Abstract
We determined the clinical effectiveness and long-term outcomes in patients with distal biliary obstruction (DBO) secondary to pancreatic carcinoma (PC) who were treated by self-expanded metallic stent (SEMS) insertion with or without high-intensity focused ultrasound (HIFU) ablation.
From January 2014 to December 2018, consecutive patients with DBO secondary to PC underwent SEMS insertion with or without HIFU ablation in our center. The long-term outcomes were compared between the 2 groups.
During the included period, 75 patients underwent SEMS insertion with (n = 34) or without (n = 41) HIFU ablation in our center. SEMS insertion was successfully performed in all patients. Liver function was significantly improved after SEMS insertion in both groups. An average of 2.9 HIFU treatment sessions per patient were performed. Twenty patients (stent + HIFU group: 7; stent-only group: 13) experienced stent dysfunction (P = .278). The clinical response rate to HIFU ablation was 79.4%. The median stent patency was significantly longer in the stent with HIFU group than in the stent-only group (175 vs 118 days, P = .005). The median survival was significantly longer in the stent with HIFU group compared with the stent-only group (211 versus 136 days, P = .004). An Eastern Cooperative Oncology Group (ECOG) Performance Status of 3 (hazard ratio: 0.300; P = .002) and subsequent HIFU ablation (hazard ratio: 0.508; P = .005) were associated with prolonged survival.
HIFU ablation following stent insertion can prolong the stent patency and survival for patients with DBO secondary to PC.
Keywords: high-intensity focused ultrasound ablation, pancreatic carcinoma, stent
1. Introduction
Pancreatic carcinoma (PC) is often associated with distal biliary obstruction (DBO) which can cause jaundice.[1–3] If the jaundice cannot be cured in a timely manner, the patients risk dying due to liver failure.[1–3] Only 10% to 20% of patients can undergo surgery once obstructive jaundice occurs, with 3- and 5-year survival rates of 18% to 52% and 5% to 31%, respectively.[4]
For inoperable patients with DBO secondary to PC, the major purpose of treatment is relief of the jaundice.[1–6] Stent insertions, which include plastic stent insertion, self-expanded metallic stent (SEMS) insertion, and covered stent insertion, have been used as palliative treatment of DBO secondary to PC.[1–6] Although many treatment strategies for DBO secondary to PC exist, there were no significant differences in survival or quality of life between surgical bypass versus stents, SEMSs versus plastic stents, or covered versus uncovered stents in such patients.[4] These results are mainly due to the fact that stent insertion alone has no additional treatment benefit with regard to the primary tumor.
To prolong stent patency and survival of patients with malignant biliary obstructions, various treatments have been used, including chemotherapy, radiotherapy, and high-intensity focused ultrasound (HIFU) ablation.[7–11] Kitano et al[1] concluded that chemotherapy prolonged the overall survival of patients with DBO secondary to PC. However, both chemotherapy and radiotherapy display treatment-related toxicity.[8] Compared with chemotherapy, radiotherapy, and other treatments such as transcatheter arterial infusion or percutaneous ablation, HIFU ablation is a noninvasive and nontoxic treatment.[10]
In this study, we compared the clinical effectiveness and long-term outcomes of SEMS insertion with or without HIFU ablation in patients with DBO secondary to PC.
2. Patients and methods
This retrospective study was approved by the institutional review board (No. QLYY-2019–0630-115), which agreed to a waiver of written informed consent for study participation. All patients provided consent for stent insertion and HIFU.
2.1. Study design
From January 2014 to December 2018, consecutive patients with DBO secondary to PC underwent SEMS insertion with or without HIFU ablation in our center. Patients were allowed to be treated with chemo- or radiotherapy before or after SEMS insertion in both groups.
Patient inclusion criteria are: a confirmed PC diagnosis; a confirmed DBO diagnosis; inoperable cases. Patient exclusion criteria are: technical failure of SEMS insertion; an Eastern Cooperative Oncology Group (ECOG) performance status ≥4.
2.2. Diagnosis
Diagnosis of DBO was made according to patients’ symptoms, computed tomography (CT), magnetic resonance cholangiopancreatography, and liver function results. Diagnosis of PC was made based on the pathology results obtained by endoscopic retrograde cholangiopancreatography and/or endoscopic ultrasound-guided fine needle aspiration.
2.3. SEMS insertion
Patients were placed in a supine position. The right intrahepatic biliary tract was punctured under combined ultrasonic and fluoroscopic guidance. A 0.035-inch normal guidewire (Terumo, Tokyo, Japan) and a 4 to 5F VER catheter (Cordis, Hialeah, FL) were used to detect the obstructed site. When the guidewire and catheter had entered the duodenum, the normal guidewire was exchanged with a 0.035-inch stiff guidewire (Cook, Bloomington, IN). The uncovered SEMS (Micro-Tech, Nanjing, China) was placed at the obstructed site via this stiff guidewire. The stents were 8 mm in diameter and 50 to 70 mm in length.
All patients received antibiotic therapy and hemostasis for 3 to 5 days after SEMS insertion
2.4. HIFU ablation
HIFU ablation was performed 1 week after SEMS insertion. The main treatment parameters of the HIFU equipment included input power, 300 to 500 W, and effective therapy depth, 2 to 15 cm. The area of each ablated dot was 6 × 4 mm.
Patients were placed in the supine position. The ultrasound imaging transducer was used to identify the tumor target. The distribution of the ablated dots was directed by the tumor size and depth. Each dot was ablated for 10 seconds.
Each patient underwent at least 2 cycles of HIFU ablation. The interval between each HIFU treatment was 1 month.
2.5. Follow-up
Routine follow-up after SEMS insertion was performed at 1, 3, and 6 months, and then every 6 months thereafter. Stent dysfunction was suspected if the patients experienced recurrence of jaundice or cholangitis. Follow-up ended at the patient's death.
2.6. Definitions
Technical success of SEMS insertion was defined as passage of the SEMS across the obstruction, along with the flow of contrast medium through the SEMS.[11] The clinical response to HIFU ablation was considered positive if contrast-enhanced CT showed necrosis or reduction of PC after 2 cycles of treatment.[12,13] Stent patency was calculated from the day of SEMS insertion to stent dysfunction or death.
2.7. Statistical analysis
Fisher exact test or the χ2 test was used for comparing categorical variables. Continuous variables are shown as means ± standard deviation, with Student t test or Mann-Whitney U test used for comparisons as appropriate. Differences before and after treatment were assessed via paired t test. Patient survival and cumulative patency were assessed using Kaplan–Meier curves. Predictors of survival were identified via Cox regression analyses. Variable with a P ≤ .1 in a univariate analysis was subsequently assessed using a multivariate model with P < .05 as the significance threshold. Statistical testing was conducted with SPSS v16.0 (SPSS Inc, Chicago, IL).
3. Results
3.1. Patients
During the included period, 75 patients with DBO secondary to PC underwent SEMS insertion with (n = 34) or without (n = 41) HIFU ablation in our center (Fig. 1). From January 2014 to December 2016, HIFU ablation was not used. From January 2017, HIFU ablation was introduced in our hospital and was used for patients with malignant tumors.
Figure 1.
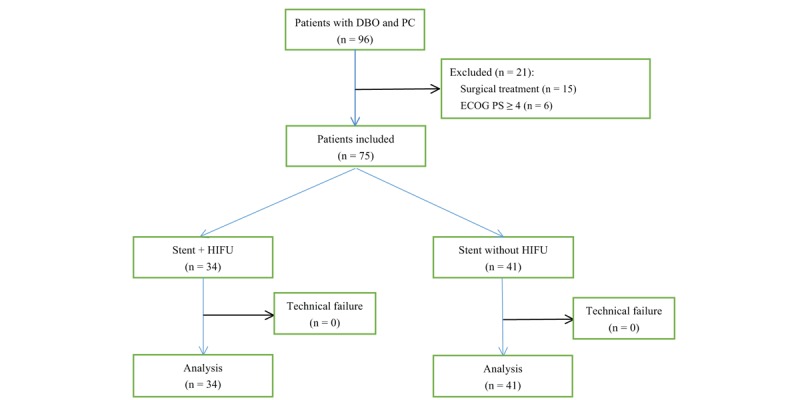
The flowchart of this study.
3.2. Effectiveness of SEMS insertion
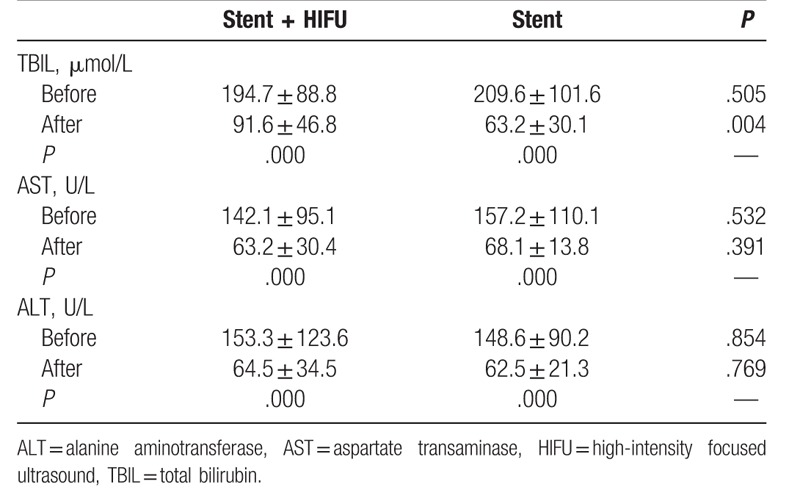
SEMS insertion was successfully performed in all patients. The baseline data of the 75 patients are shown in Table 1. There were 12 patients (Stent + HIFU group: 7; Stent group: 5) with stage II PC. These patients did not undergo surgical resection because of their older age or poor body condition. None of the patients suffered procedure-related complications. All patients underwent repeated liver function tests 1 week after SEMS insertion. The improvements of liver function are shown in Table 2.
Table 1.
Patients’ characteristics.
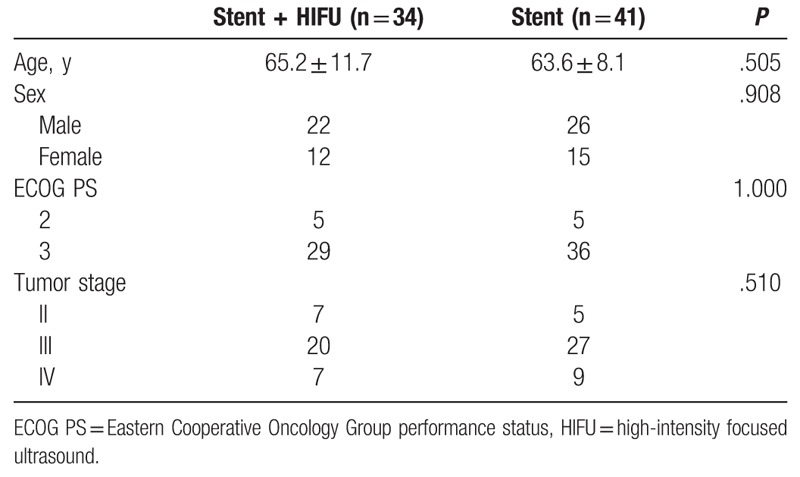
Table 2.
Improvements of liver function in 2 groups.
3.3. Effectiveness of HIFU ablation
A total of 100 HIFU treatment sessions were performed for the 34 patients (average of 2.9 sessions per patient) in the stent with HIFU group. HIFU was well tolerated by all patients. Ten, 16, and 8 patients received 2, 3, and 4 treatment sessions, respectively. The response rate to HIFU ablation was 79.4% (27/34).
3.4. Patency
Twenty patients (stent + HIFU group: 7; stent group: 13) experienced stent dysfunction (P = .278, Table 3). All cases of stent dysfunction were caused by tumor ingrowth and these patients received a repeat SEMS insertion. The median stent patency was significantly longer in the stent with HIFU group compared with the stent-only group (175 vs 118 days, respectively, P = .005, Fig. 2).
Table 3.
Complications and outcomes.
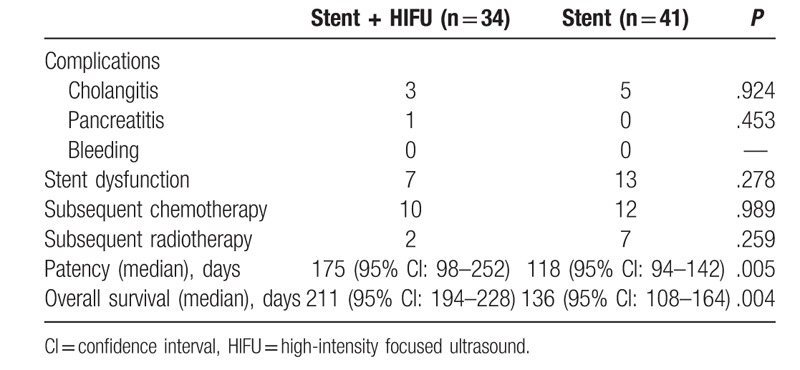
Figure 2.
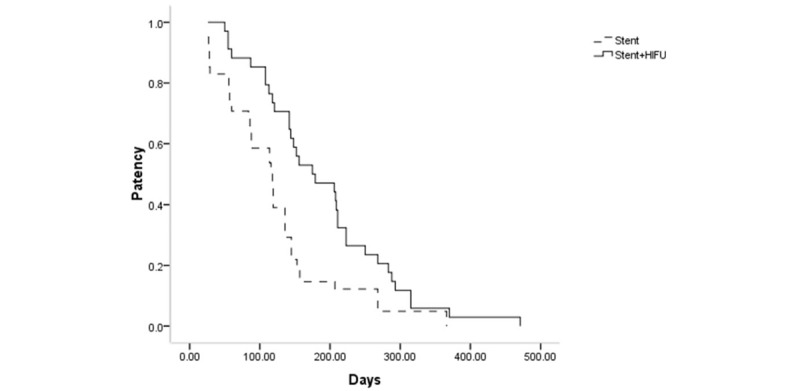
The comparison of stent patency between 2 groups.
3.5. Survival
Follow-up lasted until all patients were dead. The causes of death included tumor progression (n = 74) and abdominal infection (n = 1). The median survival time was significantly longer in the stent with HIFU group compared with the stent-only group (211 vs 136 days, respectively, P = .004, Fig. 3). In the stent with HIFU group, 10 and 2 patients received chemotherapy or radiotherapy, respectively. In the stent-only group, 12 and 7 patients received chemotherapy or radiotherapy, respectively. The remaining patients did not receive chemotherapy or radiotherapy because they could not afford it. When we removed the patients who underwent either chemotherapy or radiotherapy during follow-up from both groups, the median survival in the stent with HIFU group and in the stent-only group were 208 and 88 days, respectively (P = .001).
Figure 3.
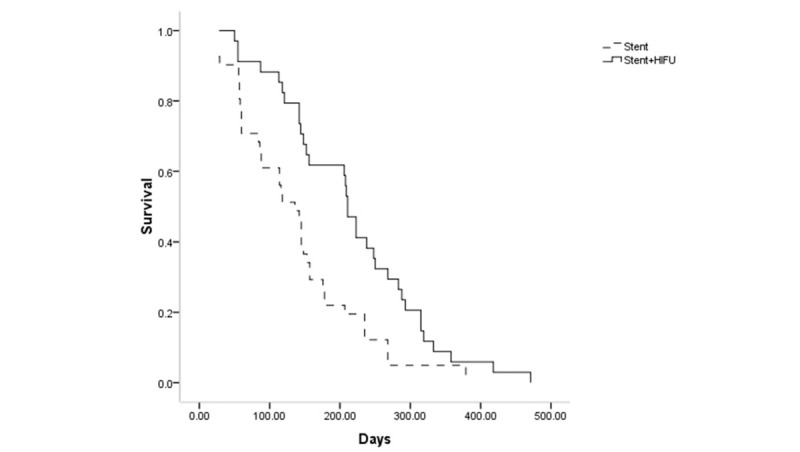
The comparison of survival between 2 groups after SEMS insertion.
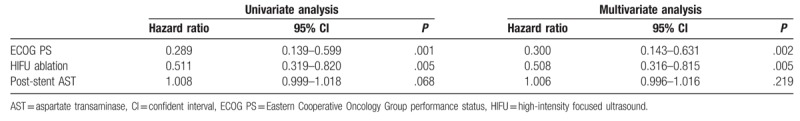
Cox regression analysis revealed that the predictors of prolonging patient survival included ECOG performance status of 3 (hazard ratio [HR]: 0.300; P = .002) and HIFU ablation (HR: 0.508; P = .005, Table 4).
Table 4.
Predictors of survival after stent insertion.
3.6. Complications
In the stent with HIFU group, 3 patients experienced cholangitis. In the stent-only group, 5 patients experienced cholangitis. In all cases, cholangitis was caused by stent dysfunction and was relieved progressively after the repeat SEMS insertion. One patient in the stent with HIFU group experienced pancreatitis and this patient was treated by conservatively. The treatment protocol included gastrointestinal decompression, antibiotic therapy, and trypsin inhibitor therapy.[14]
4. Discussion
PC is a common disease which can lead to DBO. SEMS insertion has been accepted as the first-line palliative treatment of DBO.[1–6] Partially or fully covered SEMSs were used to prevent tumor ingrowth, the main cause of stent dysfunction in uncovered SEMSs. Recently, partially covered SEMSs are more frequently used than fully covered SEMSs to prevent stent migration.[2] However, whether it is an uncovered, fully covered, or partially covered, SEMS by itself has no anticancer effect.[2]
In this study, the significant decrease of aspartate transaminase, alanine aminotransferase, and total bilirubin were achieved in both groups within 7 days. This result may indicate that SEMS can provide a rapid improvement of liver function. However, the postoperative total bilirubin level was significant higher in the stent with HIFU group than in the stent-only group (91.6 ± 46.8 μmol/L vs 63.2 ± 30.1 μmol/L, P = .004). This result may be attributed to the individual differences of each patient.
Postoperative anticancer treatments can effectively prolong the stent patency and survival of patients with malignant biliary obstruction.[8] Li et al[8] also found that different anticancer protocols (chemotherapy, radiotherapy, or combined) did not differ from each other in promoting stent patency and survival. Recently, a multicenter randomized trial proved the superiority of irradiation stents over conventional stents in terms of stent patency (212 vs 104 days, P = .01) and survival (202 vs 140 days, P = .02) for patients with unresectable malignant biliary obstruction.[15] However, both chemotherapy and radiotherapy are associated with treatment-related toxicities, which usually reduce the patients’ quality of life.
HIFU ablation has been used for treating various malignant or benign tumors due to its noninvasive and nontoxic nature.[12,13,16] The HIFU equipment can focus ultrasound energy from an extracorporeal source that is targeted within the body, resulting in thermally induced necrosis and apoptosis.[16] The temperature of the ablated area can reach up to 42oC to 45oC.[16] In this study, the clinical response rate of HIFU ablation was 79.4%, which is comparable to that (72%) in a previous study of HIFU ablation for inoperable PC.[13]
In this study, all stent dysfunctions were caused by tumor ingrowth. This study revealed that median stent patency in the stent with HIFU group was 175 days, significantly longer than the control group (median patency period of 118 days). However, the stent dysfunction rates were not significantly different between these 2 groups. These results indicated that although HIFU ablation cannot prevent stent dysfunction, it fulfilled one of the main purposes of HIFU ablation, that is, to increase stent patency by inhibiting tumor growth.
The patient survival was significantly longer in the stent with HIFU group compared with the stent-only group. This finding is similar to previous findings that patient survival can be prolonged by the addition of anticancer treatment to SEMS.[8,15] In addition, the median survival in the stent with HIFU group was 211 days. When we removed those patients who underwent postoperative chemotherapy or radiotherapy, the median survival in the stent with HIFU group remained almost unchanged at 208 days. These survival durations were comparable to those found in previous studies regarding stent insertion accompanied by chemotherapy or radiotherapy for patients with malignant biliary obstruction.[1,15] These results may indicate that the effectiveness of HIFU ablation was equivalent to that of chemotherapy or radiotherapy.
We found, that an ECOG performance status of 3 and HIFU ablation were predictors of increased patient survival following SEMS insertion. A lower ECOG performance status usually predicts longer survival.[11] In this study, the number of patients with ECOG performance status of 3 was much larger than that of patients with ECOG performance status of 2. The reason that a relatively high ECOG performance status was associated with a good outcome in this study may be mainly attributed to the small number of patients with ECOG performance status of 2. Moreover, there is only 1 grade between ECOG performance status of 2 and 3 and there may not exist a critical difference in body condition between patients with ECOG performance status of 2 and 3.
This study has some limitations. First, it is a retrospective study and a selective bias does exist. Second, a subset of patients also received chemotherapy or radiotherapy during the follow-up, and this may also cause a selective bias. Obviously, we cannot deny patients’ appropriate alternative anticancer treatments. Therefore, we also compared the survival between the 2 groups after removing the patients who underwent chemotherapy or radiotherapy from both groups and found that this made the survival difference between the 2 groups even greater. Third, this is a single-center study and the sample size was not large. In addition, the sample size has not been calculated and this added another bias to this study. Therefore, the results need to be confirmed in a multicenter trial with a larger sample size.
In conclusion, although further clinical studies are needed, our results indicate that HIFU ablation following SEMS insertion can prolong stent patency and survival of patients with DBO secondary to PC.
Author contributions
Conceptualization: Feng-Fei Xia.
Data curation: Fen Liu.
Formal analysis: Fen Liu.
Methodology: Yi Liu.
Software: Feng-Fei Xia.
Supervision: Yu-Fei Fu.
Writing – original draft: Shu-Ying Yang.
Writing – review & editing: Shu-Ying Yang.
Yu-Fei Fu orcid: 0000-0002-6772-6557.
Footnotes
Abbreviations: CT = computed tomography, DBO = distal biliary obstruction, ECOG = Eastern Cooperative Oncology Group, HIFU = high-intensity focused ultrasound ablation, PC = pancreatic carcinoma, SEMS = self-expanded metallic stent.
How to cite this article: Yang SY, Liu F, Liu Y, Xia FF, Fu YF. Stent insertion with high-intensity focused ultrasound ablation for distal biliary obstruction secondary to pancreatic carcinoma. Medicine. 2020;99:6(e19099).
References
- [1].Kitano M, Yamashita Y, Tanaka K, et al. Covered self-expandable metal stents with an anti-migration system improve patency duration without increased complications compared with uncovered stents for distal biliary obstruction caused by pancreatic carcinoma: a randomized multicenter trial. Am J Gastroenterol 2013;108:1713–22. [DOI] [PubMed] [Google Scholar]
- [2].Yokota Y, Fukasawa M, Takano S, et al. Partially covered metal stents have longer patency than uncovered and fully covered metal stents in the management of distal malignant biliary obstruction: a retrospective study. BMC Gastroenterol 2017;17:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Isayama H, Nakai Y, Kogure H, et al. Biliary self-expandable metallic stent for unresectable malignant distal biliary obstruction: which is better: covered or uncovered? Dig Endosc 2013;25:71–4. [DOI] [PubMed] [Google Scholar]
- [4].Zhu HD, Guo JH, Zhu GY, et al. A novel biliary stent loaded with (125)I seeds in patients with malignant biliary obstruction: preliminary results versus a conventional biliary stent. J Hepatol 2012;56:1104–11. [DOI] [PubMed] [Google Scholar]
- [5].Yang MJ, Kim JH, Yoo BM, et al. Partially covered versus uncovered self-expandable nitinol stents with anti-migration properties for the palliation of malignant distal biliary obstruction: a randomized controlled trial. Scand J Gastroenterol 2015;50:1490–9. [DOI] [PubMed] [Google Scholar]
- [6].Isayama H, Mukai T, Itoi T, et al. Comparison of partially covered nitinol stents with partially covered stainless stents as a historical control in a multicenter study of distal malignant biliary obstruction: the WATCH study. Gastrointest Endosc 2012;76:84–92. [DOI] [PubMed] [Google Scholar]
- [7].Qian XJ, Zhai RY, Dai DK, et al. Treatment of malignant biliary obstruction by combined percutaneous transhepatic biliary drainage with local tumor treatment. World J Gastroenterol 2006;12:331–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Li TF, Chen C, Han XW, et al. Clinical efficacy of metallic biliary stents combined with different anti-cancer treatments in the management of bile duct cancer. Hepatogastroenterology 2014;61:22–6. [PubMed] [Google Scholar]
- [9].Li MQ, Zhang JX, Lu CH, et al. Long-term outcome of interventional therapy for malignant biliary obstruction: a retrospective analysis of 109 cases. Zhonghua Yi Xue Za Zhi 2008;88:2743–7. [PubMed] [Google Scholar]
- [10].Wu F. High intensity focused ultrasound: a noninvasive therapy for locally advanced pancreatic cancer. World J Gastroenterol 2014;20:16480–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Niu S, Cheng L, Qiao Y, et al. Combined stent insertion and high-intensity focused ultrasound ablation for patients with malignant obstructive jaundice. Surg Laparosc Endosc Percutan Tech 2016;26:488–92. [DOI] [PubMed] [Google Scholar]
- [12].Ning Z, Xie J, Chen Q, et al. HIFU is safe, effective, and feasible in pancreatic cancer patients: a monocentric retrospective study among 523 patients. Onco Targets Ther 2019;12:1021–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li PZ, Zhu SH, He W, et al. High-intensity focused ultrasound treatment for patients with unresectable pancreatic cancer. Hepatobiliary Pancreat Dis Int 2012;11:655–60. [DOI] [PubMed] [Google Scholar]
- [14].Group of Pancreas Surgery, Chinese Society of Surgery, Chinese Medical Association The guideline of diagnosis and treatment of severe acute pancreatitis. Zhonghua Wai Ke Za Zhi 2007;45:727–9. [PubMed] [Google Scholar]
- [15].Zhu HD, Guo JH, Huang M, et al. Irradiation stents vs. conventional metal stents for unresectable malignant biliary obstruction: a multicenter trial. J Hepatol 2018;68:970–7. [DOI] [PubMed] [Google Scholar]
- [16].Zhou YF. High intensity focused ultrasound in clinical tumor ablation. World J Clin Oncol 2011;2:8–27. [DOI] [PMC free article] [PubMed] [Google Scholar]


