A genetic variant in the endocannabinoid system enhances vulnerability to THC reward in adolescent females.
Abstract
Adolescence represents a developmental period with the highest risk for initiating cannabis use. Little is known about whether genetic variation in the endocannabinoid system alters mesolimbic reward circuitry to produce vulnerability to the rewarding properties of the exogenous cannabinoid Δ9-tetrahydrocannabinol (THC). Using a genetic knock-in mouse model (FAAHC/A) that biologically recapitulates the human polymorphism associated with problematic drug use, we find that in adolescent female mice, but not male mice, this FAAH polymorphism enhances the mesolimbic dopamine circuitry projecting from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) and alters cannabinoid receptor 1 (CB1R) levels at inhibitory and excitatory terminals in the VTA. These developmental changes collectively increase vulnerability of adolescent female FAAHC/A mice to THC preference that persists into adulthood. Together, these findings suggest that this endocannabinoid genetic variant is a contributing factor for increased susceptibility to cannabis dependence in adolescent females.
INTRODUCTION
Adolescence represents a critical neurodevelopmental period characterized by dynamic changes in the structure and function of the mesolimbic dopamine pathway, including increased dopamine availability and increased engagement of downstream striatal pathways during reward processing (1, 2). The endocannabinoid system fine-tunes the mesolimbic dopamine pathway by affecting firing rates of ventral tegmental area (VTA) dopaminergic neurons and dopamine levels in downstream projection regions such as the nucleus accumbens (NAc), in addition to affecting reward-associated behaviors (1–3). The endocannabinoid system reaches peak expression and activity throughout the brain during adolescence (4). This positions the endocannabinoid system as a key modulator of developmental processes during adolescence such as mesolimbic reward circuitry and associated reward behaviors including vulnerability to drug addiction (5).
The levels of anandamide (AEA), a primary endocannabinoid in the brain that directly binds to cannabinoid receptor 1 (CB1R), are tightly regulated by the catabolic enzyme, fatty acid amide hydrolase (FAAH) (6). FAAH is highly expressed in brain regions implicated in reward and addiction and exerts widespread modulatory influences on molecular and behavioral responses to drugs of abuse (1). For example, pharmacological inhibition or genetic ablation of FAAH in rodents increases preference for ethanol and nicotine, self-administration of ethanol, and psychomotor sensitization to cocaine (1, 3, 7). Cellular studies have revealed that FAAH inhibition diminishes nicotine- and cocaine-induced dopamine levels and cocaine-induced NAc activity (1).
In humans, a common single-nucleotide polymorphism (SNP) in the FAAH gene (C385A; rs324420), of which 38% of individuals of European descent are carriers (8), results in destabilization of the FAAH protein and increased AEA levels (9). Human studies demonstrate that FAAH C385A SNP carriers display increased striatal activity and increased impulsivity during a reward behavioral task compared to individuals without the SNP (10), an endophenotype associated with addiction disorders. Many studies have also linked the FAAH C385A SNP to problem drug use (1, 9, 11) and greater bias to appetitive cues after Δ9-tetrahydrocannabinol (THC) administration in humans (12). While this SNP in humans has been associated with increased likelihood to try cannabis (13), the influence on the progression to cannabis dependence has led to inconclusive results (1).
Recently, we developed a knock-in mouse model (FAAHC/A) that biologically recapitulates the FAAH polymorphism and is thus characterized by decreased brain levels of FAAH protein and increased levels of AEA (14). Furthermore, both humans and mice carrying the FAAH C385A SNP display enhanced frontoamygdala and frontolimbic connectivity in addition to changes in fear-related behaviors compared to humans and mice without the SNP (FAAHC/C) (14, 15). In addition, FAAHC/A mice exhibit greater alcohol intake (7); however, the contribution of this FAAH SNP to other types of addiction, such as cannabis dependence, remains unknown.
Cannabis dependence continues to increase in prevalence as a major world health problem and as the most common substance use disorder in the United States (16), with most of the users initiating use before the age of 18 (17). Cannabis use during adolescence is associated with a higher risk for developing substance use disorders in adulthood (5, 18), including cannabis dependence (19). THC, the psychoactive ingredient of cannabis, directly acts on CB1R in the VTA, resulting in an indirect activation of the mesolimbic reward pathway originating in the VTA and projecting to the NAc (20, 21). Chronic exposure to cannabis has a prominent role in modulating brain reward function in response to drugs of abuse by increasing VTA dopamine activity and subsequent dopamine release in the NAc (22), in addition to persistent alterations of endocannabinoid activity (23).
Although females are less likely to use cannabis compared to males, a select population of females are more sensitive to the effects of cannabis and demonstrate a quicker progression to cannabis dependence (24), potentially due to an altered brain reward response (25). Epidemiological studies continue to attempt to evaluate underlying genetic and behavioral factors that may predispose individuals to become dependent on cannabis (11), but the use of animal models provides the opportunity to determine whether specific genetic factors can predict the rewarding effects of THC via direct neurobiological alterations.
In this study, we investigated whether genetic variation in FAAH can alter structure and function of reward pathways in a sex-dependent manner during adolescence. Here, we describe the anatomical and functional characterization of the mesolimbic pathway in adolescent female and male mice expressing the human FAAH C385A SNP. In addition, we examined the effect of altered mesolimbic activity on THC preference. We determined that this circuit alteration is a contributing factor to the vulnerability of adolescent female mice for preference of THC. In summary, we find a sex- and age-dependent contribution of this FAAH polymorphism to an enhanced adolescent THC preference that is persistently expressed into adulthood.
RESULTS
FAAH SNP selectively increases VTA-NAc connectivity in adolescent female mice
As the endocannabinoid system is an important contributor to the development and regulation of the mesolimbic dopamine circuits (1, 26), we aimed to determine whether the FAAH SNP results in altered structural and functional connectivity within the mesolimbic dopamine reward pathway in male and female mice during adolescence. To this end, we first performed connectivity analyses by injecting an anterograde tracer, phytohemagglutinin-L (PHA-L), into the VTA of male and female mice that are heterozygous for the FAAH SNP (FAAHC/A) and that do not express the FAAH SNP (FAAHC/C) at age postnatal day (P) 25 (Fig. 1A). At P35, the NAc and another target of the VTA, the medial prefrontal cortex (mPFC), which mainly includes the prelimbic region of the prefrontal cortex (PrL) and infralimbic prefrontal cortex (IL), were imaged and analyzed for tracer-labeled fiber density using a stereological method. These tract-tracing experiments revealed increased fiber density in the NAc of adolescent female FAAHC/A mice compared to male FAAHC/C mice, male FAAHC/A mice, and female FAAHC/C mice (Fig. 1, B and C). This genotypic difference was selective to VTA-NAc projections, as examination of fiber density in the mPFC revealed no difference between male FAAHC/C, male FAAHC/A, female FAAHC/C, and female FAAHC/A mice (Fig. 1, D and E), providing structural evidence that the FAAH SNP selectively results in the enhancement of VTA projections to the NAc in female adolescent FAAHC/A mice.
Fig. 1. Adolescent female mice, but not adolescent male mice, carrying the FAAH SNP demonstrate increased VTA-NAc connectivity and TH-labeled cells in the VTA.
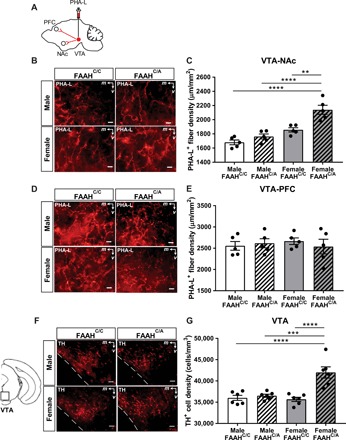
(A) Schematic of PHA-L injected into the VTA of male and female FAAHC/C and FAAHC/A mice at P25. NAc and mPFC were analyzed for fiber density at P35. (B) Representative images of PHA-L projection labeling in the NAc of adolescent male and female FAAHC/C and FAAHC/A mice injected with the anterograde tracer, PHA-L, in the VTA. Scale bars, 10 μm. (C) Adolescent female FAAHC/A mice show increased density of PHA-L projection labeling in the NAc compared to adolescent female FAAHC/C mice, male FAAHC/C mice, and male FAAHC/A mice [one-way analysis of variance (ANOVA): F3,16 = 22.02, P < 0.0001, n = 5 per group, **P < 0.01, ****P < 0.0001 post hoc Bonferroni tests]. (D) Representative images of PHA-L projection labeling in the mPFC of adolescent male and female FAAHC/C mice and FAAHC/A mice injected with the anterograde tracer, PHA-L, in the VTA. Scale bars, 10 μm. (E) Adolescent male and female FAAHC/C and FAAHC/A mice show similar density of PHA-L projection labeling in the mPFC (one-way ANOVA: F3,16 = 0.2351, P = 0.8706, n = 5 per group). (F) Representative images of the VTA showing cells positive for TH immunoreactivity. (G) Adolescent female FAAHC/A mice show increased density of TH-labeled neurons compared to male FAAHC/C mice, male FAAHC/A mice, and female FAAHC/C mice (one-way ANOVA: F3,20 = 14.51, P < 0.0001, n = 6 per group, ***P < 0.001 post hoc test, ****P < 0.0001 Bonferroni test).
To determine whether the increase in VTA-NAc projections is accompanied by an increase in dopamine neurons, brain sections from the VTA of a separate cohort of male and adolescent female mice with and without the FAAH SNP were examined for immunolabeling of tyrosine hydroxylase (TH), the rate-limiting enzyme for dopamine synthesis (Fig. 1F). Immunohistochemistry revealed that female FAAHC/A mice showed higher density of TH-containing cells within the VTA compared to male FAAHC/C, male FAAHC/A, and female FAAHC/C mice (Fig. 1, F and G). Together, the observed structural and neurochemical findings indicate that adolescent female mice carrying the FAAH SNP display hyperconnectivity between the VTA and NAc and increased TH+ cells in the VTA as compared to male FAAHC/C, male FAAHC/A, and female FAAHC/C mice, highlighting the impact of the FAAH SNP on the mesolimbic pathway in adolescent female mice.
CB1R labeling is elevated on inhibitory-type and blunted on excitatory-type axon terminals in the VTA of adolescent female FAAHC/A mice
We next wanted to determine an underlying mechanism that may be resulting in hyperconnectivity of the mesolimbic pathway observed in adolescent female FAAHC/A mice compared to female FAAHC/C mice. Given that the FAAH SNP has been shown in humans and mice to increase AEA levels (9, 14), we next sought to examine its effect on CB1Rs, the primary target of AEA (21), within adolescent female FAAHC/A and FAAHC/C mice. Endocannabinoids have been shown to provide control over the excitability of VTA dopamine cells by acting on inhibitory CB1Rs located on both GABAergic and glutamatergic presynaptic terminal inputs that synapse onto dopamine cells in the VTA (1, 20–22).
Therefore, we investigated whether the FAAH SNP induces any changes in CB1R protein levels located on inhibitory- or excitatory-type terminals in the VTA of adolescent female mice. Immunoelectron microscopy was used to detect the presence of membrane-bound or cytoplasmic CB1Rs on terminals forming symmetric (inhibitory-type) or asymmetric (excitatory-type) synapses, which are characteristic of GABAergic and glutamatergic neurons, respectively, using known parameters (27). The paranigral and parabrachial subregions of the VTA were used for identification of these synapses because of their distinct projections to the NAc core/medial shell and NAc lateral shell, respectively (28).
In the paranigral subregion, adolescent female FAAHC/A mice demonstrated a higher percentage of CB1R-labeled terminals forming symmetric synapses than that seen in female FAAHC/C mice (Fig. 2A). In parallel, female FAAHC/A mice demonstrated a lower percentage of CB1R-labeled terminals forming asymmetric synapses than female FAAHC/C mice (Fig. 2B). This bias in labeling toward symmetric synapses as a result of the SNP was also reflected in a higher density ratio of membrane-bound symmetric versus asymmetric labeling in the female FAAHC/A mice compared to female FAAHC/C mice (Fig. 2C).
Fig. 2. Adolescent female mice carrying the FAAH SNP demonstrate increased CB1R-labeled terminals forming symmetric synapses and decreased CB1R-labeled terminals forming asymmetric synapses in the paranigral subregion of the VTA.
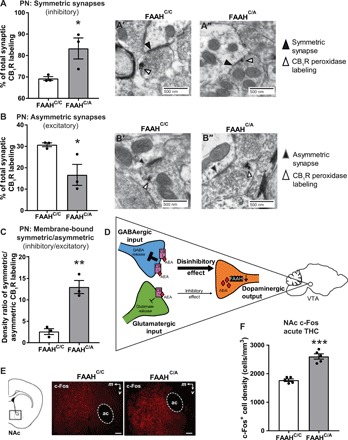
(A) Adolescent female FAAHC/A mice (A″) have more CB1R-labeled terminals forming symmetric synapses compared to adolescent female FAAHC/C mice (A′) (unpaired t test, t4 = 2.819, P = 0.0479, N = 3 animals, n = 50 to 87 labels characterized per animal; FAAHC/A: N = 3 animals, n = 39 to 76 labels characterized per animal). (B) Adolescent female FAAHC/A mice (B″) have less CB1R-labeled terminals forming asymmetric synapses compared to adolescent female FAAHC/C mice (B′) (unpaired t test, t4 = 2.819, P = 0.0479; FAAHC/C: N = 3 animals, n = 50 to 87 labels characterized per animal; FAAHC/A: N = 3 animals, n = 39 to 76 labels characterized per animal). (C) Adolescent female FAAHC/A mice have a higher density ratio of membrane-bound CB1R on terminals forming symmetric synapses versus asymmetric synapses compared to adolescent female FAAHC/C mice (unpaired t test, t4 = 6.218, P = 0.0034; FAAHC/C: N = 3 animals, n = 50 to 87 labels characterized per animal; FAAHC/A: N = 3 animals, n = 39 to 76 labels characterized per animal). (D) Schematic of cell-specific CB1R action in the VTA. (E) Representative images of c-Fos immunoreactivity in the NAc of mice euthanized 90 min following acute THC. Scale bars, 120 μm. (F) Adolescent female FAAHC/A mice show increased c-Fos labeling in the NAc compared to adolescent female FAAHC/C mice (unpaired t test, t8 = 7.985, ***P < 0.001, n = 5 per group).
In the parabrachial subregion, female FAAHC/A mice demonstrated no genotypic difference in percent of CB1R-labeled terminals forming either symmetric synapses (fig. S1A) or asymmetric synapses (fig. S1B) compared to FAAHC/C mice. In addition, there was no genotypic difference in the density ratio of membrane-bound CB1R labeling forming symmetric versus asymmetric synapses (fig. S1C).
These ultrastructural analyses of CB1R indicated that adolescent female FAAHC/A mice have higher CB1R labeling in GABAergic terminals and lower CB1R labeling in glutamatergic terminals than female FAAHC/C littermates. Because of the known inhibitory actions of CB1R, this observation suggests that activation of CB1R would result in greater inhibition of GABAergic synapses and reduced inhibition of glutamatergic synapses with a net disinhibitory effect on dopaminergic cells of the VTA (Fig. 2D).
To test this possibility, we examined the levels of c-Fos protein in the NAc as a proxy for neural activity, following acute administration of THC [5 mg/kg, intraperitoneally (ip)] that targets VTA CB1Rs (20). THC significantly increased the density of cells immunoreactive for c-Fos in the NAc of adolescent female FAAHC/A mice compared to female FAAHC/C mice (Fig. 2, E and F), supporting enhanced activity between the VTA and NAc in adolescent female FAAHC/A mice. These findings are consistent with previous data showing increased ventral striatum activity of human FAAH C385A carriers during a reward task (10).
To provide additional evidence for the cell type–specific alterations in CB1R labeling, we used THC-induced hypolocomotion (Fig. 3A), which has been shown to be driven by the inhibitory effect of VTA CB1Rs (29). As expected, acute THC (5 mg/kg, ip) administration in adolescent female FAAHC/C mice resulted in hypolocomotion compared to vehicle-treated mice (Fig. 3, B and C) that was absent in FAAHC/A mice (Fig. 3, D and E). This lack of hypolocomotion provides a functional readout to support our findings that adolescent female FAAHC/A mice have lower levels of CB1R in glutamatergic terminals and higher levels of CB1R in GABAergic terminals in the VTA, resulting in a net disinhibitory effect. Together, these data support the previous structural and neurochemical data that demonstrated that adolescent female mice with the FAAH SNP may have an enhanced VTA-NAc pathway, both basally and following activation by THC.
Fig. 3. Female mice with the FAAH SNP do not display THC-induced hypolocomotion.
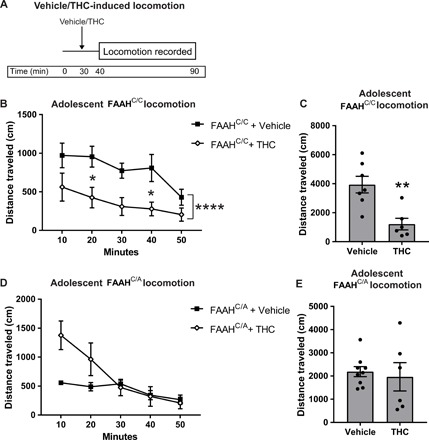
(A) Experimental protocol of locomotion testing in FAAHC/C and FAAHC/A mice that consists of 30 min of habituation in the locomotor activity box before an acute systemic vehicle or THC injection. Locomotion data are recorded 10 min after injection for 50 min. (B and C) Adolescent FAAHC/C mice show a decrease in binned locomotion (B) (two-way ANOVA, main effect of time: F4,55 = 3.323, P = 0.0165; main effect of treatment: F1,55 = 26.15, P < 0.0001, *P < 0.05, Bonferroni post hoc test) and cumulative locomotion (C) (unpaired t test, t11 = 3.761, **P = 0.0031, n = 7 vehicle and n = 6 THC) following a systemic THC injection compared to mice receiving a systemic vehicle injection. (D and E) Adolescent FAAHC/A mice show a nonsignificant increase in locomotion in first 10 min of binned data (D) (two-way ANOVA, significant interaction, time × treatment: F4,65 = 4.904, P = 0.0016; main effect of time: F4,65 = 10.82, P < 0.0001; main effect of treatment: F1,65 = 8.138, P = 0.0058) but no change in cumulative locomotion (E) (unpaired t test, t13 = 0.4037, P = 0.6930, n = 9 vehicle and n = 6 THC) following a systemic THC injection compared to mice receiving a systemic vehicle injection.
Adolescent female FAAHC/A mice demonstrate vulnerability to THC preference
Cannabis-dependent humans show a hyperconnectivity between the VTA and NAc, particularly in those who begin using in adolescence (30). In addition, human carriers of the FAAH SNP are more likely to be problem drug users (9), although previous studies have not specifically assessed cannabis dependence. As adolescent female FAAHC/A mice demonstrate hyperconnectivity between the VTA and NAc, we sought to determine whether the FAAH SNP affects preference for THC in adolescent female mice.
Adolescent female mice with and without the FAAH SNP were tested in THC conditioned place preference (CPP), a behavioral measure to assess rewarding or aversive drug effects, using a modified version of a previously published protocol (31), as outlined in Fig. 4A. At a dose of 1 mg/kg, ip, adolescent female FAAHC/C mice showed decreased preference for the THC-paired chamber, indicating conditioned place aversion (CPA) (Fig. 4B). In contrast, adolescent female FAAHC/A mice demonstrated increased preference for the THC-paired chamber, indicating CPP (Fig. 4B). This genotypic difference was additionally reflected by comparing the CPP difference score (test day preference score − baseline preference score), which showed that FAAHC/A mice had a significantly higher difference score than FAAHC/C mice (Fig. 4C), demonstrating that the FAAH SNP increases preference for THC. Neither genotype demonstrated CPA or CPP at doses of 0.1 mg/kg THC (fig. S2A), 0.5 mg/kg THC (fig. S2B), and 3 mg/kg THC (fig. S2C). On the basis of these findings, we used a dosing regimen of 1 mg/kg for the subsequent THC CPP studies.
Fig. 4. Adolescent female mice carrying the FAAH SNP display a preference for THC.
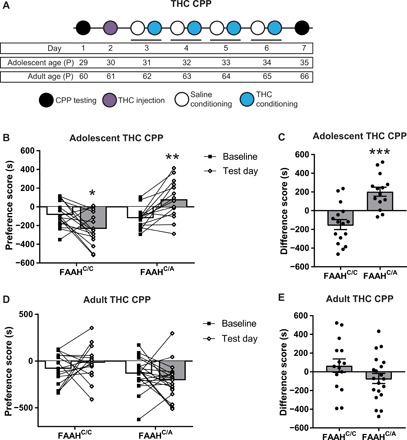
(A) Experimental and developmental timeline for THC CPP protocol in adolescent and adult mice. (B) Adolescent female FAAHC/C mice show significantly lower preference for the THC-paired chamber on the test day compared to the baseline test (paired t test, t15 = 2.782, *P < 0.05, n = 16), while adolescent female FAAHC/A mice show significantly higher preference for the THC-paired chamber on the test day compared to the baseline test (paired t test, t13 = 4.183, **P < 0.01, n = 14). (C) Adolescent female FAAHC/A mice show a significantly higher difference score than adolescent female FAAHC/C mice (unpaired t test, t28 = 4.82, ***P < 0.001, FAAHC/C: n = 16; FAAHC/A: n = 14). (D) Adult female FAAHC/C mice and adult female FAAHC/A mice show no change in preference for the THC-paired chamber on the test day compared to the baseline test (FAAHC/C: paired t test, t14 = 0.9102, P = 0.3781, n = 15; FAAHC/A: paired t test, t19 = 1.316, P = 0.2037, n = 20). (E) Adult female FAAHC/A mice show similar difference scores compared to adult female FAAHC/C mice (unpaired t test, t33 = 1.55, P = 0.1306, FAAHC/C: n = 15; FAAHC/A: n = 20).
To determine whether the FAAH SNP selectively increased vulnerability to THC or whether this also affected preference for the psychostimulant cocaine, we trained and tested adolescent female FAAHC/C and FAAHC/A mice in cocaine CPP. Both female FAAHC/C and FAAHC/A mice demonstrated preference for the cocaine-paired chamber (fig. S3A) with similar difference scores (fig. S3B), indicating that the FAAH SNP did not affect cocaine preference in female adolescent mice.
To directly test whether FAAH activity during CPP training drives THC preference as seen in adolescent female FAAHC/A mice, we used a pharmacological approach to inhibit FAAH before THC exposure. Adolescent female C57BL/6J wild-type (WT) mice were tested in THC CPP, as outlined in fig. S4A. During THC conditioning, mice received an injection of either the FAAH inhibitor (PF-3845) or vehicle 2 hours before each injection of THC. PF-3845 had no effect on THC preference. Neither vehicle-treated nor PF-3845–treated WT mice demonstrated a preference for THC (fig. S4B), indicating that the THC preference seen in adolescent female FAAHC/A mice may be due to preestablished developmental neurobiological changes as a consequence of the FAAH SNP.
Next, to determine whether the vulnerability to THC preference in female FAAHC/A mice was specific to THC preference during adolescence, we tested adult female mice in THC CPP using the protocol outlined in Fig. 4A. Adult female FAAHC/C and FAAHC/A mice were trained and tested in THC CPP. Neither genotype demonstrated a significant increase or decrease in THC preference, indicating a lack of CPP or CPA, respectively (Fig. 4D). In addition, difference scores were similar between adult female FAAHC/C and FAAHC/A mice (Fig. 4E). Thus, preference for THC in female mice carrying the FAAH SNP is only evident when trained during adolescence, supporting adolescence as a sensitive period for the rewarding effects of THC (2, 5, 18, 23).
To determine whether the FAAH SNP affected preference for the psychostimulant cocaine, we trained and tested adult female FAAHC/C and FAAHC/A mice in cocaine CPP. As opposed to lack of THC preference, both female FAAHC/C and FAAHC/A mice demonstrated preference for the cocaine-paired chamber (fig. S3C) with similar difference scores (fig. S3D), indicating that the FAAH SNP did not affect cocaine preference in female adult mice.
As exposure to drugs of abuse during adolescence can result in persistent substance use behavior in adulthood (18), we investigated whether THC CPP training during adolescence affected THC preference later in adulthood. Adolescent female FAAHC/C and FAAHC/A mice that were trained in THC CPP were tested in adulthood as outlined (Fig. 5A). Adolescent-trained female FAAHC/A mice tested during adulthood continued to demonstrate increased preference for the THC-paired chamber and thus demonstrated preference for THC that persisted into adulthood (Fig. 5B). In contrast, adolescent-trained female FAAHC/C mice tested in THC CPP during adulthood demonstrated no change in preference for THC (Fig. 5B), indicating that THC CPP training during adolescence had no impact on THC preference in FAAHC/C mice. When comparing the CPP difference score between genotypes, we also found that female FAAHC/A mice had a higher difference score that was trending in significance than female FAAHC/C mice (Fig. 5C), demonstrating that female FAAHC/A mice continue to have a preference for THC compared to female FAAHC/C mice when trained in adolescence and tested later in adulthood.
Fig. 5. Preference for THC as a result of the FAAH SNP persists into adulthood when trained in adolescence.
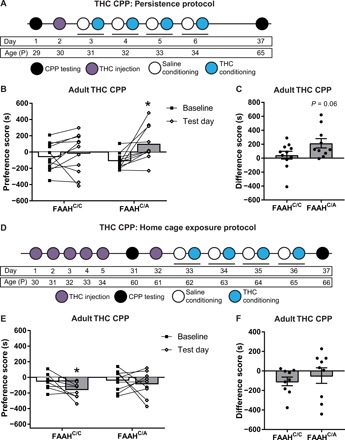
(A) Experimental timeline for persistence of THC CPP protocol. Mice were trained in THC CPP during adolescence and tested in adulthood. (B) Female FAAHC/A mice, but not female FAAHC/C mice, that had been trained in THC CPP during adolescence and then tested in THC CPP during adulthood continue to show a preference for THC, as shown by a significant increase in preference score on test day compared to baseline day (FAAHC/C mice: paired t test, t10 = 0.76, P = 0.4648, n = 11; FAAHC/A mice: paired t test, t9 = 3.164, *P < 0.05, n = 10). (C) Female FAAHC/A mice showed a trending increase in difference score compared to female FAAHC/C mice when trained in THC CPP during adolescence and retested in adulthood (unpaired t test, t19 = 1.958, P = 0.0650, n = 11 FAAHC/C and n = 11 FAAHC/A). (D) Experimental timeline for home cage exposure of THC. Mice were treated during adolescence and trained and tested in THC CPP later in adulthood. (E) Female FAAHC/C mice, when exposed to THC in their home cage during adolescence, develop an aversion for THC in adulthood (paired t test, t8 = 2.438, P = 0.0407, n = 9). In contrast, female FAAHC/A mice do not show a preference for THC as demonstrated by a lack of change in preference scores on adult test day compared to baseline day (paired t test, t8 = 0.5965, P = 0.5673, n = 9). (F) Female FAAHC/C and FAAHC/A mice show similar difference scores when exposed to home cage THC during adolescence and trained and tested in THC CPP during adulthood (unpaired t test, t16 = 0.6575, P = 0.5202, n = 9 FAAHC/C and n = 9 FAAHC/A).
Next, to test whether THC exposure alone in the absence of context-specific THC-associated learning is sufficient to induce persistent vulnerability to THC preference, adolescent female FAAHC/C and FAAHC/A mice received home cage THC injections at the same age and with the same dosage as used for the THC CPP protocol (1 mg/kg, ip), as outlined in Fig. 5D, followed by THC CPP training and testing during adulthood. Female FAAHC/C mice that received home cage THC exposure during adolescence showed an aversion to THC during adulthood, as demonstrated by a significantly lower preference for THC, while FAAHC/A mice showed no preference for THC in adulthood (Fig. 5E). Comparing difference scores of female FAAHC/A mice and FAAHC/C mice revealed no significant difference between genotypes (Fig. 5F). Home cage THC exposure in adult FAAHC/C and FAAHC/A mice using the protocol outlined in fig. S5A did not produce THC preference or aversion when tested in adulthood 30 days later (fig. S5B). These findings suggest that learning of context-specific drug-reward associations during adolescence contributes to increased vulnerability to the persistent preference for THC into adulthood.
VTA-NAc pathway activity drives persistent expression of THC preference in female FAAHC/A mice
Given the findings that female FAAHC/A mice demonstrate persistent preference for THC if trained in THC CPP during adolescence, we sought to determine whether activity of the VTA-NAc pathway is required for persistence of THC preference. We used a chemogenetic approach to inhibit VTA-NAc projection activity in combination with the adolescent persistence protocol wherein the VTA-NAc pathway was inhibited immediately before the adult THC CPP test (Fig. 6A). First, adolescent female FAAHC/A mice were trained in THC CPP. Following THC CPP training, hM4Di inhibitory DREADD (Designer Receptor Exclusively Activated by Designer Drug) was selectively expressed in VTA neurons projecting to the NAc by bilaterally injecting a Cre-dependent virus expressing hM4Di (AAV2-hSyn-DIO-hM4D-Gi-mCherry) into the VTA and a retrograde virus expressing Cre recombinase in the NAc (CAV2-Cre) (Fig. 6B). Control mice either underwent sham surgery without viral infusions or did not receive surgery. Once mice reached adulthood, 45 min before THC CPP test, hM4Di DREADD mice were injected with clozapine N-oxide (CNO) (3 mg/kg, ip) to activate hM4Di DREADD and selectively inhibit VTA-NAc pathway. Control mice were also injected with CNO to control for off-target behavioral effects of CNO administration. Control groups showed no statistical differences in behavior [difference scores, sham surgery: 132.7 ± 73.32 (n = 6) versus no surgery: 208.4 ± 37.78 (n = 3), unpaired t test, t7 = 0.687, P = 0.5141] and were thus collapsed into one group. Control female FAAHC/A mice continued to show a preference for THC in adulthood (Fig. 6C), as previously observed in female FAAHC/A mice (Fig. 5B). In contrast, chemogenetic inhibition of the VTA-NAc pathway in female FAAHC/A mice resulted in an attenuation of THC preference (Fig. 6C). This differential behavioral response was additionally reflected by comparing the CPP difference score between treatment groups, which showed that hM4Di DREADD female FAAHC/A mice had a significantly lower difference score than CNO control female FAAHC/A mice (Fig. 6D), demonstrating that inhibition of the VTA-NAc projection is sufficient to dampen the preference for THC in female FAAHC/A mice. These data indicate that the persistent expression of THC preference observed in female FAAHC/A mice is driven by enhanced function of the VTA to the NAc pathway.
Fig. 6. Activity of the VTA-NAc pathway is required for persistence of THC CPP in female mice carrying the FAAH SNP.
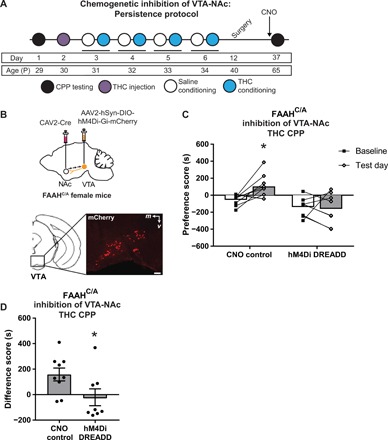
(A) Experimental timeline for surgery, chemogenetic manipulation, and THC CPP. FAAHC/A mice underwent THC CPP training during adolescence, followed by injection of mCherry-tagged Cre-dependent AAV expressing the inhibitory DREADD hM4Di (AAV2-hSyn-DIO-hM4Di-Gi-mCherry) into bilateral VTA and a retrograde CAV2 expressing Cre recombinase into bilateral NAc. Control mice either underwent sham surgery without viral infusions or did not receive surgery. All mice were tested in THC CPP in adulthood following a CNO injection. (B) Representative image of injection site in VTA with mCherry-tagged virus-labeled cells. (C) Control FAAHC/A mice that received CNO demonstrated increased preference for the THC-paired side, as demonstrated by a significantly increased preference score on adult test day compared to baseline day (paired t test, t8 = 3.147, *P = 0.0137, n = 9). FAAHC/A mice injected with hM4Di DREADD demonstrated no THC preference, as shown by a similar preference score on adult test day compared to baseline (paired t test, t7 = 0.3208, P = 0.7577, n = 8). (D) FAAHC/A mice injected with hM4Di DREADD that received CNO demonstrated a significantly lower difference score than control FAAHC/A mice that received CNO (unpaired t test, t15 = 2.189, *P < 0.05, n = 9 CNO control and n = 8 hM4Di DREADD).
DISCUSSION
The underlying mechanisms that dictate why only a fraction of those individuals experimenting with cannabis become dependent remain unknown. Substantial evidence implicates genetic factors mediating the risk for drug dependence, including cannabis use disorder, in susceptible individuals (1, 11, 13, 32). In addition, adolescence, particularly in female mice, represents a highly vulnerable developmental period in which cannabis use predicts a higher risk of dependence compared to adulthood (24, 25). Our findings in this study provide the first evidence that the FAAH C385A polymorphism is a contributing factor toward a preference for THC in adolescent female mice that persists into adulthood if the individual receives THC CPP training during adolescence. We find that compared to adolescent female FAAHC/C mice that find THC aversive, FAAHC/A mice demonstrate a preference for THC, and if exposed to THC during adolescence, this preference for THC persists into adulthood. Our mouse data are in line with the human studies demonstrating the influence of this SNP on increased impulsivity during a reward behavioral task (10), greater subjective response to the acute effects of cannabis (33), and increased problem drug use (1, 9, 11), although not segregated by gender. In addition, consistent with greater response to substances of abuse during adolescence (2), adolescent, but not adult, female FAAHC/A mice demonstrated a preference for THC. Our observations in FAAHC/A female mice are in line with the clinical observation that a select population of females demonstrate a quick progression to cannabis dependence and are very sensitive to the acute effects of cannabis (24). These findings may extend to other drugs of abuse as seen for alcohol, wherein FAAHC/A mice demonstrate higher alcohol binge drinking (7).
Genetic variants can play a key role in sculpting the brain during development. In particular, adolescence represents a period of high activity in the endocannabinoid system, reflected in FAAH activity, AEA levels, and CB1R expression (4). As a result, brain development during this period may be particularly sensitive to the FAAH C385A SNP and the resulting increase in AEA levels during adolescence. It is important to note that other FAAH substrates such as oleamide, which modulates the serotonin system in a CB1R-independent fashion, may also be increased in these mice.
Previous studies using this mouse line have identified hyperconnectivity between frontolimbic regions during the same developmental period. Adolescent-specific behavioral adaptations observed in the present study and in a previous study suggest that the developing central nervous system may be especially sensitive to differential expression of FAAH and thus heightened AEA tone (15). Consistent with female-specific effects of the FAAH SNP on mesolimbic projections and previously reported changes in endocannabinoid activity during adolescence, the FAAH SNP may have a significant impact on the development of the mesolimbic dopamine pathway that drives reward behavior to drugs such as THC (21). Our observation of hyperconnectivity of the mesolimbic pathway in adolescent female FAAHC/A mice is also in line with the observation that chronic THC during adolescence causes a hyperdopaminergic state in the VTA (22). In addition, as THC preference was not altered following administration of a FAAH inhibitor in mice lacking the FAAHC/A SNP, the FAAH SNP is likely inducing developmental changes in circuit function before THC CPP training.
Altered adolescent brain development in response to the FAAH C385A SNP may be more impactful in females because of the earlier rate of maturation of this system compared to males (4). In both sexes, peak expression of the endocannabinoid system occurs just before the onset of puberty, which is P35 in female rodents and P40 in male rodents (4). Changes in gonadal hormone functioning associated with puberty influence endocannabinoid signaling through a feedback loop involving limbic regions (4). As a result, enhanced endocannabinoid signaling during adolescence may interact with gonadal hormone fluctuations to produce changes in limbic activity during this developmental stage. Future studies will examine the interaction between gonadal hormones and the FAAH C385A SNP on sex differences in THC-related behaviors.
The further enhancement of the endocannabinoid system during adolescence as a result of the FAAH C385A SNP may affect the mesolimbic pathway similar to what has been observed with chronic THC exposure during adolescence, leading to persistent changes in levels and activity of CB1Rs in the VTA of adolescent females, but not males (24), and a persistent imbalance of the glutamate/GABA systems (34). Supporting these observations, we find that the FAAH SNP in adolescent female mice affects CB1R protein levels with higher levels at GABAergic terminals and lower levels at glutamatergic terminals in the VTA compared to FAAHC/C mice that could affect the development of the VTA-NAc pathway. Although the tools do not currently exist to selectively manipulate CB1Rs in a terminal-specific manner in the VTA, future studies should investigate whether these VTA cell type–specific changes in CB1R levels are sufficient to drive acute responses to THC- and reward-related behaviors, as well as the higher THC-induced c-Fos levels observed in the NAc of FAAHC/A mice.
Contexts associated with initiation of drug use can precipitate drug craving and contribute significantly to drug dependence (35). For example, social contexts, such as the presence of peers, can considerably influence the addictive process particularly in adolescence and predict the initiation and maintenance of drug use (36). Previous research in nonhuman primates and rodents also demonstrates that pairing of contextual cues contributes to THC-associated reward behavior by CB1R-dependent dopamine activity (22, 35). In the current study, we find that persistent THC preference in female FAAHC/A mice is dependent on context-drug association during adolescence, as mice exposed to THC in the home cage during adolescence do not exhibit persistent THC preference. Mice harboring the FAAH C385A SNP may be particularly influenced by context-paired THC behavior because CB1R expression in female FAAH C385A mice biases toward enhanced dopamine response.
In summary, we have identified a genetic variant in the endocannabinoid system that leads to enhanced risk for persistent THC reward in a developmental and sex-specific manner. This study provides a further understanding on how genetic variants can affect adolescent brain circuits and influence drug dependence. In addition, this study highlights how phenotypic expression of a genetic polymorphism varies as a function of developmental changes in gene expression and neural circuit maturation, leading to increased vulnerability to cannabis dependence. In addition, the present findings can directly inform current human clinical trials with FAAH inhibitors, which are being explored for the treatment of cannabis dependence and other neuropsychiatric disorders, with regard to sex-dependent and developmental effects.
MATERIALS AND METHODS
Animals
Mice heterozygous for the FAAH C385A mutation (FAAHC/A) were generated as previously described. Male and female mice were mated to produce mice heterozygous for the FAAH C385A mutation (FAAHC/A) and control mice without the FAAH C385A mutation (FAAHC/C) (14). Adolescent male and female C57BL/6 mice (Charles River) were used for select experiments as outlined in Results. The Weill Cornell Medicine Institutional Animal Care and Use Committee approved all procedures. Experiments were compliant with the 2011 eighth edition of the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Anterograde tracer injections and analysis
Brain region–specific tracer injections were achieved using stereotactic surgical procedures, as previously described (15). At age P25, mice were microinjected with PHA-L (20 nl per hemisphere; AS-2300, Vector Laboratories) into the VTA using coordinates adopted from the Mouse Brain Atlas (37): antero-posterior (AP) = −3.3 mm; dorso-ventral (DV) = −4.5 mm; medio-lateral (ML) = ±0.3 mm. Following 10-day survival after PHA-L injections, at age P35, animals were deeply anesthetized and perfused. Tissue was processed and stained using immunofluorescence. NAc and mPFC were imaged and analyzed for tracer-labeled fiber density using a stereological method described below and elsewhere (15).
TH immunohistochemistry
All experiments were conducted at P35, as this equated to the same age as “test day” in the CPP behavioral protocol for adolescent cohorts. Euthanasia, perfusion, and immunohistochemistry were performed in a serial manner, as described (15). Briefly, brain sections were treated using the Mouse on Mouse Kit (1:100; Vector Laboratories) and incubated in mouse anti-TH primary antibody (1:1000; Sigma-Aldrich) for 24 hours at 4°C followed by donkey anti-mouse secondary antibody labeled by Alexa Fluor 555 (1:500; Thermo Fisher). Sections were then counterstained by green Nissl for architecture and border identification.
Stereological estimation of cell and fiber density
Stereological estimation of cell and fiber density was performed using Stereo Investigator 9.0 software (MicroBrightfield, USA) (14). After systematic random sampling, sampled serial sections (every third section—120 μm) that include interested brain regions were contoured. Total area of brain regions was estimated by drawn contours.
Detection of fiber density was performed using perimetrics method. Briefly, sections were traced under a 4× lens, and then perimetrics probe analysis was performed under a 40× lens. Counting frame was set to 25 μm by 25 μm, and radius of the Merz coherent test system was set to 5 μm. Total length of all sampling sites was automatically calculated. For each animal, fiber density was obtained from the sum of the lengths divided by the sum of area for all included sections.
Estimation of cell density of TH-positive cells in VTA was performed using a fractionator, with a counting frame size of 25 μm by 25 μm by 40 μm and a sampling grid size of 100 μm by 100 μm. Individual cell density was calculated for each mouse by dividing the total sampled cell numbers by the total volume of the region.
Immunoelectron microscopy
Immunoelectron microscopy studies were performed in naïve FAAHC/C and FAAHC/A mice at age P35 to examine the subcellular distribution of CB1R immunoreactivity within terminals of the VTA. Mice were anesthetized with sodium pentobarbital (150 mg/kg, ip) and transcardially perfused with normal saline containing 2% heparin, followed by 30 ml of 3.75% acrolein and 2% paraformaldehyde in phosphate buffer (38). Brains were processed for immunoelectron microscopy using previously described procedures (38). To ensure identical labeling conditions between groups, the sections were coded with hole punches and groups were pooled into single containers. Brain sections were incubated in rabbit anti-CB1R (1:800, L15) antisera (39), washed in tris-saline, incubated for 30 min in a donkey anti-rabbit secondary antiserum (1:400; Vector Laboratories), and further processed using the peroxidase labeling procedure as described. Ultrathin sections (70 nm) through the VTA were cut using a diamond knife (Electron Microscopy Sciences) and counterstained with UranyLess (Electron Microscopy Sciences) and lead citrate. Sections were examined on a CM10 electron microscope (FEI).
An investigator blinded to the experimental conditions collected and analyzed all electron microscopic data. Each VTA section was examined at low magnification (×3400) divided into paranigral and parabrachial subregions. Images of each subregion were randomly photographed at ×23,000. Peroxidase-labeled axon terminals in the VTA were collected from three animals per genotype using established methods (38).
CB1R labeling was analyzed in presynaptic terminals and further characterized by synapse type, which was defined by established morphological criteria (27). In particular, symmetric synapses were defined by the presence of pleomorphic vesicles in the axon terminal in addition to the absence of a prominent postsynaptic density. Asymmetric synapses were defined by the presence of spherical vesicles in the axon terminal in addition to a wide cleft with a prominent dense coating on its cytoplasmic surface (27). Nonsynaptic labeling was excluded from the analysis. Labeling was further characterized as cytoplasmic and membrane bound. The total percent of peroxidase particles in each synapse type (cytoplasmic and membrane-bound) in addition to the density ratio of membrane-bound labeling was compared statistically by genotype.
Drugs
For acute THC-induced c-Fos and locomotion experiments, THC (25 mg/ml; Sigma-Aldrich) was dissolved in a vehicle solution composed of 5% Tween 80 and 95% saline to a final concentration of 0.5 mg/ml. The diluted THC was injected by intraperitoneal route at 10 ml/kg for a final dosage of 5 mg/kg. For CPP experiments, THC was dissolved in a vehicle solution composed of 5% Tween 80 and 95% saline to a final concentration of 0.1 mg/ml. The diluted THC was injected by intraperitoneal route at 10 ml/kg for a final dosage of 1 mg/kg. The FAAH inhibitor PF-3845 (Cayman Chemical) was resuspended in dimethyl sulfoxide (DMSO) to a concentration of 10 mg/ml. The concentrated PF-3845 was diluted 1:1:8 (PF-3845/Tween 80/saline) to a final concentration of 1 mg/ml. The diluted PF-3845 was injected by intraperitoneal route 2 hours before each THC injection at 10 ml/kg for a final dosage of 10 mg/kg.
Acute THC-induced c-Fos immunohistochemistry
Adolescent P35 mice were given an acute injection of THC (5 mg/kg, ip) and then returned to their home cage. Ninety minutes following treatment, mice were euthanized and perfused, as described (15). Immunohistochemistry was performed using rabbit anti–c-Fos primary antibody (sc-52, Santa Cruz Biotechnology) diluted 1:1000 and Alexa Fluor–labeled donkey anti-rabbit immunoglobulin G secondary antibody (Alexa Fluor 555) diluted 1:500. Estimation of cell density of c-Fos–positive cells in NAc was performed as described in stereological methods above.
THC-induced locomotion
Adolescent P35 mice were placed into a locomotion chamber (Med Associates Inc., St. Albans, VT). Following a 30-min habituation period, mice were injected with vehicle (90% saline, 5% DMSO, 5% Tween 80, 0.01 ml/g body weight, ip) and immediately placed back into the locomotion chamber. Following a 10-min recovery period, horizontal locomotor activity was measured for 50 min using computer-assisted activity monitoring software (Med Associates Inc.). In a separate group of P35 aged mice, mice were placed into the locomotion chamber, allowed to habituate for 30 min, injected with THC (5 mg/kg, ip), and immediately placed back into the locomotion chamber. After a 10-min recovery period in the chamber, horizontal locomotor activity was measured for 50 min.
CPP behavioral protocol
CPP was performed using a biased place conditioning procedure with a three-chamber place preference apparatus (Med Associates Inc.). The protocol for THC CPP was modified from a previous publication (Fig. 4A) (31), and cocaine CPP was performed as previously published in our laboratory (40). For all adolescent experiments, mice began the protocol at age P29. For all adult experiments, mice began the protocol at age P65. During the first preconditioning testing session (baseline test), mice were initially placed in the central gray chamber for a 1-min acclimation period, followed by free access to all three chambers for 20 min. Time spent in each chamber was recorded. For THC CPP, the next day, mice were given a systemic injection of THC (1 mg/kg at 0.01 ml/g body weight) in their home cage 24 hours before CPP training. For THC CPP, training was run on the following 4 days in which mice were given a systemic injection of vehicle (90% saline, 5% DMSO, 5% Tween 80, 0.01 ml/g body weight) in the morning session and confined to the most preferred chamber for 1 hour and then given a systemic injection of THC (1 mg/kg at 0.01 ml/g body weight) in the afternoon session and confined to the opposite least preferred chamber for 1 hour. For cocaine CPP, training was run on the following 3 days following baseline testing in which mice were given a systemic injection of saline in the morning session and confined to the most preferred chamber for 20 min and then given a systemic injection of cocaine (10 mg/kg at 0.01 ml/g body weight) in the afternoon session and confined to the opposite least preferred chamber for 20 min. For the test day session, mice were placed in the central chamber without drug treatment for a 1-min acclimation period before being allowed free access to all three chambers for a 20-min time period. Preference scores were calculated by subtracting the time spent in the saline-paired chamber from time spent in the drug-paired chamber. Difference scores were calculated by subtracting the preference score on baseline testing day from the preference score on test day. The THC CPP protocol was optimized and confirmed to produce a THC CPP response in C57BL/6J adolescent male mice [baseline: −167.4 ± 34.98 (SEM) versus test day: −43.15 ± 28.49 (SEM), paired t test, t13 = 2.666, *P < 0.05, n = 14].
To test the persistence of THC CPP response, adolescent mice trained in THC CPP were returned to their home cage for 30 days. Following this period of abstinence, mice were again placed in the central chamber without drug treatment for a 1-min acclimation period before being allowed free access to all three chambers for a 20-min time period. Preference scores were calculated as described above. Difference scores were calculated by subtracting the preference score on baseline testing day from the preference score on new testing day.
To test the effect of THC home cage exposure during adolescence on THC CPP response in adulthood, mice were given a daily intraperitoneal injection of THC (1 mg/kg at 0.01 ml/g body weight) from P30 to P34 (Fig. 5D) to replicate the same THC exposure timeline as mice tested in THC CPP. Once mice reached adulthood (P60), mice were tested for THC CPP as described above.
Pharmacological FAAH inhibition and THC CPP
To test the effect of FAAH inhibition on THC CPP, adolescent female C57BL/6J mice (WT) were treated with either vehicle (1:1:8, DMSO/Tween 80/saline) or the FAAH inhibitor PF-3845 (10 mg/kg, ip; Cayman Chemical), 2 hours before each THC injection during THC CPP training sessions. Mice received no PF-3845 treatment on test day.
Chemogenetic manipulation of neural circuitry
DREADD was used to manipulate VTA-NAc projection during behavioral testing. Following THC CPP training during adolescence, mice underwent surgery at P40. A Cre-dependent adeno-associated virus (AAV) expressing the hM4Di inhibitory DREADD (AAV2-hSyn-DIO-hM4DGi-mCherry, Addgene viral prep #44362; www.addgene.org/44362/) (41) was injected into bilateral VTA (AP = −3.0 mm; DV = −4.8 mm; ML = ±0.5 mm; 400 nl per side), and a retrograde transported canine adenovirus type 2 vector expressing CAVCre (CAV2-Cre, Plateforme de Vectorologie de Montpellier, Plateau IGMM; www.pvm.cnrs.fr/plateau-igmm/?vector=cav-cre) (42) was injected bilaterally into the NAc (AP = −1.0 mm; DV = −4.5 mm; ML = ±1.5 mm; 400 nl per side). Control groups either received sham surgery without viral infusions or did not undergo surgery. Before CPP testing, all mice were injected with CNO (3 mg/kg, ip).
Statistics
For all experiments, data were first analyzed for normality using the Shapiro-Wilk normality test. Anatomical data comparing across all four groups of male FAAHC/C, male FAAHC/A, female FAAHC/C, and female FAAHC/A were analyzed with a one-way analysis of variance (ANOVA). All cumulative locomotion data and CPP behavioral data were analyzed within genotype by a parametric paired t test. Binned locomotion data were analyzed by a two-way repeated-measures ANOVA followed by Bonferroni post hoc tests. When two independent groups were compared, experimental data were analyzed by a parametric independent-samples t test. Statistical analyses were conducted using GraphPad Prism version 6.0 for Mac (GraphPad Software, La Jolla, CA) and were considered to be statistically significant for values when P ≤ 0.05.
Supplementary Material
Acknowledgments
We thank F. Yu for technical assistance. We thank B. J. Casey and C. Glatt for helpful discussions and F. Yu and J. Chan for technical assistance. Funding: This work was supported by the NIH (grant T32DA039080 to C.E.B.; grants R01DA08259, R01HL098351, and R01HL136520 to T.A.M.; grant R01DA042943 to V.M.P.; grant R01NS052819 to F.S.L.; and grant R01DA029122 to A.M.R.), the Weill Cornell’s Mowrer Memorial Graduate Student Fellowship (C.E.B.), the New York–Presbyterian Youth Anxiety Center (F.S.L.), the Pritzker Neuropsychiatric Disorders Research Consortium (F.S.L.), the DeWitt-Wallace Fund of the New York Community Trust (F.S.L.), and the Paul Fund (A.M.R.). Author contributions: Conceptualization: C.E.B., M.N.H., V.M.P., F.S.L., and A.M.R.; investigation: C.E.B., D.J., R.Y., C.H., and T.A.M.; material creation: K.M.; writing: C.E.B., F.S.L., and A.M.R. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/7/eaay1502/DC1
Fig. S1. Adolescent female mice carrying the FAAH SNP demonstrate no change in CB1R-labeled terminals forming symmetric or asymmetric synapses in the parabrachial subregion of the VTA.
Fig. S2. THC CPP dose response in adolescent female mice carrying the FAAH SNP.
Fig. S3. Adolescent and adult FAAHC/C and FAAHC/A mice show preference for cocaine CPP.
Fig. S4. Pharmacological inhibition of FAAH in WT mice is not sufficient to reproduce rewarding effect of THC seen in adolescent female mice with the FAAH SNP.
Fig. S5. THC CPP during adulthood does not result in a preference for THC in female mice carrying the FAAH SNP.
REFERENCES AND NOTES
- 1.Parsons L. H., Hurd Y. L., Endocannabinoid signalling in reward and addiction. Nat. Rev. Neurosci. 16, 579–594 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gee D. G., Bath K. G., Johnson C. M., Meyer H. C., Murty V. P., van den Bos W., Hartley C. A., Neurocognitive development of motivated behavior: Dynamic changes across childhood and adolescence. J. Neurosci. 38, 9433–9445 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solinas M., Yasar S., Goldberg S. R., Endocannabinoid system involvement in brain reward processes related to drug abuse. Pharmacol. Res. 56, 393–405 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyer H. C., Lee F. S., Gee D. G., The role of the endocannabinoid system and genetic variation in adolescent brain development. Neuropsychopharmacology 43, 21–33 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hurd Y. L., Michaelides M., Miller M. L., Jutras-Aswad D., Trajectory of adolescent cannabis use on addiction vulnerability. Neuropharmacology 76 (Pt B), 416–424 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hillard C. J., The endocannabinoid signaling system in the CNS: A primer. Int. Rev. Neurobiol. 125, 1–47 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Y., Huang T., Lee F., Kreek M. J., Involvement of endocannabinoids in alcohol “Binge” drinking: Studies of mice with human fatty acid amide hydrolase genetic variation and after CB1 receptor antagonists. Alcohol. Clin. Exp. Res. 40, 467–473 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.1000 Genomes Project Consortium, Abecasis G. R., Auton A., Brooks L. D., DePristo M. A., Durbin R. M., Handsaker R. E., Kang H. M., Marth G. T., McVean G. A., An integrated map of genetic variation from 1,092 human genomes. Nature 491, 56–65 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiang K. P., Gerber A. L., Sipe J. C., Cravatt B. F., Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: Evidence for a link between defects in the endocannabinoid system and problem drug use. Hum. Mol. Genet. 13, 2113–2119 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Hariri A. R., Gorka A., Hyde L. W., Kimak M., Halder I., Ducci F., Ferrell R. E., Goldman D., Manuck S. B., Divergent effects of genetic variation in endocannabinoid signaling on human threat- and reward-related brain function. Biol. Psychiatry 66, 9–16 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.López-Moreno J. A., Echeverry-Alzate V., Bühler K. M., The genetic basis of the endocannabinoid system and drug addiction in humans. J. Psychopharmacol. 26, 133–143 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Hindocha C., Freeman T. P., Schafer G., Gardner C., Bloomfield M. A. P., Bramon E., Morgan C. J. A., Curran H. V., Acute effects of cannabinoids on addiction endophenotypes are moderated by genes encoding the CB1 receptor and FAAH enzyme. Addict. Biol., e12762 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Tyndale R. F., Payne J. I., Gerber A. L., Sipe J. C., The fatty acid amide hydrolase C385A (P129T) missense variant in cannabis users: Studies of drug use and dependence in Caucasians. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 144B, 660–666 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Dincheva I., Drysdale A. T., Hartley C. A., Johnson D. C., Jing D., King E. C., Ra S., Gray J. M., Yang R., DeGruccio A. M., Huang C., Cravatt B. F., Glatt C. E., Hill M. N., Casey B. J., Lee F. S., FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nat. Commun. 6, 6395 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gee D. G., Fetcho R. N., Jing D., Li A., Glatt C. E., Drysdale A. T., Cohen A. O., Dellarco D. V., Yang R. R., Dale A. M., Jernigan T. L., Lee F. S., Casey B. J.; PING Consortium , Individual differences in frontolimbic circuitry and anxiety emerge with adolescent changes in endocannabinoid signaling across species. Proc. Natl. Acad. Sci. U.S.A. 113, 4500–4505 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Budney A. J., Sofis M. J., Borodovsky J. T., An update on cannabis use disorder with comment on the impact of policy related to therapeutic and recreational cannabis use. Eur. Arch. Psychiatry Clin. Neurosci. 269, 73–86 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Center for Behavioral Health Statistics and Quality, Behavioral Health Trends in the United States: Results from the 2014 National Survey on Drug Use and Health (Center for Behavioral Health Statistics and Quality, 2015). [Google Scholar]
- 18.Taylor M., Collin S. M., Munafò M. R., MacLeod J., Hickman M., Heron J., Patterns of cannabis use during adolescence and their association with harmful substance use behaviour: Findings from a UK birth cohort. J. Epidemiol. Community Health 71, 764–770 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winters K. C., Lee C. Y., Likelihood of developing an alcohol and cannabis use disorder during youth: Association with recent use and age. Drug Alcohol Depend. 92, 239–247 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bloomfield M. A., Ashok A. H., Volkow N. D., Howes O. D., The effects of Δ9-tetrahydrocannabinol on the dopamine system. Nature 539, 369–377 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fitzgerald M. L., Shobin E., Pickel V. M., Cannabinoid modulation of the dopaminergic circuitry: Implications for limbic and striatal output. Prog. Neuropsychopharmacol. Biol. Psychiatry 38, 21–29 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oleson E. B., Cheer J. F., A brain on cannabinoids: The role of dopamine release in reward seeking. Cold Spring Harb. Perspect. Med. 2, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schoch H., Huerta M. Y., Ruiz C. M., Farrell M. R., Jung K. M., Huang J. J., Campbell R. R., Piomelli D., Mahler S. V., Adolescent cannabinoid exposure effects on natural reward seeking and learning in rats. Psychopharmacology 235, 121–134 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper Z. D., Craft R. M., Sex-dependent effects of cannabis and cannabinoids: A translational perspective. Neuropsychopharmacology 43, 34–51 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiers C. E., Shokri-Kojori E., Wong C. T., Abi-Dargham A., Demiral S. B., Tomasi D., Wang G.-J., Volkow N. D., Cannabis abusers show hypofrontality and blunted brain responses to a stimulant challenge in females but not in males. Neuropsychopharmacology 41, 2596–2605 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Covey D. P., Mateo Y., Sulzer D., Cheer J. F., Lovinger D. M., Endocannabinoid modulation of dopamine neurotransmission. Neuropharmacology 124, 52–61 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.A. Peters, S. Palay, H. Webster, The Fine Structure of the Nervous System (Oxford Univ. Press, 1991). [Google Scholar]
- 28.Lammel S., Lim B. K., Malenka R. C., Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology 76 (Pt B), 351–359 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han X., He Y., Bi G.-H., Zhang H.-Y., Song R., Liu Q.-R., Egan J. M., Gardner E. L., Li J., Xi Z.-X., CB1 receptor activation on VgluT2-expressing glutamatergic neurons underlies Δ9-tetrahydrocannabinol (Δ9-THC)-induced aversive effects in mice. Sci. Rep. 7, 12315 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manza P., Tomasi D., Volkow N. D., Subcortical local functional hyperconnectivity in cannabis dependence. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3, 285–293 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valjent E., Maldonado R., A behavioural model to reveal place preference to Δ9-tetrahydrocannabinol in mice. Psychopharmacology 147, 436–438 (2000). [DOI] [PubMed] [Google Scholar]
- 32.Melroy-Greif W. E., Wilhelmsen K. C., Ehlers C. L., Genetic variation in FAAH is associated with cannabis use disorders in a young adult sample of Mexican Americans. Drug Alcohol Depend. 166, 249–253 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schacht J. P., Selling R. E., Hutchison K. E., Intermediate cannabis dependence phenotypes and the FAAH C385A variant: An exploratory analysis. Psychopharmacology 203, 511–517 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higuera-Matas A., Miguéns M., Coria S. M., Assis M. A., Borcel E., del Olmo N., Ambrosio É., Sex-specific disturbances of the glutamate/GABA balance in the hippocampus of adult rats subjected to adolescent cannabinoid exposure. Neuropharmacology 62, 1975–1984 (2012). [DOI] [PubMed] [Google Scholar]
- 35.Panlilio L. V., Justinova Z., Preclinical studies of cannabinoid reward, treatments for cannabis use disorder, and addiction-related effects of cannabinoid exposure. Neuropsychopharmacology 43, 116–141 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beattie M. C., Meta-analysis of social relationships and posttreatment drinking outcomes: Comparison of relationship structure, function and quality. J. Stud. Alcohol 62, 518–527 (2001). [DOI] [PubMed] [Google Scholar]
- 37.G. Paxinos, K. Franklin, The Mouse Brain in Stereotaxic Coordinates (Academic Press, 2004). [Google Scholar]
- 38.Milner T. A., Waters E. M., Robinson D. C., Pierce J. P., Degenerating processes identified by electron microscopic immunocytochemical methods. Methods Mol. Biol. 793, 23–59 (2011). [DOI] [PubMed] [Google Scholar]
- 39.Bodor Á. L., Katona I., Nyíri G., Mackie K., Ledent C., Hájos N., Freund T. F., Endocannabinoid signaling in rat somatosensory cortex: Laminar differences and involvement of specific interneuron types. J. Neurosci. 25, 6845–6856 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burgdorf C. E., Schierberl K. C., Lee A. S., Fischer D. K., Van Kempen T. A., Mudragel V., Huganir R. L., Milner T. A., Glass M. J., Rajadhyaksha A. M., Extinction of contextual cocaine memories requires Cav1.2 within D1R-expressing cells and recruits hippocampal Cav1.2-dependent signaling mechanisms. J. Neurosci. 37, 11894–11911 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krashes M. J., Koda S., Ye C., Rogan S. C., Adams A. C., Cusher D. S., Maratos-Flier E., Roth B. L., Lowell B. B., Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest. 121, 1424–1428 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soudais C., Laplace-Builhe C., Kissa K., Kremer E. J., Preferential transduction of neurons by canine adenovirus vectors and their efficient retrograde transport in vivo. FASEB J. 15, 2283–2285 (2001). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/7/eaay1502/DC1
Fig. S1. Adolescent female mice carrying the FAAH SNP demonstrate no change in CB1R-labeled terminals forming symmetric or asymmetric synapses in the parabrachial subregion of the VTA.
Fig. S2. THC CPP dose response in adolescent female mice carrying the FAAH SNP.
Fig. S3. Adolescent and adult FAAHC/C and FAAHC/A mice show preference for cocaine CPP.
Fig. S4. Pharmacological inhibition of FAAH in WT mice is not sufficient to reproduce rewarding effect of THC seen in adolescent female mice with the FAAH SNP.
Fig. S5. THC CPP during adulthood does not result in a preference for THC in female mice carrying the FAAH SNP.


