Abstract
Glucose is the required metabolic substrate for the brain. Yet the brain stores very little glucose. Therefore, the brain continuously monitors glucose availability to detect hypoglycemia and to mobilize system-wide responses to protect and restore euglycemia. Catecholamine (CA) neurons in the hindbrain are critical elements of the brain’s glucoregulatory mechanisms. They project widely throughout the brain and spinal cord, innervating sites controlling behavioral, endocrine and visceral responses. Hence, CA neurons are capable of triggering a rapid, coordinated and multifaceted response to glucose challenge. This article reviews experimental data that has begun to elucidate the importance of CA neurons for glucoregulation, the functions of specific CA subpopulations in the ventrolateral medulla, and the extended circuitry through which they engage other levels of the nervous system to accomplish their essential glucoregulatory task. Hopefully, this review also suggests the vast amount of work yet to be done in this area and the justification for engaging in that effort.
Keywords: catecholamine neurons, glucoprivation, hindbrain, counter-regulatory responses, food intake, adrenal medulla, corticosterone
1. Introduction
Glucose is the required metabolic substrate for the brain. Because the brain stores only small amounts of glycogen, brain glucose deficit is an emergency situation. The response to this emergency must rapidly engage both central and peripheral mechanisms to protect and restore the brain’s glucose supply. To do this, the brain deploys mechanisms to sense glucose deficit and to initiate responses, known as counterregulatory responses (or CRRs) (Cryer, 1993). These CRRs include increased secretion of adrenal medullary epinephrine (E), adrenocortical corticosterone (CORT), pancreatic glucagon and pituitary growth hormone. Additional responses include increased gastric motility, estrus suppression and increased food intake. In our lexicon, increased food intake (FI) in response to glucose deficit also is a critical CRR, as FI is the sole mechanism for restoration of depleted glucose stores.
Understanding the anatomical, physiological and biochemical substrates responsible for CCRs is medically significant. Hypoglycemia associated autonomic failure (HAAF) is a potentially lethal condition in which CCRs are not elicited or are blunted, apparently due to failure of the brain to detect glucose deficits (Cryer, 1993). HAAF occurs in the aftermath of a recent severe hypoglycemic episode or after recurrent hypoglycemic episodes. Due to the risk of iatrogenic hypoglycemic episodes, HAAF is a constant threat for diabetic individuals on insulin therapy. The worldwide increase in the prevalence of diabetes and obesity in both adults and children (Wild, Roglic, Green, Sicree, & King, 2004) has resulted in increased incidence of HAAF, underscoring its medical significance. The pathogenic mechanisms causing HAAF have been intensively investigated but are not yet understood and cannot be determined without identification of the specific receptors and neural circuitry that elicit the essential life-supporting CRRs that occur in response to glucoprivic conditions.
Several decades of investigation have revealed that glucose monitoring occurs in multiple cell and tissue types both centrally and peripherally. Moreover, cellular glucose monitoring, which may detect increased as well as decreased glucose availability, may utilize different biochemical mechanisms, and can serve to protect or facilitate multiple physiological processes. Therefore, it is important to acknowledge at the outset that not all glucose monitoring is directly involved in elicitation of CRRs (Levin, 2001, 2002; Levin, Routh, Kang, Sanders, & Dunn-Meynell, 2004; S. Ritter, 2017; Routh, Donovan, & S.Ritter, 2012) The intentional focus of this article will be to review our own work demonstrating the importance of hindbrain catecholamine (CA) neurons as essential contributors to glucoregulatory CRRs. Specifically, we will highlight work revealing that CA neurons in the ventrolateral medulla are necessary and sufficient for elicitation of three essential CRRs to glucose deficit: adrenal medullary elevation of blood glucose (BG), secretion of corticosterone (CORT) and increased food intake (FI). Briefly, adrenal medullary epinephrine (E) secretion promotes glucagon secretion and mobilizes glucose primarily from stored liver glycogen. CORT secretion (among other functions), by switching peripheral metabolism from glucose to fat, facilitates conservation of glucose for use by the brain. Finally, increased feeding replaces depleted glucose. We will summarize results indicating that subpopulations of hindbrain catecholamine (CA) neurons are required for elicitation of these particular CRRs. A schematic diagram showing the anatomical locations of CA cell groups in the dorsomedial and ventrolateral medulla (those forming the focus of this review) is shown in Figure 1. We will discuss emerging evidence of the functional connectivity of the hindbrain CA neurons with sites in the hypothalamus, dorsal hindbrain and spinal cord that contribute to their efficacy as glucose conservators.
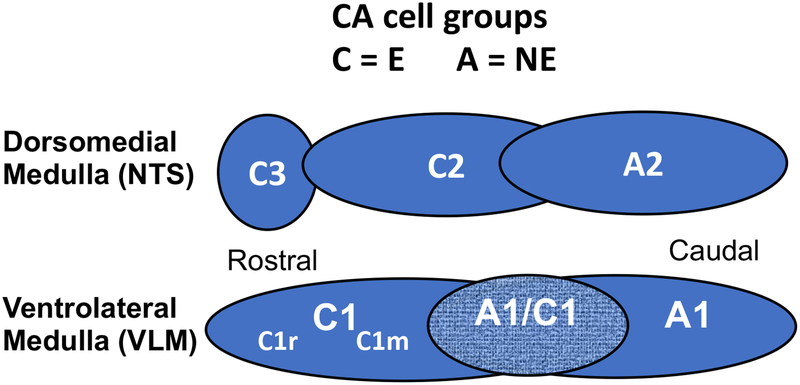
Figure 1.
Diagram depicts the organization of hindbrain CA neurons in the dorsomedial and ventrolateral medulla in sagittal plane. Groups designated as “A” or “C” are composed of norepinephrine (NE) or epinephrine (E) expressing neurons, respectively. The A1/C1 designation refers to the area of overlap of rostral A1 and caudal C1 and contains both NE and E phenotypes. The terms C1r and C1m are applied to differentiate rostral and middle areas of C1, respectively.
2. Importance of the Hindbrain in Eliciting Glucoregulatory Responses
Harvey Grill and his students found that consummatory feeding (Flynn & Grill, 1983) and blood glucose responses (DiRocco & Grill, 1979) could be elicited by glucoprivation in unanesthetized, chronically maintained decerebrate rats in which all neural connections between hindbrain and forebrain were severed. Their results clearly demonstrated that the hindbrain contains sensory and motor circuits sufficient to mobilize these responses. This conclusion was corroborated by R.C. Ritter and colleagues in intact, freely moving rats (R.C. Ritter, Slusser, & Stone, 1981). They found that acute blockade of the cerebral aqueduct, which reduced flow of cerebrospinal fluid from the forebrain to hindbrain ventricles, blocked the feeding and blood glucose responses to lateral ventricular, but not fourth ventricular, injections of the glucoprivic agent 5-thioglucose (5TG). These results indicated that hindbrain, but not forebrain, glucoprivation is necessary and sufficient to evoke feeding and sympathoadrenal CRRs.
Early work indicated that CAs, both epinephrine (E) and norepinephrine (NE), are potently orexigenic when injected into the brain (Grossman, 1960; Leibowitz, 1978; Stanley & Leibowitz, 1985). Moreover, pharmacological alteration of central CA neurotransmission alters food intake (FI) (Goldman, Marino, & Leibowitz, 1985; Leibowitz, Brown, Tretter, & Kirschgessner, 1985; McCabe, DeBellis, & Leibowitz, 1984; S. Ritter, Wise, & Stein, 1975). Later, cannula mapping experiments in our lab revealed that BG, CORT, glucagon and FI could be increased by injection of nanoliter volumes of the glucoprivic agent, 5TG, into specific hindbrain tissue sites using doses that were completely ineffective at hypothalamic tissue sites (Andrew, Dinh, & Ritter, 2007; S. Ritter, Dinh, & Zhang, 2000). Importantly, injection sites that were effective for eliciting CRRs were concentrated in or near hindbrain CA cell groups. Finally, studies using c-Fos as a marker of cellular activation showed that subpopulations of CA neurons in the hindbrain are activated by glucose deficit and that activated neurons are most prevalent in the ventrolateral medulla (VLM), compared to the dorsomedial medulla (S. Ritter, Llewellyn-Smith, & Dinh, 1998). Thus, on the basis of all of the above data, it is reasonable to hypothesize that hindbrain CA neurons contribute importantly to the mediation of responses to glucoprivation, including those now described as CRRs.
2a. Anti-Dopamine-Beta-Hydroxylase Saporin (DSAP) Reveals That CA Neurons Are Required for Elicitation of Key CRRs
While recognizing that the importance of CA neurons in glucoregulation and initiation of CRRs is strongly supported by existing data, it also is important to emphasize that not all hindbrain CA neurons are involved in CRRs. Distinct subpopulations of CA neurons that participate in different and equally critical homeostatic functions responses, including ventilatory control, blood pressure control, endocrine secretions and some “nonglucoprivic” controls of FI (see review: (Guyenet et al., 2013; Rinaman et al., 1995)) often are intermingled with CA neurons that mediate CRRs, and may even have overlapping projections with these neurons. For these reasons, traditional pharmacological, tract tracing and lesion approaches have not provided adequate neuronal selectivity for identification of the specific CA neurons involved in CRRs. Nevertheless, development of the targeted toxin, antidopamine beta-hydroxylase saporin (DSAP), has enabled important steps forward in untangling CA neuron function and circuitry as they relate to glucoregulation and CRR initiation.
DSAP is a conjugate of the ribosomal disaggregating toxin, saporin (SAP), and a monoclonal antibody against the NE and E biosynthetic enzyme, dopamine-beta-hydroxylase (DBH). The DSAP conjugate binds to DBH and is selectively internalized by NE and E neurons. DSAP does not lesion dopamine neurons, as they do not contain DBH. When injected into a CA terminal area, DSAP is retrogradely transported to the CA cell bodies, resulting in selective destruction of the CA neurons innervating the injection site (Blessing, Lappi, & Wiley, 1998; Wiley & Kline, 2000; Wrenn, Picklo, Lappi, Robertson, & Wiley, 1996). DSAP is also internalized and lesions DBH-positive neurons when injected directly into the areas containing CA cell bodies, as opposed to terminals (Madden, Ito, Rinaman, Wiley, & Sved, 1999; Rinaman, 2003).
We found that DSAP injections into the paraventricular hypothalamic nucleus (PVH), which is densely innervated by CA neurons, destroyed the majority of known rostrally-projecting CA neurons in both the dorsal and ventral medulla (Figure 2) and abolished the feeding response to insulin-induced hypoglycemia and systemic injections of the antiglycolytic agent, 2-deoxy-D-glucose (2DG) (S. Ritter, Bugarith, & Dinh, 2001) (Figure 3). The Increased secretion of CORT, a response typically elicited by systemic glucoprivation also was eliminated by PVH DSAP injections (S. Ritter, Watts, Dinh, Sanchez-Watts, & Pedrow, 2003) (Figure 4). This finding is consistent with established localization of corticotropin releasing hormone neurons in the PVH and their heavy innervation by CA neurons (Cunningham & Sawchenko, 1988; Kaminski & Watts, 2012; Swanson et al., 1981). In contrast, we found that DSAP injections into the thoracic spinal cord, where preganglionic sympathetic neurons receive dense terminal innervation from CA neurons in the rostral VLM, lesioned a different population of CA neurons, nearly all of which were C1 neurons in the rostral VLM. This lesion eliminated glucoprivation-induced activation of the adrenal medulla (indicated by lack of c-Fos expression), reduced adrenal epinephrine (E) secretion, and eliminated the blood glucose response to 2DG (S. Ritter et al., 2001). Importantly, spinal DSAP injections had no direct effect on glucoprivically-evoked FI or CORT secretion. Results obtained in PVH and spinal DSAP injected rats using 2DG to produce glucoprivation were replicated using insulin-induced hypoglycemia (S. Ritter et al., 2001; S. Ritter et al., 2003). Thus, lesions produced by DSAP are selective for CA neurons at or with projections to or through the DSAP injection site, demonstrating that rostrally and spinally projecting CA neurons comprise functionally distinct CA populations that mediate different CRRs.
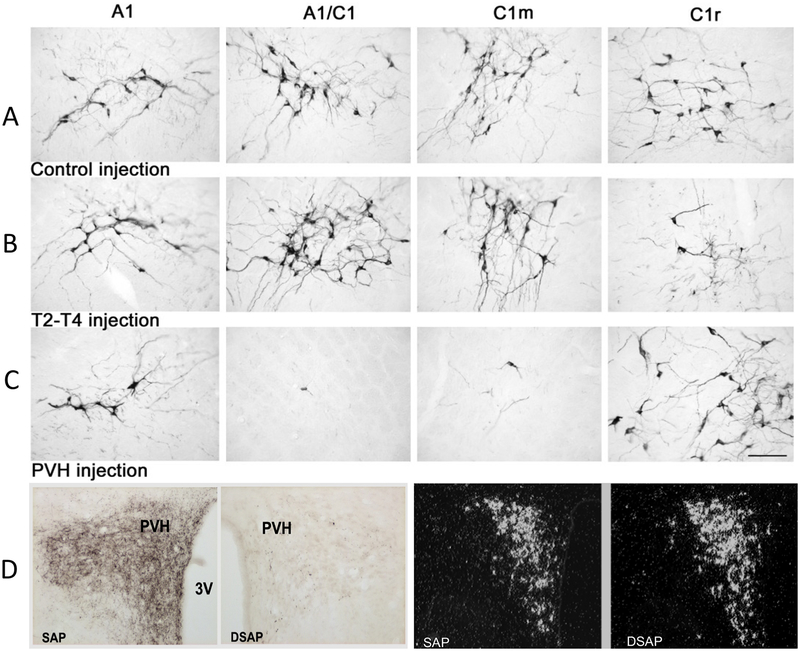
Figure 2.
Catecholamine neurons in ventrolateral medullary cell groups A1, A1/C1, C1m and C1r in control rats (A), rats given DSAP injection into the intermediolateral column at spinal level T2-T4 (B) or into the PVH (C). Tissue was stained using a monoclonal Dbh antibody and shows the effect of retrograde lesioning by DSAP. CA cell bodies are lost in areas containing spinally projecting (B) or rostrally projecting (C) CA neurons. In D, DBH-ir terminals in the PVH are shown for a PVH injected control rat (first panel) and DSAP-injected rat (second panel). The third and fourth panels in D show corticotropin releasing hormone (CRH) mRNA in PVH SAP and DSAP-injected rats. These sections reveal that DSAP injections in the PVH produced highly-selective lesions of CA neurons or their terminals with very little non-specific damage at the injection site. (Ritter, S., Bugarith, K., & Dinh, T. T. (2001). Immunotoxic destruction of distinct catecholamine subgroups produces selective impairment of glucoregulatory responses and neuronal activation. J Comp Neurol, 432(2), 197–216.)
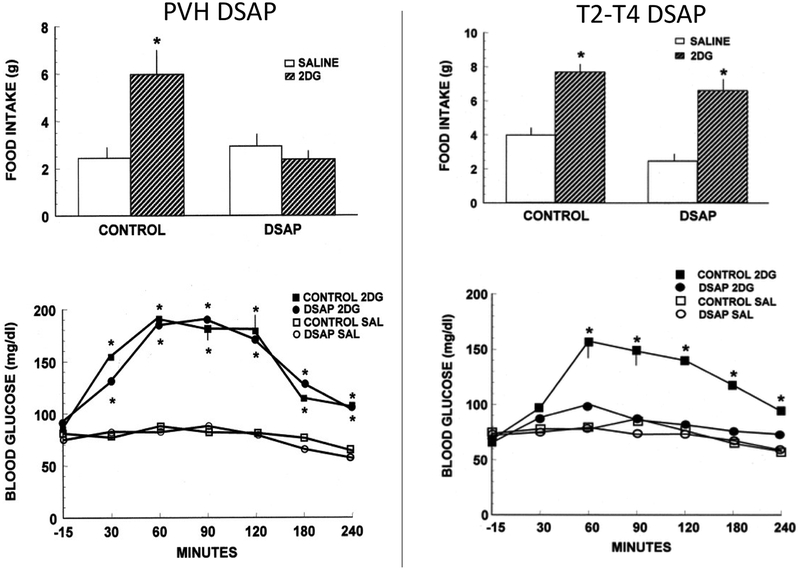
Figure 3.
Intake of pelleted rat chow (top panels) in 4-hour tests after subcutaneous administration of 2DG (200 mg/kg) or saline in rats injected previously into the PVH or spinal level T2-T4 with DSAP or control solution. 2DG or saline injections were given immediately before presentation of food. Means and standard errors are shown. PVH DSAP, but not spinal DSAP injections abolished 2DG-induced feeding. Bottom panels show blood glucose concentrations after subcutaneous administration of 2DG (200 mg/kg) or saline in the same rats as above. 2DG or saline injections were given 15 minutes after the first blood sample was drawn. The hyperglycemic response was tested in the absence of food. Means and standard errors ≥ 10 mg/dl are shown. PVH DSAP did not alter 2DG-induced hyperglycemia. Spinal cord DSAP abolished 2DG-induced hyperglycemia. *, P < 0.001 compared with saline baseline in the same group. (Ritter, S., Bugarith, K., & Dinh, T. T. (2001). Immunotoxic destruction of distinct catecholamine subgroups produces selective impairment of glucoregulatory responses and neuronal activation. J Comp Neurol, 432(2), 197–216.)
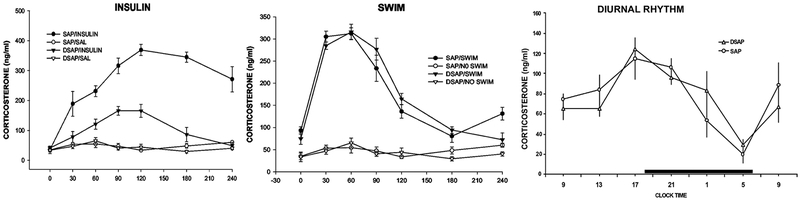
Figure 4.
Corticosterone levels in response to insulin-induced hypoglycemia (1.5 U/kg), forced swim stress and the diurnal rhythm of CORT secretion are shown (left to right) from rats injected previously into the PVH with SAP control or DSAP. All blood samples were collected by remote sampling from a jugular catheter. For the swim stress test, rats were placed individually for 5 min in a bucket of water maintained at 370 C. Blood samples were collected at 30, 60, 90, 120, and 240 min after the start of the swim. DSAP severely impaired hypoglycemia-induced, but not stress-induced, CORT secretion and did not alter the diurnal rhythm of CORT secretion. Ritter, S., Watts, A. G., Dinh, T. T., SanchezWatts, G., & Pedrow, C. (2003). Immunotoxin lesion of hypothalamically projecting norepinephrine and epinephrine neurons differentially affects circadian and stressor-stimulated corticosterone secretion. Endocrinology, 144(4), 1357–1367. doi:10.1210/en.2002-221076
2b. DSAP lesion impairs responses that are selectively elicited by glucoprivic stimulation
Although the PVH DSAP may damage rostrally-projecting CA neurons that mediate functions other than CRR initiation, our studies indicate that the CRR deficits we observe are not due damage to CA neurons involved in non-CRR functions or to nonspecific damage. For example, the feeding response to 2DG or insulin was eliminated following PVH DSAP, while daily spontaneous feeding or feeding induced by mercaptoacetate (an orexigenic drug that does not produce glucoprivation (Li, Wiater, Wang, Wank, & Ritter, 2016)) remained functional (S. Ritter et al., 2003). Glucoprivation-induced increase in CORT secretion was severely impaired in the DSAP-lesioned rats, while neither the circadian rhythm of CORT secretion nor the CORT response to swim stress was altered (S. Ritter et al., 2003) (Figure. 4). Similarly, suppression of estrus, a well-known response to glucoprivation (I’Anson, Starer, & Bonnema, 2003), was blocked in PVH DSAP-injected rats, but the normal estrous cycle of euglycemic rats was not altered (I’Anson, Sundling, Roland, & Ritter, 2003).
Despite the apparent selectivity of the deficits following DSAP lesions, the functions mediated by the lesioned CA neurons may not be limited to initiation of CRRs. For example, in a long-term study, PVH DSAP injected rats maintained on standard pelleted rodent diet gained approximately 50 g more than SAP controls over a period of 6 months (S. Ritter et al., 2001), suggesting that this lesion somehow impacts control of body weight. Recently this hypothesis has been confirmed and expanded by results showing that, compared to SAP-injected controls, PVH DSAP-injected rats dramatically increased body weight in only 8 weeks when maintained on a high fat-high sucrose diet. Increased body weight was attributable primarily to increased visceral adiposity (Lee, Jokiaho, Sanchez-Watts, & Watts, 2018).
These effects of PVH DSAP lesions on body weight are consistent with our experiments examining the metabolic consequences of this lesion using indirect calorimetry (Li et al., 2013). These experiments showed that DSAP and SAP injected rats did not differ in locomotor activity or energy expenditure under any test conditions. However, DSAP rats had a persistently higher respiratory exchange ratio (RER) than SAPs, even under basal conditions, indicating persistently higher utilization of carbohydrates, compared to fat. In addition, systemic 2DG rapidly decreased RER in SAP injected rats, but did not alter RER in DSAP rats, suggesting that the PVH DSAP lesion impairs the ability to switch from carbohydrate to fat metabolism, a response elicited by the CORT CRR that conserves glucose for use by the brain during glucoprivic challenge. Therefore, the effect of the DSAP lesion on daily food intake and accumulation of body fat may be related to chronic impairment of CA-mediated CORT secretion in PVH DSAP lesioned rats (S. Ritter et al., 2003). This interpretation is consistent with our findings that adrenalectomy, but not adrenal denervation, abolished the decrease in RER in SAP controls and that dexamethasone, a synthetic glucocorticoid, decreased RER in both SAP and DSAP rats (Li et al., 2013). Thus, it is conceivable that CA-induced control of CORT secretion may occur in response to blood glucose variations within the range of those occurring during a normal intermeal interval. In addition, it is important to recall that PVH DSAP injection lesions all CA neurons that project to or through the injection site, and therefore may lesion ascending CA neurons that control a CORT response that is not part of the CRR.
2c. CA Neurons Mediate Both Appetitive and Consummatory Responses to Glucoprivation
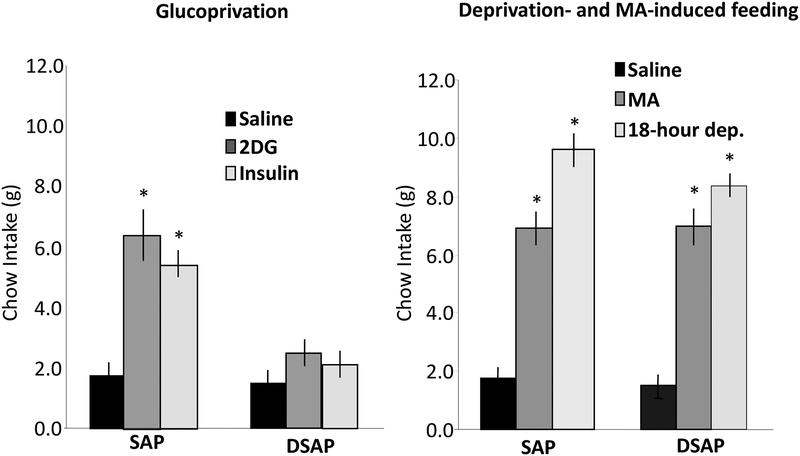
As discussed above, in rats decerebrated at the level of the midbrain, completely separating the hindbrain and forebrain, consummatory feeding responses are still elicited by glucoprivation, although appetitive responses are abolished (Flynn & Grill, 1983). That is, a decerebrated rat is not able to search for food but will eat food placed in its mouth and will increase the amount consumed in response to glucoprivic stimulation. These observations indicate that the neural circuitry for eliciting consummatory responses to glucose deficit remains intact, even though motivated behavioral responses to obtain food are incapacitated. In contrast to decerebrated rats, PVH DSAP injections in otherwise normal rats eliminated both appetitive and consummatory feeding responses to glucoprivation (Hudson & Ritter, 2004). Unlike SAP controls, that increased intake under both appetitive and consummatory protocols, DSAP-injected rats did not increase their food intake in response to 2DG or insulin-induced glucoprivation when tested with pelleted food (requiring appetitive responses) or when liquid food was provided intraorally through a chronic cheek fistula (requiring only a consummatory response). However, in the same protocols, the DSAP rats did not differ from SAP controls in their ingestive responses to either food deprivation or systemic mercaptoacetate (Figure 5). These results indicate that DSAP did not impair the underlying circuitry required for either appetitive or consummatory responding but eliminated the mechanism for control of this circuitry specifically by glucoprivation.
Figure 5.
Left panel: Total 4-h intake of pelleted rat food following subcutaneous injection of saline (1 ml/kg), 2DG (200 mg/kg), or insulin (2 units/kg) in SAP control and DSAP-immunolesioned rats. DSAP rats did not increase their food intake in response to either 2DG-induced glucoprivation or to hypoglycemic doses of insulin, even when food was infused via the cheek cannula. Right panel: Total 4-h intake of pelleted food following saline (1 ml/kg), MA (68 mg/kg), or overnight food deprivation (18 h) in SAP controls and DSAPimmunolesioned animals. DSAP rats increased their food intake significantly above control in response to both MA and food deprivation. Data are expressed as mean 4-h food intake ± S.E.M. (*P<.001 vs. intake after saline). Hudson, B., & Ritter, S. (2004). Hindbrain catecholamine neurons mediate consummatory as well as appetitive responses to glucoprivation. Physiol Behav, 82(2–3), 241–250. doi:10.1016/j.physbeh.2004.03.032
3. Activation of VLM CA Neurons is Sufficient for Elicitation of CRRs
The selectivity of DSAP toxicity for lesioning CA neurons clearly supports the hypothesis that CA neurons are necessary for elicitation of key glucoregulatory responses. However, an important question is which CA neurons. Because a large percentage of hindbrain CA neurons in both the dorsal and ventral medulla project densely to medial hypothalamic nuclei, DSAP injections retrogradely lesion many CA cell bodies in both dorsal and ventral sites. In addition, CA cell groups project to other CA cell groups, such that injections targeting a specific group may lesion CA neurons in other cell groups as well. Finally, individual cell groups appear to be populated with multiple functional phenotypes. We have attempted to overcome these obstacles using chemogenetic tools (Roth, 2016; Witten et al., 2011). This approach offers more selectivity than DSAP injections, because (1) it does not require administration of a glucoprivic agent that potentially alters glucose metabolism in all cells contacted by the drug, and (2) only neurons specifically transfected with a DREADD (designer receptor exclusively activated by a designer drug) construct are activated by a DREADD receptor agonist.
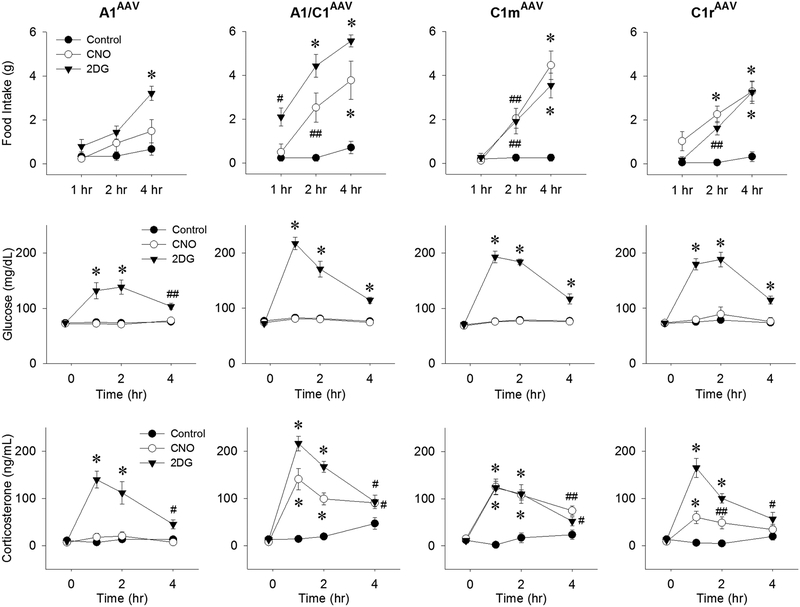
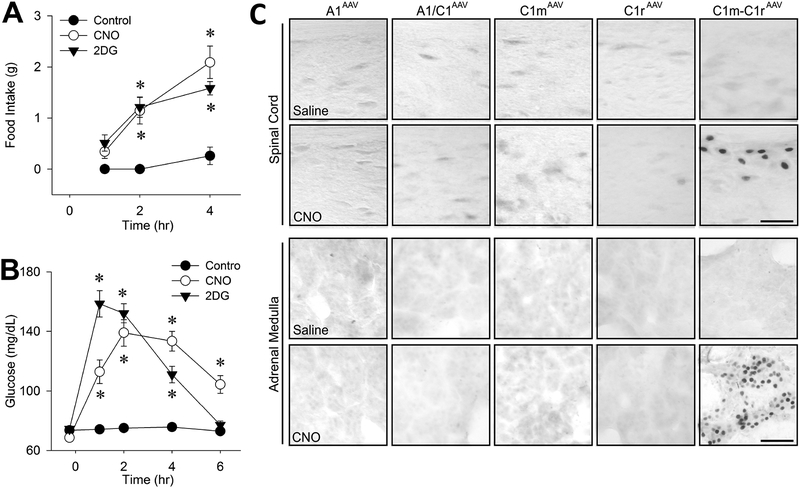
Using transgenic rats expressing Cre recombinase under the control of the tyrosine hydroxylase promoter, we transfected A1/C1 CA neurons with a DREADD construct, AAV2-DIOhSyn-hM3D(Gq)-mCherry and verified that the transfection was selective for CA neurons at the injection site (Li, Wang, Elsarelli, Brown, & S. Ritter, 2015; Li, Wang, & S. Ritter, 2018b). These results enabled us to use this approach to determine whether CA neurons that elicit different glucoregulatory responses can be distinguished by their location within the VLM (Li et al., 2018b). We mapped FI, elevation of BG and CORT secretion in response to systemic CNO in rats transfected with the DREADD construct at one of the following rostrocaudal levels of the VLM: rostral C1 (C1r), middle C1 (C1m), the area of A1 and C1 overlap (A1/C1) or the A1 area caudal to C1 (A1) (Li et al., 2018b). We found that the transfections were highly selective for CA neurons and that systemic administration of CNO was sufficient to increase FI, BG, and CORT in CA neuron-transfected rats, but not in nontransfected rats. In addition, different transfection sites were differentially effective with regard to CNO’s ability to evoke each of these three CRRs (Figure 6). CNO stimulated FI in rats that were DREADD transfected in C1r, C1m or A1/C1, but not in rats in which DREADD transfection was limited to A1. CNO increased CORT levels most effectively when C1m or A1/C1 CA neurons were transfected, but not when C1r or caudal A1 CA neurons were transfected. CNO increased BG only in rats that had dual transfection of both C1m and C1r (Figure 7). Accordingly, c-Fos expression was increased in the adrenal medulla and intermediolateral column of the spinal cord only in rats transfected simultaneously at both C1m and C1r. Collectively, these results suggest that CA neurons mediating the BG response are distributed in rostral half of C1 (i.e., C1r and C1m), while those mediating FI and CORT secretion are concentrated in C1m and A1/C1. The finding that elicitation of a BG response required transfection of two adjacent sites in the VLM, while FI and CORT required only one transfection site, may indicate that CA neurons mediating the BG response are more diffusely distributed.
Figure 6.
Responses of male Th-Cre+ transgenic rats to activation of CA neurons at different rostrocaudal levels of the VLM to systemic injection of the glucoprivic agent 2DG or the DREADD receptor agonist clozapine-N-oxide (CNO). Rats were transfected bilaterally by injection of AAV-hM3D into A1, A1/C1, C1m, or C1r. Responses were measured approximately 5 weeks after transfection during the 4-hour period following injection of control (0.9% saline intraperitoneally), CNO (1 mg/kg intraperitoneally), or 2DG (250 mg/kg subcutaneously). Food intake, blood glucose, and plasma CORT levels are shown in the upper, middle and lower panels, respectively. Food intake and CORT levels were increased maximally by CNO in rats transfected at specific VLM sites, but glucose was not altered in rats transfected at any single site. #P < 0.05; ##P < 0.01; *P < 0.001 vs saline control at the same time points by post hoc Student-Newman-Keuls test after two-way repeated-measures analysis of variance (six to eight rats for each treatment). Li, A. J., Wang, Q., & Ritter, S. (2018). Selective Pharmacogenetic Activation of Catecholamine Subgroups in the Ventrolateral Medulla Elicits Key Glucoregulatory Responses. Endocrinology, 159(1), 341–355. doi:10.1210/en.2017-00630
Figure 7.
Food intake (A), blood glucose responses (B) and c-Fos expression in spinal cord and adrenal medulla (C) in female Th-Cre+ rats transfected unilaterally with AAV-hM3D into both C1m and C1r (C1m+C1rAAV-hM3D). In C, VLM injection sites are shown for each column. Tests were conducted approximately 5–9 weeks after transfection. Feeding and glucose responses were measured following administration of control (intraperitoneal saline), CNO (1 mg/kg intraperitoneally) or 2DG (250 mg/kg subcutaneously), using 6–8 rats per treatment. *P < 0.001 vs saline control at the same time point by (post hoc Student-Newman-Keuls test after two-way repeated-measures analysis of variance. cFos in the intermediolateral thoracic spinal cord at T6 and in adrenal medulla were evaluated 2 hrs after intraperitoneal saline or CNO (1 mg/kg intraperitoneally). Li, A. J., Wang, Q., & Ritter, S. (2018). Selective Pharmacogenetic Activation of Catecholamine Subgroups in the Ventrolateral Medulla Elicits Key Glucoregulatory Responses. Endocrinology, 159(1), 341–355. doi:10.1210/en.2017-00630
At the time of this review, we have not been able to identify a viral construct that selectively transfects CA neurons in the dorsal medulla (NTS), so we cannot use the currently available DREADD approaches to examine their potential contribution to CRR elicitation. However, a variety of functions mediated in the dorsal hindbrain appear to be modulated by glucose (Cherian & Briski, 2012; Moriyama, Reyes, Tsukamura, & Maeda, 2003). Electrophysiological recordings identify both glucose-excited and glucose-inhibited neurons in the NTS (Balfour, Hansen, & Trapp, 2006). Calcium imaging approaches have demonstrated glucoprivation-induced activation of both astrocytes and CA neurons in the dorsomedial medulla, suggesting a role for astrocytes in activation of CRR circuitry (Rogers & Hermann, 2019; Rogers, McDougal, S. Ritter, Qualls-Creekmore, & Hermann, 2018). Other work shows that glucose alters the activity of some NTS neurons via glucose-excited vagal afferents (Roberts, Zhu, Zhao, Dillon, & Appleyard, 2017). These studies provide evidence that neurons in the dorsomedial medulla also contribute to aspects of glucoregulation, but these neurons require further characterization with respect to nature of their involvement in CRRs.
4. VLM CA Neurons are activated by Multiple Glucose Sensing Mechanisms
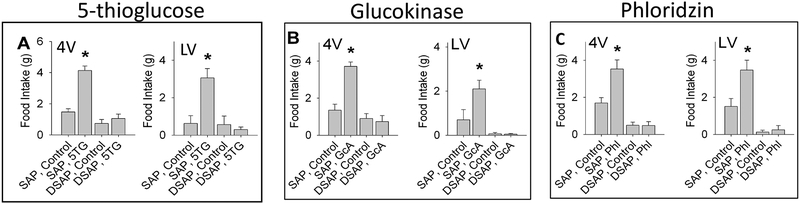
Not only are glucoprivically driven FI and BG responses mediated by different CA neuron subpopulations, they also appear be mediated by different glucose sensing mechanisms. A difference between potential mechanisms mediating increased FI and those mediating elevation of BG was observed by S.C. Woods et al. in 1978 (Woods & McKay, 1978). Specifically, they reported that central injection of alloxan, which subsequently has been determined to be a glucokinase inhibitor, stimulated FI but did not alter BG (see also: (S. Ritter, Murnane, & Ladenheim, 1982; S. Ritter & Strang, 1982)). Similarly, phloridzin, a sodium-glucose linked transporter (SGLT) inhibitor, stimulated FI, but did not elevate BG when injected either into the lateral or 4th ventricle (Flynn & Grill, 1985). To determine whether feeding induced by central phloridzin, glucosamine (a glucokinase inhibitor) and 5TG require CA neurons, we injected these agents into the lateral or 4th ventricle in separate groups of rats (Li, Wang, Dinh, Powers, & S. Ritter, 2014). Regardless of ventricular injection site, all three agents stimulated feeding responses that were abolished by PVH injection of DSAP (Figure 8). The underlying mechanisms that account for the differences in responsiveness of BG and FI controls to these mechanisms have yet to be determined.
Figure 8.
Effects of three drugs, glucosamine (GcA), phloridzin (Phl) and 5-thio-D-glucose (5TG), that stimulate food intake but interfere in different ways with cellular glucose utilization or transport. Male rats previously injected into the hypothalamic paraventricular nucleus with anti-dopamine beta hydroxylase conjugated to saporin (DSAP), a retrogradely transported immunotoxin that selectively lesions noradrenergic and adrenergic neurons, or with unconjugated saporin (SAP) control. Beginning several days later, food intake and blood glucose were measured in separate tests in response to 5TG, GcA and Phl (only food intake is shown here). All stimulated feeding in SAP controls whether injected into the lateral or fourth ventricle (LV or 4V), but responses to all three agents were eliminated in DSAP treated rats. (Li AJ, Wang Q, Dinh TT, Powers BR, Ritter S. Stimulation of feeding by three different glucose-sensing mechanisms requires hindbrain catecholamine neurons. Am J Physiol Regul Integr Comp Physiol 306: R257–R264, 2014. First published December 31, 2013; doi:10.1152/ajpregu.00451.2013).
Additional large and important gaps still exist in our understanding of the glucose-sensing mechanisms involved in elicitation of CRRs. For example, it is not known with certainty whether CA neurons mediating CRRs are themselves glucose sensing or are dependent on neighboring glucose sensing cells. Moreover, if the glucose sensors are not CA neurons, it is possible that they are not neurons at all, but rather are neighboring non-neuronal cells such as glia, astrocytes, tanocytes or all of these. Indeed, some recent work has suggested that astrocytes are glucosensors responsible for activating dorsal hindbrain CA neurons that mediate CRRs in response to glucose deficit (McDougal, Hermann, & Rogers, 2013; Rogers & Hermann, 2019) and that the GLUT2 transporter is an essential component of the astrocyte glucodetection mechanism (Klip & Hawkins, 2005; Marty et al., 2005; Marty, Dallaporta, & Thorens, 2007).
Identifying particular glucodetection mechanisms associated with particular neurons is a difficult problem in part because all neurons require glucose and may express one or more glucose sensing mechanism, some of which may manage cellular functions other than CRR initiation. This question is particularly challenging in VLM CA neurons. These neurons are relative inaccessible to electrophysiological approaches due to the density of the fiber projections that embed the cell bodies. However, we can conclude on the basis of DSAP data that stimulation of hindbrain CA neurons in the VLM, however it is achieved, is necessary for elicitation of the CRRs under investigation in our studies. Moreover, based on DREADD results, CA neuron activation also is sufficient for elicitation of these responses. It is also worth noting that the potency of the antiglycolytic agents, 5TG and 2DG, in stimulating feeding, CORT and adrenal medullary responses clearly indicates that glucose metabolism, rather than glucose itself, provides the major signal controlling these CRRs. Additional signaling mechanisms, however, cannot be disregarded.
5. Extended Circuitry of CA Neurons Involved in Responses to Glucoprivation
While it seems clear that hindbrain CA neurons are necessary for elicitation of glucoprivic feeding, the forebrain circuitry activated by these neurons to engage appetitive food-seeking behaviors is incompletely understood. Because the hypothalamus contains multiple mechanisms for detection and integration of metabolic cues and is heavily innervated by CA neurons, we investigated candidate neuropeptides in hypothalamic sites, including the paraventricular hypothalamus (PVH), arcuate nucleus (Arc) and perifornical lateral hypothalamus (PfLH), as potential downstream targets of CA neurons involved in glucoregulatory feeding. In additional (unpublished) work, we found that injections of DSAP into the lateral parabrachial nucleus, A6 (locus coeruleus), ventral tegmental area (avoiding overlying NE and E fiber bundles), did not reduce feeding or adrenal medullary responses to 2DG. DSAP injections into the bed nucleus of the stria terminalis did not alter feeding, glucose or corticosterone responses to systemic 2DG. DSAP injections into the medial accumbens shell, but not ventral or core regions, significantly reduced 2DG-induced feeding, but not feeding induced by MA or overnight food deprivation.
5a. Neuropeptide Y.
NPY is a potently orexigenic peptide implicated in counterregulatory feeding by results showing that global deletion of the NPY gene impairs glucoprivic feeding (Sindelar et al., 2004). NPY is co-expressed in many, perhaps most, of the CA neurons that project to the hypothalamus from the A1, A1/C1 and C1m areas (Sawchenko et al., 1985). Both NPY and DBH gene expression are increased in the A1/C1 area in response to glucoprivation (Li & S. Ritter, 2004; Li, Wang, & S. Ritter, 2006). In addition, simultaneous co-silencing (but not separate silencing) of DBH and NPY genes in the A1/C1 area impairs glucoprivic feeding, strongly implicating cooperation of both CAs and NPY in this response (Li, Wang, Dinh, & S. Ritter, 2009).
Despite the well-known importance of the PVN for feeding and the potency of NPY in eliciting food intake, early work of Calingasan et al. (Calingasan & Ritter, 1992) revealed the surprising finding that glucoprivic feeding was not impaired by bilateral electrolytic lesions that totally destroyed the PVN, indicating that NPY neurons or terminals located there are not required for glucoprivic feeding. Subsequently, we injected the NPY-saporin (NPY-SAP) conjugate into the basomedial hypothalamus to target and lesion neurons expressing NPY receptors at that site. Unlike DSAP, NPY-SAP is not retrogradely transported, but binds to NPY receptors and destroys known NPY receptor expressing neurons at the injection site. When injected into the basomedial hypothalamus, this toxin destroyed neurons expressing NPY/Agouti gene related peptide (AgRP) co-expressing neurons, proopiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript (CART) neurons and impaired the feeding responses to intraventricular ghrelin and leptin, but it did not impair feeding responses to intraventricular NPY, systemic cholecystokinin (CCK), or 2DG (Bugarith, Dinh, Li, Speth, & S. Ritter, 2005). Arcuate NPY neurons were also ruled out as required mediators of glucoprivic feeding by experiments using AgrpDTR/+ transgenic mice to selectively target and lesion Agrp/NPY co-expressing neurons in the arcuate nucleus, the sole location of AgRP neurons (Luquet, Phillips, & Palmiter, 2007). Although these Agrp/NPY deficient mice had deficits in some aspects of feeding, including their response to ghrelin, they had normal feeding responses to glucose deficit. Together, these data suggest that the action of NPY on the glucoprivic feeding response is not dependent on NPY neurons or other NPY receptor-expressing neurons in the PVH or Arc. However, these responses may be mediated by NPY projections to non-hypothalamic sites by non-hypothalamic NPY neurons, such as CA/NPY co-expressing neurons in the hindbrain, as suggested by our Dbh/Npy gene silencing data discussed above (Li et al., 2009).
5b. Orexin Neurons.
Orexin neurons have been extensively investigated with regard to their role in metabolic homeostasis and food intake (among other functions) (Yamanaka, Beuckmann, et al., 2003). Orexin cell bodies are concentrated in the PeFLH and project widely throughout the central nervous system, innervating many sites that are also innervated by CA neurons (Baldo, Daniel, Berridge, & Kelley, 2003). In addition, orexin neurons are densely innervated by VLM CA neurons, primarily of C1 origin, that form asymmetrical (excitatory) synapses on orexin neurons (Bochorishvili et al., 2014), and orexin neurons are activated by glucoprivation (Briski & Sylvester, 2001; Cai et al., 2001; Griffond, Risold, Jacquemard, Colard, & Fellmann, 1999; Li, Wang, Elsarelli, et al., 2015; Moriguchi, Sakurai, Nambu, Yanagisawa, & Goto, 1999).
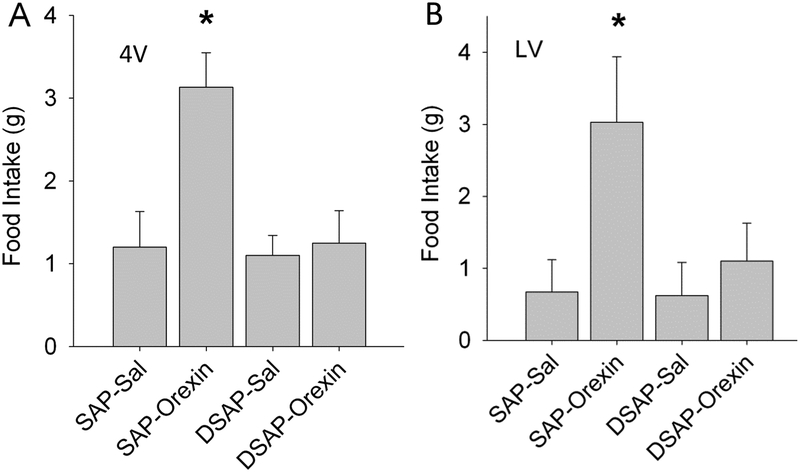
Activation of orexin neurons facilitates food anticipatory activity, promotes food-related visual, olfactory, spatial memory functions and reward – all of which are important in appetite and foraging for food (Barson, 2018; Yamanaka, Beuckmann, et al., 2003). Orexin neurons may therefore contribute importantly to appetitive responses, such as food seeking, during glucoprivation. But do orexin neurons drive glucoprivic feeding or CA neuron activation? Although orexinergic fiber projections are prevalent in all hindbrain CA cell groups and form close contacts with CA neurons, previous immunohistochemical and electron microscopic analyses have not identified orexinergic synaptic contacts on C1, C2 or C3 CA neurons (Puskas, Papp, Gallatz, & Palkovits, 2010). In fact, very few asymmetric (excitatory) synapses were observed in any of the CA cell groups, with the exception of the locus coeruleus (A6) and subcoeruleus, leading to the conclusion that most orexinergic fibers in the vicinity of the CA neurons are en route to other sites. Nevertheless, fourth ventricular (4V) orexin injections that increase FI also increase c-Fos expression in VLM CA neurons, primarily in A1 and C1 neurons (Li, Wang, Davis, Wang, & S. Ritter, 2015). Both LV and 4V feeding stimulated by orexin A were abolished by retrogradely transported DSAP delivered into either the PVH or perifornical LHA (Figure 9), although the DSAP injection did not damage orexin neurons themselves (Li, Wang, Elsarelli, et al., 2015). Thus, CA neurons are not only involved in control of orexin neurons, as suggested previously (Yamanaka et al., 2006; Yamanaka, Muraki, Tsujino, Goto, & Sakurai, 2003), but may in fact be necessary for activation of orexin neurons during glucoprivation.
Fig. 9.
Effects of orexin-A on food intake in rats pre-treated by injection of DSAP or SAP into the PVH. For feeding tests, orexin-A (0.5 nmol/rat) or saline (Sal) was injected into fourth ventricle (A) or lateral ventricle (B) in cannulated rats using the same protocol for both ventricular sites. Food intake measured during the 4 hours after the injection are shown. In A, N = 8 rats per group. *P < 0.01, vs. SAP Sal control. In B, N = 5 or 6 rats per group. *P < 0.05, vs. SAP Sal control. Retrograde lesion of hindbrain CA neurons by PVH DSAP injection was confirmed by testing feeding induced by 2DG (200 mg/kg, i.p.), which was significantly inhibited in DSAP rats (*P < 0.001, vs SAP rats), and by post mortem immunohistochemistry, which showed that hindbrain CA neurons, but not PeFLH orexin neurons, were lesioned by PVH DSAP injections (these data are not shown here). Loss of orexininduced feeding responses at both the 4V and the LV injection sites in DSAP lesioned rats indicates dependence of these responses on CA neurons. Hindbrain Catecholamine Neurons Activate Orexin Neurons During Systemic Glucoprivation in Male Rats. Endocrinology, 156(8), 2807–2820. doi:10.1210/en.2015-1138
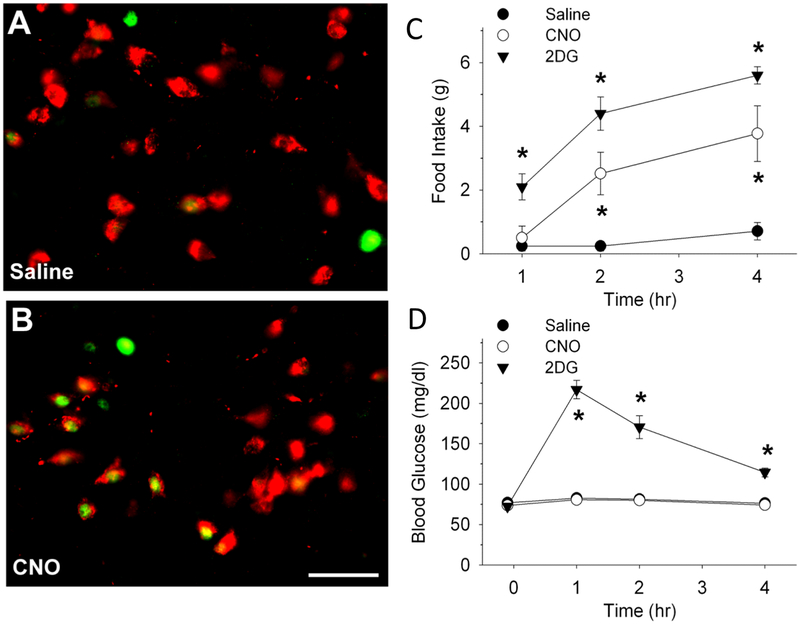
We specifically examined the role of VLM CA neurons in the response of orexin neurons to glucoprivation, as our previous work has demonstrated the importance of these particular neurons in glucoprivation induced responses. Th-Cre transgenic rats were transfected in A1/C1 with the DREADD-Gq receptor, enabling us to stimulate these neurons selectively in the absence of glucoprivation. When injected peripherally with CNO, both c-Fos expression in PeFLH orexin neurons and food intake were increased in the transfected rats (Li, Wang, Elsarelli, et al., 2015), suggesting that stimulation of A1/C1 neurons is sufficient for downstream activation of orexin neurons (Figure 10).
Fig. 10.
A and B, representative images showing c-Fos-ir (green) and orexin-A-ir (red) in PeFLH area 2 hours after a saline or CNO (1 mg/kg, ip) injection in TH-Cre+ rats, transfected bilaterally in A1/C1 regions with AAV2-DIO-hSyn-hM3D(Gq)-mCherry 6 wks prior to the feeding and blood glucose tests (Bar, 25 μm). Food intake and blood glucose levels are shown in panels C and D. cFos in orexin neurons and food intake, but not blood glucose, was increased by systemic CNO in the transfected rats. Li, A. J., Wang, Q., Elsarelli, M. M., Brown, R. L., & Ritter, S. (2015). Hindbrain Catecholamine Neurons Activate Orexin Neurons During Systemic Glucoprivation in Male Rats. Endocrinology, 156(8), 2807–2820. doi:10.1210/en.2015-1138
In addition to their contribution to FI, recent data have shown that adrenal medullary output controlled by rostral VLM CA neurons is increased by activation of orexin type 2 receptors (Korim, Bou Farah, McMullan, & Verberne, 2014; Korim, Llewellyn-Smith, & Verberne, 2016) and inhibited by activation of μ-opioid receptors (Kakall, Nedoboy, Farnham, & Pilowsky, 2018) in rostral VLM. Together these findings indicate that orexin and other hypothalamic neurons may contribute importantly to glucose homeostasis, but their specific functions and the nature of their interactions with VLM CA neurons for elicitation of CRRs require further investigation.
5c. CCK-responsive CA neurons.
CA neurons in both the dorsal and ventral hindbrain participate in control of food intake. In contrast to VLM CA neurons that are activated by glucose deficit and stimulate feeding, a subpopulation of A2 neurons in the nucleus of the solitary tract are activated by vagal afferent neurons that respond to cholecystokinin (CCK) and inhibit feeding (Rinaman, 2003; Rinaman et al., 1995). CCK is a peptide secreted from enteroendocrine cells when alimentation is adequate or in response to increased gastric distension, stress and malaise – conditions that reduce food intake (de La Serre, Kim, Moran, & Bi, 2016; R. C. Ritter, 2004a, 2004b; Smith & Gibbs, 1975, 1992). These particular NTS CA neurons thus mediate responses that are diametrically opposed to those in the VLM that increase food intake during glucoprivation.
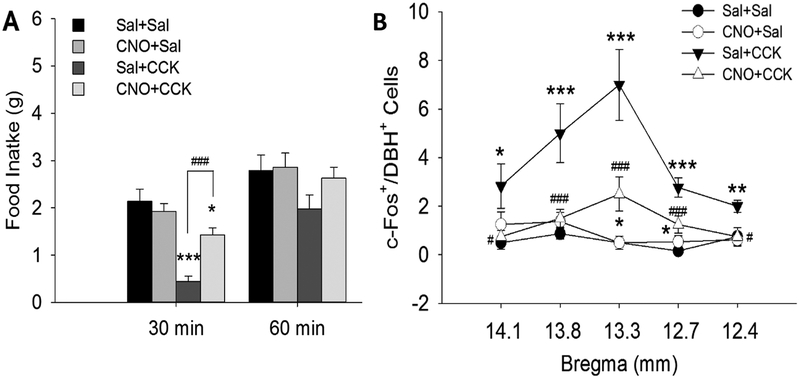
We used chemogenetic technology described above to determine whether CNO activation of DREADD-transfected VLM CA neurons alters the activity of NTS CA neurons that respond to CCK (Li, Wang, & S. Ritter, 2018a). We found that in these transfected rats, systemic CNO activated VLM CA neurons, while reducing CCK-induced c-Fos expression in A2 neurons and attenuating reduction of food intake by systemic CCK (Figure 11). Systemic 2DG produced similar effects. Glucose deficit increased c-Fos expression by A1 and C1 neurons, while reducing CCK-induced c-Fos expression in A2 neurons. These results support the hypothesis that activation of VLM CA neurons attenuates satiety during glucoprivation by inhibiting CCK-activated A2 neurons, possibly via their projections to the A2 area. This mechanism may ensure that feeding is not terminated by activation of this satiety response prior to restoration of normoglycemic conditions. If so, this action would be of particular importance since CCK is secreted in response to nutrients such as fatty acids and proteins that do not replenish brain glucose supply. We anticipate that similar interactions between glucoprivation and factors that modulate appetite will be revealed by future work.
Fig. 11.
Effects of CCK and the DREADD agonist, clozapine- N-oxide (CNO), on food intake and c-Fos expression in NE neurons in the nucleus of the solitary tract after A1/C1 transfection with AAV2DIO-hMD3. Rats were food deprived overnight (18 hrs) and then treated with the following drug combinations: Sal+Sal, CNO+Sal, Sal+CCK, CNO+CCK. A, Food intake was suppressed during the 30 min post-injection period in Sal+CCK injected rats, compared to Sal+Sal, but was not suppressed after CCK+CNO injections. B, Cell counts of double stained (c-Fos/DBH) cells are shown at five rostrocaudal levels of the NTS (~14.1, 13.8, 13.3, 12.9 and 12.5 mm caudal to bregma), indicated on X axis. c-Fos in NTS CA neurons was increased 90 min after CCK in Sal+CCK treated rats, but not after CNO+CCK treatment. Data shown are mean ± SEM from 3 – 4 rats/treatment. n = 6 – 8 sections/group/subregion (n = 9 – 12 sections for NTS at bregma −12.9 mm). *P < 0.05, **P < 0.01, ***P < 0.001, vs. Sal+Sal; #P < 0.05, ##P < 0.01, ###P < 0.001, CNO+CCK vs. Sal+CCK. (by post hoc Student-Newman-Keuls test after two-way ANOVA). Data indicate that both CCK’s satiety effects and its activation of A2 CA neurons is suppressed by CNO activation of A1/C1 CA neurons, even in the absence of glucoprivation. Li, A. J., Wang, Q., & Ritter, S. (2018a). Activation of catecholamine neurons in the ventral medulla reduces CCK-induced hypophagia and c-Fos activation in dorsal medullary catecholamine neurons. Am J Physiol Regul Integr Comp Physiol. doi:10.1152/ajpregu.00107.2018
6. Peripheral Glucoregulatory Controls
This review has emphasized the importance of CA neurons in the VLM for elicitation of CRRs. It is important, however, to remember that significant controls of blood glucose also are mediated by peripheral glucose sensors. For example, suppression of insulin secretion and stimulation of glucagon secretion in response to reduced blood glucose levels are fundamental controls that operate peripherally and maintain plasma glucose concentrations within the normal range. However, secretion of glucagon, considered to be a CRR, is also controlled by the brain, and the ventromedial hypothalamus appears to be of particular importance for this control (Routh, 2010; Routh et al., 2012). In addition, a population of peripheral glucose-sensing cells has been detected in the portomesenteric venous circulation (Bohland et al., 2014; Jokiaho, Donovan, & Watts, 2014). In contrast to the catecholaminergic glucoregulatory system that is able to generate emergency CRRs, the portomesenteric system elicits glucoprotective responses during slowly declining glucose levels. This system may contribute to maintenance of glucose during the intermeal interval or may control the timing of a subsequent meal. The identity, phenotype and properties of the cells responsible for this very interesting control are not yet known.
7. Concluding Remarks
Multiple widely distributed and diverse glucose sensing mechanisms exist in brain and peripheral tissues. In this review we have focused specifically on our attempts to clarify mechanisms and circuitry that mediate particular glucose CRRs. From results discussed in this review, it is clear that CA neurons in the VLM are necessary and sufficient for elicitation of CRRs in response to acute and profound glucose deficit. CA neurons elicit CRRs via a variety of brain circuits with diverse behavioral and physiological functions that contribute to glucoregulation. Activation of VLM CA neurons reduces satiety responses mediated by CCK-activated CA neurons in the dorsomedial medulla; VLM CA projections to the PfLH activate orexin neurons known to modulate arousal, reward and motivation; VLM CA projections to PVH neurons that control CORT secretion that reduces peripheral glucose utilization and promotes fat utilization; and finally, a distinct population of spinally projecting CA neurons mediates sympathoadrenal activation during glucoprivation. Thus, multiple widely distributed and diverse glucose sensing mechanisms exist in brain and peripheral tissues and a big challenge for future work is to understand each of these systems in more detail and to determine when, where and how they might contribute to overall glucose homeostasis.
Our data depict the VLM CA system as being essential for responses to glucoprivic emergency. Indeed, the data strongly support that role. However, that characterization may not fully describe the contributions of this system in other controls of feeding and body weight. A more complete understanding of glucoregulation under both glucoprivic and nonglucoprivic conditions, and how CA neurons contribute to the broader task of energy homeostasis is necessary. Challenges include determination of the glucose-sensing mechanisms essential for the required responses and the identification and localization of the cells (CA, other neurons or astrocytes) that express them. How these mechanisms differ between CA neurons controlling feeding and those controlling BG and other CRRs also remains to be determined. How these responses are integrated with other systems to maintain appetite and body weight also is important but unknown. Finally, a crucial challenge will be to determine how the circuitry required for elicitation of CRRs is incapacitated by repeated prior glucoprivation, very possibly contributing significantly to HAAF.
Highlights.
Because glucose is the required metabolic substrate for the brain and is stored by the brain in very small quantities, glucose availability is closely monitored. Deficits in the ability to detect or respond to severe glucose deficit, as too frequently occur in diabetic patients on insulin therapy, can be lethal.
In response to hypoglycemia the brain must initiate rapid and robust responses (i.e., “counterregulatory responses”) to restore euglycemia. Hindbrain catecholamine neurons are essential components of the glucoregulatory circuitry.
Selective lesion of hindbrain catecholamine neurons abolishes glucoprivic elicitation of key counterregulatory responses. Selective chemogenetic activation of specific catecholamine populations elicits these responses.
Circuitry and potential glucose-sensing mechanisms that contribute to the functions of glucoregulatory catecholamine neurons in the ventrolateral medulla are discussed.
Acknowledgements:
We are grateful to all the hardworking, talented and devoted colleagues who contributed to this research over the years.
This work was supported by PHS grants DK 040498 and DK 114187 and ADA grant 1-18-IBS-156 (to S. Ritter).
References
- Andrew SF, Dinh TT, & Ritter S (2007). Localized glucoprivation of hindbrain sites elicits corticosterone and glucagon secretion. Am J Physiol Regul Integr Comp Physiol, 292(5), R1792–1798. doi: 10.1152/ajpregu.00777.2006 [DOI] [PubMed] [Google Scholar]
- Baldo BA, Daniel RA, Berridge CW, & Kelley AE (2003). Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J Comp Neurol, 464(2), 220–237. doi: 10.1002/cne.10783 [DOI] [PubMed] [Google Scholar]
- Balfour RH, Hansen AM, & Trapp S (2006). Neuronal responses to transient hypoglycaemia in the dorsal vagal complex of the rat brainstem. J Physiol, 570(Pt 3), 469–484. doi: 10.1113/jphysiol.2005.098822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR (2018). Orexin/hypocretin and dysregulated eating: Promotion of foraging behavior.Brain Res. doi: 10.1016/j.brainres.2018.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessing WW, Lappi DA, & Wiley RG (1998). Destruction of locus coeruleus neuronal perikarya after injection of anti-dopamine-B-hydroxylase immunotoxin into the olfactory bulb of the rat. Neurosci Lett, 243(1–3), 85–88. [DOI] [PubMed] [Google Scholar]
- Bochorishvili G, Nguyen T, Coates MB, Viar KE, Stornetta RL, & Guyenet PG (2014). The orexinergic neurons receive synaptic input from C1 cells in rats. J Comp Neurol, 522(17), 3834–3846. doi: 10.1002/cne.23643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohland M, Matveyenko AV, Saberi M, Khan AM, Watts AG, & Donovan CM (2014). Activation of hindbrain neurons is mediated by portal-mesenteric vein glucosensors during slow-onset hypoglycemia. Diabetes, 63(8), 2866–2875. doi: 10.2337/db13-1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briski KP, & Sylvester PW (2001). Hypothalamic orexin-A-immunpositive neurons express Fos in response to central glucopenia. Neuroreport, 12(3), 531–534. [DOI] [PubMed] [Google Scholar]
- Bugarith K, Dinh TT, Li AJ, Speth RC, & Ritter S (2005). Basomedial hypothalamic injections of neuropeptide Y conjugated to saporin selectively disrupt hypothalamic controls of food intake. Endocrinology, 146(3), 1179–1191. doi: 10.1210/en.2004-1166 [DOI] [PubMed] [Google Scholar]
- Cai XJ, Evans ML, Lister CA, Leslie RA, Arch JR, Wilson S, & Williams G (2001). Hypoglycemia activates orexin neurons and selectively increases hypothalamic orexin-B levels: responses inhibited by feeding and possibly mediated by the nucleus of the solitary tract. Diabetes, 50(1), 105–112. [DOI] [PubMed] [Google Scholar]
- Calingasan NY, & Ritter S (1992). Hypothalamic paraventricular nucleus lesions do not abolish glucoprivic or lipoprivic feeding. Brain Res, 595(1), 25–31. [DOI] [PubMed] [Google Scholar]
- Cherian AK, & Briski KP (2012). A2 noradrenergic nerve cell metabolic transducer and nutrient transporter adaptation to hypoglycemia: impact of estrogen. J Neurosci Res, 90(7), 1347–1358. doi: 10.1002/jnr.23032 [DOI] [PubMed] [Google Scholar]
- Cryer PE (1993). Glucose counterregulation: prevention and correction of hypoglycemia in humans. Am J Physiol, 264(2 Pt 1), E149–155. doi: 10.1152/ajpendo.1993.264.2.E149 [DOI] [PubMed] [Google Scholar]
- Cunningham ET Jr., & Sawchenko PE (1988). Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J Comp Neurol, 274(1), 60–76. doi: 10.1002/cne.902740107 [DOI] [PubMed] [Google Scholar]
- de La Serre CB, Kim YJ, Moran TH, & Bi S (2016). Dorsomedial hypothalamic NPY affects cholecystokinin-induced satiety via modulation of brain stem catecholamine neuronal signaling. Am J Physiol Regul Integr Comp Physiol, 311(5), R930–R939. doi: 10.1152/ajpregu.00184.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRocco RJ, & Grill HJ (1979). The forebrain is not essential for sympathoadrenal hyperglycemic response to glucoprivation. Science, 204(4397), 1112–1114. [DOI] [PubMed] [Google Scholar]
- Flynn FW, & Grill HJ (1983). Insulin elicits ingestion in decerebrate rats. Science, 221(4606), 188–190. [DOI] [PubMed] [Google Scholar]
- Flynn FW, & Grill HJ (1985). Fourth ventricular phlorizin dissociates feeding from hyperglycemia in rats. Brain Res, 341(2), 331–336. [DOI] [PubMed] [Google Scholar]
- Goldman CK, Marino L, & Leibowitz SF (1985). Postsynaptic alpha 2-noradrenergic receptors mediate feeding induced by paraventricular nucleus injection of norepinephrine and clonidine. Eur J Pharmacol, 115(1), 11–19. [DOI] [PubMed] [Google Scholar]
- Griffond B, Risold PY, Jacquemard C, Colard C, & Fellmann D (1999). Insulin-induced hypoglycemia increases preprohypocretin (orexin) mRNA in the rat lateral hypothalamic area. Neurosci Lett, 262(2), 77–80. [DOI] [PubMed] [Google Scholar]
- Grossman SP (1960). Eating or drinking elicited by direct adrenergic or cholinergic stimulation of hypothalamus. Science, 132(3422), 301–302. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bochorishvili G, Depuy SD, Burke PG, & Abbott SB (2013). C1 neurons: the body’s EMTs. Am J Physiol Regul Integr Comp Physiol, 305(3), R187–204. doi: 10.1152/ajpregu.00054.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson B, & Ritter S (2004). Hindbrain catecholamine neurons mediate consummatory responses to glucoprivation. Physiol Behav, 82(2–3), 241–250. doi: 10.1016/j.physbeh.2004.03.032 [DOI] [PubMed] [Google Scholar]
- I’Anson H, Starer CA, & Bonnema KR (2003). Glucoprivic regulation of estrous cycles in the rat. Horm Behav, 43(3), 388–393. [DOI] [PubMed] [Google Scholar]
- I’Anson H, Sundling LA, Roland SM, & Ritter S (2003). Immunotoxic destruction of distinct catecholaminergic neuron populations disrupts the reproductive response to glucoprivation in female rats. Endocrinology, 144(10), 4325–4331. doi: 10.1210/en.2003-0258 [DOI] [PubMed] [Google Scholar]
- Jokiaho AJ, Donovan CM, & Watts AG (2014). The rate of fall of blood glucose determines the necessity of forebrain-projecting catecholaminergic neurons for male rat sympathoadrenal responses. Diabetes, 63(8), 2854–2865. doi: 10.2337/db13-1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakall ZM, Nedoboy PE, Farnham MMJ, & Pilowsky PM (2018). Activation of micro-opioid receptors in the rostral ventrolateral medulla blocks the sympathetic counterregulatory response to glucoprivation. Am J Physiol Regul Integr Comp Physiol, 315(6), R1115–R1122. doi: 10.1152/ajpregu.00248.2018 [DOI] [PubMed] [Google Scholar]
- Kaminski KL, & Watts AG (2012). Intact catecholamine inputs to the forebrain are required for appropriate regulation of corticotrophin-releasing hormone and vasopressin gene expression by corticosterone in the rat paraventricular nucleus. J Neuroendocrinol, 24(12), 1517–1526. doi: 10.1111/j.1365-2826.2012.02363.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klip A, & Hawkins M (2005). Desperately seeking sugar: glial cells as hypoglycemia sensors. J Clin Invest, 115(12), 3403–3405. doi: 10.1172/JCI27208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korim WS, Bou Farah L, McMullan S, & Verberne AJ (2014). Orexinergic activation of medullary premotor neurons modulates the adrenal sympathoexcitation to hypothalamic glucoprivation. Diabetes, 63(6), 1895–1906. doi: 10.2337/db13-1073 [DOI] [PubMed] [Google Scholar]
- Korim WS, Llewellyn-Smith IJ, & Verberne AJ (2016). Activation of Medulla-Projecting Perifornical Neurons Modulates the Adrenal Sympathetic Response to Hypoglycemia: Involvement of Orexin Type 2 (OX2-R) Receptors. Endocrinology, 157(2), 810–819. doi: 10.1210/en.2015-1712 [DOI] [PubMed] [Google Scholar]
- Lee SJ, Jokiaho AJ, Sanchez-Watts G, & Watts AG (2018). Catecholaminergic projections into an interconnected forebrain network control the sensitivity of male rats to diet-induced obesity. Am J Physiol Regul Integr Comp Physiol, 314(6), R811–R823. doi: 10.1152/ajpregu.00423.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz SF (1978). Adrenergic stimulation of the paraventricular nucleus and its effects on ingestive behavior as a function of drug dose and time of injection in the light-dark cycle. Brain Res Bull, 3(4), 357–363. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF, Brown O, Tretter JR, & Kirschgessner A (1985). Norepinephrine, clonidine, and tricyclic antidepressants selectively stimulate carbohydrate ingestion through noradrenergic system of the paraventricular nucleus. Pharmacol Biochem Behav, 23(4), 541–550. [DOI] [PubMed] [Google Scholar]
- Levin BE (2001). Glucosensing neurons do more than just sense glucose. Int J Obes Relat Metab Disord, 25 Suppl 5, S68–72. doi: 10.1038/sj.ijo.0801916 [DOI] [PubMed] [Google Scholar]
- Levin BE (2002). Metabolic sensors: viewing glucosensing neurons from a broader perspective. Physiol Behav, 76(3), 397–401. [DOI] [PubMed] [Google Scholar]
- Levin BE, Routh VH, Kang L, Sanders NM, & Dunn-Meynell AA (2004). Neuronal glucosensing: what do we know after 50 years? Diabetes, 53(10), 2521–2528. [DOI] [PubMed] [Google Scholar]
- Li AJ, & Ritter S (2004). Glucoprivation increases expression of neuropeptide Y mRNA in hindbrain neurons that innervate the hypothalamus. Eur J Neurosci, 19(8), 2147–2154. doi: 10.1111/j.1460-9568.2004.03287.x [DOI] [PubMed] [Google Scholar]
- Li AJ, Wang Q, Davis H, Wang R, & Ritter S (2015). Orexin-A enhances feeding in male rats by activating hindbrain catecholamine neurons. Am J Physiol Regul Integr Comp Physiol, 309(4), R358–367. doi: 10.1152/ajpregu.00065.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AJ, Wang Q, Dinh TT, Powers BR, & Ritter S (2014). Stimulation of feeding by three different glucose-sensing mechanisms requires hindbrain catecholamine neurons. Am J Physiol Regul Integr Comp Physiol, 306(4), R257–264. doi: 10.1152/ajpregu.00451.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AJ, Wang Q, Dinh TT, & Ritter S (2009). Simultaneous silencing of Npy and Dbh expression in hindbrain A1/C1 catecholamine cells suppresses glucoprivic feeding. J Neurosci, 29(1), 280–287. doi: 10.1523/JNEUROSCI.4267-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AJ, Wang Q, Dinh TT, Wiater MF, Eskelsen AK, & Ritter S (2013). Hindbrain catecholamine neurons control rapid switching of metabolic substrate use during glucoprivation in male rats. Endocrinology, 154(12), 4570–4579. doi: 10.1210/en.2013-1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AJ, Wang Q, Elsarelli MM, Brown RL, & Ritter S (2015). Hindbrain Catecholamine Neurons Activate Orexin Neurons During Systemic Glucoprivation in Male Rats. Endocrinology, 156(8), 2807–2820. doi: 10.1210/en.2015-1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AJ, Wang Q, & Ritter S (2006). Differential responsiveness of dopamine-beta-hydroxylase gene expression to glucoprivation in different catecholamine cell groups. Endocrinology, 147(7), 3428–3434. doi: 10.1210/en.2006-0235 [DOI] [PubMed] [Google Scholar]
- Li AJ, Wang Q, & Ritter S (2018a). Activation of catecholamine neurons in the ventral medulla reduces CCK-induced hypophagia and c-Fos activation in dorsal medullary catecholamine neurons. Am J Physiol Regul Integr Comp Physiol doi: 10.1152/ajpregu.00107.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AJ, Wang Q, & Ritter S (2018b). Selective Pharmacogenetic Activation of Catecholamine Subgroups in the Ventrolateral Medulla Elicits Key Glucoregulatory Responses. Endocrinology, 159(1), 341–355. doi: 10.1210/en.2017-00630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AJ, Wiater MF, Wang Q, Wank S, & Ritter S (2016). Deletion of GPR40 fatty acid receptor gene in mice blocks mercaptoacetate-induced feeding. Am J Physiol Regul Integr Comp Physiol, 310(10), R968–974. doi: 10.1152/ajpregu.00548.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luquet S, Phillips CT, & Palmiter RD (2007). NPY/AgRP neurons are not essential for feeding responses to glucoprivation. Peptides, 28(2), 214–225. doi: 10.1016/j.peptides.2006.08.036 [DOI] [PubMed] [Google Scholar]
- Madden CJ, Ito S, Rinaman L, Wiley RG, & Sved AF (1999). Lesions of the C1 catecholaminergic neurons of the ventrolateral medulla in rats using anti-DbetaH-saporin. Am J Physiol, 277(4), R1063–1075. doi: 10.1152/ajpregu.1999.277.4.R1063 [DOI] [PubMed] [Google Scholar]
- Marty N, Dallaporta M, Foretz M, Emery M, Tarussio D, Bady I, … Thorens B (2005). Regulation of glucagon secretion by glucose transporter type 2 (glut2) and astrocytedependent glucose sensors. J Clin Invest, 115(12), 3545–3553. doi: 10.1172/JCI26309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty N, Dallaporta M, & Thorens B (2007). Brain glucose sensing, counterregulation, and energy homeostasis. Physiology (Bethesda), 22, 241–251. doi: 10.1152/physiol.00010.2007 [DOI] [PubMed] [Google Scholar]
- McCabe JT, DeBellis M, & Leibowitz SF (1984). Clonidine-induced feeding: analysis of central sites of action and fiber projections mediating this response. Brain Res, 309(1), 85–104. [DOI] [PubMed] [Google Scholar]
- McDougal DH, Hermann GE, & Rogers RC (2013). Astrocytes in the nucleus of the solitary tract are activated by low glucose or glucoprivation: evidence for glial involvement in glucose homeostasis. Front Neurosci, 7, 249. doi: 10.3389/fnins.2013.00249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T, Sakurai T, Nambu T, Yanagisawa M, & Goto K (1999). Neurons containing orexin in the lateral hypothalamic area of the adult rat brain are activated by insulin-induced acute hypoglycemia. Neurosci Lett, 264(1–3), 101–104. [DOI] [PubMed] [Google Scholar]
- Moriyama R, Reyes BA, Tsukamura H, & Maeda K (2003). Glucoprivation-induced Fos expression in the hypothalamus and medulla oblongata in female rats. J Reprod Dev, 49(2), 151–157. [DOI] [PubMed] [Google Scholar]
- Puskas N, Papp RS, Gallatz K, & Palkovits M (2010). Interactions between orexinimmunoreactive fibers and adrenaline or noradrenaline-expressing neurons of the lower brainstem in rats and mice. Peptides, 31(8), 1589–1597. doi: 10.1016/j.peptides.2010.04.020 [DOI] [PubMed] [Google Scholar]
- Rinaman L (2003). Hindbrain noradrenergic lesions attenuate anorexia and alter central cFos expression in rats after gastric viscerosensory stimulation. J Neurosci, 23(31), 10084–10092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L, Hoffman GE, Dohanics J, Le WW, Stricker EM, & Verbalis JG (1995). Cholecystokinin activates catecholaminergic neurons in the caudal medulla that innervate the paraventricular nucleus of the hypothalamus in rats. J Comp Neurol, 360(2), 246–256. doi: 10.1002/cne.903600204 [DOI] [PubMed] [Google Scholar]
- Ritter RC (2004a). Gastrointestinal mechanisms of satiation for food. Physiol Behav, 81(2), 249–273. doi: 10.1016/j.physbeh.2004.02.012 [DOI] [PubMed] [Google Scholar]
- Ritter RC (2004b). Increased food intake and CCK receptor antagonists: beyond abdominal vagal afferents. Am J Physiol Regul Integr Comp Physiol, 286(6), R991–993. doi: 10.1152/ajpregu.00116.2004 [DOI] [PubMed] [Google Scholar]
- Ritter RC, Slusser PG, & Stone S (1981). Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science, 213(4506), 451–452. [DOI] [PubMed] [Google Scholar]
- Ritter S (2017). Monitoring and Maintenance of Brain Glucose Supply: Importance of Hindbrain Catecholamine Neurons in This Multifaceted Task In nd & Harris RBS (Eds.), Appetite and Food Intake: Central Control (pp. 177–204). Boca Raton (FL). [PubMed] [Google Scholar]
- Ritter S, Bugarith K, & Dinh TT (2001). Immunotoxic destruction of distinct catecholamine subgroups produces selective impairment of glucoregulatory responses and neuronal activation. J Comp Neurol, 432(2), 197–216. [DOI] [PubMed] [Google Scholar]
- Ritter S, Dinh TT, & Zhang Y (2000). Localization of hindbrain glucoreceptive sites controlling food intake and blood glucose. Brain Res, 856(1–2), 37–47. [DOI] [PubMed] [Google Scholar]
- Ritter S, Llewellyn-Smith I, & Dinh TT (1998). Subgroups of hindbrain catecholamine neurons are selectively activated by 2-deoxy-D-glucose induced metabolic challenge. Brain Res, 805(1–2), 41–54. [DOI] [PubMed] [Google Scholar]
- Ritter S, Murnane JM, & Ladenheim EE (1982). Glucoprivic feeding is impaired by lateral or fourth ventricular alloxan injection. Am J Physiol, 243(3), R312–317. doi: 10.1152/ajpregu.1982.243.3.R312 [DOI] [PubMed] [Google Scholar]
- Ritter S, & Strang M (1982). Fourth ventricular alloxan injection causes feeding but not hyperglycemia in rats. Brain Res, 249(1), 198–201. [DOI] [PubMed] [Google Scholar]
- Ritter S, Watts AG, Dinh TT, Sanchez-Watts G, & Pedrow C (2003). Immunotoxin lesion of hypothalamically projecting norepinephrine and epinephrine neurons differentially affects circadian and stressor-stimulated corticosterone secretion. Endocrinology, 144(4), 1357–1367. doi: 10.1210/en.2002-221076 [DOI] [PubMed] [Google Scholar]
- Ritter S, Wise D, & Stein L (1975). Neurochemical regulation of feeding in the rat: facilitation by alpha-noradrenergic, but not dopaminergic, receptor stimulants. J Comp Physiol Psychol, 88(2), 778–784. [DOI] [PubMed] [Google Scholar]
- Roberts BL, Zhu M, Zhao H, Dillon C, & Appleyard SM (2017). High glucose increases action potential firing of catecholamine neurons in the nucleus of the solitary tract by increasing spontaneous glutamate inputs. Am J Physiol Regul Integr Comp Physiol, 313(3), R229–R239. doi: 10.1152/ajpregu.00413.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RC, & Hermann GE (2019). Hindbrain astrocytes and glucose counter-regulation. Physiol Behav, 204, 140–150. doi: 10.1016/j.physbeh.2019.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RC, McDougal DH, Ritter S, Qualls-Creekmore E, & Hermann GE (2018). Response of catecholaminergic neurons in the mouse hindbrain to glucoprivic stimuli is astrocyte dependent. Am J Physiol Regul Integr Comp Physiol. doi: 10.1152/ajpregu.00368.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL (2016). DREADDs for Neuroscientists. Neuron, 89(4), 683–694. doi: 10.1016/j.neuron.2016.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routh VH (2010). Glucose sensing neurons in the ventromedial hypothalamus. Sensors (Basel), 10(10), 9002–9025. doi: 10.3390/s101009002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routh VH, Donovan CM, & Ritter S (2012). 2. Hypoglycemia Detection. Transl Endocrinol Metab, 3(4), 47–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW, Grzanna R, Howe PR, Bloom SR, & Polak JM (1985). Colocalization of neuropeptide Y immunoreactivity in brainstem catecholaminergic neurons that project to the paraventricular nucleus of the hypothalamus. J Comp Neurol, 241(2), 138–153. doi: 10.1002/cne.902410203 [DOI] [PubMed] [Google Scholar]
- Sindelar DK, Ste Marie L, Miura GI, Palmiter RD, McMinn JE, Morton GJ, & Schwartz MW (2004). Neuropeptide Y is required for hyperphagic feeding in response to neuroglucopenia. Endocrinology, 145(7), 3363–3368. doi: 10.1210/en.2003-1727 [DOI] [PubMed] [Google Scholar]
- Smith GP, & Gibbs J (1975). Cholecystokinin: a putative satiety signal. Pharmacol Biochem Behav, 3(1 Suppl), 135–138. [PubMed] [Google Scholar]
- Smith GP, & Gibbs J (1992). Role of CCK in satiety and appetite control. Clin Neuropharmacol, 15 Suppl 1 Pt A, 476A. [DOI] [PubMed] [Google Scholar]
- Stanley BG, & Leibowitz SF (1985). Neuropeptide Y injected in the paraventricular hypothalamus: a powerful stimulant of feeding behavior. Proc Natl Acad Sci U S A, 82(11), 3940–3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Berod A, Hartman BK, Helle KB, & Vanorden DE (1981). An immunohistochemical study of the organization of catecholaminergic cells and terminal fields in the paraventricular and supraoptic nuclei of the hypothalamus. J Comp Neurol, 196(2), 271–285. doi: 10.1002/cne.901960207 [DOI] [PubMed] [Google Scholar]
- Wild S, Roglic G, Green A, Sicree R, & King H (2004). Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care, 27(5), 1047–1053. [DOI] [PubMed] [Google Scholar]
- Wiley RG, & Kline IR (2000). Neuronal lesioning with axonally transported toxins. J Neurosci Methods, 103(1), 73–82. [DOI] [PubMed] [Google Scholar]
- Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, Brodsky M, … Deisseroth K (2011). Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron, 72(5), 721–733. doi: 10.1016/j.neuron.2011.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SC, & McKay LD (1978). Intraventricular alloxan eliminates feeding elicited by 2deoxyglucose. Science, 202(4373), 1209–1211. [DOI] [PubMed] [Google Scholar]
- Wrenn CC, Picklo MJ, Lappi DA, Robertson D, & Wiley RG (1996). Central noradrenergic lesioning using anti-DBH-saporin: anatomical findings. Brain Res, 740(1–2), 175–184. [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, … Sakurai T (2003). Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron, 38(5), 701–713. [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Muraki Y, Ichiki K, Tsujino N, Kilduff TS, Goto K, & Sakurai T (2006). Orexin neurons are directly and indirectly regulated by catecholamines in a complex manner. J Neurophysiol, 96(1), 284–298. doi: 10.1152/jn.01361.2005 [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Muraki Y, Tsujino N, Goto K, & Sakurai T (2003). Regulation of orexin neurons by the monoaminergic and cholinergic systems. Biochem Biophys Res Commun, 303(1), 120–129. [DOI] [PubMed] [Google Scholar]