Abstract
When the 5-fluorouracil, leucovorin, and oxaliplatin (FOLFOX) chemotherapy regimen is used to treat colorectal cancer (CRC), chemotherapy-induced peripheral neuropathy (CIPN) caused by oxaliplatin can substantially affect quality of life (QOL) in the CRC patients. This study compared emotional distress and QOL during FOLFOX in CRC patients with and without CIPN symptoms.
This cross-sectional, descriptive, and comparative study recruited 68 CRC patients receiving FOLFOX at a local teaching hospital and at a medical center in southern Taiwan. Self-reported structured questionnaires (oxaliplatin-associated neuropathy questionnaire, profile of mood states short form, and the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire, Core 30, version 3.0) were used for 1-time data collection. The Chi-square test, Fisher exact test, and Mann–Whitney U test were used to analyze data, and a P-value < .05 was considered statistically significant.
The CIPN group had 45 (66.2%) patients, and the non-CIPN group had 23 (33.8%) patients. The 5 most common symptoms were coldness-related burning sensation or discomfort in the upper limbs, numbness in the upper limbs, tingling in the upper limbs, impairment of vision, and discomfort in the throat. The CIPN group had more females (P = .013), a more advanced stage of CRC (P = .04) and a higher chemotherapy dosage (P = .006). The 2 groups did not significantly differ in anxiety (P = .065) or depression (P = .135). Compared to the non-CIPN group, the CIPN group had significantly lower functioning (P = .001) and global health status (P < .001) and significantly more symptoms (P < .001).
The CIPN group had significantly lower QOL compared to the non-CIPN group. However, the CIPN group did not have lower emotional distress compared to the non-CIPN group. The results of this study demonstrate the need for in-service courses specifically designed to train health professionals in assessing and managing CIPN symptoms to improve QOL in CRC patients receiving FOLFOX.
Keywords: CIPN, colorectal cancer, emotional distress, FOLFOX, quality of life
1. Introduction
Colorectal cancer (CRC) is the third most common cancer in the United States and the most common cancer in Taiwan.[1,2] Although chemotherapy prolongs survival in cancer patients, some chemotherapeutic agents can cause chemotherapy-induced peripheral neuropathy (CIPN), which can affect quality of life (QOL).[3,4] Commonly used chemotherapy agents known to cause CIPN include
-
(1)
platinum compounds,
-
(2)
vinca alkaloids,
-
(3)
taxanes, and
-
(4)
others, including thalidomide, lenalidomide, and bortezomib.[5]
Notably, CIPN is a major side effect of oxaliplatin in the chemotherapy regimen comprising oxaliplatin, 5-fluorouracil, and leucovorin (FOLFOX) in CRC patients with stage III and IV. In Seretny et al, a review of 12 studies of CRC patients receiving oxaliplatin revealed that 40.6% to 93.7% of the CRC patients developed CIPN symptoms.[6] Another study reported that 65% to 98% of CRC patients suffer acute neurotoxicity after oxaliplatin infusion.[7] However, the incidence and severity of neurotoxicity are significantly lower when the duration of chemotherapy is short.[8] A 3-month course of FOLFOX is apparently sufficient for low-risk CRC patients (T1–T3 or N1). However, in high-risk CRC patients (T4 or N2) treated with FOLFOX, the 3-year disease-free survival rate after 6 months of therapy exceeds that after 3 months of therapy.[8]
Several studies have investigated how dose de-escalation can maintain QOL in CRC patients undergoing FOLFOX chemotherapy.[9,10] In CRC patients undergoing chemotherapy, those with CIPN are usually given a reduced dose, even if chemotherapy is effective. The reduced dose can then potentially delay or even prevent achievement of treatment goals.[3] The severity of CIPN usually correlates positively with the cumulative dose of oxaliplatin. The current clinical recommendation is that the cumulative oxaliplatin dose should not exceed 850 mg/m.[1,3,5,11] Additionally, oxaliplatin should be discontinued in the event of severe neurotoxicity.
The platinum contained in oxaliplatin induces chronic CIPN by forming complexes (adducts) with DNA in neurons of the dorsal root ganglion. The adducts then inhibit DNA replication and cause apoptosis.[12] In most cases of oxaliplatin-induced CIPN, neuropathy occurs mainly in the sensory nerves.[3] Motor symptoms are relatively less common. Acute symptoms that may occur from several hours to several days after injection include numbness, tingling, coldness-induced paresthesia of the peripheral limbs, paresthesia around the mouth, difficulty swallowing while drinking cold water, shortness of breath, numbness, cramps, jaw stiffness, and changes in auditory and visual receptive fields.[5,13] An oxaliplatin dose exceeding 175 to 200 mg/m2 can cause hypoesthesia unrelated to coldness and paresthesia of the distal ends of limbs.[14] Chronic symptoms include sensory loss, reduction of deep tendon reflexes, and abnormal proprioception. Pachman et al (2015) investigated acute and chronic neuropathy experienced by 308 patients after 1 to 12 cycles of adjuvant FOLFOX. The authors reported that the severity of acute neuropathy symptoms tend to be more severe with cycle 2 than with cycle 1, and then have similar patterns for cycles 3 to 12.[15]
Studies show CIPN symptoms resulting in discomfort, functional limitations, anxiety, and depression can have significant negative impacts on daily activity, social life, ability to perform family functions, ability to perform job duties, and overall QOL.[3] However, patients are usually willing to tolerate these symptoms to avoid further deterioration of their condition caused by interruptions or changes in treatment. Health professionals often overlook CIPN symptoms and lack the knowledge needed to administer appropriate care.
Until now, most studies of QOL in CRC patients have focused on factors relevant to QOL before and after treatment.[11,16] Some studies have explored how CIPN affects QOL in CRC patients.[17] For example, 1 qualitative study explored the emotional response to CIPN in CRC patients.[18] In another study, the relationship between CIPN and psychological distress was compared between CRC survivors with high and low CIPN levels. However, no studies have compared emotional distress and QOL during FOLFOX in CRC patients with and without CIPN. Thus, the aims of this study were to
-
(1)
investigate demographic characteristics, disease characteristics, emotional distress, and QOL during FOLFOX in CRC patients with CIPN (CIPN group) and without CIPN (non-CIPN group);
-
(2)
characterize CIPN in the CIPN group; and
-
(3)
compare demographic characteristics, disease characteristics, emotional distress, and QOL between the CIPN group and the non-CIPN group.
The empirical data obtained by this study were expected to be useful for designing appropriate interventions for improving care quality and QOL in CRC patients with CIPN.
2. Methods
2.1. Design, setting, and sample
In this comparative cross-sectional study with purposive sampling, data collection was performed by structured questionnaires. The data collection settings were the departments of gastroenterology and general surgery at Kaohsiung Medical University Hospital and at Kaohsiung Municipal Hsiao-Kang Hospital in southern Taiwan. Data were collected from December, 2013 to April, 2015. The inclusion criteria were
-
(1)
age 20 years or older,
-
(2)
CRC diagnosis,
-
(3)
completion of at least 1 FOLFOX chemotherapy cycle before initiation of the study,
-
(4)
current FOLFOX treatment as in-patient, and
-
(5)
ability to communicate in Mandarin.
Each patient completed the questionnaire package 1 time (oxaliplatin-associated neuropathy questionnaire, short form of profile of mood states [POMS-S] and European Organization for Research and Treatment of Cancer [EORTC] quality of life questionnaire, core 30 [QLQ-C30], version 3.0) during any chemotherapy cycle from 2 to 12. Patients with 1 or more CIPN symptoms of oxaliplatin-associated neuropathy were enrolled in the CIPN group, and the remaining patients were enrolled in the non-CIPN group. Exclusion criteria included colostomy, cognitive impairment or other mental disorders, alcohol abuse, dialysis, and other causes of peripheral neuropathy such as diabetes. Patients on a FOLFOX regimen were scheduled to receive intravenous oxaliplatin every 2 weeks. The initial dose of 85 mg/m2 over 4 hours was administered with intravenous leucovorin 200 mg/m2 and was followed by a continuous 42-hour intravenous infusion of 5-fluorouracil infusion for a total dose of 2800 mg/m2.
2.2. Measure
2.2.1. Oxaliplatin-associated neuropathy questionnaire
The Chinese version of the oxaliplatin-associated neuropathy questionnaire was used to assess the incidence of CIPN caused by oxaliplatin in CRC patients and to collect data needed to evaluate symptom severity.[19] The questionnaire was divided into 3 parts for assessing CIPN in the upper limbs, lower limbs, and oral/facial regions. The score for each part ranged from 1 to 5 points for low to high severity of CIPN and its impacts on daily activities, respectively. Symptom severity score was converted to neurotoxicity grade as follows: no symptoms for severity score of 0, grade 1 for severity score of 1 to 2, grade 2 for severity score of 3, grade 3 for severity score of 4, and grade 4 for severity score of 5. Neurotoxicity grades 1 to 4 were defined as follows: grade 1, symptoms that had a short duration and did not interfere with activities of daily living (ADL); grade 2, symptoms that interfered with certain functions but did not interfere with ADL; grade 3, pain or functional impairment that interfered with ADL; and grade 4, persistent symptoms that were are disabling or life-threatening. Tests of content validity by 3 experts obtained a content validity index (CVI) of 0.92 in this study.
2.2.2. POMS-S
Yang translated and modified the 37-item POMS-S to measure the most common emotional responses (anxiety and depression) in a Taiwan population of cancer patients.[20] In the CRC patients investigated in the current study, emotional distress experienced during chemotherapy completed in the past 7 days was investigated using the 6-item tension-anxiety subscale and the 8-item depression-dejection subscale developed by.[21] These subscales measure tension-anxiety and depression-dejection using a 5-point Likert scale from 0 to 4 (none, a little, some, moderate, and extreme, respectively); total scores ranges are 0 to 24 in the Tension Anxiety subscale, 0 to 32 in the depression-dejection subscale, and 0 to 56 for both subscales summed together. A high score indicates a high severity of emotional distress.[21] In the Chinese version of the POMS-S used in this study, the tension-anxiety and depression-dejection subscales obtained CVI values of 0.94 and 0.99 and Cronbach alpha values of 0.85 and 0.93, respectively.
2.2.3. QOL questionnaire
This study used a Chinese version of the EORTC QLQ-C30, version 3.0.[17,22] This 30-item questionnaire assesses functional status, global health status, and symptoms in the past week. The instrument includes a global health status dimension (2 questions about global health status/QOL), a functional dimension (5 questions about physical functioning, 2 questions about role functioning, 4 questions about emotional functioning, 2 questions about cognitive functioning, 2 questions about social functioning), and a symptoms dimension (13 questions regarding symptom scales/items).[23] Questions about functioning and symptoms are answered with a Likert scoring system from 1 to 4 (not at all, only a little, some, a lot, respectively). Questions about global health status are answered using a Likert scoring system from 1 point (very poor) to 7 points (very good). The QOL was self-assessed by patients. For each part of the instrument, scores were added to obtain a total score. The scores for the EORTC QLQ-C30 were converted to standard scores of 0 to 100. High scores for the functioning and global health status/QOL groups were interpreted as better functioning and better QOL, respectively. High scores for the symptom scales group or for the single questions were interpreted as high severity of symptoms.[23] The QLQ-C30 scale used in this study had a CVI of 0.94 and a Cronbach α of 0.85.
2.3. Procedure
After obtaining approval from the internal review board at this institution (KMUH-IRB-20130275), a pilot study was performed to evaluate the instrument and procedure used for data collection. The data collection settings were the departments of gastroenterology and general surgery at a local teaching hospital and at a medical center in southern Taiwan. The aims, procedures, and approaches of the study were explained to all patients before enrollment in the study. The patients were also assured that participation in the study would not affect their rights to receive medical care. Upon agreement, the patients were asked to complete the consent form and questionnaires. A researcher was available to assist participants who could not complete the forms due to illiteracy or physical impairment. After completing the questionnaire, all participants received a gift as a token of appreciation for participating in the study.
2.4. Statistical analysis
Emotional distress and QOL during FOLFOX have not been compared between CRC patients with and without CIPN. Therefore, the results of the pilot study performed in December, 2013, were used to estimate sample size. Out of 48 patients who met the criteria, 1 patient with diabetes mellitus was excluded, and 47 patients were enrolled. The CIPN criteria were used to divide the patients into a CIPN group (32 patients) and a non-CIPN group (15 patients). The G POWER statistical analysis (version 3.1.7) software was used to estimate sample size. Independent t tests of anxiety scores and depression scores revealed a 2-point difference between the CIPN group and the non-CIPN group. Thus, the settings were an effect size of 0.8, a power level of 0.8, and an α level of 0.05. The ratio of sample numbers for the CIPN group versus the non-CIPN group was set to 2. The non-CIPN group required 19 samples, and the CIPN group required 39 samples. Therefore, 58 total samples were required. The data were entered and analyzed by SPSS software (version 21.0, SPSS Inc., Chicago), and a P-value < .05 was considered statistically significant. Categorical variables were presented as frequency and percentage, and continuous variables were described as the mean and standard deviation. The Shapiro–Wilks test was used to test the normality of variables. Chi-square test was used to compare categorical data for demographic characteristics and disease characteristics between the 2 groups. According to the literature, if the number of samples is too small or if the expected number is less than 5 in 20% of cells in a crossover design, the chi-square test is unsuitable, and the Fisher exact test should be used for verification.[11] Two-way analysis of variance (ANOVA) was used to test whether the effect of 1 independent variable on the dependent variable was the same for all values of other independent variables. Mann–Whitney U test was used to compare non-normally distributed anxiety, depression, and QOL data between the CIPN group and the non-CIPN group.
3. Results
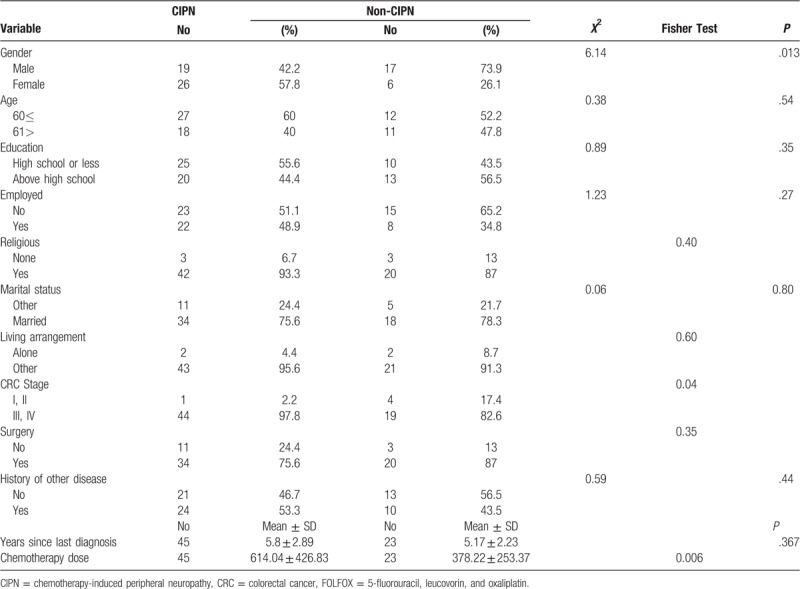
Of the 71 patients who met the inclusion criteria, 3 declined to participate (1 did not feel well, and 2 were reluctant to disclose personal information). Therefore, 68 subjects completed the questionnaires, which was a 95.8% completion rate. According to the oxaliplatin-associated neuropathy questionnaire, 45 of 68 subjects (66.2%) who presented with CIPN-associated symptoms were assigned to the CIPN group, and the remaining 23 subjects (33.8%) were assigned to the non-CIPN group. The patients had an average age of 57.75 years (± 10.87). In both groups, the median number of completed treatment cycles was 4 (range, 2–12 cycles completed in CIPN group and 2–8 cycles completed in non-CIPN group). Table 1 shows the relevant demographic and disease characteristics. Comparisons of demographic variables showed that the groups significantly differed in gender (χ2 = 6.14, P = .013). In both the CIPN and non-CIPN groups, the 2-way ANOVA results showed no significant gender differences in emotional distress, that is, anxiety (P = .93) or depression (P = .63). In terms of QOL, neither group showed significant gender differences in global health status (P = .91), functioning (P = .58), or symptoms (P = .87). Additionally, neither group had significant gender differences in emotional distress (anxiety, depression) or QOL (global health status, functioning, symptoms). However, the CIPN group and the non-CIPN group significantly differed in cancer stage (P = .04) and total chemotherapy dose (P = .006) (Table 1). In a 2-way ANOVA with control for the effects of total chemotherapy dose, cancer stage showed no association with emotional distress or with QOL in either the CIPN group or non-CIPN group.
Table 1.
Comparison of demographic and disease characteristics between CIPN and non-CIPN CRC patients receiving FOLFOX (N = 68).
3.1. CIPN
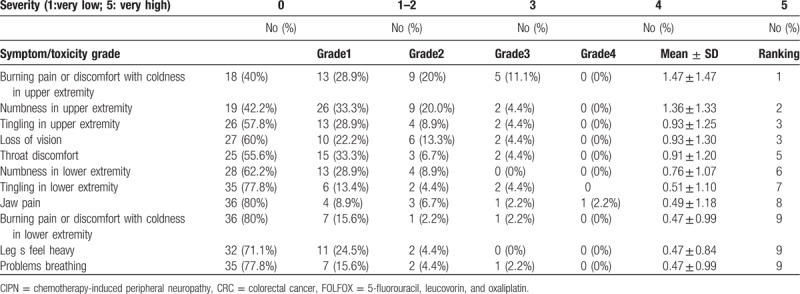
Most CIPN symptoms affected the upper limbs. The 5 most common symptoms were coldness-related burning sensation or discomfort in the upper limbs, numbness in the upper limbs, tingling in the upper limbs, impairment of vision, and discomfort in the throat. The results showed that all symptoms had low average values, and 40% of the patients had no symptoms. Additionally, symptoms were lower than grade 2 in patients who did have symptoms (Table 2).
Table 2.
Ten most common CIPN symptoms in CRC patients undergoing FOLFOX (CIPN group, n = 45).
3.2. Emotional distress
Both groups had low scores for emotional distress-anxiety and depression. The 2 groups did not significantly differ in anxiety (P = .065) or in depression (P = .135) Table 3.
Table 3.
Comparison of emotional distress between CIPN and non-CIPN CRC patients receiving FOLFOX (N = 68).

3.3. QOL
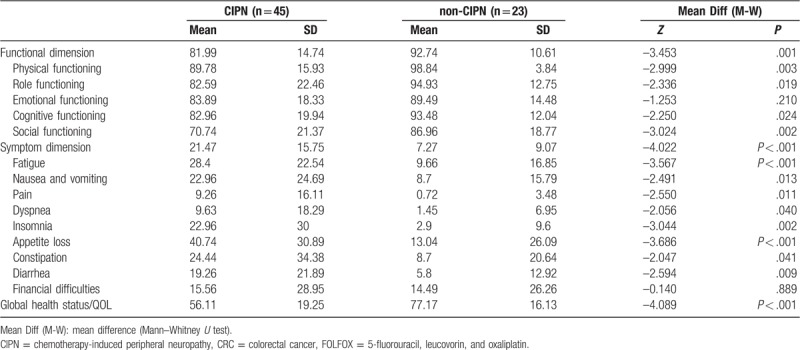
In both the CIPN and the non-CIPN groups, the highest QOL score was in physical functioning, and the lowest QOL score was in social functioning (Table 4). Appetite loss had the highest score and was the most severe symptom in both groups. The CIPN and non-CIPN groups significantly differed in functioning (P = .001), symptoms (P < .001) and global health status of QOL (P < .001). Compared to the CIPN group, the non-CIPN group had higher scores for functioning and global health status, which indicated a better overall functional status and global health status. In the functional dimension, the non-CIPN group also had significantly higher scores for physical functioning (P = . 003), role functioning (P = .019), cognitive functioning (P = .024), and social functioning (P = .002) compared to the CIPN group. However, the 2 groups did not significantly differ in scores for emotional functioning (P = .207) (Table 4). Regarding symptoms, the CIPN group had significantly higher scores for fatigue (P = .001), nausea and vomiting (P = .013), pain (P = .011), dyspnea (P = .040), insomnia (P = .002), appetite loss (P < .001), constipation (P = .041), and diarrhea (P = .009). However, the scores for economic difficulties did not significantly differ between the 2 groups.
Table 4.
Comparison of quality of life between CIPN and non-CIPN CRC patients receiving FOLFOX (N = 68).
4. Discussion
The demographic data showed that the subjects of this study had an average age of 57.75 years, which approximates the average age of 53 to 63 years reported in other studies performed in Taiwan and elsewhere.[11,13,24] The subjects of our study were predominantly male, which is also consistent with most CRC studies.[16] The most common education category was high school, and the second most common was primary school. Most subjects were unemployed/retired, not religious, married, and living with their families, which is consistent with the literature in Taiwan and in other countries. The most common disease category was stage III CRC followed by stage IV CRC, which is consistent with Lu (2012) and is consistent with the NCCN (2018) clinical guideline that patients in CRC stages III and IV should have priority for FOLFOX chemotherapy.[24,25] The number of cycles and cumulative dose of chemotherapy have a well-established relationship with CIPN symptom severity.[3,26] The average values for all symptoms were low in this study, possibly because a large portion of patients (51.1%, 23 patients) in the CIPN group had only completed 1 to 3 cycles of chemotherapy. Therefore, the average cumulative dose in the CIPN group was lower than the averages reported in the literature (eg, 695–1379 mg/m2) but was comparable to the average cumulative dose of 607.18 mg/m2 reported in the literature.[27,28]
Many studies have characterized oxiplatin-induced neurotoxicity in terms of its unique sensory and motor symptoms (cold sensitivity, throat discomfort, muscle cramps, perioral numbness, etc),[3,13,15] which occur within hours to days after treatment with a drug similar to that used in our study. In our study, patients with oxiplatin-induced neurotoxicity often complained of pain from touching cold objects or drinking cold water. Other symptoms commonly experienced by the CIPN patients in our study included numbness and tingling. Upper extremity symptoms were more common than lower extremity symptoms. These results are consistent with other studies.[15] However, impairment of vision was less common in our study compared to previous reports.[29] Patients with CIPN endure and manage their side effects in different ways to restore normality in their daily lives. The responsibility of the nurse is to support patients in developing the self-care ability needed to maintain their health. Specific guidelines for assessing and managing CIPN have emerged. For example, patients who present with numbness or cold-induced tingling in the hands are advised to avoid touching cold objects and drinking cold water and are advised to wear gloves or scarves to protect the hands and throat.
Symptoms of emotional distress such as anxiety and depression are common in CRC patients and may increase the difficulty and complexity of treatment. A qualitative study by Kanda et al (2017) explored the emotional response to CIPN in CRC patients who had completed 6 cycles of FOLFOX6. Patients reported suffering emotional distress (anxiety, helplessness, fear) caused by inability to cope with numbness, inability to perform ADL independently, fear for physical safety, fear of the threat of cancer, and fear of the inability to tolerate side effects of treatment such as numbness.[18] Additionally, Bonhof et al (2019) examined the relationships between CIPN and psychological distress (ie, anxiety and depression) and fatigue. The authors reported that the CRC survivors with high CIPN scores (upper 30%; n = 172) had more anxiety, depressive symptoms, and fatigue compared to those with low CIPN scores (lower 30%; n = 299). In our study, anxiety and depression did not significantly differ between CRC patients with CIPN and CRC patients without CIPN because those with CIPN had low average values and because the sample size was small.
A CIPN can significantly affect QOL.[30] In an earlier study, Mols et al (2013) used the EORTC QLQ-CIPN20 and QLQ-C30 questionnaires to investigate 26 CRC patients.[31] Patients with the highest 10% of scores for sensory neurological symptoms were assigned to the high score group, and the remaining patients were assigned to the low score group. As in our patients, the low score group had significantly higher QOL compared to the high score group.[31] In the functioning dimension of QOL, the scores for physical functioning, role functioning, cognitive functioning, and social functioning were significantly higher in the non-CIPN group than in the CIPN group. Patients with CIPN have various ADL challenges, including fastening buttons, holding cups, using chopsticks, opening jars, writing, turning pages, holding pots and pans, and walking.[18] Severe loss of function may cause weak dorsiflexion of the feet with subsequent foot drop. Some patients report that they often stumble when walking and that they need to hold handrails to avoid losing balance when using stairs. Symptoms of CIPN can disrupt ADL, functions, and behaviors, and patients often express feelings of frustration and depression when their symptoms prevent them from enjoying various activities.[29] Tabata et al (2018) investigated upper extremity changes, ADL, and QOL(QLQ-C30) at baseline and before the second cycle of drug therapy in 38 CRC patients undergoing FOLFOX or capecitabine-oxaliplatin therapy. After the first cycle, loss of light tough sensation, loss of physical function and role function, nausea and vomiting, and dyspnea significantly increased in severity. However, objective assessments in Tabata et al (2018) showed no worsening of upper extremity function since the total chemotherapy dose was low.
Notably, social functioning had the lowest score in the functioning dimension in both groups, and social functioning was significantly lower in the CIPN group compared to the non-CIPN group, which indicated that CIPN symptoms had significant negative effects on family life and social activities. However, the CIPN symptoms did not significantly affect emotional function evaluated in terms of tension, worry, irritability, and depression. Symptoms of CIPN can also negatively affect relationships with family, friends, and colleagues, which then impairs social function. An earlier study by Kim et al (2011) in South Korea administered the QLQ-C30 questionnaire in a sample of 93 CRC patients with CIPN, which included 53 patients who had received FOLFOX25. As in Kim et al (2011), the score for social functioning in our patients was the lowest score for the functioning dimensions.
Regarding symptoms, the CIPN group had significantly higher scores for fatigue, nausea and vomiting, pain, dyspnea, insomnia, appetite loss, constipation, and diarrhea compared to the non-CIPN group. The highest symptom scores were for appetite loss followed by fatigue and insomnia, which is consistent with Kim et al.[32] However, financial difficulties did not significantly differ between the 2 groups in this study. Since no standard treatment for CIPN has emerged, current strategies usually focus on protective managements (eg, patients are advised to wear gloves, avoid touching cold objects, etc) that have a limited financial impact on the patient and/or healthcare system.
5. Conclusions
5.1. Study limitations
A noted limitation of this study is that its cross-sectional design may have had limited accuracy in identifying the peak period of CIPN and may not have clearly depicted changing trends in emotional distress and QOL during the course of chemotherapy. The sample was recruited from a local teaching hospital and a local medical center, which limits the potential generalizability of the results. Thus, the results should be replicated in larger samples of CRC patients from various hospitals. Another limitation is that the short-term injection treatment administered in the hospital greatly increased the difficulty of recruiting subjects. In future studies, data should be collected in the first cycle of oxaliplatin-related combination chemotherapy and in each cycle thereafter until the end of treatment to obtain accurate data for identifying trends and changes in CIPN symptoms, emotional distress, and QOL.
5.2. Clinical implications
The results of this study suggest that, when FOLFOX is the selected treatment regimen for CRC patients who require ongoing treatment, the stop-and-go strategy proposed by Tournigand et al (2006) should be applied. High-risk patients (with T4, N2, or both), however, may require longer treatment.[8] Therefore, appropriate prevention and treatment strategies may improve CIPN in CRC patients undergoing FOLFOX. For example, physical therapy may be suggested to improve daily function and QOL in CIPN patients by strengthening muscles, by maintaining fine motor function in the upper limbs, which is essential for activities such as writing and threading a needle,[26] and by maintaining coarse motor function in the lower limbs, which is essential for a normal gait and balance.[33] Balance training can induce neuronal adaptations and can improve postural control by increasing muscle strength.[30] Additionally, physical activity (eg, bicycling, somatic yoga and meditation[30,34] and complementary therapies (eg, acupuncture[35]; may help to prevent or reduce CIPN symptom).[36] Some patients may benefit from a combined strategy (eg, physical activity, advance planning, and simplified ADL) to deal with CIPN symptoms.[18] For treating CIPN, some evidence suggests that medications such as Duloxetine may be effective[37] and that antioxidants and herbal extracts may be effective (eg, N-acetylcysteine, Goshajinkigan,[38] Jianwei Hiangqi Guizhi Wuwu Decoction, and glutamine).[36] Finally, Wesselink et al (2018) reported that magnesium supplements can significantly decrease the prevalence of CIPN.[39]
The results of this study demonstrate the need for in-service courses specifically designed to train health professionals in assessing and managing CIPN symptoms, emotional distress, and QOL in CRC patients undergoing combination chemotherapy that includes oxaliplatin-related agents. Patients should be educated to recognize CIPN symptoms and to recognize sensory abnormalities induced by cold temperature in the hands, feet, mouth, throat, and so on. Moreover, patients should be reminded to note details of symptoms such as the time of occurrence, the duration, and any changes in severity. Healthcare professionals can also minimize anxiety and depression in patients undergoing chemotherapy by clearly explaining the course of treatment and by addressing their concerns about CIPN side effects. A careful assessment of the psychological condition of patients can help to identify contributors to anxiety and depression, and highly distressed patients can be referred for psychological consultation. To maximize care quality and assistance for CRC patients with CIPN, further longitudinal studies are needed to collect empirical data that can be used to design interventions for improving QOL in these patients.
Acknowledgment
The authors thank Professor Yong-Yuan Chang for assistance with statistical analysis.
Author contributions
Conceptualization: Hsin-Tien Hsu, Pi-Ling Chou.
Data curation: Chiung-Hui Juan, Yu-Yen Huang.
Formal analysis: Li-Min Wu, Pei-Chao Lin.
Funding acquisition: Hsin-Tien Hsu.
Investigation: Hsin-Tien Hsu, Chiung-Hui Juan, Yu-Yen Huang.
Methodology: Hsin-Tien Hsu.
Project administration: Hsin-Tien Hsu.
Resources: Chiung-Hui Juan, Yu-Yen Huang.
Software: Li-Min Wu, Pei-Chao Lin.
Supervision: Hsin-Tien Hsu.
Validation: Hsin-Tien Hsu, Pi-Ling Chou.
Writing – original draft: Hsin-Tien Hsu, Li-Min Wu, Pei-Chao Lin.
Writing – review & editing: Hsin-Tien Hsu, Pi-Ling Chou, Jyu-Lin Chen.
Hsin-Tien Hsu orcid: 0000-0001-9638-1589.
Footnotes
Abbreviations: ANOVA = analysis of variance, CIPN = chemotherapy-induced peripheral neuropathy, CRC = colorectal cancer, CVI = content validity index, EORTC QLQ-C30 = the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30, FOLFOX = folinic acid, fluorouracil, and oxaliplatin, POMS-S = profile of mood states short form, QOL = quality of life.
How to cite this article: Hsu HT, Wu LM, Lin PC, Juan CH, Huang YY, Chou PL, Chen JL. Emotional distress and quality of life during folinic acid, fluorouracil, and oxaliplatin in colorectal cancer patients with and without chemotherapy-induced peripheral neuropathy: A cross-sectional study. Medicine. 2020;99:6(e19029).
This research was funded by the Ministry of Science and Technology, Taiwan (grant no. MOST 103-2314-B-037-056-) and by the Kaohsiung Medical University, Taiwan (grant no. KMU-M107016).
The authors report no conflicts of interest.
References
- [1].Ministry of Health and Welfare Taiwan Cancer registry 2014. Taipei, Taiwan: Health Promotion Administration, Ministry of Health and Welfare; 2017. [Google Scholar]
- [2].American Cancer Society Cancer Facts & Figures 2017. Atlanta, Ga: American Cancer Society; 2017. [Google Scholar]
- [3].Pulvers JN, Marx G. Factors associated with the development and severity of oxaliplatin-induced peripheral neuropathy: a systematic review. Asia Pac J Clin Oncol 2017;13:345–55. [DOI] [PubMed] [Google Scholar]
- [4].Babiker HM, Green MR, Nelson MA, et al. Oxaliplatin-induced neuropathy: a tale of two electrolytes. Support Care Cancer 2015;23:1483–5. [DOI] [PubMed] [Google Scholar]
- [5].Kandula T, Farrar MA, Kiernan MC, et al. Neurophysiological and clinical outcomes in chemotherapy-induced neuropathy in cancer. Clin Neurophysiol 2017;128:1166–75. [DOI] [PubMed] [Google Scholar]
- [6].Seretny M, Currie GL, Sena ES, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain 2014;155:2461–70. [DOI] [PubMed] [Google Scholar]
- [7].Zajaczkowska R, Kocot-Kepska M, Leppert W, et al. Mechanisms of chemotherapy-induced peripheral neuropathy. Int J Mol Sci 2019;20:E1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Grothey A, Sobrero AF, Shields AF, et al. Duration of adjuvant chemotherapy for stage III colon cancer. N Engl J Med 2018;378:1177–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tournigand C, Cervantes A, Figer A, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer–a GERCOR study. J Clin Oncol 2006;24:394–400. [DOI] [PubMed] [Google Scholar]
- [10].Nakayama G, Kodera Y, Yokoyama H, et al. Modified FOLFOX6 with oxaliplatin stop-and-go strategy and oral S-1 maintenance therapy in advanced colorectal cancer: CCOG-0704 study. Int J Clin Oncol 2011;16:506–11. [DOI] [PubMed] [Google Scholar]
- [11].Zhang M, Peng L, Liu W, et al. Physical and psychological predictors of quality of life in chinese colorectal cancer patients during chemotherapy. Cancer Nurs 2015;38:312–21. [DOI] [PubMed] [Google Scholar]
- [12].Cascella M. Chemotherapy-induced peripheral neuropathy: limitations in current prophylactic strategies and directions for future research. Curr Med Res Opin 2017;33:981–4. [DOI] [PubMed] [Google Scholar]
- [13].Argyriou AA, Cavaletti G, Briani C, et al. Clinical pattern and associations of oxaliplatin acute neurotoxicity: a prospective study in 170 patients with colorectal cancer. Cancer 2013;119:438–44. [DOI] [PubMed] [Google Scholar]
- [14].Wickham R. Chemotherapy-induced peripheral neuropathy: a review and implications for oncology nursing practice. Clin J Oncol Nurs 2007;11:361–76. [DOI] [PubMed] [Google Scholar]
- [15].Pachman DR, Qin R, Seisler DK, et al. Clinical course of oxaliplatin-induced neuropathy: results from the randomized phase III trial N08CB (alliance). J Clin Oncol 2015;33:3416–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gozdziewicz B, Strugala M, Talarska D, et al. Functioning of people with colorectal cancer during chemotherapy. Demographic and clinical determinants of quality of life of patients with colorectal cancer receiving chemotherapy. Pilot study. Eur J Cancer Care 2017;26:e12616. [DOI] [PubMed] [Google Scholar]
- [17].Magaji BA, Moy FM, Roslani AC, et al. Psychometric validation of the malaysian chinese version of the EORTC QLQ-C30 in colorectal cancer patients. Asian Pac J Cancer Prev 2015;16:8107–12. [DOI] [PubMed] [Google Scholar]
- [18].Kanda K, Fujimoto K, Kyota A. Emotional responses to persistent chemotherapy-induced peripheral neuropathy experienced by patients with colorectal cancer in Japan. Asia Pac J Oncol Nurs 2017;4:233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Leonard GD, Wright MA, Quinn MG, et al. Survey of oxaliplatin-associated neurotoxicity using an interview-based questionnaire in patients with metastatic colorectal cancer. BMC cancer 2005;5:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yang WQ. Self-care, Cocial Support, and Biopsychosocial Distress in Hematologic Malignant Patients Receiving Chemotherapy Industry. Taipei: College of Nursing, National Yang-Ming University; 1996. [Google Scholar]
- [21].Guo SL. The Effects of Pain Education and Relaxation Training in Patients With Cancer Pain. Taipei: College of Nursing, Taipei Medical University; 2002. [Google Scholar]
- [22].Zhao H, Kanda K. Testing psychometric properties of the standard Chinese version of the European Organization for Research and Treatment of Cancer Quality of Life Core Questionnaire 30 (EORTC QLQ-C30). J Epidemiol 2004;14:193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76. [DOI] [PubMed] [Google Scholar]
- [24].Lu LC. The Relationships of Chemotherapy Induced Peripheral Neuropathic Pain, Activities of Daily Lives, Mood, and Quality of Life In patient With Colorectal Cancers. Taipei: Nursing National Taipei University Nursing And Health Science; 2012. [Google Scholar]
- [25].National Comprehensive Cancer Network. (2018). NCCN Guidelines for Patient: Colon Cancer, 2018. Plymouth Meeting, PA: National Comprehensive Cancer Network, Inc. [Google Scholar]
- [26].Miltenburg NC, Boogerd W. Chemotherapy-induced neuropathy: a comprehensive survey. Cancer Treat Rev 2014;40:872–82. [DOI] [PubMed] [Google Scholar]
- [27].Beijers A, Mols F, Dercksen W, et al. Chemotherapy-induced peripheral neuropathy and impact on quality of life 6 months after treatment with chemotherapy. J Commun Support Oncol 2014;12:401–6. [DOI] [PubMed] [Google Scholar]
- [28].Beijers AJ, Mols F, Tjan-Heijnen VC, et al. Peripheral neuropathy in colorectal cancer survivors: the influence of oxaliplatin administration. Results from the population-based PROFILES registry. Acta Oncol 2015;54:463–9. [DOI] [PubMed] [Google Scholar]
- [29].Chan CW, Cheng H, Au SK, et al. Living with chemotherapy-induced peripheral neuropathy: uncovering the symptom experience and self-management of neuropathic symptoms among cancer survivors. Eur J Oncol Nurs 2018;36:135–41. [DOI] [PubMed] [Google Scholar]
- [30].Kneis S, Wehrle A, Muller J, et al. It's never too late - balance and endurance training improves functional performance, quality of life, and alleviates neuropathic symptoms in cancer survivors suffering from chemotherapy-induced peripheral neuropathy: results of a randomized controlled trial. BMC Cancer 2019;19:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mols F, Beijers T, Lemmens V, et al. Chemotherapy-induced neuropathy and its association with quality of life among 2- to 11-year colorectal cancer survivors: results from the population-based PROFILES registry. J Clin Oncol 2013;31:2699–707. [DOI] [PubMed] [Google Scholar]
- [32].Kim JH, Choi KS, Kim TW, et al. Quality of life in colorectal cancer patients with chemotherapy-induced peripheral neuropathy. J Korean Oncol Nurs 2011;11:254–62. [Google Scholar]
- [33].Driessen CM, de Kleine-Bolt KM, Vingerhoets AJ, et al. Assessing the impact of chemotherapy-induced peripheral neurotoxicity on the quality of life of cancer patients: the introduction of a new measure. Support Care Cancer 2012;20:877–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Galantino ML, Tiger R, Brooks J, et al. Impact of somatic yoga and meditation on fall risk, function, and quality of life for chemotherapy-induced peripheral neuropathy syndrome in cancer survivors. Integr Cancer Ther 2019;18:1534735419850627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Baviera AF, Olson K, Paula JM, et al. Acupuncture in adults with chemotherapy-induced peripheral neuropathy: a systematic review. Rev Lat Am Enfermagem 2019;27:e3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Derksen TM, Bours MJ, Mols F, et al. Lifestyle-related factors in the self-management of chemotherapy-induced peripheral neuropathy in colorectal cancer: a systematic review. Evid Based Complement Alternat Med 2017;2017:7916031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hou S, Huh B, Kim HK, et al. Treatment of chemotherapy-induced peripheral neuropathy: systematic review and recommendations. Pain Phys 2018;21:571–92. [PubMed] [Google Scholar]
- [38].Kuriyama A, Endo K. Goshajinkigan for prevention of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Support Care Cancer 2018;26:1051–9. [DOI] [PubMed] [Google Scholar]
- [39].Wesselink E, Winkels RM, van Baar H, et al. Dietary intake of magnesium or calcium and chemotherapy-induced peripheral neuropathy in colorectal cancer patients. Nutrients 2018;10:E398. [DOI] [PMC free article] [PubMed] [Google Scholar]


