans, fish, and wildlife and uses a pathway-based approach in which potential lead compounds are identified based on their interactions with endocrine pathways without relying on the observation of an adverse response or phenotypic end point (Laws et al. 2011).
The EDSP uses a two-tier framework. Tier 1 was designed as a screening tier, comprising multiple in vitro and in vivo test systems, which screen for a compound’s potential to interact with endocrine pathways without considering potential adverse effects. Tier 2 was designed to test established adverse effects and the dose–response relationships of hits identified in Tier 1 of the program (Fenner-Crisp et al. 2000). Using a similar approach, epigenetic modifiers may first be identified based on their impact on epigenetic enzyme activity or chromatin states, using techniques that are readily implemented in a high-throughput setting. The idea is that given the importance of epigenetic machinery in determining cell fate and function, evidence that a compound is capable of influencing epigenetic enzyme activity sufficiently warrants additional research and testing. This approach is particularly useful when applied to epigenetic modifiers because of the breadth of possible health outcomes they could inflict.
Some limitations to this approach are that it is uncertain whether it allows for adequately sensitive detection (Coady et al. 2017). Moreover, without prior knowledge of apical or adverse outcomes, it is uncertain whether Tier 2 testing would adequately capture the toxicity profile of the test compounds and whether it accounts for differences in species and life stages (Coady et al. 2017). Some measures can be taken to at least in part mitigate the issue of sensitivity. For example, the U.S. EPA suggested in a report that assay sensitivity could be maximized while permitting an acceptable level of false positives, based on the assumption that false positives could be detected and eliminated in the second tier of the screen (U.S. EPA 2006). Other approaches are to use multiple assays for each end point, from which weighted data can be determined (Rotroff et al. 2013); to use a screening strategy based on concentration–response curves as opposed to single-dose assays (Inglese et al. 2006); and to use a compound set enrichment approach, whereby potential active chemicals are identified in sets rather than as individual compounds (Varin et al. 2010).
Adverse Outcome Pathways and Systems Toxicology—Filling in the Gaps
A potential solution to the limitations discussed above is to implement the adverse outcome pathway (AOP) paradigm (Ankley et al. 2010). An AOP is a collection of data that connects an exposure to an adverse effect through a series of separate but overlapping pieces of evidence. In an AOP, a molecular initiating event (exposure to a toxicant) must be shown to induce a series of key events (e.g., DNA methylation change, transcriptional changes, sequence changes, cell proliferation), which in turn are shown to result in an adverse outcome (e.g., cancer, a disease state).
Although an AOP is itself a collection of correlative evidence, it provides a framework for organizing data across multiple disciplines in a biologically plausible and evidence-based manner. The AOP approach enables the integration of findings from epidemiology studies, which are generally observational associations between an exposure and adverse health outcomes, and in vitro and in vivo studies. The totality of this evidence can be used to establish a causative link between the exposure and the adverse outcome.
An application of AOPs is the systems toxicology strategy, which is based on the idea that any functional perturbation is a result of changes in genomic, proteomic, and metabolic states (Sturla et al. 2014). Systems toxicology integrates classical toxicology with multiple levels of molecular data, with the aim of establishing causality via a chain of molecular events. Systems toxicology aims to describe adverse biological effects as perturbed networks, as opposed to empirical end points (Hartung et al. 2017). This strategy has been made possible with advances in computational biology and molecular biology techniques.
However, the successful implementation and practice of systems toxicology requires characterization of exposures with regard to composition, dose, and duration, as well as extensive phenotypic and molecular profiling of standard biological model systems. Many of the building blocks for the widespread implementation of systems toxicology are already in place. For instance, the L1000 and Connectivity Map resources developed through the Library of Integrated Network-Based Cellular Signatures (LINCS) project enable the prediction of mechanisms of action based on the gene expression profile induced by a particular exposure (Li et al. 2019). The connectivity framework has also been applied to define and compare proteomic and histone mark signatures (Litichevskiy et al. 2018). Other valuable resources include the Toxin and Toxin-Target Database (T3DB), which makes gene expression data on more than 3,600 compounds publicly available (Wishart et al. 2015), and the ENCODE project, which provides systematic maps of regions of transcription, transcription factor association, chromatin structure, and histone modification (ENCODE Project Consortium 2012).
Chemical Mixtures—Something from Nothing?
An additional consideration is the fact that real-life exposures typically occur as mixtures, as opposed to single agents (Kienzler et al. 2016). There have thus been concerns that the risks of complex chemical mixtures are not adequately assessed (European Commission 2012; Kortenkamp 2014). A systematic review aimed at quantifying synergetic toxicity in mixtures identified evidence of synergistic interactions in a small subset of pesticides, metals, and antifoulant mixtures (Cedergreen 2014). Cedergreen (2014) defined synergetic mixtures as those which elicited an observed effect that was at least 2-fold greater than the predicted effect, using concentration addition as a reference model.
As reviewed extensively by Bopp et al. (2019), the challenge in realistically assessing exposure to mixtures is taking into account the target population(s), the timing of exposure, or whether individuals or organisms are exposed to mixtures simultaneously or sequentially (Bopp et al. 2019). Kienzler et al. (2016) described current and potential future tools for modeling mixture effects but indicated that further guidance, data, and expertise are required before they can be fully applied (Kienzler et al. 2016). However, the authors did note that there is still merit to assessing single substances because a thorough characterization of single agents could minimize uncertainties and the requirement for assumptions in the risk assessment of chemical mixtures (Kienzler et al. 2016).
An interesting proposal by Bornehag et al. (2019) suggests that exposure mixtures should first be identified and reconstructed based on evidence from epidemiological and biomonitoring data (Bornehag et al. 2019). These mixtures can then be tested with in vivo experiments to determine a point of departure associated with the studied adverse health outcome, and the final step is to compare the similarity of the experimental data with outcomes measured in the human population (Bornehag et al. 2019). In addition to taking into account mixtures in the risk assessment process, this approach could enable the systematic integration of epidemiological and experimental evidence (Bornehag et al. 2019). We note that with this proposed notion, the mechanistic effects of combinations of chemicals—whether independent, additive, or synergistic—are not a primary concern; the main concern is that these mixtures are relevant based on epidemiological and biomonitoring data.
We also note that there could be merit to the screening of mixtures at the preliminary in vitro screening stage. This could, at least in part, enable the detection of synergetic xenobiotic combinations that might have been missed if the constituent chemicals were analyzed individually.
Bringing It All Together
Applying a combination of these approaches could prove useful in screening for epigenetic modifiers. For instance, we envision that assays for global changes in epigenetic or chromatin sates could be used in a manner analogous to the EDSP Tier 1 screening method. This screen may include mixtures of chemicals that have been identified and reconstructed through epidemiological observations or biomonitoring data, as described by Bornehag et al. (2019). Hits identified in Tier 1 screens can be further examined with more sensitive epigenome-wide techniques, followed by the identification of phenotypic and molecular perturbations incurred by the exposure. Tier 2 screens may also include time-dependent, early life experiments in order to establish the full epigenotoxicity profile of the exposures in question. Pathway-enrichment technologies may then be applied to predict for epigenotoxicity, followed by AOP to organize preliminary data into coherent chains of evidence, and to identify areas in which further research is warranted. Finally, hit compounds may be characterized using systems toxicology approaches, ultimately resulting in comprehensive portraits of exposures and disease states (Figure 3B).
Perspectives for the Future of the Field
As with many aspects of molecular science, the field of epigenetics has benefited greatly from computational and analytical advances (Cazaly et al. 2019; Lim et al. 2010; Mensaert et al. 2014). Our view is that the future iterations of toxicology are unlikely to consider genetic, epigenetic, or proteomic factors individually, with more calls to consider the totality of these mechanisms using multi-omics analyses. This new horizon of possibilities allows for more optimistic and ambitious concepts for the future of toxicology.
Another interesting concept is to generate fingerprints of exposure to environmental toxicants using evolving -omics technologies (Messerlian et al. 2017) and apply these signatures as biomarkers and predictors of response. An important step toward this is to establish robust and universally recognized epimutation signatures, analogous to the mutational signatures established by genome sequencing (Kucab et al. 2019). Our view is that a compendium of epigenomic signatures will enable systematic comparisons between test compounds. An educated guess of an unknown compound’s mechanism of action could be made, for example, based on the epimutation signature it induces in reference to known entries. With additional information on toxicity and adverse outcomes, it may be possible to make calls on how hazardous a compound is based on its epigenomic and mutational signature. Libraries of phosphoproteomics and chromatin profiles are already in development, using the connectivity framework developed with the LINCS Connectivity Map in the context of epigenetics (Creech et al. 2015; Litichevskiy et al. 2018). The availability and growth of these resources represent exciting opportunities for the future of epigenomic research.
In an extension of -omics analyses, one of the most ambitious concepts is that of the exposome, which is defined as a summation of an individual’s experiences over the course of his or her lifetime, and the biological response to these exposures (Wild 2005). The exposome is represented by molecular measurements influenced by a lifetime of exposure to a variety of factors such as diet, lifestyle, infections, and psychosocial factors (Wild 2005). The Human Exposome Project, the environmental analog of the Human Genome Project, is a mammoth undertaking, because the possible range of exposures and individual response is immensely complex (Niedzwiecki and Miller 2017). Exposome research projects include the Human Early Life Exposome (HELIX) (Vrijheid et al. 2014), enhanced exposure assessment and omic profiling for high priority environmental exposures in Europe (EXPOsOMICS) (Vineis et al. 2017), Health and Exposome Research Center: Understanding Lifetime Exposures (HERCULES) (Niedzwiecki and Miller 2017), and Health and Environment-wide Associations based on Large population Surveys (HEALS) (http://www.heals-eu.eu/; Sarigiannis 2014) projects. Although many of the proposed ideas are not feasible at present, the concept of the exposome has important implications for the assessment of exposure in human health research; for instance, considerations that environmental exposures do not exist in isolation and that individual response to exposures can be influenced by a multitude of inherent or acquired factors.
In our view, another major issue to consider in the future is how well current assays are adapted for measuring the environmental impact of chemical agents resulting from human activity. Currently, most high-throughput assays are based on end point measures relevant to humans or mammals. Although this is of clear importance, a holistic assessment of the impact of chemical agents on the environment would require the development of robust nonmammalian or cross-species assays (Coady et al. 2017).
Conclusion
Significant advancements in analytical technologies are now enabling more robust testing and risk assessment approaches in evaluating the hazards of environmental chemical exposure. With accumulating evidence that environmental chemicals are at least partially responsible for the onset of various noncommunicable diseases, there is an urgent need to understand the mechanisms underlying chemical-induced adverse outcomes. Considering epigenetic modifications in the risk assessment process could lead to the development of more sensitive and predictive assays for detecting chemical-induced changes. In view of the impact that environmental chemicals could have on the environment, and on current and future generations, increased efforts in research and regulation are certainly warranted.
Acknowledgments
The work in the Epigenetics Group at the International Agency for Research on Cancer (IARC) is supported by grants from the Institut National du Cancer (INCa, France), the European Commission (EC) Seventh Framework Programme (FP7) Translational Cancer Research (TRANSCAN) Framework, the Foundation ARC pour la Recherche sur le Cancer (France), and Plan Cancer-Eva-Inserm research grant to Z.H. The preparation of this paper was undertaken during the tenure of a postdoctoral fellowship (to F.C.) from the IARC, partially supported by the EC FP7 Marie Curie Actions—People—Co-funding of regional, national, and international programs (COFUND). F.F.-L.C. and Z.H. are also supported by the French ministry of Foreign Affairs (the Hubert Curien Partnership—Hibiscus Mobility grant). The authors certify that their freedom to design, conduct, interpret, and publish research was not compromised by any controlling sponsor.
Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization.
Footnotes
The authors declare they have no actual or potential competing financial interests.
Note to readers with disabilities: EHP strives to ensure that all journal content is accessible to all readers. However, some figures and Supplemental Material published in EHP articles may not conform to 508 standards due to the complexity of the information being presented. If you need assistance accessing journal content, please contact ehponline@niehs.nih.gov. Our staff will work with you to assess and meet your accessibility needs within 3 working days.
References
- ACOG Committee Opinion No. 575. 2013. Exposure to toxic environmental agents. Fertility and Sterility 100:931–934, PMID: 24070500, 10.1016/j.fertnstert.2013.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adjakly M, Bosviel R, Rabiau N, Boiteux J-P, Bignon Y-J, Guy L, et al. 2011. DNA methylation and soy phytoestrogens: quantitative study in DU-145 and PC-3 human prostate cancer cell lines. Epigenomics 3(6):795–803, PMID: 22126297, 10.2217/epi.11.103. [DOI] [PubMed] [Google Scholar]
- Almeida S, Raposo A, Almeida-González M, Carrascosa C. 2018. Bisphenol A: food exposure and impact on human health. Compr Rev Food Sci Food Saf 17(6):1503–1517, 10.1111/1541-4337.12388. [DOI] [PubMed] [Google Scholar]
- Alofe O, Kisanga E, Inayat-Hussain SH, Fukumura M, Garcia-Milian R, Perera L, et al. 2019. Determining the endocrine disruption potential of industrial chemicals using an integrative approach: public databases, in vitro exposure, and modeling receptor interactions. Environ Int 131:104969, PMID: 31310931, 10.1016/j.envint.2019.104969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambatipudi S, Cuenin C, Hernandez-Vargas H, Ghantous A, Le Calvez-Kelm F, Kaaks R, et al. 2016. Tobacco smoking-associated genome-wide DNA methylation changes in the EPIC study. Epigenomics 8(5):599–618, PMID: 26864933, 10.2217/epi-2016-0001. [DOI] [PubMed] [Google Scholar]
- Andersen AM, Dogan MV, Beach SRH, Philibert RA. 2015. Current and future prospects for epigenetic biomarkers of substance use disorders. Genes (Basel) 6(4):991–1022, PMID: 26473933, 10.3390/genes6040991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson OS, Kim JH, Peterson KE, Sanchez BN, Sant KE, Sartor MA, et al. 2017. Novel epigenetic biomarkers mediating bisphenol A exposure and metabolic phenotypes in female mice. Endocrinology 158(1):31–40, PMID: 27824486, 10.1210/en.2016-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson OS, Nahar MS, Faulk C, Jones TR, Liao C, Kannan K, et al. 2012. Epigenetic responses following maternal dietary exposure to physiologically relevant levels of bisphenol A. Environ Mol Mutagen 53(5):334–342, PMID: 22467340, 10.1002/em.21692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angrand P-O, Vennin C, Le Bourhis X, Adriaenssens E. 2015. The role of long non-coding RNAs in genome formatting and expression. Front Genet 6:165, PMID: 25972893, 10.3389/fgene.2015.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, et al. 2010. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem 29(3):730–741, PMID: 20821501, 10.1002/etc.34. [DOI] [PubMed] [Google Scholar]
- Ankolkar M, Deshpande SS, Balasinor NH. 2018. Systemic hormonal modulation induces sperm nucleosomal imbalance in rat spermatozoa. Andrologia 50(8):e13060, PMID: 29920734, 10.1111/and.13060. [DOI] [PubMed] [Google Scholar]
- Anway MD, Cupp AS, Uzumcu M, Skinner MK. 2005. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308(5727):1466–1469, PMID: 15933200, 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashe A, Sapetschnig A, Weick E-M, Mitchell J, Bagijn MP, Cording AC, et al. 2012. piRNAs can trigger a multigenerational epigenetic memory in the germline of C. elegans. Cell 150(1):88–99, PMID: 22738725, 10.1016/j.cell.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba Y, Karube I, Yoshida W. 2019. Global DNA methylation level monitoring by methyl-CpG binding domain-fused luciferase. Anal Lett 52(5):754–760, 10.1080/00032719.2018.1494739. [DOI] [PubMed] [Google Scholar]
- Bai W, Chen Y, Yang J, Niu P, Tian L, Gao A. 2014a. Aberrant miRNA profiles associated with chronic benzene poisoning. Exp Mol Pathol 96(3):426–430, PMID: 24780745, 10.1016/j.yexmp.2014.04.011. [DOI] [PubMed] [Google Scholar]
- Bai W, Yang J, Yang G, Niu P, Tian L, Gao A. 2014b. Long non-coding RNA NR_045623 and NR_028291 involved in benzene hematotoxicity in occupationally benzene-exposed workers. Exp Mol Pathol 96(3):354–360, PMID: 24613687, 10.1016/j.yexmp.2014.02.016. [DOI] [PubMed] [Google Scholar]
- Bansal A, Rashid C, Xin F, Li C, Polyak E, Duemler A, et al. 2017. Sex- and dose-specific effects of maternal bisphenol A exposure on pancreatic islets of first- and second-generation adult mice offspring. Environ Health Perspect 125(9):097022, PMID: 29161229, 10.1289/EHP1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouki R, Melén E, Herceg Z, Beckers J, Chen J, Karagas M, et al. 2018. Epigenetics as a mechanism linking developmental exposures to long-term toxicity. Environ Int 114:77–86, PMID: 29499450, 10.1016/j.envint.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JR. 2017. Programming the future: epigenetics in the context of DOHaD. Environ Health Perspect 125(4):A72, PMID: 28362622, 10.1289/ehp.125-A72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak S, Srinivas V, Duttaroy AK. 2018. Bisphenol-A impairs cellular function and alters DNA methylation of stress pathway genes in first trimester trophoblast cells. Reprod Toxicol 82:72–79, PMID: 30352284, 10.1016/j.reprotox.2018.10.009. [DOI] [PubMed] [Google Scholar]
- Bastos Sales L, Kamstra JH, Cenijn PH, van Rijt LS, Hamers T, Legler J. 2013. Effects of endocrine disrupting chemicals on in vitro global DNA methylation and adipocyte differentiation. Toxicol In Vitro 27(6):1634–1643, PMID: 23603478, 10.1016/j.tiv.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Baylin SB, Jones PA. 2011. A decade of exploring the cancer epigenome—biological and translational implications. Nat Rev Cancer 11(10):726–734, PMID: 21941284, 10.1038/nrc3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck D, Sadler-Riggleman I, Skinner MK. 2017. Generational comparisons (F1 versus F3) of vinclozolin induced epigenetic transgenerational inheritance of sperm differential DNA methylation regions (epimutations) using MeDIP-Seq. Environ Epigenet 3:dvx016, PMID: 29147574, 10.1093/eep/dvx016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Maamar M, Sadler-Riggleman I, Beck D, Skinner MK. 2018. Epigenetic transgenerational inheritance of altered sperm histone retention sites. Sci Rep 8(1):5308, PMID: 29593303, 10.1038/s41598-018-23612-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bend EG, Aref-Eshghi E, Everman DB, Rogers RC, Cathey SS, Prijoles EJ, et al. 2019. Gene domain-specific DNA methylation episignatures highlight distinct molecular entities of ADNP syndrome. Clin Epigenetics 11(1):64, PMID: 31029150, 10.1186/s13148-019-0658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhan A, Hussain I, Ansari KI, Bobzean SAM, Perrotti LI, Mandal SS. 2014. Bisphenol-A and diethylstilbestrol exposure induces the expression of breast cancer associated long noncoding RNA HOTAIR in vitro and in vivo. J Steroid Biochem Mol Biol 141:160–170, PMID: 24533973, 10.1016/j.jsbmb.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco-Miotto T, Craig JM, Gasser YP, van Dijk SJ, Ozanne SE. 2017. Epigenetics and DOHaD: from basics to birth and beyond. J Dev Orig Health Dis 8(5):513–519, PMID: 28889823, 10.1017/S2040174417000733. [DOI] [PubMed] [Google Scholar]
- Bijlsma N, Cohen MM. 2016. Environmental chemical assessment in clinical practice: unveiling the elephant in the room. Int J Environ Res Public Health 13(2):181, PMID: 26848668, 10.3390/ijerph13020181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bintu B, Mateo LJ, Su J-H, Sinnott-Armstrong NA, Parker M, Kinrot S, et al. 2018. Super-resolution chromatin tracing reveals domains and cooperative interactions in single cells. Science 362(6413):eaau1783, PMID: 30361340, 10.1126/science.aau1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bintu L, Yong J, Antebi YE, McCue K, Kazuki Y, Uno N, et al. 2016. Dynamics of epigenetic regulation at the single-cell level. Science 351(6274):720–724, PMID: 26912859, 10.1126/science.aab2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. 2002. DNA methylation patterns and epigenetic memory. Genes Dev 16(1):6–21, PMID: 11782440, 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- Bjornsson HT, Sigurdsson MI, Fallin MD, Irizarry RA, Aspelund T, Cui H, et al. 2008. Intra-individual change over time in DNA methylation with familial clustering. JAMA 299(24):2877–2883, PMID: 18577732, 10.1001/jama.299.24.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blewitt M, Whitelaw E. 2013. The use of mouse models to study epigenetics. Cold Spring Harb Perspect Biol 5(11):a017939, PMID: 24186070, 10.1101/cshperspect.a017939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLUEPRINT Consortium. 2016. Quantitative comparison of DNA methylation assays for biomarker development and clinical applications. Nat Biotechnol 34(7):726–737, PMID: 27347756, 10.1038/nbt.3605. [DOI] [PubMed] [Google Scholar]
- Bojesen SE, Timpson N, Relton C, Davey Smith G, Nordestgaard BG. 2017. AHRR (cg05575921) hypomethylation marks smoking behaviour, morbidity and mortality. Thorax 72(7):646–653, PMID: 28100713, 10.1136/thoraxjnl-2016-208789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollati V, Baccarelli A, Hou L, Bonzini M, Fustinoni S, Cavallo D, et al. 2007. Changes in DNA methylation patterns in subjects exposed to low-dose benzene. Cancer Res 67(3):876–880, PMID: 17283117, 10.1158/0008-5472.CAN-06-2995. [DOI] [PubMed] [Google Scholar]
- Bopp SK, Kienzler A, Richarz A-N, van der Linden SC, Paini A, Parissis N, et al. 2019. Regulatory assessment and risk management of chemical mixtures: challenges and ways forward. Crit Rev Toxicol 49(2):174–189, PMID: 30931677, 10.1080/10408444.2019.1579169. [DOI] [PubMed] [Google Scholar]
- Bornehag C-G, Kitraki E, Stamatakis A, Panagiotidou E, Rudén C, Shu H, et al. 2019. A novel approach to chemical mixture risk assessment—linking data from population-based epidemiology and experimental animal tests. Risk Anal 39(10):2259–2271, PMID: 31173660, 10.1111/risa.13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosviel R, Dumollard E, Déchelotte P, Bignon Y-J, Bernard-Gallon D. 2012. Can soy phytoestrogens decrease DNA methylation in BRCA1 and BRCA2 oncosuppressor genes in breast cancer? OMICS 16(5):235–244, PMID: 22339411, 10.1089/omi.2011.0105. [DOI] [PubMed] [Google Scholar]
- Braconi C, Huang N, Patel T. 2010. MicroRNA-dependent regulation of DNA methyltransferase-1 and tumor suppressor gene expression by interleukin-6 in human malignant cholangiocytes. Hepatology 51(3):881–890, PMID: 20146264, 10.1002/hep.23381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breeze CE, Paul DS, van Dongen J, Butcher LM, Ambrose JC, Barrett JE, et al. 2016. eFORGE: a tool for identifying cell type-specific signal in epigenomic data. Cell Rep 17(8):2137–2150, PMID: 27851974, 10.1016/j.celrep.2016.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton CV, Marsit CJ, Faustman E, Nadeau K, Goodrich JM, Dolinoy DC, et al. 2017. Small-magnitude effect sizes in epigenetic end points are important in children’s environmental health studies: the Children’s Environmental Health and Disease Prevention Research Center’s Epigenetics Working Group. Environ Health Perspect 125(4):511–526, PMID: 28362264, 10.1289/EHP595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieño-Enríquez MA, García-López J, Cárdenas DB, Guibert S, Cleroux E, Děd L, et al. 2015. Exposure to endocrine disruptor induces transgenerational epigenetic deregulation of microRNAs in primordial germ cells. PLoS One 10(4):e0124296, PMID: 25897752, 10.1371/journal.pone.0124296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromer JG, Wu J, Zhou Y, Taylor HS. 2009. Hypermethylation of homeobox A10 by in utero diethylstilbestrol exposure: an epigenetic mechanism for altered developmental programming. Endocrinology 150(7):3376–3382, PMID: 19299448, 10.1210/en.2009-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromer JG, Zhou Y, Taylor MB, Doherty L, Taylor HS. 2010. Bisphenol-A exposure in utero leads to epigenetic alterations in the developmental programming of uterine estrogen response. FASEB J 24(7):2273–2280, PMID: 20181937, 10.1096/fj.09-140533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. 1995. Race, class, and environmental health: a review and systematization of the literature. Environ Res 69(1):15–30, PMID: 7588491, 10.1006/enrs.1995.1021. [DOI] [PubMed] [Google Scholar]
- Brzóska K, Grądzka I, Kruszewski M. 2019. Silver, gold, and iron oxide nanoparticles alter miRNA expression but do not affect DNA methylation in HepG2 cells. Materials (Basel) 12(7):1038, PMID: 30934809, 10.3390/ma12071038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley BA, Burkhart KB, Gu SG, Spracklin G, Kershner A, Fritz H, et al. 2012. A nuclear Argonaute promotes multigenerational epigenetic inheritance and germline immortality. Nature 489(7416):447–451, PMID: 22810588, 10.1038/nature11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buenrostro JD, Wu B, Chang HY, Greenleaf WJ. 2015. ATAC-seq: a method for assaying chromatin accessibility genome-wide. Curr Protoc Mol Biol 109(1):21.29.21–21.29.29, PMID: 25559105, 10.1002/0471142727.mb2129s109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujold D, Morais DAL, Gauthier C, Côté C, Caron M, Kwan T, et al. 2016. The International Human Epigenome Consortium Data Portal. Cell Syst 3(5):496–499.e2, PMID: 27863956, 10.1016/j.cels.2016.10.019. [DOI] [PubMed] [Google Scholar]
- Burton NO, Burkhart KB, Kennedy S. 2011. Nuclear RNAi maintains heritable gene silencing in Caenorhabditis elegans. Proc Natl Acad Sci USA 108(49):19683–19688, PMID: 22106253, 10.1073/pnas.1113310108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun H-M, Benachour N, Zalko D, Frisardi MC, Colicino E, Takser L, et al. 2015. Epigenetic effects of low perinatal doses of flame retardant BDE-47 on mitochondrial and nuclear genes in rat offspring. Toxicology 328:152–159, PMID: 25533936, 10.1016/j.tox.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong L-Y, Reidy JA, Needham LL. 2008. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect 116(1):39–44, PMID: 18197297, 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campa CC, Weisbach NR, Santinha AJ, Incarnato D, Platt RJ. 2019. Multiplexed genome engineering by Cas12a and CRISPR arrays encoded on single transcripts. Nat Methods 16(9):887–893, PMID: 31406383, 10.1038/s41592-019-0508-6. [DOI] [PubMed] [Google Scholar]
- Casati L, Sendra R, Poletti A, Negri-Cesi P, Celotti F. 2013. Androgen receptor activation by polychlorinated biphenyls: epigenetic effects mediated by the histone demethylase Jarid1b. Epigenetics 8(10):1061–1068, PMID: 23907094, 10.4161/epi.25811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo P, Ibáñez F, Guajardo A, Llanos MN, Ronco AM. 2012. Impact of cadmium exposure during pregnancy on hepatic glucocorticoid receptor methylation and expression in rat fetus. PLoS One 7(9):e44139, PMID: 22957049, 10.1371/journal.pone.0044139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazaly E, Saad J, Wang W, Heckman C, Ollikainen M, Tang J. 2019. Making sense of the epigenome using data integration approaches. Front Pharmacol 10:126, PMID: 30837884, 10.3389/fphar.2019.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedergreen N. 2014. Quantifying synergy: a systematic review of mixture toxicity studies within environmental toxicology. PLoS One 9(5):e96580, PMID: 24794244, 10.1371/journal.pone.0096580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-H, Jiang SS, Chang IS, Wen H-J, Sun C-W, Wang S-L. 2018. Association between fetal exposure to phthalate endocrine disruptor and genome-wide DNA methylation at birth. Environ Res 162:261–270, PMID: 29367177, 10.1016/j.envres.2018.01.009. [DOI] [PubMed] [Google Scholar]
- Chen J, Wu S, Wen S, Shen L, Peng J, Yan C, et al. 2015. The mechanism of environmental endocrine disruptors (DEHP) induces epigenetic transgenerational inheritance of cryptorchidism. PLoS One 10(6):e0126403, PMID: 26035430, 10.1371/journal.pone.0126403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M-L, Shen F, Huang W, Qi J-H, Wang Y, Feng Y-Q, et al. 2013. Quantification of 5-methylcytosine and 5-hydroxymethylcytosine in genomic DNA from hepatocellular carcinoma tissues by capillary hydrophilic-interaction liquid chromatography/quadrupole TOF mass spectrometry. Clin Chem 59(5):824–832, PMID: 23344498, 10.1373/clinchem.2012.193938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Li H, Cai J, Wang C, Lin Z, Liu C, et al. 2018. Fine particulate air pollution and the expression of microRNAs and circulating cytokines relevant to inflammation, coagulation, and vasoconstriction. Environ Health Perspect 126(1):017007, PMID: 29342453, 10.1289/EHP1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Li S, Subramaniam S, Shyy JYJ, Chien S. 2017. Epigenetic regulation: a new frontier for biomedical engineers. Annu Rev Biomed Eng 19:195–219, PMID: 28301736, 10.1146/annurev-bioeng-071516-044720. [DOI] [PubMed] [Google Scholar]
- Cheng ASL, Culhane AC, Chan MWY, Venkataramu CR, Ehrich M, Nasir A, et al. 2008. Epithelial progeny of estrogen-exposed breast progenitor cells display a cancer-like methylome. Cancer Res 68(6):1786–1796, PMID: 18339859, 10.1158/0008-5472.CAN-07-5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Hu P, Wen W, Liu L. 2018. Relative miRNA and mRNA expression involved in arsenic methylation. PLoS One 13(12):e0209014, PMID: 30543710, 10.1371/journal.pone.0209014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong A, Johnson SA, Howald EC, Ellersieck MR, Camacho L, Lewis SM, et al. 2018. Gene expression and DNA methylation changes in the hypothalamus and hippocampus of adult rats developmentally exposed to bisphenol A or ethinyl estradiol: a CLARITY-BPA consortium study. Epigenetics 13(7):704–720, PMID: 30001178, 10.1080/15592294.2018.1497388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou W-C, Lee P-H, Tan Y-Y, Lin H-C, Yang C-W, Chen K-H, et al. 2017. An integrative transcriptomic analysis reveals bisphenol A exposure-induced dysregulation of microRNA expression in human endometrial cells. Toxicol In Vitro 41:133–142, PMID: 28238728, 10.1016/j.tiv.2017.02.012. [DOI] [PubMed] [Google Scholar]
- Coady KK, Biever RC, Denslow ND, Gross M, Guiney PD, Holbech H, et al. 2017. Current limitations and recommendations to improve testing for the environmental assessment of endocrine active substances. Integr Environ Assess Manag 13(2):302–316, PMID: 27791330, 10.1002/ieam.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corces MR, Granja JM, Shams S, Louie BH, Seoane JA, Zhou W, et al. 2018. The chromatin accessibility landscape of primary human cancers. Science 362(6413):eaav1898, PMID: 30361341, 10.1126/science.aav1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter JB, O’Driscoll CM, Bressler JP. 2013. Hydroquinone increases 5-hydroxymethylcytosine formation through ten eleven translocation 1 (TET1) 5-methylcytosine dioxygenase. J Biol Chem 288(40):28792–28800, PMID: 23940045, 10.1074/jbc.M113.491365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creech AL, Taylor JE, Maier VK, Wu X, Feeney CM, Udeshi ND, et al. 2015. Building the connectivity map of epigenetics: chromatin profiling by quantitative targeted mass spectrometry. Methods 72:57–64, PMID: 25448295, 10.1016/j.ymeth.2014.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronican AA, Fitz NF, Carter A, Saleem M, Shiva S, Barchowsky A, et al. 2013. Genome-wide alteration of histone H3K9 acetylation pattern in mouse offspring prenatally exposed to arsenic. PLoS One 8(2):e53478, PMID: 23405071, 10.1371/journal.pone.0053478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crujeiras AB, Morcillo S, Diaz-Lagares A, Sandoval J, Castellano-Castillo D, Torres E, et al. 2019. Identification of an episignature of human colorectal cancer associated with obesity by genome-wide DNA methylation analysis. Int J Obes (Lond) 43(1):176–188, PMID: 29717273, 10.1038/s41366-018-0065-6. [DOI] [PubMed] [Google Scholar]
- Curtis SW, Cobb DO, Kilaru V, Terrell ML, Kennedy EM, Marder ME, et al. 2019. Exposure to polybrominated biphenyl (PBB) associates with genome-wide DNA methylation differences in peripheral blood. Epigenetics 14(1):52–66, PMID: 30676242, 10.1080/15592294.2019.1565590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Assis S, Warri A, Cruz MI, Laja O, Tian Y, Zhang B, et al. 2012. High-fat or ethinyl-oestradiol intake during pregnancy increases mammary cancer risk in several generations of offspring. Nat Commun 3:1053, PMID: 22968699, 10.1038/ncomms2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de FC Lichtenfels AJ, van der Plaat DA, de Jong K, van Diemen CC, Postma DS, Nedeljkovic I, et al. 2018. Long-term air pollution exposure, genome-wide DNA methylation and lung function in the LifeLines cohort study. Environ Health Perspect 126(2):027004, PMID: 29410382, 10.1289/EHP2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jager PL, Srivastava G, Lunnon K, Burgess J, Schalkwyk LC, Yu L, et al. 2014. Alzheimer’s disease: early alterations in brain DNA methylation at ANK1, BIN1, RHBDF2 and other loci. Nat Neurosci 17(9):1156–1163, PMID: 25129075, 10.1038/nn.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degli Esposti D, Sklias A, Lima SC, Beghelli-de la Forest Divonne S, Cahais V, Fernandez-Jimenez N, et al. 2017. Unique DNA methylation signature in HPV-positive head and neck squamous cell carcinomas. Genome Med 9(1):33, PMID: 28381277, 10.1186/s13073-017-0419-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker J, Belmont AS, Guttman M, Leshyk VO, Lis JT, Lomvardas S, et al. 2017. The 4D nucleome project. Nature 549(7671):219–226, PMID: 28905911, 10.1038/nature23884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfosse V, Grimaldi M, le Maire A, Bourguet W, Balaguer P. 2014. Nuclear receptor profiling of bisphenol-A and its halogenated analogues. In: Vitamins and Hormones, vol. 94: Litwack G, ed. Boston, MA: Academic Press, 229–251. [DOI] [PubMed] [Google Scholar]
- Ding T, Mokshagundam S, Rinaudo PF, Osteen KG, Bruner-Tran KL. 2018. Paternal developmental toxicant exposure is associated with epigenetic modulation of sperm and placental Pgr and Igf2 in a mouse model. Biol Reprod 99(4):864–876, PMID: 29741588, 10.1093/biolre/ioy111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Seebeck T, Feng Y, Jiang Y, Davis GD, Chen F. 2019. Improving CRISPR-Cas9 genome editing efficiency by fusion with chromatin-modulating peptides. CRISPR J 2:51–63, PMID: 31021236, 10.1089/crispr.2018.0036. [DOI] [PubMed] [Google Scholar]
- Ding Z-M, Jiao X-F, Wu D, Zhang J-Y, Chen F, Wang Y-S, et al. 2017. Bisphenol AF negatively affects oocyte maturation of mouse in vitro through increasing oxidative stress and DNA damage. Chem Biol Interact 278:222–229, PMID: 29102535, 10.1016/j.cbi.2017.10.030. [DOI] [PubMed] [Google Scholar]
- Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, et al. 2012. Landscape of transcription in human cells. Nature 489(7414):101–108, PMID: 22955620, 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty LF, Bromer JG, Zhou Y, Aldad TS, Taylor HS. 2010. In utero exposure to diethylstilbestrol (DES) or bisphenol-A (BPA) increases EZH2 expression in the mammary gland: an epigenetic mechanism linking endocrine disruptors to breast cancer. Horm Cancer 1(3):146–155, PMID: 21761357, 10.1007/s12672-010-0015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi T, Puri P, McCann A, Bannigan J, Thompson J. 2011. Epigenetic effect of cadmium on global de novo DNA hypomethylation in the cadmium-induced ventral body wall defect (VBWD) in the chick model. Toxicol Sci 120(2):475–480, PMID: 21278052, 10.1093/toxsci/kfr022. [DOI] [PubMed] [Google Scholar]
- Dolinoy DC, Huang D, Jirtle RL. 2007. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci USA 104(32):13056–13061, PMID: 17670942, 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doshi T, D’Souza C, Dighe V, Vanage G. 2012. Effect of neonatal exposure on male rats to bisphenol A on the expression of DNA methylation machinery in the postimplantation embryo. J Biochem Mol Toxicol 26(9):337–343, PMID: 22730197, 10.1002/jbt.21425. [DOI] [PubMed] [Google Scholar]
- Doshi T, Mehta SS, Dighe V, Balasinor N, Vanage G. 2011. Hypermethylation of estrogen receptor promoter region in adult testis of rats exposed neonatally to bisphenol A. Toxicology 289(2–3):74–82, PMID: 21827818, 10.1016/j.tox.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Drobná Z, Henriksen AD, Wolstenholme JT, Montiel C, Lambeth PS, Shang S, et al. 2018. Transgenerational effects of bisphenol A on gene expression and DNA methylation of imprinted genes in brain. Endocrinology 159(1):132–144, PMID: 29165653, 10.1210/en.2017-00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENCODE Project Consortium. 2012. An integrated encyclopedia of DNA elements in the human genome. Nature 489(7414):57–74, PMID: 22955616, 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esner M, Meyenhofer F, Bickle M. 2018. Live-cell high content screening in drug development. In: High Content Screening: A Powerful Approach to Systems Cell Biology and Phenotypic Drug Discovery Johnston PA, Trask OJ, eds. New York, NY: Springer New York, 149–164. [DOI] [PubMed] [Google Scholar]
- Espín-Pérez A, Krauskopf J, Chadeau-Hyam M, van Veldhoven K, Chung F, Cullinan P, et al. 2018. Short-term transcriptome and microRNAs responses to exposure to different air pollutants in two population studies. Environ Pollut 242(Pt A):182–190, PMID: 29980036, 10.1016/j.envpol.2018.06.051. [DOI] [PubMed] [Google Scholar]
- Esteller M. 2011. Non-coding RNAs in human disease. Nat Rev Genet 12(12):861–874, PMID: 22094949, 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- European Commission. 2012. Toxicity and assessment of chemical mixtures Brussels: European Commission. https://ec.europa.eu/health/scientific_committees/environmental_risks/docs/scher_o_155.pdf [accessed 2 January 2020].
- Fabbri M, Garzon R, Cimmino A, Liu Z, Zanesi N, Callegari E, et al. 2007. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci USA 104(40):15805–15810, PMID: 17890317, 10.1073/pnas.0707628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhy C, Hariharan S, Ylanko J, Orozco L, Zeng F-Y, Pass I, et al. 2019. Improving drug discovery using image-based multiparametric analysis of the epigenetic landscape. eLife 8:e49683, PMID: 31637999, 10.7554/eLife.49683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasanelli F, Giraudo MT, Vineis P, Fiano V, Fiorito G, Grasso C, et al. 2019. DNA methylation, colon cancer and Mediterranean diet: results from the EPIC-Italy cohort. Epigenetics 14(10):977–988, PMID: 31179817, 10.1080/15592294.2019.1629230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulk C, Kim JH, Jones TR, McEachin RC, Nahar MS, Dolinoy DC, et al. 2015. Bisphenol A-associated alterations in genome-wide DNA methylation and gene expression patterns reveal sequence-dependent and non-monotonic effects in human fetal liver. Environ Epigenet 1(1):dvv006, PMID: 27358748, 10.1093/eep/dvv006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil R, Fraga MF. 2012. Epigenetics and the environment: emerging patterns and implications. Nat Rev Genet 13(2):97–109, PMID: 22215131, 10.1038/nrg3142. [DOI] [PubMed] [Google Scholar]
- Fenner-Crisp PA, Maciorowski AF, Timm GE. 2000. The endocrine disruptor screening program developed by the U.S. Environmental Protection Agency. Ecotoxicology 9(1–2):85–91, 10.1023/A:1008972330318. [DOI] [Google Scholar]
- Fernández JM, de la Torre V, Richardson D, Royo R, Puiggròs M, Moncunill V, et al. 2016. The BLUEPRINT Data Analysis Portal. Cell Syst 3(5):491–495.e5, PMID: 27863955, 10.1016/j.cels.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga MF. 2009. Genetic and epigenetic regulation of aging. Curr Opin Immunol 21(4):446–453, PMID: 19500963, 10.1016/j.coi.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Fudhaili A, Yoon NA, Kang S, Ryu J, Jeong JY, Lee DH, et al. 2019. Resveratrol epigenetically regulates the expression of zinc finger protein 36 in non-small cell lung cancer cell lines. Oncol Rep 41(2):1377–1386, PMID: 30535453, 10.3892/or.2018.6898. [DOI] [PubMed] [Google Scholar]
- Fustinoni S, Rossella F, Polledri E, Bollati V, Campo L, Byun H-M, et al. 2012. Global DNA methylation and low-level exposure to benzene. Med Lav 103(2):84–95, PMID: 22619984. [PubMed] [Google Scholar]
- Gao Y, Zhao Y, Zhang H, Zhang P, Liu J, Feng Y, et al. 2019. Pubertal exposure to low doses of zearalenone disrupting spermatogenesis through ERα related genetic and epigenetic pathways. Toxicol Lett 315:31–38, PMID: 31419471, 10.1016/j.toxlet.2019.08.007. [DOI] [PubMed] [Google Scholar]
- Garzon R, Liu S, Fabbri M, Liu Z, Heaphy CEA, Callegari E, et al. 2009. MicroRNA-29b induces global DNA hypomethylation and tumor suppressor gene reexpression in acute myeloid leukemia by targeting directly DNMT3A and 3B and indirectly DNMT1. Blood 113(25):6411–6418, PMID: 19211935, 10.1182/blood-2008-07-170589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghantous A, Saffery R, Cros M-P, Ponsonby A-L, Hirschfeld S, Kasten C, et al. 2014. Optimized DNA extraction from neonatal dried blood spots: application in methylome profiling. BMC Biotechnol 14:60–60, PMID: 24980254, 10.1186/1472-6750-14-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh K, Chatterjee B, Maheswari U, Athifa M, Kanade SR. 2019. 4-Nonylphenol-enhanced EZH2 and RNF2 expression, H3K27me3 and H2AK119ub1 marks resulting in silencing of p21CDKN1A in vitro. Epigenomics 11(8):899–916, PMID: 31144530, 10.2217/epi-2018-0175. [DOI] [PubMed] [Google Scholar]
- González-Rojo S, Lombó M, Fernández-Díez C, Herráez MP. 2019. Male exposure to bisphenol A impairs spermatogenesis and triggers histone hyperacetylation in zebrafish testes. Environ Pollut 248:368–379, PMID: 30818116, 10.1016/j.envpol.2019.01.127. [DOI] [PubMed] [Google Scholar]
- Goodrich J, Burmeister M, Shao J, Xu L, Xia Y, Silver M, et al. 2019. Epigenetic influence of lead, pesticides and iron deficiency in China on newborn blood. Eur Neuropsychopharmacol 29(suppl 3):S747, 10.1016/j.euroneuro.2017.06.086. [DOI] [Google Scholar]
- Goodrich JM, Dolinoy DC, Sánchez BN, Zhang Z, Meeker JD, Mercado-Garcia A, et al. 2016. Adolescent epigenetic profiles and environmental exposures from early life through peri-adolescence. Environ Epigenet 2(3):dvw018, PMID: 29492298, 10.1093/eep/dvw018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson WH III, Lowe L, Carpenter DO, Gilbertson M, Manaf Ali A, Lopez de Cerain Salsamendi A, et al. 2015. Assessing the carcinogenic potential of low-dose exposures to chemical mixtures in the environment: the challenge ahead. Carcinogenesis 36(suppl 1):S254–S296, PMID: 26106142, 10.1093/carcin/bgv039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Walker DM, Zama AM, Armenti AE, Uzumcu M. 2011. Early life exposure to endocrine-disrupting chemicals causes lifelong molecular reprogramming of the hypothalamus and premature reproductive aging. Mol Endocrinol 25(12):2157–2168, PMID: 22016562, 10.1210/me.2011-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal D, Limesand SW, Goyal R. 2019. Epigenetic responses and the developmental origins of health and disease. J Endocrinol 242(1):T105–T119, PMID: 31091503, 10.1530/JOE-19-0009. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Bellanger M. 2017. Calculation of the disease burden associated with environmental chemical exposures: application of toxicological information in health economic estimation. Environ Health 16(1):123, PMID: 29202828, 10.1186/s12940-017-0340-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg MVC, Bourc’his D. 2019. The diverse roles of DNA methylation in mammalian development and disease. Nat Rev Mol Cell Biol 20(10):590–607, PMID: 31399642, 10.1038/s41580-019-0159-6. [DOI] [PubMed] [Google Scholar]
- Grindler NM, Vanderlinden L, Karthikraj R, Kannan K, Teal S, Polotsky AJ, et al. 2018. Exposure to phthalate, an endocrine disrupting chemical, alters the first trimester placental methylome and transcriptome in women. Sci Rep 8(1):6086, PMID: 29666409, 10.1038/s41598-018-24505-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Bosagna C, Covert TR, Haque MM, Settles M, Nilsson EE, Anway MD, et al. 2012. Epigenetic transgenerational inheritance of vinclozolin induced mouse adult onset disease and associated sperm epigenome biomarkers. Reprod Toxicol 34(4):694–707, PMID: 23041264, 10.1016/j.reprotox.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Bosagna C, Settles M, Lucker B, Skinner MK. 2010. Epigenetic transgenerational actions of vinclozolin on promoter regions of the sperm epigenome. PLoS One 5(9):e13100, PMID: 20927350, 10.1371/journal.pone.0013100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillette LJ Jr, Parrott BB, Nilsson E, Haque MM, Skinner MK. 2016. Epigenetic programming alterations in alligators from environmentally contaminated lakes. Gen Comp Endocrinol 238:4–12, PMID: 27080547, 10.1016/j.ygcen.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez García AK, Choudhury M, De Leon Rodriguez A. 2019. Diisononyl phthalate differentially affects sirtuin expression in the HepG2 cell line. Chem Res Toxicol 32(9):1863–1870, PMID: 31423773, 10.1021/acs.chemrestox.9b00206. [DOI] [PubMed] [Google Scholar]
- Han W, Shi M, Spivack SD. 2013. Site-specific methylated reporter constructs for functional analysis of DNA methylation. Epigenetics 8(11):1176–1187, PMID: 24004978, 10.4161/epi.26195. [DOI] [PubMed] [Google Scholar]
- Hanly DJ, Esteller M, Berdasco M. 2018. Interplay between long non-coding RNAs and epigenetic machinery: emerging targets in cancer? Phil Trans R Soc Lond B Biol Sci 373(1748):20170074, PMID: 29685978, 10.1098/rstb.2017.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung T, FitzGerald RE, Jennings P, Mirams GR, Peitsch MC, Rostami-Hodjegan A, et al. 2017. Systems toxicology: real world applications and opportunities. Chem Res Toxicol 30(4):870–882, PMID: 28362102, 10.1021/acs.chemrestox.7b00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori N, Ushijima T. 2016. Epigenetic impact of infection on carcinogenesis: mechanisms and applications. Genome Med 8(1):10, PMID: 26823082, 10.1186/s13073-016-0267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson-Smith A, Fisch KM, Hua J, Liu G, Ricciardelli E, Jepsen K, et al. 2019. DNA methylation changes associated with Parkinson’s disease progression: outcomes from the first longitudinal genome-wide methylation analysis in blood. Epigenetics 14(4):365–382, PMID: 30871403, 10.1080/15592294.2019.1588682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herceg Z, Ghantous A, Wild CP, Sklias A, Casati L, Duthie SJ, et al. 2018. Roadmap for investigating epigenome deregulation and environmental origins of cancer. Int J Cancer 142(5):874–882, PMID: 28836271, 10.1002/ijc.31014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herceg Z, Lambert M-P, van Veldhoven K, Demetriou C, Vineis P, Smith MT, et al. 2013. Towards incorporating epigenetic mechanisms into carcinogen identification and evaluation. Carcinogenesis 34(9):1955–1967, PMID: 23749751, 10.1093/carcin/bgt212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herceg Z, Vaissière T. 2011. Epigenetic mechanisms and cancer: an interface between the environment and the genome. Epigenetics 6(7):804–819, PMID: 21758002, 10.4161/epi.6.7.16262. [DOI] [PubMed] [Google Scholar]
- Ho S-M, Cheong A, Lam H-M, Hu W-Y, Shi G-B, Zhu X, et al. 2015. Exposure of human prostaspheres to bisphenol A epigenetically regulates SNORD family noncoding RNAs via histone modification. Endocrinology 156(11):3984–3995, PMID: 26248216, 10.1210/en.2015-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S-M, Tang W-Y, Belmonte de Frausto J, Prins GS. 2006. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res 66(11):5624–5632, PMID: 16740699, 10.1158/0008-5472.CAN-06-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houseman EA, Kile ML, Christiani DC, Ince TA, Kelsey KT, Marsit CJ. 2016. Reference-free deconvolution of DNA methylation data and mediation by cell composition effects. BMC Bioinformatics 17:259, PMID: 27358049, 10.1186/s12859-016-1140-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Ma H, Zhang W, Yu Z, Sheng G, Fu J. 2014. Effects of benzene and its metabolites on global DNA methylation in human normal hepatic L02 cells. Environ Toxicol 29(1):108–116, PMID: 21953684, 10.1002/tox.20777. [DOI] [PubMed] [Google Scholar]
- Huang W, Zhao C, Zhong H, Zhang S, Xia Y, Cai Z. 2019. Bisphenol S induced epigenetic and transcriptional changes in human breast cancer cell line MCF-7. Environ Pollut 246:697–703, PMID: 30616060, 10.1016/j.envpol.2018.12.084. [DOI] [PubMed] [Google Scholar]
- Hung C-H, Yang S-N, Kuo P-L, Chu Y-T, Chang H-W, Wei W-J, et al. 2010. Modulation of cytokine expression in human myeloid dendritic cells by environmental endocrine-disrupting chemicals involves epigenetic regulation. Environ Health Perspect 118(1):67–72, PMID: 20056579, 10.1289/ehp.0901011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain I, Bhan A, Ansari KI, Deb P, Bobzean SAM, Perrotti LI, et al. 2015. Bisphenol-A induces expression of HOXC6, an estrogen-regulated homeobox-containing gene associated with breast cancer. Biochim Biophys Acta 1849(6):697–708, PMID: 25725483, 10.1016/j.bbagrm.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer). 2018. Benzene. IARC Monographs on the Evaluation of Carcinogenic Risk to Humans, vol. 120. http://publications.iarc.fr/_publications/media/download/5630/48902d7035dcd9f6f64ac780ac64cabaf033871b.pdf [accessed 2 January 2020].
- Inglese J, Auld DS, Jadhav A, Johnson RL, Simeonov A, Yasgar A, et al. 2006. Quantitative high-throughput screening: a titration-based approach that efficiently identifies biological activities in large chemical libraries. Proc Natl Acad Sci USA 103(31):11473–11478, PMID: 16864780, 10.1073/pnas.0604348103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal K, Tran DA, Li AX, Warden C, Bai AY, Singh P, et al. 2015. Deleterious effects of endocrine disruptors are corrected in the mammalian germline by epigenome reprogramming. Genome Biol 16:59, PMID: 25853433, 10.1186/s13059-015-0619-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Yaoi T, Fushiki S. 2012. Bisphenol A, an endocrine-disrupting chemical, and brain development. Neuropathology 32(4):447–457, PMID: 22239237, 10.1111/j.1440-1789.2011.01287.x. [DOI] [PubMed] [Google Scholar]
- Jadhav RR, Santucci-Pereira J, Wang YV, Liu J, Nguyen TD, Wang J, et al. 2017. DNA methylation targets influenced by bisphenol A and/or genistein are associated with survival outcomes in breast cancer patients. Genes (Basel) 8(5):144, PMID: 28505145, 10.3390/genes8050144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R, Bird A. 2003. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet (suppl 33):245–254, PMID: 12610534, 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- Jefferson WN, Chevalier DM, Phelps JY, Cantor AM, Padilla-Banks E, Newbold RR, et al. 2013. Persistently altered epigenetic marks in the mouse uterus after neonatal estrogen exposure. Mol Endocrinol 27(10):1666–1677, PMID: 24002655, 10.1210/me.2013-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Z, Zhang L, Peng V, Ren X, McHale CM, Smith MT. 2010. A comparison of the cytogenetic alterations and global DNA hypomethylation induced by the benzene metabolite, hydroquinone, with those induced by melphalan and etoposide. Leukemia 24(5):986–991, PMID: 20339439, 10.1038/leu.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z, Liang Y, Ma B, Xu X, Xiong J, Duan L, et al. 2017. A 5-mC dot blot assay quantifying the DNA methylation level of chondrocyte dedifferentiation in vitro. J Vis Exp 123:e55565, PMID: 28570531, 10.3791/55565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Cao L, Wang F, Ge H, Wu P-C, Li X-W, et al. 2016. Accelerated reduction of serum thyroxine and hippocampal histone acetylation links to exacerbation of spatial memory impairment in aged CD-1 mice pubertally exposed to bisphenol-A. Age (Dordr) 38(5–6):405–418, PMID: 27631330, 10.1007/s11357-016-9947-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo WJ, Ren X, Chu F, Aleshin M, Wintz H, Burlingame A, et al. 2009. Acetylated H4K16 by MYST1 protects UROtsa cells from arsenic toxicity and is decreased following chronic arsenic exposure. Toxicol Appl Pharmacol 241(3):294–302, PMID: 19732783, 10.1016/j.taap.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas S, Izaurralde E. 2015. Towards a molecular understanding of microRNA-mediated gene silencing. Nat Rev Genet 16(7):421–433, PMID: 26077373, 10.1038/nrg3965. [DOI] [PubMed] [Google Scholar]
- Jorgensen EM, Alderman MH III, Taylor HS. 2016. Preferential epigenetic programming of estrogen response after in utero xenoestrogen (bisphenol-A) exposure. FASEB J 30(9):3194–3201, PMID: 27312807, 10.1096/fj.201500089R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kala R, Tollefsbol TO. 2016. A novel combinatorial epigenetic therapy using resveratrol and pterostilbene for restoring estrogen receptor-α (ERα) expression in ERα-negative breast cancer cells. PLoS One 11(5):e0155057, PMID: 27159275, 10.1371/journal.pone.0155057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaman EF, Ozden S. 2019. Alterations in global DNA methylation and metabolism-related genes caused by zearalenone in MCF7 and MCF10F cells. Mycotoxin Res 35(3):309–320, PMID: 30953299, 10.1007/s12550-019-00358-8. [DOI] [PubMed] [Google Scholar]
- Karimi M, Johansson S, Stach D, Corcoran M, Grandér D, Schalling M, et al. 2006. LUMA (LUminometric Methylation Assay)—a high throughput method to the analysis of genomic DNA methylation. Exp Cell Res 312(11):1989–1995, PMID: 16624287, 10.1016/j.yexcr.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Kelly TK, De Carvalho DD, Jones PA. 2010. Epigenetic modifications as therapeutic targets. Nat Biotechnol 28(10):1069–1078, PMID: 20944599, 10.1038/nbt.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienzler A, Bopp SK, van der Linden S, Berggren E, Worth A. 2016. Regulatory assessment of chemical mixtures: requirements, current approaches and future perspectives. Regul Toxicol Pharmacol 80:321–334, PMID: 27211294, 10.1016/j.yrtph.2016.05.020. [DOI] [PubMed] [Google Scholar]
- Kitraki E, Nalvarte I, Alavian-Ghavanini A, Rüegg J. 2015. Developmental exposure to bisphenol A alters expression and DNA methylation of Fkbp5, an important regulator of the stress response. Mol Cell Endocrinol 417:191–199, PMID: 26427651, 10.1016/j.mce.2015.09.028. [DOI] [PubMed] [Google Scholar]
- Klukovich R, Nilsson E, Sadler-Riggleman I, Beck D, Xie Y, Yan W, et al. 2019. Environmental toxicant induced epigenetic transgenerational inheritance of prostate pathology and stromal-epithelial cell epigenome and transcriptome alterations: ancestral origins of prostate disease. Sci Rep 9(1):2209, PMID: 30778168, 10.1038/s41598-019-38741-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobets T, Iatropoulos MJ, Williams GM. 2019. Mechanisms of DNA-reactive and epigenetic chemical carcinogens: applications to carcinogenicity testing and risk assessment. Toxicol Res (Camb) 8(2):123–145, PMID: 30997017, 10.1039/c8tx00250a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochmanski J, Montrose L, Goodrich JM, Dolinoy DC. 2017. Environmental deflection: the impact of toxicant exposures on the aging epigenome. Toxicol Sci 156(2):325–335, PMID: 28087834, 10.1093/toxsci/kfx005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortenkamp A. 2014. Low dose mixture effects of endocrine disrupters and their implications for regulatory thresholds in chemical risk assessment. Curr Opin Pharmacol 19:105–111, PMID: 25244397, 10.1016/j.coph.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Krauskopf J, de Kok TM, Hebels DG, Bergdahl IA, Johansson A, Spaeth F, et al. 2017. MicroRNA profile for health risk assessment: environmental exposure to persistent organic pollutants strongly affects the human blood microRNA machinery. Sci Rep 7(1):9262, PMID: 28835693, 10.1038/s41598-017-10167-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucab JE, Zou X, Morganella S, Joel M, Nanda AS, Nagy E, et al. 2019. A compendium of mutational signatures of environmental agents. Cell 177(4):821–836.e16, PMID: 30982602, 10.1016/j.cell.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto T, Oshio S. 2013. Effect of fetal exposure to bisphenol A on brain mediated by X-chromosome inactivation. J Toxicol Sci 38(3):485–494, PMID: 23719926, 10.2131/jts.38.485. [DOI] [PubMed] [Google Scholar]
- Kumar D, Thakur MK. 2017. Effect of perinatal exposure to bisphenol-A on DNA methylation and histone acetylation in cerebral cortex and hippocampus of postnatal male mice. J Toxicol Sci 42(3):281–289, PMID: 28496034, 10.2131/jts.42.281. [DOI] [PubMed] [Google Scholar]
- Kumar N, Hori Y, Kikuchi K. 2018. Live-cell imaging of DNA methylation based on synthetic-molecule/protein hybrid probe. Chem Rec 18(12):1672–1680, PMID: 29863802, 10.1002/tcr.201800039. [DOI] [PubMed] [Google Scholar]
- Kundakovic M, Gudsnuk K, Franks B, Madrid J, Miller RL, Perera FP, et al. 2013. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc Natl Acad Sci USA 110(24):9956–9961, PMID: 23716699, 10.1073/pnas.1214056110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladd-Acosta C. 2015. Epigenetic signatures as biomarkers of exposure. Curr Environ Health Rep 2(2):117–125, PMID: 26231361, 10.1007/s40572-015-0051-2. [DOI] [PubMed] [Google Scholar]
- Laing LV, Viana J, Dempster EL, Trznadel M, Trunkfield LA, Uren Webster TM, et al. 2016. Bisphenol A causes reproductive toxicity, decreases dnmt1 transcription, and reduces global DNA methylation in breeding zebrafish (Danio rerio). Epigenetics 11(7):526–538, PMID: 27120497, 10.1080/15592294.2016.1182272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaKind JS, Overpeck J, Breysse PN, Backer L, Richardson SD, Sobus J, et al. 2016. Exposure science in an age of rapidly changing climate: challenges and opportunities. J Expo Sci Environ Epidemiol 26(6):529–538, PMID: 27485992, 10.1038/jes.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb YN, Dhillon S. 2017. Epi proColon® 2.0 CE: a blood-based screening test for colorectal cancer. Mol Diagn Ther 21(2):225–232, PMID: 28155091, 10.1007/s40291-017-0259-y. [DOI] [PubMed] [Google Scholar]
- LaRocca J, Binder AM, McElrath TF, Michels KB. 2014. The impact of first trimester phthalate and phenol exposure on IGF2/H19 genomic imprinting and birth outcomes. Environ Res 133:396–406, PMID: 24972507, 10.1016/j.envres.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRocca J, Binder AM, McElrath TF, Michels KB. 2016. First-trimester urine concentrations of phthalate metabolites and phenols and placenta miRNA expression in a cohort of U.S. women. Environ Health Perspect 124(3):380–387, PMID: 26090578, 10.1289/ehp.1408409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laws SC, Riffle Bw, Stoker TE, Goldman JM, Wilson V, Gray E Jr, et al. 2011. The U.S. EPA Endocrine Disruptor Screening Program: the Tier 1 Screening Battery. In: Developmental and Reproductive Toxicology: A Practical Approach. 3rd ed Hood R, ed. New York, NY: Informa Healthcare, 388–408. [Google Scholar]
- Lee HJ, Hore TA, Reik W. 2014. Reprogramming the methylome: erasing memory and creating diversity. Cell Stem Cell 14(6):710–719, PMID: 24905162, 10.1016/j.stem.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JT, Bartolomei MS. 2013. X-inactivation, imprinting, and long noncoding RNAs in health and disease. Cell 152(6):1308–1323, PMID: 23498939, 10.1016/j.cell.2013.02.016. [DOI] [PubMed] [Google Scholar]
- Lee S-H, Oh T. 2012. Genome-wide analysis of epigenetic changes in mouse sperm by maternal exposure to endocrine disruptor vinclozolin. Mol Cell Toxicol 8:43–52, 10.1007/s13273-012-0006-8. [DOI] [Google Scholar]
- Lee S-W, Chatterjee N, Im J-E, Yoon D, Kim S, Choi J. 2018. Integrated approach of eco-epigenetics and eco-metabolomics on the stress response of bisphenol-A exposure in the aquatic midge Chironomus riparius. Ecotoxicol Environ Saf 163:111–116, PMID: 30041127, 10.1016/j.ecoenv.2018.06.084. [DOI] [PubMed] [Google Scholar]
- Lei Q, Liu X, Fu H, Sun Y, Wang L, Xu G, et al. 2016. miR-101 reverses hypomethylation of the PRDM16 promoter to disrupt mitochondrial function in astrocytoma cells. Oncotarget 7(4):5007–5022, PMID: 26701852, 10.18632/oncotarget.6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Lu X, Natoli T, Bittker J, Sipes NS, Subramanian A, et al. 2019. The carcinogenome project: in vitro gene expression profiling of chemical perturbations to predict long-term carcinogenicity. Environ Health Perspect 127(4):47002, PMID: 30964323, 10.1289/EHP3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Chang H, Xia W, Mao Z, Li Y, Xu S. 2014. F0 maternal BPA exposure induced glucose intolerance of F2 generation through DNA methylation change in Gck. Toxicol Lett 228(3):192–199, PMID: 24793715, 10.1016/j.toxlet.2014.04.012. [DOI] [PubMed] [Google Scholar]
- Li J, Chen P, Sinogeeva N, Gorospe M, Wersto RP, Chrest FJ, et al. 2002. Arsenic trioxide promotes histone H3 phosphoacetylation at the chromatin of CASPASE-10 in acute promyelocytic leukemia cells. J Biol Chem 277(51):49504–49510, PMID: 12388546, 10.1074/jbc.M207836200. [DOI] [PubMed] [Google Scholar]
- Li J, Xing X, Zhang X, Liang B, He Z, Gao C, et al. 2018. Enhanced H3K4me3 modifications are involved in the transactivation of DNA damage responsive genes in workers exposed to low-level benzene. Environ Pollut 234:127–135, PMID: 29175474, 10.1016/j.envpol.2017.11.042. [DOI] [PubMed] [Google Scholar]
- Li J, Zhang X, He Z, Sun Q, Qin F, Huang Z, et al. 2017. MGMT hypomethylation is associated with DNA damage in workers exposed to low-dose benzene. Biomarkers 22(5):470–475, PMID: 28001451, 10.1080/1354750X.2016.1274335. [DOI] [PubMed] [Google Scholar]
- Li Y, Duan F, Zhou X, Pan H, Li R. 2018. Differential responses of GC-1 spermatogonia cells to high and low doses of bisphenol A. Mol Med Rep 18(3):3034–3040, PMID: 30015891, 10.3892/mmr.2018.9256. [DOI] [PubMed] [Google Scholar]
- Lim SJ, Tan TW, Tong JC. 2010. Computational epigenetics: the new scientific paradigm. Bioinformation 4(7):331–337, PMID: 20978607, 10.6026/97320630004331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lithwick-Yanai G, Dromi N, Shtabsky A, Morgenstern S, Strenov Y, Feinmesser M, et al. 2017. Multicentre validation of a microRNA-based assay for diagnosing indeterminate thyroid nodules utilising fine needle aspirate smears. J Clin Pathol 70(6):500–507, PMID: 27798083, 10.1136/jclinpath-2016-204089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litichevskiy L, Peckner R, Abelin JG, Asiedu JK, Creech AL, Davis JF, et al. 2018. A library of phosphoproteomic and chromatin signatures for characterizing cellular responses to drug perturbations. Cell Syst 6(4):424–443.e7, PMID: 29655704, 10.1016/j.cels.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Cui Y, Ren W, Irudayaraj J. 2019. Epigenetic biomarker screening by FLIM-FRET for combination therapy in ER+ breast cancer. Clin Epigenetics 11(1):16, PMID: 30700309, 10.1186/s13148-019-0620-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Yan, Zhang Y, Tao S, Guan Y, Zhang T, Wang Z. 2016. Global DNA methylation in gonads of adult zebrafish Danio rerio under bisphenol A exposure. Ecotoxicol Environ Saf 130:124–132, PMID: 27101439, 10.1016/j.ecoenv.2016.04.012. [DOI] [PubMed] [Google Scholar]
- Liu Yang, Chen X, Bian Q, Shi Y, Liu Q, Ding L, et al. 2016. Analysis of plasma microRNA expression profiles in a Chinese population occupationally exposed to benzene and in a population with chronic benzene poisoning. J Thorac Dis 8(3):403–414, PMID: 27076935, 10.21037/jtd.2016.02.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombó M, González-Rojo S, Fernández-Díez C, Herráez MP. 2019. Cardiogenesis impairment promoted by bisphenol A exposure is successfully counteracted by epigallocatechin gallate. Environ Pollut 246:1008–1019, PMID: 31126002, 10.1016/j.envpol.2019.01.004. [DOI] [PubMed] [Google Scholar]
- Lou X-D, Wang H-D, Xia S-J, Skog S, Sun J. 2014. Effects of resveratrol on the expression and DNA methylation of cytokine genes in diabetic rat aortas. Arch Immunol Ther Exp (Warsz) 62(4):329–340, PMID: 24496569, 10.1007/s00005-014-0271-4. [DOI] [PubMed] [Google Scholar]
- Low FM, Gluckman PD. 2018. Epigenetic mechanisms. In: Neonatology: A Practical Approach to Neonatal Diseases. Buonocore G, Bracci R, Weindling M, eds. Cham, Switzerland: Springer International Publishing, 41–48. [Google Scholar]
- Lu W, Zhang R, Jiang H, Zhang H, Luo C. 2018. Computer-aided drug design in epigenetics. Front Chem 6:57, PMID: 29594101, 10.3389/fchem.2018.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lungu C, Pinter S, Broche J, Rathert P, Jeltsch A. 2017. Modular fluorescence complementation sensors for live cell detection of epigenetic signals at endogenous genomic sites. Nat Commun 8(1):649, PMID: 28935858, 10.1038/s41467-017-00457-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macalino SJY, Gosu V, Hong S, Choi S. 2015. Role of computer-aided drug design in modern drug discovery. Arch Pharm Res 38(9):1686–1701, PMID: 26208641, 10.1007/s12272-015-0640-5. [DOI] [PubMed] [Google Scholar]
- Machtinger R, Zhong J, Mansur A, Adir M, Racowsky C, Hauser R, et al. 2018. Placental lncRNA expression is associated with prenatal phthalate exposure. Toxicol Sci 163(1):116–122, PMID: 29385630, 10.1093/toxsci/kfy013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillot G, Lacroix-Triki M, Pierredon S, Gratadou L, Schmidt S, Bénès V, et al. 2009. Widespread estrogen-dependent repression of microRNAs involved in breast tumor cell growth. Cancer Res 69(21):8332–8340, PMID: 19826037, 10.1158/0008-5472.CAN-09-2206. [DOI] [PubMed] [Google Scholar]
- Majid S, Dar AA, Shahryari V, Hirata H, Ahmad A, Saini S, et al. 2010. Genistein reverses hypermethylation and induces active histone modifications in tumor suppressor gene B-cell translocation gene 3 in prostate cancer. Cancer 116(1):66–76, PMID: 19885928, 10.1002/cncr.24662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majid S, Kikuno N, Nelles J, Noonan E, Tanaka Y, Kawamoto K, et al. 2008. Genistein induces the p21WAF1/CIP1 and p16INK4a tumor suppressor genes in prostate cancer cells by epigenetic mechanisms involving active chromatin modification. Cancer Res 68(8):2736–2744, PMID: 18413741, 10.1158/0008-5472.CAN-07-2290. [DOI] [PubMed] [Google Scholar]
- Mancini M, Mandruzzato M, Garzia AC, Sahnane N, Magnani E, Macchi F, et al. 2017. In vitro hydroquinone–induced instauration of histone bivalent mark on human retroelements (LINE-1) in HL60 cells. Toxicol In Vitro 40:1–10, PMID: 27979589, 10.1016/j.tiv.2016.12.007. [DOI] [PubMed] [Google Scholar]
- Manikkam M, Haque MM, Guerrero-Bosagna C, Nilsson EE, Skinner MK. 2014. Pesticide methoxychlor promotes the epigenetic transgenerational inheritance of adult-onset disease through the female germline. PLoS One 9(7):e102091, PMID: 25057798, 10.1371/journal.pone.0102091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. 2013. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS One 8(1):e55387, PMID: 23359474, 10.1371/journal.pone.0055387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z, Xia W, Chang H, Huo W, Li Y, Xu S. 2015. Paternal BPA exposure in early life alters Igf2 epigenetic status in sperm and induces pancreatic impairment in rat offspring. Toxicol Lett 238(3):30–38, PMID: 26276081, 10.1016/j.toxlet.2015.08.009. [DOI] [PubMed] [Google Scholar]
- Marczylo EL, Jacobs MN, Gant TW. 2016. Environmentally induced epigenetic toxicity: potential public health concerns. Crit Rev Toxicol 46(8):676–700, PMID: 27278298, 10.1080/10408444.2016.1175417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsit CJ. 2015. Influence of environmental exposure on human epigenetic regulation. J Exp Biol 218(Pt 1):71–79, PMID: 25568453, 10.1242/jeb.106971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EM, Fry RC. 2018. Environmental influences on the epigenome: exposure-associated DNA methylation in human populations. Annu Rev Public Health 39:309–333, PMID: 29328878, 10.1146/annurev-publhealth-040617-014629. [DOI] [PubMed] [Google Scholar]
- Martinez-Arguelles DB, Papadopoulos V. 2015. Identification of hot spots of DNA methylation in the adult male adrenal in response to in utero exposure to the ubiquitous endocrine disruptor plasticizer di-(2-ethylhexyl) phthalate. Endocrinology 156(1):124–133, PMID: 25330100, 10.1210/en.2014-1436. [DOI] [PubMed] [Google Scholar]
- Martínez-Ibarra A, Martínez-Razo LD, Vázquez-Martínez ER, Martínez-Cruz N, Flores-Ramírez R, García-Gómez E, et al. 2019. Unhealthy levels of phthalates and bisphenol A in Mexican pregnant women with gestational diabetes and its association to altered expression of miRNAs involved with metabolic disease. Int J Mol Sci 20(13):3343, PMID: 31284700, 10.3390/ijms20133343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massy R, Jacobs M. 2013. Trends and indicators. In: Global Chemicals Outlook: Towards Sound Management of Chemicals. Kemf E, ed. Nairobi, Kenya: United Nations Environment Programme, 1–8. [Google Scholar]
- Matsumoto Y, Hannigan B, Crews D. 2014. Embryonic PCB exposure alters phenotypic, genetic, and epigenetic profiles in turtle sex determination, a biomarker of environmental contamination. Endocrinology 155(11):4168–4177, PMID: 25105783, 10.1210/en.2014-1404. [DOI] [PubMed] [Google Scholar]
- McCarrey JR, Lehle JD, Raju SS, Wang Y, Nilsson EE, Skinner MK. 2016. Tertiary epimutations—a novel aspect of epigenetic transgenerational inheritance promoting genome instability. PLoS One 11(12):e0168038, PMID: 27992467, 10.1371/journal.pone.0168038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney DL, Hillary RF, Stevenson AJ, Ritchie SJ, Walker RM, Zhang Q, et al. 2018. Epigenetic prediction of complex traits and death. Genome Biol 19(1):136, PMID: 30257690, 10.1186/s13059-018-1514-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor K, Bernatsky S, Colmegna I, Hudson M, Pastinen T, Labbe A, et al. 2016. An evaluation of methods correcting for cell-type heterogeneity in DNA methylation studies. Genome Biol 17:84, PMID: 27142380, 10.1186/s13059-016-0935-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHale CM, Zhang L, Lan Q, Li G, Hubbard AE, Forrest MS, et al. 2009. Changes in the peripheral blood transcriptome associated with occupational benzene exposure identified by cross-comparison on two microarray platforms. Genomics 93(4):343–349, PMID: 19162166, 10.1016/j.ygeno.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHale CM, Zhang L, Lan Q, Vermeulen R, Li G, Hubbard AE, et al. 2011. Global gene expression profiling of a population exposed to a range of benzene levels. Environ Health Perspect 119(5):628–634, PMID: 21147609, 10.1289/ehp.1002546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meakin CJ, Martin EM, Szilagyi JT, Nylander-French LA, Fry RC. 2019. Inorganic arsenic as an endocrine disruptor: modulation of the glucocorticoid receptor pathway in placental cells via CpG methylation. Chem Res Toxicol 32(3):493–499, PMID: 30746931, 10.1021/acs.chemrestox.8b00352. [DOI] [PubMed] [Google Scholar]
- Meissner A, Gnirke A, Bell GW, Ramsahoye B, Lander ES, Jaenisch R. 2005. Reduced representation bisulfite sequencing for comparative high-resolution DNA methylation analysis. Nucleic Acids Res 33(18):5868–5877, PMID: 16224102, 10.1093/nar/gki901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Men Y, Zhao Y, Zhang P, Zhang H, Gao Y, Liu J, et al. 2019. Gestational exposure to low-dose zearalenone disrupting offspring spermatogenesis might be through epigenetic modifications. Basic Clin Pharmacol Toxicol 125(4):382–393, PMID: 31058416, 10.1111/bcpt.13243. [DOI] [PubMed] [Google Scholar]
- Mensaert K, Denil S, Trooskens G, Van Criekinge W, Thas O, De Meyer T. 2014. Next-generation technologies and data analytical approaches for epigenomics. Environ Mol Mutagen 55(3):155–170, PMID: 24327356, 10.1002/em.21841. [DOI] [PubMed] [Google Scholar]
- Meruvu S, Zhang J, Bedi YS, Choudhury M. 2016. Mono-(2-ethylhexyl) phthalate induces apoptosis through miR-16 in human first trimester placental cell line HTR-8/SVneo. Toxicol In Vitro 31:35–42, PMID: 26597031, 10.1016/j.tiv.2015.11.010. [DOI] [PubMed] [Google Scholar]
- Messerlian C, Martinez RM, Hauser R, Baccarelli AA. 2017. ‘Omics’ and endocrine-disrupting chemicals—new paths forward. Nat Rev Endocrinol 13(12):740–748, PMID: 28707677, 10.1038/nrendo.2017.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier L, Siddeek B, Vega A, Lakhdari N, Inoubli L, Bellon RP, et al. 2012. Perinatal programming of adult rat germ cell death after exposure to xenoestrogens: role of microRNA miR-29 family in the down-regulation of DNA methyltransferases and Mcl-1. Endocrinology 153(4):1936–1947, PMID: 22334722, 10.1210/en.2011-1109. [DOI] [PubMed] [Google Scholar]
- Miao M, Zhou X, Li Y, Zhang O, Zhou Z, Li T, et al. 2014. LINE-1 hypomethylation in spermatozoa is associated with bisphenol A exposure. Andrology 2(1):138–144, PMID: 24293158, 10.1111/j.2047-2927.2013.00166.x. [DOI] [PubMed] [Google Scholar]
- Milesi MM, Varayoud J, Ramos JG, Luque EH. 2017. Uterine ERα epigenetic modifications are induced by the endocrine disruptor endosulfan in female rats with impaired fertility. Mol Cell Endocrinol 454:1–11, PMID: 28559116, 10.1016/j.mce.2017.05.028. [DOI] [PubMed] [Google Scholar]
- Molden RC, Garcia BA. 2014. Middle-down and top-down mass spectrometric analysis of co-occurring histone modifications. Curr Protoc Protein Sci 77:23.27.21–23.27.28, PMID: 25081742, 10.1002/0471140864.ps2307s77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal A, Alonso-Magdalena P, Ripoll C, Fuentes E. 2005. Disentangling the molecular mechanisms of action of endogenous and environmental estrogens. Pflugers Arch 449(4):335–343, PMID: 15517344, 10.1007/s00424-004-1343-9. [DOI] [PubMed] [Google Scholar]
- Niedzwiecki MM, Miller GW. 2017. The exposome paradigm in human health: lessons from the Emory Exposome Summer Course. Environ Health Perspect 125(6):064502, PMID: 28669935, 10.1289/EHP1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson E, King SE, McBirney M, Kubsad D, Pappalardo M, Beck D, et al. 2018. Vinclozolin induced epigenetic transgenerational inheritance of pathologies and sperm epimutation biomarkers for specific diseases. PLoS One 13(8):e0202662, PMID: 30157260, 10.1371/journal.pone.0202662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIOSH (National Institute for Occupational Safety and Health). 2019. Benzene. https://www.cdc.gov/niosh/topics/benzene/default.html [accessed 3 January 2020].
- Novo M, Verdú I, Trigo D, Martínez-Guitarte JL. 2018. Endocrine disruptors in soil: effects of bisphenol A on gene expression of the earthworm Eisenia fetida. Ecotoxicol Environ Saf 150:159–167, PMID: 29275183, 10.1016/j.ecoenv.2017.12.030. [DOI] [PubMed] [Google Scholar]
- O’Brien J, Hayder H, Zayed Y, Peng C. 2018. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol (Lausanne) 9:402, PMID: 30123182, 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD (Organisation for Economic Co-operation and Development). 2012. OECD Environmental Outlook to 2050: The Consequences of Inaction. https://www.oecd.org/g20/topics/energy-environment-green-growth/oecdenvironmentaloutlookto2050theconsequencesofinaction.htm [accessed 3 January 2020].
- Otsuka S, Ishihara A, Yamauchi K. 2014. Ioxynil and tetrabromobisphenol A suppress thyroid-hormone-induced activation of transcriptional elongation mediated by histone modifications and RNA polymerase II phosphorylation. Toxicol Sci 138(2):290–299, PMID: 24449421, 10.1093/toxsci/kfu012. [DOI] [PubMed] [Google Scholar]
- Padmanabhan R, Hendry IR, Knapp JR, Shuai B, Hendry WJ. 2017. Altered microRNA expression patterns during the initiation and promotion stages of neonatal diethylstilbestrol-induced dysplasia/neoplasia in the hamster (Mesocricetus auratus) uterus. Cell Biol Toxicol 33(5):483–500, PMID: 28265775, 10.1007/s10565-017-9389-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel BB, Raad M, Sebag IA, Chalifour LE. 2013. Lifelong exposure to bisphenol A alters cardiac structure/function, protein expression, and DNA methylation in adult mice. Toxicol Sci 133(1):174–185, PMID: 23418087, 10.1093/toxsci/kft026. [DOI] [PubMed] [Google Scholar]
- Perrier F, Viallon V, Ambatipudi S, Ghantous A, Cuenin C, Hernandez-Vargas H, et al. 2019. Association of leukocyte DNA methylation changes with dietary folate and alcohol intake in the EPIC study. Clin Epigenetics 11(1):57, PMID: 30940212, 10.1186/s13148-019-0637-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot-Applanat M, Kolf-Clauw M, Michel C, Beausoleil C. 2018. Alteration of mammary gland development by bisphenol A and evidence of a mode of action mediated through endocrine disruption. Mol Cell Endocrinol 475:29–53, PMID: 30048677, 10.1016/j.mce.2018.06.015. [DOI] [PubMed] [Google Scholar]
- Philbrook NA, Winn LM. 2015. Investigating the effects of in utero benzene exposure on epigenetic modifications in maternal and fetal CD-1 mice. Toxicol Appl Pharmacol 289(1):12–19, PMID: 26341289, 10.1016/j.taap.2015.08.019. [DOI] [PubMed] [Google Scholar]
- Philibert RA, Beach SRH, Brody GH. 2012. Demethylation of the aryl hydrocarbon receptor repressor as a biomarker for nascent smokers. Epigenetics 7(11):1331–1338, PMID: 23070629, 10.4161/epi.22520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philibert R, Hollenbeck N, Andersen E, McElroy S, Wilson S, Vercande K, et al. 2016. Reversion of AHRR demethylation is a quantitative biomarker of smoking cessation. Front Psychiatry 7:55, PMID: 27092088, 10.3389/fpsyt.2016.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietryk EW, Clement K, Elnagheeb M, Kuster R, Kilpatrick K, Love MI, et al. 2018. Intergenerational response to the endocrine disruptor vinclozolin is influenced by maternal genotype and crossing scheme. Reprod Toxicol 78:9–19, PMID: 29535025, 10.1016/j.reprotox.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilsner JR, Hu H, Ettinger A, Sánchez BN, Wright RO, Cantonwine D, et al. 2009. Influence of prenatal lead exposure on genomic methylation of cord blood DNA. Environ Health Perspect 117(9):1466–1471, PMID: 19750115, 10.1289/ehp.0800497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins GS, Tang W-Y, Belmonte J, Ho S-M. 2008. Developmental exposure to bisphenol A increases prostate cancer susceptibility in adult rats: epigenetic mode of action is implicated. Fertil Steril 89(suppl 2):e41, PMID: 18308059, 10.1016/j.fertnstert.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priya ES, Kumar TS, Singh PR, Balakrishnan S, Arunakaran J. 2018. Impact of lactational exposure to polychlorinated biphenyl causes epigenetic modification and impairs Sertoli cells functional regulators in F1 progeny. Reprod Sci 25(6):818–829, PMID: 28359186, 10.1177/1933719117699707. [DOI] [PubMed] [Google Scholar]
- Prüss-Ustün A, Wolf J, Corvalan C, Bos R, Neira M. 2016. Preventing Disease through Healthy Environments, a Global Assessment of the Burden of Disease from Environmental Risks. Geneva, Switzerland: World Heath Organization; http://apps.who.int/iris/bitstream/10665/204585/1/9789241565196_eng.pdf?ua=1 [accessed 3 January 2020]. [Google Scholar]
- Qin W, Zhu W, Shi H, Hewett JE, Ruhlen RL, MacDonald RS, et al. 2009. Soy isoflavones have an antiestrogenic effect and alter mammary promoter hypermethylation in healthy premenopausal women. Nutr Cancer 61(2):238–244, PMID: 19235040, 10.1080/01635580802404196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasoulpour RJ, LeBaron MJ, Ellis-Hutchings RG, Klapacz J, Gollapudi BB. 2011. Epigenetic screening in product safety assessment: are we there yet? Toxicol Mech Methods 21(4):298–311, PMID: 21495868, 10.3109/15376516.2011.557883. [DOI] [PubMed] [Google Scholar]
- Ray PD, Yosim A, Fry RC. 2014. Incorporating epigenetic data into the risk assessment process for the toxic metals arsenic, cadmium, chromium, lead, and mercury: strategies and challenges. Front Genet 5:201, PMID: 25076963, 10.3389/fgene.2014.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud L, Silveira WAD, Hazard ES, Simpson J, Falcinelli S, Chung D, et al. 2017. The plasticizer bisphenol A perturbs the hepatic epigenome: a systems level analysis of the miRNome. Genes (Basel) 8(10):269, PMID: 29027980, 10.3390/genes8100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roadmap Epigenomics Consortium, Kundaje A, Meuleman W, Ernst J, Bilenky M, Yen A, et al. 2015. Integrative analysis of 111 reference human epigenomes. Nature 518(7539):317–330, PMID: 25693563, 10.1038/nature14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts CE, Raner GM, Isaacs GD. 2018. High performance liquid chromatography separation of epigenetic cytosine variants. Methods Protoc 1(2):10, PMID: 31164555, 10.3390/mps1020010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roetker NS, Pankow JS, Bressler J, Morrison AC, Boerwinkle E. 2018. Prospective study of epigenetic age acceleration and incidence of cardiovascular disease outcomes in the ARIC study (Atherosclerosis Risk in Communities). Circ Genom Precis Med 11(3):e001937, PMID: 29555670, 10.1161/CIRCGEN.117.001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronco AM, Llaguno E, Epuñan MJ, Llanos MN. 2010. Effect of cadmium on cortisol production and 11β-hydroxysteroid dehydrogenase 2 expression by cultured human choriocarcinoma cells (JEG-3). Toxicol In Vitro 24(6):1532–1537, PMID: 20624455, 10.1016/j.tiv.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Rose NR, Klose RJ. 2014. Understanding the relationship between DNA methylation and histone lysine methylation. Biochim Biophys Acta 1839(12):1362–1372, PMID: 24560929, 10.1016/j.bbagrm.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotroff DM, Dix DJ, Houck KA, Knudsen TB, Martin MT, McLaurin KW, et al. 2013. Using in vitro high throughput screening assays to identify potential endocrine-disrupting chemicals. Environ Health Perspect 121(1):7–14, PMID: 23052129, 10.1289/ehp.1205065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santangeli S, Maradonna F, Gioacchini G, Cobellis G, Piccinetti CC, Dalla Valle L, et al. 2016. BPA-induced deregulation of epigenetic patterns: effects on female zebrafish reproduction. Sci Rep 6:21982, PMID: 26911650, 10.1038/srep21982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarigiannis D. 2014. Deliverable 3.2—Report on Conceptual Framework of HEALS WP 3 Definition of Methodological Framework. HEALS: Health and Environment-wide Associations based on Large population Surveys. FP7-ENV-2013- 603946. http://www.heals-eu.eu/wp-content/uploads/2013/08/HEALS_D3.2.pdf [accessed 3 January 2020].
- Sato K, Fukata H, Kogo Y, Ohgane J, Shiota K, Mori C. 2006. Neonatal exposure to diethylstilbestrol alters the expression of DNA methyltransferases and methylation of genomic DNA in the epididymis of mice. Endocr J 53(3):331–337, PMID: 16714842, 10.1507/endocrj.k06-009. [DOI] [PubMed] [Google Scholar]
- Sato K, Fukata H, Kogo Y, Ohgane J, Shiota K, Mori C. 2009. Neonatal exposure to diethylstilbestrol alters expression of DNA methyltransferases and methylation of genomic DNA in the mouse uterus. Endocr J 56(1):131–139, PMID: 18997445, 10.1507/endocrj.k08e-239. [DOI] [PubMed] [Google Scholar]
- Schmidl C, Rendeiro AF, Sheffield NC, Bock C. 2015. ChIPmentation: fast, robust, low-input ChIP-seq for histones and transcription factors. Nat Methods 12(10):963–965, PMID: 26280331, 10.1038/nmeth.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster A, Skinner MK, Yan W. 2016. Ancestral vinclozolin exposure alters the epigenetic transgenerational inheritance of sperm small noncoding RNAs. Environ Epigenet 2(1):dvw001, PMID: 27390623, 10.1093/eep/dvw001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears ME, Genuis SJ. 2012. Environmental determinants of chronic disease and medical approaches: recognition, avoidance, supportive therapy, and detoxification. J Environ Public Health 2012:356798, PMID: 22315626, 10.1155/2012/356798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A, Heredia N, Senut M-C, Land S, Hollocher K, Lu X, et al. 2015. Multigenerational epigenetic inheritance in humans: DNA methylation changes associated with maternal exposure to lead can be transmitted to the grandchildren. Sci Rep 5:14466, PMID: 26417717, 10.1038/srep14466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senyildiz M, Karaman EF, Bas SS, Pirincci PA, Ozden S. 2017. Effects of BPA on global DNA methylation and global histone 3 lysine modifications in SH-SY5Y cells: an epigenetic mechanism linking the regulation of chromatin modifiying genes. Toxicol In Vitro 44:313–321, PMID: 28765096, 10.1016/j.tiv.2017.07.028. [DOI] [PubMed] [Google Scholar]
- Seow WJ, Pesatori AC, Dimont E, Farmer PB, Albetti B, Ettinger AS, et al. 2012. Urinary benzene biomarkers and DNA methylation in Bulgarian petrochemical workers: study findings and comparison of linear and beta regression models. PLoS One 7(12):e50471, PMID: 23227177, 10.1371/journal.pone.0050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoulars K, Rodriguez MA, Thompson T, Turk J, Crowley J, Markaverich BM. 2008. Regulation of the nitric oxide pathway genes by tetrahydrofurandiols: microarray analysis of MCF-7 human breast cancer cells. Cancer Lett 264(2):265–273, PMID: 18346845, 10.1016/j.canlet.2008.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NP, Miranda K, Singh UP, Nagarkatti P, Nagarkatti M. 2018. Diethylstilbestrol (DES) induces autophagy in thymocytes by regulating Beclin-1 expression through epigenetic modulation. Toxicology 410:49–58, PMID: 30153466, 10.1016/j.tox.2018.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MK, Anway MD, Savenkova MI, Gore AC, Crews D. 2008. Transgenerational epigenetic programming of the brain transcriptome and anxiety behavior. PLoS One 3(11):e3745, PMID: 19015723, 10.1371/journal.pone.0003745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeester L, Rager JE, Bailey KA, Guan X, Smith N, García-Vargas G, et al. 2011. Epigenetic changes in individuals with arsenicosis. Chem Res Toxicol 24(2):165–167, PMID: 21291286, 10.1021/tx1004419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CM, Vera MKM, Bhandari RK. 2019. Developmental and epigenetic effects of Roundup and glyphosate exposure on Japanese medaka (Oryzias latipes). Aquat Toxicol 210:215–226, PMID: 30875550, 10.1016/j.aquatox.2019.03.005. [DOI] [PubMed] [Google Scholar]
- Solomon O, Yousefi P, Huen K, Gunier RB, Escudero-Fung M, Barcellos LF, et al. 2017. Prenatal phthalate exposure and altered patterns of DNA methylation in cord blood. Environ Mol Mutagen 58(6):398–410, PMID: 28556291, 10.1002/em.22095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Wu N, Wang S, Gao M, Song P, Lou J, et al. 2014. Transgenerational impaired male fertility with an Igf2 epigenetic defect in the rat are induced by the endocrine disruptor p,pʹ-DDE. Hum Reprod 29(11):2512–2521, PMID: 25187598, 10.1093/humrep/deu208. [DOI] [PubMed] [Google Scholar]
- Song Y, Yang L. 2018. Transgenerational impaired spermatogenesis with sperm H19 and Gtl2 hypomethylation induced by the endocrine disruptor p,pʹ-DDE. Toxicol Lett 297:34–41, PMID: 30153481, 10.1016/j.toxlet.2018.08.015. [DOI] [PubMed] [Google Scholar]
- Sonkar R, Powell CA, Choudhury M. 2016. Benzyl butyl phthalate induces epigenetic stress to enhance adipogenesis in mesenchymal stem cells. Mol Cell Endocrinol 431:109–122, PMID: 27164441, 10.1016/j.mce.2016.04.025. [DOI] [PubMed] [Google Scholar]
- Soubry A, Hoyo C, Butt CM, Fieuws S, Price TM, Murphy SK, et al. 2017. Human exposure to flame-retardants is associated with aberrant DNA methylation at imprinted genes in sperm. Environ Epigenet 3(1):dvx003, PMID: 29492305, 10.1093/eep/dvx003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer Y, Shivalila CS, Soldner F, Markoulaki S, Jaenisch R. 2015. Tracing dynamic changes of DNA methylation at single-cell resolution. Cell 163(1):218–229, PMID: 26406378, 10.1016/j.cell.2015.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenz L, Escoffier J, Rahban R, Nef S, Paoloni-Giacobino A. 2017. Testicular dysgenesis syndrome and long-lasting epigenetic silencing of mouse sperm genes involved in the reproductive system after prenatal exposure to DEHP. PLoS One 12(1):e0170441, PMID: 28085963, 10.1371/journal.pone.0170441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone A, Valdés-Mora F, Gee JMW, Farrow L, McClelland RA, Fiegl H, et al. 2012. Tamoxifen-induced epigenetic silencing of oestrogen-regulated genes in anti-hormone resistant breast cancer. PLoS One 7(7):e40466, PMID: 22808167, 10.1371/journal.pone.0040466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouder C, Paoloni-Giacobino AP-G. 2011. Specific transgenerational imprinting effects of the endocrine disruptor methoxychlor on male gametes. Reproduction 141(2):207–216, PMID: 21062904, 10.1530/REP-10-0400. [DOI] [PubMed] [Google Scholar]
- Stouder C, Paoloni-Giacobino A. 2010. Transgenerational effects of the endocrine disruptor vinclozolin on the methylation pattern of imprinted genes in the mouse sperm. Reproduction 139(2):373–379, PMID: 19887539, 10.1530/REP-09-0340. [DOI] [PubMed] [Google Scholar]
- Stueve TR, Marconett CN, Zhou B, Borok Z, Laird-Offringa IA. 2016. The importance of detailed epigenomic profiling of different cell types within organs. Epigenomics 8(6):817–829, PMID: 27305639, 10.2217/epi-2016-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturla SJ, Boobis AR, FitzGerald RE, Hoeng J, Kavlock RJ, Schirmer K, et al. 2014. Systems toxicology: from basic research to risk assessment. Chem Res Toxicol 27(3):314–329, PMID: 24446777, 10.1021/tx400410s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susiarjo M, Sasson I, Mesaros C, Bartolomei MS. 2013. Bisphenol A exposure disrupts genomic imprinting in the mouse. PLoS Genet 9(4):e1003401, PMID: 23593014, 10.1371/journal.pgen.1003401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W-Y, Morey LM, Cheung YY, Birch L, Prins GS, Ho S-M. 2012. Neonatal exposure to estradiol/bisphenol A alters promoter methylation and expression of Nsbp1 and Hpcal1 genes and transcriptional programs of Dnmt3a/b and Mbd2/4 in the rat prostate gland throughout life. Endocrinology 153(1):42–55, PMID: 22109888, 10.1210/en.2011-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W-Y, Newbold R, Mardilovich K, Jefferson W, Cheng RYS, Medvedovic M, et al. 2008. Persistent hypomethylation in the promoter of nucleosomal binding protein 1 (Nsbp1) correlates with overexpression of Nsbp1 in mouse uteri neonatally exposed to diethylstilbestrol or genistein. Endocrinology 149(12):5922–5931, PMID: 18669593, 10.1210/en.2008-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani H, Onuma Y, Ito Y, Torimura M. 2014. Long non-coding RNAs as surrogate indicators for chemical stress responses in human-induced pluripotent stem cells. PLoS One 9(8):e106282, PMID: 25171338, 10.1371/journal.pone.0106282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Shioda K, Mitsunaga S, Yawata S, Angle BM, Nagel SC, et al. 2018. Prenatal exposure to bisphenol A disrupts naturally occurring bimodal DNA methylation at proximal promoter of fggy, an obesity-relevant gene encoding a carbohydrate kinase, in gonadal white adipose tissues of CD-1 mice. Endocrinology 159(2):779–794, PMID: 29220483, 10.1210/en.2017-00711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirlwell C, Eymard M, Feber A, Teschendorff A, Pearce K, Lechner M, et al. 2010. Genome-wide DNA methylation analysis of archival formalin-fixed paraffin-embedded tissue using the Illumina Infinium HumanMethylation27 BeadChip. Methods 52(3):248–254, PMID: 20434562, 10.1016/j.ymeth.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Tindula G, Murphy SK, Grenier C, Huang Z, Huen K, Escudero-Fung M, et al. 2018. DNA methylation of imprinted genes in Mexican-American newborn children with prenatal phthalate exposure. Epigenomics 10(7):1011–1026, PMID: 29957030, 10.2217/epi-2017-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonge DP, Gant TW. 2016. What is normal? Next generation sequencing-driven analysis of the human circulating miRNAOme. BMC Mol Biol 17:4, PMID: 26860190, 10.1186/s12867-016-0057-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryndyak VP, Kovalchuk O, Muskhelishvili L, Montgomery B, Rodriguez-Juarez R, Melnyk S, et al. 2007. Epigenetic reprogramming of liver cells in tamoxifen-induced rat hepatocarcinogenesis. Mol Carcinog 46(3):187–197, PMID: 17219426, 10.1002/mc.20263. [DOI] [PubMed] [Google Scholar]
- Tryndyak VP, Muskhelishvili L, Kovalchuk O, Rodriguez-Juarez R, Montgomery B, Churchwell MI, et al. 2006. Effect of long-term tamoxifen exposure on genotoxic and epigenetic changes in rat liver: implications for tamoxifen-induced hepatocarcinogenesis. Carcinogenesis 27(8):1713–1720, PMID: 16632870, 10.1093/carcin/bgl050. [DOI] [PubMed] [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency). 2000. Reregistration Eligibility Decision (RED): Vinclozolin. EPA 738-R-00-023. Washington, DC: U.S. EPA, Office of Prevention, Pesticides and Toxic Substances. [Google Scholar]
- U.S. EPA. 2006. Validation of Screening and Testing Assays Proposed for the EDSP. Washington, DC: U.S. Environmental Protection Agency. [Google Scholar]
- Ueda J, Maehara K, Mashiko D, Ichinose T, Yao T, Hori M, et al. 2014. Heterochromatin dynamics during the differentiation process revealed by the DNA methylation reporter mouse, MethylRO. Stem Cell Reports 2(6):910–924, PMID: 24936475, 10.1016/j.stemcr.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urich MA, Nery JR, Lister R, Schmitz RJ, Ecker JR. 2015. MethylC-seq library preparation for base-resolution whole-genome bisulfite sequencing. Nat Protoc 10(3):475–483, PMID: 25692984, 10.1038/nprot.2014.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzumcu M, Suzuki H, Skinner MK. 2004. Effect of the anti-androgenic endocrine disruptor vinclozolin on embryonic testis cord formation and postnatal testis development and function. Reprod Toxicol 18(6):765–774, PMID: 15279874, 10.1016/j.reprotox.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Vaissière T, Sawan C, Herceg Z. 2008. Epigenetic interplay between histone modifications and DNA methylation in gene silencing. Mutat Res 659(1–2):40–48, PMID: 18407786, 10.1016/j.mrrev.2008.02.004. [DOI] [PubMed] [Google Scholar]
- van der Weijden VA, Flöter VL, Ulbrich SE. 2018. Gestational oral low-dose estradiol-17β induces altered DNA methylation of CDKN2D and PSAT1 in embryos and adult offspring. Sci Rep 8(1):7494, PMID: 29748642, 10.1038/s41598-018-25831-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN. 2013. Non-monotonic dose responses in studies of endocrine disrupting chemicals: bisphenol A as a case study. Dose Response 12(2):259–276, PMID: 24910584, 10.2203/dose-response.13-020.Vandenberg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr, Lee D-H, et al. 2012. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev 33(3):378–455, PMID: 22419778, 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Welshons WV, vom Saal FS, Toutain P-L, Myers JP. 2014. Should oral gavage be abandoned in toxicity testing of endocrine disruptors? Environ Health 13(1):46, PMID: 24961440, 10.1186/1476-069X-13-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhees K, Coort S, Ruijters EJB, Godschalk RWL, van Schooten FJ, Barjesteh van Waalwijk van Doorn-Khosrovani S. 2011. Epigenetics: prenatal exposure to genistein leaves a permanent signature on the hematopoietic lineage. FASEB J 25(2):797–807, PMID: 21048042, 10.1096/fj.10-172155. [DOI] [PubMed] [Google Scholar]
- Varin T, Gubler H, Parker CN, Zhang J-H, Raman P, Ertl P, et al. 2010. Compound set enrichment: a novel approach to analysis of primary HTS data. J Chem Inf Model 50(12):2067–2078, PMID: 21073183, 10.1021/ci100203e. [DOI] [PubMed] [Google Scholar]
- Venturelli S, Berger A, Böcker A, Busch C, Weiland T, Noor S, et al. 2013. Resveratrol as a pan-HDAC inhibitor alters the acetylation status of histone [corrected] proteins in human-derived hepatoblastoma cells. PLoS One 8(8):e73097, PMID: 24023672, 10.1371/journal.pone.0073097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbanck M, Canouil M, Leloire A, Dhennin V, Coumoul X, Yengo L, et al. 2017. Low-dose exposure to bisphenols A, F and S of human primary adipocyte impacts coding and non-coding RNA profiles. PLoS One 12(6):e0179583, PMID: 28628672, 10.1371/journal.pone.0179583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma M. 2015. Toxicoepigenomics and cancer: implications for screening. In: Cancer Epigenetics: Risk Assessment, Diagnosis, Treatment, and Prognosis. Verma M, ed. New York, NY: Springer New York, 355–367. [Google Scholar]
- Vilahur N, Bustamante M, Byun H-M, Fernandez MF, Santa Marina L, Basterrechea M, et al. 2014. Prenatal exposure to mixtures of xenoestrogens and repetitive element DNA methylation changes in human placenta. Environ Int 71:81–87, PMID: 24980756, 10.1016/j.envint.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vineis P, Chadeau-Hyam M, Gmuender H, Gulliver J, Herceg Z, Kleinjans J, et al. 2017. The exposome in practice: design of the EXPOsOMICS project. Int J Hyg Environ Health 220(2 Pt A):142–151, PMID: 27576363, 10.1016/j.ijheh.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal FS. 2019. Flaws in design, execution and interpretation limit CLARITY-BPA’s value for risk assessments of bisphenol A. Basic Clin Pharmacol Toxicol 125(Suppl 3):32–43, PMID: 30589220, 10.1111/bcpt.13195. [DOI] [PubMed] [Google Scholar]
- VonHandorf A, Sánchez-Martín FJ, Biesiada J, Zhang H, Zhang X, Medvedovic M, et al. 2018. Chromium disrupts chromatin organization and CTCF access to its cognate sites in promoters of differentially expressed genes. Epigenetics 13(4):363–375, PMID: 29561703, 10.1080/15592294.2018.1454243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijheid M, Slama R, Robinson O, Chatzi L, Coen M, van den Hazel P, et al. 2014. The Human Early-Life Exposome (HELIX): project rationale and design. Environ Health Perspect 122(6):535–544, PMID: 24610234, 10.1289/ehp.1307204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Trevino LS, Wong RLY, Medvedovic M, Chen J, Ho S-M, et al. 2016. Reprogramming of the epigenome by MLL1 links early-life environmental exposures to prostate cancer risk. Mol Endocrinol 30(8):856–871, PMID: 27219490, 10.1210/me.2015-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Han J, Duan X, Xiong B, Cui X-S, Kim N-H, et al. 2016. The toxic effects and possible mechanisms of bisphenol A on oocyte maturation of porcine in vitro. Oncotarget 7(22):32554–32565, PMID: 27086915, 10.18632/oncotarget.8689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Pehrsson EC, Purushotham D, Li D, Zhuo X, Zhang B, et al. 2018. The NIEHS TaRGET II Consortium and environmental epigenomics. Nat Biotechnol 36(3):225–227, PMID: 29509741, 10.1038/nbt.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Zhang J, Tan K, Sun R, Yin L, Pu Y. 2015. Benzene-induced aberrant miRNA expression profile in hematopoietic progenitor cells in C57BL/6 mice. Int J Mol Sci 16(11):27058–27071, PMID: 26569237, 10.3390/ijms161126001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhouse C, Bergin IL, Harris C, Dolinoy DC. 2015. Stat3 is a candidate epigenetic biomarker of perinatal bisphenol A exposure associated with murine hepatic tumors with implications for human health. Epigenetics 10(12):1099–1110, PMID: 26542749, 10.1080/15592294.2015.1107694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild CP. 2005. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev 14(8):1847–1850, PMID: 16103423, 10.1158/1055-9965.EPI-05-0456. [DOI] [PubMed] [Google Scholar]
- Winterbottom EF, Moroishi Y, Halchenko Y, Armstrong DA, Beach PJ, Nguyen QP, et al. 2019. Prenatal arsenic exposure alters the placental expression of multiple epigenetic regulators in a sex-dependent manner. Environ Health 18(1):18, PMID: 30819207, 10.1186/s12940-019-0455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart D, Arndt D, Pon A, Sajed T, Guo AC, Djoumbou Y, et al. 2015. T3DB: the toxic exposome database. Nucleic Acids Res 43(Database issue):D928–D934, PMID: 25378312, 10.1093/nar/gku1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CCY, Caspi A, Williams B, Craig IW, Houts R, Ambler A, et al. 2010. A longitudinal study of epigenetic variation in twins. Epigenetics 5(6):516–526, PMID: 20505345, 10.4161/epi.5.6.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong NC, Morley R, Saffery R, Craig JM. 2008. Archived Guthrie blood spots as a novel source for quantitative DNA methylation analysis. BioTechniques 45(4):423–430, PMID: 18855769, 10.2144/000112945. [DOI] [PubMed] [Google Scholar]
- Woo HD, Fernandez-Jimenez N, Ghantous A, Degli Esposti D, Cuenin C, Cahais V, et al. 2018. Genome-wide profiling of normal gastric mucosa identifies Helicobacter pylori- and cancer-associated DNA methylome changes. Int J Cancer 143(3):597–609, PMID: 29574700, 10.1002/ijc.31381. [DOI] [PubMed] [Google Scholar]
- Wu H, Estill MS, Shershebnev A, Suvorov A, Krawetz SA, Whitcomb BW, et al. 2017. Preconception urinary phthalate concentrations and sperm DNA methylation profiles among men undergoing IVF treatment: a cross-sectional study. Hum Reprod 32(11):2159–2169, PMID: 29024969, 10.1093/humrep/dex283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SC, Zhang Y. 2010. Active DNA demethylation: many roads lead to Rome. Nat Rev Mol Cell Biol 11(9):607–620, PMID: 20683471, 10.1038/nrm2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia B, Wang Y, Wang X, Wu J, Song Q, Sun Z, et al. 2018. In utero and lactational exposure of DEHP increases the susceptibility of prostate carcinogenesis in male offspring through PSCA hypomethylation. Toxicol Lett 292:78–84, PMID: 29689378, 10.1016/j.toxlet.2018.04.022. [DOI] [PubMed] [Google Scholar]
- Xie Q, Bai Q, Zou L-Y, Zhang Q-Y, Zhou Y, Chang H, et al. 2014. Genistein inhibits DNA methylation and increases expression of tumor suppressor genes in human breast cancer cells. Genes Chromosomes Cancer 53(5):422–431, PMID: 24532317, 10.1002/gcc.22154. [DOI] [PubMed] [Google Scholar]
- Yang AS, Estécio MRH, Doshi K, Kondo Y, Tajara EH, Issa J-PJ. 2004. A simple method for estimating global DNA methylation using bisulfite PCR of repetitive DNA elements. Nucleic Acids Res 32(3):e38, PMID: 14973332, 10.1093/nar/gnh032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Bai W, Niu P, Tian L, Gao A. 2014. Aberrant hypomethylated STAT3 was identified as a biomarker of chronic benzene poisoning through integrating DNA methylation and mRNA expression data. Exp Mol Pathol 96(3):346–353, PMID: 24613686, 10.1016/j.yexmp.2014.02.013. [DOI] [PubMed] [Google Scholar]
- Yang PJ, Hou MF, Tsai EM, Liang SS, Chiu CC, Ou-Yang F, et al. 2018. Breast cancer is associated with methylation and expression of the a disintegrin and metalloproteinase domain 33 (ADAM33) gene affected by endocrinedisrupting chemicals. Oncol Rep 40(5):2766–2777, PMID: 30226539, 10.3892/or.2018.6675. [DOI] [PubMed] [Google Scholar]
- Yang Y-M, Sun D, Kandhi S, Froogh G, Zhuge J, Huang W, et al. 2018. Estrogen-dependent epigenetic regulation of soluble epoxide hydrolase via DNA methylation. Proc Natl Acad Sci USA 115(3):613–618, PMID: 29295935, 10.1073/pnas.1716016115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Tang Y, Xiong Y, Feng L, Li X. 2019. Bisphenol A exposure alters placentation and causes preeclampsia-like features in pregnant mice involved in reprogramming of DNA methylation of WNT2. FASEB J 33(2):2732–2742, PMID: 30303745, 10.1096/fj.201800934RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida W, Baba Y, Karube I. 2016. Global DNA methylation detection system using MBD-fused luciferase based on bioluminescence resonance energy transfer assay. Anal Chem 88(18):9264–9268, PMID: 27541340, 10.1021/acs.analchem.6b02565. [DOI] [PubMed] [Google Scholar]
- Yuan Z-F, Arnaudo AM, Garcia BA. 2014. Mass spectrometric analysis of histone proteoforms. Annu Rev Anal Chem (Palo Alto Calif) 7:113–128, PMID: 24896311, 10.1146/annurev-anchem-071213-015959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zama AM, Uzumcu M. 2009. Fetal and neonatal exposure to the endocrine disruptor methoxychlor causes epigenetic alterations in adult ovarian genes. Endocrinology 150(10):4681–4691, PMID: 19589859, 10.1210/en.2009-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Ding S, Qiao P, Dong L, Yu M, Wang C, et al. 2016. n-Butylparaben induces male reproductive disorders via regulation of estradiol and estrogen receptors. J Appl Toxicol 36(9):1223–1234, PMID: 26888303, 10.1002/jat.3291. [DOI] [PubMed] [Google Scholar]
- Zhang R, Liu L, Yao Y, Fei F, Wang F, Yang Q, et al. 2017. High resolution imaging of DNA methylation dynamics using a zebrafish reporter. Sci Rep 7(1):5430, PMID: 28710355, 10.1038/s41598-017-05648-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Zhou X, Li D-K, Yang F, Pan H, Li T, et al. 2017. Genome-wide alteration in DNA hydroxymethylation in the sperm from bisphenol A-exposed men. PLoS One 12(6):e0178535, PMID: 28582417, 10.1371/journal.pone.0178535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Baccarelli AA, Mansur A, Adir M, Nahum R, Hauser R, et al. 2019. Maternal phthalate and personal care products exposure alters extracellular placental miRNA profile in twin pregnancies. Reprod Sci 26(2):289–294, PMID: 29690855, 10.1177/1933719118770550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Li Q, Arita A, Sun H, Costa M. 2009. Effects of nickel, chromate, and arsenite on histone 3 lysine methylation. Toxicol Appl Pharmacol 236(1):78–84, PMID: 19371620, 10.1016/j.taap.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Liu H, Wang C, Lu Q, Huang Q, Zheng C, et al. 2015. Long non-coding RNAs as novel expression signatures modulate DNA damage and repair in cadmium toxicology. Sci Rep 5:15293, PMID: 26472689, 10.1038/srep15293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Li Y, Lou D, Gao Y, Yu J, Kong D, et al. 2018. Sodium arsenite exposure inhibits histone acetyltransferase p300 for attenuating H3K27ac at enhancers in mouse embryonic fibroblast cells. Toxicol Appl Pharmacol 357:70–79, PMID: 30130555, 10.1016/j.taap.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zober A, Hoffmann G, Ott MG, Will W, Germann C, van Ravenzwaay B. 1995. Study of morbidity of personnel with potential exposure to vinclozolin. Occup Environ Med 52(4):233–241, PMID: 7795738, 10.1136/oem.52.4.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoghbi HY, Beaudet AL. 2016. Epigenetics and human disease. Cold Spring Harb Perspect Biol 8(2):a019497, PMID: 26834142, 10.1101/cshperspect.a019497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Lippert C, Heckerman D, Aryee M, Listgarten J. 2014. Epigenome-wide association studies without the need for cell-type composition. Nat Methods 11(3):309–311, PMID: 24464286, 10.1038/nmeth.2815. [DOI] [PubMed] [Google Scholar]