Abstract
Background:
Epidemiological evidence on the health effects of ultrafine particles (UFP) remains insufficient to infer a causal relationship that is largely due to different size ranges and exposure metrics examined across studies. Moreover, evidence regarding the association between UFP and cardiovascular disease at a sub-daily timescale is lacking.
Objective:
We investigated the relationship between different particle metrics, including particle number (PNC), length (PLC), and surface area (PSC) concentrations, and myocardial infarction (MI) at an hourly timescale.
Methods:
We collected hourly air pollution and meteorological data from fixed urban background monitoring sites and hourly nonfatal MI cases from a MI registry in Augsburg, Germany, during 2005–2015. We conducted a time-stratified case-crossover analysis with conditional logistic regression to estimate the association between hourly particle metrics and MI cases, adjusted for air temperature and relative humidity. We also examined the independent effects of a certain particle metric in two-pollutant models by adjusting for copollutants, including particulate matter (PM) with an aerodynamic diameter of or ( and , respectively), nitrogen dioxide, ozone, and black carbon.
Results:
Overall, a total of 5,898 cases of nonfatal MI cases were recorded. Exploratory analyses showed similar associations across particle metrics in the first 6–12 h. For example, interquartile range increases in PNC within the size range of , PLC, and PSC were associated with an increase of MI 6 h later by 3.27% [95% confidence interval (CI): 0.27, 6.37], 5.71% (95% CI: 1.79, 9.77), and 5.84% (95% CI: 1.04, 10.87), respectively. Positive, albeit imprecise, associations were observed for PNC within the size range of and . Effect estimates for PLC and PSC remained similar after adjustment for PM and gaseous pollutants.
Conclusions:
Transient exposure to particle number, length, and surface area concentrations or other potentially related exposures may trigger the onset of nonfatal myocardial infraction. https://doi.org/10.1289/EHP5478
Introduction
Ultrafine particles (UFP) are hypothesized to have adverse health effects due to their small size, their large active surface areas, and their ability to penetrate into the alveoli and to translocate in the systemic circulation (HEI Review Panel on Ultrafine Particles 2013). Toxicological studies have provided early evidence that UFP might have higher toxicity per mass unit than larger particles (HEI Review Panel on Ultrafine Particles 2013). However, epidemiological evidence on the association between UFP exposure and cardiovascular effects remains inconclusive and insufficient to infer a causal relationship despite an increasing number of studies over the past decade (HEI Review Panel on Ultrafine Particles 2013; Ohlwein et al. 2019; Rückerl et al. 2011).
The paucity of consistent findings across epidemiological studies may be in part due to the different size ranges and exposure metrics examined to characterize ambient UFP exposure (Baldauf et al. 2016; HEI Review Panel on Ultrafine Particles 2013; Ohlwein et al. 2019). Conventionally, UFP are defined as particles in diameter, which consist of both primary particles () emitted directly from vehicle exhaust (Aitken mode) and newly formed secondary particles () from mostly nucleation processes (nucleation mode) (Brines et al. 2015; Morawska et al. 2008). Particle number concentration (PNC) is assumed to be dominated by particles , which could comprise more than 75% of total PNC (Evans et al. 2014; Iskandar et al. 2012). Thus, two-thirds of recent studies investigated UFP exposure using PNC within the size range up to (Ohlwein et al. 2019). However, recent studies have reported that exposure to particles in the accumulation mode () could also be associated with daily cardiovascular mortality (Hennig et al. 2018; Meng et al. 2013), morbidity (Andersen et al. 2008; Braniš et al. 2010; Rosenthal et al. 2013), and subclinical outcomes (Chen et al. 2015; Han et al. 2016; Sun et al. 2015). Moreover, UFP metrics other than PNC have been rarely applied in epidemiological studies to assess the health effects of UFP exposure. Alternative physical attributes of UFP, especially particle surface area concentration (PSC), may be more biologically effective in determining the pulmonary toxicity such as inflammatory potential or oxidative stress (Sager and Castranova 2009; Valavanidis et al. 2008). Previous epidemiological studies have found that PSC and particle length concentration (PLC) were more closely associated with daily mortality and inflammatory blood markers than PNC (Hennig et al. 2018; Rückerl et al. 2016). Thus, to improve the investigation of UFP-related health effects and to determine whether other particle metrics besides particulate matter (PM) with an aerodynamic diameter impact public health, there is an urgent need to explore alternative metrics and different size ranges, including particles (Baldauf et al. 2016; Ohlwein et al. 2019).
A major challenge of determining an association between UFP exposure and health outcomes is relying on just a single monitor site to assess UFP exposure, which has high spatial and temporal variability. Despite the high spatial variability, moderately good temporal correlations between UFP number concentrations at multiple locations in some cities might be sufficient to support epidemiological studies on the short-term effects of UFP on health (HEI Review Panel on Ultrafine Particles 2013). Another challenge is to differentiate its health effects from effects associated with other copollutants. The few studies that adjusted for copollutants generally found attenuated effect estimates for UFP after adjustment for PM with an aerodynamic diameter of (), (), or nitrogen dioxide () (HEI Review Panel on Ultrafine Particles 2013; Ohlwein et al. 2019). Because traffic emission is a major source of UFP in urban areas (Morawska et al. 2008), it is of particular importance to assess whether UFP exert health effects independent of copollutants in the traffic pollutant mixture.
Acute myocardial infarction (MI) is a major cause of death from cardiovascular disease, which can be triggered by environmental factors such as PM (Claeys et al. 2017; Peters et al. 2001). The evidence on the association between short-term exposure to UFP and MI is relatively scarce and inconsistent. Using daily average PNC as the exposure metric, some studies have reported a null association (Belleudi et al. 2010; Delfino et al. 2011), whereas some have observed a positive, albeit imprecise [i.e., wide confidence interval (CI)], association (Gardner et al. 2014; Link et al. 2013; von Klot et al. 2005) and others have found positive associations among fatal cases of age (Lanki et al. 2006), recurrent cases (Wolf et al. 2015), and out-of-hospital cardiac arrest (Rosenthal et al. 2013). In addition, due to the limited monitoring of UFP, most of the previous epidemiological studies focused on a daily timescale, whereas only a small body of work has revealed a potential association of MI with exposure to PM or, more generally, traffic within a few hours (Bhaskaran et al. 2011; Peters et al. 2001, 2004).
In this study, we aimed to assess the association between the hourly onset of nonfatal MI and exposure to UFP using different particle metrics, including PNC, PLC, and PSC, in Augsburg, Germany. We conducted a time-stratified case-crossover analysis using data from a validated, complete, and detailed MI registry from 2005 to 2015. We also evaluated the independence of UFP effects by adjusting for copollutants and conducted subgroup analyses to identify potentially susceptible subpopulations.
Methods
Study Population
As part of the WHO MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) project, the population-based Augsburg MI registry was founded in 1984 (Löwel et al. 2005). Since 1996, it has been continued as part of the KORA (Cooperative Health Research in the Augsburg Region) research program. The study area includes the city of Augsburg and the two adjacent rural counties of Augsburg and Aichach-Friedberg. The registry has been continuously recording all cases of nonfatal MI in eight hospitals in the study area and coronary deaths occurring among residents 25–74 years of age (about 400,000 inhabitants). From 2009 on, patients with MI and 75–84 years of age have also been enrolled in the registry. Over the whole study period, we used a consistent MONICA definition for MI diagnosis (Wolf et al. 2009). Within the MONICA-defined MI events, additional nonST segment elevation MI (NSTEMI) cases were categorized since 2000 if symptomatic patients had elevated troponin concentrations and no typical electrocardiographic changes (Löwel et al. 2005). Following the MONICA protocol, MI patients who survived at least 24 h after hospitalization were interviewed about the event, demographic information, comorbidities, medication, and family history. Clinical history of diabetes mellitus and hypertension were obtained from patient interview and chart review during the hospital stay (Kirchberger et al. 2014). If a patient survived the 28th day after hospital admission, the MI was identified as nonfatal, otherwise as fatal. Patients who were registered for the first time and did not have a history of previous MI events were defined as incident events, whereas those having a readmission at least 28 d after the first MI were defined as recurrent events. For a nonfatal event, the hour of onset was defined as the time of the onset of chest pain that lasted at least 20 min or the time of the severest symptoms, with further validation from the patients’ medical record (Peters et al. 2004). The present study included all nonfatal MI cases for patients 25–74 years of age between 2005 and 2015. More details of the Augsburg MI registry have been described elsewhere (Kuch et al. 2008; Löwel et al. 2005). This study was approved by the ethics committee of the Bavarian Chamber of Physicians and performed in accordance with the Declaration of Helsinki.
Air Pollution and Meteorological Data
Hourly averages of PNC, PLC, PSC, , , and black carbon (BC) were obtained from a single aerosol monitoring station located southeast of the city center and considered as representative for the urban background in Augsburg (Cyrys et al. 2008). This measurement station was established in fall 2004 by Helmholtz Zentrum München in cooperation with the University of Augsburg, and it has been designed for the collection of numerous physical and chemical aerosol parameters, which are generally not routinely measured at monitoring sites operated by the official network (Gu et al. 2011, 2012; Pitz et al. 2008a, 2008b; Rückerl et al. 2016; Sun et al. 2019). Size-fractionated PNCs between 3 and (mobility diameter) were measured with a Twin Differential Mobility Particle Sizer (TDMPS) system (Birmili et al. 1999). Because there are substantial diffusion losses of particles in the mobility particle size spectrometers (Wiedensohler et al. 2012; Zhang and Flagan 1996), we excluded the size range from our analysis. To explore the health effects of different particle sizes, we investigated the following particle size fractions, including (reflecting nucleation mode), (reflecting Aitken mode), (reflecting the usually defined range of UFP), and (reflecting accumulation mode). We did not investigate the size range of because most (99.8%) of PNC was in the size range of (see Figure S1).
To explore the representativeness of using a central monitoring site to reflect the temporal fluctuations in PNC measurement, we compared the hourly total PNC measured in the central site with those from an intensive sampling campaign in the Augsburg region between March 2014 and April 2015 (Pilz et al. 2018; Wolf et al. 2017). A total of 20 sampling sites were selected based on the spatial variation of air pollution and included a mixture of urban traffic (6), urban background (5), regional traffic (4), regional background (4), and industry area (1) monitoring sites (see Figure S3). For each site, PNC was measured for three periods of 2 weeks in the warm, cold, and one intermediate (spring or fall) season. Total PNC () were measured by three GRIMM ultrafine particle counters (model EDM 465 UFPC; GRIMM aerosol) and a NANOSCAN SMPS Nanoparticle Sizer (Nanoscan, model 3910; TSI) (Wolf et al. 2017).
As the total length of an imaginary chain of particles, PLC between 10 and in aerodynamic diameter (or in mobility equivalent diameter) was measured by an electrical aerosol detector (EAD, model 3070A; TSI). The total active (Fuchs) surface of particles, PSC, in the size range of in aerodynamic diameter was measured by a Diffusion Charging Particle Sensor (DCPS, model LQ1; Matter Aerosol AG). The upper cut point for PLC and PSC size range ( in aerodynamic diameter or in mobility diameter) was the same as that for the total PNC. Thus, PLC and PSC were considered to represent physical attributes of particles within the ultrafine and quasi-ultrafine range. Because of a DCPS equipment malfunction after 30 April 2012 (see Figure S2), PSC measurements from 1 January 2005 to 30 April 2012 were used in this study. and were measured by two independent tapered element oscillating microbalances (TEOM, model 1400ab; Thermo Fisher Scientific) equipped with the Filter Dynamics Measurement System (FDMS, model 8500b; Thermo Fisher Scientific). BC was measured with an Aethalometer (model series 8,100; Thermo Fisher Scientific). A detailed description of measurement methods can be found elsewhere (Gu et al. 2012; Pitz et al. 2008a).
Hourly averages of nitrogen dioxide (), ozone (), and meteorological parameters were collected separately from a single urban background monitoring site operated by the Bavarian Environment Agency (LfU, Bayerisches Landesamt für Umwelt) in the city of Augsburg. The meteorological variables (air temperature and relative humidity) and concentrations were obtained from the official urban background monitoring site located on the premises of the LfU, approximately south of the city center. Hourly averages of were obtained from another official urban background monitoring site at Bourgesplatz, which is located approximately north of the city center.
Statistical Analysis
We applied a time-stratified case-crossover design to estimate the association between hourly particle metrics and MI cases. The case-crossover design is a type of self-matched case–control study in which each individual serves as his or her own control (Janes et al. 2005). For each individual, air pollution exposure in the case period (i.e., the hour of MI onset) was compared with exposures in a series of control periods within a fixed time strata that were not associated with the event of interest (Bhaskaran et al. 2011). In this study, we used calendar month as the fixed time strata and selected the same hour in the same day of the week within the same time strata (i.e., calendar month) as the control periods, including periods both before and after the event. This approach thus controls for long-term time trends, seasonality, day of the week, intra-day variation, and confounders that do not vary within a month, such as time-invariant individual-level characteristics (e.g., occupation, socioeconomic status, and preexisting cardiovascular disease).
We used a conditional logistic regression model to compare particle exposure on case and control hours. We investigated particle exposure at both single-hour lags from the same hour up to 6 h preceding the onset time (lag0–lag6) and moving average lags for the period 1–6, 7–12, 13–18, 19–24, and 25–72 h (Bhaskaran et al. 2011). To facilitate comparison with findings from daily time-series studies, we also explored moving averages of 1–24, 1–48, and 1–72 h before the onset of MI. We further adjusted for potential time-varying confounders by using natural cubic splines with 4 degrees of freedom (df) for air temperature and relative humidity at lag 1–72 h (Li et al. 2016). Based on the literature examining the effects of short-term exposure to particles on MI (Bhaskaran et al. 2011; Peters et al. 2001; Zanobetti and Schwartz 2005), we used a linear term for each of the particle metrics in the single-pollutant model. To evaluate the potential confounding of copollutants, we explored two-pollutant models using pairs among the particle metrics (, , PLC, PSC, , and ). We also investigated whether the estimates for particle metrics were independent of gaseous pollutants ( and ) and BC. In two-pollutant models, we excluded pairs of pollutants that were highly correlated with a . We checked the multicollinearity of the remaining pairs by calculating variance inflation factor (VIF) for each pollutant (Fox and Monette 1992). A cut off value of 3 for VIF was used to avoid multicollinearity (Wang et al. 2014). The exclusion of highly correlated pollutants in two-pollutant models may also help ease the concern of overadjustment for particle metrics highly correlated with other metrics, as is usually the case when examining the effects of a specific constituent after adjusting for mass (Mostofsky et al. 2012).
To identify potential susceptible subgroups, we performed stratified analyses for different groups: sex (male and female), age groups (25- to 64- and 65- to 74-y-olds), living alone, history of diabetes mellitus, history of hypertension, education level [low (primary school), medium (high school), and high (university)], smoking status (smoker, ex-smoker, and nonsmoker), and obesity (). We further stratified the nonfatal MI cases by admission type (incident and recurrent events) and infarction type [ST segment elevation MI (STEMI) and NSTEMI]; we did not examine patients with bundle branch blocks due to the small sample size. Moreover, we assessed effect modification by high () or low () levels of hourly temperature and copollutants. The 75th percentile was chosen based on a previous study (Hennig et al. 2018) that had yielded comparable sample sizes (hence similar width of CI) at high and low levels of copollutants. The statistical significance of effect modification was tested by calculating the 95% CI of the difference between effect estimates within each subgroup or effect modifier (Chen et al. 2018; Zeka et al. 2006). Odds ratios estimated from logistic regression are presented as percent differences (95% CI) in hourly MI cases per interquartile range (IQR) increase in the respective air pollutant.
We conducted several sensitivity analyses to assess the robustness of our results: a) we increased the population age limit from 74 y to 84 y by including additional patients 75–84 years of age from 2009 to 2015 (1,377 MI cases); b) we used an alternative 5-d average lag for temperature as reported in a previous analysis (Wolf et al. 2009); c) we used two temperature terms to account for both the current temperature and lagged temperature (1–24 h, 1–48 h, or 1–72 h), as well as the concurrent temperature with the same lag as particle metrics and lagged temperature at the previous 2–3 d (25–72 h); d) we redefined the control periods by changing the fixed time strata from calendar month to a recurring 3-week period (e.g., first to third week of the year) to assess the influence of time strata (Kim et al. 2018); e) we redefined the high and low levels of copollutants and air temperature, using the 50th percentile as the cut off value in the effect modification analyses; and f) we explored the exposure–response relationships between particle metrics and MI by applying a natural cubic spline with 4 df to the pollutant term (rather than a linear term). Akaike information criteria (AIC) values were used to compare the model fits between model with this nonlinear pollutant term and the main model with a linear pollutant term.
Results
Descriptive Statistics
A total of 5,898 nonfatal MIs were recorded between 2005 and 2015 in Augsburg (Table 1). Most of these cases were incident events (82.9%). For infarction type, STEMI and NSTEMI events accounted for 39.6% and 51.7%, respectively. The remaining 8.7% included bundle branch blocks and cases with missing information. Patients with MI were predominantly male, and more than half of them were of age. A larger proportion of cases were patients not living alone, without diabetes or obesity, having hypertension, and with low education level.
Table 1.
Characteristics of survivors of myocardial infarction (MI) in Augsburg, Germany, from 2005 to 2015 ().
| Characteristic | (%) |
|---|---|
| Male | 4,495 (76.2) |
| Female | 1,403 (23.8) |
| Age (y) | |
| 25–59 | 2,424 (41.1) |
| 60–74 | 3,474 (58.9) |
| Missing | 0 (0) |
| Admission type | |
| Incident MI | 4,890 (82.9) |
| Recurrent MI | 1,006 (17.1) |
| Missing | 2 (0.0) |
| Infarction type | |
| STEMI | 2,333 (39.6) |
| NSTEMI | 3,048 (51.7) |
| Bundle branch blocks | 368 (6.2) |
| Missing | 149 (2.5) |
| Living alone | |
| Yes | 1,107 (18.8) |
| No | 4,543 (77.0) |
| Missing | 248 (4.2) |
| History of disease | |
| Diabetes | 1,797 (30.5) |
| Hypertension | 4,528 (76.8) |
| Education level | |
| Low (primary school) | 3,460 (58.7) |
| Medium (high school) | 1,060 (18.0) |
| High (university) | 629 (10.7) |
| Missing | 749 (12.6) |
| Obesity () | |
| Yes | 1,615 (27.4) |
| No | 3,975 (67.4) |
| Missing | 308 (5.2) |
| Smoking status | |
| Smoker | 2,208 (37.4) |
| Ex-smoker | 1,845 (31.3) |
| Nonsmoker | 1,519 (25.8) |
| Missing | 326 (5.5) |
Note: BMI, body mass index; NSTEMI, non-ST segment elevation MI; STEMI, ST segment elevation MI.
During the study period, the mean number concentration of hourly exposure to UFP () was , with slightly larger contribution from the Aitken mode (, ) than that from the nucleation mode (, ) (Table 2). Mean levels of hourly PLC, PSC, , , , , BC, air temperature, and relative humidity were , , , , , , , 10.0°C, and 75.6%, respectively. There was 9.4% of PSC measurements missing, which was mainly due to the DCPS equipment malfunction during 29 May 2015 and 23 November 2015 (accounting for 6.7% of missing PSC data) (see Figure S2). Compared with 20 sampling sites throughout the study region, the hourly PNC measured at the central site showed high correlations with urban background , urban traffic (), and industry area () sites and moderate correlations with regional background () and regional traffic () sites (see Table S6).
Table 2.
Summary statistics for hourly air pollutants and meteorology in Augsburg, Germany, from 2005 to 2015 (data on 96,408 consecutive hours).
| Variable | Missing (%) | Min | P25 | P50 | P75 | Max | IQR | |
|---|---|---|---|---|---|---|---|---|
| PNC () | ||||||||
| 4.6 | 0 | 1,689 | 2,828 | 4,733 | 934,944 | 3,044 | ||
| 4.6 | 0 | 2,036 | 3,320 | 5,643 | 291,464 | 3,607 | ||
| 4.6 | 0 | 3,911 | 6,333 | 10,440 | 1,009,751 | 6,528 | ||
| 4.8 | 0 | 811 | 1,301 | 2,074 | 33,335 | 1,263 | ||
| PLC () | 6.1 | 0 | 0.3 | 0.5 | 0.7 | 7.3 | 0.4 | |
| PSC ()a | 9.4 | 0.3 | 21.5 | 34.1 | 53.4 | 416.4 | 31.9 | |
| () | 0.4 | 0 | 6.4 | 11.6 | 19.6 | 156.4 | 13.2 | |
| () | 0.4 | 0 | 9.3 | 15.9 | 25.1 | 386.5 | 15.8 | |
| () | 0.6 | 1.0 | 18.5 | 28.0 | 41.0 | 189.0 | 22.5 | |
| () | 0.6 | 0 | 16.0 | 42.5 | 66.0 | 196.0 | 50.0 | |
| BC () | 8.4 | 0.2 | 1.0 | 1.4 | 2.2 | 25.0 | 1.2 | |
| Air temperature (°C) | 0.1 | 3.0 | 9.7 | 16.1 | 41.1 | 13.1 | ||
| Relative humidity (%) | 0.3 | 17.8 | 62.4 | 82.0 | 91.5 | 99.9 | 29.1 | |
Note: BC, black carbon; IQR, interquartile range; , nitrogen dioxide; , ozone; PLC, particle length concentration; , particulate matter with an aerodynamic diameter below ; , particulate matter with an aerodynamic diameter below ; PNC, particle number concentration; , PNC of particles with mobility diameter; , PNC of particles with mobility diameter; , PNC of particles with mobility diameter; , PNC of particles with mobility diameter; PSC, particle surface concentration; SD, standard deviation.
For PSC, data on 64,248 consecutive hours during 1 January 2005–30 April 2012 were used.
Overall, hourly PNC within different size fractions were highly positively correlated, except for with moderate correlations with and (Table 3). Exposure to UFP () was highly positively correlated with PLC, moderately positively correlated with PSC, , , , and BC, moderately negatively correlated with , and weakly correlated with air temperature and relative humidity. High correlations were observed for with PLC, PSC, , , and BC. PLC, PSC, and BC were also highly correlated. No or weak correlations were present between particle metrics and meteorological variables. Pairs of pollutants with high correlations () were excluded from the two-pollutant analyses (e.g., PLC-PSC, , , and PSC-).
Table 3.
Spearman correlation coefficients between hourly particle metrics and air pollutants in Augsburg, Germany, from 2005 to 2015.
| PLC | PSCa | BC | Air Temperature | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.93 | 1 | |||||||||||
| 0.94 | 0.76 | 1 | ||||||||||
| 0.67 | 0.46 | 0.78 | 1 | |||||||||
| PLC | 0.77 | 0.61 | 0.82 | 0.83 | 1 | |||||||
| PSCa | 0.67 | 0.50 | 0.75 | 0.85 | 0.92 | 1 | ||||||
| 0.39 | 0.25 | 0.47 | 0.76 | 0.68 | 0.78 | 1 | ||||||
| 0.42 | 0.29 | 0.50 | 0.77 | 0.70 | 0.77 | 0.92 | 1 | |||||
| 0.67 | 0.58 | 0.67 | 0.68 | 0.71 | 0.70 | 0.56 | 0.57 | 1 | ||||
| 1 | ||||||||||||
| BC | 0.58 | 0.45 | 0.65 | 0.81 | 0.77 | 0.82 | 0.75 | 0.75 | 0.74 | 1 | ||
| Air temperature | 0.55 | 1 | ||||||||||
| Relative humidity | 0.04 | 0.08 | 0.11 | 0.08 | 0.14 | 0.14 | 0 | 0.13 | 0.26 |
Note: BC, black carbon; , nitrogen dioxide; , ozone; PLC, particle length concentration; , particulate matter with an aerodynamic diameter below ; , particulate matter with an aerodynamic diameter below ; PNC, particle number concentration; , PNC of particles with mobility diameter; , PNC of particles with mobility diameter; , PNC of particles with mobility diameter; , PNC of particles with mobility diameter; PSC, particle surface concentration.
For PSC, measurements during 1 January 2005–30 April 2012 were used.
Hourly Association between Particle Metrics and MI
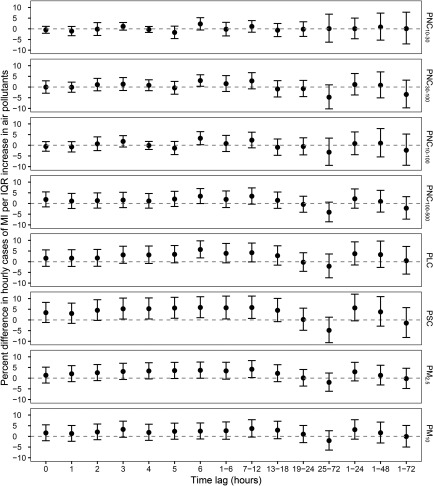
Figure 1 shows the estimated associations between hourly exposure to particle metrics and nonfatal MI in single-pollutant models (see Table S1 for numeric data). Effect estimates of particle metrics were mostly positive within 12 h and then decreased to be null or negative at longer lag hours (13–72 h). Overall patterns of associations across particle number, length, and surface area metrics were similar and comparable to those of particle mass metrics, showing transient positive associations with nonfatal MI in the first 6–12 h. An IQR increase in , (UFP), PLC, and PSC at lag6 h were associated with an increased MI risk of 3.02% (95% CI: 0.36, 5.75), 3.27% (95% CI: 0.27, 6.37), 5.71% (95% CI: 1.79, 9.77), and 5.84% (95% CI: 1.04, 10.87), respectively. We also found positive, albeit imprecise, associations of MI with exposure to , , and at lag6 h. The association between and MI were strongest at lag7–12 h, leading to an increased 4.18% (95% CI: 0.28, 8.23) of MI cases. For all size fractions of PNC, no increased risks of MI were found for daily average exposures (1–24, 1–48, and 1–72 h).
Figure 1.
Percent difference (95% CI) in hourly cases of myocardial infarction (MI) per interquartile range (IQR) increase in particle metrics in Augsburg, Germany from 2005 to 2015. A time-stratified case-crossover design with a conditional logistic model was used to derive the estimates while adjusting for natural splines of air temperature and relative humidity at lag 1–72 h (each with 4 degrees of freedom). Short-term associations were estimated at single-hour lags (lag0–lag6) and for moving averages (lag1–6, lag7–12, lag13–18, lag19–24, lag25–72, lag1–24, lag1–48, and lag1–72). Corresponding numeric data are provided in Table S1. For PSC, measurements during 1 January 2005–30 April 2012 were used. Note: PLC, particle length concentration; , particulate matter with an aerodynamic diameter ; , particulate matter with an aerodynamic diameter . PNC, particle number concentration; , PNC of particles with mobility diameter; , PNC of particles with mobility diameter; , PNC of particles with mobility diameter; , PNC of particles with mobility diameter; PSC, particle surface concentration.
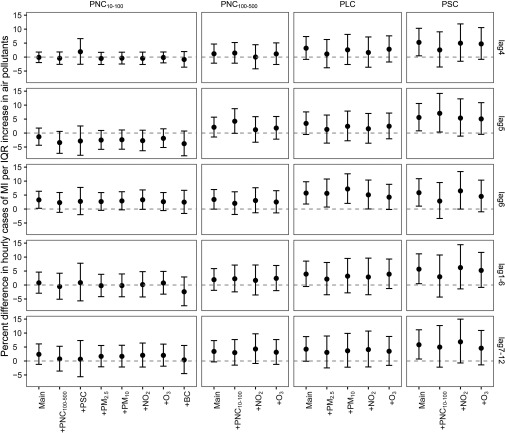
In the two-pollutant models, exposure to and at lag 6 remained positively associated with hourly onset of MI, although effect estimates slightly decreased and became less precise (Figure 2; see also Table S2 for numeric data). Effect estimates for PLC and PSC remained similar when adjusted for , , , and . For example, after adjustment for , an IQR increase in PLC at lag6 h was associated with an increased MI risk of 5.61% (95% CI: 0.72, 10.75). The hourly association between PSC and MI persisted after adjustment for UFP () at lag5 or adjustment for at lag6. The VIFs were in all two-pollutant models (see Table S3), indicating limited evidence of multicollinearity. Although strong associations were observed for BC in the first 18 h [e.g., 4.06% (95% CI: 0.98, 7.24) increase in MI events per IQR increase in BC at lag hour 6], its effect estimates decreased and became less precise after adjustment for (see Figure S4). No clear associations were observed between and MI in either single or two-pollutant models (see Figure S5).
Figure 2.
Percent difference (95% CI) in hourly cases of myocardial infarction (MI) per interquartile range (IQR) increase in particle metrics with additional adjustment for copollutants in Augsburg, Germany from 2005 to 2015. A time-stratified case-crossover design with a conditional logistic model was used to derive the estimates while adjusting for natural splines of air temperature and relative humidity at lag 1–72 h (each with 4 degrees of freedom). Short-term hourly associations (lag4, lag5, lag6, lag1–6, and lag7–12) were estimated in two-pollutant models. Corresponding numeric data are provided in Table S2. For PSC, measurements during 1 January 2005–30 April 2012 were used. Note: BC, black carbon; CI, confidence interval; , nitrogen dioxide; , ozone; PLC, particle length concentration; , particulate matter with an aerodynamic diameter ; , particulate matter with an aerodynamic diameter ; PNC, particle number concentration; , PNC of particles with mobility diameter; , PNC of particles with mobility diameter; , PNC of particles with mobility diameter; , PNC of particles with mobility diameter; PSC, particle surface concentration.
We found no evidence of effect modification by copollutants or air temperature (see Figure S6). Effect estimates for UFP (), , PLC, and PSC at lag 6 h remained similar at different levels of , , , and BC but were generally larger at low levels () of and air temperature.
Subgroup Analysis
In subgroup analysis, we found no evidence of effect modification by individual characteristics or subtypes of MI (see Table S4). There was a consistent pattern of larger and significant risks for , , PLC, PSC, and among male, nondiabetic people, and patients with hypertension. Effect estimates for PLC and PSC were also stronger and more precise for the elderly, patients with their first MI (incident), and those with NSTEMI events. However, there were no significant differences when comparing effect estimates between different subgroups (e.g., male vs. female).
Sensitivity Analysis
Our findings were fairly robust with regard to a) including additional patients 75–84 years of age; b) extending the temperature lag period to 5 d; c) using both current/concurrent temperature and lagged temperature; d) applying shorter strata (3 weeks) for selecting control cases (see Table S5); and e) using an alternative cut off value (50th percentile) to define the high and low levels of copollutants and air temperature (see Figure S7). The exposure–response curves for associations between hourly onset of MI and all particle metrics were essentially linear (see Figure S8).
Discussion
In this registry-based time-stratified case-crossover study, we found that elevated concentrations of particle number (), length, and surface area concentrations were associated with the onset of MI within 6–12 h afterward. These transient risk estimates decreased at longer lag hours, leading to a null association of MI cases with any particle metrics for a 72-h or longer period after exposure. We found evidence of positive associations for exposure to PNC within the Aitken (), nucleation (), and accumulation () modes, although effect estimates were less precise for the nucleation and accumulation modes. Similar pattern of associations was found across particle metrics, whereas risk estimates for PLC and PSC were larger than for PNC within the ultrafine range () and remained similar after adjustment for PM and gaseous pollutants. We found no evidence of significant effect modification by individual characteristics, copollutants, or air temperature.
Our study revealed a transient increase in the risk of MI after 6 h of exposure to PNC within the ultrafine range. Only a small number of epidemiological studies have evaluated the associations of MI with hourly exposure to UFP (using PNC as surrogate) and the results were quite mixed (Delfino et al. 2011; Gardner et al. 2014; Link et al. 2013; Rosenthal et al. 2013). Some studies reported positive but insignificant hourly associations for PNC within both ultrafine and accumulation mode ranges (Gardner et al. 2014; Rosenthal et al. 2013), whereas others found no association for total PNC (size up to ) (Delfino et al. 2011; Link et al. 2013; Peters et al. 2005). Similarly, previous daily time-series studies have found that generally had the strongest effects on cardiovascular mortality (Hennig et al. 2018; Meng et al. 2013). Several panel studies also showed that hourly effects of PNC on circulating biomarkers, respiratory inflammation, and autonomic dysfunction would decrease with increasing particle size, with the strongest effects occurring for particles (Han et al. 2016; Rich et al. 2012; Sun et al. 2015) or (Chen et al. 2015) in size. Comparison of short-term studies on UFP health effects is complicated by the fact that most studies only use a single monitor that might have good characterization for temporal variations of UFP exposure in some (e.g., Augsburg), but not all, cities (HEI Review Panel on Ultrafine Particles 2013). Differences in the hourly health effects of different size-fractionated PNC may be in part due to different emission sources. In Augsburg, particles within the ultrafine ranges () are mainly from traffic emissions, whereas larger particles are strongly associated with stationary combustion (), secondary aerosols (), long-range transported dust (), or resuspended dust () (Gu et al. 2012). Source apportionment studies in other cities have also consistently identified traffic emission as the major source of PNC (Brines et al. 2015; Vu et al. 2015). When using exposure to traffic (e.g., cars, buses, or motorcycles) as an alternative estimate of UPF exposure, our previous study conducted in Augsburg during 1999–2001 also found an increased risk of MI 1 h after being in traffic (Peters et al. 2004).
We found that PLC and PSC metrics tended to have a larger and more precise effect estimates than PNC in both single- and two-pollutant models (Figure 2). This is in line with findings from a previous panel study in Augsburg showing stronger positive associations with inflammatory blood biomarkers for PLC and PSC than for PNC (Rückerl et al. 2016). Given that PLC was highly correlated () with PNC and PSC, we were not able to disentangle its independent effects. PSC was moderately correlated with PNC (), and its effect estimate on MI at lag 5 h remained similar after adjustment for (Figure 2). A recent daily time-series study from the Ruhr Area, Germany, found similar effects for lung-deposited PSC and on cardiovascular mortality, albeit a strong correlation () between PSC and (Hennig et al. 2018). Toxicological studies also suggested that PSC might be more biologically relevant than number or mass metrics for nanoparticle toxicology in the lung (Sager and Castranova 2009; Schmid and Stoeger 2016). This may be because the particle surface is where components of UFP interact directly with bodily fluids and tissue (Schmid and Stoeger 2016). Greater surface area may increase the surface reactivity and therefore the oxidative stress in pro-inflammatory effects (Hussain et al. 2009). Given the high correlation between PLC and PSC and their similar effect estimates, it is likely that PLC and PSC might capture the same underlying properties of UFP (Rückerl et al. 2016).
A major barrier in understanding the health effects of UFP lies in the independence of its effects within the ambient pollution mixture (Baldauf et al. 2016). In most previous epidemiological studies, adjustment for generally largely attenuated the effect estimates of PNC because both pollutants originate from similar sources, such as traffic emissions, and feature similar spatial-temporal distributions (Ohlwein et al. 2019). Our results indicated effects of PLC and PSC remained similar when additionally adjusting for , , , or (Figure 2). Thus, PSC and PLC might reflect the independent adverse health effects of particle properties better than PNC.
We found that a few hours of exposure to UFP (i.e., 6 h for and PLC, and 3–6 h for PSC) could result in increased risks of nonfatal MI events (Figure 1). It is biologically plausible that very short-term exposure to UFP may trigger the onset of MI. A few hours of exposure to UFP may activate neural reflexes in the respiratory tract, provoke imbalance of the autonomic nervous system, and initiate cardiac arrhythmias (Brook et al. 2010). Recent panel studies have reported associations between UFP and decreased heart rate variability within hours (Breitner et al. 2019; Rich et al. 2012; Sun et al. 2015) or even minutes (Hampel et al. 2014; Peters et al. 2015) of increased exposure. Moreover, short-term exposure to UFP may result in systemic oxidative stress and inflammation, thus leading to impaired vascular function and thrombosis (Brook et al. 2010). In a controlled human exposure study, 1-h postexposure to ultrafine concentrated ambient particles () was associated with increased urinary 8-hydroxydeoxyguanosine, a systemic oxidative stress biomarker, and lasted (Liu et al. 2015). A panel study of healthy young adults in Shanghai, China, reported significant associations between 2 h of exposure to and increases in circulating biomarkers of inflammation, such as intercellular adhesion molecule-1 and P-selectin; these associations were mostly restricted to the first 12–24 h (Chen et al. 2015). Another panel study of cardiac patients in Rochester, New York, observed positive associations between UFP exposure in the previous 12 h and increased levels of fibrinogen (Croft et al. 2017).
To the best of our knowledge, this is the first epidemiological study to investigate the effect of UFP and MI using particle number, length, and surface area concentrations at an hourly temporal resolution. A major strength of this study is the validated, complete, and detailed registration of all nonfatal MI cases by the MONICA/KORA MI registry. The time of MI onset was validated against the medical records, instead of using patient self-reported time upon arrival of hospital admission or time of receiving the emergency call (Gardner et al. 2014; Rosenthal et al. 2013). Other strengths include the characterization of UFP exposure with novel metrics and size-fractionated number concentrations, the adjustment for key copollutants in the traffic pollutant mixture, and the time-stratified cased-crossover design that controls for long-term time trends, seasonality, day of the week effect, and time-invariant individual-level confounders.
Our study also has some limitations. First, exposure measurement error is inevitable given that we only used one fixed urban background monitoring site for each air pollutant. This poses a particular challenge of exposure assessment for UFP due to its higher spatial variability within cities (HEI Review Panel on Ultrafine Particles 2013). However, a previous study reported high temporal correlations of PNC at four background sites across Augsburg (Cyrys et al. 2008). A further analysis using 20 sampling sites throughout the Augsburg region during 2014–2015 also showed moderate-to-high temporal correlations of hourly PNC measurements with the central site used in this study, suggesting that using one single background monitoring site might be adequate to characterize the temporal variability of short-term exposure to PNC in this area. Because there were no additional monitoring sites measuring particle length and surface area concentrations in Augsburg, the assumption that the temporal fluctuations in PLC and PSC measured at a central site monitor are reflective throughout the city warrants further investigation. Second, although we adjusted for copollutants in two-pollutant models, we cannot rule out the possibility of residual confounding by other traffic-related pollutants that may have complex spatiotemporal variations in the city or other unmeasured temporal variables such as noise. Third, we are unable to differentiate the health effects of PLC, PSC, and BC because of their high correlations. Fourth, we examined only the nonfatal MIs because the hour of onset information was not available for fatal events. Thus, our findings may not be applicable to fatal events, albeit no significant differences in UFP effects were observed between fatal and nonfatal MIs at the daily timescale (Wolf et al. 2015). Finally, our results are confined to a single location in Germany and may not be applicable to other cities and other countries with different emission sources as well as different demographic and socioeconomic conditions. Further studies from other locations with similar study designs are warranted to confirm our findings.
In conclusion, exposure to PNC within the ultrafine size range (), PLC, and PSC were associated with an increased risk of nonfatal MI in the first 6–12 h in Augsburg during 2005–2015. The effects of PLC and PSC were stronger than the ones of PNC and remained similar after adjustment for PM or gaseous pollutants. These exploratory findings suggest that UFP or potentially other related exposures could trigger the onset of nonfatal MI at a sub-daily timescale and particle length and surface area concentrations might serve as alternative exposure metrics in epidemiological studies examining the health effects of UFP.
Supplementary Material
Acknowledgments
The KORA-Study Group consists of A. Peters (speaker), H. Schulz, L. Schwettmann, R. Leidl, M. Heier, K. Strauch, and their coworkers, who are responsible for the design and conduct of the KORA studies. The KORA research platform and the MONICA Augsburg studies were initiated and financed by the Helmholtz Zentrum München, German Research Center for Environmental Health, which is funded by the German Federal Ministry of Education, Science, Research, and Technology and by the State of Bavaria. Since 2000, the MI data collection has been cofinanced by the German Federal Ministry of Health and Social Security to provide population-based MI morbidity data for the official German Health Report (see http://www.gbe-bund.de).
Footnotes
Supplemental Material is available online (https://doi.org/10.1289/EHP5478).
The authors declare they have no actual or potential competing financial interests.
Note to readers with disabilities: EHP strives to ensure that all journal content is accessible to all readers. However, some figures and Supplemental Material published in EHP articles may not conform to 508 standards due to the complexity of the information being presented. If you need assistance accessing journal content, please contact ehponline@niehs.nih.gov. Our staff will work with you to assess and meet your accessibility needs within 3 working days.
References
- Andersen ZJ, Wahlin P, Raaschou-Nielsen O, Ketzel M, Scheike T, Loft S. 2008. Size distribution and total number concentration of ultrafine and accumulation mode particles and hospital admissions in children and the elderly in Copenhagen, Denmark. Occup Environ Med 65(7):458–466, PMID: 17989204, 10.1136/oem.2007.033290. [DOI] [PubMed] [Google Scholar]
- Baldauf RW, Devlin RB, Gehr P, Giannelli R, Hassett-Sipple B, Jung H, et al. . 2016. Ultrafine particle metrics and research considerations: review of the 2015 UFP workshop. Int J Environ Res Public Health 13(11):1054, PMID: 27801854, 10.3390/ijerph13111054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belleudi V, Faustini A, Stafoggia M, Cattani G, Marconi A, Perucci CA, et al. . 2010. Impact of fine and ultrafine particles on emergency hospital admissions for cardiac and respiratory diseases. Epidemiology 21(3):414–423, PMID: 20386174, 10.1097/EDE.0b013e3181d5c021. [DOI] [PubMed] [Google Scholar]
- Bhaskaran K, Hajat S, Armstrong B, Haines A, Herrett E, Wilkinson P, et al. . 2011. The effects of hourly differences in air pollution on the risk of myocardial infarction: case crossover analysis of the MINAP database. BMJ 343:d5531, PMID: 21933824, 10.1136/bmj.d5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmili W, Stratmann F, Wiedensohler A. 1999. Design of a DMA-based size spectrometer for a large particle size range and stable operation. J Aerosol Sci 30(4):549–553, 10.1016/S0021-8502(98)00047-0. [DOI] [Google Scholar]
- Braniš M, Vyškovská J, Malý M, Hovorka J. 2010. Association of size-resolved number concentrations of particulate matter with cardiovascular and respiratory hospital admissions and mortality in Prague, Czech Republic. Inhal Toxicol 22(suppl 2):21–28, PMID: 20718587, 10.3109/08958378.2010.504758. [DOI] [PubMed] [Google Scholar]
- Breitner S, Peters A, Zareba W, Hampel R, Oakes D, Wiltshire J, et al. . 2019. Ambient and controlled exposures to particulate air pollution and acute changes in heart rate variability and repolarization. Sci Rep 9(1):1946, PMID: 30760868, 10.1038/s41598-019-38531-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brines M, Dall’Osto M, Beddows DCS, Harrison RM, Gómez-Moreno F, Núñez L, et al. . 2015. Traffic and nucleation events as main sources of ultrafine particles in high-insolation developed world cities. Atmos Chem Phys 15(10):5929–5945, 10.5194/acp-15-5929-2015. [DOI] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA III, Brook JR, Bhatnagar A, Diez-Roux AV, et al. . 2010. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the AmericanHeart Association. Circulation 121(21):2331–2378, PMID: 20458016, 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Chen K, Wolf K, Breitner S, Gasparrini A, Stafoggia M, Samoli E, et al. . 2018. Two-way effect modifications of air pollution and air temperature on total natural and cardiovascular mortality in eight European urban areas. Environ Int 116:186–196, PMID: 29689465, 10.1016/j.envint.2018.04.021. [DOI] [PubMed] [Google Scholar]
- Chen R, Zhao Z, Sun Q, Lin Z, Zhao A, Wang C, et al. . 2015. Size-fractionated particulate air pollution and circulating biomarkers of inflammation, coagulation, and vasoconstriction in a panel of young adults. Epidemiology 26(3):328–336, PMID: 25738902, 10.1097/EDE.0000000000000273. [DOI] [PubMed] [Google Scholar]
- Claeys MJ, Rajagopalan S, Nawrot TS, Brook RD. 2017. Climate and environmental triggers of acute myocardial infarction. Eur Heart J 38(13):955–960, PMID: 27106953, 10.1093/eurheartj/ehw151. [DOI] [PubMed] [Google Scholar]
- Croft DP, Cameron SJ, Morrell CN, Lowenstein CJ, Ling F, Zareba W, et al. . 2017. Associations between ambient wood smoke and other particulate pollutants and biomarkers of systemic inflammation, coagulation and thrombosis in cardiac patients. Environ Res 154:352–361, PMID: 28167447, 10.1016/j.envres.2017.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyrys J, Pitz M, Heinrich J, Wichmann H-E, Peters A. 2008. Spatial and temporal variation of particle number concentration in Augsburg, Germany. Sci Total Environ 401(1–3):168–175, PMID: 18511107, 10.1016/j.scitotenv.2008.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Gillen DL, Tjoa T, Staimer N, Polidori A, Arhami M, et al. . 2011. Electrocardiographic ST-segment depression and exposure to traffic-related aerosols in elderly subjects with coronary artery disease. Environ Health Perspect 119(2):196–202, PMID: 20965803, 10.1289/ehp.1002372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans KA, Halterman JS, Hopke PK, Fagnano M, Rich DQ. 2014. Increased ultrafine particles and carbon monoxide concentrations are associated with asthma exacerbation among urban children. Environ Res 129:11–19, PMID: 24528997, 10.1016/j.envres.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J, Monette G. 1992. Generalized collinearity diagnostics. J Am Stat Assoc 87(417):178–183, 10.2307/2290467. [DOI] [Google Scholar]
- Gardner B, Ling F, Hopke PK, Frampton MW, Utell MJ, Zareba W, et al. . 2014. Ambient fine particulate air pollution triggers ST-elevation myocardial infarction, but not non-ST elevation myocardial infarction: a case-crossover study. Part Fibre Toxicol 11(1):1, PMID: 24382024, 10.1186/1743-8977-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Pitz M, Breitner S, Birmili W, von Klot S, Schneider A, et al. . 2012. Selection of key ambient particulate variables for epidemiological studies—applying cluster and heatmap analyses as tools for data reduction. Sci Total Environ 435–436:541–550, PMID: 22895165, 10.1016/j.scitotenv.2012.07.040. [DOI] [PubMed] [Google Scholar]
- Gu J, Pitz M, Schnelle-Kreis J, Diemer J, Reller A, Zimmermann R, et al. . 2011. Source apportionment of ambient particles: comparison of positive matrix factorization analysis applied to particle size distribution and chemical composition data. Atmos Environ 45(10):1849–1857, 10.1016/j.atmosenv.2011.01.009. [DOI] [Google Scholar]
- Hampel R, Rückerl R, Yli-Tuomi T, Breitner S, Lanki T, Kraus U, et al. . 2014. Impact of personally measured pollutants on cardiac function. Int J Hyg Environ Health 217(4–5):460–464, PMID: 24231411, 10.1016/j.ijheh.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Han Y, Zhu T, Guan T, Zhu Y, Liu J, Ji Y, et al. . 2016. Association between size-segregated particles in ambient air and acute respiratory inflammation. Sci Total Environ 565:412–419, PMID: 27179679, 10.1016/j.scitotenv.2016.04.196. [DOI] [PubMed] [Google Scholar]
- HEI Review Panel on Ultrafine Particles. 2013. Understanding the Health Effects of Ambient Ultrafine Particles. HEI Perspectives 3. Boston, MA: Health Effects Institute; https://www.healtheffects.org/system/files/Perspectives3.pdf [accessed 16 December 2019]. [Google Scholar]
- Hennig F, Quass U, Hellack B, Küpper M, Kuhlbusch TA, Stafoggia M, et al. . 2018. Ultrafine and fine particle number and surface area concentrations and daily cause-specific mortality in the Ruhr Area, Germany, 2009–2014. Environ Health Perspect 126(2):027008, PMID: 29467106, 10.1289/EHP2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain S, Boland S, Baeza-Squiban A, Hamel R, Thomassen LC, Martens JA, et al. . 2009. Oxidative stress and proinflammatory effects of carbon black and titanium dioxide nanoparticles: role of particle surface area and internalized amount. Toxicology 260(1–3):142–149, PMID: 19464580, 10.1016/j.tox.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Iskandar A, Andersen ZJ, Bønnelykke K, Ellermann T, Andersen KK, Bisgaard H. 2012. Coarse and fine particles but not ultrafine particles in urban air trigger hospital admission for asthma in children. Thorax 67(3):252–257, PMID: 22156960, 10.1136/thoraxjnl-2011-200324. [DOI] [PubMed] [Google Scholar]
- Janes H, Sheppard L, Lumley T. 2005. Case–crossover analyses of air pollution exposure data: referent selection strategies and their implications for bias. Epidemiology 16(6):717–726, PMID: 16222160, 10.1097/01.ede.0000181315.18836.9d. [DOI] [PubMed] [Google Scholar]
- Kim Y, Ng CFS, Chung Y, Kim H, Honda Y, Guo YL, et al. . 2018. Air pollution and suicide in 10 cities in northeast Asia: a time-stratified case-crossover analysis. Environ Health Perspect 126(3):037002, PMID: 29529596, 10.1289/EHP2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchberger I, Meisinger C, Golüke H, Heier M, Kuch B, Peters A, et al. . 2014. Long-term survival among older patients with myocardial infarction differs by educational level: results from the MONICA/KORA myocardial infarction registry. Int J Equity Health 13:19, PMID: 24552463, 10.1186/1475-9276-13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuch B, Heier M, von Scheidt W, Kling B, Hoermann A, Meisinger C. 2008. 20-year trends in clinical characteristics, therapy and short-term prognosis in acute myocardial infarction according to presenting electrocardiogram: the MONICA / KORA AMI registry (1985–2004). J Intern Med 264(3):254–264, PMID: 18397247, 10.1111/j.1365-2796.2008.01956.x. [DOI] [PubMed] [Google Scholar]
- Lanki T, Pekkanen J, Aalto P, Elosua R, Berglind N, D’Ippoliti D, et al. . 2006. Associations of traffic related air pollutants with hospitalisation for first acute myocardial infarction: the HEAPSS study. Occup Environ Med 63(12):844–851, PMID: 16912091, 10.1136/oem.2005.023911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Guo Y, Williams G. 2016. Acute impact of hourly ambient air pollution on preterm birth. Environ Health Perspect 124(10):1623–1629, PMID: 27128028, 10.1289/EHP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link MS, Luttmann-Gibson H, Schwartz J, Mittleman MA, Wessler B, Gold DR, et al. . 2013. Acute exposure to air pollution triggers atrial fibrillation. J Am Coll Cardiol 62(9):816–825, PMID: 23770178, 10.1016/j.jacc.2013.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Urch B, Poon R, Szyszkowicz M, Speck M, Gold DR, et al. . 2015. Effects of ambient coarse, fine, and ultrafine particles and their biological constituents on systemic biomarkers: a controlled human exposure study. Environ Health Perspect 123(6):534–540, PMID: 25616223, 10.1289/ehp.1408387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löwel H, Meisinger C, Heier M, Hörmann A. 2005. The population-based acute myocardial infarction (AMI) registry of the MONICA/KORA study region of Augsburg. Gesundheitswesen 67(suppl 1):S31–S37, PMID: 16032515, 10.1055/s-2005-858241. [DOI] [PubMed] [Google Scholar]
- Meng X, Ma Y, Chen R, Zhou Z, Chen B, Kan H. 2013. Size-fractionated particle number concentrations and daily mortality in a Chinese city. Environ Health Perspect 121(10):1174–1178, PMID: 23942310, 10.1289/ehp.1206398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska L, Ristovski Z, Jayaratne ER, Keogh DU, Ling X. 2008. Ambient nano and ultrafine particles from motor vehicle emissions: characteristics, ambient processing and implications on human exposure. Atmos Environ 42(35):8113–8138, 10.1016/j.atmosenv.2008.07.050. [DOI] [Google Scholar]
- Mostofsky E, Schwartz J, Coull BA, Koutrakis P, Wellenius GA, Suh HH, et al. . 2012. Modeling the association between particle constituents of air pollution and health outcomes. Am J Epidemiol 176(4):317–326, PMID: 22850792, 10.1093/aje/kws018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlwein S, Kappeler R, Kutlar Joss M, Künzli N, Hoffmann B. 2019. Health effects of ultrafine particles: a systematic literature review update of epidemiological evidence. Int J Public Health 64(4):547–559, PMID: 30790006, 10.1007/s00038-019-01202-7. [DOI] [PubMed] [Google Scholar]
- Peters A, Dockery DW, Muller JE, Mittleman MA. 2001. Increased particulate air pollution and the triggering of myocardial infarction. Circulation 103(23):2810–2815, PMID: 11401937, 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- Peters A, Hampel R, Cyrys J, Breitner S, Geruschkat U, Kraus U, et al. . 2015. Elevated particle number concentrations induce immediate changes in heart rate variability: a panel study in individuals with impaired glucose metabolism or diabetes. Part Fibre Toxicol 12:7, PMID: 25888845, 10.1186/s12989-015-0083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, von Klot S, Heier M, Trentinaglia I, Cyrys J, Hörmann A, et al. . 2005. Air pollution, personal activities, and onset of myocardial infarction in a case-crossover study. Res Rep Health Eff Inst 124:1–66, PMID: 17153517. [PubMed] [Google Scholar]
- Peters A, von Klot S, Heier M, Trentinaglia I, Hörmann A, Wichmann HE, et al. . 2004. Exposure to traffic and the onset of myocardial infarction. N Engl J Med 351(17):1721–1730, PMID: 15496621, 10.1056/NEJMoa040203. [DOI] [PubMed] [Google Scholar]
- Pilz V, Wolf K, Breitner S, Rückerl R, Koenig W, Rathmann W, et al. . 2018. C-reactive protein (CRP) and long-term air pollution with a focus on ultrafine particles. Int J Hyg Environ Health 221(3):510–518, PMID: 29428699, 10.1016/j.ijheh.2018.01.016. [DOI] [PubMed] [Google Scholar]
- Pitz M, Birmili W, Schmid O, Peters A, Wichmann H, Cyrys J. 2008a. Quality control and quality assurance for particle size distribution measurements at an urban monitoring station in Augsburg, Germany. J Environ Monit 10(9):1017–1024, PMID: 18728893, 10.1039/b807264g. [DOI] [PubMed] [Google Scholar]
- Pitz M, Schmid O, Heinrich J, Birmili W, Maguhn J, Zimmermann R, et al. . 2008b. Seasonal and diurnal variation of PM2.5 apparent particle density in urban air in Augsburg, Germany. Environ Sci Technol 42(14):5087–5093, PMID: 18754352, 10.1021/es7028735. [DOI] [PubMed] [Google Scholar]
- Rich DQ, Zareba W, Beckett W, Hopke PK, Oakes D, Frampton MW, et al. . 2012. Are ambient ultrafine, accumulation mode, and fine particles associated with adverse cardiac responses in patients undergoing cardiac rehabilitation? Environ Health Perspect 120(8):1162–1169, PMID: 22542955, 10.1289/ehp.1104262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal FS, Kuisma M, Lanki T, Hussein T, Boyd J, Halonen JI, et al. . 2013. Association of ozone and particulate air pollution with out-of-hospital cardiac arrest in Helsinki, Finland: evidence for two different etiologies. J Expo Sci Environ Epidemiol 23(3):281–288, PMID: 23361443, 10.1038/jes.2012.121. [DOI] [PubMed] [Google Scholar]
- Rückerl R, Schneider A, Breitner S, Cyrys J, Peters A. 2011. Health effects of particulate air pollution: a review of epidemiological evidence. Inhal Toxicol 23(10):555–592, PMID: 21864219, 10.3109/08958378.2011.593587. [DOI] [PubMed] [Google Scholar]
- Rückerl R, Schneider A, Hampel R, Breitner S, Cyrys J, Kraus U, et al. . 2016. Association of novel metrics of particulate matter with vascular markers of inflammation and coagulation in susceptible populations—results from a panel study. Environ Res 150:337–347, PMID: 27344265, 10.1016/j.envres.2016.05.037. [DOI] [PubMed] [Google Scholar]
- Sager TM, Castranova V. 2009. Surface area of particle administered versus mass in determining the pulmonary toxicity of ultrafine and fine carbon black: comparison to ultrafine titanium dioxide. Part Fibre Toxicol 6:15, PMID: 19413904, 10.1186/1743-8977-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid O, Stoeger T. 2016. Surface area is the biologically most effective dose metric for acute nanoparticle toxicity in the lung. J Aerosol Sci 99:133–143, 10.1016/j.jaerosci.2015.12.006. [DOI] [Google Scholar]
- Sun J, Birmili W, Hermann M, Tuch T, Weinhold K, Spindler G, et al. . 2019. Variability of black carbon mass concentrations, sub-micrometer particle number concentrations and size distributions: results of the German Ultrafine Aerosol Network ranging from city street to high alpine locations. Atmos Environ 202:256–268, 10.1016/j.atmosenv.2018.12.029. [DOI] [Google Scholar]
- Sun Y, Song X, Han Y, Ji Y, Gao S, Shang Y, et al. . 2015. Size-fractioned ultrafine particles and black carbon associated with autonomic dysfunction in subjects with diabetes or impaired glucose tolerance in Shanghai, China. Part Fibre Toxicol 12:8, PMID: 25884677, 10.1186/s12989-015-0084-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valavanidis A, Fiotakis K, Vlachogianni T. 2008. Airborne particulate matter and human health: toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 26(4):339–362, PMID: 19034792, 10.1080/10590500802494538. [DOI] [PubMed] [Google Scholar]
- von Klot S, Peters A, Aalto P, Bellander T, Berglind N, D’Ippoliti D, et al. . 2005. Ambient air pollution is associated with increased risk of hospital cardiac readmissions of myocardial infarction survivors in five European cities. Circulation 112(20):3073–3079, PMID: 16286602, 10.1161/CIRCULATIONAHA.105.548743. [DOI] [PubMed] [Google Scholar]
- Vu TV, Delgado-Saborit JM, Harrison RM. 2015. Review: particle number size distributions from seven major sources and implications for source apportionment studies. Atmos Environ 122:114–132, 10.1016/j.atmosenv.2015.09.027. [DOI] [Google Scholar]
- Wang M, Beelen R, Bellander T, Birk M, Cesaroni G, Cirach M, et al. . 2014. Performance of multi-city land use regression models for nitrogen dioxide and fine particles. Environ Health Perspect 122(8):843–849, PMID: 24787034, 10.1289/ehp.1307271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedensohler A, Birmili W, Nowak A, Sonntag A, Weinhold K, Merkel M, et al. . 2012. Mobility particle size spectrometers: harmonization of technical standards and data structure to facilitate high quality long-term observations of atmospheric particle number size distributions. Atmos Meas Tech 5(3):657–685, 10.5194/amt-5-657-2012. [DOI] [Google Scholar]
- Wolf K, Cyrys J, Harciníková T, Gu J, Kusch T, Hampel R, et al. . 2017. Land use regression modeling of ultrafine particles, ozone, nitrogen oxides and markers of particulate matter pollution in Augsburg, Germany. Sci Total Environ 579:1531–1540, PMID: 27916311, 10.1016/j.scitotenv.2016.11.160. [DOI] [PubMed] [Google Scholar]
- Wolf K, Schneider A, Breitner S, Meisinger C, Heier M, Cyrys J, et al. . 2015. Associations between short-term exposure to particulate matter and ultrafine particles and myocardial infarction in Augsburg, Germany. Int J Hyg Environ Health 218(6):535–542, PMID: 26013401, 10.1016/j.ijheh.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Wolf K, Schneider A, Breitner S, von Klot S, Meisinger C, Cyrys J, et al. . 2009. Air temperature and the occurrence of myocardial infarction in Augsburg, Germany. Circulation 120(9):735–742, PMID: 19687361, 10.1161/CIRCULATIONAHA.108.815860. [DOI] [PubMed] [Google Scholar]
- Zanobetti A, Schwartz J. 2005. The effect of particulate air pollution on emergency admissions for myocardial infarction: a multicity case-crossover analysis. Environ Health Perspect 113(8):978–982, PMID: 16079066, 10.1289/ehp.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeka A, Zanobetti A, Schwartz J. 2006. Individual-level modifiers of the effects of particulate matter on daily mortality. Am J Epidemiol 163(9):849–859, PMID: 16554348, 10.1093/aje/kwj116. [DOI] [PubMed] [Google Scholar]
- Zhang S-H, Flagan RC. 1996. Resolution of the radial differential mobility analyzer for ultrafine particles. J Aerosol Sci 27(8):1179–1200, 10.1016/0021-8502(96)00036-5. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.