Abstract
Background:
Lead can adversely affect maternal and child health across a wide range of exposures; developing fetuses and breastfeeding infants may be particularly vulnerable. We describe the distribution of blood lead levels (BLLs) in U.S. women of childbearing age and associations with sociodemographic, reproductive, smoking, and housing characteristics over a 40-y period.
Methods:
Data from the National Health and Nutrition Examination Survey (NHANES) II, NHANES III Phase I and Phase II, and 1999–2016 continuous NHANES were used to describe the distribution of BLLs (given in micrograms per deciliter; ) in U.S. women 15–49 years of age between 1976 and 2016. For all women with valid BLLs (), geometric mean (GM) BLLs and estimated prevalence of BLLs were calculated overall and by selected demographic characteristics. For NHANES II, estimated prevalence of BLLs and were also calculated.
Results:
The most recent GM BLLs (2007–2010 and 2011–2016, respectively) were [95% confidence interval (CI): 0.79, 0.84] and (95% CI: 0.59, 0.64). In comparison, GM BLLs in earlier periods (1976–1980, 1988–1991, and 1991–1994) were (95% CI: 9.95, 10.79), (95% CI: 1.75, 1.94), and (95% CI: 1.45, 1.60), respectively. In 2011–2016, 0.7% of women of childbearing age had BLLs , and higher BLLs were associated with older age, other race/ethnicity, birthplace outside the United States, four or more live births, exposure to secondhand tobacco smoke, and ever pregnant or not currently pregnant.
Discussion:
Lead exposure in U.S. women of childbearing age is generally low and has substantially decreased over this 40-y period. However, based on these estimates, there are still at least 500,000 U.S. women being exposed to lead at levels that may harm developing fetuses or breastfeeding infants. Identifying high-risk women who are or intend to become pregnant remains an important public health issue. https://doi.org/10.1289/EHP5925
Introduction
Despite overall declines in environmental sources, lead exposure remains an important public health problem for certain groups, including women of childbearing age, due to the potential exposure to the developing fetus and breastfeeding infant (CDC 2010). Women of childbearing age (15–49 y old) represent 23% of the total U.S. population and 46% of females (U.S. Census Bureau 2017), and, at any given time, about 9% are pregnant (Crocetti et al. 1990). Lead readily crosses the placenta by passive diffusion, and fetal blood lead concentrations are highly correlated with maternal blood lead concentrations (Goyer 1990). Prenatal lead exposure has known influences on maternal health, infant birth, and neurodevelopmental outcomes across a wide range of exposure levels (Bellinger 2005). No safe level of exposure to lead in children has been identified, and toxic effects of lead have been identified even at lower levels of exposure than previously considered harmful (NTP 2012). In addition, declines in environmental sources of lead highlight maternal bone as a continuing endogenous source of exposure (Hu and Hernandez-Avila 2002). Bone lead stores are mobilized during periods of increased bone turnover, such as during pregnancy and lactation (Gulson et al. 2016b, 2003), and lead is released into maternal blood and breastmilk (Ettinger et al. 2014; Gulson et al. 2016a). Since cumulative bone lead stores persist for years to decades, women and their infants may be at risk for lead exposure long after environmental sources have been abated.
In general, U.S. population lead exposures, which are estimated by blood lead levels (BLLs) measured in the National Health and Nutrition Examination Survey (NHANES), have declined over time (Raymond et al. 2014). This is due, in large part, to successful policies aimed at controlling sources of lead in the environment, including removal of lead from gasoline, paint, plumbing fixtures, and consumer products (Brown and Margolis 2012). However, numerous lead hazards still exist, including deteriorating lead-based paint and dust in housing built before the 1978 ban on lead in residential paint, as well as from drinking water provided by pipes made from lead, lead solder, and plumbing fixtures containing lead. Occupational and take-home exposures from workers or household members exposed to lead in the workplace remain an important preventable source of lead exposure, as occupational exposure accounts for over 90% of known lead exposure among adults (Alarcon 2016). Other preventable sources of lead exposure include soil, traditional/folk medicines, fishing sinkers, bullets, materials used to make ceramics and stained glass (Alarcon 2016), and consumer products, including certain foods, spices, medicines, cosmetics, and vitamins (Pfadenhauer et al. 2016).
Little is known about the full extent of lead exposure among U.S. women of childbearing age and, more importantly, among pregnant and lactating women who may pass lead to their unborn baby or breastfeeding infant, since adult women are not routinely tested (CDC 2010). Some population subgroups may be highly exposed, particularly foreign-born recent immigrants (Graber et al. 2006; Klitzman et al. 2002; Pezzi et al. 2019), workers in several high-risk occupations (Alarcon 2016; CDC 2007), and those practicing high-risk behaviors, such as pica of lead-contaminated objects, smoking or exposure to secondhand smoke, or renovation of older homes (CDC 2010; Richter et al. 2013; Thihalolipavan et al. 2013). Women living near hazardous waste sites or active smelters (García Vargas et al. 2001) and who are residents in countries where leaded gasoline is used or only has recently been phased out may also be exposed to relatively high levels of lead in the environment (Bede-Ojimadu et al. 2018; Forsyth et al. 2018; Prihartono et al. 2019).
We analyzed 40 y of NHANES data (1976–2016) to describe the distribution of BLLs in U.S. women of childbearing age (15–49 years of age) and the association with sociodemographic, reproductive, smoking, and housing characteristics.
Methods
NHANES Sample Design
NHANES is a cross-sectional, nationally representative survey designed to monitor the health and nutritional status of the U.S. noninstitutionalized population. Prior to 1999, NHANES was conducted on a periodic basis. The NHANES II and NHANES III Phase I and Phase II survey designs and blood lead components have been described in detail previously (Brody et al. 1994; CDC 1985; Pirkle et al. 1994, 1998). Since 1999, NHANES has been a continuous survey conducted every 2 y on an ongoing basis among a representative sample of all ages, as previously described (CDC 2019). A stratified, multistage probability sampling design is used to select approximately 10,000 participants every 2 y for a personal interview and a standardized physical examination conducted in specially designed and outfitted mobile examination centers. The survey collects information on chronic disease prevalence (including undiagnosed conditions), risk factors, diet and nutritional status, immunization status, infectious disease prevalence, health insurance, and measures of environmental exposures. The household interview includes questions about sociodemographic characteristics, health history, health-related behaviors, and access to health care.
Blood Lead Measurements
During the physical examination, participants 1 year of age and older are eligible for blood lead testing by venipuncture (Paschal et al. 1995). Laboratory methods for NHANES II (CDC 1985), NHANES III (Gunter et al. 1996), and NHANES 1999–2016 have been described previously (Jones et al. 2009). The blood lead detection limit decreased from in 1976–1980 to in 1988–1994, to in 1999–2010, to in 2011–2012, and finally to in 2013–2016 as technology improved. Results below the lower detection limit are imputed by NHANES and replaced with a value equal to the detection limit divided by the square root of 2 (CDC 2009). Whole-blood specimens were analyzed for lead concentration using inductively coupled argon plasma mass spectrometry with isotope dilution by the Division of Laboratory Sciences at the National Center for Environmental Health of the Centers for Disease Control and Prevention (CDC).
Sociodemographic Characteristics
Age was categorized into 15–24 y, 25–34 y, 35–44 y, and 45–49 y for all analysis periods. Race/ethnicity for NHANES II and NHANES III was categorized as non-Hispanic white, non-Hispanic black, and Mexican American. For NHANES III, “other race” was not included due to small sample sizes. For NHANES 1999–2016, we reported “other race” and “other Hispanic” in the tables in addition to the three categories mentioned previously. These categories were included in the race/ethnicity survey response choices starting in 1999. Birthplace was categorized as the United States, Mexico, or other.
Socioeconomic status was assessed using the poverty-to-income ratio (PIR) (calculated as total family income divided by the federal poverty threshold for the year of interview) stratified as and to be consistent with government assistance programs that use a PIR of 1.3 to determine eligibility (Pirkle et al. 1998). The Medicaid status variable was defined as whether a woman was enrolled in Medicaid at the time of the questionnaire after indicating that she was covered by some health insurance plan.
Reproductive Characteristics
Self-reported reproductive characteristics assessed include: have you ever been pregnant, how many live births have you had, have you ever breastfed, and were you currently pregnant at the time of interview. “Ever breastfed” represents women who reported ever breastfeeding and includes those who reported current breastfeeding. Breastfeeding was not assessed in NHANES II, and it was not consistently assessed between 2011 and 2016.
Smoking Characteristics
Smoking status and secondhand smoke exposure were defined according to the methods of Dietz et al. (2011). We analyzed the association between self-reported smoking status (current, former, or never) on BLLs for women 15–49 years of age (NHANES III, NHANES II) and for women 20–49 years of age (continuous NHANES cycles). Different questions on tobacco use were asked of those women of age for the continuous NHANES (1999–2016) cycles, and, therefore, the information is not directly comparable to tobacco use information for women 20–49 y old. Additionally, we analyzed the association between self-reported secondhand smoke exposure (yes, no) on BLLs for women 15–49 years of age (NHANES III) and for women 20–49 years of age (continuous NHANES cycles). Environmental tobacco smoke exposure was not assessed in NHANES II.
Housing Age
Responses for “year housing was built” used different categories for NHANES III and the NHANES 1999–2010 cycles. In NHANES III, housing age was reported as pre-1946, 1946–1973, 1973 to present, and unknown. From 1999–2010, housing age was reported as pre-1950, 1950–1977, 1978 to present, and unknown. We defined risk based on the most similar housing age categories; observations for which housing age was unavailable were recorded as unknown. Housing age was not collected in NHANES II or in the 2011–2016 survey periods.
Statistical Analysis
This analysis includes data from NHANES II, NHANES III Phase I, NHANES III Phase II, and the 18 y of NHANES data from 1999 to 2016 for women 15–49 years of age with valid blood lead measurements. Participants were grouped according to the survey period as follows: NHANES II (1976–1980), NHANES III (1988–1991), NHANES III (1991–1994), NHANES (1999–2002), NHANES (2003–2006), NHANES (2007–2010), and NHANES (2011–2016). Using 4-y intervals and a 6-y interval for 2011–2016 provided a greater number of women tested and yielded more stable estimates.
All statistical analyses were completed using SAS (version 9.3; SAS Institute Inc.) and SAS-callable SUDAAN statistical software packages (version 11.0.0; RTI International). All analyses were performed incorporating the sample weights and respecting the complex sample design of NHANES. Pregnancy variables for women 15–19 and 45–49 years of age and BLLs (NHANES 1999–2000 only) are restricted variables. These variables were accessed through the Research Data Center at the National Center for Health Statistics (NCHS). Estimates were produced using the examination sampling weight to represent U.S. adults in order to account for unequal probabilities of selection, oversampling, and survey nonresponse. The sample weights were poststratified to the U.S. population as estimated by the U.S. Bureau of the Census. BLLs were not rounded before statistical analysis.
We computed actual sample size, geometric mean (GM) BLL, and the estimated prevalence of women with BLLs for each NHANES survey period and by select sociodemographic, reproductive, and housing characteristics. Formal statistical testing for significant BLL differences for each variable of interest was not completed. Potential differences in variables are noted for nonoverlapping confidence intervals (CIs). For NHANES II, the estimated prevalence of women with BLLs and also were calculated. Although there is no safe BLL for women of childbearing age, the use of dichotomous BLL thresholds is advantageous for assessing trends over time. These cut points may be more easily understood than statistically derived cut points such as quartiles. NCHS Data Standards include criteria for evaluating estimated means based on the relative standard error (RSE) (CDC 2018a). According to these criteria, prevalence estimates were considered statistically unreliable if the RSE of the estimate (expressed as a percent) was greater than 30% and results from sample sizes less than five were suppressed.
Results
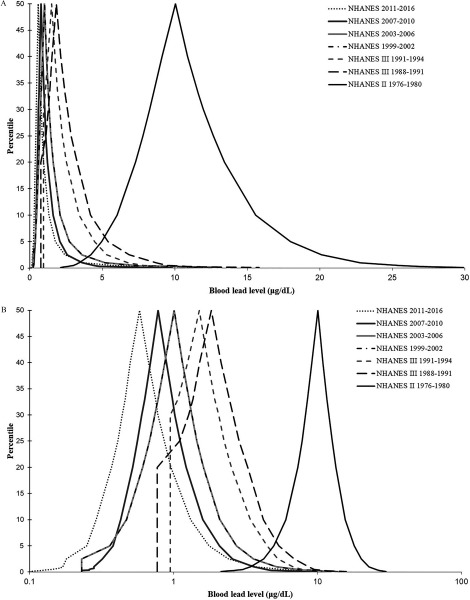
Table 1 shows the sample size by NHANES period for all women 15–49 years of age, all women 15–49 years of age with available BLLs, women with BLLs , and all women 15–49 years of age with available BLLs and current pregnancy status. Approximately 4.5–16.5% of women 15–49 years of age with complete blood lead data were currently pregnant at the time of survey for each of the survey periods. Figure 1 shows the shift in distribution of BLLs among U.S. women of childbearing age over the 40-y period from 1976 to 2016.
Table 1.
Blood lead levels (BLLs) and pregnancy status among U.S. women 15–49 years of age by the National Health and Nutrition Examination Survey (NHANES) survey period from 1976 to 2016.
| Survey cycle | Years | All women 15–49 years of age () | Women 15–49 years of age with data available on BLLs | Women 15–49 years of age with data available on BLLs and current pregnancy status | ||
|---|---|---|---|---|---|---|
| Total [ (%)]a | BLL [ (%)]b | Total [ (%)]c | Pregnant [ (%)]c | |||
| NHANES II | 1976–1980 | 4,892 | 1,820 (37.2) | 1,791 (98.4) | 1,146 (63.0) | 59 (5.1) |
| NHANES III | 1988–1991 | 2,993 | 2,616 (87.4) | 268 (10.2) | 2,250 (86.1) | 154 (6.8) |
| NHANES III | 1991–1994 | 3,527 | 3,201 (90.8) | 167 (5.2) | 2,812 (87.8) | 172 (6.1) |
| NHANES | 1999–2002 | 4,384 | 3,914 (89.3) | 54 (1.4) | 3,545 (90.6) | 585 (16.5) |
| NHANES | 2003–2006 | 4,251 | 3,830 (90.1) | 35 (0.9) | 3,830 (100.0) | 608 (15.9) |
| NHANES | 2007–2010 | 3,889 | 3,577 (91.9) | 13 (0.4) | 2,422 (67.7) | 115 (4.7) |
| NHANES | 2011–2016 | 5,750 | 3,450 (60.0) | 25 (0.7) | 2,284 (66.2) | 103 (4.5) |
Note: BLL, blood lead level; , sample size, where is the number of participants, not a weighted population estimate.
Unweighted percentage of all women 15–49 years of age.
Unweighted percentage of women 15–49 years of age with data available on BLL.
Unweighted percentage of women 15–49 years of age with data available on BLL and current pregnancy status.
Figure 1.
(A) Distribution of blood lead levels among U.S. women 15–49 years of age by National Health and Nutrition Examination Survey (NHANES) survey period 1976–2016; the peak of the curve corresponds to the median (50th percentile) of blood lead level distribution. (B) Distribution of blood lead levels among U.S. women 15–49 years of age by the NHANES survey period from 1976 to 2016, plotted on log scale.
BLLs were highest among women 15–49 years of age in NHANES II (1976–1980). During this period, the GM BLL was (95% CI: 9.95, 10.79), and 98.3% of women of childbearing age had BLLs (95% CI: 97.1, 99.2), 61.4% had BLLs (95% CI: 55.8, 66.8), and 3.6% had BLLs (95% CI: 2.1, 5.5) (Table 2).
Table 2.
Geometric means (GM) [95% confidence interval (CI)] and estimated prevalence (95% CI) of blood lead levels (BLLs) , , and among U.S. women 15–49 years of age for selected characteristics in National Health and Nutrition Examination Survey (NHANES) II, 1976–1980.
| NHANES II, 1976–1980 | |||||
|---|---|---|---|---|---|
| Variablea | GM (95% CI) | BLL [% (95% CI)] | BLL [% (95% CI)] | BLL [% (95% CI)] | |
| Overall | 1,820 | 10.37 (9.95, 10.79) | 98.3 (97.1, 99.2) | 61.4 (55.8, 66.8) | 3.6 (2.1, 5.5) |
| Age (y) | |||||
| 15–24 | 692 | 9.88 (9.37, 10.39) | 97.9 (95.9, 99.2) | 56.3 (48.6, 63.9) | 1.5 (0.5, 2.9)b |
| 25–34 | 539 | 10.11 (9.65, 10.58) | 97.8 (95.8, 99.1) | 58.1 (52.2, 63.9) | 3.7 (1.5, 6.6)b |
| 35–44 | 411 | 10.85 (10.31, 11.40) | 99.2 (98.1, 99.8) | 66.7 (60.9, 72.3) | 4.6 (2.4, 7.5) |
| 45–49 | 178 | 11.93 (11.24, 12.63) | 99.6 (98.3, 100.0) | 77.4 (68.3, 85.4) | 8.5 (4.8, 13.3) |
| Race/ethnicity | |||||
| Non-Hispanic white | 1,548 | 10.23 (9.79, 10.66) | 98.1 (96.6, 99.1) | 60.1 (54.1, 65.9) | 3.3 (1.9, 5.0) |
| Non-Hispanic black | 229 | 11.52 (10.71, 12.33) | 99.7 (98.6, 100.0) | 72.1 (63.1, 80.4) | 5.9 (1.5, 12.9)b |
| Mexican American | 43 | 10.04 (9.02, 11.06) | 100.0 (N/A, N/A) | 56.6 (38.4, 74.0) | 3.1 (0.3, 15.7)b |
| Birthplace | |||||
| United States | 1,719 | 10.38 (9.94, 10.82) | 98.2 (96.9, 99.2) | 61.5 (55.9, 67.0) | 3.8 (2.2, 5.8) |
| Mexico | N/A | N/A | N/A | N/A | N/A |
| Other | 101 | 10.13 (9.34, 10.91) | 99.4 (97.5, 100.0) | 60.4 (46.5, 73.5) | 0.5 (0.0, 2.4)b |
| Ever pregnant | |||||
| Yes | 1,160 | 10.62 (10.14, 11.10) | 98.4 (97.0, 99.4) | 64.5 (58.8, 70.0) | 4.0 (2.5, 5.9) |
| No | 653 | 9.91 (9.43, 10.39) | 98.1 (96.2, 99.3) | 55.6 (48.5, 62.7) | 2.9 (1.2, 5.4)b |
| Number of live births | |||||
| Never pregnant | 653 | 9.91 (9.43, 10.39) | 98.1 (96.2, 99.3) | 55.6 (48.5, 62.7) | 2.9 (1.2, 5.4)b |
| 0 | 91 | 10.33 (9.63, 11.04) | 100.0 (N/A, N/A) | 62.4 (53.7, 70.8) | 3.0 (0.5, 7.6)b |
| 1 | 254 | 10.34 (9.51, 11.17) | 97.3 (93.9, 99.3) | 66.1 (55.6, 75.9) | 4.1 (1.9, 7.1)b |
| 2–3 | 533 | 10.57 (10.05, 11.09) | 98.2 (96.5, 99.4) | 62.3 (56.4, 68.0) | 4.0 (2.1, 6.7) |
| 4 or more | 279 | 11.09 (10.52, 11.66) | 99.4 (98.2, 100.0) | 68.0 (60.9, 74.7) | 4.3 (2.0, 7.4)b |
| Poverty-to-income ratio | |||||
| 438 | 10.44 (9.84, 11.05) | 98.8 (97.3, 99.7) | 63.1 (56.2, 70.7) | 3.5 (1.6, 6.0)b | |
| 1,337 | 10.38 (9.94, 10.81) | 98.2 (97.0, 99.1) | 61.2 (55.2, 66.9) | 3.5 (1.9, 5.6) | |
| Currently pregnant | |||||
| Yes | 59 | 8.54 (7.64, 9.44) | 98.8 (94.6, 100.0) | 45.3 (30.1, 60.9) | N/A |
| No | 1,087 | 10.75 (10.26, 11.25) | 98.5 (97.0, 99.5) | 65.6 (59.7, 71.2) | 4.3 (2.6, 6.3) |
| Smoking status | |||||
| Current smoker | 636 | 11.52 (10.94, 12.09) | 99.3 (98.2, 99.9) | 72.5 (66.4, 78.2) | 7.1 (4.1, 10.8) |
| Former smoker | 206 | 10.05 (9.37, 10.72) | 98.3 (94.6, 99.9) | 58.2 (48.6, 67.6) | 1.9 (0.3, 4.7)b |
| Never smoker | 777 | 9.78 (9.30, 10.26) | 97.7 (95.9, 98.9) | 55.1 (48.1, 62.1) | 2.0 (0.9, 3.5)b |
Note: N/A, not applicable; indicates that the data were not collected in the survey cycle.
Housing age, Medicaid, ever breastfed, and secondhand tobacco smoke are not included because these variables were not assessed in NHANES II.
Relative standard error (RSE) .
GMs, sample size, and estimated prevalence of BLL by select characteristics are presented in Tables 3 and 4. BLLs were higher among women 15–49 years of age in NHANES III (1988–1991, 1991–1994) than in more recent continuous survey years (1999–2016). From 1991 to 1994, the GM BLL was (95% CI: 1.45, 1.60), 3.8% had BLLs (95% CI: 2.4, 5.6), and 0.3% had BLLs (95% CI: 0.2, 0.5) (Table 3). From 1988 to 1991, the GM BLL was (95% CI: 1.75, 1.94), 6.6% had BLLs (95% CI: 5.4, 7.9), and 0.4% had BLLs (95% CI: 0.1, 0.9) (Table 3).
Table 3.
Geometric means (GM) [95% confidence interval (CI)] and estimated prevalence (95% CI) of blood lead levels (BLLs) among U.S. women 15–49 years of age for selected characteristics in National Health and Nutrition Examination Survey (NHANES) III, 1988–1994.
| NHANES III | ||||||
|---|---|---|---|---|---|---|
| 1988–1991 | 1991–1994 | |||||
| Variable | GM (95% CI) | BLL [% (95% CI)] | GM (95% CI) | BLL [% (95% CI)] | ||
| Overall | 2,616 | 1.85 (1.75, 1.94) | 6.6 (5.4, 7.9) | 3,201 | 1.53 (1.45, 1.60) | 3.8 (2.4, 5.6) |
| Age (y) | ||||||
| 15–24 | 881 | 1.43 (1.31, 1.56) | 2.6 (1.4, 4.2) | 997 | 1.22 (1.14, 1.31) | 1.3 (0.4, 2.6)a |
| 25–34 | 778 | 1.80 (1.68, 1.93) | 5.2 (3.2, 7.6) | 984 | 1.46 (1.38, 1.54) | 3.3 (2.0, 5.0) |
| 35–44 | 713 | 2.18 (2.02, 2.35) | 10.3 (7.7, 13.2) | 944 | 1.71 (1.59, 1.83) | 5.2 (2.7, 8.4) |
| 45–49 | 244 | 2.48 (2.25, 2.71) | 11.7 (7.2, 17.0) | 276 | 2.11 (1.80, 2.41) | 7.6 (2.9, 14.1)a |
| Race/ethnicity | ||||||
| Non-Hispanic white | 888 | 1.76 (1.65, 1.86) | 5.2 (4.2, 6.4) | 910 | 1.46 (1.38, 1.53) | 3.4 (1.9, 5.2) |
| Non-Hispanic black | 746 | 2.16 (1.95, 2.37) | 12.0 (8.5, 15.9) | 1,206 | 1.75 (1.62, 1.87) | 6.5 (4.3, 9.0) |
| Mexican American | 878 | 2.04 (1.73, 2.35) | 11.4 (8.0, 15.3) | 915 | 1.67 (1.55, 1.78) | 6.4 (5.0, 8.0) |
| Birthplace | ||||||
| United States | 2,035 | 1.80 (1.70, 1.90) | 6.3 (5.2, 7.5) | 2,480 | 1.47 (1.39, 1.55) | 3.8 (2.3, 5.7) |
| Mexico | 430 | 2.60 (2.11, 3.10) | 18.8 (11.3, 27.8) | 450 | 2.09 (1.95, 2.23) | 10.6 (7.6, 14.0) |
| Other | 144 | 2.10 (1.84, 2.35) | 7.9 (2.9, 15.1)a | 263 | 1.83 (1.61, 2.04) | 3.9 (1.3, 8.0)a |
| Ever pregnant | ||||||
| Yes | 1,873 | 1.98 (1.87, 2.10) | 7.1 (5.8, 8.5) | 2,335 | 1.61 (1.51, 1.70) | 4.0 (2.5, 5.9) |
| No | 665 | 1.50 (1.40, 1.60) | 4.5 (2.7, 6.7) | 787 | 1.31 (1.21, 1.42) | 3.3 (1.3, 6.4)a |
| Number of live births | ||||||
| Never pregnant | 665 | 1.50 (1.40, 1.60) | 4.5 (2.7, 6.7) | 787 | 1.31 (1.21, 1.42) | 3.3 (1.3, 6.4)a |
| 0 | 180 | 1.88 (1.58, 2.17) | 8.3 (4.1, 13.8) | 202 | 1.49 (1.34, 1.63) | 2.5 (0.5, 5.9)a |
| 1 | 469 | 1.90 (1.75, 2.05) | 6.6 (4.2, 9.6) | 581 | 1.45 (1.30, 1.60) | 2.4 (1.1, 4.2)a |
| 2–3 | 888 | 1.98 (1.85, 2.10) | 6.1 (4.0, 8.5) | 1,181 | 1.68 (1.55, 1.82) | 4.4 (2.2, 7.5) |
| 4 or more | 336 | 2.37 (1.92, 2.82) | 11.8 (6.2, 18.8) | 371 | 1.77 (1.58, 1.95) | 8.2 (3.3, 14.9)a |
| Ever Breastfed | ||||||
| Yes | 790 | 1.88 (1.75, 2.02) | 5.3 (3.6, 7.2) | 1,011 | 1.55 (1.41, 1.68) | 3.4 (1.4, 6.1)a |
| No | 902 | 2.13 (2.00, 2.25) | 8.7 (6.3, 11.5) | 1,121 | 1.71 (1.58, 1.85) | 5.2 (3.1, 7.8) |
| Poverty-to-income ratio | ||||||
| 839 | 2.10 (1.87, 2.33) | 9.8 (7.2, 12.8) | 1,241 | 1.66 (1.54, 1.78) | 5.2 (3.3, 7.6) | |
| 1,519 | 1.77 (1.67, 1.86) | 5.7 (4.2, 7.4) | 1,735 | 1.48 (1.40, 1.55) | 3.5 (1.9, 5.6) | |
| Medicaid | ||||||
| Yes | 344 | 2.19 (1.91, 2.48) | 11.3 (6.6, 17.0) | 552 | 1.74 (1.58, 1.91) | 4.7 (2.6, 7.3) |
| No | 2,078 | 1.81 (1.71, 1.91) | 6.3 (4.9, 7.8) | 2,647 | 1.50 (1.42, 1.58) | 3.8 (2.3, 5.5) |
| Housing age | ||||||
| Pre-1946 | 461 | 2.16 (1.94, 2.39) | 9.3 (6.4, 12.8) | 510 | 1.59 (1.44, 1.74) | 4.9 (1.6, 9.8)a |
| 1946–1973 | 1,188 | 1.79 (1.67, 1.91) | 7.0 (5.3, 8.9) | 1,225 | 1.57 (1.46, 1.67) | 4.1 (2.4, 6.3) |
| 1973 to present | 696 | 1.71 (1.56, 1.85) | 4.7 (2.4, 7.6) | 993 | 1.40 (1.29, 1.51) | 2.5 (1.1, 4.6)a |
| Unknown | 129 | 2.30 (1.98, 2.63) | 6.6 (3.0, 11.5) | 426 | 1.77 (1.57, 1.96) | 5.5 (2.7, 9.2) |
| Currently pregnant | ||||||
| Yes | 154 | 1.41 (1.20, 1.61) | 1.4 (0.5, 2.9)a | 172 | 1.27 (1.11, 1.43) | 2.0 (0.2, 5.9)a |
| No | 2,096 | 1.97 (1.87, 2.07) | 7.4 (6.1, 8.8) | 2,640 | 1.59 (1.50, 1.68) | 4.3 (2.8, 6.3) |
| Smoking status | ||||||
| Current smoker | 644 | 2.45 (2.27, 2.63) | 10.3 (7.8, 13.1) | 697 | 1.95 (1.80, 2.10) | 6.9 (4.2, 10.4) |
| Former smoker | 306 | 1.74 (1.58, 1.90) | 5.5 (2.8, 9.1) | 340 | 1.62 (1.48, 1.76) | 2.9 (0.7, 6.6)a |
| Never smoker | 1,489 | 1.66 (1.55, 1.76) | 4.5 (3.3, 6.0) | 1,943 | 1.37 (1.31, 1.44) | 2.8 (1.8, 4.1) |
| Secondhand tobacco smoke | ||||||
| Yes | 1,152 | 2.15 (2.03, 2.27) | 9.2 (7.6, 11.0) | 1,135 | 1.77 (1.65, 1.89) | 6.0 (3.7, 8.8) |
| No | 1,461 | 1.63 (1.55, 1.71) | 3.9 (3.1, 4.9) | 2,065 | 1.41 (1.34, 1.49) | 2.6 (1.5, 4.1) |
Relative standard error (RSE) .
Table 4.
Geometric means (GMs) [95% confidence intervals (CI)] and estimated prevalence (95% CI) of blood lead levels (BLLs) among U.S. women 15–49 years of age for selected characteristics, NHANES, 1999–2016.
| Continuous NHANES | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1999–2002 | 2003–2006 | 2007–2010 | 2011–2016 | |||||||||
| Variable | GM (95% CI) | BLL [% (95% CI)] | GM (95% CI) | BLL [% (95% CI)] | GM (95% CI) | BLL [% (95% CI)] | GM (95% CI) | BLL % [% (95% CI)] | ||||
| Overall | 3,914 | 1.05 (1.01, 1.09) | 1.3 (0.7, 2.0) | 3,830 | 0.91 (0.88, 0.95) | 0.7 (0.4, 1.1) | 3,577 | 0.81 (0.79, 0.84) | 0.3 (0.1, 0.5)a | 3,450 | 0.61 (0.59, 0.64) | 0.7 (0.4, 1.2) |
| Age (y) | ||||||||||||
| 15–24 | 1,813 | 0.85 (0.80, 0.90) | 1.1 (0.1, 3.0)a | 1,772 | 0.74 (0.71, 0.77) | 0.4 (0.1, 1.0)a | 1,123 | 0.66 (0.63, 0.69) | 0.2 (0.0, 0.7)a | 1,144 | 0.46 (0.43, 0.49) | 0.2 (0.0, 0.4)a |
| 25–34 | 910 | 0.98 (0.92, 1.04) | 0.6 (0.0, 2.0)a | 939 | 0.83 (0.78, 0.88) | 0.3 (0.1, 0.7)a | 924 | 0.77 (0.75, 0.79) | 0.3 (0.0, 0.9)a | 907 | 0.57 (0.54, 0.60) | 0.5 (0.1, 1.0)a |
| 35–44 | 827 | 1.20 (1.13, 1.27) | 0.8 (0.3, 1.6)a | 755 | 1.01 (0.95, 1.07) | 0.9 (0.5, 1.5) | 1,017 | 0.87 (0.82, 0.91) | 0.3 (0.1, 0.5) | 941 | 0.72 (0.67, 0.76) | 1.4 (0.5, 2.7)a |
| 45–49 | 364 | 1.36 (1.24, 1.47) | 3.8 (1.7, 6.7)a | 364 | 1.27 (1.16, 1.37) | 1.5 (0.4, 3.1)a | 513 | 1.17 (1.11, 1.23) | 0.4 (0.0, 1.2)a | 458 | 0.87 (0.82, 0.93) | 0.9 (0.1, 2.6)a |
| Race/ethnicity | ||||||||||||
| Non-Hispanic white | 1,452 | 1.01 (0.96, 1.05) | 1.1 (0.4, 2.3)a | 1,516 | 0.86 (0.83, 0.89) | 0.2 (0.0, 0.5)a | 1,476 | 0.76 (0.74, 0.79) | 0.1 (0.0, 0.3)a | 1,126 | 0.59 (0.56, 0.62) | 0.5 (0.1, 1.3)a |
| Non-Hispanic black | 869 | 1.21 (1.12, 1.30) | 1.2 (0.5, 2.4)a | 1,014 | 1.03 (0.92, 1.14) | 2.0 (0.7, 3.8)a | 689 | 0.92 (0.88, 0.96) | 0.2 (0.0, 0.6)a | 798 | 0.61 (0.57, 0.65) | 0.6 (0.1, 1.3)a |
| Mexican American | 1,214 | 1.19 (1.09, 1.29) | 2.9 (1.9, 4.1) | 981 | 1.05 (0.98, 1.12) | 2.1 (0.9, 3.9)a | 751 | 0.88 (0.83, 0.94) | 0.6 (0.2, 1.3)a | 557 | 0.61 (0.56, 0.65) | 2.1 (0.8, 4.1)a |
| Other race | 146 | 0.98 (0.83, 1.13) | 0.5 (0.0, 0.9)a | 178 | 1.04 (0.92, 1.16) | 1.1 (0.0, 4.6)a | 206 | 1.06 (0.93, 1.19) | 1.6 (0.2, 4.0)a | 613 | 0.80 (0.74, 0.86) | 0.4 (0.1, 1.1)a |
| Other Hispanic | 233 | 1.09 (0.97, 1.20) | 1.2 (0.0, 5.0)a | 141 | 0.89 (0.75, 1.03) | 1.6 (0.2, 4.1)a | 455 | 0.84 (0.78, 0.91) | 0.6 (0.0, 1.8)a | 356 | 0.58 (0.53, 0.63) | 0.5 (0.0, 1.6)a |
| Birthplace | ||||||||||||
| United States | 3,011 | 1.01 (0.97, 1.06) | 1.2 (0.6, 2.1)a | 3,021 | 0.87 (0.83, 0.90) | 0.4 (0.2, 0.7)a | 2,659 | 0.78 (0.75, 0.80) | 0.2 (0.1, 0.4)a | 2,466 | 0.58 (0.55, 0.60) | 0.5 (0.2, 1.1)a |
| Mexico | 589 | 1.52 (1.39, 1.65) | 4.5 (2.8, 6.6) | 492 | 1.35 (1.25, 1.45) | 3.8 (1.6, 6.9)a | 406 | 1.11 (1.02, 1.18) | 1.1 (0.3, 2.3)a | 0 | — | 0.0 (N/A, N/A) |
| Other | 313 | 1.21 (1.12, 1.30) | 0.2 (0.0, 1.0)a | 317 | 1.14 (1.05, 1.22) | 1.7 (0.4, 3.9)a | 510 | 1.03 (0.96, 1.10) | 0.5 (0.0, 1.6)a | 983 | 0.79 (0.75, 0.83) | 1.5 (0.7, 2.7)a |
| Ever pregnant | ||||||||||||
| Yes | 2,339 | 1.13 (1.07, 1.18) | 1.6 (0.8, 2.7) | 2,189 | 0.98 (0.94, 1.02) | 0.8 (0.5, 1.3) | 2,125 | 0.89 (0.86, 0.92) | 0.1 (0.0, 0.3)a | 1,603 | 0.62 (0.59, 0.65) | 0.6 (0.2, 1.1)a |
| No | 1,316 | 0.88 (0.83, 0.93) | 0.3 (0.1, 0.7)a | 1,347 | 0.77 (0.72, 0.81) | 0.5 (0.1, 1.4)a | 1,032 | 0.68 (0.65, 0.71) | 0.3 (0.0, 0.7)a | 908 | 0.51 (0.48, 0.55) | 0.2 (0.0, 0.4)a |
| Number of live births | ||||||||||||
| Never pregnant | 1,316 | 0.88 (0.83, 0.93) | 0.3 (0.1, 0.7)a | 1,347 | 0.77 (0.72, 0.81) | 0.5 (0.1, 1.4)a | 1,032 | 0.68 (0.65, 0.71) | 0.3 (0.0, 0.7)a | 908 | 0.51 (0.48, 0.55) | 0.2 (0.0, 0.4)a |
| 0 | 184 | 1.13 (0.95, 1.31) | 2.6 (0.2, 7.7)a | 155 | 0.95 (0.84, 1.05) | 0.0 (N/A, N/A) | 9 | 1.14 (0.70, 1.58) | 0.0 (NA, NA) | 5 | 0.47 (0.13, 0.80) | 0.0 (N/A, N/A) |
| 1 | 659 | 1.06 (0.98, 1.14) | 1.0 (0.1, 3.2)a | 586 | 0.92 (0.87, 0.98) | 1.1 (0.5, 2.0)a | 504 | 0.84 (0.80, 0.88) | 0.2 (0.0, 0.6)a | 408 | 0.59 (0.53, 0.65) | 0.2 (0.0, 0.6)a |
| 2-3 | 1,021 | 1.13 (1.07, 1.20) | 1.6 (0.4, 3.4)a | 989 | 1.01 (0.96, 1.05) | 0.8 (0.4, 1.5)a | 1,172 | 0.89 (0.85, 0.94) | 0.0 (0.0, 0.1)a | 829 | 0.63 (0.59, 0.67) | 0.8 (0.2, 1.8)a |
| 4 or more | 276 | 1.35 (1.20, 1.49) | 3.1 (0.8, 6.6)a | 208 | 1.18 (1.07, 1.28) | 1.3 (0.2, 3.6)a | 269 | 1.05 (0.97, 1.14) | 0.8 (0.1, 2.1)a | 217 | 0.68 (0.62, 0.74) | 0.8 (0.0, 2.5)a |
| Ever breastfedb | ||||||||||||
| Yes | 1,244 | 1.07 (1.02, 1.12) | 0.8 (0.3, 1.5)a | 1,204 | 0.97 (0.93, 1.02) | 0.8 (0.3, 1.5)a | 1,217 | 0.87 (0.84, 0.91) | 0.1 (0.0, 0.3)a | N/A | — | — |
| No | 712 | 1.25 (1.15, 1.34) | 2.9 (1.1, 5.4)a | 579 | 1.05 (0.98, 1.12) | 1.3 (0.8, 2.1) | 727 | 0.93 (0.90, 0.97) | 0.2 (0.0, 0.5)a | N/A | — | — |
| Poverty-to-income ratio | ||||||||||||
| 1,287 | 1.17 (1.08, 1.25) | 2.4 (0.9, 4.6)a | 1,348 | 1.02 (0.95, 1.10) | 1.4 (0.6, 2.6)a | 1,316 | 0.90 (0.85, 0.95) | 0.4 (0.1, 1.0)a | 1,243 | 0.63 (0.60, 0.66) | 1.2 (0.6, 2.1)a | |
| 2,278 | 0.99 (0.95, 1.03) | 0.8 (0.4, 1.4)a | 2,304 | 0.87 (0.84, 0.91) | 0.4 (0.2, 0.8)a | 1,972 | 0.78 (0.76, 0.81) | 0.1 (0.0, 0.3)a | 1,967 | 0.60 (0.57, 0.63) | 0.4 (0.1, 0.9)a | |
| Medicaid | ||||||||||||
| Yes | 484 | 1.11 (0.98, 1.23) | 2.0 (0.0, 6.9)a | 590 | 0.98 (0.89, 1.07) | 1.2 (0.4, 2.5)a | 479 | 0.82 (0.78, 0.87) | 0.4 (0.0, 1.1)a | 597 | 0.61 (0.56, 0.66) | 0.8 (0.2, 1.6)a |
| No | 2,383 | 0.99 (0.95, 1.03) | 0.8 (0.3, 1.4)a | 2,792 | 0.87 (0.84, 0.90) | 0.4 (0.2, 0.8)a | 3,091 | 0.82 (0.79, 0.84) | 0.3 (0.1, 0.6)a | 2,843 | 0.61 (0.59, 0.64) | 0.7 (0.3, 1.2)a |
| Housing age | ||||||||||||
| Pre-1950 | 600 | 1.15 (1.05, 1.26) | 1.3 (0.4, 2.7)a | 572 | 0.99 (0.92, 1.07) | 1.0 (0.9, 2.6)a | 576 | 0.88 (0.83, 0.94) | 0.1 (0.0, 0.5)a | N/A | — | — |
| 1950–1977 | 919 | 1.04 (0.97, 1.11) | 1.7 (0.6, 3.6)a | 872 | 0.90 (0.85, 0.95) | 0.6 (0.2, 1.2)a | 829 | 0.80 (0.76, 0.84) | 0.1 (0.0, 0.4)a | N/A | — | — |
| 1978 to present | 1,253 | 0.95 (0.90, 1.00) | 0.3 (0.1, 0.7)a | 1,201 | 0.83 (0.80, 0.87) | 0.3 (0.0, 0.8)a | 1,286 | 0.77 (0.74, 0.80) | 0.3 (0.1, 0.7)a | N/A | — | — |
| Unknown | 1,104 | 1.20 (1.10, 1.30) | 2.6 (0.8, 5.5)a | 1,150 | 1.03 (0.98, 1.08) | 1.6 (0.9, 2.4) | 863 | 0.91 (0.86, 0.96) | 0.6 (0.1, 1.6)a | N/A | — | — |
| Currently pregnant | ||||||||||||
| Yes | 585 | 0.80 (0.71, 0.88) | 0.4 (0.1, 0.9)a | 608 | 0.65 (0.60, 0.70) | 0.4 (0.0, 1.5)a | 115 | 0.63 (0.57, 0.69) | 0.0 (N/A, N/A) | 103 | 0.48 (0.39, 0.57) | 2.8 (0.0, 11.0)a |
| No | 2,960 | 1.08 (1.03, 1.13) | 1.4 (0.7, 2.2) | 3,222 | 0.93 (0.89, 0.97) | 0.7 (0.4, 1.2) | 2,307 | 0.81 (0.78, 0.83) | 0.3 (0.1, 0.5)a | 2,181 | 0.61 (0.59, 0.64) | 0.7 (0.3, 1.2)a |
| Smoking statusc | ||||||||||||
| Current smoker | 548 | 0.99 (0.94, 1.04) | 4.3 (1.9, 7.5)a | 572 | 1.24 (1.16, 1.31) | 1.0 (0.3, 2.2)a | 723 | 1.08 (1.02, 1.13) | 0.4 (0.1, 1.1)a | 510 | 0.82 (0.77, 0.88) | 1.6 (0.3, 4.0)a |
| Former smoker | 398 | 1.02 (0.93, 1.10) | 0.1 (0.0, 0.4)a | 387 | 0.90 (0.83, 0.97) | 0.1 (0.0, 0.6)a | 358 | 0.82 (0.77, 0.88) | 0.2 (0.0, 0.7)a | 320 | 0.64 (0.60, 0.69) | 0.1 (0.0, 0.4)a |
| Never smoker | 1,666 | 1.49 (1.38, 1.60) | 0.6 (0.3, 1.0) | 1,608 | 0.86 (0.81, 0.90) | 0.9 (0.4, 1.4) | 1,854 | 0.79 (0.76, 0.82) | 0.2 (0.1, 0.5)a | 2,054 | 0.60 (0.57, 0.63) | 0.7 (0.3, 1.3)a |
| Secondhand tobacco smokec | ||||||||||||
| Yes | 833 | 1.34 (1.24, 1.43) | 3.4 (1.4, 6.3)a | 763 | 1.16 (1.08, 1.24) | 1.4 (0.5, 2.9)a | 640 | 1.01 (0.95, 1.06) | 0.3 (0.0, 0.7)a | 438 | 0.79 (0.70, 0.87) | 2.5 (0.5, 5.9)a |
| No | 3,042 | 0.97 (0.93, 1.01) | 0.6 (0.3, 1.0) | 3,030 | 0.85 (0.82, 0.89) | 0.5 (0.2, 0.9) | 2,917 | 0.78 (0.76, 0.81) | 0.3 (0.1, 0.6)a | 1,681 | 0.62 (0.59, 0.66) | 0.4 (0.1, 0.7)a |
Note: —, no data; N/A, not applicable; indicates that the data are not available in the survey cycle or that the upper/lower limits of a confidence interval could not be derived due to small sample sizes.
Relative standard error (RSE) .
Ever breastfed includes women who answered yes to currently breastfeeding.
Restricted to women 20–49 years of age because women of age were not asked the same tobacco questions as older women and therefore were not included in this analysis.
From 1999 to 2002 (Table 4), the GM BLL was (95% CI: 1.01, 1.09) in women 15–49 years of age, and 1.3% of women of childbearing age (15–49 y) had BLLs (95% CI: 0.7, 2.0). Higher BLLs were associated with older age, non-Hispanic black, or Mexican-American race/ethnicity, birthplace outside of the United States, ever being pregnant, a higher number of live births, never breastfed, lower PIR (), not being currently pregnant, current smoking cigarettes, and secondhand smoke exposure.
From 2003 to 2006 (Table 4), the GM BLL was (95% CI: 0.88, 0.95) in women 15–49 years of age, and 0.7% of women of childbearing age (15–49 y old) had BLLs (95% CI: 0.4, 1.1). Higher BLLs were associated with older age, birthplace in Mexico, ever pregnant, a higher number of live births, never breastfed, and not currently pregnant.
From 2007 to 2010 (Table 4), the GM BLL was (95% CI: 0.79, 0.84) in women 15–49 years of age, and 0.3% of women of childbearing age (15–49 y old) had BLLs (95% CI: 0.1, 0.5). Higher BLLs were associated with older age, birthplace outside the United States, ever pregnant, a higher number of live births, lower PIR (), and not currently pregnant. In the most recent survey years (2011–2016) (Table 4), the GM BLL was (95% CI: 0.59, 0.64) in women 15–49 years of age, and 0.7% of women of childbearing age (15–49 y old) had BLLs (95% CI: 0.4, 1.2), which represents over 500,000 U.S. women (U.S. Census Bureau 2017). Higher BLLs were associated with older age, other race/ethnicity, ever pregnant, and not currently pregnant.
Discussion
The GM BLL among U.S women of childbearing age has declined substantially from 10.37 to over the 40-y period of this analysis (1976–2016). The reported generational decline in BLLs corresponds to successful policy initiatives over this period that reduced sources of lead in gasoline, paint, plumbing fixtures, and other consumer products (Dignam et al. 2019). In NHANES II, the first to measure BLLs, an astonishing 98.3% of U.S. women 15–49 years of age had BLLs compared to less than 1% with BLLs in the most recent survey period of this analysis (2011–2016). However, despite the dramatic declines in population BLLs over time, this still represents over 500,000 women of childbearing age in the United States who have exposure to lead above the CDC action level, BLLs for pregnant women (Ettinger and Wengrovitz 2010; U.S. Census Bureau 2017). A BLL of or greater in a pregnant woman flags the occurrence of prior or ongoing lead exposure that may not otherwise be recognized. The vulnerability of a developing fetus to adverse effects of lead and the possibility of preventing additional postnatal exposures justify intervention for pregnant women showing evidence of lead exposure at these levels.
Epidemiologic and experimental evidence suggest that lead is a potent reproductive and developmental toxicant (Landrigan et al. 2000; NTP 2012), but the biological mechanisms of effect are not fully understood. Maternal exposure to high levels of lead (BLL of ) have been consistently linked to an increased risk for spontaneous abortion (Hertz-Picciotto 2000). Lead also has been identified as a statistically significant predictor of maternal blood pressure (Rothenberg et al. 1999) and associated with increased risk for gestational hypertension (Karumanchi et al. 2005; Kosnett et al. 2007; Rothenberg et al. 2002). It remains uncertain at what exposure level risk begins to increase and whether or not lead-induced increases in blood pressure during pregnancy may lead to severe hypertension or preeclampsia.
Elevated BLLs among pregnant and lactating women are a particular problem due to an increased risk for exposure to developing fetuses and breastfeeding infants during key stages of development. There is no safe level of lead exposure in children (Advisory Committee on Childhood Lead Poisoning Prevention 2012). Although data are inconsistent, maternal lead exposure may increase the risk for preterm delivery, fetal growth restriction, and decreased postnatal growth (Cheng et al. 2017; González-Cossío et al. 1997; Greene and Ernhart 1991; Ingelfinger and Schnaper 2005; Sanin et al. 2001). Fetal lead exposure is predictive of adverse neurodevelopment later in life (Schnaas et al. 2006; Wasserman et al. 2000), and these effects are independent of the effects of postnatal exposure (Gomaa et al. 2002; Hu et al. 2006).
There is increasing awareness that unintended exposure to environmental contaminants, such as lead, may be adversely affecting maternal and infant health, including the ability to become pregnant, maintain a healthy pregnancy, and have a healthy baby. Women and children may be disproportionately affected by environmental risk factors that are associated with poverty and social injustice, thus making them particularly vulnerable to adverse health effects of such environmental exposures (Silbergeld and Patrick 2005). Maternal lead exposure is a serious public health concern because lead remains a widespread environmental health hazard, and current efforts at primary prevention have focused almost entirely on children. Identifying women at risk and implementing effective strategies for prevention of fetal and early infant lead exposure is an important public health priority. In fact, it may be necessary to consider prepregnancy interventions among certain high-risk groups because research suggests that screening and intervention after than the first trimester may be too late to prevent fetal neurotoxic effects (Hu et al. 2006).
In particular, lead exposure remains a concern for women in certain population subgroups at increased risk for exposure, such as foreign-born recent immigrants, and those with specific opportunity for exposure, such as individuals who may be exposed in the workplace or through the use of certain consumer products that contain lead. The U.S. Occupational Safety and Health Administration (OSHA) Lead Standard 1910.1025, established in the 1970s and updated in the 1980s, requires workers be removed from lead exposure when BLLs are equal to or greater than (construction industry) or (general industry), and allows workers to return to work when the BLL is below (OSHA 2012). However, current guidelines for medical management of lead-exposed adults (Kosnett et al. 2007) recommend that it is advisable for pregnant women to avoid occupational or avocational exposures that would result in blood lead concentrations , and, in 2015, NIOSH designated BLLs as elevated (CDC 2018b).
Lee et al. (2005) studied determinants of blood lead in U.S. women of childbearing age using data from NHANES III (1988–1994) and found a number of factors associated with higher BLLs, including age, black or Hispanic race/ethnicity, living in the northeast region or in urban areas, lower educational level, poverty, lower hematocrit, alcohol use, cigarette smoking, and serum protoporphyrin level (Lee et al. 2005). In the more recent years, highlighted by our current analysis, several of these risk factors remain, including increased maternal age, race/ethnicity, poverty, and birth outside the United States, suggesting that lead exposure persists in certain segments of the population due to sociodemographic disparities.
Interestingly, we found that women “not currently pregnant” had higher BLLs than pregnant women in most survey cycles, which is counterintuitive given bone lead mobilization during pregnancy. However, this group of “not currently pregnant” includes women of different ages, parities, lead exposure histories, and other characteristics that may also be associated with higher BLLs (and bone lead levels). We did not conduct multivariate analyses, so it is not possible to tease out the reasons for this paradoxical finding.
The major limitation of this analysis is the incomplete ability to conduct subgroup and multivariate analyses due to small cell sizes, particularly at higher BLLs. Despite combining 40 y of survey data, the population subsample with valid blood lead test results is limited within survey periods. Therefore, several estimates, as evaluated by the RSE according to NHANES analytic guidelines, are unstable and should be reviewed with caution. Nonetheless, our analysis provides important information on long-term trends in BLLs among women of childbearing age in the United States.
The U.S. Preventive Services Task Force recently highlighted key gaps in the evidence that is insufficient to either support or recommend against blood lead screening in pregnant women and children (Cantor et al. 2019; Curry et al. 2019; Jin 2019; Spanier et al. 2019). The American College of Obstetricians and Gynecologists (ACOG), following the CDC’s 2010 guidelines (CDC 2010), recommends that providers ask about common risk factors for lead exposure and perform blood lead testing of pregnant women if risk factors are identified (ACOG 2012).
Overall, current lead exposure in U.S. women of childbearing age is generally low. The estimates presented here, based on a nationally representative sample, can be generalized to the U.S. population. However, an increasing number of reports indicate that highly exposed women do still exist, and local surveillance may identify additional risk factors for specific population subgroups. The identification of high-risk women who are pregnant or intend to become pregnant before they pass their own lead burden on to their developing fetus or breastfeeding infant is important, as there is no apparent threshold for the adverse effects of lead in children. BLLs remain higher for certain children at risk, particularly those in minority populations, from low-income families, and/or who live in older homes (CDC 2018b, 2018c). Some of these children may begin their lead exposure in utero. Continued efforts to preemptively eliminate or control sources of lead, particularly in high-risk communities, screen persons at highest risk for exposure, and provide timely interventions for those identified with elevated BLLs will help to protect future generations. The prevention of lead exposure and its adverse health effects remains an important public health issue.
Acknowledgments
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the CDC and Prevention or the Agency for Toxic Substances and Disease Registry. Data files were created by Karon C. Lewis at the NCHS Research Data Center.
Footnotes
The authors declare they have no actual or potential competing financial interests.
Note to readers with disabilities: EHP strives to ensure that all journal content is accessible to all readers. However, some figures and Supplemental Material published in EHP articles may not conform to 508 standards due to the complexity of the information being presented. If you need assistance accessing journal content, please contact ehponline@niehs.nih.gov. Our staff will work with you to assess and meet your accessibility needs within 3 working days.
References
- ACOG (American College of Obstetricians and Gynecologists). 2012. Committee opinion No. 533: lead screening during pregnancy and lactation. Obstet Gynecol 120(2 Pt 1):416–420, PMID: 22825110, 10.1097/AOG.0b013e31826804e8. [DOI] [PubMed] [Google Scholar]
- Advisory Committee on Childhood Lead Poisoning Prevention. 2012. Low Level Lead Exposure Harms Children: A Renewed Call for Primary Prevention. Atlanta, GA: Centers for Disease Control and Prevention. [Google Scholar]
- Alarcon WA. 2016. Elevated blood lead levels among employed adults - United States, 1994–2013. MMWR Morb Mortal Wkly Rep 63(55):59–65, PMID: 27736830, 10.15585/mmwr.mm6355a5. [DOI] [PubMed] [Google Scholar]
- Bede-Ojimadu O, Amadi CN, Orisakwe OE. 2018. Blood lead levels in women of child-bearing age in Sub-Saharan Africa: a systematic review. Front Public Health 6:367, PMID: 30619808, 10.3389/fpubh.2018.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DC. 2005. Teratogen update: lead and pregnancy. Birth Defects Res Part A Clin Mol Teratol 73(6):409–420, PMID: 15880700, 10.1002/bdra.20127. [DOI] [PubMed] [Google Scholar]
- Brody DJ, Pirkle JL, Kramer RA, Flegal KM, Matte TD, Gunter EW, et al. . 1994. Blood lead levels in the US population. Phase 1 of the third National Health and Nutrition Examination Survey (NHANES III, 1988 to 1991). JAMA 272(4):277–283, PMID: 8028140, 10.1001/jama.272.4.277. [DOI] [PubMed] [Google Scholar]
- Brown MJ, Margolis S. 2012. Lead in drinking water and human blood lead levels in the United States. MMWR Suppl 61(4):1–9, PMID: 22874873, https://www.cdc.gov/mmwr/preview/mmwrhtml/su6104a1.htm?s_cid=su6104a1_w. [PubMed] [Google Scholar]
- Cantor AG, Hendrickson R, Blazina I, Griffin J, Grusing S, McDonagh MS. 2019. Screening for elevated blood lead levels in childhood and pregnancy: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 321(15):1510–1526, PMID: 30990555, 10.1001/jama.2019.1004. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 1985. Laboratory Procedures Used by the Clinical Chemistry Division, Centers for Disease Control, for the Second Health and Nutrition Examination Survey (NHANES II) 1976–1980. Atlanta, GA: Centers for Disease Control and Prevention. [Google Scholar]
- CDC. 2007. Lead exposure among females of childbearing age–United States, 2004. MMWR Morb Mortal Wkly Rep 56(16):397–400, PMID: 17464282, https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5616a4.htm. [PubMed] [Google Scholar]
- CDC. 2009. National Health and Nutrition Examination Survey (NHANES) Laboratory Procedures Manual, July 2009. Atlanta, GA: Centers for Disease Control and Prevention. [Google Scholar]
- CDC. 2010. Guidelines for the Identification and Management of Lead Exposure in Pregnant And Lactating Women. Atlanta, GA: Centers for Disease Control and Prevention. [Google Scholar]
- CDC. 2018a. National Health and Nutrition Examination Survey Analytic Guidelines, 2011–2014 and 2015–2016. Atlanta, GA: Centers for Disease Control and Prevention. [Google Scholar]
- CDC. 2018b. Reference blood lead levels (BLLS) for adults in the US. https://www.cdc.gov/niosh/topics/ables/ReferenceBloodLevelsforAdults.html [accessed 3 May 2019].
- CDC. 2018c. Blood lead levels (μg/dl) among U.S. children < 72 months of age, by state, year, and blood lead level (BLL) group. https://www.cdc.gov/nceh/lead/data/CBLS-National-Table-508.pdf [accessed 7 November 2018].
- CDC. 2019. National Health and Nutrition Examination Survey. https://www.cdc.gov/nchs/nhanes/about_nhanes.htm [accessed 8 November 2018].
- Cheng L, Zhang B, Huo W, Cao Z, Liu W, Liao J, et al. . 2017. Fetal exposure to lead during pregnancy and the risk of preterm and early-term deliveries. Int J Hyg Environ Health 220(6):984–989, PMID: 28619549, 10.1016/j.ijheh.2017.05.006. [DOI] [PubMed] [Google Scholar]
- Crocetti AF, Mushak P, Schwartz J. 1990. Determination of numbers of lead-exposed women of childbearing age and pregnant women: an integrated summary of a report to the U.S. Congress on childhood lead poisoning. Environ Health Perspect 89:121–124, PMID: 2088737, 10.1289/ehp.9089121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry SJ, Krist AH, Owens DK, Barry MJ, Cabana M, et al. . 2019. Screening for elevated blood lead levels in children and pregnant women: US Preventive Services Task Force recommendation statement. JAMA 321(15):1502–1509, PMID: 30990556, 10.1001/jama.2019.3326. [DOI] [PubMed] [Google Scholar]
- Dietz PM, Homa D, England LJ, Burley K, Tong VT, Dube SR, et al. . 2011. Estimates of nondisclosure of cigarette smoking among pregnant and nonpregnant women of reproductive age in the United States. Am J Epidemiol 173(3):355–359, PMID: 21178103, 10.1093/aje/kwq381. [DOI] [PubMed] [Google Scholar]
- Dignam T, Kaufmann RB, LeStourgeon L, Brown MJ. 2019. Control of lead sources in the United States, 1970–2017: public health progress and current challenges to eliminating lead exposure. J Public Health Manag Pract 25(suppl 1):S13–S22, PMID: 30507765, 10.1097/PHH.0000000000000889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger AS, Roy A, Amarasiriwardena CJ, Smith D, Lupoli N, Mercado-Garcia A, et al. . 2014. Maternal blood, plasma, and breast milk lead: lactational transfer and contribution to infant exposure. Environ Health Perspect 122(1):87–92, PMID: 24184948, 10.1289/ehp.1307187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth JE, Saiful Islam M, Parvez SM, Raqib R, Sajjadur Rahman M, Marie Muehe E, et al. . 2018. Prevalence of elevated blood lead levels among pregnant women and sources of lead exposure in rural Bangladesh: a case control study. Environ Res 166:1–9, PMID: 29804028, 10.1016/j.envres.2018.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García Vargas GG, Rubio Andrade M, Del Razo LM, Borja Aburto V, Vera Aguilar E, Cebrián ME. 2001. Lead exposure in children living in a smelter community in Region Lagunera, Mexico. J Toxicol Environ Health Part A 62(6):417–429, PMID: 11289316, 10.1080/00984100150501150. [DOI] [PubMed] [Google Scholar]
- Gomaa A, Hu H, Bellinger D, Schwartz J, Tsaih SW, Gonzalez-Cossio T, et al. . 2002. Maternal bone lead as an independent risk factor for fetal neurotoxicity: a prospective study. Pediatrics 110(1 Pt 1):110–118, PMID: 12093955, 10.1542/peds.110.1.110. [DOI] [PubMed] [Google Scholar]
- González-Cossío T, Peterson KE, Sanin LH, Fishbein E, Palazuelos E, Aro A, et al. . 1997. Decrease in birth weight in relation to maternal bone-lead burden. Pediatrics 100(5):856–862, PMID: 9346987, 10.1542/peds.100.5.856. [DOI] [PubMed] [Google Scholar]
- Goyer RA. 1990. Transplacental transport of lead. Environ Health Perspect 89:101–105, PMID: 2088735, 10.1289/ehp.9089101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graber N, Gabinskaya T, Forman J, Gertner M. 2006. Prenatal lead exposure in New York City immigrant communities [poster]. In: Pediatric Academic Societies (PAS) 2006 Annual Meeting, 29 April–02 May 2006, San Francisco. [Google Scholar]
- Greene T, Ernhart CB. 1991. Prenatal and preschool age lead exposure: relationship with size. Neurotoxicol Teratol 13(4):417–427, PMID: 1921921, 10.1016/0892-0362(91)90091-a. [DOI] [PubMed] [Google Scholar]
- Gulson B, Mizon K, Korsch M, Taylor A. 2016a. Revisiting mobilisation of skeletal lead during pregnancy based on monthly sampling and cord/maternal blood lead relationships confirm placental transfer of lead. Arch Toxicol 90(4):805–816, PMID: 25877328, 10.1007/s00204-015-1515-8. [DOI] [PubMed] [Google Scholar]
- Gulson B, Taylor A, Eisman J. 2016b. Bone remodeling during pregnancy and post-partum assessed by metal lead levels and isotopic concentrations. Bone 89:40–51, PMID: 27233973, 10.1016/j.bone.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Gulson BL, Mizon KJ, Korsch MJ, Palmer JM, Donnelly JB. 2003. Mobilization of lead from human bone tissue during pregnancy and lactation–a summary of long-term research. Sci Total Environ 303(1–2):79–104, PMID: 12568766, 10.1016/s0048-9697(02)00355-8. [DOI] [PubMed] [Google Scholar]
- Gunter EW, Lewis BG, Koncikowski SM. 1996. Laboratory Procedures Used for the Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994. Atlanta, GA: Centers for Disease Control and Prevention. [Google Scholar]
- Hertz-Picciotto I. 2000. The evidence that lead increases the risk for spontaneous abortion. Am J Ind Med 38(3):300–309, PMID: 10940968, . [DOI] [PubMed] [Google Scholar]
- Hu H, Hernandez-Avila M. 2002. Invited commentary: lead, bones, women, and pregnancy–the poison within? Am J Epidemiol 156(12):1088–1091, PMID: 12480652, 10.1093/aje/kwf164. [DOI] [PubMed] [Google Scholar]
- Hu H, Téllez-Rojo MM, Bellinger D, Smith D, Ettinger AS, Lamadrid-Figueroa H, et al. . 2006. Fetal lead exposure at each stage of pregnancy as a predictor of infant mental development. Environ Health Perspect 114(11):1730–1735, PMID: 17107860, 10.1289/ehp.9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingelfinger JR, Schnaper HW. 2005. Renal endowment: developmental origins of adult disease. J Am Soc Nephrol 16(9):2533–2536, PMID: 16049065, 10.1681/ASN.2005060622. [DOI] [PubMed] [Google Scholar]
- Jin J. 2019. Screening for high blood lead levels in children and pregnant women. JAMA 321(15):1542, PMID: 30990551, 10.1001/jama.2019.2944. [DOI] [PubMed] [Google Scholar]
- Jones RL, Homa DM, Meyer PA, Brody DJ, Caldwell KL, Pirkle JL, et al. . 2009. Trends in blood lead levels and blood lead testing among us children aged 1 to 5 years, 1988–2004. Pediatrics 123(3):e376–385, PMID: 19254973, 10.1542/peds.2007-3608. [DOI] [PubMed] [Google Scholar]
- Karumanchi SA, Maynard SE, Stillman IE, Epstein FH, Sukhatme VP. 2005. Preeclampsia: a renal perspective. Kidney Int 67(6):2101–2113, PMID: 15882253, 10.1111/j.1523-1755.2005.00316.x. [DOI] [PubMed] [Google Scholar]
- Klitzman S, Sharma A, Nicaj L, Vitkevich R, Leighton J. 2002. Lead poisoning among pregnant women in New York city: risk factors and screening practices. J Urban Health 79(2):225–237, PMID: 12023498, 10.1093/jurban/79.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosnett MJ, Wedeen RP, Rothenberg SJ, Hipkins KL, Materna BL, Schwartz BS, et al. . 2007. Recommendations for medical management of adult lead exposure. Environ Health Perspect 115(3):463–471, PMID: 17431500, 10.1289/ehp.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan PJ, Boffetta P, Apostoli P. 2000. The reproductive toxicity and carcinogenicity of lead: a critical review. Am J Ind Med 38(3):231–243, PMID: 10940961, . [DOI] [PubMed] [Google Scholar]
- Lee MG, Chun OK, Song WO. 2005. Determinants of the blood lead level of US women of reproductive age. J Am Coll Nutr 24(1):1–9, PMID: 15670978, 10.1080/07315724.2005.10719436. [DOI] [PubMed] [Google Scholar]
- NTP (National Toxicology Program). 2012. NTP monograph on health effects of low-level lead. NTP Monogr (1):148, PMID: 23964424. [PubMed] [Google Scholar]
- OSHA (Occupational Safety and Health Administration). 2012. Standard Number 1910.1025—Lead Washington, DC: U.S. Department of Labor; https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.1025. [Google Scholar]
- Paschal DC, Caldwell KL, Ting BG. 1995. Determination of lead in whole blood using inductively coupled argon plasma mass spectrometry with isotope dilution. J Anal At Spectrom 10(5):367–370, 10.1039/ja9951000367. [DOI] [Google Scholar]
- Pezzi C, Lee D, Kennedy L, Aguirre J, Titus M, Ford R, et al. . 2019. Blood lead levels among resettled refugee children in select US states, 2010–2014. Pediatrics 143(5):e20182591, PMID: 30996119, 10.1542/peds.2018-2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfadenhauer LM, Burns J, Rohwer A, Rehfuess EA. 2016. Effectiveness of interventions to reduce exposure to lead through consumer products and drinking water: a systematic review. Environ Res 147:525–536, PMID: 26990846, 10.1016/j.envres.2016.03.004. [DOI] [PubMed] [Google Scholar]
- Pirkle JL, Brody DJ, Gunter EW, Kramer RA, Paschal DC, Flegal KM, et al. . 1994. The decline in blood lead levels in the United States. The National Health and Nutrition Examination Surveys (NHANES). JAMA 272(4):284–291, PMID: 8028141, 10.1001/jama.1994.03520040046039. [DOI] [PubMed] [Google Scholar]
- Pirkle JL, Kaufmann RB, Brody DJ, Hickman T, Gunter EW, Paschal DC. 1998. Exposure of the U.S. population to lead, 1991–1994. Environ Health Perspect 106(11):745–750, PMID: 9799191, 10.1289/ehp.98106745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prihartono NA, Djuwita R, Mahmud PB, Haryanto B, Helda H, Wahyono TYM, et al. . 2019. Prevalence of blood lead among children living in battery recycling communities in Greater Jakarta, Indonesia. Int J Environ Res Public Health 16(7):E1276, PMID: 30974753, 10.3390/ijerph16071276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond J, Wheeler W, Brown MJ. 2014. Lead screening and prevalence of blood lead levels in children aged 1-2 years–child blood lead surveillance system, United States, 2002–2010 and National Health and Nutrition Examination Survey, United States, 1999–2010. MMWR Suppl 63(2):36–42, PMID: 25208256. [PubMed] [Google Scholar]
- Richter PA, Bishop EE, Wang J, Kaufmann R. 2013. Trends in tobacco smoke exposure and blood lead levels among youths and adults in the United States: the National Health and Nutrition Examination Survey, 1999–2008. Prev Chronic Dis 10:E213, PMID: 24355106, 10.5888/pcd10.130056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg SJ, Kondrashov V, Manalo M, Jiang J, Cuellar R, Garcia M, et al. . 2002. Increases in hypertension and blood pressure during pregnancy with increased bone lead levels. Am J Epidemiol 156(12):1079–1087, PMID: 12480651, 10.1093/aje/kwf163. [DOI] [PubMed] [Google Scholar]
- Rothenberg SJ, Manalo M, Jiang J, Cuellar R, Reyes S, Sanchez M, et al. . 1999. Blood lead level and blood pressure during pregnancy in South Central Los Angeles. Arch Environ Health 54(6):382–389, PMID: 10634227, 10.1080/00039899909603369. [DOI] [PubMed] [Google Scholar]
- Sanin LH, González-Cossío T, Romieu I, Peterson KE, Ruíz S, Palazuelos E, et al. . 2001. Effect of maternal lead burden on infant weight and weight gain at one month of age among breastfed infants. Pediatrics 107(5):1016–1023, PMID: 11331680, 10.1542/peds.107.5.1016. [DOI] [PubMed] [Google Scholar]
- Schnaas L, Rothenberg SJ, Flores MF, Martinez S, Hernandez C, Osorio E, et al. . 2006. Reduced intellectual development in children with prenatal lead exposure. Environ Health Perspect 114(5):791–797, PMID: 16675439, 10.1289/ehp.8552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbergeld EK, Patrick TE. 2005. Environmental exposures, toxicologic mechanisms, and adverse pregnancy outcomes. Am J Obstet Gynecol 192(suppl 5):S11–S21, PMID: 15891707, 10.1016/j.ajog.2004.06.117. [DOI] [PubMed] [Google Scholar]
- Spanier AJ, McLaine P, Gilden RC. 2019. Screening for elevated blood lead levels in children and pregnant women. JAMA 321(15):1464–1465, PMID: 30990534, 10.1001/jama.2019.2594. [DOI] [PubMed] [Google Scholar]
- Thihalolipavan S, Candalla BM, Ehrlich J. 2013. Examining pica in NYC pregnant women with elevated blood lead levels. Matern Child Health J 17(1):49–55, PMID: 22302239, 10.1007/s10995-012-0947-5. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau. 2017. Annual estimates of the resident population for selected age groups by sex for the United States: April 1, 2010 to July 1, 2016. https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?src=bkmk [accessed 24 February 2019].
- Wasserman GA, Liu X, Popovac D, Factor-Litvak P, Kline J, Waternaux C, et al. . 2000. The Yugoslavia Prospective Lead Study: contributions of prenatal and postnatal lead exposure to early intelligence. Neurotoxicol Teratol 22(6):811–818, PMID: 11120386, 10.1016/s0892-0362(00)00106-9. [DOI] [PubMed] [Google Scholar]