Abstract
Adult-onset Still disease (AOSD), a systemic inflammatory disorder, is characterized by high fever, evanescent rash, arthritis, and hyperferritinaemia. AOSD is also reported to be associated with other skin lesions, including persistent pruritic papules and plaques. This study aimed to assess the significance of dyskeratotic skin lesions in Japanese AOSD patients.
We retrospectively assessed the histology of persistent pruritic skin lesions and evanescent rashes and the relationship between dyskeratotic cells, serum markers, and outcomes in 20 Japanese AOSD patients, comparing AOSD histology with that of dermatomyositis (DM), drug eruptions, and graft-versus-host disease (GVHD).
As the results, Persistent pruritic lesions were characterized by scattered single keratinocytes with an apoptotic appearance confined to the upper layer of the epidermis and horny layer without inflammatory infiltrate. In contrast to AOSD, the histology of DM, drug eruption, and GVHD demonstrated dyskeratotic cells in all layers of the epidermis with inflammatory infiltrate. AOSD with evanescent rash showed no dyskeratotic cells. The dyskeratotic cells in pruritic AOSD lesions stained positive for ssDNA and terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling, indicating apoptosis. Serum IL-18 was significantly higher in AOSD patients with dyskeratotic cells than those without, and generally required higher doses of glucocorticoids, immunosuppressants, and biologic agents. Two of ten AOSD patients with dyskeratotic cells died from hemophagocytic lymphohistiocytosis.
In conclusion, Persistent pruritic AOSD skin lesions are characterized by dyskeratotic cells with apoptotic features, involving the upper layers of the epidermis. There may be a link to elevated IL-18. This dyskeratosis may be a negative prognostic indicator.
Keywords: adult-onset Still disease, apoptosis, dyskeratosis, IL-18
Key Messages
Unlike the more common evanescent rash, persistent pruritic lesions can be seen in AOSD.
The pruritic lesions have dyskeratotic cells, which are apoptotic and confined to the upper epidermis.
Pruritic lesions, often accompanied by elevated IL-18, may be a negative prognostic indicator.
1. Introduction
Adult-onset Still disease (AOSD) is an acute systemic inflammatory disorder that is characterized by high spiking fevers, an evanescent salmon-colored rash, arthralgia/arthritis, and hyperferritinemia. Diagnosis is difficult, and sometimes delayed. AOSD is a diagnosis of exclusion, after infection, malignancy, and other connective tissue diseases have been ruled out. The typical evanescent salmon-colored rash is said to occur in about 73% of affected patients.[1] When present, this is considered an important finding and is accepted as one of the Yamaguchi classification criteria.[2] The rash usually coincides with fever.
However, there are also some less typical rashes seen with AOSD. There have been recent reports of persistent pruritic papules and plaques associated with AOSD. One report found the evanescent rash in 86% of AOSD patients, and persistent pruritic eruptions in 78%.[3] Another report found atypical skin lesions in 34.6% of patients.[4] It has been suggested that dyskeratotic cells, scattered single keratinocytes with an apoptotic appearance, in skin lesions of AOSD are pathologically characteristic, but the clinical significance has not been defined.[5]
Hyperferritinaemia has been considered the hallmark of AOSD activity.[6] Aberrant induction of several cytokines (interleukin-1β [IL-1β], IL-6, IL-18, tumor necrosis factor-α [TNF-α], interferon-γ [IFNγ]) may be involved in the pathologic mechanisms of AOSD.[7] Among them, IL-18, a member of the IL-1 family, was first identified as a potent inducer of IFNγ from Th1 lymphocytes[8] and is similar to IL-1β for being processed by caspase 1 to an 18 kDa-biologically active mature form. IL-18 binds the ligand receptor IL-18 receptor α chain (IL-18Rα), inducing the recruitment of IL-18 receptor β chain (IL-18Rβ) to form a high affinity receptor. The Toll-IL-1R Receptor (TIR) domains approximate and allow the binding of MyD88, which then induces a pro-inflammatory signal to the cells which terminates in NF-κB activation. In addition to NF-κB, the IL-18/IL-18Rα/ IL-18Rβ complex has been shown to induce phosphorylation of STAT3 in NK cells and the p38 MAP kinase pathway in neutrophils,[9] similar to the abnormal kinetics of canonical STAT3 and MAP kinase phosphorylation induced by granulocyte-colony stimulating factor (G-CSF)/G-CSF receptor.[10–12] Moreover, IL-18 can also activate macrophages directly to induce chemokine secretion.[9] Therefore, IL-18 may be the main contributor to the development of inflammation in AOSD.[13]
In this study, we assessed clinical and histological findings in Japanese patients with AOSD skin lesions, including persistent pruritic skin lesions and evanescent rash, and compared the dyskeratotic cells found in the upper epidermis in AOSD with the dyskeratosis in dermatomyositis (DM), drug eruptions, and graft-vs-host disease (GVHD).
2. Patients and methods
We retrospectively assessed clinical and histologic findings before treatment in skin lesions, including persistent pruritic skin lesions (n = 12) and evanescent rash (n = 8) in 20 Japanese patients with AOSD at Kochi Medical School Hospital and Kochi Red Cross Hospital. To assess whether the dyskeratotic cells in AOSD were apoptotic, we employed single-stranded DNA (ssDNA) staining and terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL). Serum levels of TNF-α, IL-6 and IL-18 were measured using an electrochemiluminescence assay (Meso Scale Discovery, MSD, Rockville, MD). We also evaluated the relationship between serum levels of IL-18 and skin lesions in AOSD with (n = 12) and without dyskeratosis (n = 8), and their relationship to disease outcome. The relationship between the appearance of dyskeratotic cells and age, sex, disease duration, white blood cell counts, hemoglobin level, serum levels of C-reactive protein (CRP), ferritin, TNF-α, IL-6, and IL-18, and erythrocyte sedimentation rate (ESR) was examined using stepwise multiple regression analysis. Finally, we assessed the relationship between the appearance of dyskeratotic cells and outcomes in AOSD.
This study was approved by the Ethics Committee of Kochi Medical School Hospital (approval #28–50) and conducted in accordance with the Declaration of Helsinki. All clinical information was obtained after the patients had given their informed consent. Age is presented as mean ± S.D. A comparison of variables between 2 groups was performed using a Mann–Whitney U test or one-way analysis of variance. Results were considered significant at P < .05.
3. Results
3.1. AOSD with persistent pruritic skin lesions histologically shows dyskeratosis
Patient age (mean ± SD) was 55 ± 18 y and male/female ratio was 1:2. AOSD cases with persistent pruritic skin lesions (n = 12) before treatment histologically showed scattered single keratinocytes with an apoptotic appearance limited to the upper layer of the epidermis and horny layer without inflammatory infiltrates, indicating dyskeratosis. AOSD cases with evanescent rash (n = 8) histologically showed no dyskeratotic cells.
A typical case of AOSD with dyskeratotic cells was a 57-year-old woman who developed high fever, persistent pruritic eruption, arthritis, and hepatic dysfunction. Laboratory examination showed high levels of serum CRP (12.3 mg/dl), ferritin (11,622 ng/ml; normal 39.4–340) and IL-18 (98,900 pg/ml). We could diagnose with AOSD after differential diagnosis. A biopsy of her persistent pruritic skin lesions showed dyskeratotic cells limited to the upper layer of the epidermis and horny layer without associated inflammatory infiltrate (Fig. 1 A and B).
Figure 1.
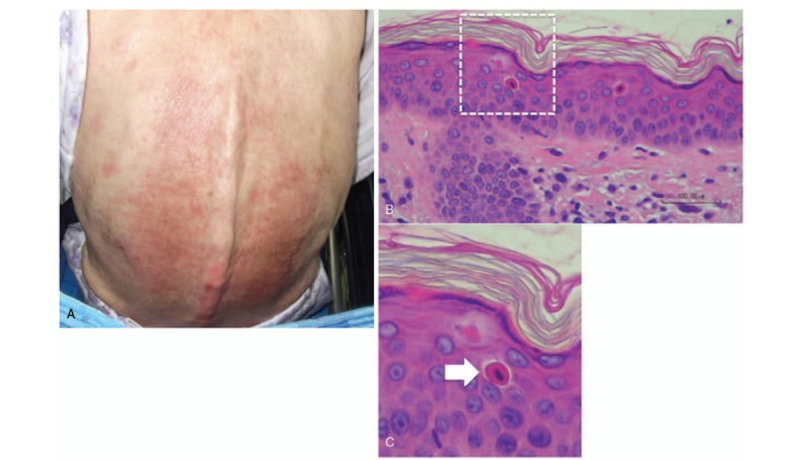
Representative example of a typical case of AOSD with persistent pruritic skin lesions. “Atypical” rash is shown (A) and histology shows dyskeratotic cells (B, C).
3.2. Dyskeratotic cells in AOSD are confined to the upper layer of the epidermis and horny layer
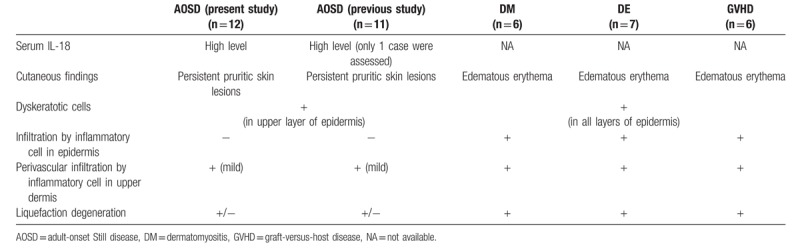
We compared the gross appearance and histological findings of AOSD with dyskeratotic cells (n = 12) with cases of other diseases associated with dyskeratotic cells, namely DM (n = 6), drug eruption (n = 7), and GVHD (n = 6), to investigate the specificity of the histological findings in AOSD. The gross appearance of the pruritic skin lesions in AOSD with dyskeratotic cells was closely similar to that of DM, drug eruption, and GVHD (Fig. 2A). Histologically, however, AOSD with persistent pruritic skin lesions showed dyskeratotic cells only in the upper layer of epidermis and horny layer and absence of inflammatory infiltrate. In contrast, the histology of DM, drug eruption and GVHD demonstrated dyskeratotic cells in all layers of the epidermis with accompanying inflammatory cell infiltration (Fig. 2B).
Figure 2.
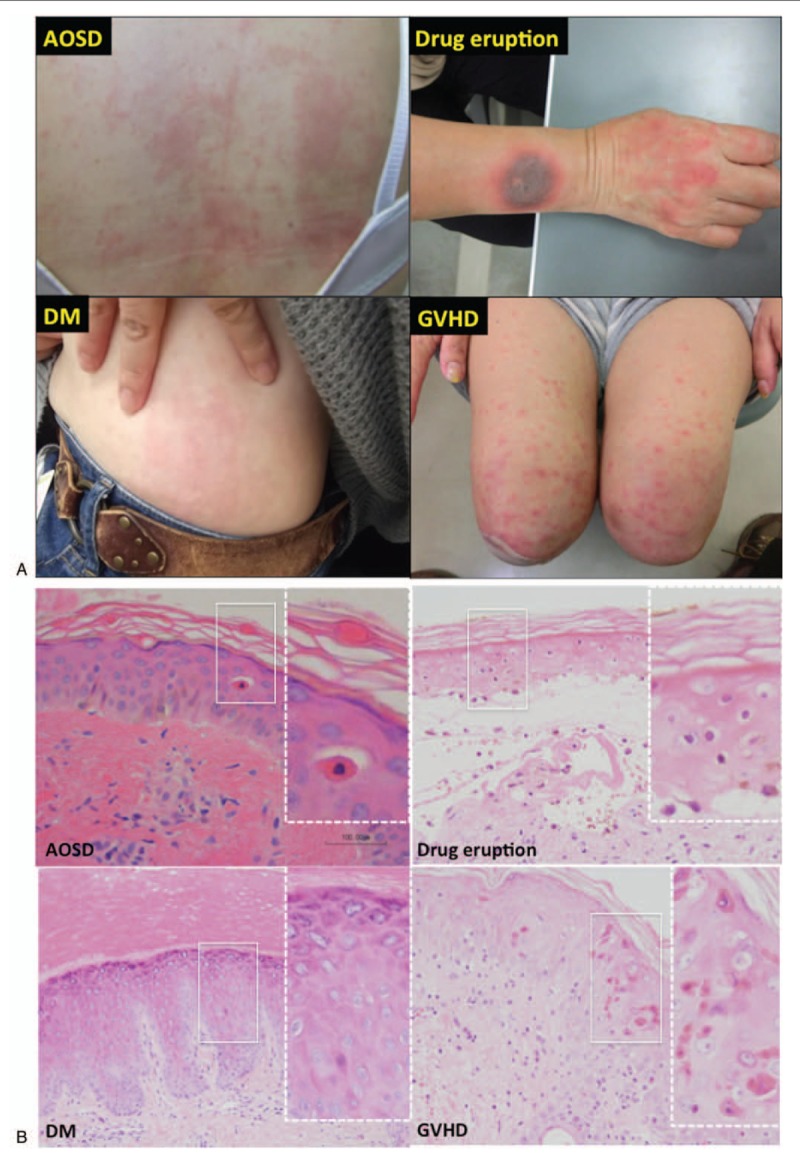
Dyskeratotic cells in AOSD are confined to the upper layer of the epidermis and horny layer. A. Comparison of gross appearances of persistent pruritic skin lesions of AOSD, DM, drug eruption, and GVHD. B. In contrast to AOSD with dyskeratotic cells, the histology of DM, drug eruption, and GVHD demonstrate dyskeratotic cells in all layers of the epidermis with accompanying inflammatory infiltrates.
3.3. Dyskeratotic cells in AOSD are apoptotic
To assess whether the dyskeratotic cells in AOSD were apoptotic or necrotic, we evaluated the specimens with ssDNA staining and TUNEL. The AOSD dyskeratotic cells (Fig. 3A) were positive on both ssDNA staining (Fig. 3B) and TUNEL (image not shown), indicating apoptosis. Normal skin showed no dyskeratotic cells and no ssDNA staining (Fig. 3C and D).
Figure 3.
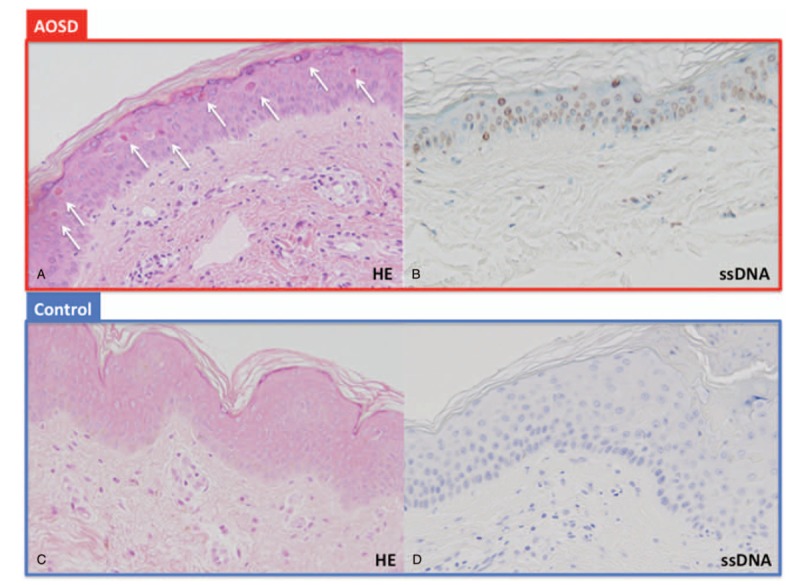
Dyskeratotic cells in AOSD are apoptotic. A. Scattered dyskeratotic cells (white arrows) without inflammatory infiltrate. B. Positive staining ssDNA of dyskeratotic cells suggests apoptosis. C, D. Control was negative.
3.4. Serum IL-18 levels in AOSD are significantly related to dyskeratosis
Noting that cases with pruritic lesions tended to have elevated levels of IL-18, we then evaluated the relationship between serum levels of IL-18 and AOSD skin lesions with and without dyskeratotic cells. Serum IL-18 levels were significantly higher in AOSD patients with dyskeratotic cells than in those without dyskeratotic cells (P = .03) (Fig. 4). The relationship between the appearance of dyskeratotic cells and age, sex, disease duration, white blood cell counts, hemoglobin level, and serum levels of CRP, ESR, ferritin, TNF-α, IL-6, and IL-18 was examined using stepwise multiple regression analysis. Although serum IL-18 level exhibited a strong correlation with levels of TNF-α (r = 0.328, P = .001) and IL-6 (r = 0.296, P = .005), serum IL-18 levels the most significantly correlated with the appearance of dyskeratotic cells (β = 0.324, F = 24.145) (table not shown).
Figure 4.
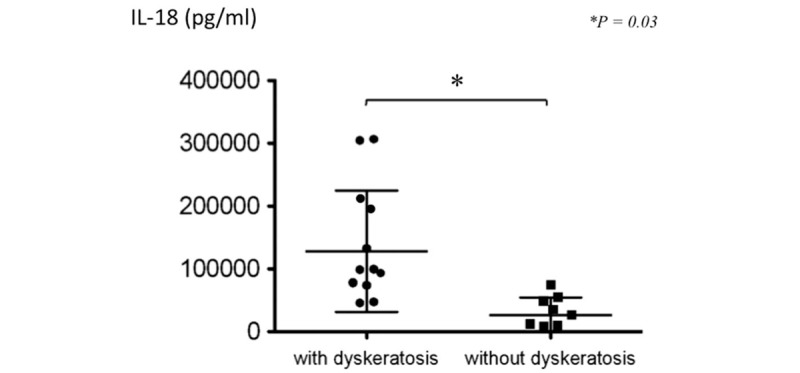
Serum IL-18 levels in AOSD with or without dyskeratosis. Serum IL-18 levels were significantly higher in AOSD patients with dyskeratotic cells (n = 10) than without dyskeratotic cells (n = 5) (P = .03).
3.5. Dyskeratosis may be a negative prognostic indicator
Finally, we assessed the relationship between the appearance of dyskeratotic cells and outcomes in AOSD. AOSD patients with persistent pruritic lesions and dyskeratotic cells required higher doses of glucocorticoids, immunosuppressants, and biologic agents compare to those without persistent pruritic lesions and dyskeratotic cells. Two of 10 AOSD patients with persistent pruritic lesions and dyskeratotic cells died of hemophagocytic lymphohistiocytosis within one year after diagnosis.
4. Discussion
This study demonstrates that persistent pruritic skin lesions in AOSD were associated with the presence of dyskeratotic cells, indicating prominent epidermal apoptosis, involving the upper layers of the epidermis. Elevated IL-18 levels were significantly correlated with dyskeratosis. The appearance of elevated serum IL-18 and persistent pruritic lesions with dyskeratosis in AOSD might be a negative prognostic indicator.
The classic rash of AOSD is evanescent and commonly observed with the fever spike, and disappears with defervescence. Pruritic, persistent skin rashes in AOSD, distinct from the classic typical evanescent rash, have been described. This type of eruption is pruritic and often has a linear configuration, possibly due to the Koebner phenomenon, and thus resembles flagellate erythema.[14] Several reports have described the unique histological features of persistent pruritic papules and plaques in AOSD patients, including dyskeratosis confined to the upper epidermis and horny layers with focal hyperkeratosis.[15] Previous reports have used terms such as “dyskeratosis”,[5] “necrotic keratinocyte”,[16] or “dyskeratotic cells”[17] (Table 1). In the present study, persistent pruritic skin lesions in AOSD revealed dyskeratotic cells limited to the upper layer of epidermis and horny layer without inflammatory infiltrates. Whether dyskeratotic cells in AOSD are necrotic or apoptotic cells is still controversial. In this study, these dyskeratotic cells were positive by ssDNA staining and TUNEL, indicating apoptosis.
Table 1.
Reviews of previous reports about atypical skin lesions of AOSD.
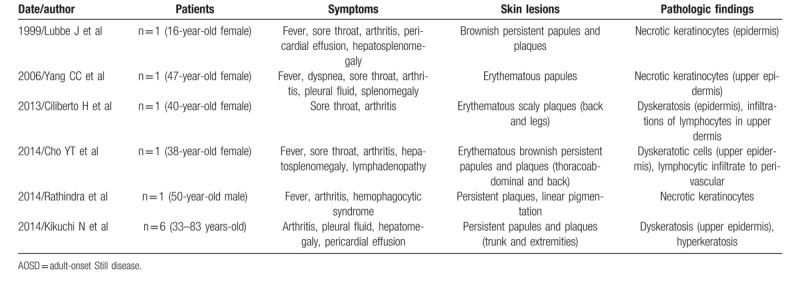
We found serum IL-18 levels to be significantly higher in AOSD patients with dyskeratotic cells than in those without dyskeratotic cells, suggesting that epidermal apoptosis of keratinocytes might correlate with elevation of serum IL-18. High levels of serum IL-18 have been reported in the acute phase of AOSD.[5,18] It has been suggested that several inflammatory cytokines such as IL-1, IL-6, IL-18, IFN-γ and TNF-α play a pathogenic role in AOSD,[19] with IL-18 the most prominent. IL-18 belongs to the IL-1 family of cytokines. IL-18 is produced and secreted by several types of cells including macrophages, osteoblasts, chondrocytes, and dendritic cells.[20] In the skin, IL-18 is also produced and secreted by keratinocytes and Langerhans cells.[21] IL-18 may induce the production of IFN-γ by Th1 cells and NK cells. Further, IL-18 induces TNF-α production, and enhances IL-17 production by Th17 cells.[20] Expression of IL-18 receptor on human keratinocytes has been shown at the mRNA level.[22] Therefore, IL-18 might combine with keratinocytes and directly induce apoptosis. It is also possible that keratinocytes, activated by activated IL-18, induce apoptosis via IFN-γ, IL-17, and TNF-α. To address these issues including IL-18/IL-18R signaling might then lead to new approaches to targeted treatment.
Considering the differential diagnosis, the gross appearance of skin lesions in AOSD patients is often similar to the skin lesions of DM, drug eruption and GVHD patients. As mentioned above, dyskeratotic cells in AOSD are limited to the upper layers of the epidermis and horny layer and are devoid of inflammatory infiltrates. In contrast to AOSD, the histology of DM, drug eruption, and GVHD demonstrates dyskeratotic cells in all layers of the epidermis accompanied by inflammatory infiltrates. These differences are useful in distinguishing AOSD from other skin lesions (Table 2) The dyskeratotic cells of DM, drug eruption, and GVHD patients might be induced by the accompanying inflammatory infiltrate.
Table 2.
Summary of histological characteristics of atypical skin lesions in AOSD, compared with DM, drug eruption, GVHD and the results from previous reports of AOSD.
We assessed the relationship between the presence of dyskeratotic cells and outcomes in AOSD. In the present cases, the majority of AOSD patients with dyskeratotic cells required higher doses of glucocorticoids, immunosuppressants, and biologic agents than those without dyskeratotic cells. Two of 10 AOSD patients with dyskeratotic cells died of hemophagocytic lymphohistiocytosis. Narvaez Garcia et al reported that atypical rash might imply persistent disease activity and the requirement for more aggressive treatment.[23] As in our study, the appearance of dyskeratotic cells in AOSD suggesting epidermal apoptosis of keratinocytes could be related to the high levels of serum IL-18 and worse prognosis in the disease course. Therefore, the recognition of dyskeratotic cells in persistent pruritic skin lesions, along with elevated IL-18 levels, is thought to be crucial for the early diagnosis of AOSD as it appears to be a negative prognostic indicator that may require that more intense therapy.
This study had several limitations. First, the treatment regimen and follow-up protocols were inconsistent among patients because of the study's retrospective and multi-institutional nature, complicating evaluation of the influence of treatment protocol differences on patient outcome. Second, although this study included more patients with long-term, appropriate radiologic follow-up over 2 years than previous studies, the number of patients was not sufficient to conclude that the identified factors were definitively significant predictors nor that the indicated cutoffs were optimal. Larger-scale prospective studies will be needed to confirm our results.
In conclusion, persistent pruritic skin lesions in AOSD are specific for the presence of dyskeratotic cells, indicating prominent epidermal apoptosis involving the upper layers of the epidermis. Finally, epidermal apoptosis of keratinocytes correlates with high levels of serum IL-18 and confers a worse prognosis in AOSD.
Acknowledgment
We thank Libby Cone, MD, MA, from DMC Corp. (www.dmed.co.jp <http://www.dmed.co.jp/>) for editing drafts of this manuscript.
Yoshinori Taniguchi Orcid: 0000-0002-8621-7790.
Footnotes
Abbreviations: AOSD = adult-onset Still disease, G-CSF = granulocyte-colony stimulating factor, IFN = interferon, IL = interleukin, MAP = mitogen-activated protein, mRNA = messenger ribonucleic acid, NF-κB = nuclear factor-kappa B, ssDNA = single-stranded deoxyribonucleic acid, STAT = signal transuducer and activator of transcription, TNF = tumor necrosis factor, TUNEL = terminal deoxynucleotidyl transferase-mediated dUTP nick end-labelling.
How to cite this article: Maeda-Aoyama N, Hamada-Ode K, Taniguchi Y, Nishikawa H, Arii K, Nakajima K, Fujimoto S, Terada Y. Dyskeratotic cells in persistent pruritic skin lesions as a prognostic factor in adult-onset Still disease. Medicine. 2020;99:6(e19051).
NMA, KHO, and YT contributed equally to this manuscript.
This work was supported by the Kochi Organization for Medical Reformation and Renewal (TY).
The authors declare no conflict of interest.
References
- [1].Bagnari V, Colina M, Ciancio G, et al. Adult-onset Still's disease. Rheumatol Int 2010;30:855–62. [DOI] [PubMed] [Google Scholar]
- [2].Yamaguchi M. Preliminary criteria for classification of adult Still's disease. J Rheumatol 1992;19:424–30. [PubMed] [Google Scholar]
- [3].Lee JY, Hsu CK, Liu MF, et al. Evanescent and persistent pruritic eruptions of adult-onset still disease: a clinical and pathologic study of 36 patients. Semin Arthritis Rheum 2012;42:317–26. [DOI] [PubMed] [Google Scholar]
- [4].Kim HA, Kwon JE, Yim H, et al. The pathologic findings of skin, lymph node, liver, and bone marrow in patients with adult-onset Still disease: a comprehensive analysis of 40 cases. Medicine (Baltimore) 2015;94:e787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kikuchi N, Satoh M, Ohtsuka M, et al. Persistent pruritic papules and plaques associated with adult-onset Still's disease: Report of six cases. J Dermatol 2014;41:407–10. [DOI] [PubMed] [Google Scholar]
- [6].Schwarz-Eywill M, Heiling B, Bauer H, et al. Evaluation of serum ferritin as a marker for adult Still's disease activity. Ann Rheum Dis 1992;51:683–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hoshino T, Ohta A, Yang D, et al. Elevated serum interleukin 6, interferon-(, and tumor necrosis factor-( levels in patients with adult Still's disease. J Rheumatol 1998;25:396–8. [PubMed] [Google Scholar]
- [8].Okamura H, Tsutsui H, Komatsu T, et al. Cloning of a new cytokine that induces IFN-ɣ production by T cells. Nature 1995;378:88–91. [DOI] [PubMed] [Google Scholar]
- [9].Kaplanski G. Interleukin-18: Biological properties and role in disease pathogenesis. Immunol Rev 2018;281:138–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dwivedi P, Muench DE, Wagner M, et al. Time resolved quantitative phosphor-tyrosine analysis reveals Bruton's Tyrosine kinase mediated signaling downstream of the mutated granulocyte-colony stimulating factor receptors. Leukemia 2019;33:75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dwivedi P, Muench DE, Wagner M, et al. Phosphoserine and threonine analysis of normal and mutated granulocyte colony stimulating factor receptors. Sci Data 2019;6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dwivedi P, Greis KD. Granulocyte colony stimulating factor receptor signaling in severe congenital neutropenia, chronic neutrophilic leukemia, and related malignancies. Exp Hematol 2017;46:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kawaguchi Y, Terajima H, Harigai M, et al. Interleukin-18 as a novel diagnostic marker and indicator of disease severity in adult-onset Still's disease. Arthritis Rheum 2001;44:1716–7. [DOI] [PubMed] [Google Scholar]
- [14].Yamamoto N, Nishioka K. Flagellate erythema. Int J Dermatol 2006;45:627–31. [DOI] [PubMed] [Google Scholar]
- [15].Fortna RR, Gudjonsson JE, Seidel G, et al. Persistent pruritic papules and plaques: a characteristic histopathologic presentation seen in a subset of patients with adult-onset and juvenile Still's disease. J Cutan Pathol 2010;37:932–7. [DOI] [PubMed] [Google Scholar]
- [16].Yang CC, Lee JY, Liu MF, et al. Adult-onset Still's disease with persistent skin eruption and fatal respiratory failure in a Taiwanese woman. Eur J Dermatol 2006;16:593–4. [PubMed] [Google Scholar]
- [17].Cho YT, Liao YH. Prurigo pigmentosa-like persistent papules and plaques in a patient with adult-onset Still's disease. Acta Derm Venereol 2014;94:102–3. [DOI] [PubMed] [Google Scholar]
- [18].Kawashima M, Yamamura M, Yamauchi H, et al. Levels of interleukin-18 and its binding inhibitors in the blood circulation of patients with adult-onset Still's disease. Arthritis Rheum 2001;44:550–60. [DOI] [PubMed] [Google Scholar]
- [19].Chen DY, Lan JL, Lin FJ, et al. Proinflammatory cytokine profiles in sera and pathological tissues of patients with active untreated adult onset Still's disease. J Rheumatol 2004;31:2189–98. [PubMed] [Google Scholar]
- [20].Carroll HP, Paunovic V, Gadina M. Signalling, inflammation and arthritis: Crossed signals: the role of interleukin-15 and -18 in autoimmunity. Rheumatology (Oxford) 2008;47:1269–77. [DOI] [PubMed] [Google Scholar]
- [21].Yamamoto T. Cutaneous manifestations associated with adult-onset Still's disease: important diagnostic values. Rheumatol Int 2012;32:2233–7. [DOI] [PubMed] [Google Scholar]
- [22].Mee JB. Human keratinocytes constitutively produce but do not process interleukin-18. Br J Dermatol 2000;143:330–6. [DOI] [PubMed] [Google Scholar]
- [23].Narvaez Garcia FJ, Pascul M, Lopez de Recalde M, et al. Adult-onset Still's disease with atypical cutaneous manifestations. Medicine (Baltimore) 2017;96:e6318. [DOI] [PMC free article] [PubMed] [Google Scholar]


