Supplemental Digital Content is available in the text.
Abstract
Background:
Breast cancer is the most prevalent cancer and second leading cause of cancer-related deaths in both the US and UK female population, a prominent cause of morbidity and cost to both health services. All surgically fit patients are offered breast reconstruction following the initial surgery, and this is traditionally an open approach: either implant-based or an autologous tissue flap. Both lead to scarring that is difficult to conceal. This paper aims to evaluate the novel minimally invasive technique of robotic-assisted surgery.
Methods:
A systematic review was conducted using Medline (OvidSP) and Embase (OvidSP) to evaluate the current application of robotic-assisted surgery in breast surgery and reconstruction.
Results:
Twenty-one articles were identified and discussed, composing of level 4 and 5 evidence comparing different surgeons' experiences, techniques, and outcomes. To date, the robotic system has been utilized to harvest the latissimus dorsi muscle for use as a tissue flap (total harvest time of 92 minutes), to perform nipple-sparing mastectomy with immediate breast reconstruction (total operation time 85 minutes) and lately to harvest a deep inferior epigastric perforator flap via an intraabdominal approach.
Conclusions:
Robotic-assisted surgery can successfully and reproducibly perform a nipple-sparing mastectomy with breast reconstruction. It can minimize the size of scarring and is superior to the laparoscopic technique, with improved 3-dimensional visualization, dexterity, and range of motion able to guide around the curvature of the breast. The main limiting factors are the lack of the US Food and Drug Administration approval, cost of the robot, and specialized skills required.
INTRODUCTION
Breast Cancer
Breast cancer is the most prevalent cancer and second leading cause of cancer-related deaths in both the US and UK female population.1 In 2018, approximately 640 and 150 new cases were diagnosed per day in the United States and United Kingdom, respectively, totalling a staggering 289,526 cases in the entire year; furthermore, the incidence rates are still predicted to increase.1,2 However, through extensive research and improved screening and detection, survival rates are improving with almost 8 out of 10 women surviving greater than 10 years from diagnosis.2
What Is the Current Practice?
After diagnosis, the treatment regime is tailored to the individual case through a multidisciplinary team approach. The majority of patients are offered surgery to remove the primary tumor along with chemotherapy and radiotherapy, depending on the stage of the cancer. Surgery can either be in the form of breast conservation therapy (BCT) or mastectomy.
The surgical procedures are disfiguring to the female body and can lead to psychological distress and depression.3,4 Taking into consideration comorbidities and metastatic disease, surgically fit patients should be offered breast reconstruction following breast cancer resection as this has been associated with a significant decrease in the incidence rates of anxiety and depression.5 Breast reconstruction can be performed immediately at the time of initial surgery or as a delayed procedure or delayed–immediate procedure.
To reconstruct the breast, the current techniques are either an implant-based procedure or an autologous tissue flap procedure. The implant-based procedure is the most common form of reconstruction, having an initially lower cost, technical ease, and no donor site morbidity, although has the prerequisite of an adequate and healthy mastectomy skin coverage. Although the autologous tissue flap can be a pedicled or free flap, the common flaps include the latissimus dorsi (LD) muscle flap, the transverse rectus abdominis muscle flap, and the deep inferior epigastric artery perforator flap. Both methods can incur complications such as flap failure, fat necrosis, infection, implant capsular contracture, and implant loss.6,7 Table 1 summarizes the key information for each technique (data from the studies by Fischer et al, Atherton et al, Xu et al, Gill et al6,8–10).
Table 1.
Cost Data Incorporate the Total Cost to the Primary Care Trust (Includes the Primary Surgery, the Hospital Stay, and an Estimate for Revision Surgery)
| Type of Reconstruction | Operation Length (Mean) | Cost (Average) | Length of Hospital Stay | Overall Complication Rate (%) |
|---|---|---|---|---|
| Implant based | 190 min | £8,034 | Mean: 4.6 d Median: 4 d |
20.8 |
| LD + implant | 297.8 min | £10,617 | Mean: 10.7 d Median: 9 d |
21.2 |
| TRAM | Free—539.2 min Pedicled—332.2 min |
£10,967 | Mean: 12 d Median: 11 d |
33.3 |
| DIEP | 276 min | £10,910 | Mean: 10.2 d Median: 8 d |
33.3 |
DIEP, deep inferior epigastric artery perforator; TRAM, transverse rectus abdominis muscle.
Traditionally, these procedures have been performed as open surgery, with an attempt to hide the scarring secondary to incisions around the areolar, within the inframammary fold or beneath the arm in the axilla. Although this is easier for some techniques, the traditional open technique (TOT) to harvest the LD muscle results in an unsightly 15–45-cm scar across the back. A laparoscopic technique has been developed to reduce the length of the scar, although it is a challenging procedure and is limited with 2-dimensional views and nonflexible instruments. Robot-assisted surgery (RAS) is the most advanced minimally invasive technique and has the potential to overcome these challenges with 3-dimensional views, greater instrument dexterity, and wider range of movement.
Robotic Surgery
The US Food and Drug Administration (FDA) first authorized the use of the da Vinci robotic system for specific abdominal surgical procedures in 2000 and then later for radical prostatectomy pelvic surgery in 2001.11 Since its inception, it is now used in a wide range of urological, gynecological, and general surgical procedures12 with FDA approval for prostatectomy, hysterectomy, and cholecystectomy. These procedures typically involve creating a cavity in which the robot can function. Within urology, the RAS is the gold standard for many conditions and has demonstrated a reduction in intraoperative blood loss, length of hospital admission, and risk of positive resection margins.13,14 These advantages could be propagated throughout the surgical field if the technology was widely adopted for the appropriate indications. Lately, its application has been evaluated in more superficial procedures, including thyroidectomies and head and neck malignancies.15,16 Although still highly experimental for many surgical specialties, could the da Vinci system now be used to advance breast cancer surgery and reconstructive procedures?
The aim of this systematic review was to identify and evaluate the current literature on the application of robotic-assisted surgery within breast cancer surgery and reconstruction.
METHODS
To evaluate the potential use of robotic surgery within breast surgery and reconstruction, a systematic review was conducted using the Medline database (OvidSP; 1946 to April 17, 2019) and Embase database (OvidSP; 1947 to April 17, 2019), adhering to the Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
The search criteria were formulated to identify articles on “Robotic Surgery” AND “Breast Reconstruction.” The base search filters for “Robotic Surgery” included {Robotics/ OR Robotic Surgical Procedures/ (MeSH terms)} OR {Robot* AND Surg* (Keyword)}. The base search filters for “Breast Reconstruction” included {Mammaplasty/ (MeSH term)} OR {Mamm?plasty OR “Breast reconstruct*” (Keyword)}.
The search criteria were restricted to the English language. Review articles were excluded. The inclusion criteria incorporated the use of a robotic system to perform breast surgery ± breast reconstruction.
RESULTS
The systematic review identified 21 articles consisting mainly of level-4 evidence human case series, only 2 were human cadaveric models (Fig. 1 and Table 2). These were composed by 10 authors (1 author described 2 procedures) describing their robotic surgical experiences for the following procedures: 2 for vessel harvest only, 3 for LD muscle flap harvest only, 4 for nipple-sparing mastectomy (NSM) with immediate breast reconstruction (IBR) using an implant, and 2 for NSM with LD flap harvest.
Fig. 1.
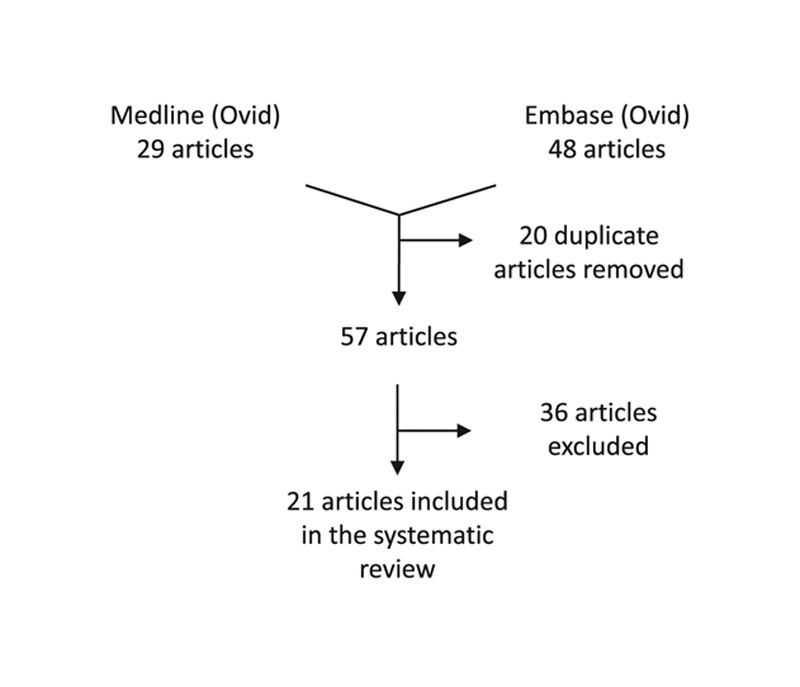
A flowchart to depict the database search and exclusion criteria identifying 21 articles.
Table 2.
Data Extraction: Breast Reconstruction Using Robotic Surgery
| Study | Title | Year | Journal | Aim | Sample Size | Robot |
|---|---|---|---|---|---|---|
| Boyd et al17 | Robotic harvest of internal mammary vessels in breast reconstruction | 2006 | J Reconstr Microsurg | To harvest the intermammary vessels using the robot (similar to standard technique in cardiac surgery), then traditional free flap approach | 22 free flaps on 20 patients | Aesop voice-activated robotic arm |
| Selber et al18 | Robotic harvest of the latissimus dorsi muscle: laboratory and clinical experience | 2012 | J Reconstr Microsurg | To evaluate the robotic harvest of the LD muscle in a cadaver model for use in patient clinical series | 8 fresh human cadavers; harvesting 10 LD muscles. Clinical series—8 LD flaps (6 for pedicled implant-based breast reconstruction) |
Da Vinci Si |
| Selber et al19 | Robotic latissimus dorsi muscle harvest: a case series | 2012 | Plast Reconstr Surg | The first clinical report of robotic harvest of the LD muscle | 7 LD muscles were harvested; 5 for breast reconstruction (3 for immediate implant based, 2 for radiated breasts exchanging for an implant | Da Vinci |
| Clemens et al20 | Robotic-assisted latissimus dorsi harvest in delayed-immediate breast reconstruction | 2014 | Semin Plast Surg | To compare outcomes of RALDH and TOT for patients undergoing delayed–immediate reconstruction following RT | 76 patients; 64 using TOT (average f/u 16.4 mo), 12 using RALDH (average f/u 12.3 mo) | Da Vinci |
| Chung et al21 | A novel technique for robot assisted latissimus dorsi flap harvest | 2015 | J Plast Reconstr Aesthet Surg | To introduce a new technique using an articulated long retractor for transaxillary gasless robot-assisted LD muscle harvest | 12 muscles flaps; mean age 35.8 y, mean BMI 23.1 | Da Vinci |
| Toesca et al23 | Preliminary report of robotic nipple-sparing mastectomy and immediate breast reconstruction with implant (poster abstract) |
2015 |
Eur J Cancer (Conference) |
“Aim of this study is to evaluate feasibility, safety, advantages and limitations of robotic surgery applied to the nipple-sparing mastectomy (NSM) and immediate breast reconstruction with implant (IBRI).” | 3 prophylactic NSM with IBR for BRCA-positive patients with prior breast cancer on contralateral side | Da Vinci Si |
| Sarfati et al29 | Robotic-assisted nipple sparing mastectomy: a feasibility study on cadaveric models | 2016 | J Plast Reconstr Aesthet Surg | To evaluate the technical feasibility of R-NSM through lateral axillary incision using cadavers | 4 breasts from 2 fresh female human cadavers | Da Vinci |
| Toesca et al25 | Robotic nipple sparing mastectomy and immediate breast reconstruction: future prospectives for breast cancer surgery (conference abstract) |
2016 |
Eur J Cancer (Conference) |
“The aim of our study is to evaluate the applicability of robotic surgery also for breast cancer patients.” | 10 patients with breast cancer or DCIS underwent 11 robotic mastectomies | Da Vinci (unknown model) |
| Toesca et al22 | Robotic nipple-sparing mastectomy and immediate breast reconstruction with implant: first report of surgical technique | 2017 | Ann Surg | To evaluate feasibility, safety, advantages, and limitations of robotic surgery to perform NSM and IBR with implant | 3 prophylactic NSM with IBR for BRCA-positive patients with prior breast cancer on contralateral side | Da Vinci Si |
| Toesca et al24 | Robotic nipple-sparing mastectomy for the treatment of breast cancer: feasibility and safety study | 2017 | Breast | To describe the outcome of the first 29 consecutive R-NSM and IBR procedures performed and assess feasibility, reproducibility and safety | 24 female patients; 18 for breast cancer, 6 for prophylaxis with BRCA mutation | Da Vinci Si |
| Sarfati et al33 | Robotic nipple-sparing mastectomy with immediate prosthetic breast reconstruction: a preliminary study (conference abstract) |
2017 | Cancer Research Conference | “The aim of this prospective study was to assess feasibility of the RNSM with immediate prosthetic breast reconstruction (IPBR) on the first 50 consecutive cases performed in Gustave Roussy.” | 50 patients with RNSM with IPBR | Unknown |
| Lai et al27 | Robotic nipple-sparing mastectomy and immediate breast reconstruction with gel implant | 2018 | Plast Reconstr Surg Glob Open | To report the preliminary experience and results of R-NSM and IBR with gel implant | 15 patients with breast cancer; mean age 46.5 y (30.8% DCIS, 30.8% stage 1, 30.8% stage 2, 7.7% stage 3) | Da Vinci |
| Lai et al26 | Robotic nipple-sparing mastectomy and immediate breast reconstruction with gel implant: technique, preliminary results and patient-reported cosmetic outcome | 2018 | Ann Surg Oncol | To report the preliminary experience and results of R-NSM and IBR with gel implant | 22 patients with 23 R-NSM and IBR. Mean age 48.9 y. | Da Vinci |
| Lai et al28 | The learning curve of robotic nipple sparing mastectomy for breast cancer: an analysis of consecutive 39 procedures with cumulative sum plot | 2018 | Eur J Surg Oncol | To report the preliminary experience of R-NSM in the management of breast cancer and analyze the learning curve from the same surgeon | 39 R-NSM from 35 patients; mean age 49.8 y | Da Vinci |
| Sarfati et al29 | Robotic da Vinci Xi-assisted nipple-sparing mastectomy: first clinical report | 2018 | Breast J | To describe the surgical technique and postoperative outcome of the first case of NSM with da Vinci robot | Case report; 46-y-old woman. Prophylactic bilateral NSM with BRCA2 positive | Da Vinci |
| Sarfati et al31 | Robotic nipple-sparing mastectomy with immediate prosthetic breast reconstruction: surgical technique | 2018 | Plast Reconstr Surg | To describe the surgical technique, the authors have developed from experience gained from over 60 procedures | 32 patients with 60 procedures | Da Vinci |
| Sarfati et al32 | Robotic prophylactic nipple-sparing mastectomy with immediate prosthetic breast reconstruction: a prospective study | 2018 | Ann Surg Oncol | To assess the feasibility and safety of R-NSM with IPBR | 33 female patients underwent 63 R-NSM with IPBR (all prophylactic except 1 for DCIS). Mean age 37 y, mean BMI 20.9. | Da Vinci |
| Lai et al35 | Technique for single axillary incision robotic assisted quadrantectomy and immediate partial breast reconstruction with robotic latissimus dorsi flap harvest for breast cancer: a case report | 2018 | Medicine (Baltimore) | To report preliminary experience and clinical outcome of RAQ and IPBR with RLDFH | Case report; 28-y-old woman. Triple-negative breast cancer T3N1M0. Final pathology T2N0M0 and stage 2A. | Da Vinci |
| Gundlapalli et al37 | Robotic-assisted deep inferior epigastric artery perforator flap abdominal harvest for breast reconstruction: a case report | 2018 | Microsurgery | To report the use of robot to harvest the DIEV in a DIEP flap breast reconstruction | Case report; 51-y-old woman | Da Vinci |
| Ahn et al34 | Early experiences with robot-assisted prosthetic breast reconstruction | 2019 | Arch Plast Surg | “We describe several patients with invasive ductal carcinoma who underwent robot-assisted nipple-sparing mastectomy and implant-based immediate breast reconstruction with satisfactory results.” | 4 patients with invasive ductal carcinoma | Da Vinci Xi |
| Houvenaeghel et al36 | Breast cancer robotic nipple sparing mastectomy: evaluation of several surgical procedures and learning curve | 2019 | World J Surg Oncol | “To report feasibility of robotic NSM and determine standard surgical procedure and learning curve threefold.” | 27 patients; 22 invasive, and 5 in situ BC | Da Vinci Si and Xi |
BMI, body mass index; DCIS, ductal carcinoma in-situ; DIEP, deep inferior epigastric artery perforator; f/u, follow up; IPBR, immediate prosthetic breast reconstruction; RAQ, robotic-assisted quadrantectomy; RLDFH, robotic LD flap harvest; RT, radiotherapy.
DISCUSSION
Robotic Surgery in Breast Cancer Surgery and Reconstruction
Boyd et al17 first demonstrated the microsurgical skills of a robotic system harvesting the intermammary vessels for breast reconstruction. The technique was similar to that used in cardiac surgery and then followed by a traditional free flap approach. It avoided the removal of intercostal cartilage, allowing a mean pedicle length of 6.7 cm to be brought through the intercostal muscle of the second intercostal space. The mean operating time to harvest the intermammary vessels was 113 minutes; postoperatively, there was a standard 3-day stay in the intensive care unit with an average hospital stay of 7 days. It did however have a high complication rate with 6 out of 22 patients having to return to theater for evacuation of a hematoma. The hematoma was compressing venous return and leading to flap compromise; this was due to the hole in the intercostal muscle through which the pedicle was brought through being too small.
As previously mentioned, the TOT to harvest the LD muscle results in a lengthy, unsightly scar. A laparoscopic technique had been developed, although limited in its application, with few surgeons adopting it. The da Vinci robotic system has improved 3-dimensional visualization and surgical dexterity with full wrist range of motion, when compared to laparoscopy; hence, it was hypothesized whether it could be utilized for the harvest of the LD muscle. To assess the feasibility, Selber et al developed and evaluated a cadaveric model for the robotic harvest of 10 LD muscle flaps.18 The average total incision length was 5 cm for 3 ports and a robot docking time of 23 minutes with an average harvest time of 68 minutes. The robotic harvest was successful, and the morbidity of the unsightly back incision was eliminated. This model was then translated into a clinical series with the successful harvest of 7 LD muscle flaps (5 for breast reconstruction), thus supporting the feasibility and reproducibility.19 In the clinical series, the docking time had remained at 23 minutes, whereas the average harvest time had increased to 111 minutes. The only complication implicated was 1 case of transient radial nerve palsy secondary to malposition.
Clemens et al20 compared the robotic-assisted LD harvest (RALDH) with TOT in 2-staged delayed IBR 7 months after radiotherapy had finished. The average RALDH operating time was longer than the TOT (92 versus 58 minutes), whereas the average hospital stay was shorter (2.7 versus 3.4 days). The overall complication rate was less in RALDH (16.7% versus 37.5%), and none required conversion to TOT. It is noted however that the study did have a small population size, compromising its statistical significance.
In an attempt to advance the LD flap harvest, a novel gasless technique was described that used 3 incisions for robot docking: 2 in the axillary and 1 in the inframammary fold21 (Fig. 2) (see figure, Supplemental Digital Content 1, which displays the LD muscle flap harvest, http://links.lww.com/PRSGO/B264).
Fig. 2.
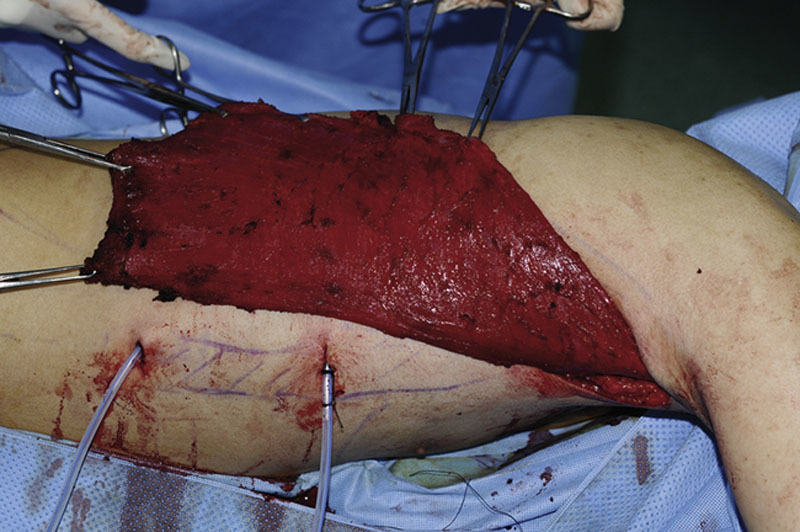
LD muscle flap harvest. The LD flap harvested entirely through the axillary incision. Reprinted with permission from J Plast Reconstr Aesthet Surg 2015;68:966–972.
The mean robotic harvest time decreased to 85.8 minutes, although the prior mean docking time had increased to 54.6 minutes. It did have excellent patient satisfaction outcomes, with patients rating the general outcome 9.6/10 and the scar 9.9/10 (Fig. 3).
Fig. 3.
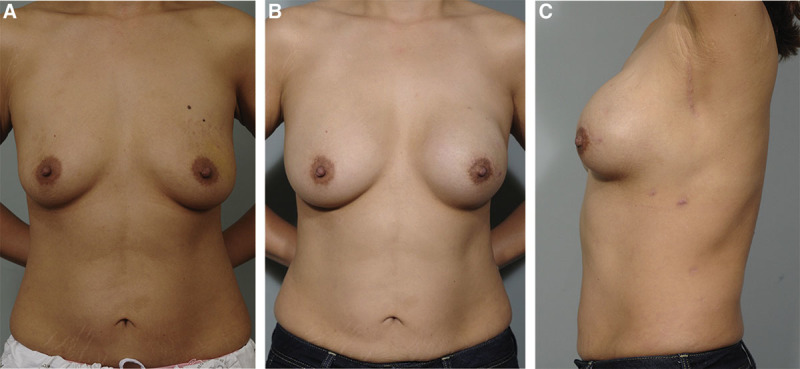
A 38-year-old patient with left-sided breast cancer. A, Before operation. B and C, At 1-year follow-up. The patient had undergone an NSM with IBR with a robotically harvested LD muscle flap and silicon implant. C, The largest incision can be well hidden in the axillary. Reprinted with permission from J Plast Reconstr Aesthet Surg 2015;68:966–972.
It was Toesca et al22,23 who proposed and first reported the robotic-assisted NSM (R-NSM) with IBR in a human model. Three female patients, positive for the BRCA mutation, underwent prophylactic R-NSM and IBR with an implant using a single port da Vinci robot. As expected, the first operation took the longest (420 minutes), learning and refining the procedure to 150 minutes for the third patient. All patients were discharged on the second postoperative day, and the only complications were a temporary strength reduction in 1 biceps brachii and some mild ecchymosis. The study was expanded to include 24 female patients24,25: 6 for prophylaxis with BRCA mutation and 18 for breast cancer. Docking of the robot had reduced from 90 to 30 minutes, and the total operating time was 180 minutes for the last case, 90 minutes for R-NSM and 60 minutes for IBR. Two cases had positive axillary lymph nodes that were operated on through the same incisions used for the robot at the end of the procedure. R-NSM and IBR continued to be safely performed with no major or systemic complications. Unfortunately, 2 cases were converted to open: the first operation to reduce operating time and 1 due to nipple-areolar complex (NAC) positivity for invading cancer.
Lai et al26,27 demonstrated reproducibility of the procedure, reporting their preliminary experience and results (Fig. 4) (see figure, Supplemental Digital Content 2, which displays a cartoon image depicting technique for NSM and IBR with gel implant, http://links.lww.com/PRSGO/B265). Describing the R-NSM and IBR as “a safe procedure” through a single port incision, their mean total operating time was 279.8 minutes (R-NSM of 118.8 minutes and IBR of 74.5 minutes plus docking time) with minimal intraoperative blood loss, zero positive resection margins, and mean hospital stay of 6.7 days. The robot was shown to efficiently perform subnipple biopsy, minimizing the risk of local recurrence, and after gaining experience with the procedure, docking could be finished in under 10 minutes and R-NSM shortened to under 100 minutes. Most importantly, the patients were satisfied with good cosmetic results, 86.7% grading the outcome as excellent and there was no local recurrence or distant metastases with mean follow-up of 6.3 months. Cumulative sum chart (CUSUM) plots analyzed the learning curve and identified that it took 13 separate procedures to refine and decrease the total operating time28 (Fig. 5).
Fig. 4.
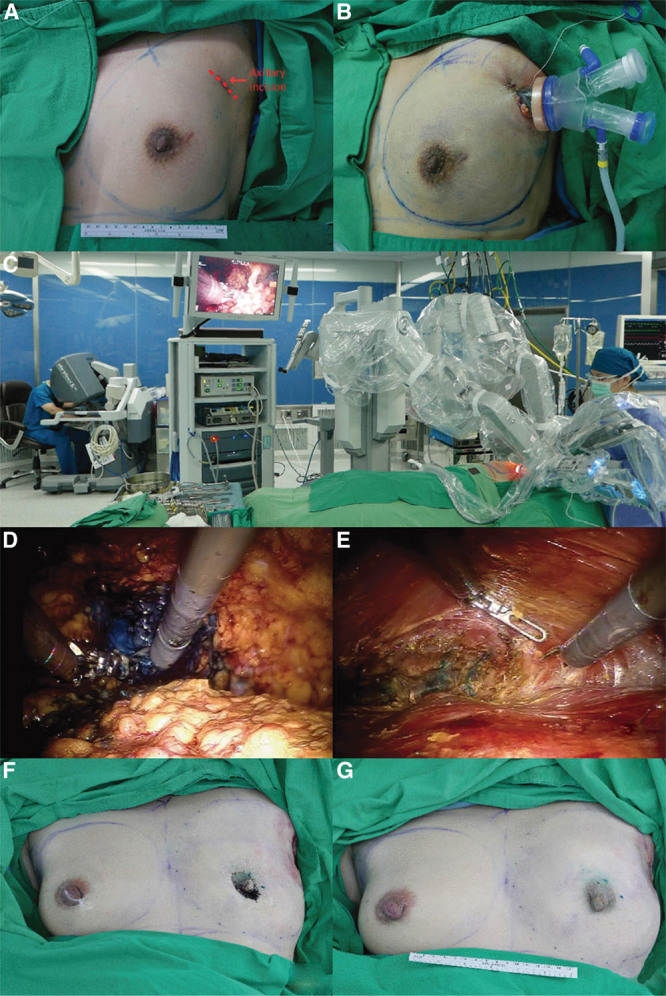
Intraoperative images of a robotic NSM and immediate reconstruction. A and B, The 3–5-cm incision with single port insertion. C, The positioning and docking of the robotic side cart posterior to the patient with the arms extending over the patient, aligned with the plane of the breast and nearly parallel to the floor. D, Superficial dissection separating the skin flap from the breast glandular tissue. E, Subpectoral pocket dissection for prosthesis insertion. F, Immediately post mastectomy and before reconstruction, followed by (G) immediate postbreast reconstruction with gel implant. Reprinted with permission from Ann Surg Oncol 2018;14:14. IPBR, immediate prosthetic breast reconstruction.
Fig. 5.
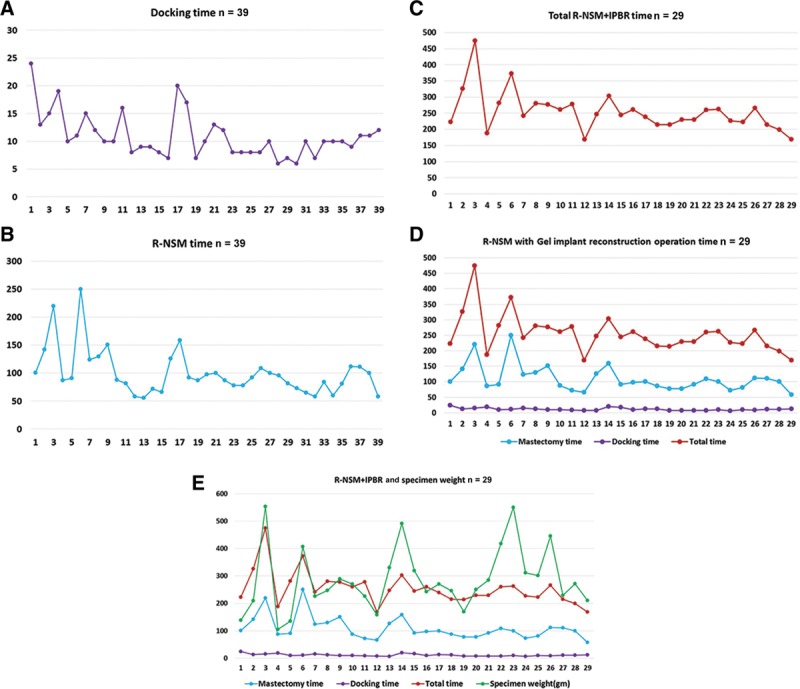
Operation time and learning curve of NSM. A, The docking time (minutes) and the chronologic case sequence demonstrated the robotic system could be fully setup in 10 minutes. B, The R-NSM time initially fluctuated and as cases accumulated, it could be performed in less than 100 minutes. C, The total time for R-NSM and IBR also initially fluctuated with the later cases completed within 250 minutes. Both (D) and (E) combine the docking time, R-NSM, and total R-NSM with IBR against the chronologic case sequence, along with considering the mastectomy tissue weight. The graphs illustrate that it took 13 procedures to refine and efficiently perform the procedure. Reprinted with permission from Eur J Surg Oncol 2018;17:17.
Following on from their initial cadaveric study demonstrating the feasibility,29 Sarfati et al30–33 went on to describe their surgical technique and report the preliminary data in a female human population with excellent cosmetic results (Fig. 6). The initial total operating time was 150 minutes with a postoperative hospital stay of 5 days and no complications reported with 3-month follow-up. After a further 33 patients who underwent 63 R-NSM and IBR (all prophylactic for BRCA2 mutation except 1 for breast cancer), the whole procedure could be performed in 85 minutes with the robotic section less than 50 minutes per breast and a median postoperative hospital stay of 6 days. Only 1 case had to be converted to an open procedure to control a bleeding internal mammary perforator. Although 3 postoperative infections occurred, 2 required washout and revision surgery with 1 unfortunately leading to implant loss.
Fig. 6.
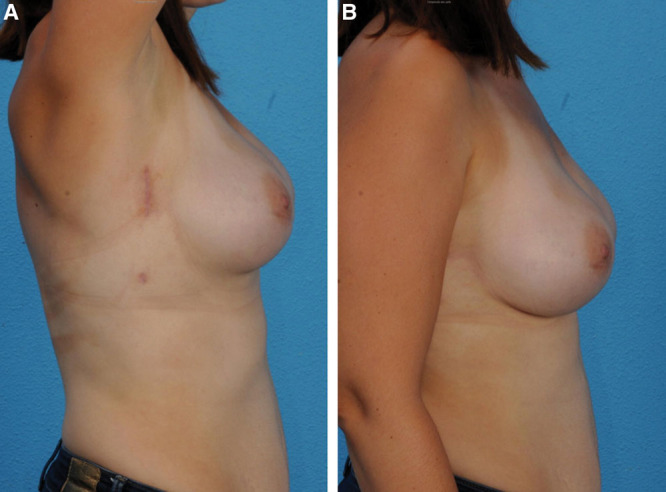
A patient at 3 months postoperatively after a bilateral NSM and IBR. A, arms fully abducted, and B, at rest. The incision scars are well hidden within the axillary. Reprinted with permission from Ann Surg Oncol 2018;25:2579–2586.
A summary comparing the R-NSM and IBR techniques of the 3 main authors can be found in Table 3.
Table 3.
Technique for R-NSM with IBR (Implant Based)
| Study | Sample Size | Robot System | Incision | Port | Technique | Implant Pocket | Operation Length | Complications |
|---|---|---|---|---|---|---|---|---|
| Toesca et al22–25 | 24 patients; 29 R-NSMs and IBR | da Vinci Xi (except for 5 procedures with da Vinci Si) | 1 cm × 3 cm incision along midaxillary line in axillary fossa | Single port with 4 mm × 5–12 mm access. Insufflator set to 8 mm Hg pressure. Camera: rigid 0-degree 12 mm diameter |
Dissection performed with 5-mm monopolar cautery with cautery spatula tip. Traction performed with 8-mm Cadiere Bipolar Forceps. R-NSM—superficial dissection of gland moving from axilla toward NAC and continued to breast fold along lateral, inferior, and internal margins. Followed by deep layer dissection posterior to gland, from lateral to medial along major pectoral fascia. Specimen removed en bloc through axillary incision. Implant inserted manually. Drains—submuscular and subcutaneous planes |
Submuscular pocket | R-NSM: 90 min. Implant placement: 60 min. Total: approximately 180 min (included extra time for docking) |
2 cases (6.9%) converted to open. 1 to reduce procedure time and 1 for NAC positivity |
| Sarfati et al29–33 | 33 patients; 63 R-NSMs and IBR | da Vinci Xi | 2 × incisions; a high vertical 3–5 cm incision within the footprint of bra and a subcentimeter vertical incision 8 cm below (both lateral thoracic wall 6 cm posterior from lateral mammary fold | 3 mm × 8 mm diameter ports via the lower incision. Insufflator set to 8 mm Hg pressure. Camera: 30-degree camera |
Dissection performed with monopolar curved scissors. Traction performed with bipolar grasping forceps. R-NSM—began with infiltration of adrenaline containing saline solution to reduce bleeding. Subcutaneous dissection with manual scissors as far as possible, linking the 2 incisions. 3 ports were inserted and fixed with stitches to the skin (2 in upper incision and 1 in lower incision). Subcutaneous dissection of gland from lateral to medial, followed by separation from pectoralis major muscle from lateral to medial. Robot undocked and ports removed, with gland removed en bloc through larger upper incision and implant insertion. Drain inserted through inferior incision. Implant pocket closed with stitches between skin and thoracic wall |
Prepectoral pocket | Nonrobotic section: approximately 45 min. Robotic section: approximately 40 min Total: approximately 85 min per breast. (plus docking approximately 10 min) |
No major complications. 3 infections (4.8%) and 1 conversion to open (1.6%) due to bleeding perforator |
| Lai et al26–28,35 | 35 patients; 39 R-NSM and IBR | da Vinci Si | 1 cm × 2.5–5 cm oblique axillary incision in the extra-mammary region | Single port. Insufflator set to 8 mm Hg pressure with CO2. Camera: 30-degree 12 mm diameter |
Dissection performed with 8-mm monopolar scissors. Traction performed with 8-mm prograsp forceps. R-NSM—began with subcutaneous infiltration with lidocaine and epinephrine saline solution to reduce bleeding. Dissection began with superficial skin flaps in all quadrants with tunneling technique with subnipple biopsy to exclude NAC involvement. Followed by peripheral and posterior detachment of gland from pectoralis major muscle. Gland removed en bloc through axillary incision |
Subpectoral pocket. | Docking time: 10 min. R-NSM time: 100 min. R-NSM + IBR time: 240 min. Total operation time: 250 min |
Overall complication rate 30.8% |
BC, breast cancer; DIEV, deep interior epigastric vessels.
The procedure gained recognition worldwide, with Ahn et al34 describing their early experiences on 4 patients with invasive ductal carcinoma. Mean operating time for R-NSM with expander insertion was 86 minutes, and although at the time of article publication only 1 patient had completed the first and second stage of prosthetic reconstruction with the remaining 3 still due the second stage, satisfaction of the aesthetic outcome was high.
So far, no one had reported breast cancer resection with immediate LD flap harvest until Lai et al35 published a case report of a 28-year-old female patient with triple-negative breast cancer. Not wanting an implant and desiring a natural result, BCT with volume replacement using an LD flap was performed robotically through 1 small inconspicuous axillary incision. Having chemotherapy before the surgery, the total operating time was 179 minutes (BCT of 82 minutes and IBR with RALDH of 97 minutes), and no local recurrence or metastases were diagnosed during the 5 months of follow-up. The patient was satisfied with the minimal scarring and aesthetic outcome. Seroma formation was noted over the back, although this resolved in the outpatient setting after repeated aspirations.
More recently, Houvenaeghel et al36 have attempted to determine a standard surgical procedure. Analyzing different techniques for the skin and NAC dissection, the authors concluded that the safest and quickest procedure was to initially use nonrobotic scissors for dissection after subcutaneous infiltration, followed by robotic dissection for the remainder. Additionally, the analysis demonstrated a learning curve, developed over 10–11 robotic mastectomies, to safely and efficiently perform the procedure.
A differing approach from those discussed so far is a case report of a 51-year-old female patient which demonstrates the use of a robot to harvest the deep inferior epigastric vessels for a deep inferior epigastric artery perforator flap breast reconstruction.37 A novel technique, the da Vinci robot, performs an intraabdominal dissection of the vessels to create the pedicle, minimizing the incision to the anterior rectus fascia and hence the disruption of innervation to the rectus abdominis muscle, thereby decreasing the risk of abdominal wall bulge and hernia. The total operation time was long (531 minutes), although the robotic dissection portion was only approximately 40 minutes. Contrary to other similar studies highlighting the cost as a major concern, this study cited the robotic approach was comparable to the open approach: $16,300 versus $14,800.
Benefits versus Costs and Potential Barriers
This review has highlighted some of the potential benefits of RAS within breast surgery and reconstruction, with reproducible techniques, a considerable reduction of scar length in the harvest of the LD flap, shorter hospital stay, and lower complication rates.
Of course, there are limitations with the majority of studies identify the capital cost of the robot as limiting its widespread use in smaller hospitals and the success of the procedures being operator dependant, requiring specialized skills and training. Unfortunately, due to the novelty of the techniques discussed and the limited data, there are no defined costs reported for each specific procedure, although it is noted that the capital cost of the da Vinci robot is a significant investment, in the order of £1.5–£2 million, with a yearly maintenance cost between £100,000 and £150,000.
Further, the application of RAS for mastectomy and breast reconstruction is “off-label” and not FDA approved with a recent statement released warning health-care providers and patients that the effectiveness and safety has yet to be established.38 This leads to issues with informed consent of the patient and to the credentialing of the operating hospital.
The traditional techniques for breast surgery and reconstruction are well established, and the above are potential barriers to adoption of the robotic technique. Robotic systems remain highly experiential and at present, a device looking for a desired indication within plastic surgery, rather than the more correct other way around.
Future of Robotic Surgery
If the robot-assisted technique is to surmount the potential barriers and gain the FDA approval, then the 30-day safety and long-term complication risk need to be assessed with comparison to their traditional counterparts. However, it is one thing gaining FDA approval and another for clinicians to change their practice and incorporate the novel technique when the traditional methods are well established. Within breast reconstruction, it may be that the only indication for RAS is if an LD flap harvest is necessary to provide implant coverage and create the breast pocket, thus eliminating the upper back scarring. However, the NSM and IBR with implant only continue to be performed by the well-established open technique.
CONCLUSIONS
Since the inception of robotic surgery, the technology has significantly advanced and its use has shown exponential growth. It is now being widely applied to more superficial procedures, and this article demonstrates its potential application within breast surgery and reconstruction. The da Vinci robotic system can successfully and reproducibly perform an NSM and IBR using an implant ± LD muscle flap.
The rather expensive initial cost of the robotic system along with the cost of the disposable instruments required for individual procedures is currently a major limiting factor. However, as new technology companies design alternative robotic systems to the Intuitive da Vinci, this will create competition and diversity, driving the cost down and possibly changing the direction of how surgery is performed.
Before it can become more widely adopted, there is a need for further research in the prospect of gaining the FDA approval. The procedure needs to be standardized in addition to comparing both the short- and long-term costs and complications compared to the TOTs.
ACKNOWLEDGMENTS
The authors would like to thank the reciprocal authors and publishers for giving full permission to reuse the figures illustrated in this article.
Supplementary Material
Footnotes
Published online 29 January 2020.
Disclosure: The article reviews the application of a robotic surgical system, including the da Vinci system. The authors have no financial interest to declare in relation to the content of this article.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Research UK. Breast cancer statistics. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer#heading-Five. Accessed December 29, 2018.
- 3.Jamison KR, Wellisch DK, Pasnau RO. Psychosocial aspects of mastectomy: I. The women’s perspective. Am J Psychiatry. 1978;135:432–436. [DOI] [PubMed] [Google Scholar]
- 4.Markopoulos C, Tsaroucha AK, Kouskos E, et al. Impact of breast cancer surgery on the self-esteem and sexual life of female patients. J Int Med Res. 2009;37:182–188. [DOI] [PubMed] [Google Scholar]
- 5.Chen W, Lv X, Xu X, et al. Meta-analysis for psychological impact of breast reconstruction in patients with breast cancer. Breast Cancer. 2018;25:464–469. [DOI] [PubMed] [Google Scholar]
- 6.Fischer JP, Nelson JA, Cleveland E, et al. Breast reconstruction modality outcome study: a comparison of expander/implants and free flaps in select patients. Plast Reconstr Surg. 2013;131:928–934. [DOI] [PubMed] [Google Scholar]
- 7.Gart MS, Smetona JT, Hanwright PJ, et al. Autologous options for postmastectomy breast reconstruction: a comparison of outcomes based on the American College of Surgeons National Surgical Quality Improvement Program. J Am Coll Surg. 2013;216:229–238. [DOI] [PubMed] [Google Scholar]
- 8.Atherton DD, Hills AJ, Moradi P, et al. The economic viability of breast reconstruction in the UK: comparison of a single surgeon’s experience of implant; LD; TRAM and DIEP based reconstructions in 274 patients. J Plast Reconstr Aesthet Surg. 2011;64:710–715. [DOI] [PubMed] [Google Scholar]
- 9.Xu F, Sun H, Zhang C, et al. Comparison of surgical complication between immediate implant and autologous breast reconstruction after mastectomy: a multicenter study of 426 cases. J Surg Oncol. 2018;118:953–958. [DOI] [PubMed] [Google Scholar]
- 10.Gill PS, Hunt JP, Guerra AB, et al. A 10-year retrospective review of 758 DIEP flaps for breast reconstruction. Plast Reconstr Surg. 2004;113:1153–1160. [DOI] [PubMed] [Google Scholar]
- 11.Meadows M. Robots lend a helping hand to surgeons. FDA Consum. 2002;36:10–15. [PubMed] [Google Scholar]
- 12.Autorino R, Kaouk JH, Stolzenburg JU, et al. Current status and future directions of robotic single-site surgery: a systematic review. Eur Urol. 2013;63:266–280. [DOI] [PubMed] [Google Scholar]
- 13.Ramsay C, Pickard R, Robertson C, et al. Systematic review and economic modelling of the relative clinical benefit and cost-effectiveness of laparoscopic surgery and robotic surgery for removal of the prostate in men with localised prostate cancer. Health Technol Assess. 2012;16:1–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang K, Jiang K, Chen H, et al. Robotic vs. Retropubic radical prostatectomy in prostate cancer: a systematic review and an meta-analysis update. Oncotarget. 2017;8:32237–32257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Son SK, Kim JH, Bae JS, et al. Surgical safety and oncologic effectiveness in robotic versus conventional open thyroidectomy in thyroid cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2015;22:3022–3032. [DOI] [PubMed] [Google Scholar]
- 16.Rinaldi V, Pagani D, Torretta S, et al. Transoral robotic surgery in the management of head and neck tumours. Ecancermedicalscience. 2013;7:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyd B, Umansky J, Samson M, et al. Robotic harvest of internal mammary vessels in breast reconstruction. J Reconstr Microsurg. 2006;22:261–266. [DOI] [PubMed] [Google Scholar]
- 18.Selber JC, Baumann DP, Holsinger CF. Robotic harvest of the latissimus dorsi muscle: laboratory and clinical experience. J Reconstr Microsurg. 2012;28:457–464. [DOI] [PubMed] [Google Scholar]
- 19.Selber JC, Baumann DP, Holsinger FC. Robotic latissimus dorsi muscle harvest: a case series. Plast Reconstr Surg. 2012;129:1305–1312. [DOI] [PubMed] [Google Scholar]
- 20.Clemens MW, Kronowitz S, Selber JC. Robotic-assisted latissimus dorsi harvest in delayed-immediate breast reconstruction. Semin Plast Surg. 2014;28:20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung JH, You HJ, Kim HS, et al. A novel technique for robot assisted latissimus dorsi flap harvest. J Plast Reconstr Aesthet Surg. 2015;68:966–972. [DOI] [PubMed] [Google Scholar]
- 22.Toesca A, Peradze N, Galimberti V, et al. Robotic nipple-sparing mastectomy and immediate breast reconstruction with implant: first report of surgical technique. Ann Surg. 2017;266:e28–e30. [DOI] [PubMed] [Google Scholar]
- 23.Toesca A, Manconi A, Peradze N, et al. Preliminary report of robotic nipple-sparing mastectomy and immediate breast reconstruction with implant. Eur J Cancer. 2015;51:S309. [DOI] [PubMed] [Google Scholar]
- 24.Toesca A, Peradze N, Manconi A, et al. Robotic nipple-sparing mastectomy for the treatment of breast cancer: feasibility and safety study. Breast. 2017;31:51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toesca A, Peradze N, Manconi A, Lichosik D, Rietjens M, Veronesi P. Robotic nipple sparing mastectomy and immediate breast reconstruction: future prospectives for breast cancer surgery. Eur J Cancer. 2016;57:S72. [Google Scholar]
- 26.Lai HW, Chen ST, Lin SL, et al. Robotic nipple-sparing mastectomy and immediate breast reconstruction with gel implant: technique, preliminary results and patient-reported cosmetic outcome. Ann Surg Oncol. 2018;14:14. [DOI] [PubMed] [Google Scholar]
- 27.Lai HW, Lin SL, Chen ST, et al. Robotic nipple-sparing mastectomy and immediate breast reconstruction with gel implant. Plast Reconstr Surg Glob Open. 2018;6:e1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai HW, Wang CC, Lai YC, et al. The learning curve of robotic nipple sparing mastectomy for breast cancer: an analysis of consecutive 39 procedures with cumulative sum plot. Eur J Surg Oncol. 2018;17:17. [DOI] [PubMed] [Google Scholar]
- 29.Sarfati B, Honart JF, Leymarie N, et al. Robotic-assisted nipple sparing mastectomy: a feasibility study on cadaveric models. J Plast Reconstr Aesthet Surg. 2016;69:1571–1572. [DOI] [PubMed] [Google Scholar]
- 30.Sarfati B, Honart JF, Leymarie N, et al. Robotic da vinci xi-assisted nipple-sparing mastectomy: first clinical report. Breast J. 2018;24:373–376. [DOI] [PubMed] [Google Scholar]
- 31.Sarfati B, Struk S, Leymarie N, et al. Robotic nipple-sparing mastectomy with immediate prosthetic breast reconstruction: surgical technique. Plast Reconstr Surg. 2018;142:624–627. [DOI] [PubMed] [Google Scholar]
- 32.Sarfati B, Struk S, Leymarie N, et al. Robotic prophylactic nipple-sparing mastectomy with immediate prosthetic breast reconstruction: a prospective study. Ann Surg Oncol. 2018;25:2579–2586. [DOI] [PubMed] [Google Scholar]
- 33.Sarfati B, Struk S, Leymarie N, et al. Robotic nipple-sparing mastectomy with immediate prosthetic breast reconstruction: a preliminary study. Paper presented at: Cancer Research Conference: San Antonio Breast Cancer Symposium; December 5-9 2017; San Antonio, TX. [Google Scholar]
- 34.Ahn SJ, Song SY, Park HS, et al. Early experiences with robot-assisted prosthetic breast reconstruction. Arch Plast Surg. 2019;46:79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai HW, Chen ST, Lin SL, et al. Technique for single axillary incision robotic assisted quadrantectomy and immediate partial breast reconstruction with robotic latissimus dorsi flap harvest for breast cancer: a case report. Medicine (Baltimore). 2018;97:e11373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Houvenaeghel G, Bannier M, Rua S, et al. Breast cancer robotic nipple sparing mastectomy: evaluation of several surgical procedures and learning curve. World J Surg Oncol. 2019;17:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gundlapalli VS, Ogunleye AA, Scott K, et al. Robotic-assisted deep inferior epigastric artery perforator flap abdominal harvest for breast reconstruction: a case report. Microsurgery. 2018;38:702–705. [DOI] [PubMed] [Google Scholar]
- 38.U. S. Food and Drug Administration. Caution when using robotically-assisted surgical devices in women’s health including mastectomy and other cancer-related surgeries: FDA safety communication. In M. D. Safety. ed. Safety communications. 2019. https://www.fda.gov/medical-devices/safety-communications/caution-when-using-robotically-assisted-surgical-devices-womens-health-including-mastectomy-and. Accessed August 8, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


