Abstract
The effectiveness of direct-acting antivirals (DAAs) against hepatitis C virus (HCV) infection is ascertained. However, some authors raised the issue of an increased incidence of de novo hepatocellular carcinoma (HCC) in patients treated with DAAs. Aim of the study was to evaluate the rate of HCC occurrence in a real-life cohort of patients who received anti-HCV treatment with DAAs.
A prospective multicentre study was conducted. All adult patients with HCV infection who received treatment between March 2015 and December 2017 in 4 hospital of Campania region (South Italy) with at least 6 months of follow-up were enrolled.
A total of 323 patients were included in the study. Most patients had HCV genotype 1b (61.8%). The overall SVR12 rate was 95.5%. Median time of observation was 10 months. The incidence rate of HCC was 0.2 per 100 person-months (crude incidence rate 3.4%, 95 confidence interval: 1.5%–5.3%). The median time for HCC occurrence was 11 months. HCC occurrence rate was significantly higher among patients who did not achieve SVR12 compared with patients who did (28.6% vs 2.8%, P < 0.05). No patient with F0-F3 fibrosis developed HCC. Among patients with cirrhosis, at the multivariate time-to-event analysis, no covariates were independently associated with the risk of HCC occurrence.
Treatment with DAAs did not increase the risk of HCC occurrence. Patients who achieved SVR12 had a lower rate of HCC occurrence. Further studies are needed to estimate the incidence and the risk for HCC in the long-term follow-up among patients undergoing treatment with DAAs.
Keywords: direct acting antivirals, HCV, hepatocellular carcinoma, real-world
1. Introduction
Direct-acting antivirals (DAAs) against hepatitis C virus (HCV) elicited great enthusiasm worldwide for their effectiveness in eradicating the infection since the first available trials.[1–3] In fact, treatment with DAAs leads to sustained virologic response at 12 weeks post-treatment (SVR12) in up to 99% of treated patients with few adverse drug reactions.[4–7] Such great enthusiasm has been undermined in the first years of DAAs availability due to the aroused question of possible high risk of hepatocellular carcinoma development during and after treatment administration.[8] Several studies indeed reported high rates of both HCC occurrence and recurrence, first related to DAAs administration.[9,10] However, all these studies had significant bias, such as the small sample size and the retrospective design. Moreover, the authors often reported incidence rates of HCC that were actually not different from the known incidence rate of HCC among the whole HCV-infected population, which is estimated to be approximately 2% to 8%.[11] In fact, in a recent review of 24 papers[12] the risk of HCC recurrence among patients who received DAAs therapy was evaluated. The authors stated that no significant conclusion can be drawn, due to the above-mentioned bias of the available studies. They also advocated the necessity of large prospective studies. For what concerns the risk of de novo HCC occurrence, the largest available study was conducted among the US veterans cohort.[13] Results from this study reported an annual incidence rate of 1.18 per 100 person-year during and after DAAs administration, but the retrospective design of the study limited generalization of the results. For such reasons, the debate on DAAs and HCC development is still open. In fact, it is not clear whether some patients’ characteristics or comorbidity may have an impact on HCC occurrence and recurrence during and after treatment with DAAs.[14] In such a doubtful setting, results from prospective real-life cohorts are needed to estimate whether a significant association between DAAs and HCC occurrence really exists. The LINA (liver network activity) is a local inter-departmental network established in 2015 with the aim of analyzing the real-life efficacy of DAAs-based interferon-free regimens in the treatment of chronic HCV infection in Campania Region,[15–17] which is a high-prevalence area for HCV infection in Southern Italy.[18] All the patients who started any DAAs-based interferon-free treatment in one of centers involved in the network, regardless HCV genotype and stage of liver cirrhosis, were included in the cohort (LINA cohort). A unique dataset was used to collect demographical, clinical and laboratory data of the included patients.
The aim of this study was to estimate the occurrence of HCC among patients with chronic HCV infection included in the LINA cohort of patients
2. Methods
We conducted a prospective observational multicentre study involving all the patients with HCV chronic infection who received a DAAs-based interferon-free treatment regimen between March 2015 and December 2017 and who referred to one of the following hospitals (LINA cohort)[16]:
-
(1)
University of Naples Federico II, Department of Clinical Medicine and Science – Section of Infectious Diseases
-
(2)
University of Campania, Luigi Vanvitelli, Infectious Diseases Unit, Department of Mental Health and Public Medicine
-
(3)
Azienda ospedaliera dei colli, HIV Unit
-
(4)
OORR Area Stabiese – P.O. Gragnano. U.O.C. Medicina Interna, Epatologia ed Ecografia Interventistica
The present analysis was conducted among the patients included in the LINA cohort and selected according to the following criteria:
Inclusion criteria for the present study were:
-
(1)
Patients with chronic HCV hepatitis
-
(2)
Treatment with DAAs started between March 2015 and December 2017
-
(3)
Age ≥18 years old
-
(4)
More than 6 months of follow-up (FU) from the beginning of DAAs treatment
Exclusion criteria were:
-
(1)
Child C cirrhosis
-
(2)
Diagnosis of active HCC at the baseline and previous HCC diagnosis
-
(3)
Consent refusal
The Metavir score was estimated with a FibroScan© exam performed within 6 months before the beginning of the antiviral treatment. Clinical cirrhosis was defined as the presence of at least 1 of the following signs in patients with a diagnosis of chronic HCV infection:
-
(1)
Ultrasonography (US) of the abdomen suggestive for liver cirrhosis (ie, hypertrophy of the caudate lobe, nodularity of the liver surfaces, altered straightness of suprahepatic veins)[19]
-
(2)
Combination of at least 2 laboratory tests suggestive for liver cirrhosis (ie, low platelets count, low plasmatic pseudocholinesterase concentrations, Aspartate Aminotransferase (AST)/Alanine Aminotransferase (ALT) ratio >1)[20–22]
-
(3)
Presence of at least 1 cirrhosis-related complication (ie, ascites, porto-systemic encephalopathy, oesophageal varices)
Patients who previously received an antiviral anti-HCV treatment (interferon-based) were defined as treatment-experienced; patients who never received a previous treatment against HCV were defined as treatment-naïve.
Diagnosis of active HCC was made by radiological, histological or cytological criteria, in accordance with American Association for the Study of Liver Diseases (AASLD) hepatocellular carcinoma guidelines.[23] All the enrolled patients underwent an US of the abdomen within 6 months before the beginning of DAAs treatment to rule out the presence of active HCC. Patients with hepatic lesions at the US, underwent a computerized tomography (CT) or a magnetic resonance (MR) to confirm the diagnosis of HCC according to the above-mentioned guidelines.[23] The indication for antiviral therapy and the choice of the interferon-free regimen was made according to international guidelines[24] and local availability. The dose of the different DAAs and the duration of the regimen were chosen according to the international guidelines.[24] Among patients in the LINA cohort,[15–17] for the present analysis we included only those who completed 6 months of follow up after the end of treatment (EOT). Moreover, patients with a history of HCC (as well as those with a diagnosis of HCC at baseline) were excluded. Finally, patients with active alcohol abuse were excluded. Scheduled FU visits were time of enrolment (TOE), 1 month after the beginning of treatment (1-month of therapy, 1MT), at the EOT and at 12 weeks after the end of treatment (12WPT). All the patients underwent a clinical exam, an US of the abdomen and laboratory tests at TOE and at each FU visit. Patients with hepatic lesions at the US, underwent a CT or a MR of the abdomen to confirm the diagnosis of HCC. Plasmatic HCV-RNA concentrations were dosed within 3 months before the enrolment and then at each FU visit. HCV-RNA detectable was defined as HCV-RNA plasmatic concentrations above the lower limit of detectability (15 IU/mL). SVR12 was defined as HCV-RNA plasmatic concentrations below the lower limit of detectability at 12WPT. Finally, after the scheduled FU visits, all the patients underwent a clinical examination and an US of the abdomen every 3 month to evaluate the occurrence/recurrence of HCC. A CT or a MR was performed to patients with hepatic lesions at the US, to confirm the diagnosis of HCC. The date of last FU was also recorded.
2.1. Study outcomes and sample size
The primary outcome of the study was to assess the incidence rate of HCC in a real-life cohort of patients with chronic HCV infections who received treatment with DAAs and who never had a HCC diagnosis (HCC occurrence).
Secondary outcomes were:
-
(1)
To compare the rate of HCC occurrence between patients who achieved SVR12 and patients who did not
-
(2)
To analyze the presence of risk factors for HCC occurrence among patients who received DAAs
Assuming the previously reported average incidence rate of 6% of HCC among patients with liver cirrhosis,[11] we hypothesized a 10% incidence rate of HCC among patients who received treatment with DAAs in our cohort. Given the above, the calculated sample size was 322, with α- and β-error of 0.05 e 0.2, respectively.
2.2. Statistical analysis
The Kolmogorov-Smirnov test was applied to quantitative variables to check for Gaussian distribution. Data are given as mean ± standard deviation or as median and interquartile range (IQR) in case of Gaussian and non-Gaussian distribution, respectively. For categorical dichotomic variables, the χ2 test (or Fisher exact test if appropriate) was used for comparisons between 2 unpaired groups. HCC incidence was calculated as the number of HCC divided by total person-months (PM) of follow-up. Kaplan–Meier curves were generated to illustrate the cumulative incidence of HCC by SVR; the log-rank test was used to compare the differences between the curves. A time-to-event analysis with the cox regression model was used to estimate the risk for HCC occurrence. Variables included in the model (and stated a priori) were: sex, age >60 years, treatment received, treatment duration, Child B cirrhosis, Model for End-Stage Liver Disease (MELD) score >10, treatment-experience, failure to achieve SVR12. The variables that showed with a P-value < .2 for HCC occurrence at the univariate model, were included in the multivariate model analysis. For all tests, a P-value < .05 at 2-sided test was considered statistically significant. Statistical analysis was carried out using the Statistical Package for the Social Sciences version 20.0 (SPSS Inc., Chicago, IL).
2.3. Ethical statement
The present prospective study was conducted in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki-Sixth Version) for experiments involving humans. The study protocol was approved by the local Ethical Committee (Prot. N° 259/18). Written informed consent was obtained for all the patients involved into the study.
3. Results
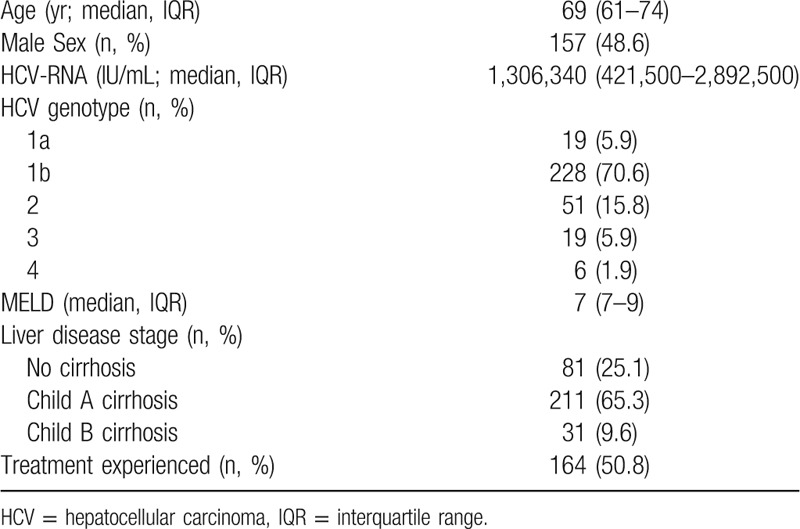
Three-hundred forty-one patients were included in the study according to the inclusion/exclusion criteria. In particular, 612 patients were excluded because they did not complete a 6 months post-treatment FU period. Moreover, 12 patients had a diagnosis of previous HCC at TOE, while 6 were alcohol abuser, thus they were all excluded. Consequently, 323 patients were included in the present analysis (Fig. 1). Clinical parameters of the included patients at TOE are reported in Table 1.
Figure 1.
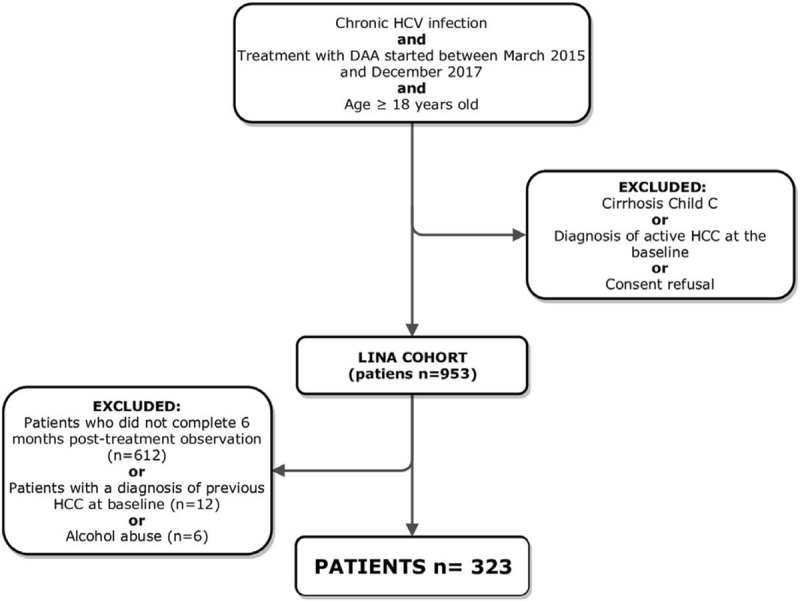
Flowchart of the cohort inclusion criteria.
Table 1.
Clinical parameters of the enrolled patients (N = 323).
3.1. Treatment efficacy
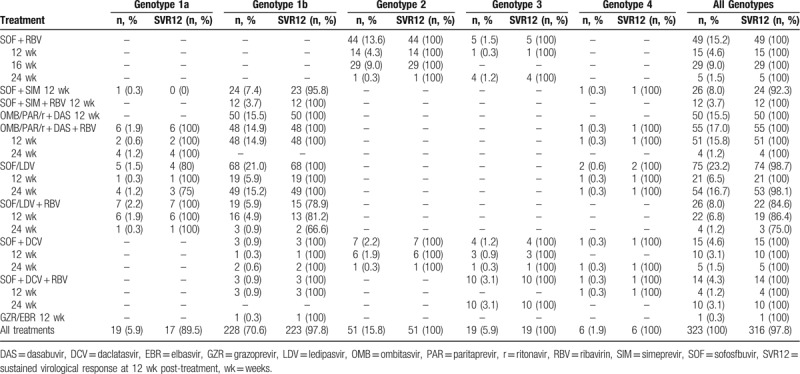
Sofosfbuvir (SOF) plus ledipasvir (LDV) for 24 weeks was the most prescribed treatment in our cohort (75/323 patients, 23.2%). Among patients with HCV genotype 2, 44 (13.6%) received SOF plus ribavirin (RBV) while 7 (2.2%) received SOF plus daclatasvir when this treatment became available for patients with this genotype and with contraindications to RBV (eg, anemia, coronary diseases, severe pulmonary diseases). All patients except 2 had HCV-RNA <15 IU/mL at 1MT (321/323, 99.4%). One patient with detectable HCV-RNA at EOT eventually achieved the SVR12, while 6 (1.9%) patients with undetectable HCV-RNA at EOT had a relapse after treatment. Therefore, the overall SVR12 rate was 95.5% (316/323 patients). The SVR12 rate was lower among patients with Child B cirrhosis compared with patients with Child A cirrhosis or with no cirrhosis (27/31, 87.1% vs 289/292, 99.0%; P < .01). Most patients (70.6%) had HCV genotype 1b infection and they achieved a 97.8% SVR12 rate (223/228). All patients with HCV genotype 2, 3, and 4 achieved the SVR12 (51/51, 19/19, and 6/6, respectively), while patients with genotype 1a (5.9% of all patients) achieved a SVR12 rates of 89.5% (17/19). Rates of SVR12 according to HCV genotype and treatment are shown in Table 2. Four out of 7 patients who failed the cure received SOF + LDV + RBV (3 of them for 12 weeks, 1 of them for 24 weeks). They all had a genotype 1b HCV infection and all but 1 was treatment-experienced. Two out of 7 patients who did not achieve SVR12 received SOF plus Simeprevir for 12 weeks (1 treatment-experienced genotype 1a and 1 treatment-naïve genotype 1b), while the remaining patient received SOF + LDV for 24 weeks; she had genotype 1a infection and she had already received a previous interferon-based anti-HCV treatment. All the 323 patients completed the treatment, no discontinuation occurred.
Table 2.
Treatment allocation of the enrolled patients and SVR12 rates (N = 323).
3.2. HCC incidence
There were 11/323 patients with a de novo diagnosis of HCC during 3926 PM (median time 10 months, IQR: 6–14). The incidence rate in our cohort was 0.2 per 100 PM (crude rate 3.4%, 95 confidence interval [CI]: 1.5%–5.3%). The median time for HCC occurrence was 11 months from the beginning of the treatment course (IQR: 6–23). HCC occurrence rate was significantly higher among patients who did not achieve SVR12 compared with the occurrence rate among patients who did (2/7, 28.6%, 1.7 per 100 PM vs 9/316, 2.8%, 0.2 per 100 PM; P < .05). SVR12 was strongly and positively associated with time until development of HCC (P < .01) (Fig. 2). All patients with a de novo diagnosis of HCC had liver cirrhosis, thus HCC incidence rate was significantly higher among patients with liver cirrhosis compared with patients without cirrhosis (11/242, 4.5%, 0.4 per 100 PM vs 0/81, 0%, 0 per 100 PM; P < .05). Patients in class B of Child-Pugh classification had a higher rate of HCC occurrence compared with patients in class A (5/31, 16.1%, 1.00 per 100 PM vs 6/211, 2.8%, 0.2 per 100 PM; P < .01). Most HCC (8/11, 72.7%) occurred after the EOT, while 3/11 (27.3%) occurred during treatment (from TOE to EOT). Finally, treatment-experienced patients had a similar HCC occurrence rate compared with treatment-naive patients (6/164, 3.7%, 0.3 per 100 PM vs 5/159, 3.1%, 0.3 PPM; P = .52). Table 3 shows the characteristics of patients who had HCC occurrence.
Figure 2.
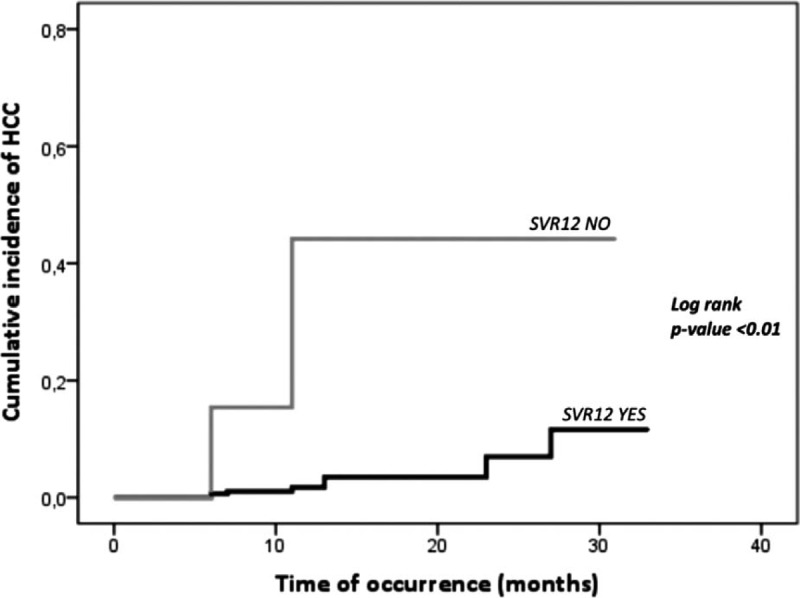
Cumulative incidence of hepatocellular carcinoma (HCC) according to sustained virologic response at 12 wk post-treatment (SVR12).
Table 3.
Clinical characteristics of the 11 patients with HCC occurrence.
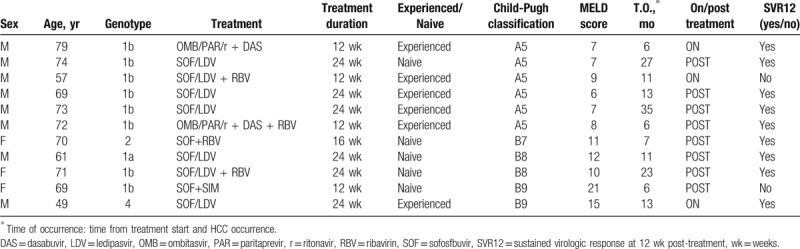
3.3. Risk factors for HCC occurrence
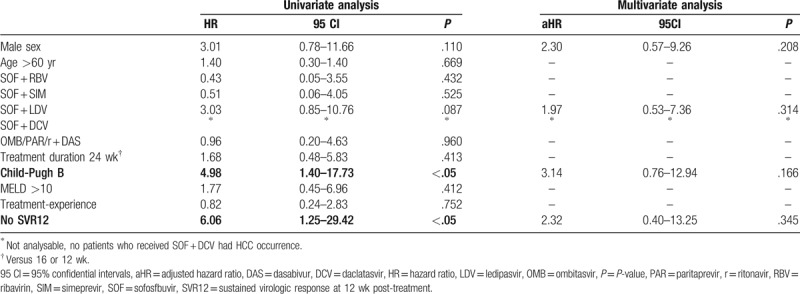
All patients with a de novo diagnosis of HCC in our cohort had liver cirrhosis (Child-Pugh A or B). Thus, the time-to-risk analysis was conducted among the 242 patients with a diagnosis of liver cirrhosis at the baseline. According to the univariate time-to-event analysis, patients with Child B cirrhosis, as well as those who failed to achieve SVR12, had a higher risk for HCC occurrence (Table 4). However, at the multivariate time-to-event analysis, when also male sex and treatment with SOF + LDV were included in the model, no covariates were independently associated with the risk of HCC occurrence.
Table 4.
Cox regression analysis for HCC occurrence among patients with cirrhosis (n = 242).
4. Discussion
DAAs have a great efficacy in the eradication of HCV infection. These treatments were also effective in reducing the incidence of several complications of cirrhosis, such as ascites and encephalopathy.[16,25] However, since the interferon era, it has been demonstrated that SVR12 achievement in patients with liver cirrhosis does not eliminate the HCC risk.[26] These data have been also confirmed in the first available real-life studies with DAAs. Moreover, in the first years of DAAs availability, some concerns were raised regarding a presumed higher incidence of HCC development in patients treated with DAAs.[9] In this study, which was carried out among 332 patients who received treatment with DAAs, we showed a crude HCC occurrence rate of 3.4% (0.2 per 100 PM) during 3926 PM. This rate is not different from the known incidence rate of HCC among patients who had never receive antiviral treatment.[27,28] Thus, our results do not confirm the hypothesis of an increased incidence of HCC occurrence in the short- and in the mid-term among patients on anti-HCV treatment. Actually, several studies and systematic reviews have already estimated the risk of both recurrence (among patients with a previous diagnosis of HCC) and de novo occurrence (among patients without a previous diagnosis of HCC) after antiviral treatment with DAAs.[12,29] However, most available data came from retrospective cohorts and several authors advocated the necessity of large prospective cohorts. Nevertheless, data about the risk of HCC occurrence are very few compared with available data on HCC recurrence. In fact, in a recent meta-analysis[29] aimed at comparing the risk of HCC occurrence and recurrence between patients treated with DAAs and those treated with interferon-free regimens, only 9 studies discussing the risk of de novo HCC occurrence were included. These studies reported results from retrospective cohorts or small prospective cohorts. Moreover, the aim of some of such studies was often different from the evaluation of HCC occurrence. The SVR12 rate of our cohort (95.5%) was similar to the rate reported in other studies and real-life cohorts.[30–32] Interestingly, the rate of HCC occurrence was significantly higher among patients who did not achieved SVR12 compared with those who did (2/7, 28.6%, 1.7 per 100 PM vs 9/316, 2.8%, 0.2 per 100 PM; P < .05). A similar difference was reported in the U.S. Veteran cohort. In fact, in the retrospective analysis conducted by Kanwal et al,[13] a considerable lower cumulative incidence of HCC among patients who achieved SVR12 compared with patients who did not, was reported (0.90 per 100 person-year [95 CI: 0.77–1.03] vs 3.45 per 100 person-year [95 CI: 2.73–4.18], P < .0001). Despite the aroused risk of HCC in patients receiving DAAs, these results suggest an association between viral clearance and a lower risk of de novo HCC, further averting a possible increased HCC risk during DAAs treatment. It is also noteworthy that, according to our univariate time-to-event analysis, the only factors significantly associated with HCC occurrence were class Child-Pugh B liver cirrhosis and failure to treatment (no SVR12 achieved). At the multivariate time-to-event model, both the covariates lose their association with HCC occurrence, hinting a mutual influence. In fact, the SVR12 rate was significantly lower among patients with Child B cirrhosis compared with patients without cirrhosis or Child A cirrhosis (27/31, 87.1% vs 289/292, 99.0%; P < .01). Supposedly, the higher risk for HCC resulted from the univariate time-to-event analysis among patients who failed to achieve SVR12 was mostly related to the decompensated phase of liver diseases, which is known to be associated with an increased incidence of HCC.[33,34] Finally, there were no significant associations between the different DAAs combinations and HCC occurrence risk. Our study had some limitations. First of all, there was no control group. While this is a main limitation of the study, it must be stated that denying an immediate antiviral-treatment to chronically HCV infected people would raise significant ethical concerns. In fact, an effective anti-HCV treatment must be provided as soon as possible both to avoid a further progression of liver diseases and to minimize the risk of HCV transmission in the general population. Given the lack of a control group, we compared our results with the previously reported occurrence of HCC in our country. Sangiovanni et al indeed showed a 3.4% rate per year of HCC occurrence among 417 patients with compensated cirrhosis who did not receive DAAs or other anti-HCV drugs.[28] This occurrence rate was similar to the rate in our cohort; moreover, it was higher compared with the occurrence rate in patients in our cohort who achieved SVR12 (2.8%). Another limitation is the short median time of FU (10 months; IQR: 6–14), which did not allow us to draw conclusions on the long-term risk of HCC among patients who receive anti-HCV treatment with DAAs. Furthermore, 64% of the initially included patients (n = 612) did not completed 6 months of post-treatment observation at the time of the analysis and they were excluded from the study. Since potential DAAs-related HCCs are unlikely to occur in a short-term period after treatment completion, these patients were excluded to avoid a selection bias that could have led to a lower, and distorted, HCC occurrence. All these patients were lost to FU after they achieved the SVR12 at the 12WPT scheduled visit. Thus, they were probably unwilling to attend the clinical follow up after the clearance of HCV infection. Given the above, the sample size may be not sufficient to highlight the presence of risk factors independently associated with HCC. However, according to our sample size calculation, it is large enough to estimate the incidence of HCC occurrence.
In conclusion, our results showed an incidence of HCC occurrence among patients who received anti-HCV treatment which is similar to the expected incidence among patients with HCV-related liver disease. We found no independent risk factors associated with HCC occurrence but we interestingly showed a higher HCC incidence in patients who did not achieve SVR12. Further studies are needed to estimate the incidence and the risk for HCC in the long-term FU among patients undergoing treatment with direct acting antivirals.
Author contributions
ARB conceived the study and the study protocol and revised the final version of the manuscript. RS filled in the dataset, performed the statistical analysis and wrote the first draft of the manuscript. CC filled in the dataset and revised the first draft of the manuscript. GV, CMR, LS, MS, FS, FP, MP, SDP, SM, GT, SN filled in the dataset and managed the enrolled patients during the treatment. BP managed the enrolled patients during the treatment and revised the first draft of the manuscript. IG and NC conceived the study and revised the final version of the manuscript.
Riccardo Scotto orcid: 0000-0002-0178-2986.
Footnotes
Abbreviations: 12WPT = 12 weeks after the end of treatment, 1MT = 1-month of treatment, CT = computerized tomography, DAAs = direct-acting antivirals, EOT = end of treatment, FU = follow-up, HCC = hepatocellular carcinoma, HCV = hepatitis C virus, IQR = interquartile range, LDV = ledipasvir, MR = magnetic resonance, PM = person-months, r = ritonavir, RBV = ribavirin, SOF = sofosbuvir, SVR12 = sustained virological response at 12 weeks of treatment, TOE = time of enrolment, US = ultrasonography.
How to cite this article: Buonomo AR, Scotto R, Coppola C, Pinchera B, Viceconte G, Rapillo CM, Staiano L, Saturnino M, Scarano F, Portunato F, Pisaturo M, De Pascalis S, Martini S, Tosone G, Nappa S, Coppola N, Gentile I. Direct acting antivirals treatment for hepatitis C virus infection does not increase the incidence of de novo hepatocellular carcinoma occurrence: Results from an Italian real-life cohort (LINA cohort). Medicine. 2020;99:6(e18948).
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Ivan Gentile was consultant for Abbvie, MSD, and Cardiome. He received a grant (in the framework of Fellowship program) from Gilead Sciences. Nicola Coppola received grants from ViiV Healthcare, Janssen-Cilag, and Gilead Sciences; personal fees from Gilead Sciences, Abbvie, Bristol-Myers Squibb and Merck Sharp & Dohme.
Conflicts of Interest: Ivan Gentile was consultant for Abbvie, MSD and Cardiome. He received a grant (in the framework of Fellowship program) from Gilead Sciences.
Nicola Coppola received grants from ViiV Healthcare, Janssen-Cilag, and Gilead Sciences; personal fees from Gilead Sciences, Abbvie, Bristol-Myers Squibb and Merck Sharp & Dohme.
References
- [1].Gentile I, Coppola N, Buonomo AR, et al. Investigational nucleoside and nucleotide polymerase inhibitors and their use in treating hepatitis C virus. Expert Opin Investig Drugs 2014;23:1211–23. [DOI] [PubMed] [Google Scholar]
- [2].Gentile I, Buonomo AR, Borgia F, et al. MK-5172: a second-generation protease inhibitor for the treatment of hepatitis C virus infection. Expert Opin Investig Drugs 2014;23:719–28. [DOI] [PubMed] [Google Scholar]
- [3].Borgia G, Maraolo AE, Buonomo AR, et al. The therapeutic potential of new investigational hepatitis C virus translation inhibitors. Expert Opin Investig Drugs 2016;25:1209–14. [DOI] [PubMed] [Google Scholar]
- [4].Gentile I, Scotto R, Zappulo E, et al. Investigational direct-acting antivirals in hepatitis C treatment: the latest drugs in clinical development. Expert Opin Investig Drugs 2016;25:557–72. [DOI] [PubMed] [Google Scholar]
- [5].Schinazi R, Halfon P, Marcellin P, et al. HCV direct-acting antiviral agents: the best interferon-free combinations. Liver Int 2014;34: Suppl 1: 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hezode C. Treatment of hepatitis C: results in real life. Liver Int 2018;38: Suppl 1: 21–7. [DOI] [PubMed] [Google Scholar]
- [7].Scotto R, Buonomo AR, Moriello NS, et al. Real-world efficacy and safety of pangenotypic direct-acting antivirals against hepatitis C virus infection. Rev Recent Clin Trials 2019;14:173–82. [DOI] [PubMed] [Google Scholar]
- [8].Buonomo AR, Gentile I, Borgia G. Direct acting antiviral agents and hepatocellular carcinoma development: don’t take it for granted. Transl Gastroenterol Hepatol 2017;2:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Reig M, Marino Z, Perello C, et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol 2016;65:719–26. [DOI] [PubMed] [Google Scholar]
- [10].Conti F, Buonfiglioli F, Scuteri A, et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol 2016;65:727–33. [DOI] [PubMed] [Google Scholar]
- [11].Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology (Baltimore, Md) 2011;53:1020–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Guarino M, Vigano L, Ponziani FR, et al. Recurrence of hepatocellular carcinoma after direct acting antiviral treatment for hepatitis C virus infection: literature review and risk analysis. Dig Liver Dis 2018;50:1105–14. [DOI] [PubMed] [Google Scholar]
- [13].Kanwal F, Kramer J, Asch SM, et al. Risk of hepatocellular cancer in HCV patients treated with direct acting antiviral agents. Gastroenterology 2017;153:996–1005.e1. [DOI] [PubMed] [Google Scholar]
- [14].Nahon P, Layese R, Bourcier V, et al. Incidence of hepatocellular carcinoma after direct antiviral therapy for HCV in patients with cirrhosis included in surveillance programs. Gastroenterology 2018;155:1436–50.e6. [DOI] [PubMed] [Google Scholar]
- [15].Gentile I, Buonomo AR, Coppola C, et al. Efficacy of the “first wave” direct acting antivirals against HCV infection: results from the Italian LINA (Liver Network Activity) cohort. New Microbiol 2019;41:94–100. [PubMed] [Google Scholar]
- [16].Gentile I, Scotto R, Coppola C, et al. Treatment with direct-acting antivirals improves the clinical outcome in patients with HCV-related decompensated cirrhosis: results from an Italian real-life cohort (Liver Network Activity-LINA cohort). Hepatol Int 2018;13:66–74. [DOI] [PubMed] [Google Scholar]
- [17].Coppola N, Portunato F, Buonomo AR, et al. Interferon-free regimens improve kidney function in patients with chronic hepatitis C infection. J Nephrol 2019;32:763–73. [DOI] [PubMed] [Google Scholar]
- [18].Buonomo AR, Scotto R, Pinchera B, et al. Epidemiology and risk factors for hepatitis C virus genotypes in a high prevalence region in Italy. New Microbiol 2018;41:26–9. [PubMed] [Google Scholar]
- [19].Procopet B, Berzigotti A. Diagnosis of cirrhosis and portal hypertension: imaging, non-invasive markers of fibrosis and liver biopsy. Gastroenterol Rep 2017;5:79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nyblom H, Berggren U, Balldin J, et al. High AST/ALT ratio may indicate advanced alcoholic liver disease rather than heavy drinking. Alcohol Alcohol 2004;39:336–9. [DOI] [PubMed] [Google Scholar]
- [21].Gopal DV, Rosen HR. Abnormal findings on liver function tests. Interpreting results to narrow the diagnosis and establish a prognosis. Postgrad Med 2000;107:100–2. 105-109, 113-104. [DOI] [PubMed] [Google Scholar]
- [22].Afdhal N, McHutchison J, Brown R, et al. Thrombocytopenia associated with chronic liver disease. J Hepatol 2008;48:1000–7. [DOI] [PubMed] [Google Scholar]
- [23].Jordi B, Morris S. Management of hepatocellular carcinoma: an update. Hepatology (Baltimore, Md) 2011;53:1020–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].EASL. Recommendations on Treatment of Hepatitis C; 2008. Available at: https://www.journal-of-hepatology.eu/article/S0168-8278(18)31968-8/pdf [Accessed Oct 2018] [Google Scholar]
- [25].Fernandez Carrillo C, Lens S, Llop E, et al. Treatment of hepatitis C virus infection in patients with cirrhosis and predictive value of model for end-stage liver disease: analysis of data from the Hepa-C registry. Hepatology (Baltimore, Md) 2017;65:1810–22. [DOI] [PubMed] [Google Scholar]
- [26].Pinzone MR, Zanghi AM, Rapisarda L, et al. Cirrhotic patients are still at risk of developing hepatocellular carcinoma despite Interferon-induced sustained virological response. Eur Rev Med Pharmacol Sci 2014;18: 2 Suppl: 11–5. [PubMed] [Google Scholar]
- [27].El-Serag HB. Hepatocellular carcinoma. New Engl J Med 2011;365:1118–27. [DOI] [PubMed] [Google Scholar]
- [28].Sangiovanni A, Del Ninno E, Fasani P, et al. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance. Gastroenterology 2004;126:1005–14. [DOI] [PubMed] [Google Scholar]
- [29].Waziry R, Hajarizadeh B, Grebely J, et al. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: a systematic review, meta-analyses, and meta-regression. J Hepatol 2017;67:1204–12. [DOI] [PubMed] [Google Scholar]
- [30].Gentile I, Borgia G. A pill a day keeps HCV away. Lancet Infect Dis 2015;15:616–7. [DOI] [PubMed] [Google Scholar]
- [31].Ozono Y, Nagata K, Hasuike S, et al. Efficacy and safety of sofosbuvir and ledipasvir in Japanese patients aged 75 years or over with hepatitis C genotype 1. World J Hepatol 2017;9:1340–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Trifan A, Stanciu C, Gheorghe L, et al. Efficacy and safety of paritaprevir/ritonavir, ombitasvir, and dasabuvir with ribavirin for the treatment of HCV genotype 1b compensated cirrhosis in patients aged 70 years or older. Medicine 2017;96:e9271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lok AS, Seeff LB, Morgan TR, et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology 2009;136:138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Fattovich G, Stroffolini T, Zagni I, et al. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 2004;127: 5 Suppl 1: S35–50. [DOI] [PubMed] [Google Scholar]


