Summary:
This article describes the triple use of autologous amnion graft as a new procedure in the treatment of myelomeningocele and in myelomeningocele with split cord malformation. The first amnion graft was used as a physical and mechanical barrier to protect the myelomeningocele (MMC) from desiccation and mechanical stress directly after birth. A second graft was used as a dura substitute to close the cerebrospinal fluid compartment. Autologous amnion seems to be the ideal dural graft for closure of an MMC and for an MMC with split cord malformation. A tension-free and watertight closure was obtained. With the epithelium side placed to the spinal cord and due to its beneficial effect on scar formation, the risk for tethering cord syndrome is reduced when using autologous amnion as a dural graft. The regenerative properties of autologous amnion may contribute to repair neural damage. Finally, a third amnion graft was placed beneath the perforator flap used to close the skin defect to provide a watertight barrier and to stimulate flap survival.
INTRODUCTION
Myelomeningocele (MMC) is with a global prevalence of 2 per 1,000 live births the most common open neural tube defect.1 MMC causes significant morbidity and mortality due to spinal cord and intracranial abnormalities. MMC can be associated with split cord malformation (SCM), a rare congenital anomaly in which a segment of the spinal cord is divided into 2 hemi-cords separated by a fibrous or bony spur.2 Both MMC and SCM are potentially causing tethered cord syndrome (TCS), a condition that arises from tension on the spinal cord due to adhesions between the spinal cord and dura. TCS may lead to progressive sensory and neuromotor deterioration causing symptoms such as motor dysfunction, pain, gait abnormality, scoliosis, orthopedic deformities, and genitourinary dysfunction.3
The goals of MMC repair are to prevent infection, eliminate cerebrospinal fluid (CSF) leakage, preservation of neural function, and to diminish late sequelae as TCS and pain over the repair side. The neural repair involves closure of the neural tube and dura creating a closed CSF compartment. There is an inherent difficulty of closing the dura in such a way that the placode is bathed in CSF and not touching the dura. Such can be even more difficult to obtain in a patient with MMC and SCM. The neural repair requires tension-free and stable soft tissue coverage. This article describes the triple use of autologous amnion as a new approach in the treatment of a newborn with MMC and for a newborn with MMC and SCM.
CASE PRESENTATION
A girl (weight 3,837 g) was delivered by elective caesarean section in week 38. Prenatal diagnostics had revealed a large thoracolumbar MMC (8 cm × 7 cm) and hydrocephalus. The MMC was covered with sterile autologous amnion harvested at delivery and cleaned from the underlying chorion by mechanical peeling (Fig. 1). The newborn had spontaneous leg movements. Within 24 hours, she was operated on for her hydrocephalus and MMC. An Ommaya reservoir was placed to reduce intracranial pressure. The placode was carefully separated from the dysplastic and rudimentary meningeal tissues, and dysplastic skin was excised. A SCM was diagnosed at the level of the placode. It was decided not to excise the bone spur. Autologous amnion was used as a dural graft. The graft was sutured in place with it epithelium side toward the spinal cord (Fig. 2). A tension-free closure of the CSF compartment was obtained. The neural repair was covered with a thoracolumbar fascia followed by another autologous amnion layer and a sensate medial dorsal intercostal artery perforator flap (Fig. 3). Postoperatively, CSF was withdrawn for the Ommaya reservoir on a daily basis to prevent a high CSF pressure. No CSF leakage occurred in the postoperative phase. A minor wound dehiscence developed. After 1 week, a ventriculoperitoneal shunt was placed, and a minor wound revision was performed. The postoperative course was uneventful. At 36-month follow-up, the patient was able to stand without braces and to walk with braces. She had intact anal sphincter function and was able to urinate spontaneously.
Fig. 1.
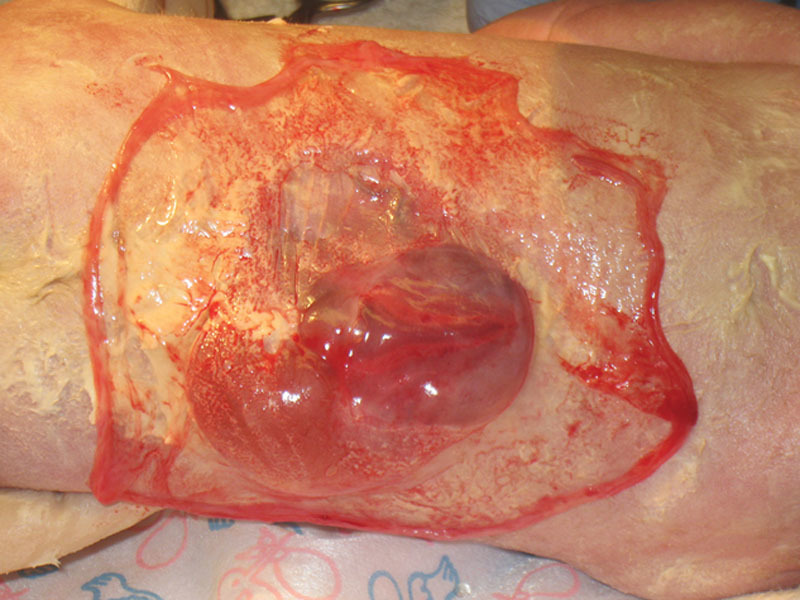
Autologous amnion is used directly after birth to cover the MMC with an associated SCM in the period before surgery.
Fig. 2.
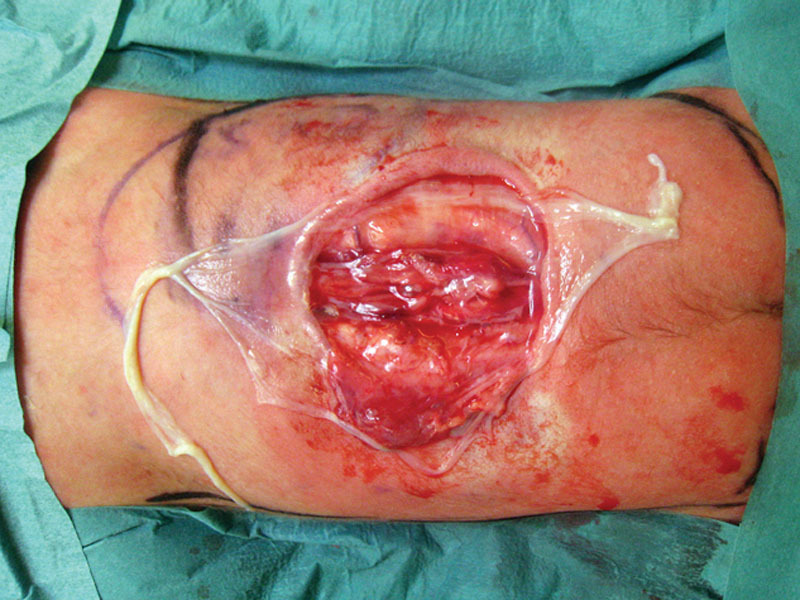
Autologous amnion is used as a dural graft with its epithelium side placed to the spinal cord and adapted to the defect.
Fig. 3.
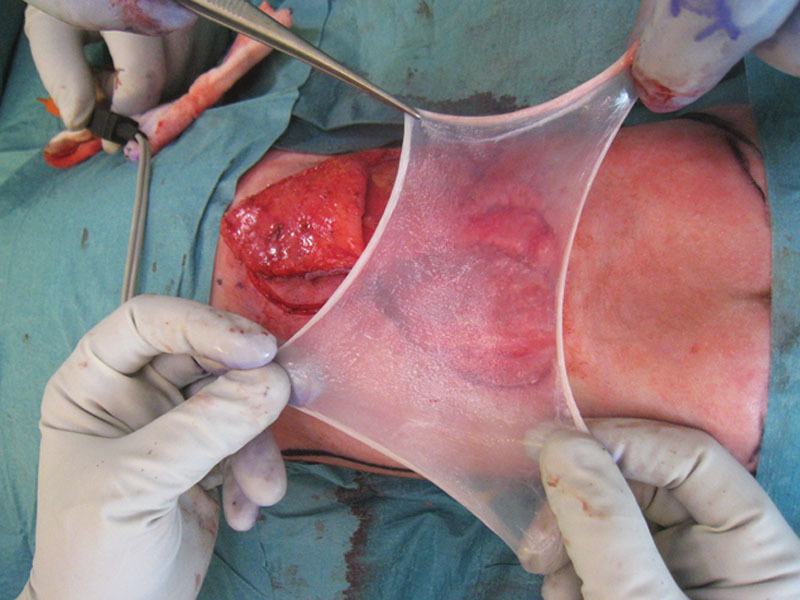
Autologous amnion is placed beneath the medial dorsal intercostal artery perforator flap as an extra watertight barrier and to contribute to flap survival.
DISCUSSION
MMC closure remains a challenge and should preferably be done within 48 hours to prevent infection and to preserve neural function.4 MMC is still associated with high mortality and devastating neurological defects in the majority of patients. Bowman et al showed that 75% of MMC children survive to adulthood but live with significant physical disabilities.5 Following delivery, it is important to prevent rupture of the sac and to protect the exposed neural elements from desiccation. The amniotic membrane is watertight and has unique tensile strength and elasticity.6 It is an ideal physical barrier to protect the sac from mechanical stress and desiccation in the period before MMC closure. In vitro studies have shown a reduced viability of Streptococcus and Staphylococcus in the presence of amnion making it also a bacterial barrier.6 Autologous amnion has a beneficial effect on scar formation and does not cause an immunological response.6 At the time of surgery, the amniotic membrane could easily be separated from the sac.
In our patient with MMC and SCM, a standard dura closure would have resulted in a tight closure over the cord and in compression of the 2 hemi-cords of the SCM to the midline spur, which increases the risk for adhesions between dura and cord and therewith causing limitations in cord movements. Using amnion as a dura substitute created a tension-free closure over the cord. Indeed, the tightest neurodural adhesions have been reported to be found at the dural suture lines.7 In a fetal lamb model, Brown et al found an increased preservation of spinal cord tissue and fewer adhesions in MMC defects prenatally repaired with autologous amnion compared to skin-only repaired lambs.8 In such, autologous amnion could possibly reduce the risk for TCS. A study by Samuels et al showed less retethering after a duraplasty.9
The epithelium layer is a source of neurotransmitters, neurotrophic factors, and neuropeptides.7 Autologous amnion with its epithelium placed toward the CSF compartment may, in contrast to denuded amnion, have a regenerative effect on damaged neural tissue. Amnion is a rich source of stem cells. Transplantation of amniotic epithelial cells improved hindlimb function in a rat model with spinal cord injury.10
To our knowledge, this is the first report using autologous amnion as dural graft in the repair of MMC in humans. This technique could be a promising technique in the treatment of MMC and in MMC with SCM.
Using the extra amnion layer beneath the perforator flap provided an extra watertight and bacterial barrier and has also shown to increase capillary proliferation, reduce neutrophilic infiltration, and improve flap survival.6 (See Video [online], which displays the patient at 28 and 30 months.)
Video 1. Video showing the patient at 28 months and 30 months. From "Triple use of autologous amnion graft in treatment of meningomyelocele and split cord malformation."
1_pnhy6n9p Kaltura
ACKNOWLEDGEMENT
We thank the parents for permission to use their video.
Footnotes
Published online 20 January 2020.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The article processing charge was funded by a grant from the publication fund of the UiT The Arctic University of Norway.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Rai SK, Singh R, Pandey S, et al. High incidence of neural tube defects in northern part of India. Asian J Neurosurg. 2016;11:352–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ansari S, Nejat F, Yazdani S, et al. Split cord malformation associated with myelomeningocele. J Neurosurg. 2007;1074 Suppl281–285. [DOI] [PubMed] [Google Scholar]
- 3.Mehta VA, Bettegowda C, Ahmadi SA, et al. Spinal cord tethering following myelomeningocele repair. J Neurosurg Pediatr. 2010;6:498–505. [DOI] [PubMed] [Google Scholar]
- 4.Attenello FJ, Tuchman A, Christian EA, et al. Infection rate correlated with time to repair of open neural tube defects (myelomeningoceles): an institutional and national study. Childs Nerv Syst. 2016;32:1675–1681. [DOI] [PubMed] [Google Scholar]
- 5.Bowman RM, McLone DG, Grant JA, et al. Spina bifida outcome: a 25-year prospective. Pediatr Neurosurg. 2001;34:114–120. [DOI] [PubMed] [Google Scholar]
- 6.Fairbairn NG, Randolph MA, Redmond RW. The clinical applications of human amnion in plastic surgery. J Plast Reconstr Aesthet Surg. 2014;67:662–675. [DOI] [PubMed] [Google Scholar]
- 7.Talamonti G, D’Aliberti G, Collice M. Myelomeningocele: long-term neurosurgical treatment and follow-up in 202 patients. J Neurosurg. 2007;1075 Suppl368–386. [DOI] [PubMed] [Google Scholar]
- 8.Brown EG, Saadai P, Pivetti CD, et al. In utero repair of myelomeningocele with autologous amniotic membrane in the fetal lamb model. J Pediatr Surg. 2014;49:133–7; discussion 137. [DOI] [PubMed] [Google Scholar]
- 9.Samuels R, McGirt MJ, Attenello FJ, et al. Incidence of symptomatic retethering after surgical management of pediatric tethered cord syndrome with or without duraplasty. Childs Nerv Syst. 2009;25:1085–1089. [DOI] [PubMed] [Google Scholar]
- 10.Wu ZY, Hui GZ, Lu Y, et al. Transplantation of human amniotic epithelial cells improves hindlimb function in rats with spinal cord injury. Chin Med J (Engl). 2006;119:2101–2107. [PubMed] [Google Scholar]


