Supplemental Digital Content is available in the text
Keywords: bivalirudin, heprin, meta-analysis, STEMI
Abstract
Background:
This meta-analysis is to evaluate the efficacy and safety of bivalirudin in patients with ST-elevation myocardial infarction (STEMI).
Methods:
PubMed, Cochrane Library, Embase, CNKI, CBMdisc, and VIP database were searched. Randomized controlled trial (RCT) was selected and the meta-analysis was conducted by RevMan 5.1. The primary efficacy endpoint was the incidence of major adverse cardiovascular events (MACE) and the primary safety endpoint was the incidence of major bleeding. Secondary efficacy endpoints were myocardial infarction (MI), target vessel revascularization (TVR), stent thrombosis (ST), stock, mortality, and thrombocytopenia. The pooled risk ratios (RRs) with the corresponding 95% confidence intervals (CI) were used to assess the efficacy and safety of bivalirudin vs heparin.
Results:
Seven RCTs met the inclusion criteria, and 16,640 patients were included. We found that bivalirudin associated with lower risk of mortality (RR = 1.05; 95% CI = 0.74–1.49; P = .03; I2 = 2%), major bleeding (RR = 0.64; 95% CI = 0.54–0.75; P < .00001; I2 = 70%) and thrombocytopenia (RR = 0.39; 95% CI = 0.25–0.61; P < .0001; I2 = 0) compared with heparin. However, the use of bivalirudin increase the risk of MI(RR = 1.37; 95% CI = 1.10–1.71; P = .004; I2 = 25%) and ST(RR = 1.61; 95% CI = 1.05–2.47; P = .03; I2 = 70%) and has similar risk of MACE (RR = 1.00; 95% CI = 0.90–1.11; P = .97; I2 = 16%), TVR (RR = 1.43; 95% CI = 0.92–2.22; P = .11; I2 = 46%) and stock (RR = 1.43; 95% CI = 0.92–2.22; P = .11; I2 = 46%) compared with heparin used in STEMI patients.
Conclusion:
Bivalirudin associated with lower risk of mortality, major bleeding and thrombocytopenia compared with heparin. However, the use of bivalirudin increase the risk of MI and ST and has similar risk of MACE, TVR and stock compared with heparin used in STEMI patients.
1. Introduction
ST segment elevation myocardial infarction (MI) refers to a typical ischemic chest pain that persists for more than 20 minutes accompanied with the serum concentration of myocardial necrosis markers rises and the electrocardiogram has a typical ST-segment elevation. The study of the Chinese disease burden in 2013 showed that the top three causes of death in the Chinese population were stroke, ischemic heart disease, and chronic obstructive pulmonary disease, and the first 2 accounted for about 90% of all cardiovascular diseases (including cerebrovascular disease).[1] Currently, the most important treatment for MI is percutaneous coronary intervention (PCI). The operation of PCI may cause platelet activation, aggregation, atherosclerotic plaque rupture, activation of exogenous coagulation system, and promote coagulation cascades, eventually leading to thrombosis. To ensure smooth operation and prevent postoperative thrombosis and embolism, anticoagulants should be used before, during, and after PCI.
Heparin is the primary choice for antithrombotic treatment. In contrast, bivalirudin is a new direct thrombin inhibitor that has been reported to have antiischemic properties and a lower risk of bleeding during PCI.[2–4] According to the ACCF/AHA guidelines recommendation that bivalirudin is superior to heparin plus platelet glycoprotein IIb/IIIa receptor inhibitor (GPI IIb/IIIa) as an anticoagulation therapy for patients with a high risk of major bleeding who undergo PCI.[5] Out meta-analysis is undertaken to evaluate the efficacy and safety of bivalirudin vs heparin in patients with ST-segment elevation MI.
2. Methods
2.1. Data sources and searches
This meta-analysis was conducted followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)[6] statement for conducting a high-quality study. Cochrane Library, EMBASE, PubMed, and Clinical Trials.gov databases were searched for RCTs which search time was set from January 1990 through April 2018. The sensitive filter for RCTs was used and following keywords were used in search strategies: “bivalirudin”; “heparin”; “STEMI”, “ST-segment elevation myocardial infarction” and “percutaneous coronary intervention”. Since animal experiment or human was not involved in this study, the ethical approval was not necessary.
2.2. Study selection
Studies were screened by 2 investigators and a third investigator was consulted when disagreements arose. The inclusion criteria are following
-
(1)
patients associated with STEMI and undertaken PCI;
-
(2)
bivalirudin was given in treatment group;
-
(3)
heparin was given in control group;
-
(4)
the clinical outcomes of major adverse cardiac events, morality, MI, target vessel revascularization (TVR), stent thrombosis (ST), stock, major bleeding and thrombocytopenia were reported;
-
(5)
RCTs conducted in human being.
The literature with the newest reported data was included if there were duplicate studies from the same trial. Reviews, meta-analyses, editorials, observational studies, and studies in which lacked a control group were excluded.
2.3. Data extraction and quality assessment
Clinical data were independently extracted by 2 independent authors using the same extraction table and a third investigator will be consulted to resolve conflicting opinions. Authors’ names, year of publication, baseline characteristics of the participants and GPI use rate was extracted from included investigations. The incidences of the following endpoints were extracted: MACE, morality, MI, TVR, ST, stock, major bleeding, and thrombocytopenia. In addition, information regarding blinding, random sequence generation, allocation concealment, indications for incomplete outcome data, indications for selective reporting, and other biases were also collected to evaluate the quality of the included investigations.
2.4. Statistical analysis
Data were analyzed according to the intention-to-treat principle. Differences of dichotomous data are reported the risk ratio and 95% confidence interval. Cochran Q test and I2 statistic was used to assessed the heterogeneity that a Cochran P < .10 and an I2 > 50 were considered to be indicative of significant heterogeneity. Random effect according to the method of Mantel–Haenszel was the primary analytic method. Fixed effect model for all safety and efficacy endpoints were also reported in Supplemental Digital Content (sFigures 1–8).[7,8] Publication bias was assessed using Begg test and sensitivity analysis was conducted by excluding each individual study. Data analyses were performed by Review Manager (RevMan) software (version 5.1; The Cochrane Collaboration, Copenhagen, Denmark) and STATA software (version 11.1; Stata Corp LP, College Station, TX).
3. Results
3.1. Search results
In the total of 959 articles that we identified, there are 7 clinical trials[9–15] satisfied our inclusion criteria finally. The selection procedure was shown in Supplemental Digital Content (sFigure 9). There are 8313 patients were randomized to a bivalirudin (experimental) group and 8327 patients were randomized to a heparin (control) group. The baseline characteristics of included studies were detailed in Table 1. The quality assessment is presented in Supplemental Digital Content (sFigures 10 and 11). All clinical trials included in our study were characterized by a low risk of blinding of participants and outcome assessment, incomplete outcome data, selective outcome reporting. In addition, 2 trials were with an unclear risk of random sequence generation, and 1 trial with unclear risk of allocation concealment. In conclusion, all trials included in the present analysis are high-quality studies.
Table 1.
Baseline characters of included studies.
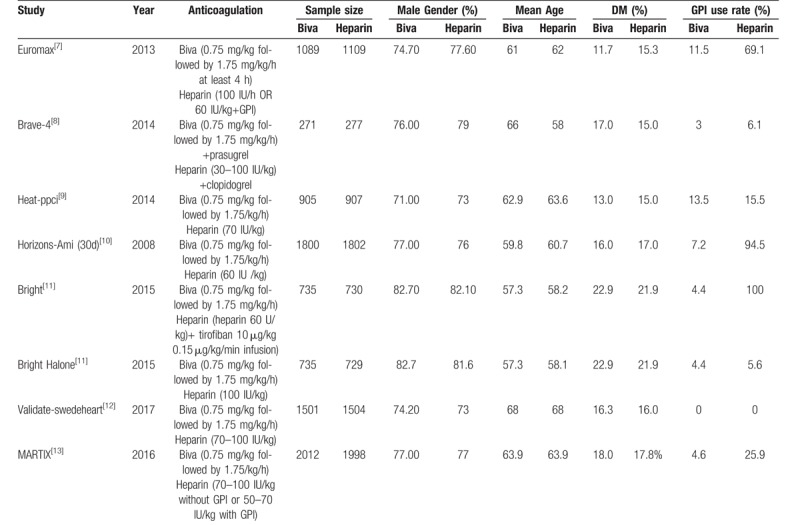
3.2. Clinical results
The primary efficacy endpoint is MACE and the rate of major bleeding was the primary safety endpoint. Secondary endpoints included MI, morality, TVR, ST, stock, and thrombocytopenia. Subgroup analysis were conducted according to the different rates of GPI use in 2 arm. When the rate of GPI use in bivalirudin arm is larger than in heparin arm define as Gureater GPI use subgroup. When the rate of GPI use in bivalirudin is equal to that in heparin define as Balance GPI use subgroup.
3.3. Efficacy outcomes
In this study, MACE is the primary efficacy endpoint and 7 clinical trials reported these results. No significant difference was founded between bivalirudin and heparin on the risk of MACE in subgroup (Gureater GPI use subgroup: RR = 0.97; 95% CI = 0.83–1.13; P = .70; I2 = 0; Balance GPI use subgroup: RR = 1.03; 95% CI = 0.77–1.39; P = .82; I2 = 59%) and overall analysis (RR = 1.00; 95% CI = 0.89–1.13; P = .71; I2 = 16%) as shown in Figure 1. Mortality was significantly decreased by bivalirudin used in Gureater GPI use subgroup (RR = 0.73; 95% CI = 0.58–0.92; P = .007; I2 = 0) and overall analysis (RR = 0.81; 95% CI = 0.67–0.99; P = .04; I2 = 2%). There are no significant difference between bivalirudin and heparin on the risk of mortality in Balance GPI use subgroup analysis (RR 1.06; 95%CI 0.74–1.50; P = 0.76; I2 = 0) as illustrated in Figure 2. Risk of MI was significantly decreased by heparin used in Gureater GPI use subgroup (RR = 1.30; 95% CI = 1.02–1.67; P = .03; I2 = 2%) and overall analysis (RR = 1.38; 95% CI = 1.04–1.84; P = .02; I2 = 25%). There are no significant difference between bivalirudin and heparin on the risk of MI in Balance GPI use subgroup analysis (RR 1.50; 95%CI 0.63 to 3.60; P = .36; I2 = 53%) as illustrated in Figure 3. Risk of ST was significantly decreased by heparin used in Gureater GPI use subgroup (RR = 1.62; 95% CI = 1.08–2.44; P = .02; I2 = 40%) and overall analysis (RR = 1.61; 95% CI = 1.05–2.47; P = .03; I2 = 70%). There are no significant difference between bivalirudin and heparin on the risk of ST in Balance GPI use subgroup analysis (RR 1.38; 95%CI 0.38–4.98; P = .63; I2 = 71%) as illustrated in Figure 4. No significant difference was found between bivalirudin and heparin on the risk of TVR in subgroup (Gureater GPI use subgroup: RR = 1.43; 95% CI = 1.01–2.02; P = .05; I2 = 0; Balance GPI use subgroup: RR = 1.34; 95% CI = 0.42–4.28; P = .62; I2 = 76%) and overall analysis (RR = 1.43; 95% CI = 0.92–2.22; P = .11; I2 = 46%) as shown in Supplemental Digital Content (sFigure 12).
Figure 1.
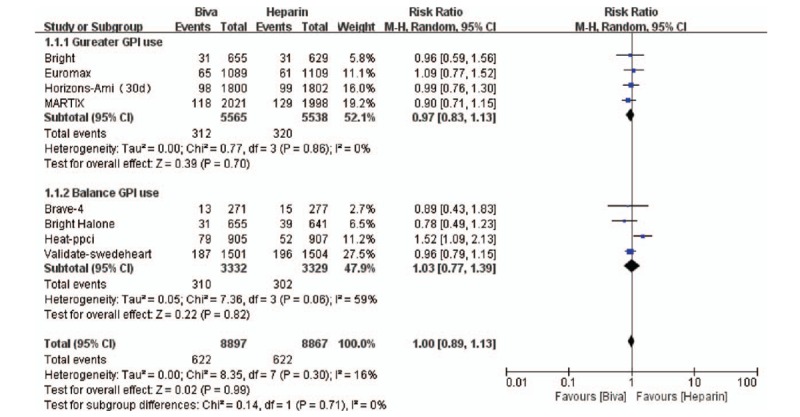
Forest plot of MACE.
Figure 2.
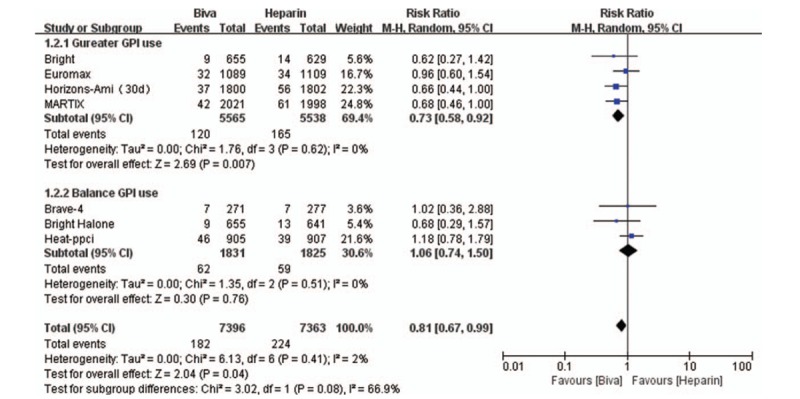
Forest plot of mortality.
Figure 3.
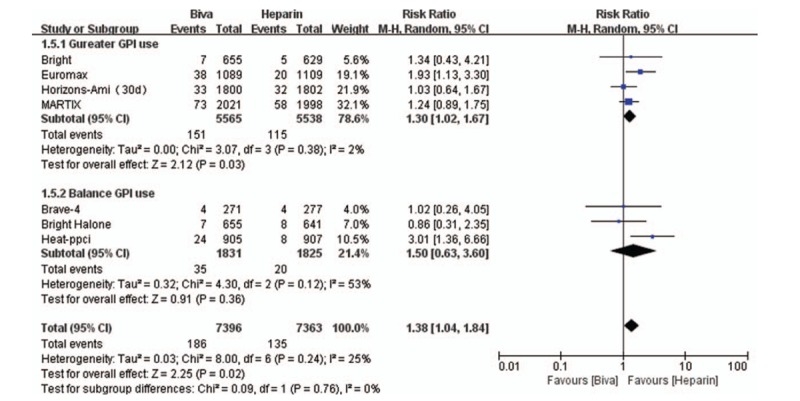
Forest plot of MI.
Figure 4.
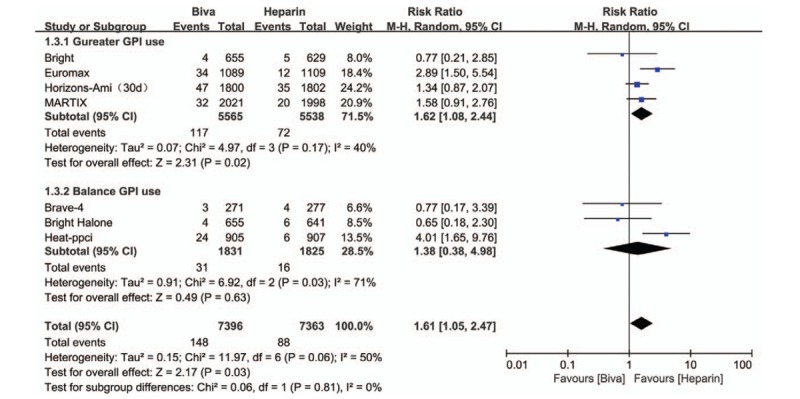
Forest plot of ST.
3.4. Safety outcomes
Major bleeding, which served as the primary safety endpoint and there are 6 clinical trials reported this result. Risk of major bleeding was significantly decreased by bivalirudin used in Gureater GPI use subgroup (RR = 0.53; 95% CI = 0.42–0.68; P < .00001; I2 = 21%) and overall analysis (RR 0.64; 95%CI 0.45–0.90; P = .031; I2 = 70%). There are no significant difference between bivalirudin and heparin on the risk of major bleeding in Balance GPI use subgroup analysis (RR = 0.98; 95% CI = 0.60–1.59; P = .93; I2 = 48%) as illustrated in Figure 5. No significant difference was found between bivalirudin and heparin on the risk of stock in subgroup (Gureater GPI use subgroup: RR = 0.74; 95% CI = 0.46–1.19; P = .21; I2 = 34%; Balance GPI use subgroup: (RR = 0.69; 95% CI = 0.27–1.77; P = .44; I2 = 0) and overall analysis (RR 0.73; 95%CI 0.48–1.11; P = .14; I2 = 5%) as illustrated in Supplemental Digital Content (sFigure 13). Risk of thrombocytopenia was significantly decreased by bivalirudin used in Gureater GPI use subgroup (RR = 0.40; 95% CI = 0.26–0.63; P < .0001; I2 = 0). There are no significant difference between bivalirudin and heparin on the risk of major bleeding in Balance GPI use subgroup (RR 1.12; 95%CI 0.89 to 1.42; P < .00001; I2 = 0%) and overall analysis (RR = 0.66; 95% CI = 0.39–1.12; P = .12; I2 = 74%) as illustrated in Supplemental Digital Content (sFigure 14).
Figure 5.
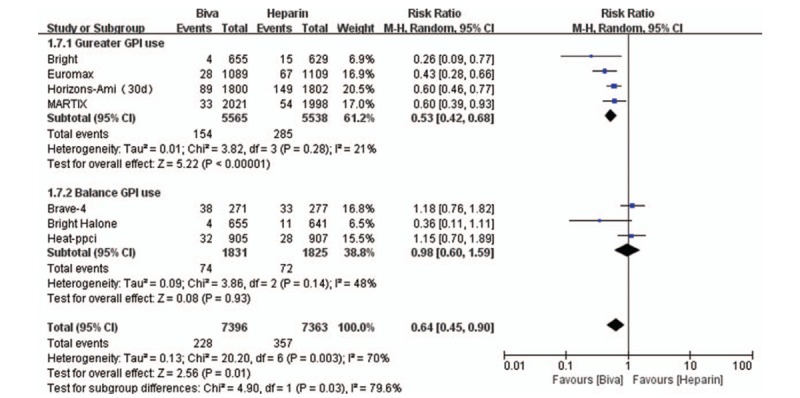
Forest plot of major bleeding.
3.5. Sensitivity and bias analysis
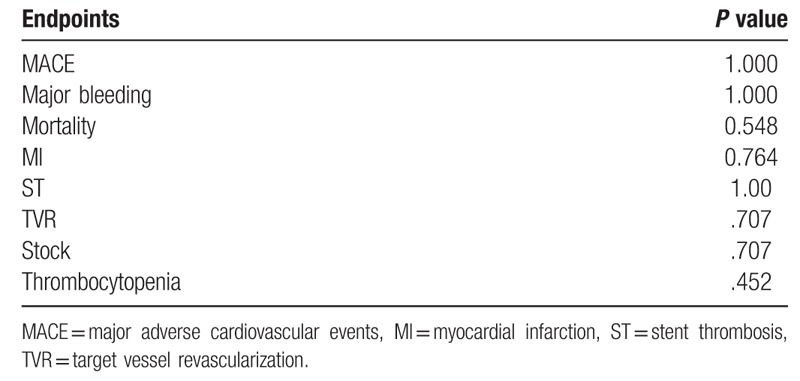
Sensitivity analysis was conducted by excluding each individual study and similar meta-analysis outcome was obtained which demonstrated that our conclusion was stable. No significant evidence of publication bias was obtained using the Begg Test in the study endpoints, as shown in Table 2.
Table 2.
Begg test results of each endpoint.
4. Discussion
This meta-analysis includes16,640 patients, randomized to a bivalirudin and a heparin group, who underwent PCI within 7 RCTs. In this meta-analysis, we found that bivalirudin associated with lower risk of mortality, major bleeding and thrombocytopenia compared with heparin in Greater GPI use group subgroup analysis and overall analysis, nevertheless, there was no significant difference on these outcomes when consider the subgroup analysis of Balance GPI use. Additionally, the use of bivalirudin associated with higher risk of MI and ST compared with heparin in Balance GPI use subgroup analysis and overall analysis, however, there was no significant difference on these outcomes when consider the subgroup analysis of Greater GPI use. Bivalirudin and heparin has similar risk of MACE, TVR and stock compared with heparin used in the subgroup analysis and overall analysis. Above all, the bivalirudin reduce the risk of mortality, major bleeding and thrombocytopenia regardless of the heparin combined application with greater GPI use.
Bivalirudin is one kind of anticoagulation strategy for patients undergoing PCI that also an alternative to heparin to reduce the risk of major bleeding events. In addition, bivalirudin was also suggested to possess a wider range of pharmacological properties than heparin.[16] Angioplasty trial[17] is the first clinical trial about bivalirudin which reported that bivalirudin reduced the ischemic events rate and the risk of bleeding. And the following clinical study HORIZON-AMI[12] which is specialized for STEMI patients obtained similar results with the above trial. The results of BRIGHT[13] trial which showed that bivalirudin did not increase the incidence of ischemic events but reduce the incidence of bleeding events in STEMI patients. The MARTIX[15] clinical trial, which showed that bivalirudin use was associated with a lower risk of death and major bleeding.
As far as we know, this is the second meta-analysis that compare the efficacy and safety of bivalirudin vs heparin used in patients with STEMI. In the previous study[18] which reported that bivalirudin was associated with comparable mortality and reduced major bleeding at the price of an increased risk of acute ST compared with heparin. However, there was non-significant differences in the overall rates of ST and reinfarction. Intended use of GPI in the heparin arm did not significantly modify the treatment effects of bivalirudin. Compare with this study, we have included 2 more latest clinical trials which completed nearly years and conducted subgroup analysis according to the GPI use rate. From the subgroup analysis, we founded that in the Greater GPI use subgroup, bivalirudin associated with lower risk of mortality, major bleeding and thrombocytopenia in the price of increasing the risk of MI and ST. These results were different with the Balance GPI use group that there are no significant difference between bivalirudin and heparin group on the risk of mortality, major bleeding, thrombocytopenia, MI and ST. This difference indicate that the higher GPI use rate may be the reason of the lower rate of mortality, major bleeding and thrombocytopenia in bivalirudin group.
Nevertheless, there are several limitations in our study. Firstly, the dosage and type of heparin were slightly different in these included clinical trials such as some patients were given enoxaparin and others were given unfractionated heparin. Secondly, individual patient-level data were could not obtain to further analysis potential limitations. Overall, the different design and characteristics of each trial including the catheter diameter used for the procedure, baseline anemia and BMI difference might have caused heterogeneity and affect clinical results.[19–21] Given the important differences between trials, further randomized trials are warranted to discriminate whether there are substantial safety and efficacy differences between these agents during primary PCI in STEMI patients.
5. Conclusion
Bivalirudin associated with lower risk of mortality, major bleeding and thrombocytopenia compared with heparin in subgroup analysis and overall analysis. However, the use of bivalirudin increase the risk of MI and ST and has similar risk of MACE, TVR and stock compared with heparin used in STEMI patients.
Author contributions
Conceptualization: Yan-Qing Wu.
Formal analysis: Xiao-Qiang Liu, Xian-Du Luo.
Methodology: Xiao-Qiang Liu, Xian-Du Luo.
Supervision: Yan-Qing Wu.
Writing – original draft: Yan-Qing Wu.
Writing – review & editing: Yan-Qing Wu.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: CI = confidence intervals, MACE = major adverse cardiovascular events, MI = myocardial infarction, PCI = percutaneous coronary intervention, RCT = randomized controlled trial, RevMan = Review Manager, RRs = the pooled risk ratios, ST = stent thrombosis, STEMI = ST-elevation myocardial infarction, TVR = target vessel revascularization.
How to cite this article: Liu XQ, Luo XD, Wu YQ. Efficacy and safety of bivalirudin vs heparin in patients with coronary heart disease undergoing percutaneous coronary intervention: A meta-analysis of randomized controlled trials. Medicine. 2020;99:6(e19064).
The authors have no conflicts of interests to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Zhou M, Wang H, Zhu J, et al. Cause-specific mortality for 240 causes in China during 1990-2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet (London, England) 2016;387:251–72. [DOI] [PubMed] [Google Scholar]
- [2].Lincoff AM, Bittl JA, Harrington RA, et al. Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. JAMA 2003;289:853–63. [DOI] [PubMed] [Google Scholar]
- [3].Stone GW, McLaurin BT, Cox DA, et al. Bivalirudin for patients with acute coronary syndromes. N Engl J Med 2006;355:2203–16. [DOI] [PubMed] [Google Scholar]
- [4].Stone GW, Witzenbichler B, Guagliumi G, et al. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med 2008;358:2218–30. [DOI] [PubMed] [Google Scholar]
- [5].Ghimire G, Gupta A, Hage FG. Guidelines in review: 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction. J Nucl Cardiol 2014;21:190–1. [DOI] [PubMed] [Google Scholar]
- [6].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. [DOI] [PubMed] [Google Scholar]
- [7].Sorrentino S, Giustino G, Mehran R, et al. Everolimus-eluting bioresorbable scaffolds versus everolimus-eluting metallic stents. J Am Coll Cardiol 2017;69:3055–66. [DOI] [PubMed] [Google Scholar]
- [8].Guedeney P, Giustino G, Sorrentino S, et al. Efficacy and safety of alirocumab and evolocumab: a systematic review and meta-analysis of randomized controlled trials. Eur Heart J 2019;ii:ehz430. [DOI] [PubMed] [Google Scholar]
- [9].Steg PG, van ’t Hof A, Hamm CW, et al. Bivalirudin started during emergency transport for primary PCI. N Engl J Med 2013;369:2207–17. [DOI] [PubMed] [Google Scholar]
- [10].Schulz S, Richardt G, Laugwitz KL, et al. Comparison of prasugrel and bivalirudin vs clopidogrel and heparin in patients with ST-segment elevation myocardial infarction: design and rationale of the Bavarian Reperfusion Alternatives Evaluation (BRAVE) 4 trial. Clin Cardiol 2014;37:270–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shahzad A, Kemp I, Mars C, et al. Unfractionated heparin versus bivalirudin in primary percutaneous coronary intervention (HEAT-PPCI): an open-label, single centre, randomised controlled trial. Lancet (London, England) 2014;384:1849–58. [DOI] [PubMed] [Google Scholar]
- [12].Mehran R, Lansky AJ, Witzenbichler B, et al. Bivalirudin in patients undergoing primary angioplasty for acute myocardial infarction (HORIZONS-AMI): 1-year results of a randomised controlled trial. Lancet (London, England) 2009;374:1149–59. [DOI] [PubMed] [Google Scholar]
- [13].Han Y, Guo J, Zheng Y, et al. Bivalirudin vs heparin with or without tirofiban during primary percutaneous coronary intervention in acute myocardial infarction: the BRIGHT randomized clinical trial. JAMA 2015;313:1336–46. [DOI] [PubMed] [Google Scholar]
- [14].Erlinge D, Omerovic E, Frobert O, et al. Bivalirudin versus heparin monotherapy in myocardial infarction. N Engl J Med 2017;377:1132–42. [DOI] [PubMed] [Google Scholar]
- [15].Leonardi S, Frigoli E, Rothenbuhler M, et al. Bivalirudin or unfractionated heparin in patients with acute coronary syndromes managed invasively with and without ST elevation (MATRIX): randomised controlled trial. BMJ (Clin Res ed) 2016;354:i4935. [DOI] [PubMed] [Google Scholar]
- [16].Lee MS, Liao H, Yang T, et al. Comparison of bivalirudin versus heparin plus glycoprotein IIb/IIIa inhibitors in patients undergoing an invasive strategy: a meta-analysis of randomized clinical trials. Int J Cardiol 2011;152:369–74. [DOI] [PubMed] [Google Scholar]
- [17].Bittl JA, Chaitman BR, Feit F, et al. Bivalirudin versus heparin during coronary angioplasty for unstable or postinfarction angina: final report reanalysis of the Bivalirudin Angioplasty Study. Am Heart J 2001;142:952–9. [DOI] [PubMed] [Google Scholar]
- [18].Capodanno D, Gargiulo G, Capranzano P, et al. Bivalirudin versus heparin with or without glycoprotein IIb/IIIa inhibitors in patients with STEMI undergoing primary PCI: an updated meta-analysis of 10,350 patients from five randomized clinical trials. Eur Heart J Acute Cardiovasc Care 2016;5:253–62. [DOI] [PubMed] [Google Scholar]
- [19].Faggioni M, Baber U, Afshar AE, et al. Effects of body mass index on clinical outcomes in female patients undergoing percutaneous coronary intervention with drug-eluting stents: results from a patient-level pooled analysis of randomized controlled trials. JACC Cardiovasc Interv 2018;11:68–76. [DOI] [PubMed] [Google Scholar]
- [20].Faggioni M, Baber U, Sartori S, et al. Influence of baseline anemia on dual antiplatelet therapy cessation and risk of adverse events after percutaneous coronary intervention. Circ Cardiovasc Interv 2019;12:e007133. [DOI] [PubMed] [Google Scholar]
- [21].Sorrentino S, Baber U, Claessen BE, et al. Determinants of significant out-of-hospital bleeding in patients undergoing percutaneous coronary intervention. Thromb Haemost 2018;118:1997–2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


