Supplemental Digital Content is available in the text.
Abstract
Background:
The risk of surgical site infection (SSI) for breast surgery in patients without additional risk factors is low, below 5%. Evidence shows the risk of SSI is significantly elevated in patients undergoing immediate breast reconstruction (IBR). However, there is no consensus regarding the use of extended antibiotic prophylaxis. We aim to determine the effect of extended antibiotic prophylaxis on the incidence of SSI after IBR.
Methods:
PubMed and Scopus were searched by 2 independent reviewers. Data abstracted included types of study, basic characteristics, detailed antibiotic prophylaxis information, SSI event, and other secondary outcomes. We calculated the risk ratio (RR) and 95% confidence interval (CI) for each study and used a random-effects model to estimate the results. Study quality, bias, and heterogeneity were also analyzed.
Results:
A total of 11 studies (15,966 mastectomy procedures) were included. We found an overall 5.99% SSI rate in our population. Three studies comparing topical antibiotics with no topical antibiotics demonstrated statistical significance (RR = 0.26, 95% CI: 0.12–0.60, P = 0.001), whereas 8 studies comparing extended systemic antibiotics with standard of care found no statistical significance (RR = 0.80, 95% CI: 0.60–1.08, P = 0.13).
Conclusions:
In the setting of IBR following mastectomy, there is insufficient evidence for the use of extended prophylactic antibiotics to reduce SSI rates. Well-designed randomized controlled trials in patients undergoing IBR should be conducted to determine the appropriate regimen and/or duration of prophylactic antibiotics on SSI outcomes.
INTRODUCTION
Breast cancer is one of the most common malignancies diagnosed in women and comprises about 18% of all female cancers.1 Surgery is the primary modality for the treatment of breast cancer, depending on tumor stage.2 An increasing number of breast cancer patients are opting for mastectomy with reconstruction for treatment.
Postmastectomy reconstruction can surgically restore the shape of the breast and provide breast cancer patients with psychological benefits.3,4 There are 2 types of postmastectomy reconstructions: autologous tissue flap and tissue expander/implant. Although autologous tissue provides the most lasting and natural outcomes, implant-based breast reconstruction is the more popular procedure, accounting for about 80% of postmastectomy reconstructions.5,6 Breast reconstruction can be divided by timing into immediate breast reconstruction (IBR) and delayed breast reconstruction (DBR). IBR, compared to DBR, offers a native inframammary fold and a pliable skin envelope for a more natural appearance.7 IBR also reduces psychological impact on patients8 and thus may be favored by some patients. However, compared to DBR, IBR is associated with greater risk of surgical site infection (SSI).9,10
SSI is defined as infection of the superficial incision, organ, and/or space after surgery. Accordingly, there are 3 categories of SSI: superficial incisional SSI, deep incisional SSI, and organ/space SSI.11 The rate of SSI is strongly associated with the type of surgical wound. The Centers for Disease Control (CDC) published a guideline in 1985,12 which classified surgical wounds into clean, clean/contaminated, contaminated, and dirty, with the SSI rate of 1%–5%, 3%–11%, 10%–17%, and over 27%, respectively.13 The occurrence of SSI can impact the postoperative recovery process and result in extra cost and rate of hospital readmissions.
Breast surgeries are classically categorized as clean,14 and according to the CDC and the Surgical Care Improvement Project, for breast surgical procedures, antibiotics should be discontinued within 24 hours after surgery.12 However, among breast surgeries, IBR with a tissue expander/implant is associated with higher SSI, with the average SSI rate ranging from 5% to as high as 35%.10,15,16
In the setting of IBR, several studies supported the use of extended prophylactic antibiotics to prevent SSI,17–19 but others stated that extended antibiotic usage could lead to systemic side effects and the development of resistant organisms.20–22 Thus, there is still no consensus regarding the extended usage, regimens, and timing of prophylactic antibiotics for mastectomy with IBR. This meta-analysis aims to determine the efficacy and safety of extended prophylactic antibiotics on SSI after mastectomy with IBR.
PATIENTS AND METHODS
Search Strategy
Our study followed guidelines published by the Centre for Reviews and Dissemination (CRD) and the Cochrane Collaboration and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses criteria.23,24 A protocol for this systematic review was registered using Prospero (CRD42019127536).
We included patients undergoing mastectomy with IBR, or mixed types (IBR and DBR) if the study had separate outcomes for IBR and DBR groups. Both randomized controlled trials (RCTs) and observational studies were included. Studies that compare pre-, peri-, postoperative extended prophylactic antibiotics with standard of care were included. Studies with no comparison group were excluded.
Two reviewers independently assessed the title and abstract of articles identified by the search described earlier. Two reviewers applied the inclusion and exclusion criteria, and disagreements were resolved by reading the full text. A third reviewer examined the article and made the final decision if still undecided. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart diagram of literature retrieval is shown in Fig. 1.
Fig. 1.
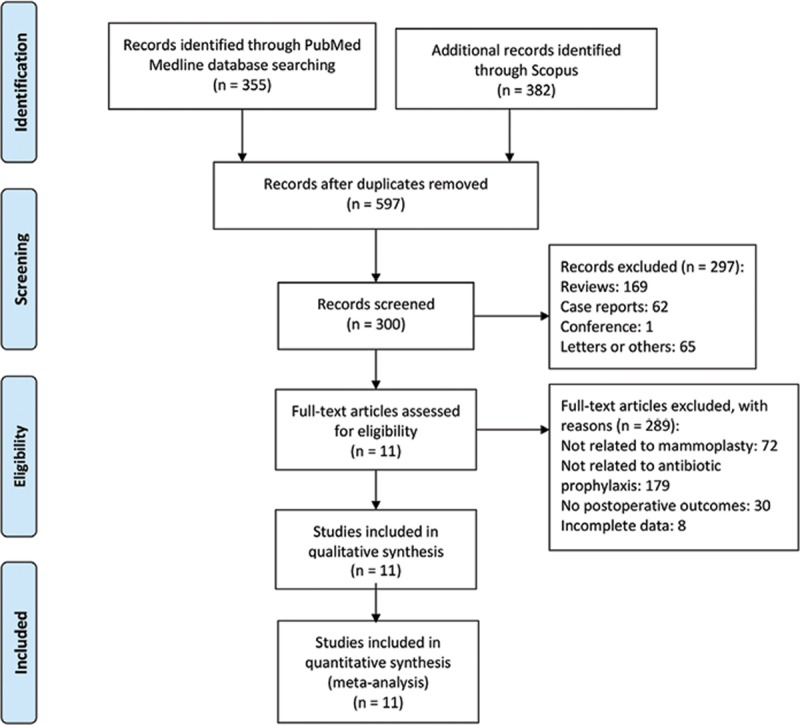
The PRISMA diagram of literature retrieved. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Data Extraction
Two reviewers independently extracted the data. Data were collected from each study: the first author, publication year, study design, number of procedures, type of IBR, antibiotics (ie, regimen name, dose, duration of treatment), and outcomes.
Quality Assessment for Included Studies
Two reviewers independently assessed the study quality using the Newcastle–Ottawa Scale.25 The Newcastle–Ottawa Scale is used to evaluate non-RCTs, with 1 version for case–control studies and the other for cohort studies. Both versions of the scale consist of 8 multiple-choice questions that address subject selection and comparability (of cases and controls in case–control studies and of cohorts in cohort studies) and the assessment of the outcome (in case–control studies) or exposure (in cohort studies).
Dealing with Missing Data
For those articles which met inclusion criteria but had incomplete data (ie, missing type of standard of care of comparison group, or no details of antibiotic use of extended antibiotics group), we emailed the corresponding author of that article. If corresponding authors did not respond after 3 weeks, the article was labeled as having incomplete data, and this would be mentioned in the results section.
Outcome Measures and Data Synthesis
All statistical analyses were performed by Review Manager V.5.3 (The Cochrane Collaboration, Software Update, Oxford, UK). We calculated the risk ratio (RR) at 95% confidence interval (CI) for each study, weighted by the number of events in each study. Statistical significance was defined as 2-tailed alpha < 0.05. Forest plots were generated for graphical presentations for clinical outcomes, and we used I2 statistics to define the heterogeneity of each study. Mantel-Haenszel method was used to conduct meta-analysis, and because I2 was >50%, we used the random-effects model instead of the fixed-effects model. We did subgroup analyses where the data were applicable.
Assessment of Heterogeneity
We assessed the heterogeneity between study results using the I2 statistics. The result is a percentage of total variation among studies due to heterogeneity. I2 is commonly divided into 3 categories—low, moderate, and high, with upper limits of 25%, 50%, and 75%, respectively.26
Sensitivity Analysis
We assessed the influence of a single study on the overall effect by sequentially removing 1 study at a time to test the robustness of the pooled results to further verify whether any study had an excessive influence on the overall results.
RESULTS
Results of the Search
A total of 597 articles were identified, of which 297 articles were excluded as they were ineligible publication types. After initial screening of the remaining 300 original articles, we further excluded 289 articles because they were irrelevant to our study topic or had incomplete data. The details of the search strategy are shown in Fig. 1.
Characteristics of Included Studies
A total of 11 studies (15,966 mastectomy procedures) were included, 10,688 in the extended antibiotics arm and 5,278 in the comparison arm. One was an RCT,21 and 10 studies17–20,22,27–31 were retrospective studies with 1 matched cohort study.31 The studies were published between 2009 and 2018, with sample sizes ranging from 112 to 7,443 mastectomies. The main characteristics of all 11 studies are presented in Table 1.
Table 1.
Characteristics of Included Studies (n = 11)
| Study | Study Design | Type of Reconstruction | No. Operations | Intervention Group Antibiotics | Intervention Group SSI (%) | Control Group Antibiotics | Control Group SSI (%) |
|---|---|---|---|---|---|---|---|
| Avashia et al17 | Retrospective | IBR TE | 138 | Postoperative > 24 h | 8/119 (6.7%) | Standard of care | 6/19 (31.6%) |
| Hunsicker et al19 | Retrospective | IBR implant | 535 | Postoperative irrigation for 96 h | 6/316 (1.9%) | Standard of care + irrigation once | 14/219 (6.4%) |
| Clayton et al18 | Retrospective | IBR implant | 250 | Postoperative > 24 h | 21/116 (18.1%) | Standard of care | 46/134 (34.3%) |
| Goh et al20 | Retrospective | IBR TE, latissimus dorsi, subpectoral | 240 | Postoperative > 24 h | 12/145 (8.3%) | Standard of care | 2/95 (2.1%) |
| Kenna et al27 | Retrospective | IBR implant | 127 | Irrigation + antibiotic beads | 1/68 (1.47%) | Standard of care | 7/59 (11.86%) |
| McCullough et al22 | Retrospective | IBR implant | 378 | Postoperative > 24 h | 24/200 (12%) | Standard of care | 24/178 (13/5%) |
| Murray et al28 | Retrospective | IBR TE | 200 | Topical ointment | 0/23 (0%) | Standard of care | 10/177 (5.65%) |
| Olsen et al29 | Retrospective | IBR implant, flap, both | 5,938 | Postoperative > 24 h | 240/3,305 (7.26%) | Standard of care | 213/2,633 (8.09%) |
| Phillips et al21 | RCT | IBR implant | 112 | Postoperative > 24 h | 11/50 (22%) | Standard of care | 12/62 (19.35%) |
| Ranganathan et al30 | Retrospective | IBR implant | 7,443 | Postoperative > 24 h | 166/6,049 (5.26%) | Standard of care | 41/1,394 (5.45%) |
| Townley et al31 | Retrospective | IBR implant | 605 | Postoperative > 24 h | 9/297 (3.03%) | Standard of care | 11/308 (3.6%) |
TE, tissue expander.
Type of Procedures
All procedures were unilateral or bilateral mastectomy with IBR. Immediate breast implant, tissue expander, and autologous flap (including latissimus dorsi flap and subpectoral flap) placement were described in 7, 3, and 2 studies, respectively.
Timing and Types of Antibiotics
Eight studies evaluated the usage of extended postoperative systemic prophylactic antibiotics compared to standard of care, where antibiotics are discontinued within 24 hours after breast surgery.12 However, the duration and types of antibiotics used vary between studies. In 5 of 8 studies,17,18,21,22,31 all patients were given pre- or perioperative antibiotics as baseline treatment, whereas the other 3 studies20,29,30 had incomplete data on antibiotic usage in their comparison groups. The study design was also different. Of the 8 studies, 1 study was an RCT, 2 studies were claims databases with a large sample size, and 5 studies were single-site retrospective studies.
Three studies evaluated the usage of postoperative topical antibiotics compared to no postoperative topical antibiotics. Two articles19,28 focused on the usage of topical mupirocin ointment and irrigation with both the extended antibiotics group and comparison arm using the same pre-/ peri- and postoperative antibiotics. The third article27 used a novel antibiotic bead compared to comparison, where both arms had the same preoperative and irrigation antibiotics.
Synthesis of Results
Incidence of SSI
We found an overall average SSI rate of 5.99% in mastectomy procedures. Heterogeneity within the interventions used in the study prevented pooling of all the studies for analysis. Analysis of 8 studies comparing extended systemic antibiotics with standard of care found no statistical significance (RR = 0.80, 95% CI: 0.60–1.07, P = 0.13) (Fig. 2); as 1 RCT and 2 claims database studies are in the group of 8 studies, we also conducted other sensitivity analysis to check whether these studies may have affected the results (see further “Sensitivity Analysis” section). For the topical antibiotics group, analysis of the 3 studies comparing topical antibiotics with no topical antibiotics demonstrated statistically significant effect of antibiotics on reducing the incidence of SSI (RR = 0.26, 95% CI: 0.12–0.60, P = 0.001) (Fig. 3) However, this statistical significance has to be interpreted with caution, as more research is needed to confirm the findings. The use of topical mupirocin ointment and the use of novel antibiotic beads may not be generalizable to many other centers’ experiences.
Fig. 2.
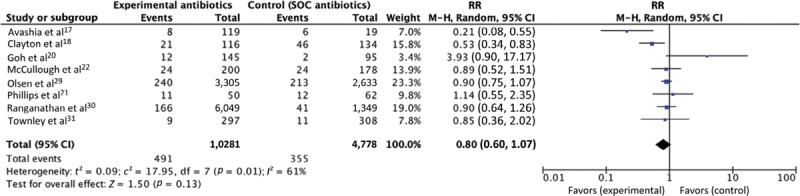
Forest plot of studies that compared systemic antibiotics to standard of care (n = 8). SOC, standard of care. RR, risk ratio. M-H, Mantel-Haenszel.
Fig. 3.
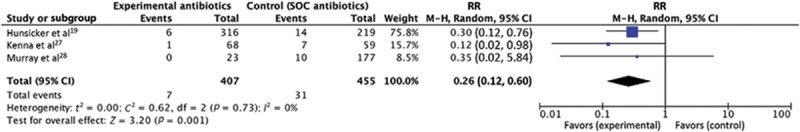
Forest plot of studies that compared topical antibiotics to standard of care (n = 3). SOC, standard of care. RR, risk ratio. M-H, Mantel-Haenszel.
Wound Complications
Wound complications included hematoma, wound dehiscence, seroma, hematoma, and mastectomy flap necrosis. Four17,19,29,30 studies measured the incidence of wound complications, with 9,789 mastectomies on extended prophylactic antibiotics and 4,220 mastectomies on standard of care. Analysis of the 4 studies comparing extended prophylactic antibiotics with standard of care found no statistically significant effect on reducing the incidence of wound complications (RR = 0.89, 95% CI: 0.78–1.03, P = 0.12). The result is presented in Supplemental Digital Content 1, which displays a forest plot of wound complications subgroup analysis, http://links.lww.com/PRSGO/B293).
Hospital Readmission
Only 1 study measured the incidence of hospital readmission, with 6,049 mastectomies on extended prophylactic antibiotics and 1,349 mastectomies on standard of care. Analysis of the study comparing extended prophylactic antibiotics with standard of care found no statistical significance in reducing the rate of hospital readmission (RR = 1.22, 95% CI: 0.85–1.74, P = 0.28). The result is presented in Supplemental Digital Content 2, which displays forest plot of readmission subgroup analysis, http://links.lww.com/PRSGO/B294).
Quality Assessment for Included Studies
We created a quality assessment figure based on the Risk of Bias Tool found in the Cochrane Handbook for Systemic Reviews of Interventions,32 and we presented the percentages of risk in each of the 9 domains. High-quality responses were marked “low risk.” The result is shown in Supplemental Digital Content 3, which displays risk of bias, http://links.lww.com/PRSGO/B295.
Sensitivity Analysis and Publication Bias
Funnel plot analysis disclosed no asymmetry around the axis, which means that publication bias was not detected (Fig. 4). No significant results were identified in the “leave one out” sensitivity test.
Fig. 4.
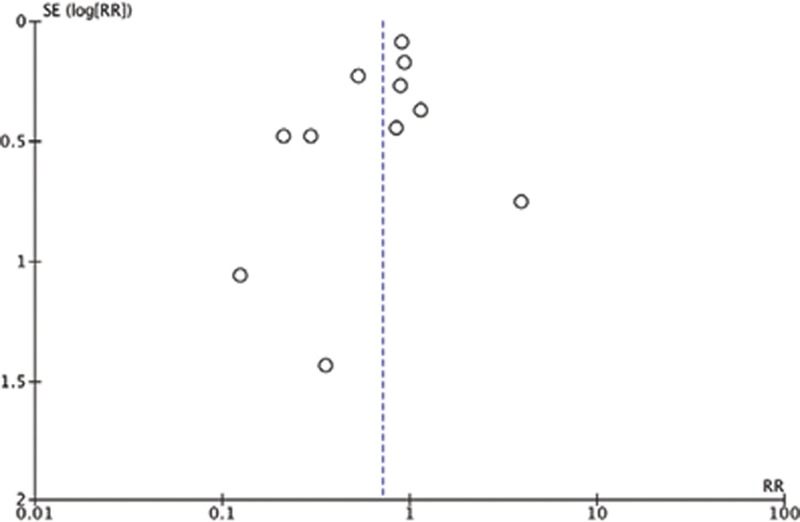
Funnel plot of included studies (n = 11). SE, standard error. RR, risk ratio.
Further Sensitivity Analysis to Understand Heterogeneity across Studies
As there were substantial differences in the types of studies within the 8 studies that investigated extended antibiotics on SSI, we conducted 2 further analyses. First, we noted that 2 studies using claims database had 88.5% of the weight (Fig. 2). We analyzed extended systemic antibiotics with and without the 2 claims database studies, resulting in 8 and 6 studies (Fig. 2) [see figure, Supplemental Digital Content 5, which displays a forest plot of extended systemic prophylactic antibiotics (without 2 claims databases), http://links.lww.com/PRSGO/B297]. In Fig. 2, RR was 0.80 (95% CI: 0.60–1.07, I2 = 61%). In Supplemental Digital Content 5, which displays a forest plot of extended systemic prophylactic antibiotics (without 2 claims databases) [http://links.lww.com/PRSGO/B297], RR was 0.93 (CI: 0.49–1.80, I2 = 79%). Both analyses showed no overall significance, but statistical heterogeneity increased slightly. We conclude that claims databases, even with their larger sample sizes, did not greatly alter the RR in this instance. We also note that a random-effects model is more accurate than a fixed-effects model due to the heterogeneity of the studies included.
There was 1 RCT among our included studies, and we were interested in its effect (or lack thereof) on the meta-analysis. Taken alone, the RCT reported no differences between 24-hour antibiotics or extended antibiotics (until drain removed), with an RR of 1.18 (95% CI: 0.47–2.95). We conducted an analysis of extended systemic antibiotics with and without this RCT, resulting in 8 and 7 studies [Fig. 2; also see figure, Supplemental Digital Content 4, which displays a forest plot of extended systemic prophylactic antibiotics (without RCT study), http://links.lww.com/PRSGO/B296]. In Fig. 2, RR was 0.80 (95% CI: 0.60–1.07, I2 = 61%). The weight of the RCT was 0.7%. In Supplemental Digital Content 4, which displays a forest plot of extended systemic prophylactic antibiotics (without RCT study) [http://links.lww.com/PRSGO/B296], RR was 0.77 (95% CI: 0.56–1.06, I2 = 65%). Overall, the RCT did not change the results of the 8 studies. We note that given the nature of the study, theoretically the RCT should have been given more weight, but our predefined statistical method did not provide allowances for ad hoc increases in statistical weight.
DISCUSSION
For patients who are opting for mastectomy with reconstruction as treatment, IBR, especially implant-based IBR, has become a common procedure to restore the shape of the breast and improve psychological well-being.33 Breast surgery is historically thought of as a “clean” procedure,14 and for clean surgical procedures, the CDC calls for the discontinuation of perioperative prophylactic antibiotics within 24 hours.12 In recent years, some have advocated breast surgeries as “clean-contaminated” procedures,34,35 noting the breast microbiome36,37 and bacteria presence on normal breast implants,38 and of contamination of breast implants even when precautions are taken.39 However, this may be more complicated than applying a blanket label for all breast surgeries, because the SSI rates after different breast surgeries have varied widely in the published literature, ranging from 0.8% to 26%.40,41 Among all types of procedures, mastectomy with implant-based IBR has a 2-fold increase in SSI incidence compared with mastectomy alone.42 Therefore, antibiotics are usually prescribed for an extended duration after mastectomy with IBR even though there is a lack of clinical evidence. Such practice has been prone to criticism by infection control officers and others who quote data from studies suggesting no corresponding decrease in rates of postoperative infection despite increasing use of prophylactic antibiotics.16,43–45 During that time, a center implemented changes to not provide postoperative prophylactic antibiotics for breast reconstruction (from previous practice of giving postoperative antibiotics until drains were removed). They reported that this is associated with an increased risk of infection.18 The group then proposed an RCT to test out the optimal duration of postoperative antibiotics. Amid the controversy, there is still no consensus for reconstructive breast surgeries, including postmastectomy implant-based IBR.
Our study aimed to examine the correlation of extended antibiotic prophylaxis (>24 hours postoperatively) and the incidence of SSI after IBR. The average SSI rate of our study population was 5.99%, ranging from 2.83% to 26.2%. Our meta-analysis found that extended postoperative systemic antibiotics had no significant effect on reducing the incidence of SSI. According to our protocol, we initially aimed to pool all the studies in a forest plot, but given the heterogeneity of the studies included, this was not statistically or clinically appropriate. Further subgroup analysis demonstrated that extended antibiotic prophylaxis showed no significant effect on reducing the incidence of wound complications and hospital readmission.
This is the first meta-analysis focusing on the effects of using extended prophylactic antibiotics for postmastectomy IBR in both topical and systemic antibiotics groups. However, there are several limitations in our meta-analysis, which are mostly related to the studies that were analyzed. First, there are missing data in some of the studies. Three out of the 11 studies lacked adequate descriptions of their antibiotic protocols for the comparison groups and most studies lacked antibiotic protocols and a defined time of discontinuation.
The second limitation was the heterogeneity of the included studies. As we mentioned earlier, we divided I2 into 3 categories—low, moderate, and high, with upper limits of 25%, 50%, and 75%. The average I2 of our studies is around 60%. According to a previous review, a quarter of meta-analyses has I2 values over 50%, and quantification of heterogeneity is only 1 component of a wider evaluation of variability among different studies.26 Thus, meta-analysts must also consider the clinical applications of the observed level of inconsistency across different studies. In particular for our studies, antibiotics were not used uniformly in terms of regimens, timing, dosing, and duration. There was no indication that there was any standardization of what constituted an SSI in the included articles. Redness, fever, requirement for intraoperative irrigation, requirement for removal of the implant or expander, and a combination of the following could technically constitute SSI.46 Thus, having clarity of what makes an SSI would be very important for any prospectively designed study. Disagreement on what constitutes an SSI is a commonly encountered problem when comparing articles discussing infection rates, as it may be field specific. We would like to engage stakeholders and experts in the area of breast reconstruction to potentially form a consensus for prospective studies that will improve evidence-based practices.
The third limitation is the type of studies included. Ten of 11 studies were retrospective studies. Since there was only 1 RCT conducted within the included studies, more well-designed RCTs should be conducted to demonstrate the effect of different regimens of prophylactic antibiotics on the SSI rate of IBR. Appropriate prophylactic antibiotic protocols should be tested. Finally, a better reporting system of the types of SSI, antibiotic regime/dosage/duration, and other complications should be used for future studies.
Future Directions
Given the paucity of prospective studies on this important topic, well-designed studies are sorely needed. However, there are many prior considerations that go into a well-designed prospective study for this particular question. Although we try not to prescribe particular rules here, the following questions (among others) are important to consider. Should patients with tissue necrosis be excluded? Should cases done by surgeons who leave completely different flap thicknesses be grouped together? Should reconstructions going under the muscle be compared to reconstructions done above the muscle? Should small areas of necrosis count the same as larger areas? Should the experience of the plastic surgeon be assessed? Should drain removal be based on drainage volume or based on duration, and if so, how much? Should patients who are discharged from hospital with a drain be grouped separately? It has been noted that these factors, while important in predicting SSI, are never available from retrospective chart reviews. Importantly, regarding antibiotic duration, the timepoints of antibiotics for 24 hours only, antibiotics until drain removal (which can vary significantly), and antibiotics for a certain duration after drain removal are essential to study and compare. Even if the sample size is relatively low, such well-controlled prospective studies will be valued. We rally surgeons in this field to consider starting prospective studies on this.
CONCLUSIONS
From our systematic review and meta-analysis, we conclude that, at this point, in part due to the lack of large prospective studies and in part due to the heterogeneity of interventions, there are insufficient data to suggest that extended antibiotics reduce the risk of SSI in patients undergoing mastectomy with IBR. Moreover, broad-spectrum antibiotics may significantly influence the normal gastrointestinal flora and lead to unfavorable clinical consequences, such as Clostridium difficile–related pseudomembranous colitis and antibiotic resistance. Therefore, we appeal for RCTs that test if there is improved efficacy and safety of extended prophylactic antibiotics on IBR. In particular, focus should be put on the choice of antibiotic regimens, the treatment duration, and a standardized clinical criterion for SSI evaluation.
ACKNOWLEDGMENT
We would like to acknowledge the critical feedback and intellectual contributions of the peer reviewers of this article.
Supplementary Material
Footnotes
Published online 27 January 2020.
Yang Hai and Weelic Chong contributed equally.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.McPherson K, Steel CM, Dixon JM. ABC of breast diseases. Breast cancer-epidemiology, risk factors, and genetics. BMJ. 2000;321:624–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Matsen CB, Neumayer LA. Breast cancer: a review for the general surgeon. JAMA Surg. 2013;148:971–979. [DOI] [PubMed] [Google Scholar]
- 3.Schmauss D, Machens HG, Harder Y. Breast reconstruction after mastectomy. Front Surg. 2015;2:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Senkus E, Kyriakides S, Ohno S, et al. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26 Suppl 5:v8–30. [DOI] [PubMed] [Google Scholar]
- 5.Hanna KR, Tilt A, Holland M, et al. Reducing infectious complications in implant based breast reconstruction: impact of early expansion and prolonged drain use. Ann Plast Surg. 2016;76 Suppl 4:S312–5. [DOI] [PubMed] [Google Scholar]
- 6.Platt J, Baxter N, Zhong T. Breast reconstruction after mastectomy for breast cancer. CMAJ. 2011;183:2109–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serletti JM, Fosnot J, Nelson JA, et al. Breast reconstruction after breast cancer. Plast Reconstr Surg. 2011;127:124e–135e. [DOI] [PubMed] [Google Scholar]
- 8.Zhong T, Hu J, Bagher S, et al. A comparison of psychological response, body image, sexuality, and quality of life between immediate and delayed autologous tissue breast reconstruction: a prospective long-term outcome study. Plast Reconstr Surg. 2016;138:772–780. [DOI] [PubMed] [Google Scholar]
- 9.Olsen MA, Nickel KB, Fox IK, et al. Comparison of wound complications after immediate, delayed, and secondary breast reconstruction procedures. JAMA Surg. 2017;152:e172338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alderman AK, Wilkins EG, Kim HM, et al. Complications in postmastectomy breast reconstruction: two-year results of the Michigan breast reconstruction outcome study. Plast Reconstr Surg. 2002;109:2265–2274. [DOI] [PubMed] [Google Scholar]
- 11.Berríos-Torres SI, Umscheid CA, Bratzler DW, et al. ; Healthcare Infection Control Practices Advisory Committee Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg. 2017;152:784–791. [DOI] [PubMed] [Google Scholar]
- 12.Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. Hospital infection control practices advisory committee. Infect Control Hosp Epidemiol. 1999;20:250–78; quiz 279. [DOI] [PubMed] [Google Scholar]
- 13.Cruse PJE, Foord R. The epidemiology of wound infection: a 10-Year Prospective Study of 62,939 wounds. Surg Clin North Am. 1980;60:27–40. [DOI] [PubMed] [Google Scholar]
- 14.Platt R, Zucker JR, Zaleznik DF, et al. Perioperative antibiotic prophylaxis and wound infection following breast surgery. J Antimicrob Chemother. 1993;31 Suppl B:43–48. [DOI] [PubMed] [Google Scholar]
- 15.Cohen JB, Carroll C, Tenenbaum MM, et al. Breast implant-associated infections: the role of the national surgical quality improvement program and the local microbiome. Plast Reconstr Surg. 2015;136:921–929. [DOI] [PubMed] [Google Scholar]
- 16.Phillips BT, Bishawi M, Dagum AB, et al. A systematic review of antibiotic use and infection in breast reconstruction: what is the evidence? Plast Reconstr Surg. 2013;131:1–13. [DOI] [PubMed] [Google Scholar]
- 17.Avashia YJ, Mohan R, Berhane C, et al. Postoperative antibiotic prophylaxis for implant-based breast reconstruction with acellular dermal matrix. Plast Reconstr Surg. 2013;131:453–461. [DOI] [PubMed] [Google Scholar]
- 18.Clayton JL, Bazakas A, Lee CN, et al. Once is not enough: withholding postoperative prophylactic antibiotics in prosthetic breast reconstruction is associated with an increased risk of infection. Plast Reconstr Surg. 2012;130:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunsicker LM, Chavez-Abraham V, Berry C, et al. Efficacy of vancomycin-based continuous triple antibiotic irrigation in immediate, implant-based breast reconstruction. Plast Reconstr Surg Glob Open. 2017;5:e1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goh SC, Thorne AL, Williams G, et al. Breast reconstruction using permanent Becker expander implants: an 18 year experience. Breast. 2012;21:764–768. [DOI] [PubMed] [Google Scholar]
- 21.Phillips BT, Fourman MS, Bishawi M, et al. Are prophylactic postoperative antibiotics necessary for immediate breast reconstruction? Results of a prospective randomized clinical trial. J Am Coll Surg. 2016;222:1116–1124. [DOI] [PubMed] [Google Scholar]
- 22.McCullough MC, Chu CK, Duggal CS, et al. Antibiotic prophylaxis and resistance in surgical site infection after immediate tissue expander reconstruction of the breast. Ann Plast Surg. 2016;77:501–505. [DOI] [PubMed] [Google Scholar]
- 23.Tacconelli E. Systematic reviews: CRD’s guidance for undertaking reviews in health care. Lancet Infect Dis. 2010;10:226. [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, et al. ; PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Plos Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wells G. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis. 2004. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed February 20, 2019.
- 26.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kenna DM, Irojah BB, Mudge K, et al. Absorbable antibiotic beads prophylaxis in immediate breast reconstruction. Plast Reconstr Surg. 2018;141:486e–492e. [DOI] [PubMed] [Google Scholar]
- 28.Murray JD, Elwood ET, Jones GE, et al. Decreasing expander breast infection: a new drain care protocol. Can J Plast Surg. 2009;17:17–21. [PMC free article] [PubMed] [Google Scholar]
- 29.Olsen MA, Nickel KB, Fraser VJ, et al. Prevalence and predictors of postdischarge antibiotic use following mastectomy. Infect Control Hosp Epidemiol. 2017;38:1048–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ranganathan K, Sears ED, Zhong L, et al. Antibiotic prophylaxis after immediate breast reconstruction: the reality of its efficacy. Plast Reconstr Surg. 2018;141:865–877. [DOI] [PubMed] [Google Scholar]
- 31.Townley WA, Baluch N, Bagher S, et al. A single pre-operative antibiotic dose is as effective as continued antibiotic prophylaxis in implant-based breast reconstruction: a matched cohort study. J Plast Reconstr Aesthet Surg. 2015;68:673–678. [DOI] [PubMed] [Google Scholar]
- 32.Armijo-Olivo S, Stiles CR, Hagen NA, et al. Assessment of study quality for systematic reviews: a comparison of the Cochrane Collaboration Risk of Bias Tool and the effective public health practice project quality assessment tool: methodological research. J Eval Clin Pract. 2012;18:12–18. [DOI] [PubMed] [Google Scholar]
- 33.Jeevan R, Mennie JC, Mohanna PN, et al. National trends and regional variation in immediate breast reconstruction rates. Br J Surg. 2016;103:1147–1156. [DOI] [PubMed] [Google Scholar]
- 34.Kataria K, Bagdia A, Srivastava A. Are breast surgical operations clean or clean contaminated? Indian J Surg. 2015;77Suppl 31360–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bartsich S, Ascherman JA, Whittier S, et al. The breast: a clean-contaminated surgical site. Aesthet Surg J. 2011;31:802–806. [DOI] [PubMed] [Google Scholar]
- 36.Urbaniak C, Gloor GB, Brackstone M, et al. The microbiota of breast tissue and its association with breast cancer. Appl Environ Microbiol. 2016;82:5039–5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hieken TJ, Chen J, Hoskin TL, et al. The microbiome of aseptically collected human breast tissue in benign and malignant disease. Sci Rep. 2016;6:30751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker JN, Pinkner CL, Pinkner JS, et al. The detection of bacteria and matrix proteins on clinically benign and pathologic implants. Plast Reconstr Surg Glob Open. 2019;7:e2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galdiero M, Larocca F, Iovene MR, et al. Microbial evaluation in capsular contracture of breast implants. Plast Reconstr Surg. 2018;141:23–30. [DOI] [PubMed] [Google Scholar]
- 40.Degnim AC, Throckmorton AD, Boostrom SY, et al. Surgical site infection after breast surgery: impact of 2010 CDC reporting guidelines. Ann Surg Oncol. 2012;19:4099–4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nahabedian MY, Tsangaris T, Momen B, et al. Infectious complications following breast reconstruction with expanders and implants. Plast Reconstr Surg. 2003;112:467–476. [DOI] [PubMed] [Google Scholar]
- 42.Olsen MA, Nickel KB, Fox IK, et al. Incidence of surgical site infection following mastectomy with and without immediate reconstruction using private insurer claims data. Infect Control Hosp Epidemiol. 2015;36:907–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lyle WG, Outlaw K, Krizek TJ, et al. Prophylactic antibiotics in plastic surgery: trends of use over 25 years of an evolving specialty. Aesthet Surg J. 2003;23:177–183. [DOI] [PubMed] [Google Scholar]
- 44.Hunter JG. Commentary on: effectiveness of prophylactic antibiotics in outpatient plastic surgery. Aesthet Surg J. 2014;34:1259–1260. [DOI] [PubMed] [Google Scholar]
- 45.Grunebaum LD, Reiter D. Perioperative antibiotic usage by facial plastic surgeons: national survey results and comparison with evidence-based guidelines. Arch Facial Plast Surg. 2006;8:88–91. [DOI] [PubMed] [Google Scholar]
- 46.Parikh RP, Sharma K, Qureshi AA, et al. Quality of surgical outcomes reporting in plastic surgery: a 15-year analysis of complication data. Plast Reconstr Surg. 2018;141:1332–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


