Abstract
Activation of the leptin receptor, LepRb, by the adipocytokine/neurotrophic factor leptin in the central nervous system has procognitive and antidepressive effects. Leptin has been shown to increase glutamatergic synaptogenesis in multiple brain regions. In contrast, mice that have a mutation in the LepRb gene show abnormal synapse development in the hippocampus as well as deficits in cognition and increased depressive-like symptoms. Leptin increases glutamatergic synaptogenesis, in part, through enhancement of N-methyl-D-aspartic acid (NMDA) receptor function; yet the underlying signaling pathway is not known. In this study, we examine how leptin regulates surface expression of NR2B-containing NMDA receptors in hippocampal neurons. Leptin stimulation increases NR2BY1472 phosphorylation, which is inhibited by the Src family kinase inhibitor, PP1. Moreover, we show that Fyn, a member of the Src family kinases, is required for leptin-stimulated NR2BY1472 phosphorylation. Furthermore, inhibiting Y1472 phosphorylation with either a dominant negative Fyn mutant or an NR2B mutant that lacks the phosphorylation site (NR2BY1472F) blocks leptin-stimulated synaptogenesis. Additionally, we show that LepRb forms a complex with NR2B and Fyn. Taken together, these findings expand our knowledge of the LepRb interactome and the mechanisms by which leptin stimulates glutamatergic synaptogenesis in the developing hippocampus. Comprehending these mechanisms is key for understanding dendritic spine development and synaptogenesis, alterations of which are associated with many neurological disorders.
Keywords: Leptin, NMDA receptor, synaptogenesis, receptor trafficking, hippocampus
Mutations in either the hormone leptin (obese, ob/ob) or the long form of its receptor (LepRb, diabetes, db/db) cause a large increase in food intake and morbid obesity in mice (1,2). In adults, leptin is produced and secreted primarily by adipocytes, circulates through the bloodstream, and is actively transported across the blood–brain barrier (3,4). It then regulates energy homeostasis and feeding behavior by binding to LepRbs expressed by neurons in multiple hypothalamic and hindbrain nuclei as well as other regions, including nuclei involved in reward such as the ventral tegmental area (5–8). While animals with these mutations have been most extensively studied for their defects in energy homeostasis and neuroendocrine function, they also show abnormalities in central nervous system (CNS) structure and other CNS behaviors, including hippocampal-dependent behaviors such as exhibiting increased depressive-like behaviors and anhedonia (9–11). Ob/ob mice have lower brain weight and cortical volume, which can be rescued with leptin injections only during the early neonatal period (12). Abnormalities in hypothalamic neural projections found in ob/ob mice can also only be rescued by leptin injections during the neonatal period (13). This suggests a neurotrophic role of leptin in early CNS development.
Circulating leptin levels surge in mice during postnatal days 7 to 14, which is also an important hippocampal developmental period (14). LepRbs are expressed in the CA1/CA3 regions and the dentate gyrus of the hippocampus (15–17). Our lab and others have shown that leptin enhances dendritic spine and synapse formation in the hippocampus as well as the hypothalamus (18–21). This enhancement in hippocampal neuronal development is dependent on LepRb expression, with both short hairpin ribonucleic acid (shRNA) knockdown of LepRb and db/db mice exhibiting decreases in dendritic spines and glutamatergic synapse density (20).
Leptin modifies long-term potentiation and trafficking of glutamate receptors while also having antidepressive and anxiolytic effects (22–27). One of the mechanisms by which leptin affects memory and synaptic plasticity in the hippocampus is by modulating N-methyl-D-aspartate receptors (NMDARs) (22,24). These receptors are heterotetramers composed of various combinations of subunits, with different combinations having diverse biophysical, pharmacological, and signaling properties (28,29). The most abundant modulatory subunits found in the cortex and hippocampus are the NR2A and NR2B subunits (30,31), with the ratio of NR2B/NR2A-containing receptors playing a critical role in synapse formation and maturation, respectively (32,33). Leptin enhances NMDAR function in the hippocampus, as evidenced by increased Ca2+ influx through NMDARs following leptin administration (22). However, the full signaling cascade by which leptin activates NMDARs involved is not known.
Leptin, through binding to LepRb, has been shown to activate multiple signaling cascades such as the janus kinase/signal transducer and activator of transcription (Jak/Stat3), mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase (PI3K), and calcium/calmodulin depdent protein kinase (CaMKK/CaMKI) pathways, which are important for its actions (21,34). Of specific interest to leptin’s regulation of NMDARs in the hippocampus are the PI3K, mitogen-activated protein kinase, and Src family kinase (SFK) signaling pathways (22), all of which have been shown to increase NMDAR function. In this study, we focus on SFK signaling, as it is required for leptin’s effects on the NMDARs (22). The 5 SFK family members that are expressed in the mammalian CNS are Src, Fyn, Yes, Lck, and Lyn (35), but it is not known which family member is involved in leptin-mediated enhancement of NMDARs in the hippocampus. The NR2B subunit of NMDARs has multiple posttranslational modulatory sites (28), with phosphorylation of the Y1472 residue by Fyn, maintaining the receptor in the synapse and preventing its translocation to extrasynaptic sites and endocytosis (36). NR2BY1472 phosphorylation by Fyn is also proposed to mediate memory formation, with fyn-deficient mice and NR2BY1472F-mutant mice showing decreased ability to form contextual and auditory fear memories (37,38).
Here we show for the first time that leptin stimulation increases the levels of surface localized NR2B-containing NMDARs. The mechanism of this enhancement includes activation of Fyn, which in turn phosphorylates the NR2BY1472 residue, promoting the maintenance of the receptor on the cell surface. Targeted inhibition of Fyn or the removal of this phosphorylation site with the NR2BY1472F mutant blocks leptin-stimulated synaptogenesis, suggesting a critical role of synaptic NR2B in leptin-stimulated synaptogenesis. We also show that NR2B and Fyn occur in a complex with LepRb and that overexpression of Fyn is sufficient to increase glutamatergic synaptogenesis, while knockdown of Fyn attenuates leptin-stimulated synapse formation. Thus, this study identifies a novel mechanism by which leptin increases surface expression of NMDARs to promote synaptogenesis in the hippocampus and increases our understanding of the LepRb signaling complex.
Materials and Methods
Drugs and DNA constructs
Full-length rat-recombinant leptin (50 nM, Peprotech) was used as described in the text and figure legends. The shRNA targeting the Fyn sequence: 5'-GCACGACAAGCTGGTGCAG-3' was cloned into the pLKO.3G vector between the EcoRI and Pac1 restriction sites. The constructs expressing shLepRb was used as previously described (20). NR2B constructs were subcloned from constructs that were a kind gift from the Barria Lab (University of Washington) (33) into pCAGGS-expressing vectors containing the designated tag using Gateway Cloning (ThermoFisher). LepRb–BioID constructs were used as previously described (39). Other constructs expressing tagged proteins were constructed by amplifying Fyn from rat complementary deoxyribonucleic acid (cDNA) and cloned into pCAGGS destination vectors containing the designated tag using Gateway Cloning. The NR2BY1472F and FynK299M mutants were constructed by mutating the respective wild-type entry vectors with NEBaseChanger and then cloned into the designated pCAGGS destination vector. NR2BΔC and LepRΔC were constructed by amplifying NR2B and LepRb without the carboxyl-terminal cytoplasmic tail domain (NR2B residues 1–838, LepRb residues 1–860) from the respective wild-type entry vectors and cloned into pCAGGS destination vectors. BioID destination vectors were constructed by subcloning myc-BioID (Addgene) into pCAGGS destination vectors using Gibson cloning (New England Biolabs). All supplementary material and figures are located in a digital research materials repository (40).
Cell culture
Procedures with animals used for hippocampal cultures were carried out in compliance with the Washington State University Institutional Animal Care and Use Committee approved protocols 03717-019 and 04409-006. Hippocampal neuronal cultures were prepared as previously described in Dhar et al (20). Hippocampal neurons used for immunostaining and fluorescent intensity imaging were transfected with various constructs on days in vitro (DIV) 6 DIV6 and then on DIV7 to 8 treated with leptin, fixed (4% paraformaldehyde in PHEMS buffer [60 mM PIPES, 25 mM HEPES, 1 mM MgCl2, 5 mM EGTA], 87.6 mM sucrose, pH 7.4) for 20 minutes at room temperature, and then immunostained and mounted on glass slides using Elvanol. Hippocampal neurons used for dendritic spine analysis, vGlut1 immunostaining, and electrophysiological analysis were transfected with various constructs on DIV6; treated with leptin (50 nM) on DIV8; and on DIV11 to 12, cells were either fixed, immunostained, and mounted as previously stated or used for electrophysiological recordings. All control conditions received the same amount of media at the time of reagent stimulation.
HEK293T cells (5 × 104 cells/cm2 for 6-well plates used for biochemistry experiments) were maintained in DMEM/high glucose (HyClone) supplemented with 10% fetal bovine serum (Atlanta Biologicals) and 1% penicillin/streptomycin (Gibco). HEK293T cells were transfected with various constructs 24 hours after plating, treated 24 to 48 hours after transfection with leptin (50 nM) in Opti-Mem (Gibco), and collected at designated time points. All control conditions received the same amount of media at the time of reagent stimulation.
Transfection
Primary hippocampal cultures were transfected with Lipofectamine 2000 (Life Technologies). Native media were collected before transfection and replaced with warm growth media. Lipofectamine 2000 and experimental DNA plasmids (0.5 µg/well for 24-well plates, and 2 µg/well for 6-well plates) were added to cells and incubated for 30 minutes. The media were then aspirated and replaced with native media. This protocol produces a transfection efficiency of only 3% to 5% of total neurons transfected.
HEK293T cells were transfected with Lipofectamine 2000 and experimental DNA plasmids (2 µg/well for 6-well plates). The mixture was added to the native media and allowed to incubate until cells were collected. This protocol produces a much higher transfection efficiency of approximately 80% of total cells transfected.
Spine quantification
Hippocampal cultures were transfected with designated DNA constructs and Clover-βactin to allow for visualization of dendritic spine density and morphology. Confocal fluorescent images were obtained using Metamorph software and a Leica DMI6000 SD confocal microscope equipped with a Yokogawa CSU-X1 spinning disk, Hamamatsu-R2 CCD camera, and a 63 × oil immersion lens (NA: 1.4). Dendritic spine density and classification were measured as previously described (20). In summary, 2 to 3 dendritic segments were counted from a minimum of 15 neurons from 3 separate independent culture preparations for each condition.
Immunocytochemistry
Transfected neurons were treated and fixed as described above. After fixation, cells were washed in phosphate buffer solution (PBS) and permeabilized with 0.1% Triton X-100 detergent (Bio-Rad Laboratories), blocked with 8% bovine serum albumin for 1 hour, incubated for 24 hours at 4°C with primary antibodies against antivesicular glutamate transporter 1 (vGlut1) (1:250, Neuromab) (41), incubated for 1 hour at room temperature with the appropriate Alexa Fluor secondary IgG antibody (Life Technologies) (42), and mounted with Elvanol. Confocal images for vGlut1 and dendritic spine juxtaposition were obtained as described earlier in the Spine quantification section. The percentage juxtaposition was manually measured from 50 to 75 spines on 10 different neurons from 3 separate cultures per condition using ImageJ 1.48. For surface staining of EGFP-NR2B, the permeabilization step was omitted, and the cells were incubated with the primary antibody against EGFP (1:250, Rockland) (43) for 1 hour at room temperature. Wide-field images of EGFP-NR2B surface staining were obtained using Slidebook 5.5 Digital Microscopy Software and an Olympus IX81 inverted microscope equipped with a Hamamatsu ORCA-ER CCD camera, and a 100 × oil immersion lens (numerical aperture: 1.4). The integrated staining density of EGFP-NR2B was measured in the entire neuron in frame from a minimum of 15 neurons from 3 separate cultures per condition using ImageJ 1.48. Background was calculated using secondary antibody incubation only and subtracted from images. Antibodies are located in the supplemental digital repository (40).
Colocalization
For NR2B/LepRb colocalization, immunostaining of surface NR2B and Flag-LepRb was performed on neurons transfected with Flag-LepRb and Clover and treated with leptin (50 nM, 2 hours). Neurons fixed and incubated with an anti-NR2B (1:100, Alomone Labs) (44) and anti-Flag (1:250, Sigma Aldrich) (45) antibody for 1 hour and then incubated with the appropriate Alexa Fluor secondary IgG antibody (42) for 1 hour at room temperature. Confocal images were acquired as described earlier in the Spine quantification section, except images were collected with an Andor iXon3 EMCCD camera and a 100 × oil immersion objective (numerical aperture: 1.47). Images were analyzed for surface NR2B/Flag-LepRb colocalization using Metamorph software. To correct for random colocalization, LepRb images were rotated 90° and colocalization with NR2B was compared to nonrotated images.
Western blotting
Protein samples were collected by lysing cells or tissue with radioimmunoprecipitation assay (RIPA) buffer (Santa Cruz Biotechnology) supplemented with phosphatase inhibitor cocktail 2 and 3 (Sigma Aldrich), protease inhibitor cocktail, PMSF (2 mM), and sodium orthovanadate (1 mM, Santa Cruz Biotechnology) and centrifuged at 16000 × g. Samples were prepared with NuPage LDS Sample Buffer (Life Technologies) and 50 mM DTT (Fisher Scientific) and heated at 75°C for 10 minutes. Equal volumes were then loaded into Bolt 4% to 12% Bis-Tris gels (Life Technologies). Proteins were transferred to a PVDF membrane (Life Technologies) overnight, blocked with 5% bovine serum albumin for phosphorylated proteins or 5% milk for nonphosphorylated proteins, incubated with primary antibodies against NR2B (1:1000, Cell Signaling) (46), NR2B phospho-Y1472 (1:1000, Sigma Aldrich) (47), Fyn (1:1000, Cell Signaling) (48), phospho-Src family Y416 (1:500, Cell Signaling) (49), MAP2B (1:1000, Sigma Aldrich) (50), c-Myc (1:1000, Sigma Aldrich) (51), Erk2 (1:1000, Santa Cruz Biotechnology) (52), or V5 (1:1000, Cell Signaling) (53) for 2 hours at room temperature and then incubated with the appropriate Alexa Fluor-647 secondary IgG F(ab')2 fragment antibody (Cell Signaling) (42) for 1 hour at room temperature. Blots were imaged using a Chemidoc MP imaging system (Bio Rad) and analyzed using the ImageJ 1.48 gel analyzer tool.
Immunoprecipitation
Protein samples were collected as described in the Western blotting section protocol from cells transfected with the designated constructs. Equal amounts of sample were incubated with 2 µL anti-Fyn antibody (Biolegend) (54) or myc-tag mouse monoclonal antibody conjugated to magnetic beads (Cell Signaling) and rotated overnight at 4°C. Fyn IP samples were then rotated with protein G magnetic beads (GenScript) for an additional 2 hours at room temperature. Samples were then washed 3 times with PBS, eluted in NuPage LDS Sample Buffer and 50 mM DTT, heated at 75°C for 10 minutes, and then loaded into Bolt 4% to 12% Bis-Tris gels. Samples were probed in Western blot analysis as previously described.
BioID assay
HEK293T cells were transfected with a V5-tagged target protein and a BioID-tagged labeling protein. Biotin (50 µM, Amresco) was added to the media at time of transfection to allow the BioID-tagged labeling protein to attach biotin to any proteins in close proximity. Protein samples were collected after 24 hours as described in the Western blotting section protocol, and equal volumes of sample were incubated with 2 µL anti-V5 antibody (Cell Signaling) (53) and rotated overnight at 4°C, after which Magne Protein G beads (Promega) were added for an additional 2 hours at room temperature to immunoprecipitate the V5-tagged target protein. Samples were washed in PBS, eluted in NuPage LDS Sample Buffer and 50 mM DTT, and probed in Western blot analysis. Biotinylated proteins were stained with streptavidin conjugated to Alexa Fluor 647 (Life Technologies) (55) and normalized to the total amount of V5-tagged protein immunoprecipitated.
Surface biotinylation
Hippocampal cultures were treated with leptin as previously described. Neurons were washed with Buffer A (1 × PBS, 1 mM CaCl2, 0.5 mM MgCl2) and sulfo-NHS-biotin (1 mg/mL in Buffer A, ThermoFisher) was added to cells and incubated on ice for 30 minutes. The reaction was quenched with Buffer B (Buffer A supplemented with 1 mM glycine), and total protein was collected. Biotinylated proteins were affinity purified by addition of streptavidin magnetic beads (Genscript), rotated overnight at 4°C, washed, eluted, and analyzed as described in the Immunoprecipitation section protocol.
Whole-cell recordings
Performed as previously described in Dhar et al (20), on DIV11 to 12 hippocampal cultures transfected with Clover-βactin and designated constructs.
Statistical analysis
All data is expressed as the mean ± standard error of the mean (SEM). Multiple comparisons were analyzed using one-way analysis of variance with post hoc Tukey analysis, and single comparisons were analyzed using a two-tailed, unpaired Student t test. Statistical significance was set to a minimum of P < 0.05.
Results
Leptin signaling increases NR2B surface localization
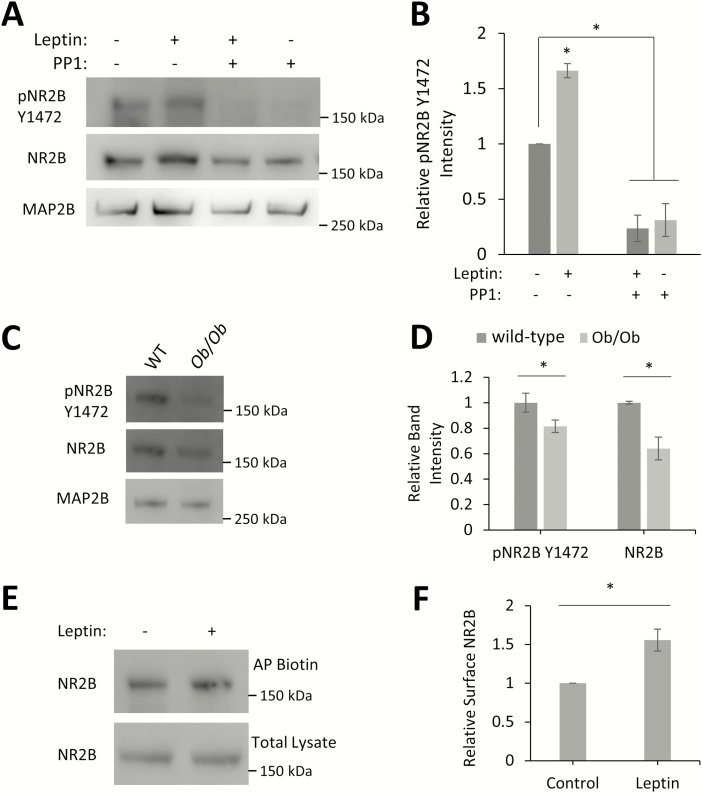
Activation of LepRb by leptin stimulates the formation of new excitatory glutamatergic synapses in hippocampal pyramidal neurons (19–21). While it is known that leptin enhances synaptic plasticity through the enhancement of NMDARs (22,24), the exact mechanism of this enhancement is not yet known. It has been hypothesized that leptin stimulation enhances the surface expression of NMDARs (56). Phosphorylation of the NR2BY1472 residue promotes the maintenance of the receptor in the synapse and prevents its endocytosis (36), so we tested the role of leptin signaling in the phosphorylation of this important NR2B residue and the regulation of NR2B surface expression by leptin and LepRb. Stimulation of cultured hippocampal neurons with leptin for 2 hours enhances (159% ± 13%, P < 0.05) phosphorylation of the NR2BY1472 residue (Fig. 1A, B). As this residue has been shown to be phosphorylated by the SFK family member Fyn, we examined the effect of pretreatment with PP1, a specific SFK inhibitor. PP1 not only blocks leptin’s ability to increase the phosphorylation of NR2BY1472 but also lowers the basal levels of phosphorylation (PP1: 31% ± 15%; PP1 + leptin: 24% ± 12%, P < 0.05) (Fig. 1A, B). To determine whether this effect occurs in vivo, we analyzed the phosphorylation of NR2B in hippocampal protein extracts from postnatal day 10 wild-type and ob/ob mice (lacking leptin) by Western blot. Postnatal day 10 hippocampi were chosen as this time point represents the peak of the postnatal leptin surge observed in mice (14). Mice lacking endogenous leptin (ob/ob) have decreased levels of NR2BY1472 phosphorylation as well as total expression of NR2B compared to wild-type mice (pNR2BY1472: 81% ± 5%, P = 0.044; NR2B: 64% ± 9%, P < 0.05) (Fig. 1C, D). A smaller decrease in the levels of NR2BY1472 phosphorylation compared with total NR2B expression is not surprising because nonphosphorylated NR2B is endocytosed and degraded (36).
Figure 1.
Leptin signaling increases pNR2BY1472 levels and surface expression. (A) Representative Western blot of hippocampal neurons treated with leptin (50 nM), PP1 (10 µM), or both for 2 hours. (B) Quantification of pNR2BY1472 intensity normalized to total NR2B intensity (n = 3). (C) Representative Western blot of hippocampal protein extracts from P10 wild-type and ob/ob mice pups (wild-type: n = 5; ob/ob: n = 5). (D) Quantification of pNR2BY1472 intensity normalized to total NR2B intensity and total NR2B intensity normalized to the neuronal marker MAP2B intensity (n = 3). (E) Representative Western blot of surface biotinylated hippocampal cultures treated with leptin (50 nM, 2 hours). Biotinylated proteins were affinity purified (AP) with streptavidin magnetic beads. (F) Quantification of biotinylated NR2B intensity normalized to NR2B intensity in total lysate (n = 3). All experiments were repeated in 3 independent culture preparations and expressed as the mean ± SEM, *P < 0.05, compared to control or wild-type.
Abbreviations: AP, affinity purify; Ob/Ob, obese/obese; pNR2B, phospho NR2B; WT, wild-type; Y1472, tyrosine 1472.
We next determined the effect of leptin on NR2B surface localization. Dissociated hippocampal neurons were stimulated with leptin and subjected to surface biotinylation. Biotinylated proteins were affinity purified and showed an increase (175% ± 14%, P < 0.05) in the level of surface localized NR2B with leptin stimulation (Fig. 1E, F). Taken together, these data suggest that leptin stimulation leads to increased phosphorylation of the NR2BY1472 residue, which correlates with increased surface localization of the receptor.
Leptin enhances Fyn activity
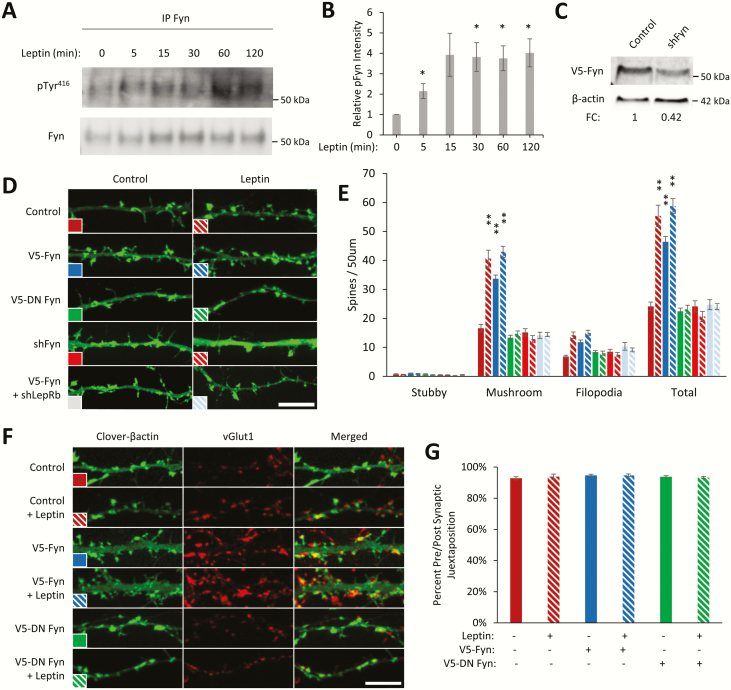
Fyn is the SFK family kinase that specifically targets and phosphorylates the NR2BY1472 residue (36). Leptin has been shown to increase levels of the activated form of Fyn in the thymus (57), but it is not known if leptin activates Fyn in the hippocampus. To determine this, we stimulated dissociated hippocampal cultures with leptin and measured the levels of the activated form of endogenous Fyn following immunoprecipitation. Leptin increases the level of FynY416 phosphorylation, the active form of Fyn (58), within 5 minutes, which persists for >2 hours (5 minutes: 214% ± 37%, P < 0.05; 15 minutes: 392% ± 106%; 30 minutes: 382% ± 71%, P < 0.05; 60 minutes: 375% ± 61%, P < 0.05; 120 minutes: 402% ± 69%, P < 0.05) (Fig. 2A, B). While there was an apparent increase of FynY416 phosphorylation at all time points, the increase was not significant at the 15-minute time point due to high variability between samples. These data suggest that leptin stimulation can enhance Fyn activity.
Figure 2.
Leptin signaling activates Fyn, which is required for synaptogenesis. (A) Representative Western blot of immunoprecipitated Fyn from hippocampal cultures treated with leptin (50 nM). (B) Quantification of pTyr416 intensity normalized to total immunoprecipitated Fyn (n = 3). (C) Representative Western blot of HEK293T cells transfected with V5-Fyn ± shFyn. (D-G) DIV6 hippocampal neurons were transfected with a fluorescent Clover-βactin and the indicated constructs. Neurons were stimulated with leptin (50 nM) on DIV8 and fixed on DIV11 to 12 for spine density or pre/post synaptic juxtaposition experiments. (D) Representative fluorescent images of dendrite segments. Dendritic spine density was measured by hand using ImageJ with the NeuronJ plugin. (E) Quantification of dendritic spine density from a minimum of 2 to 3 dendritic segments from 15 neurons. (F) Representative fluorescent images of hippocampal cultures immunostained for the presynaptic marker vGlut1. (G) Quantification of juxtaposition of dendritic spines and vGlut1 puncta averaged from 30 to 80 spines from 10 neurons. All experiments were repeated in 3 independent culture preparations and expressed as the mean ± SEM, *P < 0.05, **P < 0.01, compared to control or wild-type. White bar = 5µm.
Abbreviations: DN, dominant negative; IP, immunoprecipitate; min, minutes; pTyr416, phosphotyrosine 416.
We have previously shown that leptin stimulates synaptogenesis in hippocampal neurons. While other kinases have been shown to be important (20,21,34), the role of Fyn is not known. To determine the role of Fyn in glutamatergic synaptogenesis, we transfected cultured hippocampal neurons with Fyn or inhibited Fyn with either a dominant negative version (FynK299M [dominant negative (DN)-Fyn]) or shRNA knockdown of Fyn (shFyn) and then examined the effect on synaptic development (59). Overexpression of Fyn in hippocampal cultures is sufficient to enhance mature mushroom spines and immature filopodia dendritic spine density, which is inhibited with shRNA knockdown of LepRb. Conversely, expression of DN-Fyn or shFyn does not alter dendritic spine density in the basal condition, but blocks leptin-induced dendritic spine formation (mushroom spine density ± SEM; control: 16.6 ± 1.3; control + leptin: 40.6 ± 2.9, P < 0.01; V5-Fyn: 33.7 ± 1.3, P < 0.01; V5-Fyn + leptin: 43 ± 1.9, P < 0.01; V5-DN Fyn: 13.4 ± 0.8; V5-DN Fyn + leptin: 14.7 ± 0.9; shFyn: 15.2 ± 1.3; shFyn + leptin: 12.8 ± 1.2; V5-Fyn + shLepRb: 14.1 ± 1.1; V5-Fyn + shLepRb + leptin: 14.4 ± 0.7) (Fig. 2D, E). To determine if Fyn induces an increase in morphological synapses (ie, contains both pre- and postsynaptic specializations), we measured the percentage of dendritic spines that are juxtaposed to the presynaptic marker vGlut1 (morphological synapses) (Fig. 2F, G). Under all conditions tested the same level of pre/postsynaptic juxtaposition occur (≈ 95% occurrence), suggesting that the dendritic spines induced by expression of Fyn form part of a morphological synapse.
NR2BY1472 phosphorylation is necessary for leptin-stimulated synapse formation
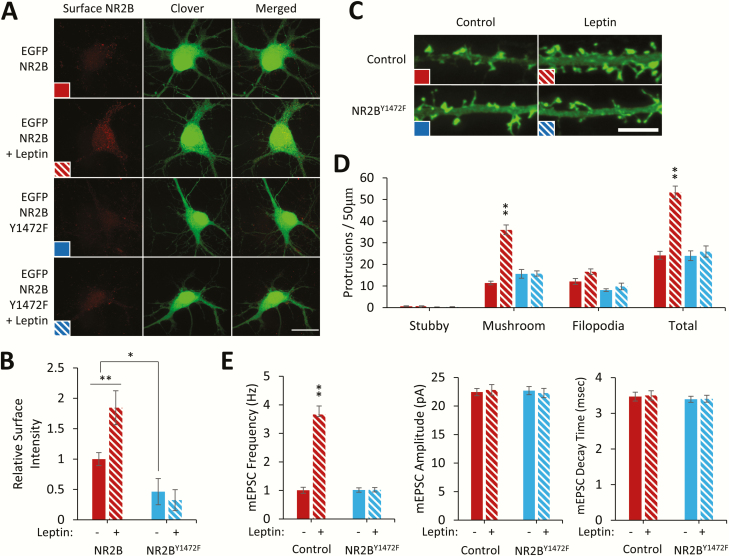
We next determined the requirement of NR2BY1472 phosphorylation for leptin-stimulated spinogenesis by expressing a phosphorylation-deficient mutant of NR2B, (NR2BY1472F) tagged extracellularly with EGFP (EGFP-NR2B-V5 and EGFP-NR2BY1472F-V5). Live staining of surface-expressed receptors shows a decrease in NR2BY1472F surface expression (46% ± 32%, P < 0.05), suggesting that the Y1472 site is critical for NR2B surface localization (Fig. 3A, B). Leptin stimulation also increases the surface localization of wild-type NR2B (184% ± 30%, P < 0.01) but not NR2BY1472F. Furthermore, cultured hippocampal neurons transfected with NR2BY1472F completely inhibit leptin’s ability to stimulate dendritic spine formation (mushroom spine density ± SEM; control: 11.4 ± 0.9; control + leptin: 36 ± 2.2, P < 0.01; NR2BY1472F: 15.6 ± 2; NR2BY1472F + leptin: 15.8 ± 1.2) (Fig. 3C, D). This finding was corroborated in electrophysiological recordings of miniature excitatory postsynaptic currents (mEPSC’s) measuring the ability of leptin to induce the formation of functional synapses (mEPSC frequency (Hz) ± SEM; control: 1.04 ± 0.11, n = 32; control + leptin: 3.66 ± 0.32, P < 0.001, n = 34; NR2BY1472: 1.01 ± 0.08, NS, n = 33; NR2BY1472 + leptin: 1.06 ± 0.08, NS, n = 33)(Fig. 3E), supporting the hypothesis that phosphorylation of NR2BY1472 is essential for leptin-stimulated synapse formation.
Figure 3.
pNR2BY1472 is necessary for leptin-stimulated spine formation. (A) Representative fluorescent images of hippocampal neurons expressing Clover and EGFP-NR2B-V5 or EGFP-NR2BY1472F-V5 ± leptin stimulation (50 nM, 2 hours) and live immunostained for surface EGFP-NR2B. White bar = 20 µm. (B) Quantification of immunostained EGFP-integrated signal density (n = 23). (C-E) Hippocampal neurons that were transfected with a fluorescent Clover-βactin and EGFP-NR2BY1472F-V5. Neurons were stimulated with leptin (50 nM) on DIV8, and on DIV11 to 12 spine density was measured by hand using ImageJ with the NeuronJ plugin (C,D), or electrophysiological recordings were performed (E). White bar = 5 µm. (D) Quantification of dendritic spine density from a minimum of 2 to 3 dendritic segments from 15 neurons. (E) Quantification of mEPSC frequency, amplitude, and decay time normalized to control condition (control: n = 32; control + leptin: n = 34; NR2BY1472F: n = 33; NR2BY1472F + leptin: n = 33). All experiments were repeated in 3 independent culture preparations and expressed as the mean ± SEM, *P < 0.05, **P < 0.01 compared to control.
Abbreviations: mEPSC, miniature excitatory postsynaptic current; Y1472F, tyrosine 1472 phenylalanine mutant.
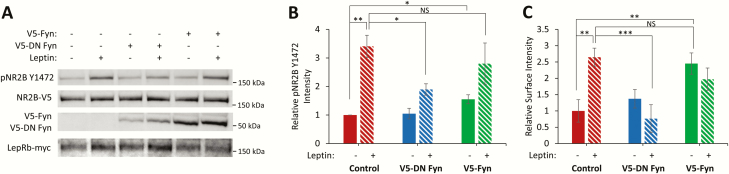
Furthermore, to determine the role of Fyn in leptin-stimulated NR2BY1472 phosphorylation and consequent surface expression, HEK239T cells were transfected with NR2B-V5, NR1, and either wild-type V5-Fyn or V5-DN Fyn. Leptin stimulation and overexpression of wild-type V5-Fyn alone in these cells increase NR2BY1472 phosphorylation, which is blocked with expression of V5-DN Fyn (control + leptin: 341% ± 39%, P < 0.01; V5-DN Fyn: 105% ± 19%; V5-DN Fyn + leptin: 190% ± 20%; V5-Fyn: 155% ± 16%, P < 0.05; V5-Fyn + leptin: 280% ± 72%)(Fig. 4A). This is recapitulated in NR2B surface expression in cultured hippocampal neurons (control + leptin: 265% ± 27%, P < 0.01; V5-DN Fyn: 137% ± 28%; V5-DN Fyn + leptin: 76% ± 42%; V5-Fyn: 245% ± 33%, P < 0.01; V5-Fyn + leptin: 197% ± 34%)((Fig. 4C). These data further support the hypothesis that leptin-induced NR2BY1472 phosphorylation and surface expression is Fyn dependent.
Figure 4.
Leptin-regulated NR2BY1472 phosphorylation and surface expression is Fyn dependent. (A) Representative Western blot of HEK293T cells transfected with NR2B-V5, NR1, LepRb-myc, and either V5-Fyn or V5-DN Fyn and treated with leptin (50 nM, 2 hours). (B) Quantification of pNR2BY1472 intensity normalized to total NR2B-V5 intensity (n = 3). (C) Hippocampal neurons were transfected with Clover and EGFP-NR2B-V5 and either V5-Fyn or V5-DN Fyn ± leptin stimulation (50 nM, 2 hours) and live immunostained for surface EGFP–NR2B. Quantification of immunostained EGFP-integrated signal density (n = 15). All experiments were repeated in 3 independent culture preparations and expressed as the mean ± SEM, *P < 0.05, **P < 0.01.
Abbreviations: DN, dominant negative; pNR2B, phospho NR2B; Y1472, tyrosine 1472.
LepRb is in a complex with NR2B and Fyn
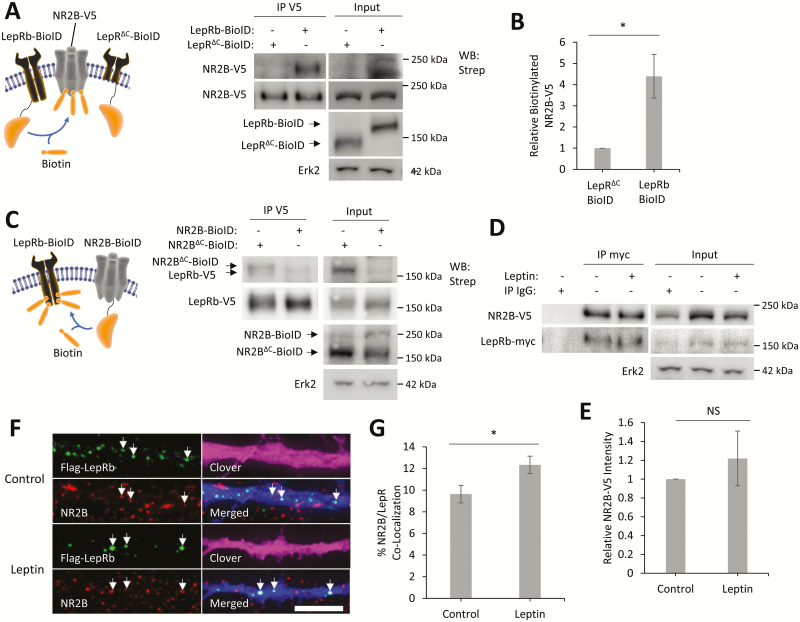
The function of NMDARs is affected by both subunit composition and protein–protein interactions (28,60). To determine if LepRb directly interacts with NR2B and Fyn, we used a variety of different biochemical methods including coimmunoprecipitation and the BioID method (61). To determine if NR2B is in close proximity to LepRb, we used the BioID method (Fig. 5A–C). This method uses a construct that fuses the promiscuous biotin ligase myc-BirA* (which will be further listed as BioID) to a protein of interest, which can then add biotin to any proteins that are in close proximity to the BioID-tagged protein (≤ 20 nm labeling radius) (62). We first expressed a LepRb–BioID fusion protein or a truncated form of LepRb (LepRΔC–BioID), which only includes the extracellular and transmembrane domain of LepRb (residues 1–860), to normalize for nonspecific biotinylation. In the presence of biotin, LepRb–BioID will covalently attach biotin to proteins that are either in a complex with LepRb–BioID or in close proximity. Since LepRΔC–BioID has no intracellular domain to form complexes with other proteins, this construct was used to normalize for nonspecific biotinylation by the BioID tag. To determine if NR2B is in a complex with LepRb, we coexpressed LepRb–BioID and NR2B-V5 in HEK293T cells and incubated them for 24 hours in media supplemented with biotin. To measure the ability of LepRb–BioID to biotinylation NR2B-V5, we immunoprecipitated NR2B-V5 and measured its biotinylation levels (Fig. 5A). LepRb–BioID robustly biotinylates NR2B-V5 (4.4 ± 1-fold over LepRΔC–BioID, P < 0.05). To further validate the interaction between LepRb and NR2B, we expressed BioID constructs on the reciprocal side of the interaction; NR2B–BioID and LepRb-V5. To normalize for nonspecific biotinylation we used a truncated NR2B (NR2BΔC–BioID) that contained the entire subunit minus the cytoplasmic carboxyl-terminal tail (residues 1–838). Interestingly, both NR2B–BioID and NR2BΔC–BioID biotinylate LepRb-V5 (Fig. 5C). As NR2BΔC–BioID is very close to the same size as LepRb-V5 and co-immunopreciptates (co-IP) with LepRb-V5, the difference between LepRb-V5 biotinylation levels could not be calculated.
Figure 5.
LepRb directly interacts with NR2B. (A) Schematic of LepRb–BioID experiment with representative Western blot of NR2B-V5 immunoprecipitated from HEK293T cells expressing the designated BioID constructs and NR2B-V5 and NR1-Clover to the right. (B) Quantification of IP biotinylated NR2B-V5 intensity normalized to total NR2B-V5 intensity in the same lane (n = 3). (C) Schematic of NR2B–BioID experiment with representative Western blot of LepRb-V5 immunoprecipitated from HEK293T cells expressing designated BioID constructs and LepRb-V5 and NR1-Clover. (D) Representative Western blot of LepRb-myc immunoprecipitated from HEK293T cells stimulated with leptin (50 nM, 2 hours) and expressing LepRb-myc, NR2B-V5, and NR1-Clover. (E) Quantification of coimmunoprecipitated NR2B-V5 intensity normalized to immunoprecipitated LepRb-myc intensity from the same lane (n = 3). (F) Representative fluorescent images of hippocampal cultures expressing Flag-LepRb and Clover. Surface Flag-LepRb and endogenous surface NR2B were live immunostained after stimulation with leptin (50 nM, 2 hours). (G) Quantification of NR2B/Flag-LepRb puncta colocalization compared to total NR2B puncta. Colocalization experiments were repeated in 2 independent hippocampal culture preparations. All BioID experiments were stimulated with biotin (50 µM) at the time of transfection. All experiments were repeated in 3 independent culture preparations and expressed as the mean ± SEM, *P < 0.05 compared to control.
Abbreviations: IP, immunoprecipitate; NS, not significant; Strep, streptavidin; WB, western blot.
To further test the interaction of NR2B with LepRb, we transiently transfect HEK293T cells with a myc-tagged LepRb (LepRb-myc) and NR2B-V5. LepRb-myc was immunoprecipitated along with any proteins in a complex with the receptor. We show that NR2B-V5 co-IP with LepRb-myc (Fig. 5D), further supporting the hypothesis that these proteins can form a complex. Leptin stimulation (2hrs) does not affect the levels of NR2B that co-IP with LepRb (Fig. 5D, E).
Lastly, we asked if surface LepRb and surface NR2B colocalize with each other in hippocampal neurons. We transiently transfected hippocampal neurons with an LepRb that expressed an extracellular Flag tag (Flag-LepRb) (Fig. 5F). These cultures were live-immunostained for surface Flag-LepRb and surface NR2B and colocalization was measured following imaging. NR2B colocalization with LepRb occurs in 9.6% [0.8%] of total surfaced-expressed NR2B receptors, which was enhanced to 12.3% [0.8%] (P < 0.05) with leptin stimulation (Fig. 5G). This further supports the idea that LepRb directly interacts with NR2B in hippocampal neurons. This data also suggest that clearly not all LepRb or NR2B are complexed together under unstimulated conditions; however, leptin significantly increases the complex formation by 25% to 30%.
We performed similar BioID and IP experiments to determine the interaction of Fyn with LepRb (Fig. 6). While LepRb–BioID did not label V5-Fyn in HEK293T cells, BioID-Fyn robustly biotinylates LepRb-V5 (6.8 ± 0.8-fold over soluble BirA*, P < 0.01). V5-Fyn also co-IPs with LepRb-myc, an effect enhanced (231% ± 23%, P < 0.01) by leptin stimulation (2 hours) (Fig. 6D, E). These data support the idea that LepRb creates a signalasome with NR2B and Fyn in hippocampal neurons through direct interaction.
Figure 6.
LepRb directly interacts with Fyn. (A) Schematic of LepRb–BioID experiment with representative Western blot of V5-Fyn immunoprecipitated from HEK293T cells expressing the designated BioID constructs and V5-Fyn. (B) Schematic of BioID-Fyn experiment with representative Western blot of LepRb-V5 immunoprecipitated from HEK293T cells expressing LepRb-V5 and either BioID-Fyn or a soluble BioID (BirA*). (C) Quantification of IP biotinylated LepRb-V5 intensity normalized to total LepRb-V5 intensity in the same lane (n = 3). (D) Representative Western blot of LepRb-myc immunoprecipitated from HEK293T cells stimulated with leptin (50 nM, 2 hours) and expressing LepRb-myc and V5-Fyn. (E) Quantification of coimmunoprecipitated V5-Fyn intensity normalized to immunoprecipitated LepRb-myc intensity from the same lane (n = 3). All BioID experiments were stimulated with biotin (50 µM) at the time of transfection. All experiments were repeated in 3 independent culture preparations and expressed as the mean ± SEM, **P < 0.01 compared to control, Abbreviations: IP, immunoprecipitate; Strep, streptavidin; WB, western blot.
Discussion
Leptin, an adipocytokine, is best characterized in the adult as a regulator of energy homeostasis and feeding behavior (6,63). However, during perinatal development and into the adult, leptin has neurotrophic actions in the CNS and is essential for proper development of multiple brain regions (64). Leptin regulates dendritic spine and glutamatergic synapse formation in brain regions such as the hypothalamus and the CA1/CA3 regions and dentate gyrus of the hippocampus (18,19–21). It also regulates NMDAR function and trafficking, which play an important role in synapse formation, function, and plasticity (22,26). However, the mechanism by which leptin regulates NMDAR was not well understood. Here, we show for the first time that leptin stimulation increases the surface expression of NR2B-containing NMDARs through the activation of the SFK, Fyn. Leptin enhances Fyn activity, which leads to the phosphorylation of NR2BY1472, maintaining the receptor at the cell surface. We also provide evidence that NR2B and Fyn are in a complex with LepRb and that their interactions are dynamic and essential for leptin-stimulated synaptogenesis.
Leptin signaling increases Fyn activation
SFKs play important roles in cellular processes such as cell growth, differentiation, survival, and synaptic function (35). Fyn specifically plays an important role in synapse formation and synaptic plasticity (65,66). Fyn-deficient mice show impairment in long-term potentiation and decreased dendritic spine density and decreased ability to form contextual fear memories (37,38,67–70). The activation of Fyn is important for its synaptic role, with the dephosphorylation of the negative regulatory residue, Y527, allowing autophosphorylation of the positive regulatory residue, Y416, enhancing kinase activity (58). Leptin was previously shown to increase Fyn activity in the thymus of rodents. Moreover, Fyn has been shown to enhance activation of STAT3 by leptin signaling in the thymus (57,71). Here we show for the first time that leptin enhances Fyn-Y416 phosphorylation in hippocampal neurons. We also show that enhanced expression of Fyn in hippocampal cultures increases dendritic spine density, while expression of a dominant negative Fyn inhibits leptin-stimulated synaptogenesis. This suggests that Fyn is not only required for leptin-induced synaptogenesis but is sufficient to increase synaptogenesis in hippocampal neurons.
Leptin signaling increases NR2B surface localization
Leptin has been shown to enhance NMDAR-mediated long-term potentiation and long-term depression (22,72). It also has been shown to increase Ca2+ influx through NMDARs in hippocampal neurons, an effect that required SFKs (22). Here we show that leptin also increases the surface expression of NR2B-containing NMDARs in hippocampal cultures, again through an SFK. We have also identified that SKF as Fyn.
NR2B-containing NMDARs are critical for normal glutamatergic synapse development (33,73,74). Here we show that phosphorylation of the Y1472 phosphorylation site is also required for leptin-stimulated dendritic spine formation; leptin fails to induce synaptogenesis when an NR2B mutant lacking this tyrosine is expressed. This suggests that not only the expression of NR2B-containing receptors are important, but the phosphorylation of NR2BY1472 by Fyn is also essential for leptin-mediated glutamatergic synapse formation in hippocampal neurons.
Leptin levels have been shown to rise rapidly postnatal day 7 to 14, which is a period of rapid postnatal synaptogenesis in the hippocampus (14,75). Furthermore, we have previously shown that mice lacking leptin receptors have fewer dendritic spines as adults, suggesting that leptin promotes the formation of spines in vivo. This new study suggests that one pathway through which leptin promotes the formation of synapses is through activation of Fyn to enhance NR2B-receptor localization to promote the formation of synapses. As leptin levels fall and synapses mature, NR2BY1472 phosphorylation decreases, and NR2B-containing NMDARs are removed from the synapse and are replaced by NR2A-containing receptors. This developmental switch between synaptic NR2B and NR2A receptors has been characterized, but the underlying mechanism(s) are not well understood (32,76–78). Given the coincidence in timing between the drop in leptin levels at the end of the leptin surge and the switch from NR2B to NR2A, it would be intriguing to examine the role of leptin in the switch in future studies.
Physiological implication of the contribution of the LepRb/NR2B/Fyn complex to the development of synapses in the hippocampus
Many NR2B-binding partners affect both the function and localization of NR2B-containing receptors. The binding of NR2B to PSD-95 promotes its synaptic localization, while binding of AP-2 to NR2B promotes its translocation from synaptic to extrasynaptic sites and eventual endocytosis (36,79). Here we show for the first time that LepRb and NR2B form a complex, which provides a mechanism by which leptin could enhance NMDAR function by bringing active Fyn into close proximity to NR2B. The finding that the NR2BΔC–BioID construct also biotinylated LepRb may indicate that LepRb interacts with NR2B domains other than the cytoplasmic carboxyl-tail or through interaction with the NR1 subunit. In contrast, the lack of the ability of LepRb–BioID to label Fyn may be due to Fyn being localized in close proximity to LepRb but possibly in an orientation that does not allow it to come into contact with the LepRb carboxyl-terminus BioID tag. This LepRb/NR2B/Fyn complex could be one mechanism by which leptin potentiates NMDAR currents to alter synaptic plasticity, which could provide a way for leptin to promote learning and memory and other hippocampus-dependent behaviors.
Multiple studies over the past decade have shown that leptin signaling has procognitive affects to enhance learning and memory (80) as well as antidepressive and anxiolytic effects (11,27). Peripheral and intrahippocampal leptin injections have positive effects on memory tasks, which are dose dependent (24,81). The actions of leptin in the CNS requires the expression of LepRb, whose expression has been observed in the hippocampus (16,82). Obese db/db mice, which lack functional LepRb receptors, show impairments in spatial learning tasks (83). This correlates well with human studies, as diet-induced obesity has been shown to be associated with a decrease in cognitive function, including effects on executive decision-making and short-term memory (84,85). These deficits could arise from altered leptin/LepRb signaling both peripherally and centrally, which has been shown to occur in diet-induced obesity (86). Furthermore, leptin signaling has been implicated in multiple neurological disorders with decreased levels of LepRb mRNA observed in the hippocampus of patients with Alzheimer disease (87). In contrast, children with autism spectrum disorders, which is associated with excess or inappropriate synapse formation, have increased levels of circulating leptin (88). Since dendritic spine and synapse number are closely correlated with cognitive function and many neurological disorders (89–91), improper leptin signaling could underlie many of these disorders, whereas restoration of normal leptin signaling could provide a potential remedy.
Improper NMDAR function and signaling are also seen in many neurological disorders. Altered NMDAR function is seen in Parkinson disease and Alzheimer disease models, while loss of NR2B-containing NMDARs in the hippocampus and cortex has negative effects on learning (92–94). It is possible that leptin stimulation could rescue some of these defects through its enhancement of NMDAR function. For example, leptin has also been shown to protect dopaminergic neurons in a Parkinson disease model (95).
In summary, we have identified a novel leptin-signaling pathway in the hippocampus that involves NR2B and Fyn. These proteins are all involved in synapse formation and occur in a complex with LepRb. Our data support the hypothesis that LepRb scaffolds Fyn and that leptin activates Fyn. Fyn in turn phosphorylates NR2B at the Y1472 residue, maintaining NR2B in the cell surface. Furthermore, we show that this signaling complex/cascade is required for leptin stimulation of glutamate synapse formation. Taken together, these results provide additional insight into mechanisms underlying leptin’s neurotrophic actions in the developing brain.
Acknowledgments
Financial Support: We thank Dr Andres Barria for the gifts of NR2B expression constructs. This work was funded by the National Institutes of Health, grant numbers MH086032 and HD09269 (G.A.W.), DK083452 (S.M.A.).
Glossary
Abbreviations
- CNS
central nervous system
- DIV
days in vitro
- DN
dominant negative
- DNA
deoxyribonucleic acid
- IP
immunoprecipitation
- LepR
leptin receptor
- NMDA
N-methyl-D-aspartic acid
- NMDAR
NMDA receptor
- PBS
phosphate buffer solution
- RNA
ribonucleic acid
- SFK
Src family kinase
- sh
short hairpin ribonucleic acid
Author Contributions: T.B., G.W., G.S.S. and J.L.R.L. conducted the experiments, analyzed the results, and wrote the initial draft of the manuscript. G.A.W. and S.M.A. assisted in the design of the study and writing the manuscript. M.Z. conducted and analyzed the electrophysiological recording experiments. C.D. prepared the primary hippocampal cultures. All authors reviewed the results and approved the final version of the manuscript.
Additional Information
Disclosure Summary: The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1. Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered. 1950;41(12):317–318. [DOI] [PubMed] [Google Scholar]
- 2. Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science. 1966;153(3740):1127–1128. [DOI] [PubMed] [Google Scholar]
- 3. Maffei M, Halaas J, Ravussin E, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1(11):1155–1161. [DOI] [PubMed] [Google Scholar]
- 4. Banks WA, Kastin AJ, Huang W, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides. 1996;17(2):305–311. [DOI] [PubMed] [Google Scholar]
- 5. Stephens TW, Basinski M, Bristow PK, et al. The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature. 1995;377(6549):530–532. [DOI] [PubMed] [Google Scholar]
- 6. Zhao S, Kanoski SE, Yan J, Grill HJ, Hayes MR. Hindbrain leptin and glucagon-like-peptide-1 receptor signaling interact to suppress food intake in an additive manner. Int J Obes (Lond). 2012;36(12):1522–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alhadeff AL, Hayes MR, Grill HJ. Leptin receptor signaling in the lateral parabrachial nucleus contributes to the control of food intake. Am J Physiol Regul Integr Comp Physiol. 2014;307(11):R1338–R1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Domingos AI, Vaynshteyn J, Voss HU, et al. Leptin regulates the reward value of nutrient. Nat Neurosci. 2011;14(12):1562–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Collin M, Håkansson-Ovesjö ML, Misane I, Ogren SO, Meister B. Decreased 5-HT transporter mRNA in neurons of the dorsal raphe nucleus and behavioral depression in the obese leptin-deficient ob/ob mouse. Mol Brain Res. 2000;81(1–2):51–61. [DOI] [PubMed] [Google Scholar]
- 10. Sharma AN, Elased KM, Garrett TL, Lucot JB. Neurobehavioral deficits in db/db diabetic mice. Physiol Behav. 2010;101(3):381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yamada N, Katsuura G, Ochi Y, et al. Impaired CNS leptin action is implicated in depression associated with obesity. Endocrinology. 2011;152(7):2634–2643. [DOI] [PubMed] [Google Scholar]
- 12. Steppan CM, Swick AG. A role for leptin in brain development. Biochem Biophys Res Commun. 1999;256(3):600–602. [DOI] [PubMed] [Google Scholar]
- 13. Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304(5667):108–110. [DOI] [PubMed] [Google Scholar]
- 14. Ahima RS, Prabakaran D, Flier JS. Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. J Clin Invest. 1998;101(5):1020–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang XF, Koutcherov I, Lin S, Wang HQ, Storlien L. Localization of leptin receptor mRNA expression in mouse brain. Neuroreport. 1996;7(15-17):2635–2638. [DOI] [PubMed] [Google Scholar]
- 16. Elmquist JK, Bjørbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395(4):535–547. [PubMed] [Google Scholar]
- 17. Caron E, Sachot C, Prevot V, Bouret SG. Distribution of leptin-sensitive cells in the postnatal and adult mouse brain. J Comp Neurol. 2010;518(4):459–476. [DOI] [PubMed] [Google Scholar]
- 18. Pinto S., Roseberry AG., Liu H., et al. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304(5667):110–115. [DOI] [PubMed] [Google Scholar]
- 19. O’Malley D, MacDonald N, Mizielinska S, Connolly CN, Irving AJ, Harvey J. Leptin promotes rapid dynamic changes in hippocampal dendritic morphology. Mol Cell Neurosci. 2007;35(4):559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dhar M, Zhu M, Impey S, et al. Leptin induces hippocampal synaptogenesis via CREB-regulated microRNA-132 suppression of p250GAP. Mol Endocrinol. 2014;28(7):1073–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dhar M, Wayman GA, Zhu M, Lambert TJ, Davare MA, Appleyard SM. Leptin-induced spine formation requires TrpC channels and the CaM kinase cascade in the hippocampus. J Neurosci. 2014;34(30):10022–10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shanley LJ, Irving AJ, Harvey J. Leptin enhances NMDA receptor function and modulates hippocampal synaptic plasticity. J Neurosci. 2001;21(24):RC186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wayner MJ, Armstrong DL, Phelix CF, Oomura Y. Orexin-A (hypocretin-1) and leptin enhance LTP in the dentate gyrus of rats in vivo. Peptides. 2004;25(6):991–996. [DOI] [PubMed] [Google Scholar]
- 24. Farr SA, Banks WA, Morley JE. Effects of leptin on memory processing. Peptides. 2006;27(6):1420–1425. [DOI] [PubMed] [Google Scholar]
- 25. Oomura Y, Hori N, Shiraishi T, et al. Leptin facilitates learning and memory performance and enhances hippocampal CA1 long-term potentiation and CaMK II phosphorylation in rats. Peptides. 2006;27(11):2738–2749. [DOI] [PubMed] [Google Scholar]
- 26. Moult PR, Harvey J. Regulation of glutamate receptor trafficking by leptin. Biochem Soc Trans. 2009;37(Pt 6):1364–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu J, Garza JC, Bronner J, Kim CS, Zhang W, Lu XY. Acute administration of leptin produces anxiolytic-like effects: a comparison with fluoxetine. Psychopharmacology (Berl). 2010;207(4):535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang JQ, Guo ML, Jin DZ, Xue B, Fibuch EE, Mao LM. Roles of subunit phosphorylation in regulating glutamate receptor function. Eur J Pharmacol. 2014;728:183–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wyllie DJ, Livesey MR, Hardingham GE. Influence of GluN2 subunit identity on NMDA receptor function. Neuropharmacology. 2013;74:4–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Laurie DJ, Seeburg PH. Regional and developmental heterogeneity in splicing of the rat brain NMDAR1 mRNA. J Neurosci. 1994;14(5 Pt 2):3180–3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Al-Hallaq RA, Conrads TP, Veenstra TD, Wenthold RJ. NMDA di-heteromeric receptor populations and associated proteins in rat hippocampus. J Neurosci. 2007;27(31):8334–8343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yashiro K, Philpot BD. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology. 2008;55(7):1081–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gambrill AC, Barria A. NMDA receptor subunit composition controls synaptogenesis and synapse stabilization. Proc Natl Acad Sci U S A. 2011;108(14):5855–5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Flak JN, Myers MG Jr. Minireview: CNS mechanisms of leptin action. Mol Endocrinol. 2016;30(1):3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Salter MW, Kalia LV. Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci. 2004;5(4):317–328. [DOI] [PubMed] [Google Scholar]
- 36. Prybylowski K, Chang K, Sans N, Kan L, Vicini S, Wenthold RJ. The synaptic localization of NR2B-containing NMDA receptors is controlled by interactions with PDZ proteins and AP-2. Neuron. 2005;47(6):845–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kojima N, Sakamoto T, Endo S, Niki H. Impairment of conditioned freezing to tone, but not to context, in Fyn-transgenic mice: relationship to NMDA receptor subunit 2B function. Eur J Neurosci. 2005;21(5):1359–1369. [DOI] [PubMed] [Google Scholar]
- 38. Nakazawa T, Komai S, Watabe AM, et al. NR2B tyrosine phosphorylation modulates fear learning as well as amygdaloid synaptic plasticity. Embo J. 2006;25(12):2867–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bland T, Sahin GS, Zhu M, et al. USP8 deubiquitinates the leptin receptor and is necessary for leptin-mediated synapse formation. Endocrinology. 2019;160(8):1982–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bland T, Zhu M, Dillion C, et al. Supplemental data for: Leptin controls glutamatergic synaptogenesis and NMDA-receptor trafficking via Fyn kinase regulation of NR2B 2019. ProMED-mail website. https://ipn.vetmed.wsu.edu/docs/librariesprovider7/faculty-docs/bland_nr2b-manuscript_supplement.pdf?s. Accessed November 6, 2019.
- 41. RRID:AB_2750766 ProMED-mail website. https://scicrunch.org/resolver/RRID:AB_2750766.
- 42. RRID:AB_10693544 ProMED-mail website. https://scicrunch.org/resolver/RRID:AB_10693544.
- 43. RRID:AB_218216 ProMED-mail website. https://scicrunch.org/resolver/RRID:AB_218216.
- 44. RRID:AB_2040028 ProMED-mail website. https://scicrunch.org/resolver/RRID:AB_2040028.
- 45. RRID:AB_259529 ProMED-mail website. https://scicrunch.org/resolver/RRID:AB_259529.
- 46. RRID:AB_1264223 ProMED-mail website. https://scicrunch.org/resolver/RRID:AB_1264223.
- 47. RRID:AB_262150 ProMED-mail website. https://scicrunch.org/resolver/RRID:AB_262150.
- 48. RRID:AB_10698604 ProMED-mail website. https://scicrunch.org/resolver/RRID:AB_10698604.
- 49. RRID:AB_331697 ProMED-mail website. https://scicrunch.org/resolver/RRID:AB_331697.
- 50. RRID:AB_477256 ProMED-mail website. https://scicrunch.org/resolver/RRID:AB_477256.
- 51. RRID:AB_439680 ProMED-mail website. https://scicrunch.org/resolver/RRID:AB_439680.
- 52. RRID:AB_627547 ProMED-mail website. https://scicrunch.org/resolver/RRID:AB_627547.
- 53. RRID:AB_2687461 ProMED-mail website. https://scicrunch.org/resolver/RRID:AB_2687461.
- 54. RRID:AB_2108785 ProMED-mail website. https://scicrunch.org/resolver/RRID:AB_2108785.
- 55. RRID:AB_2336066 ProMED-mail website. https://scicrunch.org/resolver/RRID:AB_2336066.
- 56. Harvey J, Solovyova N, Irving A. Leptin and its role in hippocampal synaptic plasticity. Prog Lipid Res. 2006;45(5):369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Girasol A, Albuquerque GG, Mansour E, et al. Fyn mediates leptin actions in the thymus of rodents. PLoS One. 2009;4(11):e7707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Engen JR, Wales TE, Hochrein JM, et al. Structure and dynamic regulation of Src-family kinases. Cell Mol Life Sci. 2008;65(19):3058–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Twamley-Stein GM, Pepperkok R, Ansorge W, Courtneidge SA. The Src family tyrosine kinases are required for platelet-derived growth factor-mediated signal transduction in NIH 3T3 cells. Proc Natl Acad Sci U S A. 1993;90(16):7696–7700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013;14(6):383–400. [DOI] [PubMed] [Google Scholar]
- 61. Roux KJ, Kim DI, Raida M, Burke B. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J Cell Biol. 2012;196(6):801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kim DI, Birendra KC, Zhu W, Motamedchaboki K, Doye V, Roux KJ. Probing nuclear pore complex architecture with proximity-dependent biotinylation. Proc Natl Acad Sci U S A. 2014;111(24):E2453–E2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Halaas JL, Gajiwala KS, Maffei M, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269(5223):543–546. [DOI] [PubMed] [Google Scholar]
- 64. Bouret SG. Neurodevelopmental actions of leptin. Brain Res. 2010;1350:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Maness PF. Nonreceptor protein tyrosine kinases associated with neuronal development. Dev Neurosci. 1992;14(4):257–270. [DOI] [PubMed] [Google Scholar]
- 66. Grant SG, Silva AJ. Targeting learning. Trends Neurosci. 1994;17(2):71–75. [DOI] [PubMed] [Google Scholar]
- 67. Huerta PT, Scearce KA, Farris SM, Empson RM, Prusky GT. Preservation of spatial learning in fyn tyrosine kinase knockout mice. Neuroreport. 1996;7(10):1685–1689. [DOI] [PubMed] [Google Scholar]
- 68. Isosaka T, Hattori K, Kida S, et al. Activation of Fyn tyrosine kinase in the mouse dorsal hippocampus is essential for contextual fear conditioning. Eur J Neurosci. 2008;28(5):973–981. [DOI] [PubMed] [Google Scholar]
- 69. Grant SG, O’Dell TJ, Karl KA, Stein PL, Soriano P, Kandel ER. Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science. 1992;258(5090):1903–1910. [DOI] [PubMed] [Google Scholar]
- 70. Kojima N, Wang J, Mansuy IM, Grant SG, Mayford M, Kandel ER. Rescuing impairment of long-term potentiation in fyn-deficient mice by introducing Fyn transgene. Proc Natl Acad Sci U S A. 1997;94(9):4761–4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jiang L, Li Z, Rui L. Leptin stimulates both JAK2-dependent and JAK2-independent signaling pathways. J Biol Chem. 2008;283(42):28066–28073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Durakoglugil M, Irving AJ, Harvey J. Leptin induces a novel form of NMDA receptor-dependent long-term depression. J Neurochem. 2005;95(2):396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gray JA, Shi Y, Usui H, During MJ, Sakimura K, Nicoll RA. Distinct modes of AMPA receptor suppression at developing synapses by GluN2A and GluN2B: single-cell NMDA receptor subunit deletion in vivo. Neuron. 2011;71(6):1085–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Akashi K, Kakizaki T, Kamiya H, et al. NMDA receptor GluN2B (GluR epsilon 2/NR2B) subunit is crucial for channel function, postsynaptic macromolecular organization, and actin cytoskeleton at hippocampal CA3 synapses. J Neurosci. 2009;29(35):10869–10882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Dailey ME, Smith SJ. The dynamics of dendritic structure in developing hippocampal slices. J Neurosci. 1996;16(9):2983–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Barria A, Malinow R. Subunit-specific NMDA receptor trafficking to synapses. Neuron. 2002;35(2):345–353. [DOI] [PubMed] [Google Scholar]
- 77. Tovar KR, Westbrook GL. The incorporation of NMDA receptors with a distinct subunit composition at nascent hippocampal synapses in vitro. J Neurosci. 1999;19(10):4180–4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12(3):529–540. [DOI] [PubMed] [Google Scholar]
- 79. Lavezzari G, McCallum J, Lee R, Roche KW. Differential binding of the AP-2 adaptor complex and PSD-95 to the C-terminus of the NMDA receptor subunit NR2B regulates surface expression. Neuropharmacology. 2003;45(6):729–737. [DOI] [PubMed] [Google Scholar]
- 80. Van Doorn C, Macht VA, Grillo CA, Reagan LP. Leptin resistance and hippocampal behavioral deficits. Physiol Behav. 2017;176:207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Haleem DJ, Haque Z, Inam QU, Ikram H, Haleem MA. Behavioral, hormonal and central serotonin modulating effects of injected leptin. Peptides. 2015;74:1–8. [DOI] [PubMed] [Google Scholar]
- 82. Mercer JG, Hoggard N, Williams LM, Lawrence CB, Hannah LT, Trayhurn P. Localization of leptin receptor mRNA and the long form splice variant (Ob-Rb) in mouse hypothalamus and adjacent brain regions by in situ hybridization. FEBS Lett. 1996;387(2-3):113–116. [DOI] [PubMed] [Google Scholar]
- 83. Li XL, Aou S, Oomura Y, Hori N, Fukunaga K, Hori T. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience. 2002;113(3):607–615. [DOI] [PubMed] [Google Scholar]
- 84. Cournot M, Marquié JC, Ansiau D, et al. Relation between body mass index and cognitive function in healthy middle-aged men and women. Neurology. 2006;67(7):1208–1214. [DOI] [PubMed] [Google Scholar]
- 85. Nguyen JC, Killcross AS, Jenkins TA. Obesity and cognitive decline: role of inflammation and vascular changes. Front Neurosci. 2014;8:375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Frederich RC, Hamann A, Anderson S, Löllmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med. 1995;1(12):1311–1314. [DOI] [PubMed] [Google Scholar]
- 87. Bonda DJ, Stone JG, Torres SL, et al. Dysregulation of leptin signaling in Alzheimer disease: evidence for neuronal leptin resistance. J Neurochem. 2014;128(1):162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Al-Zaid FS, Alhader AA, Al-Ayadhi LY. Altered ghrelin levels in boys with autism: a novel finding associated with hormonal dysregulation. Sci Rep. 2014;4:6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kaufmann WE, Moser HW. Dendritic anomalies in disorders associated with mental retardation. Cereb Cortex. 2000;10(10):981–991. [DOI] [PubMed] [Google Scholar]
- 90. Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci. 2011;14(3):285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kulkarni VA, Firestein BL. The dendritic tree and brain disorders. Mol Cell Neurosci. 2012;50(1):10–20. [DOI] [PubMed] [Google Scholar]
- 92. Brigman JL, Wright T, Talani G, et al. Loss of GluN2B-containing NMDA receptors in CA1 hippocampus and cortex impairs long-term depression, reduces dendritic spine density, and disrupts learning. J Neurosci. 2010;30(13):4590–4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Feng ZJ, Zhang X, Chergui K. Allosteric modulation of NMDA receptors alters neurotransmission in the striatum of a mouse model of Parkinson’s disease. Exp Neurol. 2014;255:154–160. [DOI] [PubMed] [Google Scholar]
- 94. Hanson JE, Pare JF, Deng L, Smith Y, Zhou Q. Altered GluN2B NMDA receptor function and synaptic plasticity during early pathology in the PS2APP mouse model of Alzheimer’s disease. Neurobiol Dis. 2015;74:254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Weng Z, Signore AP, Gao Y, et al. Leptin protects against 6-hydroxydopamine-induced dopaminergic cell death via mitogen-activated protein kinase signaling. J Biol Chem. 2007;282(47):34479–34491. [DOI] [PubMed] [Google Scholar]