Abstract
Background:
We aimed to assess whether our novel Nanofat grafting procedure improves skin quality while yielding a regenerative effect and whether this novel technique can also achieve a lifting effect.
Methods:
Patients who requested nonsurgical facial rejuvenation were enrolled between June 2018 and December 2018. Fat was aspirated from the medial thigh, inner part of the knee, or lower abdomen regions. Following aspiration and flushing, microfat was obtained after washing with saline. This microfat was emulsified to obtain a Nanofat suspension, which was injected using a 25-G cannula into the subcutaneous layer at different facial sites. Images were obtained before and at 1, 3, and 6 months after facial rejuvenation. Patients were also administered a survey. Characterization of the isolated stromal vascular fraction (3 patients), and before/after biopsies were performed.
Results:
Fifty patients were included (2 men and 48 women; mean age, 35–65 years; mean follow-up, 9 months). The clinical results were apparent between 2 and 4 weeks after injection, and improvements were continuously observed until 6 months postoperatively. All patients confirmed an improvement in skin quality. A lifting effect was also observed. The data confirm that the Nanofat procedure does not damage cells, maintaining cell viability, and number of adipose-derived stem cells. Biopsies showed an increased dermal cellularity, vascular density, and elastic and collagen fiber density.
Conclusion:
Facial rejuvenation with subcutaneous Nanofat injections appears to be an effective method, representing a skin rejuvenation effect by modifying the pattern of the dermis, although additional studies are necessary.
INTRODUCTION
The demand for nonsurgical facial restoration has been constantly increasing in the past few decades. The Nanofat grafting procedure is a promising technique that could meet this demand and yields a marked improvement in skin quality. (See Video [online], which displays facelift by Nanofat grafting.)
Video 1. This video shows facial lift with nanofat grafting. From “Subcutaneous injections of Nanofat adipose derived stem cells grafting in facial rejuvenation”
1_30tqoazn Kaltura
In 2013, Tonnard et al introduced a Nanofat grafting technique, wherein mechanical emulsification was used to mechanically dissociate adipose tissue while retaining the extracellular matrix with all nucleated cells including the already present stem cells. This sample was then injected using a 30-G needle into the fine lines of the face.1 This method took advantage of the release of growth factors from cells present in the tissue and the regenerative capacities of adipose-derived stem cells (ASCs).2,3 On exposure to tissue-specific biochemical signals, ASCs could differentiate into different tissues. This grafting procedure frees the cells present in the adipose tissue to have a biologic effect in the process of reshaping the face without a change in volume.
In the present study, we aimed to assess whether the Nanofat grafting procedure improves skin quality and whether the product issued from this process of mechanical dissociation maintains the cells present in the tissue alive and in a reasonable amount. Finally, we also sought to determine whether this novel technique can also achieve a lifting effect.
MATERIALS AND METHODS
Fat Collection
We enrolled patients who requested nonsurgical facial rejuvenation between June 2018 and December 2018. The Nanofat grafting procedure was performed under local anesthesia. In brief, following disinfection with povidone iodine, fat was aspirated at the most superficial level possible to recover the maximum number of stem cells from the medial thigh, inner part of the knee, or lower abdomen. Anesthesia was induced with infiltration with Klein solution (500-mL normal saline, 50-mL 2% lidocaine, 1-mL 1:1,000 adrenaline, and 15-mL 8.4% sodium bicarbonate) between 1 and 4 areas are injected with 20–40 mL. For fat collection, we used the Hapi Fat kit (Benewmedical, Geneva, Switzerland); a 14-G 130-mm cannula was connected to a 10-mL Luer Lock syringe, and small adipocyte lobules were harvested under low negative pressure via a fatlock connector valve system and a tube attached to a PureGraft 50 pocket.
Pure Graft Procedure
The procedure was conducted in a closed system, and the quantity of fat harvested ranged from 30 to 50 mL. Each 10-mL sample of fat aspirated and flushed into the Pure Graft pocket was washed with 10 mL of sterile saline. At the end of the procedure, 40 mL of fat was collected to obtain approximately 20 mL of microfat tissue.
Emulsification of the microfat to obtain a Nanofat suspension was achieved with the Tulip kit (Tulip Medical products, San Diego, CA); this included 3 filters (2.4, 1.4, and 1.2 mm) and involved shifting between two 20-mL syringes connected to each other by a female-to-female Luer-lock connector for >30 passes for each filter. The final filter included a Tulip nanotransfer 600/400 μm device, and only 1 pass was used to obtain a pure product with the least number of fibers possible; the product could easily be injected through a 25-G cannula or a 27-G needle.
A total of 15 mL of Nanofat suspension was expected to be recovered from 20 mL of microfat. To this suspension, we added 20% platelet-rich plasma (PRP), which was prepared from the native blood of the patient with a 2-step centrifugation Duografter II (Proteal, Madrid, Spain), after the platelets were counted with a Horiba ABS Micro device (Axonlab, Baden, Switzerland) to ensure appropriate quality for Nanofat grafting.
The Nanofat-PRP suspension was finally transferred into individual 1-mL syringes and could be injected into the recipient area. The emulsion was injected with a fanning technique, using a 25-G cannula into the subcutaneous layer at various sites on the face via 4 entry points: orbital area, cheek, jaw line, and temporal area. Nanofat grafting was first performed at the lower lid and orbital area 1 mL, followed by the upper lip 1 mL, lower lip 1 mL, chin 1 mL, cheek 1 mL, nasolabial fold 1 mL, jaw line 1 mL, parotid area 1 mL, and temporal area 1 mL. Nanofat was applied from a single syringe in each area (Fig. 1).
Fig. 1.
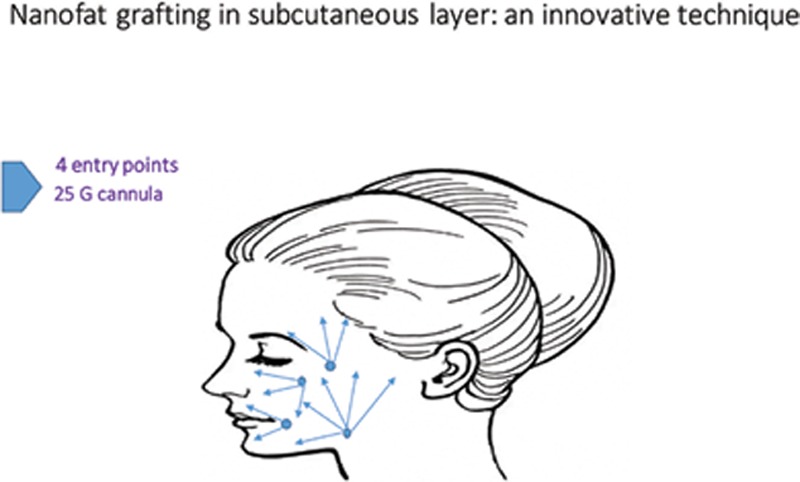
Graph of injection sides.
Standardized photographs were obtained for all patients before the procedure, and at 1, 3, and 6 months after facial rejuvenation. All the patients were self-evaluated using a visual scale, graded from 0 to 10. The survey concerned the following clinical aspects of the face: luminosity, firmness, superficial and deep wrinkles, folds, volume, and overall rejuvenation. The subjects were also asked to grade the overall appreciation by their social circle (Fig. 2).
Fig. 2.
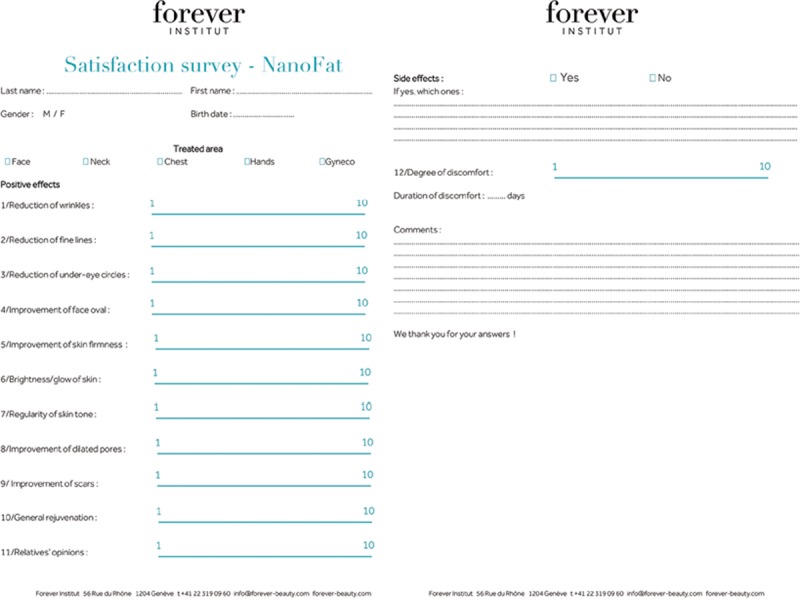
Satisfaction survey—Nanofat.
Characterization of the Isolated Stromal Vascular Fraction
The fraction produced by the Nanofat procedure was characterized for cell counting, viability, and ASC presence by enzymatically extracting the Nanofat product. The sample was washed with 40-mL Dulbecco’s Deprieved Phosphate Buffered Saline (DPBS; with Ca2+ and Mg2+; PAA Laboratories, Pasching, Austria) by gentle agitation. The syringe was held vertically in the support stand for a few minutes to allow for the separation of the phases, then the lower aqueous phase was discarded by pushing the piston. The sample was washed twice. To free the cells in the aqueous phase, the washed adipose tissue must be digested with the appropriate amount of Liberase MTF-S (Roche Applied Science, Basel, Switzerland) at a final concentration of 0.28 Wünsch U/mL diluted in 10-mL DPBS (with Ca2+ and Mg2+). The sample was incubated for 45 minutes at 37°C under constant but gentle agitation. Enzymatic reaction was stopped by aspiration of 30 mL of injectable 5% human albumin solution (CSL Behring AG, Bern, Switzerland) in the syringe. The syringe was then put back in vertical position to allow the separation of the phases. The lower layer, which now contained the stromal vascular fraction (SVF) cells, was carefully poured out into a conical 50-mL centrifuge tube (TPP, Trasadingen, Switzerland). The extracted cells were washed again with 40-mL 5% human albumin solution to increase cell yield. Finally, after filtration through a 100- and 40-μm sieve (Cell Strainer, BD Falcon, Basel, Switzerland), SVF was centrifuged 400g, 5-minute room temperature (RT) and the pellet suspended another time in DPBS (without Ca2+ and Mg2+; PAA Laboratories) or in tissue culture medium. The SVF was then analyzed for cell count and viability using an automated propidium iodide-based cell counting device (Nucleocounter NC-100, Chemometec A/S, Denmark). The cells of the SVF were also characterized by cytofluorimetric analysis using a 10-channel Navios cytometer [Beckman Coulter (BC), Nyon, Switzerland). Briefly, roughly 500,000 cells from fresh SVF preparation were taken and centrifuged 5 minutes at 400g. The pellet was resuspended in 220 μL of PBS without Ca2+/Mg2+ (Eurobio, CS1PBS01) with 1% human converted AB serum (PAA, C11-021), and the cells were stained for control antibodies IgG2a-PE (BC, A12695), IgG1-KRO (BC, A96415), IgG1-APC-A750 (BC, A71120), and Syto 40 (Invitrogen, S11351) on the one hand and CD146-PE (BC, A07483), CD45-KRO (BC, A96416), CD34-APC-A750 (BC, A89309), 7-amino-actinomycin D, and Syto 40 on the other hand for sample counting, respectively. The data were analyzed with the Kaluza software (BC). Briefly, the DNA marker Syto 40 was used to exclude cellular debris (ie, negative) and 7-amino-actinomycin D was used for dead and live cell discrimination and therefore for assessing the cellular viability. ASCs were identified in the CD45- and CD146-negative and CD34-positive fraction. Finally, flow-count fluorospheres were used to directly determinate the absolute number of ASCs by applying the formula: absolute count (cells/μL) = (total number of cells counted/total number of fluorospheres counted) × flow-count fluorospheres assayed concentration.
Histology
Histologic examination of the skin before treatment and 6 months after treatment was performed. The biopsy was performed on the forearm 2 cm × 1 cm before, and after injection of 1-mL Nanofat suspension, before adding PRP, in the subcutaneous layer, at a distance of 5 cm. The biopsy material was fixed in formalin, embedded in paraffin, cut at 5 µm and stained with hematoxylin–eosin, Trichrome-Masson and Miller, and immunohistochemistry was performed for CD31 (DakoCytomation, Glostrup, Denmark) according to standard procedures. We counted the number of dermal cells, blood vessels, and elastic fibers of each slide per 3 high-power fields (×40). Statistical analysis was performed by using a Student’s t test.
RESULTS
The 50 patients who underwent Nanofat grafting for facial rejuvenation included 2 men and 48 women aged 35–65 years. The mean follow-up period was 9 months (range, 6–12 months). At least 18 mL of Nanofat-PRP suspension was injected into the subcutaneous layer with the 25-G cannula over different facial sites. Patients received no other concomitant procedures or topical therapies during the follow-up period. The clinical results were apparent between 2 and 4 weeks after injection, and improvements were continuously observed over time until 6 months postoperatively (Figs. 3–6).
Fig. 3.
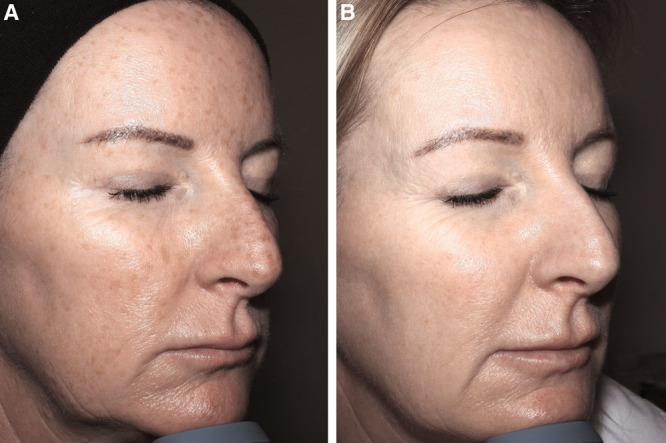
Photographs of the face before (A) and 6 months after (B) 18-mL Nanofat grafting of a 42-year-old patient.
Fig. 6.
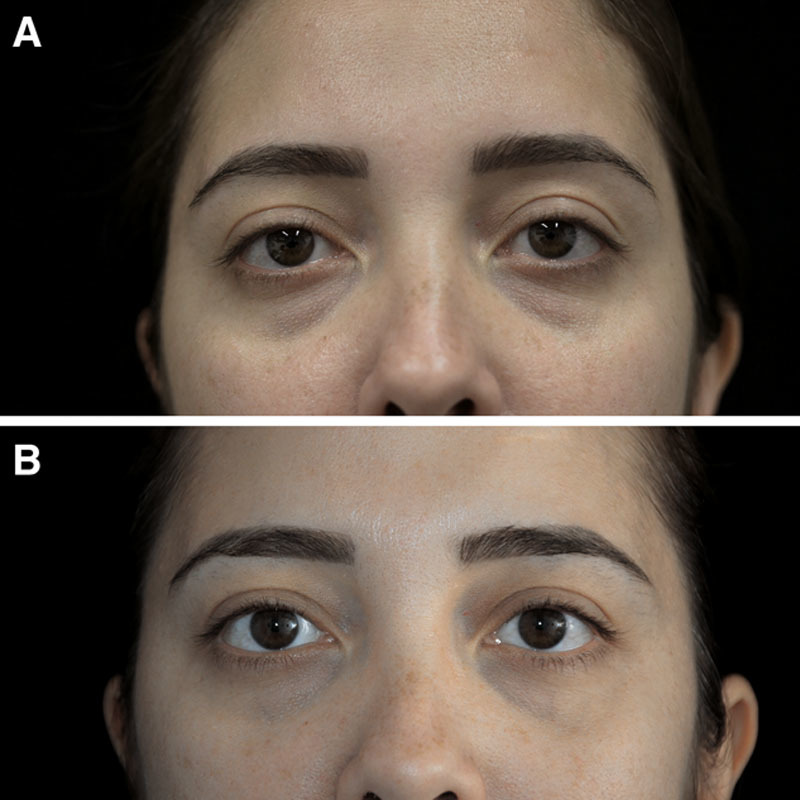
Photographs of the face before (A) and after (B) 6 months 18-mL Nanofat grafting of a 35-year-old patient.
Fig. 4.
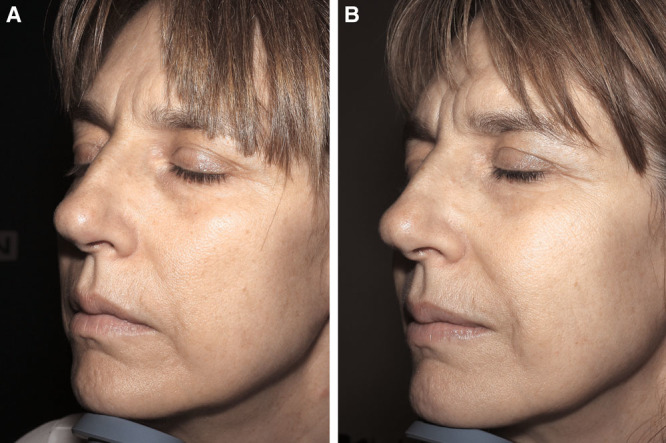
Photographs of the face before (A) and 6 months after (B) 18-mL Nanofat grafting of a 48-year-old patient.
Fig. 5.
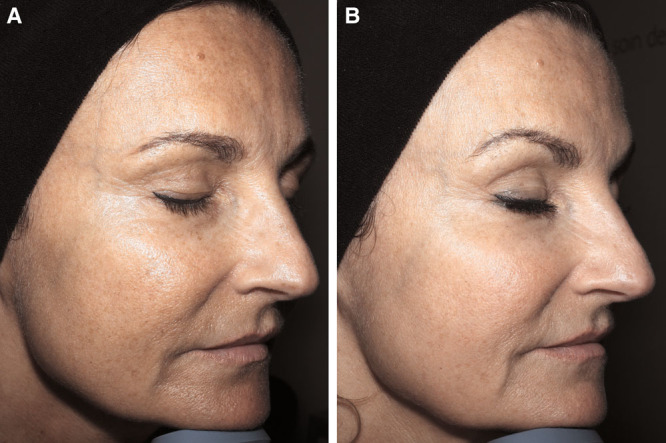
Photographs of the face before (A) and 6 months after (B) 18-mL Nanofat grafting of a 52-year-old patient.
An analysis of preprocedural and postprocedural images indicated that a lifting effect of the face was achieved with the procedure. In the survey (Fig. 7), most patients answered that they now did not need to apply makeup or skin care daily. All patients confirmed an improvement in texture, elasticity, glow, firmness, fine wrinkles, and skin hydration, along with a regenerative effect. Moreover, all patients exhibited a considerable improvement with regard to skin glow and regularity, and more than 80% of patients exhibited a large improvement in facial shape and opinions of relatives.
Fig. 7.
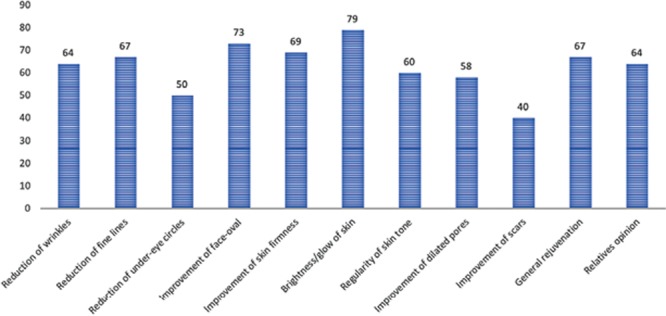
Patients’ answers to questions about skin improvement.
No major side effects were observed in the present study. Minor complications, including redness and edema between 2 and 4 days, some bruises, and pain at the donor sites, were noted in all the patients. Only 1 patient developed nodules at lower lid, although this condition healed spontaneously after 1 month. No infections, fat cysts, or granulomas were observed after treatment and during the follow-up period.
To assess the quality of Nanofat product, we extracted the SVF of 3 samples after Nanofat processing. Data are shown in Fig. 8. As can be observed, the viability of the cells is maintained after this procedure of mechanical disruption with more than 90% of cell viable. Total nucleated cells counts are also in the range of what is obtained by regular SVF extraction without Nanofat procedure, where, on more than 300 samples processed under GMP guidelines, we found a comparable mean value. Finally, the number of ASCs is also similar to that obtained without the Nanofat procedure.
Fig. 8.
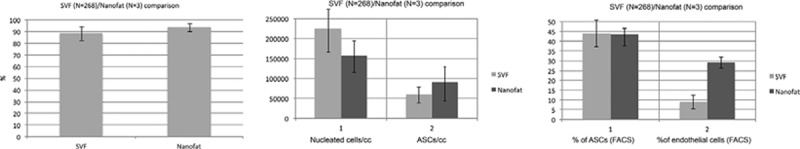
Comparison of N = 3 patients, where adipose samples have been extracted before and after Nanofat processing. In both cases, cells were extracted by manual processing following enzymatic digestion and then counted for total nucleated cells and ASCs.
Overall, the data confirm that the Nanofat procedure does not damage cells contained in the adipose tissue, maintaining cell viability, and number of ASCs.
Histologic analyses were performed to assess the histologic changes of the skin after injection of Nanofat grafting.
Skin biopsy performed on the posttreatment zone showed an increase in collagen (denser and broader collagen fibers) and elastic fibers (control: 171 ± 9.5; Nanofat: 266 ± 17.3; P < 0.005) in the superficial dermis (Fig. 9). Treated zones also displayed an increased dermal cellularity (mainly fibroblasts) (control: 59.6 ± 6.8; Nanofat: 90.6 ± 3.05; P < 0.03) and vascular density (control: 15.6 ± 0.5; Nanofat: 26.6 ± 3.05; P < 0.01) in the superficial dermis (Fig. 10). No modification was observed in epidermal thickness or in mucin content of the dermis. There was no significant inflammation in the treated zones.
Fig. 9.
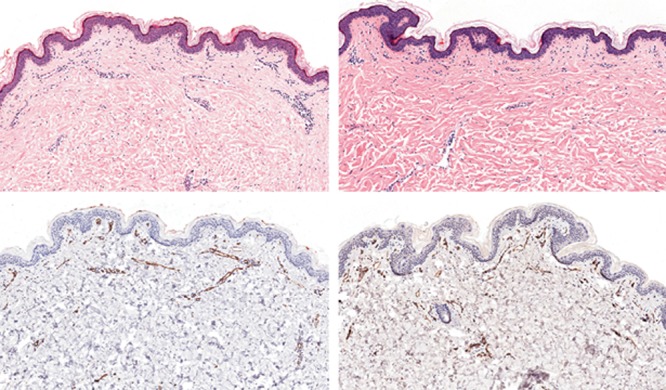
Hematoxylin–eosin staining (A, B) and CD31 immunohistochemistry (C, D) of untreated (A, C) and Nanofat-treated (B, D) skin (original magnification, 20×). Note the increased density of dermal cellularity (mainly fibroblasts) (B) and dermal vessels (D) in the treated skin.
Fig. 10.
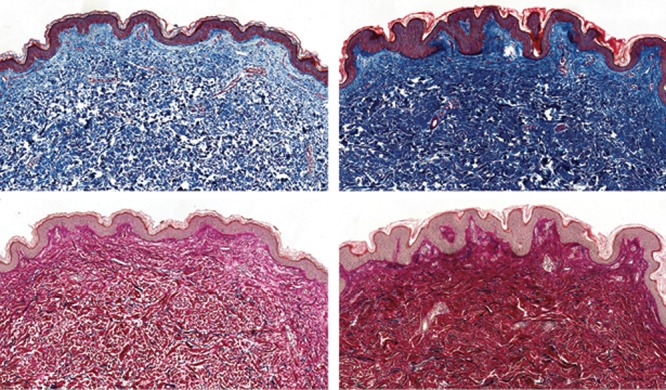
Trichrome-Masson (A, B) and Miller (C, D) stainings of untreated (A, C) and Nanofat-treated (B, D) skin (original magnification, 20×). Note the increased density of collagen (B) and elastic fibers (D) in the treated skin.
DISCUSSION
The quality of an individual’s skin communicates several details about their habits. There are 2 major factors that form the foundation of patients’ attitudes toward skin quality: health (radiant, glow, natural, nourished) and youth (no wrinkles, smooth, firm, plump, elastic). Patients believe that skin beauty plays a role in self-confidence and success.
The Nanofat grafting technique seeks to increase vascularization, neocollagenesis, and tissue regeneration and also to improve skin physiology. In particular, ASCs play a vital role in skin rejuvenation,2 although their multipotent nature may not be the only advantage. ASCs also secrete stimulatory factors, such as cytokines, chemotactic factors, extracellular remodelers, growth factors, and their inhibitors. These factors are capable of very complex functions involving several bioactive factors and reactivities.3 ASCs not only help in skin regeneration but also in the repair of various functions such as melanocyte action. ASCs inhibit melanocyte proliferation and melanin synthesis by downregulating melanogenic enzymes through an interleukin-6-mediated mechanism.4,5 This leads to good results for the treatment of dark coloration of lower eyelid skin.6
In a previous study, Tonnard et al quantified the cells from the SVF and its CD34+ subfraction; the researchers assessed stem cell quality by culturing the cells in standard and adipogenic media.1 In the emulsion, the normal fat tissue structure is lost, but mesenchymal stem cells remain intact as they were in the undissociated adipose tissue.7 In the 3 samples analyzed, we found higher number of ASCs than microfat samples (Fig. 7). Moreover, these cells are associated with increased regenerative capacity, which may have contributed to the observed lifting effect.8 Nanofat processing is more than simply breaking down adipocytes; in fact, mechanical emulsification leads to upregulation of multipotent and pluripotent markers, which induce the regenerative capacity.1 In addition to skin regeneration, the product also created a lifting effect through the injection of the product subdermally in many areas of the face. This technique led to improvements in facial appearance without a change of volume.1
Hyaluronic acid has been used as an appropriate treatment for improving skin quality. In addition, lipofilling, PRP, mesotherapy with peptides and vitamins, lasers, and laser-based devices such as ultrasound and radiofrequency are also considered appropriate options. However, these modalities only have a moderate impact on skin quality, and fillers have also been reported to cause complications, lumps, bruising, and irregularities.9
Additional studies should be conducted to test a control group with hyaluronic acid or PRP alone. Liang et al showed that both Nanofat-plasma rich fibrin (PRF) and hyaluronic acid injection improve facial skin status without serious complications with greater patient satisfaction, implying that Nanofat-PRF injection is a safe, highly effective, and long-lasting method for skin rejuvenation.10
We believe that the lower third of the aging face (lips and immediate perioral area) deserves as much attention as the midface and that the temporal area should be covered with the injections. The injection of Nanofat in the subcutaneous layer could enable expansion of this intervention to sensitive areas, including skin rejuvenation from the zygomatic arch to the temporal area, without side effects.
In the Nanofat procedure, the time required for infiltration, harvesting, and emulsification ranged from 30 to 45 minutes; to reduce process time, PRP was centrifuged before fat harvesting. The injection time with the cannula remained the same as for treatment with fillers. The Nanofat grafting procedure may cause swelling for 2–4 days, as well as bruising and pain at the donor site for 1 week. However, no delayed reactions have been noted with this procedure over 5 years, as noted through studies in the literature. To avoid vascular complications, the product was injected with a cannula; if a needle was used, small aliquots would need to be injected under low pressure.
The disadvantages of the Nanofat technique include the necessity of a surgical procedure, as some discomfort is involved in extracting fat from the donor site. Moreover, the site and manner in which the product is injected are important factors for obtaining a good result, but there are other factors involved as well. Each step in the process should be carefully monitored, beginning with the choice of donor site, followed by harvesting, processing, and injection techniques.11–14 Disinfection is very important, although Pallua et al showed that local antiseptic treatment can affect ASC viability. In particular, octenidine has been found to decrease ASC viability, even at the lowest concentrations.15 Povidone iodine and polyhexanide also decrease ASC viability and significantly reduce the expression of stem cell markers (CD29, CD34, CD73, CD90, CD105). Polyhexanide reduces the levels of CD34 alone, but mafenide does not alter ASC viability, even at high concentrations, and does not affect stem cell marker levels. Thus, mafenide is a good antiseptic for such purposes. However, in the present study, we used povidone iodine as it was easy to procure and then washed the area carefully with saline solution, including the entry points, to avoid any introduction of antiseptic inside the tissues.15
Furthermore, Pallua et al indicated that the abdominal wall is the optimal site for harvesting due to the high number of SVF cells, as well as high levels of MMP-9, IGF-1bFGF, and PDGF. However, there was no significant difference in the ASC count (CD90+, CD105+, CD31−) among the harvest sites, and the proliferation, differentiation, and SVF cell/ASC counts did not significantly differ among the harvest sites.16 In addition, the amount of anesthesia administered should be limited to protect the stem cells, as lidocaine has been found to be toxic.17
Tonnard et al indicated that microfat should be harvested with barbed (rasping) cannulas, as they contain a greater number of ASCs as compared to fat harvested with smooth multiholed cannulas.12 In the present study, we used a microcannula as it represented a minimally invasive method to obtain clinically relevant numbers of stromal and vascular cells for tissue regeneration and neovascularization.18 Furthermore, the collected material had to be clear and washed carefully. Condé-Green et al observed that washed fat grafts contained higher amounts of ASCs, as compared to decanted and centrifuged fat grafts.19 By adding PRP, we enhance cell graft survival and maintenance.20,21
PRP alone is effective on skin rejuvenation, but a minimum of 3 sessions are still necessary to obtain results.22 In a thesis work on PRP as potentialisator of cell therapy in the field of plastic surgery, 20% is the number allowing the best efficiency.23
Some data suggest that lesions could be treated by injection of ASCs. They are capable to differentiate in other cells giving them the first role in regenerative medicine. They also have an important paracrine activity and modulate immune tolerance and inflammation.24 The effects of Nanofat are visible in the epidermis also if the injection is in the subcutaneous layer. We need however more studies to understand all sequences of events. It is interesting to note that treatment with Nanofat induces histologic modification quite similar to those induced by SVF. It modifies the pattern of collagen fibers and elastic fibers in the dermis.
Biopsy was performed in the inner part of the forearm because biopsy on the face was not acceptable for patients. The skin is not similar in all parts of the body, even in the face. Distribution of skin thickness (diameter of the epidermis and dermis) differs in the face from 2.5 mm in the chin to 0.5 mm in the eyelids. The number of hair follicles per square centimeter differs too, from 700 on the chin to 1,200 on the nose. But the layers, the structure, function, and number of fibroblasts are the same in all the skin types.25
Fat grafting techniques are generally based on the volume and goal needed, including the Coleman technique [small volume fat grafting (<150 mL) for facial rejuvenation and regeneration], Khouri technique [arge-volume fat grafting (>150 mL) for breast contouring and buttock augmentation], and Nanofat technique by Tonnard [very small volume (<15 mL) for facial skin rejuvenation with a small and sharp needle for intradermal injections).
At the Forever Institut (Geneva, Switzerland), we developed a novel technique wherein Nanofat (18 mL) was subcutaneously injected at many facial sites using a 25-G cannula, and good results were noted in terms of skin quality and a lifting effect. The duration for which the effects of Nanofat grafting persist is unclear, as this procedure needs to be assessed further. Hence, additional studies should be conducted to test the significant effect of Nanofat grafting1 and should include objective skin quality tests with a Cutometer, to measure improvement, elasticity, and firmness.
CONCLUSIONS
Although the current modalities are all suitable for improving skin quality, facial rejuvenation with subcutaneous Nanofat injections appears to be a very effective method, by modifying the pattern of dermis. Nanofat grafting is a regenerative technique, involves an autologous procedure, enables easy collection, and involves single-day ambulatory treatment. In addition, the results of clinical trials indicated the safety of the method. Moreover, Nanofat naturally integrates into host tissues without producing any major side effects. The long-term effects need to be further evaluated, but Nanofat technology can likely be expanded to cover indications for hair or genital rejuvenation; the initial results for those indications appear very promising.
Footnotes
Published online 21 January 2020.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Tonnard P, Verpaele A, Peeters G, et al. Nanofat grafting: basic research and clinical applications. Plast Reconstr Surg. 2013;132:1017. [DOI] [PubMed] [Google Scholar]
- 2.Charles-de-Sa L, Gontijo-de-Amorin NF, Maeda Takiya C, et al. Antiaging treatment of the facial skin by fat graft and adipose-derived stem cells. Plast Reconstr Surg. 2015;135:999–1009. [DOI] [PubMed] [Google Scholar]
- 3.Caplan A, Yap S, Casteilla L, et al. Perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. [DOI] [PubMed] [Google Scholar]
- 4.Lin JY, Fisher DE. Melanocyte biology and skin pigmentation. Nature. 2007;445:843–850. [DOI] [PubMed] [Google Scholar]
- 5.Deok-Woo K, Byung Joon J, Na Hyun H, et al. Adipose derived stem cells inhibit epidermal melanocytes through an interleukin-6-mediated mechanism. Plast Reconstr Surg. 2014;134:470–480. [DOI] [PubMed] [Google Scholar]
- 6.Dong Seok O, Dae Hwa K, Tai Suk R, et al. Correction of dark coloration of the lower eyelid skin with Nanofat grafting. Arch Aesthetic Plast Surg. 2014;20:92–96. [Google Scholar]
- 7.Memar O, Nezamabadi A, Milani BY, et al. Nanofat grafting: basic research and clinical application. Plast Reconstr Surg. 2014;133:728e. [DOI] [PubMed] [Google Scholar]
- 8.Banyard DA, Sarantopoulos CN, Borovikova AA, et al. Phenotypic analysis of stromal vascular fraction after mechanical shear reveals stress-induced progenitor populations. Plast Reconstr Surg. 2016;138:237e–247e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goisis M, Stella E, Di Petrillo A, et al. Nanofat grafting compared to hyaluronic acid and PRP treatment of the lower lid and tear trough: clinical outcome. JSM Ophtalmol. 2015;3:1057. [Google Scholar]
- 10.Liang ZJ, Lu X, Li DQ, et al. Precise intradermal injection of nanofat-derived stromal cells combined with platelet-rich fibrin improves the efficacy of facial skin rejuvenation. Cell Physiol Biochem. 2018;47:316–329. [DOI] [PubMed] [Google Scholar]
- 11.Alharbi Z, Opländer C, Almakadi S, et al. Conventional vs. micro-fat harvesting: how fat harvesting technique affects tissue-engineering approaches using adipose tissue-derived stem/stromal cells. J Plast Reconstr Aesthet Surg. 2013;66:1271–1278. [DOI] [PubMed] [Google Scholar]
- 12.Caggiati A, Germani A, Di Carlo A, et al. Naturally adipose stromal cell-enriched fat graft: comparative polychromatic flow cytometry study of fat harvested by barbed or blunt multihole cannula. Aesthet Surg J. 2017;37:591–602. [DOI] [PubMed] [Google Scholar]
- 13.Charles-de-Sá L, Gontijo-de-Amorim NF, Maeda Takiya C, et al. Antiaging treatment of the facial skin by fat graft and adipose derived stem cells. Plast Reconstr Surg. 2015;135:999–1009. [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez RL, Condé-Green A. Reply: adipocyte damage in relation to different pressures generated during manual lipoaspiration with a syringe. Plast Reconstr Surg. 2013;131:646e–647e. [DOI] [PubMed] [Google Scholar]
- 15.Kim BS, Ott V, Boecker AH, et al. The effect of antiseptics on adipose-derived stem cells. Plast Reconstr Surg. 2017;139:625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kakagia D, Pallua N. Autologous fat grafting: in search of the optimal technique. Surg Innov. 2014;21:327–336. [DOI] [PubMed] [Google Scholar]
- 17.Wu T, Smith J, Nie H, et al. Cytotoxicity of local anesthetics in mesenchymal stem cells. Am J Phys Med Rehabil. 2018;97:50–55. [DOI] [PubMed] [Google Scholar]
- 18.Trivisonno A, Di Rocco G, Cannistra C, et al. Harvest of superficial layers of fat with a microcannula and isolation of adipose tissue-derived stromal and vascular cells. Aesthet Surg J. 2014;34:601–613. [DOI] [PubMed] [Google Scholar]
- 19.Condé-Green A, de Amorim NF, Pitanguy I. Influence of decantation, washing and centrifugation on adipocyte and mesenchymal stem cell content of aspirated adipose tissue: a comparative study. J Plast Reconstr Aesthet Surg. 2010;63:1375–1381. [DOI] [PubMed] [Google Scholar]
- 20.Liao HT, James IB, Marra KG, et al. The effects of platelet-rich plasma on cell proliferation and adipogenic potential of adipose-derived stem cells. Tissue Eng Part A. 2015;21:2714–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin R, Zhang L, Zhang YG. Does platelet-rich plasma enhance the survival of grafted fat? An update review. Int J Clin Exp Med. 2013;6:252–258. [PMC free article] [PubMed] [Google Scholar]
- 22.Fisher GJ, Wang ZQ, Datta SC, et al. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med. 1997;337:1419–1428. [DOI] [PubMed] [Google Scholar]
- 23.Hersant B. Role of PRP as potentiator of cell therapy in the field of plastic surgery [unpublished thesis]. [Google Scholar]
- 24.Lee SH, Jin SY, Song JS, et al. Paracrine effects of adipose-derived stem cells on keratinocytes and dermal fibroblasts. Ann Dermatol. 2012;24:136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubin MG, Schurer, Wiest LG, et al. Illustrated guide to chemical peels: basics indications, uses. Batavia, IL: Quintessence Publishing Group. [Google Scholar]


