Abstract
Breast reconstruction has undergone significant innovation over the past 50 years. Both the development of nipple sparing mastectomy and the use of acellular dermal matrices have facilitated the concept of direct to implant (DTI) reconstruction. The next step in this evolution is further limiting the length of incisions as well as placing access in a more remote location. A robot-assisted surgical approach for DTI reconstruction (R-DTI) with an acellular dermal matrix scaffold is feasible and addresses limitations with open approaches and ergonomics. The authors performed a cadaveric exploration to demonstrate proof of concept and feasibility for an R-DTI following a robot-assisted nipple sparing mastectomy. Tremor stabilization, direct visualization, endo-wristed robotic instrumentation, and exposure were noted as key benefits over existing open DTI reconstruction techniques. Additionally, the ability to have a more remote access to entry at the perimeter of the breast eliminated incisional tension which can jeopardize reconstructive results. Further exploration and procedure refinements are warranted.
INTRODUCTION
The first report of implant-based reconstruction was described by Cronin and Gerow in 1963 as a delayed technique utilizing silicone gel.1 By 1971, Snyderman and Guthrie published their experience utilizing a direct to implant (DTI) reconstruction under the remaining chest wall skin immediately following the mastectomy.2 Despite these innovative approaches, first generation silicone implants (consisting of a thin shell and soft gel) were fraught with complications, including capsular contracture, implant malposition, and asymmetries resulting in immediate reconstruction failure rates of 26%.3
By the 1980s, many breast surgeons were performing modified radical mastectomies and preserving the pectoralis muscle. The development of the Radovan tissue expander was a significant innovation, shifting the reconstructive paradigm from an immediate to a 2-stage approach.4 Various creative methods to obtain partial and or total muscle coverage evolved during these early years and included the use of pectoralis major and serratus muscles in whole or in part. By the early 2000s, plastic surgeons began utilizing acellular dermal matrices (ADMs) in delayed reconstruction.5–11 The use of ADM in breast reconstruction is an off-label practice and should be conducted under specific clinical trials approved by the board of the concerned hospital and in some countries by the clinical authority. This technique improved soft tissue support and allowed for compartmentalization of implants, control of the inferior position of the pectoralis, and overall recreation of the breast footprint. The use of ADM further allowed for both consideration of DTI reconstruction which has become more popular in the last 15 years, as well as secondary management of hyper animation deformity seen in retropectoral implant placement in breast reconstruction after mastectomy.12,13
With larger and thicker sheets of ADM available, along with an increased prevalence in nipple sparing approaches to mastectomy, plastic surgeons have now reverted back to prepectoral DTI based reconstruction, the technique previously highlighted 50 years prior by Snyderman and Guthrie.2 The combination of DTI placement and ADM selection relies on proper patient selection as a fundamental component for optimal outcomes: healthy, nonsmokers, non-diabetics, and no immunosuppression. Additional considerations include current breast shape and size, degree of ptosis, and need for adjuvant therapies. While prepectoral DTI reconstruction results in less pain and less animation deformity, surgeons are quick to point out its shortcomings including implant malposition, contour irregularities (rippling), and the expense of unplanned secondary surgeries. Additionally, the incisional approach for mastectomy (periareola or inframammary fold), initial avascularity of ADM, and underlying implant tension theoretically leaves the mastectomy flap vulnerable to wound breakdown and puts the entire underlying reconstruction at risk of infection and total loss.
Given the recent success of Safarti and Toesca in their development of robot-assisted nipple sparing mastectomy (R-NSM) utilizing remote lateral incisions, the next natural evolution of robot-assisted breast surgery includes DTI reconstructive solutions.14,15 No prior authors have demonstrated a reproducible method of DTI reconstruction utilizing ADM. Our novel technique aims to standardize R-DTI reconstruction in an appropriate subset of patients.
The authors explored the concept of a robotic direct to implant (R-DTI) ADM reconstruction in a cadaveric model. They specifically chose to explore R-DTI with the da Vinci Single Port (SP) robotic platform (Intuitive Surgical; Sunnyvale, Calif.) so that a robotic nipple sparing mastectomy (R-NSM) and R-DTI could be performed through the same mid-axillary 3 cm incision. The authors hypothesized that the initial R-NSM would provide for a standardized mastectomy flap dissection with direct visualization of surgical planes as well as flap thickness. Additionally, R-DTI would utilize the same oblique mid-axillary incision which would reduce flap tension and wound complications associated with traditional DTI approaches, recreate the breast footprint with ADM by allowing for direct fixation to the chest wall and enable a no-touch technique for implant deployment potentially decreasing capsular contracture.
MATERIALS AND METHODS
The da Vinci SP surgical robotic system was utilized in a female cadaveric exploration over a 2-day period. The procedure explorations were led by the primary and senior author following an extensive discussion on the history, challenges, and unmet needs for DTI based reconstruction. Nipple sparing mastectomy, skin flap creation, and ADM fixation to the chest wall were performed entirely with the robotic system. The primary and senior author fabricated the ADM pocket ex-vivo on the back table to be ready for insertion at the completion of the mastectomy. The robot was undocked briefly for removal of the en-bloc mastectomy specimen, and to allow insertion of the ADM. Redocking of the robot followed to allow for visualization of flap perfusion, hemostasis, and robotic fixation of the ADM. A Keller Funnel (Allergan Inc.; Dublin Ireland) was utilized for implant deployment within the carefully constructed ADM scaffold.
RESULTS
The female cadaveric specimen was placed in a lateral decubitus position at the edge of the operating room table with the arm placed and secured across the patient’s body to allow tension-free safe docking of the robotic SP arm. After initially attempting a trans-axillary incision high in the axilla, the authors experienced a number of challenges with clearance of the robotic instrumentation due to external collisions. After a white board discussion and brainstorming, the authors elected to then perform both the nipple sparing mastectomy and DTI reconstruction through a lower mid-axillary incision using a slightly oblique incision conforming to Langer’s lines. A 3-cm incision was created along with a 1.5-cm subcutaneous circumferential dissection to allow for deployment of a GelPOINT wound retractor Advanced Access Platform (Applied Medical Resources Corporation; Rancho Santa Margarita, Calif.). The GelPOINT cover was then attached followed by introduction of the SP cannula. The da Vinci SP patient side cart was then docked (Fig. 1). Pneumo-insufflation was then established to aid with space creation. A meticulous nipple sparing mastectomy dissection was then performed with SP instrumentation utilizing the Cadiere and Hot ShearsTM (monopolar curved shears). The surgeons elected to perform the posterior (chest wall plane) dissection first to use both gravity and pneumo-insufflation to their advantage. After completion of the dissection of the breast gland off of the pectoralis fascia, the authors then proceed with the anterior dissection (skin flap adjacent portion) of the breast gland. Direct and magnified visualization of cooper’s ligament and the subcutaneous fat layer proved to be advantageous with the SP system. An assistant was present adjacent to the robot to ensure orientation and depth of flap dissection.
Fig. 1.
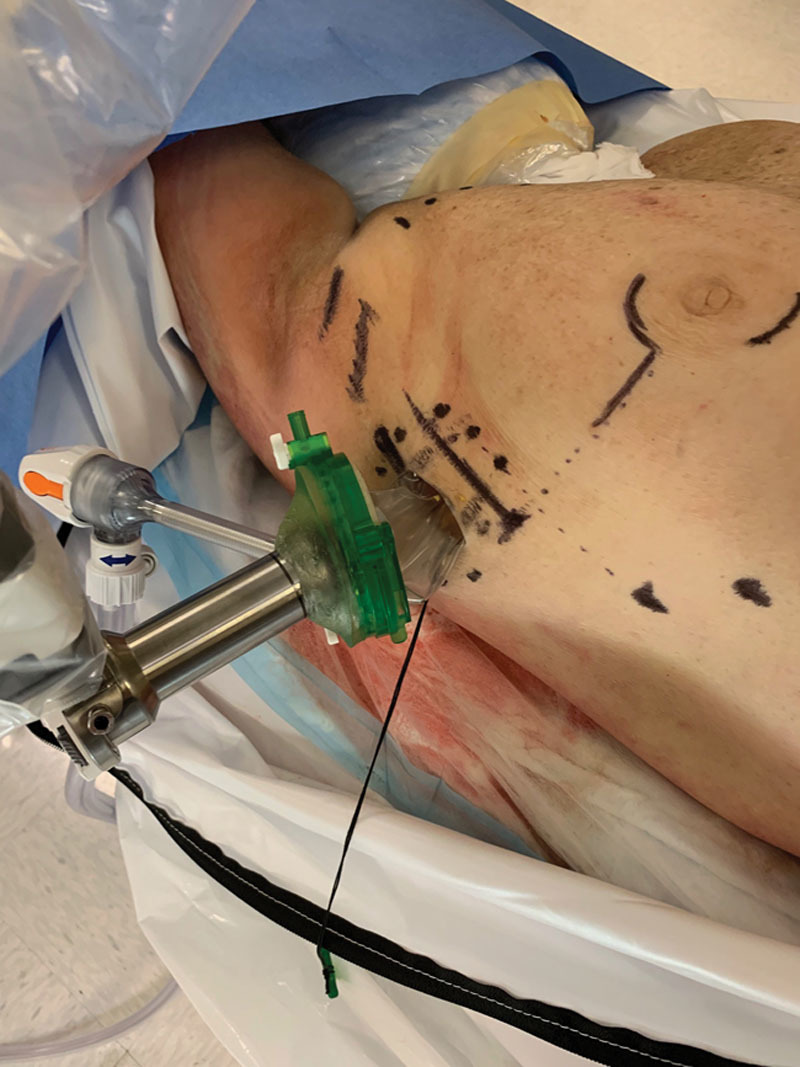
Docking to entry guide to facilitate robot-assisted nipple sparing mastectomy performed with da Vinci SP. Site chosen for best visualization, lack of skin flap tension, and lack of external collisions.
During the final phases of the mastectomy and removal of the breast gland, the plastic surgeons prepared the reconstruction construct of ADM on the back table fabricating a complete envelope around the implant sizer: having already estimated the most optimal implant for the reconstruction based on patient’s base diameter and overall breast shape and size (Fig. 2). The robot was undocked to allow insertion of the ADM into the cavity. The plastic surgeons re-docked the robot and re-insufflated the mastectomy cavity. Using the robot, the plastic surgeons initially attempted direct placement of interrupted sutures along the medial and inferior borders of the chest wall to fixate the ADM scaffolding and recreate the footprint of the breast gland. An initial attempt at fixating the ADM posteriorly along the medial border and inframammary fold was significantly limited with cavity pneumo-insufflation alone; it proved ineffective to manipulate, elevate, and mobilize the ADM construct during suturing. Following this initial attempt, the plastic surgeons deployed percutaneous placement of 2 temporary stay centering sutures under direct robotic guidance to anchor the anterior portion of the ADM scaffolding and prop up the leading proximal edges of ADM within the pocket (Figs. 3 and 4) While the pneumo-insufflation allowed for insufflation of the nipple sparing mastectomy flap itself, the percutaneous through and through sutures were foundational and enabled the plastic surgeons to strategically place the 5 interrupted braided absorbable sutures along the pre-marked medial and inferior borders of the scaffold and into the corresponding chest wall locations (Fig. 5). The ADM was fabricated to include an oblique laterally located opening, which, with the robot undocked, allowed for ease of final smooth surface implant deployment utilizing a no-touch technique into the ADM pocket via a Keller Funnel. A drain theoretically may be placed through the lateral incision. Final suturing of this 3-cm opening was addressed through the robot port and the skin incision was then closed.
Fig. 2.
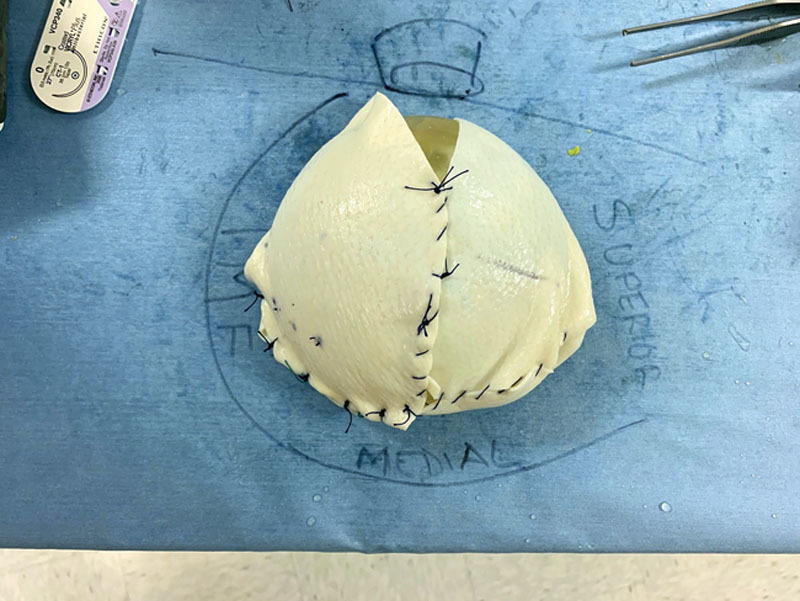
ADM wrapped breast implant sizer prefabrication on the back table.
Fig. 3.
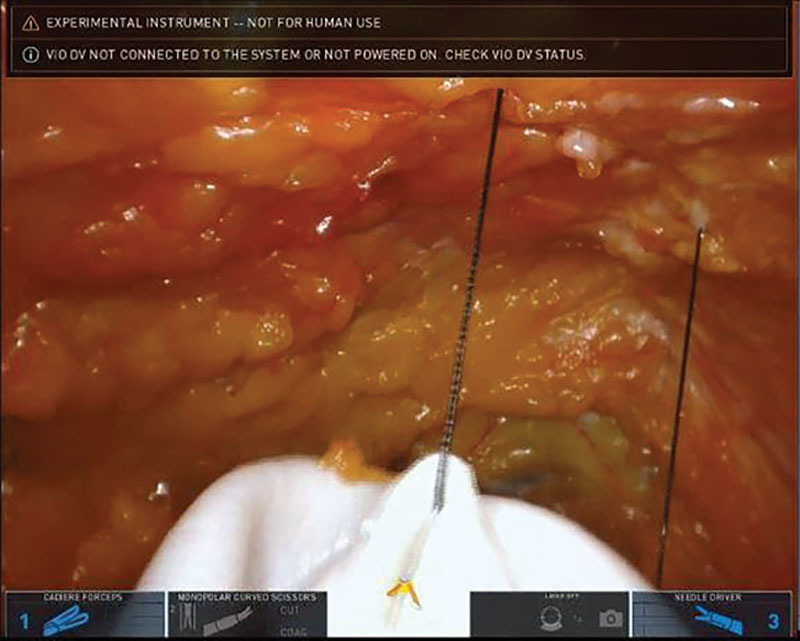
Percutaneous through and through centering suture placement.
Fig. 4.
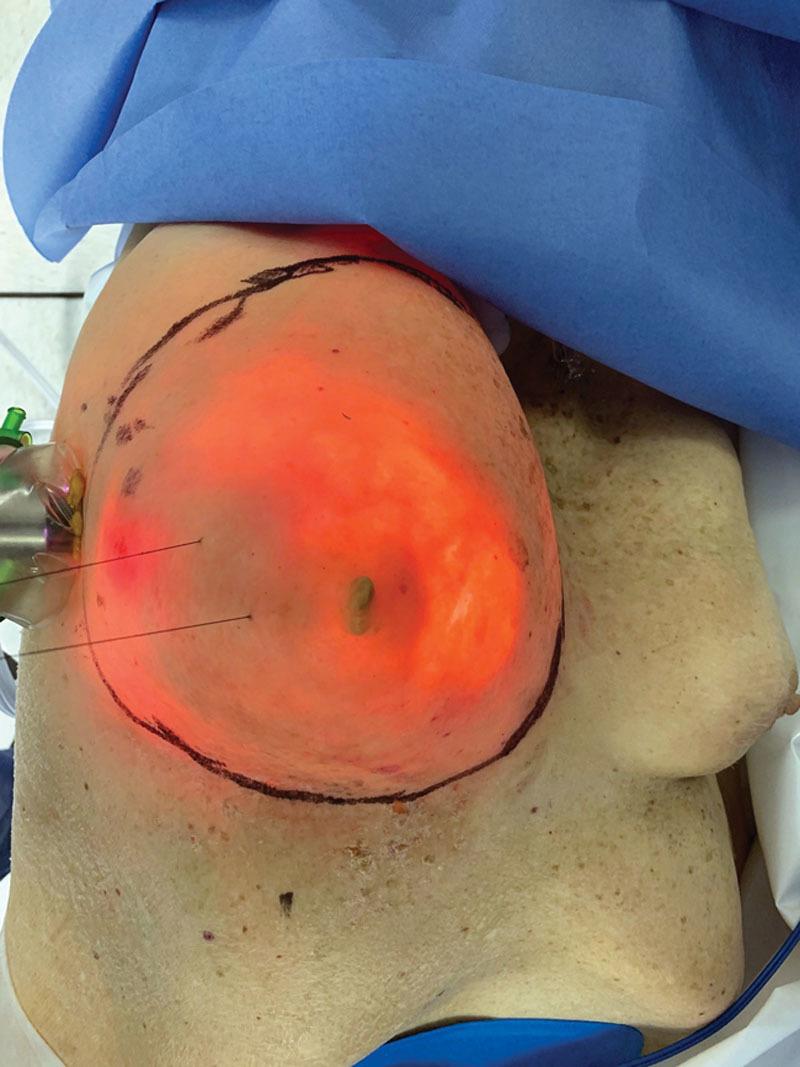
External view of percutaneous temporary stay sutures.
Fig. 5.
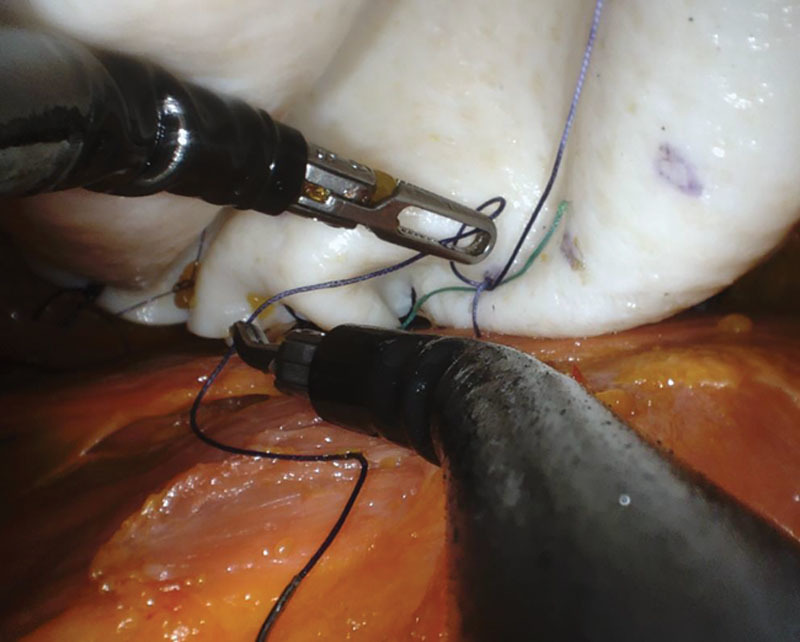
Robot-assisted DTI reconstruction with fixation of ADM to chest wall to recreate breast footprint.
DISCUSSION
In 2018, there were an estimated 266,120 new cases of breast cancer diagnosed in the United States with an additional 63,960 new cases of noninvasive (in-situ) breast cancer.16 Current evidence estimates that 1 in 8 US women (~12.4%) will develop invasive breast cancer over the course of their lifetime.17 In 2016, the American Society of Plastic Surgeons (ASPS) estimated that 109,256 reconstructive procedures were performed for reconstructive surgery following mastectomy including both unilateral and bilateral procedures. Of those reconstructions, implant-based reconstruction was chosen in 77% of cases.18 The ASPS further reported that 58,310 of the cases utilized ADM.
Given the current state of breast reconstruction outcomes, the authors developed a feasibility study to address the unmet need for a minimally invasive solution that can address problems with DTI based reconstruction: malposition, capsular contracture, skin flap tension and incision placement leading to wound healing delays and potentials loss of implant and need for additional surgeries. Further, the authors sought to develop a minimally invasive robot-assisted technique that can also address the ergonomics and limitations associated with open approaches.
The plastic surgeons were able to follow the R-NSM with an effective, reproducible R-DTI reconstruction that recreated and stabilized the footprint of the breast with ADM fixation. Robotic fixation of the ADM allows for precise placement not currently achievable through remote or limited length incisional approaches and could help prevent implant migration and malposition, exposure, rippling, and capsular contracture. Further, the ADM scaffolding can be internally fixated in a way that the tension is evenly distributed over the base of the chest wall. A remote access incision (modified mid-axillary) may theoretically avoid the tension and flap compromise that currently utilized approaches may influence.
Precision of implant placement was exact in presurgical markings and found to be reproducible in 2 breasts on a first attempt. When this lateral incision is combined with a no-touch Keller Funnel deployment technique, the authors hypothesize that surgical site infections and wound complications will be improved as well. The use of a Keller Funnel and direct deployment and fixation of the ADM scaffolding within the flap itself avoided any lengthening of the mastectomy incision that may be required with traditional approaches.
With every new technique or use of new technology comes a learning curve and along with it initially longer surgery. The use of Robotic Surgery in gynecology, urology, and general surgery has proven to be time and cost effective. This technique has the potential to result in less overall surgery with decreased revision rates. Additional benefits may include decreased postoperative burden on the patient and office staff by reducing the need for frequent office visits (as seen during tissue expansion), as well as decreased overall expense to the payer and healthcare system.
CONCLUSIONS
Key learnings from the cadaveric laboratory exploration include the necessity for optimal patient selection, positioning and incision placement to avoid external collisions between the robotic SP arm and the patient’s shoulder.
While the study showed proof-of-concept and feasibility for R-DTI based reconstruction with an ADM scaffolding, next steps include further exploration to further refine techniques. The authors also plan to evaluate the learning curve of this approach in comparison to current practices being utilized by Plastic and Reconstructive Surgeons.
ACKNOWLEDGMENT
The authors thank Intuitive Surgical, Inc., for their laboratory, technical, hardware, and software support.
Footnotes
Published online 24 January 2020.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
Off Label Disclosure: Da Vinci systems (Xi and SP) are not cleared for breast surgery or reconstruction in the United States or EU. Performing robot-assisted breast or reconstructive surgery is an off-label practice and should be conducted under specific clinical trials approved by the board of the concerned hospital and in some countries by the clinical authority.
REFERENCES
- 1.Cronin TD, Gerow FJ. Augmentation mammaplasty: a new ‘‘natural feel’’ prosthesis. 1963Paper Presented at: Transactions of the Third International Congress of Plastic and Reconstructive Surgery. Amsterdam Excerpta Medica. [Google Scholar]
- 2.Snyderman RK, Guthrie RH. Reconstruction of the female breast following radical mastectomy. Plast Reconstr Surg. 1971;47:565–567. [DOI] [PubMed] [Google Scholar]
- 3.Nahai F, Bostwick J., III. Aesthetic aspects of breast reconstruction. Aesthetic Plast Surg. 1982;6:61–67. [DOI] [PubMed] [Google Scholar]
- 4.Radovan C. Breast reconstruction after mastectomy using the temporary expander. Plast Reconstr Surg. 1982;69:195–208. [DOI] [PubMed] [Google Scholar]
- 5.Breuing KH, Colwell AS. Inferolateral alloderm hammock for implant coverage in breast reconstruction. Ann Plast Surg. 2007;59:250–255. [DOI] [PubMed] [Google Scholar]
- 6.Spear SL, Parikh PM, Reisin E, et al. Acellular dermis-assisted breast reconstruction. Aesthetic Plast Surg. 2008;32:418–425. [DOI] [PubMed] [Google Scholar]
- 7.Nahabedian MY, Mesbahi AN. Nahabedian MY. Breast reconstruction with tissue expanders and implants. In: Cosmetic and Reconstructive Breast Surgery. 2009:London: Elsevier; 1–20. [Google Scholar]
- 8.Salzberg AC. Nonexpansive immediate breast reconstruction using human acellular tissue matrix graft (AlloDerm). Ann Plast Surg. 2006;57:1–5. [DOI] [PubMed] [Google Scholar]
- 9.Zienowicz RJ, Karacaoglu E. Implant-based breast reconstruction with allograft. Plast Reconstr Surg. 2007;120:373–381. [DOI] [PubMed] [Google Scholar]
- 10.Namnoum JD. Expander/implant reconstruction with alloderm: recent experience. Plast Reconstr Surg. 2009;124:387–394. [DOI] [PubMed] [Google Scholar]
- 11.Bindingnavele V, Gaon M, Ota KS, et al. Use of acellular cadaveric dermis and tissue expansion in postmastectomy breast reconstruction. J Plast Reconstr Aesthet Surg. 2007;60:1214–1218. [DOI] [PubMed] [Google Scholar]
- 12.Jones G, Yoo A, King V, et al. Prepectoral immediate direct-to-implant breast reconstruction with anterior alloderm coverage. Plast Reconstr Surg. 2017;1406S Prepectoral Breast Reconstruction31S–38S. [DOI] [PubMed] [Google Scholar]
- 13.Gabriel A, Sigalove S, Sigalove NM, et al. Prepectoral revision breast reconstruction for treatment of implant-associated animationdeformity: a review of 102 reconstructions. Aesthet Surg J. 2018;38:519–526. [DOI] [PubMed] [Google Scholar]
- 14.Toesca A, Peradze N, Galimberti V, et al. Robotic nipple-sparing mastectomy and immediate breast reconstruction with implant: first report of surgical technique. Ann Surg. 2017;266:e28–e30. [DOI] [PubMed] [Google Scholar]
- 15.Sarfati B, Struk S, Leymarie N, et al. Robotic prophylactic nipple-sparing mastectomy with immediate prosthetic breast reconstruction: a prospective study. Ann Surg Oncol. 2018;25:2579–2586. [DOI] [PubMed] [Google Scholar]
- 16.National Cancer Institute. Surveillance, Epidemiology and End Results Program. 2019. https://seer.cancer.gov/statfacts/html/breast.html
- 17.DeSantis C, Ma J, Bryan L, et al. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64:52–62. [DOI] [PubMed] [Google Scholar]
- 18.Plastic Surgery Statistics Report 2016. ASPS National Clearinghouse of Plastic Surgery Procedural Statistics.


