Abstract
Upper extremity vein thrombosis (UE-VT) are more and more frequent pathologies and yet little studied. The aim is to describe the clinical and ultrasound features, UE-VT-related diseases, and the prevalence of pulmonary embolism (PE) and associated deaths.
All UE-VT patients diagnosed by Doppler-ultrasound in Nantes University Hospital, from January 2015 to December 2017, were included retrospectively. UE-VT suspicion patterns, clinical features, UE-VT topography, and prevalence of PE and death were analyzed.
Seven hundred and fifty-five UE-VT were analyzed, including 427 deep thrombosis (UE-DVT) and 328 superficial thrombosis (UE-SVT). In 86.2% (n = 651) UE-VT were related to endovascular devices. Among these thrombosis, one third is in connection with a PICC LINE and one quarter with a peripheral venous line. Forty nine percent (n = 370) of the patients had solid neoplasia or hematological malignancies. An inflammatory or systemic infectious context was found in 40.8% (n = 308) of the cases. The most frequently observed clinical sign at the UE-VT diagnosis was edema (28.6%). Among the UE-SVT it was the presence of an indurated cord (33.2%) and among the UE-DVT the indication of the Doppler-ultrasound was mainly a suspicion of infection on endovascular device (35.1%). In 10.6% (n = 80) of the cases the UE-VT were asymptomatic. The most frequently thrombosed veins were brachial basilic veins (16.7% of all thrombosed segments) followed by jugular (13%) and subclavian (12.3%) veins; 61.3% (n = 463) of UE-VT were in the right upper extremity; 63.3% (n = 478) UE-VT were occlusive. The occurrence of PE is 4% and the death rate is 10.2%, mainly related to the severe comorbidities of patients with UE-VT.
UE-VT occurs in particular clinical contexts (hematological malignancies, solid cancers, systemic infections) and in the majority of endovascular devices (86.2%). The occurrence of PE is low.
Keywords: catheter, Doppler-ultrasound, peripherally inserted central catheter – line, pulmonary embolism, upper extremity venous thrombosis
1. Introduction
Venous thromboembolism (VTE) is a major public health problem because of its prevalence and severity. It is one of the leading causes of morbidity and mortality in hospitalized patients.[1]
Venous thrombosis of the upper extremity (UE-VT) is an increasingly common pathology; in the 2000s, it accounted for 1% to 4% of all cases of venous thrombosis,[2] today it represents about 10%.[3] This is mainly explained by the increasing use of central venous catheters (CVC) and in particular the use of PICC LINE (Peripherally Inserted Central Catheter – Line).
The major UE-VT-related diseases and conditions described in the literature are: venous catheter[4] and in particular a PICC LINE[5]; a solid neoplasia[6] or a hematological malignancy[7]; a thoracic outlet syndrome (TOS) or effort thrombosis (Paget-Schroetter syndrome)[8]; an estrogenic hormonal impregnation (pregnancy, contraceptive pill[9]), a medically assisted procreation protocol[10] with or without ovarian hyperstimulation[11]; hereditary or acquired biological thrombophilia (antiphospholipid syndrome (APLS), deficiency of anticoagulant factors (protein C, protein S, antithrombin, factor II or factor V mutations))[12]; severe kidney failure[13]; or other situations such as flares of inflammatory diseases (hemorrhagic rectocolitis, Crohn, Behcet, Buerger).[14]
The pathophysiology, epidemiology and management of UE-VT, although much less studied than the lower extremities, have long been considered similar to lower extremity vein thrombosis (LE-VT), yet it is a particular form of VTE: diagnostic elements, clinical features, risk factors and evolutive risks seem different between these two types of thrombosis.[15] UE-VT characteristics are poorly known, indeed few recent studies have been done on UE-VT and they only concerned deep thrombosis.[16–18] The purpose of this study was to describe the UE-VT presentations in a large cohort and to compare the deep and superficial UE-VT features.
2. Methods
We conducted a descriptive, retrospective, monocentric study. Patients were identified by performing at least one Doppler ultrasound of the upper extremity at Nantes University Hospital Center during the period from January 1, 2015 to December 31, 2017. The included patients had a UE-VT defined on the Doppler ultrasound by a hypo or isoechoic image, without Doppler flow, associated with incompressibility of the vein. UE-VT linked to the central venous catheter was defined by a thrombus facing the catheter pathway with a wall adherent thrombus whose major axis was > 5 mm.
The topography of UE-VT is described according to the most proximal thrombosed venous segment. This description distinguishes upper extremity deep vein thrombosis (UE-DVT) from upper extremity superficial vein thrombosis (UE-SVT). The innominate vein, the internal jugular vein, the subclavian vein and the axillary vein belong to the deep proximal venous network. The humeral (or brachial) vein, ulnar veins and radial veins belong to the deep distal network. Brachial and antebrachial cephalic veins, brachial and antebrachial basilic veins, dorsal veins of the hand and their collaterals belong to the superficial network.
The included patients had an acute UE-VT episode diagnosed by Doppler ultrasound. If the patient had multiple episodes of UE-VT distinct in time, each episode was included.
Pulmonary embolism (PE) diagnosed by thoracic angioscan or ventilated perfusion scan in the 14 days prior to UE-VT diagnosis or within 90 days of diagnosis, and all deaths within 90 days of diagnosis were analyzed.
Based on the patient's computerized care record, a standardized collection chart made it possible to evaluate the demographic characteristics of the patients, the presence or absence of factors favoring thrombosis, the indication of the Doppler ultrasound and the clinical signs at diagnosis, the characteristics of thrombosis, as well as the occurrence of PE and / or death. Patients for whom the medical record was incomplete were excluded. Isolated superior vena cava thrombosis were excluded. Episodes of recurrence or extension of thrombosis already diagnosed before January 1, 2015 were also excluded.
The study was approved by the “Groupe Nantais d’Ethique dans le Domaine de la Santé” (GNEDS), the ethics committee of the Nantes university hospital, and complied with the requirements of the “Commission Nationale de l’Informatique et des Libertés”, in accordance with current French legislation.
Continuous data are presented as the means ± standard deviation or, for non-normal distributions, as medians with minimum and maximum. Categorical data are given as count and percentage. Continuous data were compared with the use of the Student's test or Mann-Whitney test; Chi-square test or Fischer's exact test were used for comparison of categorical data. All P values are two-sided; P < .05 indicated a statistically significant difference, no correction for multiple comparisons. All statistical analyses were performed using Prism6 (San Diego).
3. Results
Seven hundred and fifty-five UE-VT were included (Fig. 1) among which 427 (56.6%) UE-DVT and 328 (43.4%) UE-SVT.
Figure 1.
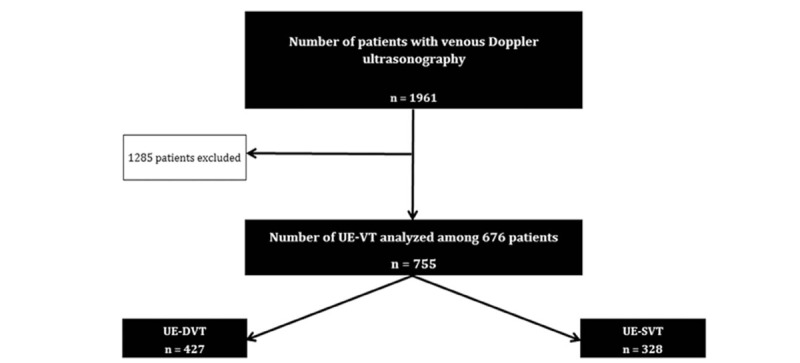
Flow chart of patient selection included with UE-VT, (UE-VT: Upper extremity vein thrombosis. UE-DVT: Upper extremity deep vein thrombosis, UE-SVT: Upper extremity superficial vein thrombosis).
Patients were included in a university hospital, consisting of an emergency department (376,908 admissions during the period from January 1, 2015 to December 31, 2017), 1,021 hospitalization beds, 568 surgical beds (138,990 surgeries over the studied period) and 155 intensive care unit beds. Also during this period, 1824 PICC LINE were inserted, 45 MID LINE, 1172 PM and 291 ICD; 8114 coronarography were performed and 1830 patients were dialyzed. There were also 12501 births. During the analyzed period, 1988 venous Doppler of the upper limb were performed, 62 patients were excluded due to insufficient information in the medical file and 755 UE-VT were diagnosed (38% positive tests).
The median age of UE-VT patients was 59 years [12 days-95 years]; 472 were men (61.2%), respectively 61.8% (n = 264) and 60.4% men (n = 198) for UE-DVT and UE-SVT.
Six hundred and seventy-six patients (34.5%) had at least one UE-VT: 90.4% (n = 611) had a single UE-VT; 8.3% (n = 56) had 2; 0.9% (n = 6) had 3; 0.1% (n = 1) had 4 and 0.3% (n = 2) had 5.
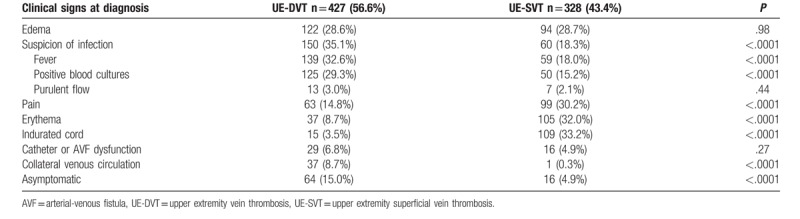
The clinical signs found at the time of diagnosis are described in Table 1. Their presence was very different depending on whether it was a UE-DVT or a UE-SVT except for catheter dysfunction, purulent flow and edema, which were present in the same proportions for UE-DVT and UE-SVT. A superior vena cava syndrome was found in 3.5% (n = 15) cases of UE-DVT.
Table 1.
Clinical signs at the diagnosis of deep and superficial venous thrombosis.
Eighty UE-VT (10.6%) were asymptomatic. In these cases, the reason for performing the Doppler ultrasound was: systematic identification before the placement of a venous device in patients with history of central catheter placement (42.5%, n = 34); accidental discovery of a suspicious image of UE-VT on CT-scan (37.5%, n = 30); after failure of catheter placement (15.0%, n = 12); in the investigation of PE (3.8%, n = 3) and thrombosis testing for heparin-induced thrombocytopenia (HIT) (1.3%, n = 1).
At diagnosis of UE-VT, hematologic or progressive solid cancer was noted in 49% of cases (n = 370). Among patients with UE-VT, 33.1% (n = 250) had hematological malignancy and 15.9% (n = 120) had solid cancer. The different types of neoplasia and the differences between UE-DVT and UE-SVT are presented in Table 2: acute leukemia was present in 14.4% of UE-VT cases (n = 109), lymphoma in 10.5% of UE-VT cases (n = 79), colonic or grelic neoplasia in 3.4% of UE-VT cases (n = 26) and pulmonary neoplasia in 2.9% of UE-VT cases (n = 22).
Table 2.
Diseases and specificities associated with upper extremity deep and superficial vein thrombosis.
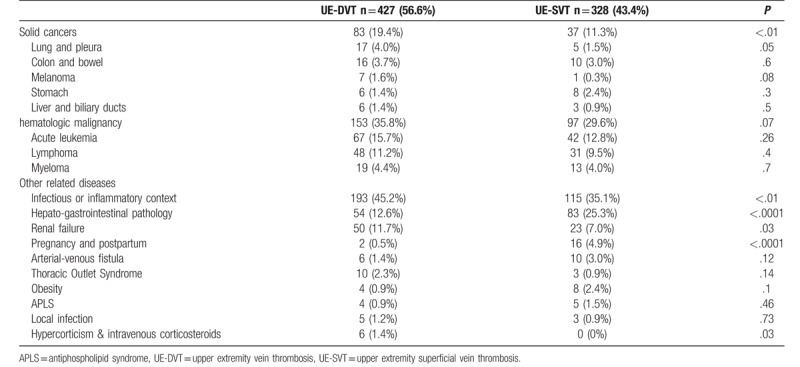
UE-VT were associated with specific clinical settings which are presented in Table 2. A systemic inflammatory or infectious context was found in 40.8% of UE-VT cases (n = 308); kidney failure in 9.7% (n = 73); arteriovenous fistula thrombosis (AVF) in 2.1% (n = 16); thoracic outlet syndrome (TOS) in 1.7% (n = 13); obesity in 1.6% (n = 12); an APLS in 1.2% (n = 9) and a local infection not related to endovenous device in 1.1% (n = 8). Shoulder surgery was found in one case of UE-DVT (0.1% of UE-VT) and two cases of arteriovenous malformation of the upper extremity resulted in 2 UE-SVT (0.3% UE-VT). Concerning inherited bleeding disorders, only one UE-DVT in a context of antithrombin deficiency as well as a UE-DVT and a UE-SVT linked to a heterozygous factor V Leiden mutation have been identified. No UE-VT occurring in the context of estrogen treatment (pill, medically-assisted procreation, menopausal hormone replacement therapy) has been identified; 2 UE-DVT and 16 UE-SVT were reported during periods of pregnancy or postpartum.
Six hundred and fifty-one thrombosis (86.2%) were associated with the presence of endovenous device including 29.1% (n = 220) of PICC LINE; 27.4% (n = 207) of peripheral venous catheters (PVC); 8.7% (n = 66) of implantable ports; 4.8% (n = 36) of dialysis catheters; 2.4% (n = 18) of MID LINE. The 75 thrombosis on CVC were exclusively UE-DVT and the same finding was made for Pace-Maker (PM) or implantable cardioverter defibrillator (ICD) thrombosis. New UE-VT (1.4%) were associated with other endovenous devices: extracorporeal membrane oxygenation (ECMO) (n = 3), venous stent (n = 1), arterial catheter implanted in radial vein (n = 1), and intravenous drug abuse context (n = 4).
One hundred and four thrombosis were not catheter-related (13.8%). The diseases and specificities associated with these thrombosis were: infectious or inflammatory context (n = 26; 25%), solid neoplasia (n = 25; 24%), haematological malignancy (n = 17; 16.3%), hepatogastro-intestinal tract disease (n = 17, 16.3%), renal failure (n = 17, 16.3%), TOS (n = 11, 10.6%), an infection on contact with thrombosis (n = 5, 4.8%) and APLS (n = 4, 3.8%).
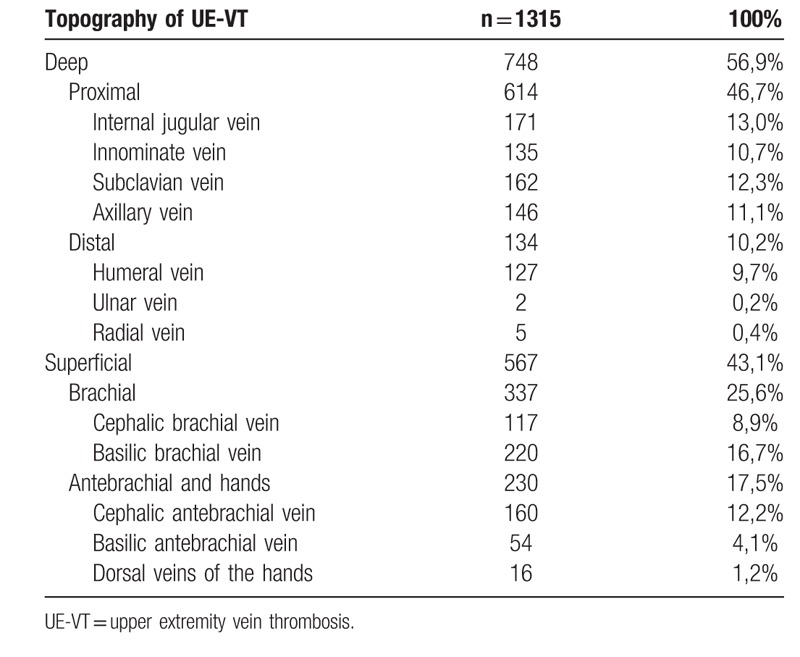
The topographic distribution of UE-VT is described in the Table 3. The median number of thrombosed venous segments was 1. The maximum number of thrombosed segments was 9. In more than one out of two cases (n = 429) the thrombus length at diagnosis was not described; the median length of the UE-VT was 5 cm, the maximum length 49 cm and the minimum length 0.5 cm. UE-DVT were shorter than UE-SVT, with a median length of 2.7 cm versus 8 cm for UE-SVT (P < .0001).
Table 3.
Topographic distribution of upper extremity vein thrombosis.
In 28% (n = 211) of the cases the UE-VT were partially occlusive, in 63% (n = 478) the UE-VT were occlusive and in 9% of the cases (n = 66) the occlusive character was not indicated.
Four hundred and sixty-three UE-VT (61.3%) were located in the right upper extremity, 261 in the upper left (34.6%) and 31 were bilateral (4.1%).
Thirty PE occurred within 15 days before or 3 months after UE-VT diagnosis with a prevalence of 4%; 23 PE were found after UE-VT diagnosis (median time of 2 days [0 days - 77 days] after UE-VT) and 7 PE before UE-VT diagnosis (median time of 7 days [13 days - 1 day] before UE-VT). Twenty PE were symptomatic (dyspnea of varying intensity, malaise, chest pain), 8 PE were asymptomatic (finding on a systematic chest CT scan) and in 2 cases it was not specified whether they were symptomatic or not. Patients with PE had significantly more UE-DVT than UE-SVT (90% and 10%, respectively) compared to patients without PE (55.2% and 44.8%, respectively) (P < .001).
Seventy-seven deaths occurred within 3 months of UE-VT diagnosis, which is 10.2% of all UE-VT in this study. The median time to death was 33 days after UE-VT [3 days - 90 days]. Five deaths (6.4%) were directly related to the occurrence of UE-VT: 2 patients died from a PE, 2 patients from gastrointestinal bleeding under curative anticoagulation and 1 patient from cerebral hemorrhage also under curative anticoagulation.
4. Discussion
This study describes, to our knowledge, the largest cohort of UE-VT with 755 cases including 427 deep and 328 superficial. For the first time the characteristics of UE-SVT are described.
4.1. Etiologies of UE-VT
UE-DVT or UE-SVT are associated with the presence of endovenous material in more than 85% of cases. The most commonly found devices are PICC LINE, followed by PVC, CVC and implantable ports. These proportions seem to be related to the number of patients carrying each of these devices in our hospital.
CVC and more specifically PICC LINE would be a major risk factor for UE-VT. Winter et al showed that the use of a central venous catheter increased the risk of UE-VT by 14-fold, but without increasing the risk of PE.[4] According to old data, PM appeared as the most thrombogenic devices (23% risk of developing thrombosis after exposure)[19] while the risk of developing UE-VT after laying a PICC LINE would be between 2.5 and 9%.[20,21]
Venous thrombosis occurs in specific clinical settings with the presence of solid cancer or hematologic disease in almost every other case, comparable to previous studies which found between 35 and 67% of UE-VT related to active neoplasia.[16,18,22]
The majority of UE-VT found in our study is of secondary origin. In addition to the presence of a catheter or active neoplasia, the most frequently found contributing factor was a systemic inflammatory or infectious context. This situation was found in more than 40% of UE-VT cases.
Little described in the literature, hepatogastro-intestinal diseases such as Crohn disease, hepatic cirrhosis, peptic ulcer, esophagitis and pancreatitis were in our study frequently found as associated with UE-VT. It is not possible to definitively determine whether this high rate was related to the pathologies themselves or to their treatments.
In rare cases, thrombosis were related to intravenous corticosteroid therapy or hypercorticism, 0.8% in this study: this can be explained by the plasma increase of coagulation factors and fibrinogen during exposure to high levels of endogenous or exogenous corticosteroids.[23]
More rarely, in the absence of a major thrombotic risk factor, UE-VT may reveal Paget-Schroetter syndrome (effort thrombosis) or TOS. These are axillary or subclavian venous thrombosis that occur during repetitive efforts of the upper extremities or during an effort in generally male subjects under age 30, with an incidence of 1/100 000 patient- year.[8,24] TOS with cervical rib, congenital fibrous band, scalene hypertrophy or abnormal insertion of the costoclavicular ligament associated with repetitive trauma of the subclavian vein endothelium are key factors in initiating thrombosis and its progression. Intense muscular arm activity is reported in approximately 25% of patients and is associated with twice the risk of primary UE-VT.[25–28] According to Lechner et al, 7% of UE-VT are related to TOS[29] compared to only 1.7% in our study.
4.2. Difference between UE-SVT and UE-DVT
Implantation of endovenous device in the superficial veins is progressing with the use of PICC LINE and MID LINE. There is very little research on UE-SVT. Yet, it occurs in particular contexts of hospitalization with peripheral or central venous route placement. They have specific clinical characteristics with more frequent presence of pains, indurated cords and local erythema; unlike UE-DVT which are more often asymptomatic or revealed by collateral circulation, superior vena cava syndrome or suspected catheter infection.[30] The presence of an upper vena cava syndrome, a collateral venous circulation, a central catheter placement failure have positive predictive values for a UE-DVT between 66.7 and 100%, whereas for the UE-SVT, the positive predictive value goes from 60.8 to 85.7% for the presence respectively of an erythema or an indurated cord.[30]
UE-VT treatments are poorly codified, as are the evolution or predictors of post-thrombotic syndrome in the upper extremity. Unlike the UE-DVT where the objectives of the anticoagulant treatment are mainly the reduction of the occurrence of pulmonary embolism and post thrombotic syndrome, the main challenge for the UE-SVT is the preservation of the venous capital. The use of peripheral venous catheters is trivialized, yet more than a quarter of UE-VT are related to PVC and the impact of these UE-SVT is underestimated in particular for patients with chronic diseases requiring intravenous treatment and for whom the residual occlusion of these veins alters the quality of life and delays the administration of treatments. To specify their evolution, prospective studies are necessary.
4.3. Difference between UE-VT and LE-VT
The diseases and contexts associated with the occurrence of UE-VT and LE-VT are different: the discovery of an occult cancer in the 12 months following the UE-VT diagnosis is 23% compared to only 11% after the diagnosis of LE-VT[31] and biological thrombophilia is found in 55.3% of LE-VT cases compared with only 34.2% of UE-VT cases.[12] For lower extremity superficial venous thrombosis (LE-SVT), the most common cases are obesity and varicose veins.[32] In the upper extremity the occurrence of venous thrombosis is mainly related to the presence of a catheter and a neoplasia.[16,18,22]
In our study, 4% of UE-VT were complicated by a PE. This rate is comparable to two American studies of 300 and 546 patients presenting only UE-DVT, respectively 2 and 5%.[33,34] In comparison with lower extremity deep venous thrombosis (LE-DVT), UE-DVT are less complicated with pulmonary embolism (5.4% versus 27.9%); however UE-VT mortality rate is higher than that of LE-DVT (7.6% versus 4.2%).[35] In our study, a minority of patients (6.4%) died of a cause directly related to the occurrence of UE-VT. The remaining deaths were overwhelmingly (93.6%) related to the progression of an underlying disease or multiple comorbidities. This result is consistent with studies showing that the occurrence of UE-VT is not associated with a major risk of death, but that it is the co-morbidities associated with the occurrence of UE-VT that explain the significant mortality rate in the aftermath of UE-VT diagnosis.[36–38]
4.4. Limitations
The limits of this work come from its lack of completeness and its retrospective nature. The frequency of asymptomatic venous thrombosis is probably underestimated since the patients were identified from the realization of a doppler ultrasound which was in the vast majority of cases performed due to symptoms especially in the EU-VT. In addition, a search for PE was not performed systematically.
5. Conclusion
UE-VT occur most often in specific clinical settings: hematological malignancy, solid neoplasia or progressive infection. For UE-DVT as for UE-SVT, endovenous device is present most of the time (in 85.5 and 87.2% of cases respectively). Edema is the most commonly found clinical sign but does not point to a deep or superficial localization. Other clinical manifestations such as fever, pain, erythema or an indurated cord are frequently found. However a significant proportion of UE-VT is asymptomatic, in particular the UE-DVT.
UE-DVT are rarely complicated by PE (4%) and even more rarely in UE-SVT (0.9%). UE-VT high mortality rate is related to the evolution of the underlying pathology and not to the evolution of VTE.
The UE-VT interest in about half of the cases the superficial network. The most frequently affected segments are the jugular veins, the innominate veins and the cephalic and brachial basilic veins.
The management of UE-VT is poorly codified and requires specific prospective studies to define the anticoagulation strategy and duration as well as to assess the post-thrombotic syndrome.
Acknowledgments
We thank Béatrice Guyomarch, Christophe Leux and Romain Dumont for their help in data recovery and statistical development, without whom this work could not have been done.
Author contributions
Conceptualization: Gaetan Ploton, Olivier Espitia.
Data curation: Gaetan Ploton, Chan Ngohou.
Formal analysis: Gaetan Ploton, Chan Ngohou, Olivier Espitia.
Funding acquisition: Gaetan Ploton.
Investigation: Gaetan Ploton, Marc-Antoine Pistorius, Alizée Raimbeau, Julien Denis Le Seve, Guillaume Bergère, Yann Goueffic, Mathieu Artifoni, Cécile Durant, Giovanni Gautier, Jérôme Connault, Olivier Espitia.
Methodology: Gaetan Ploton, Olivier Espitia.
Project administration: Gaetan Ploton.
Resources: Gaetan Ploton.
Software: Gaetan Ploton.
Supervision: Olivier Espitia.
Validation: Gaetan Ploton.
Visualization: Gaetan Ploton.
Writing – original draft: Gaetan Ploton, Olivier Espitia.
Writing – review & editing: Olivier Espitia.
Gaetan Ploton orcid: 0000-0003-0524-2335.
Footnotes
Abbreviations: APLS = antiphospholipid syndrome, AVF = arteriovenous fistula, CT Scan = computerized tomography scan, CVC = central venous catheter, ECMO = extracorporeal membrane oxygenation, GNEDS = Groupe Nantais d’Ethique dans le Domaine de la Santé, HIT = heparin-induced thrombocytopenia, ICD = implantable cardioverter defibrillator, LE-DVT = lower extremity deep vein thrombosis, LE-SVT = lower extremity superficial vein thrombosis, LE-VT = lower extremity vein thrombosis, PE = pulmonary embolism, PICC LINE = peripherally inserted central catheter – Line, PM = pace maker, PVC = peripheral venous catheter, TOS = thoracic outlet syndrome, UE-DVT = upper extremity deep vein thrombosis, UE-SVT = upper extremity superficial vein thrombosis, UE-VT = upper extremity vein thrombosis, VTE = venous thromboembolism.
How to cite this article: Ploton G, Pistorius MA, Raimbeau A, Seve JD, Bergère G, Ngohou C, Goueffic Y, Artifoni M, Durant C, Gautier G, Connault J, Espitia O. A STROBE cohort study of 755 deep and superficial upper-extremity vein thrombosis. Medicine. 2020;99:6(e18996).
In this cohort study of 755 patients, upper extremity venous thrombosis occurs in 86.2% of cases in the presence of endovascular device. This is deep vein thrombosis in 56.6% of cases.
This study suggests that upper extremity venous thrombosis (UE-VT) occur in particular clinical contexts (hematological malignancies, solid cancers, systemic infections). The occurrence of pulmonary embolism is less important than for lower extremity vein thrombosis and the death rate is high, it is mainly related to the severe comorbidities of patients with UE-VT.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Olie V, Chin F, De Peretti C. La maladie veineuse thromboembolique: patients hospitalisés et mortalité en France en 2010. J Mal Vasc 2013;38:308. [Google Scholar]
- [2].Kommareddy A, Zaroukian MH, Hassouna HI. Upper extremity deep venous thrombosis. Semin Thromb Hemost 2002;28:89–99. [DOI] [PubMed] [Google Scholar]
- [3].Feinberg J, Nielsen EE, Jakobsen JC. Thrombolysis for acute upper extremity deep vein thrombosis. Cochrane Database Syst Rev 2017;12:CD012175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Winters JP, Callas PW, Cushman M, et al. Central venous catheters and upper extremity deep vein thrombosis in medical inpatients: the Medical Inpatients and Thrombosis (MITH) Study. J Thromb Haemost 2015;13:2155–60. [DOI] [PubMed] [Google Scholar]
- [5].Chopra V, Anand S, Hickner A, et al. Risk of venous thromboembolism associated with peripherally inserted central catheters: a systematic review and meta-analysis. Lancet 2013;382:311–25. [DOI] [PubMed] [Google Scholar]
- [6].Bleker SM, van Es N, van Gils L, et al. Clinical course of upper extremity deep vein thrombosis in patients with or without cancer: a systematic review. Thromb Res 2016;140: Suppl 1: S81–88. [DOI] [PubMed] [Google Scholar]
- [7].Helley D. Thrombosis and malignant hemopathies: truths and realities. Hematologie 2011;183–8. 5-6. [Google Scholar]
- [8].Illig KA, Doyle AJ. A comprehensive review of Paget-Schroetter syndrome. J Vasc Surg 2010;51:1538–47. [DOI] [PubMed] [Google Scholar]
- [9].Villani M, Dentali F, Colaizzo D, et al. Pregnancy-related venous thrombosis: comparison between spontaneous and ART conception in an Italian cohort. BMJ Open 2015;5:e008213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bar-On S, Cohen A, Levin I, et al. Upper extremity deep vein thrombosis following ovarian stimulation. Harefuah 2011;150:849–51. 875. [PubMed] [Google Scholar]
- [11].Salomon O, Schiby G, Heiman Z, et al. Combined jugular and subclavian vein thrombosis following assisted reproductive technology—new observation. Fertil Steril 2009;92:620–5. [DOI] [PubMed] [Google Scholar]
- [12].Linnemann B, Meister F, Schwonberg J, et al. Hereditary and acquired thrombophilia in patients with upper extremity deep-vein thrombosis. Results from the MAISTHRO registry. Thromb Haemost 2008;100:440–6. [PubMed] [Google Scholar]
- [13].Baumann Kreuziger L, Cote L, Verhamme P, et al. A RIETE registry analysis of recurrent thromboembolism and hemorrhage in patients with catheter-related thrombosis. J Vasc Surg Venous Lymphat Disord 2015;3:243–50. .e1. [DOI] [PubMed] [Google Scholar]
- [14].Somaï M, Toujani S, El Ouni A, et al. Les thromboses veineuses du membre supérieur [Internet]. Available from: https://www.em-consulte.com/es/revue/REVMED/38/S1/table-des-matieres/ [access date May 8, 2019 ] [Google Scholar]
- [15].Robert-Ebadi H, Becker F, Righini MP. Thrombose veineuse profonde du membre supérieur: une forme particulière de maladie thromboembolique veineuse - Revue Médicale Suisse [Internet]. Available from: https://www.revmed.ch/RMS/2015/RMS-N-460/Thrombose-veineuse-profonde-du-membre-superieur-une-forme-particuliere-de-maladie-thromboembolique-veineuse [access date May 8, 2019 ] [PubMed] [Google Scholar]
- [16].Delluc A, Le Mao R, Tromeur C, et al. Incidence of upper-extremity deep vein thrombosis in western France: a community-based study. Haematologica 2019;104:e29–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].ALKindi SY, Chai-Adisaksopha C, Cheah M, et al. Management of cancer-associated upper extremity deep vein thrombosis with and without venous catheters at a tertiary care center. Thromb Res 2018;166:92–5. [DOI] [PubMed] [Google Scholar]
- [18].Cote LP, Greenberg S, Caprini JA, et al. Comparisons between upper and lower extremity deep vein thrombosis: a review of the RIETE registry. Clin Appl Thromb 2017;23:748–54. [DOI] [PubMed] [Google Scholar]
- [19].van Rooden CJ, Molhoek SG, Rosendaal FR, et al. Incidence and risk factors of early venous thrombosis associated with permanent pacemaker leads. J Cardiovasc Electrophysiol 2004;15:1258–62. [DOI] [PubMed] [Google Scholar]
- [20].Saber W, Moua T, Williams EC, et al. Risk factors for catheter-related thrombosis (CRT) in cancer patients: a patient-level data (IPD) meta-analysis of clinical trials and prospective ... - PubMed - NCBI [Internet]. Available from: https://www.ncbi.nlm.nih.gov/pubmed/21040443. [access date May 8, 2019 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chemaly RF, de Parres JB, Rehm SJ, et al. Venous thrombosis associated with peripherally inserted central catheters: a retrospective analysis of the Cleveland Clinic experience. - PubMed - NCBI [Internet]. Available from: https://www.ncbi.nlm.nih.gov/pubmed/11941543. [access date May 8, 2019 ] [DOI] [PubMed] [Google Scholar]
- [22].Muñoz FJ, Mismetti P, Poggio R, et al. Clinical outcome of patients with upper-extremity deep vein thrombosis: results from the RIETE Registry. Chest 2008;133:143–8. [DOI] [PubMed] [Google Scholar]
- [23].van Zaane B, Nur E, Squizzato A, et al. Systematic review on the effect of glucocorticoid use on procoagulant, anti-coagulant and fibrinolytic factors. J Thromb Haemost JTH 2010;8:2483–93. [DOI] [PubMed] [Google Scholar]
- [24].Lindblad B, Tengborn L, Bergqvist D. Deep vein thrombosis of the axillary-subclavian veins: Epidemiologic data, effects of different types of treatment and late sequele. Eur J Vasc Surg 1988;2:161–5. [DOI] [PubMed] [Google Scholar]
- [25].Blom JW, Doggen CJM, Osanto S, et al. Old and new risk factors for upper extremity deep venous thrombosis. J Thromb Haemost 2005;3:2471–8. [DOI] [PubMed] [Google Scholar]
- [26].Martinelli I, Battaglioli T, Bucciarelli P, et al. Risk factors and recurrence rate of primary deep vein thrombosis of the upper extremities. Circulation 2004;110:566–70. [DOI] [PubMed] [Google Scholar]
- [27].Héron E, Lozinguez O, Alhenc-Gelas M, et al. Hypercoagulable states in primary upper-extremity deep vein thrombosis. Arch Intern Med 2000;160:382–6. [DOI] [PubMed] [Google Scholar]
- [28].Vaya A, Martinez Triguero M, Romagnoli M, et al. Lack of association between hemorheological alterations and upper-extremity deep vein thrombosis. Clin Hemorheol Microcirc 2009;41:279–85. [DOI] [PubMed] [Google Scholar]
- [29].Lechner D, Wiener C, Weltermann A, et al. Comparison between idiopathic deep vein thrombosis of the upper and lower extremity regarding risk factors and recurrence. J Thromb Haemost 2008;6:1269–74. [DOI] [PubMed] [Google Scholar]
- [30].Drouin L, Pistorius M-A, Lafforgue A, et al. Upper-extremity venous thrombosis: a retrospective study about 160 cases. Rev Med Interne 2019;40:9–15. [DOI] [PubMed] [Google Scholar]
- [31].Girolami A, Prandoni P, Zanon E, et al. Venous thromboses of upper limbs are more frequently associated with occult cancer as compared with those of lower limbs. Blood Coagul Fibrinolysis Int J Haemost Thromb 1999;10:455–7. [DOI] [PubMed] [Google Scholar]
- [32].Decousus H, Prandoni P, Mismetti P, et al. Fondaparinux for the treatment of superficial-vein thrombosis in the legs. N Engl J Med 2010;363:1222–32. [DOI] [PubMed] [Google Scholar]
- [33].Levy MM, Albuquerque F, Pfeifer JD. Low incidence of pulmonary embolism associated with upper-extremity deep venous thrombosis. Ann Vasc Surg 2012;26:964–72. [DOI] [PubMed] [Google Scholar]
- [34].Hingorani A, Ascher E, Markevich N, et al. Risk factors for mortality in patients with upper extremity and internal jugular deep venous thrombosis. J Vasc Surg 2005;41:476–8. [DOI] [PubMed] [Google Scholar]
- [35].Williams GW, Giri S, Siwakoti K, et al. Incidence, Risk Factors and Outcomes of Upper Extremity Deep Venous Thrombosis Among Hospitalized Patients in the United States. In American Thoracic Society; 2016 A7768-A7768. (American Thoracic Society International Conference Abstracts). Available from: https://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2016.193.1_MeetingAbstracts.A7768 [access date May 27, 2019 ] [Google Scholar]
- [36].Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet Lond Engl 1999;353:1386–9. [DOI] [PubMed] [Google Scholar]
- [37].Bĕlohlávek J, Dytrych V, Linhart A. Pulmonary embolism, part I: Epidemiology, risk factors and risk stratification, pathophysiology, clinical presentation, diagnosis and nonthrombotic pulmonary embolism. Exp Clin Cardiol 2013;18:129–38. [PMC free article] [PubMed] [Google Scholar]
- [38].Engelberger RP, Kucher N. Management of deep vein thrombosis of the upper extremity. Circulation 2012;126:768–73. [DOI] [PubMed] [Google Scholar]


