Abstract
The objective of this study was based on the formulation development of fast dispersible Aceclofenac tablets (100 mg) and to evaluate the influence of pharmaceutical mixtures of directly compressible Avicel PH102 with Mannitol and Ac-di-sol on the compressional, mechanical characteristics and drug release properties. Fast dispersible Aceclofenac formulations were developed by central composite design (CCD). Among them the best possible formulation was selected on the basis of micromeritic properties, appropriate tablet weight and disintegration time for further study. Tablets were directly compressed using manual hydraulic press with a compressional force ranging from 7.2 to 77.2 MN/m2. Pre and post compression studies were performed and the compressed formulations (FA-FF) were assessed for different quality tests. The Heckel and Kawakita equations were applied for determination of compressional behavior of formulations. The quality attributes suggested that formulation (FB) containing avicel PH 102 (20%), mannitol (25%) and ac-di-sol (3%) as best optimized formulation showing better mechanical strength i.e. hardness 35.40 ± 6.93N, tensile strength 0.963 MN/m2, and friability 0.68%. Furthermore, compressional analysis of FB showed lowest PY value 59.520 MN/m2 and Pk value 1.040 MN/m2 indicating plasticity of the material. Formulation FB disintegrated rapidly within 21 seconds and released 99.92% drug after 45 min in phosphate buffer pH 6.8. Results of drug release kinetics showed that all formulations followed Weibull and First-order models in three different dissolution media. Avicel PH102 based formulation mixture exhibit excellent compactional strength with rapid disintegration and quick drug release.
Introduction
Advancement in tablet manufacturing technology has offered viable dosage alternatives for those patients who are facing problem related to compliance with conventional dosage forms. One such alternative dosage form is fast dispersible tablets [1]. These tablets are of two types: first type is taken in mouth without water to disintegrate rapidly or disperse readily and the second type of tablets form dispersion or solution in water to be taken by patients [2, 3]. These tablets are usually developed by direct compression method. Aceclofenac is a Cycloxygenase inhibitor having analgesic and anti-inflammatory activity. Due to its short half-life (4hr) and twice daily dose, it is considered a suitable candidate for fast dispersible tablets [4, 5].
Avicel®, the first commercialized brand of microcrystalline cellulose (MCC), is introduced by FMC Corporation as a direct compression tableting ingredient. MCC is a partially depolymerized cellulose that is obtained as pulp by mineral acid treatment of alpha cellulose type lb of fibrous plant material. Cellulose is the most abundant natural polymer having linear chains of b-1, 4-D anhydroglucopyranosyl units. Pharmaceutical MCC is most commonly obtained from wood where cellulose chains are packed in layers held together by strong hydrogen bonds and lignin (cross-linking polymer). The primary particles of all MCC types (101, 102 and 200) are about 50μm but the difference in the larger 2%, 2% aggregated particle numbers. Type 102 has a median particle size of about 100μm indicating adequate flow properties for successful tableting. MCC deforms plastically and maximizes the interparticle bonding area during compression. It forms strong and cohesive compacts even under low compression pressure due to the formation of numerous hydrogen bonds. Tableting is further enhanced by mechanical interlocking of elongated and irregularly shaped particles [6].
Formulations designed for fast disintegration require sugar-based diluents to impart pleasant taste, water solubility, and ability to mask the bitterness of medicament. Mannitol was used in this study to improve the mouthfeel of fast dispersible tablets [7]. A combination of avicel PH102 with water soluble mannitol exhibits shorter disintegration time and increased water solubility of mannitol. It may lead to the formation of pores in the tablets matrix, causing capillary action for water permeation into the tablet matrix; resulting in fast disintegration [8].
Ac-Di-Sol (i.e. super disintegrant) exerts its action by wicking and swelling of the tablet. The porous nature of Ac-Di-Sol provides access for water diffusion in tablets, resulting in wicking and the faster disintegration of tablets. It is recommended to be used in 0.5–3% concentration for directly compressible tablets [9–12].
During tablet manufacturing, compression of powder shows a reduction in the volume of powder bed by the application of compressional pressure in a confined space. As a result, there is a formation of strong inter-particle bonds that produce a compact mass and built inherent strength in the compact to increase the overall mechanical strength of the dosage unit. This analysis is significant in understanding the behavior of poorly compactable powders during the manufacturing of the tablets[13]. Heckel and Kawakita equations have been largely employed to understand this relationship between applied compressional pressure and mechanical strength of the compact.
The aim of the present study was to prepare fast dispersible Aceclofenac tablets and to evaluate the compressional behavior of the newly developed tablets using Heckel and Kawakita analysis.
Formulations were designed by Central Composite Design (CCD) using varying concentrations of avicel PH102, mannitol, and ac-di-sol. All the formulations were developed by direct compression method.
Materials
Aceclofenac was gifted by Sami Pharmaceutical (Pvt.) Limited. Avicel PH-102, mannitol, ac-di-sol, aspartame, talc, and vanilla flavor were purchased from FMC Corporation, USA.
Methods
Experimental design
Twenty fast dispersible Aceclofenac tablet formulations (100mg) were designed with the help of Central Composite Design using Design Expert®10.0 software (Stat–Ease, Inc, Minneapolis, MN 55413, USA). The selected independent variables were avicel PH102 (20–35%), mannitol (10–25%) and ac-di-sol (0.5–3%). Excipients such as aspartame, vanilla flavor and talc were used at a fixed concentration i.e. 2%, 1%, and 2% respectively. Disintegration time, percentage friability and hardness of formulations were selected as the dependent variables. The run type was arranged and recoded as F1 = FA, F2 = FB, …………. F15 = FO. The composition of each formulation in % and mg is mentioned in Table 1. There was six center points out of 20 formulations (i.e. F3 = center point formulation) with the same composition. These six formulations showed almost the same responses (disintegration time, friability and hardness) therefore results of only one center point formulation (i.e. F3 = Centre point formulation) is mentioned in proceeding text.
Table 1. Composition of fast dispersible Aceclofenac formulations using central composite design.
| Ingredients amount in % | Ingredients amount in mg | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Run | Formulation Codes | Avicel PH102 | Mannitol | Ac-di-sol | Aspartame | Talc | Flavor | Drug | Avicel PH102 | Mannitol | Ac-di-sol | Aspartame | Talc | Flavor | Drug | Tablet weight |
| X1 (%) | X2 (%) | X3 (%) | (%) | (%) | (%) | (%) | X1 (mg) | X2 (mg) | X3 (mg) | (mg) | (mg) | (mg) | (mg) | (mg/tab) | ||
| F1 | FA | 20.00 | 25.00 | 0.50 | 2 | 2 | 1 | 49.50 | 40.80 | 51.00 | 1.02 | 4.08 | 4.08 | 2.04 | 100 | 203.02 |
| F2 | FB | 20.00 | 25.00 | 3.00 | 2 | 2 | 1 | 47.00 | 42.55 | 53.00 | 6.36 | 4.25 | 4.25 | 2.12 | 100 | 212.53 |
| *F3 | FC | 27.50 | 17.50 | 1.75 | 2 | 2 | 1 | 48.25 | 57.20 | 36.45 | 3.64 | 4.16 | 4.16 | 2.08 | 100 | 207.69 |
| F4 | FD | 27.50 | 17.50 | 3.39 | 2 | 2 | 1 | 46.61 | 58.91 | 37.45 | 7.10 | 4.28 | 4.28 | 2.14 | 100 | 214.16 |
| F5 | FE | 35.00 | 10.00 | 0.50 | 2 | 2 | 1 | 49.50 | 71.42 | 20.40 | 1.02 | 4.08 | 4.08 | 2.04 | 100 | 203.04 |
| F6 | FF | 35.00 | 10.00 | 3.00 | 2 | 2 | 1 | 47.00 | 74.20 | 21.20 | 6.36 | 4.25 | 4.25 | 2.12 | 100 | 212.38 |
| F7 | FG | 20.00 | 10.00 | 0.50 | 2 | 2 | 1 | 64.50 | 31.24 | 15.62 | 0.78 | 3.12 | 3.12 | 1.56 | 100 | 155.44 |
| F8 | FH | 20.00 | 10.00 | 3.00 | 2 | 2 | 1 | 62.00 | 32.20 | 16.10 | 4.83 | 3.22 | 3.22 | 1.61 | 100 | 161.18 |
| F9 | FI | 27.50 | 07.63 | 1.75 | 2 | 2 | 1 | 58.12 | 47.52 | 13.18 | 3.02 | 3.45 | 3.45 | 1.72 | 100 | 172.34 |
| F10 | FJ | 17.63 | 17.50 | 1.75 | 2 | 2 | 1 | 58.12 | 30.46 | 30.24 | 3.02 | 3.45 | 3.45 | 1.72 | 100 | 172.34 |
| F11 | FK | 35.00 | 25.00 | 3.00 | 2 | 2 | 1 | 32.00 | 109.37 | 78.00 | 9.36 | 6.25 | 6.25 | 3.12 | 100 | 312.35 |
| F12 | FL | 27.50 | 27.37 | 1.75 | 2 | 2 | 1 | 38.38 | 72.05 | 71.70 | 4.58 | 5.24 | 5.24 | 2.62 | 100 | 261.43 |
| F13 | FM | 35.00 | 25.00 | 0.50 | 2 | 2 | 1 | 34.50 | 102.90 | 73.50 | 1.47 | 5.88 | 5.88 | 2.94 | 100 | 292.57 |
| F14 | FN | 37.37 | 17.50 | 1.75 | 2 | 2 | 1 | 38.38 | 98.00 | 45.89 | 4.58 | 5.24 | 5.24 | 2.62 | 100 | 261.57 |
| F15 | FO | 27.50 | 17.50 | 0.10 | 2 | 2 | 1 | 49.89 | 50.09 | 31.88 | 0.191 | 3.64 | 3.64 | 1.82 | 100 | 191.26 |
*F3 = Centre point formulation
Response surface methodology (RSM) was used to explore the interaction of avicel PH102, mannitol and ac-di-sol to establish the appropriate amount of excipient for optimized fast dispersible formulation. On the basis of the fit summary, ANOVA and multiple correlation coefficient appropriate model was selected.
Pre-compression studies
Assessment of powder densities and flow characteristics
Bulk density of all formulations was evaluated using a measuring cylinder. The cylinder’s weight was tare to zero and then filled with certain amount of formulation blend and reweighed. The following formula was used to determine the bulk density (g/cm3).
| Eq 1 |
Where ρbulk is the bulk density, and bulk volume is the initial volume occupied by the formulation blend in the cylinder.
The cylinder was tapped 100 times and reduction in formulation bed’s volume was recorded as tapped volume and the following formula was used to calculate the tapped density (g/cm3).
| Eq 2 |
Formulation blends were also assessed by Hausner’s Ratio (HR) and angle of repose (ϴ) using the following equations [14]:
| Eq 3 |
| Eq 4 |
In the above equation θ is the angle of repose, ‘h’ is the height of powder heap and ‘D’ is the diameter of the heap formed. True density (ρt) of all powder blends were determined by liquid displacement method using xylene as displacement liquid with the help of a pycnometer. The difference between empty pycnometer (W) and xylene filled pycnometer (W1) was calculated as the weight of xylene (W2). Approximately 2 g sample of each formulation was weighed (W3) and transferred to the pycnometer along with xylene and weighed again (W4). True density (g/cm3) was calculated using the following equation [15]:
Weight of empty pycnometer = W
Weight of pycnometer filled with xylene = W1
Weight of xylene W2 = W1-W
Weight of formulation blend = W3
Weight of pycnometer filled with xylene and formulation blend = W4
| Eq 5 |
Compression of powder blends
All the ingredients were weighed and passed through 20 mesh sieve separately and mixed for six minutes (optimized mixing time) by tumbling in a polybag. After mixing all the ingredients, talc was added and mixed for a further five minutes. Blends were compressed by manual filling of the die cavity. Tablets were compressed by applying varied compressional pressure 7.72, 23.16, 30.88, 38.0, 46.32, 54.04, 61.76, 69.48 and 77.2 MN/m2) using a manual hydraulic tableting machine (locally manufactured) fitted with pressure gauge. Tablets were stored in a desiccator for 24 h over silica gel for elastic recovery and hardening.
Evaluation of physicochemical parameters of Aceclofenac formulations
Compressed tablets were weighed and their thickness and diameter were accurately measured with digital vernier caliper (Digital Caliper: Seiko brand). Tablet hardness was determined by using hardness tester (OSK Fujiwara, Ogawa Seiki Co. Ltd., Tokyo, Japan). Tensile strength of round flat-faced tablets was calculated using the given equation [16]:
| Eq 6 |
Where, F (N) is the crushing load applied on tablets (i.e. hardness), ‘H’ is the thickness (mm) and ‘D’ is the diameter (mm) of tablets. Percentage friability was determined using Roche type Friabilator (H. Jurgens Gmbh H and Co- Bremen, D2800, Germany) with the help of following formula:
| Eq 7 |
Where Wo is the initial weight and Wt is the final weight of tablets after 100 rotations. For the disintegration test of fast dispersible tablets, 900 mL distilled water was maintained at 37°C. Tablets were disintegrated in seconds [17]. The assay assessment was carried out using UV-Spectrophotometer (UV-1800 Shimadzu Corporation Kyoto, Japan) at 274 nm absorbance [18, 19]. Similarly, the dissolution test was performed using 0.1N HCI, phosphate buffer pH 4.5 and pH 6.8.
Evaluation of compressional behavior
Heckel analysis. Mathematically Heckel equation is expressed as follows:
| Eq 8 |
This equation is used to relate the powder bed’s relative density (ρr) to the applied pressure during compression which is the ratio of apparent density (ρA) of the tablet and the true density (ρT) of powder blend:
| Eq 9 |
The apparent density ρA of tablet is calculated using the weight (g), radius (cm) and thickness (cm) of the tablet, whereas true density of the powder blend is determined by liquid displacement method (g/cm3).
| Eq 10 |
By plotting the values of and applied pressure ‘P’, linear portion of the plot provides the values of slope and intercept (i.e. K and A). The value of K determines the mean yield pressure (1/K = PY). This mean yield pressure PY indicates the plasticity of material under compression. Smaller the value of 1/K, higher the plasticity of material. Using the value of intercept ‘A’ densification of powder at different stages (i.e. Do, DA, and DB) is determined [15, 20, 21]. The value of Do (relative density = bulk density / true density) shows the powder densification at die filling stage. Since it measures the packing characteristics of powder, thus high value of Do indicates high dense packing of powder blend [22, 23]. The DB value indicates densification of powder when it is compressed and particles show movement and re-arrangement. Magnitude of rearrangement based on the theoretical point of densification at which deformation of particles started [24]. The value of DB is calculated as follows:
| Eq 11 |
The DA value is the final compact densification (DA = ρr) and it is calculated from intercept ‘A’ of the Heckel plot:
| Eq 12 |
Kawakita equation
Kawakita equation describes the compressional behavior of powders, either by tapping or from continuous compression experiments [25]. This equation explains the relationship between the degree of volume reduction ‘C’ upon the application of compressional pressure ‘P’. The linear expression of the Kawakita equation is given below:
or
| Eq 13 |
Where Vo is the initial bulk volume of the powder, Vp is the volume of the powder after compression, a is the total volume reduction for the powder bed or minimum porosity before compression and ‘b’ is the powder’s plasticity [26]. A graph of versus P was plotted for all formulations and constants a and b were determined from slope and intercept of the plot. Reciprocal of slope produced the value of a (i.e. a = 1/slope) and reciprocal of the intercept yielded, ab (i.e. ab = 1/Intercept).
Reciprocal of b is a pressure (PK) which reduced the thickness of powder bed 50%.
Evaluation of in-vitro release behavior
Six tablets of each test (FA-FH) and immediate release marketed (reference) formulations were placed in the dissolution apparatus (USP Apparatus-II: Paddle Stirring Element) containing 900 mL of dissolution media at 37± 0.5°C at 50 rpm. Multiple points sampling was conducted in different dissolution media i.e. 0.1N HCl, phosphate buffer pH 4.5 and 6.8. 10 ml sample was withdrawn at 5, 10, 15, 20, 30, 45, 60, 90 and 120 min interval and substituted with fresh 10 ml of same solution. Each test and reference solutions were diluted, filtered and analyzed spectrophotometrically at 274nm [19].
Release kinetics
Model- dependent method. Various kinetic models were used for the evaluation of release patternas mention in below equations [27, 28]:
First order kinetics. Explains that the drug release from systems is reliant on concentration, which represents time vs. log cumulative percentage drug remaining [27, 28].
| Eq 14 |
Where Qt is the collective amount of drug release at time t, Qo is the initial concentration of drug, k1 is the First—Order rate constant [29]
Weibull model. Weibull model expresses the fraction of drug release (m) in solution at time t, by following equation:
| Eq 15 |
or
Where, Ti is the lag time, in various cases zero, α, is the time process and b is the shape parameter [30] (b = 1) illustrates the curve as exponential, (b < 1) parabolic with the elevated early slope and after that constant with the exponential, (b > 1) shows S-shaped with increasing curve followed by turning point.
Hixson–crowell model. Following equation represents his model:
| Eq 16 |
Where Q0 = initial concentration of drug, Qt = drug concentration at time t and KHC is the Hixson—Crowell rate constant [28].
Higuchi model. Based on diffusion process, the drug release can be described by Higuchi model Equation of Higuchi model is expressed as follows:
| Eq 17 |
Where k is the Higuchi release rate constant and t is the time in hr [31].
Results and discussion
The purpose of this study was to prepare fast dispersible tablets of Aceclofenac and to evaluate the effect of different concentrations of avicel PH102 on the compressional behavior of newly developed formulations. The central composite design was used for formulation design. These formulations were arranged and assigned with specific codes for identification purposes and are presented in Table 1.
Flow properties
Powder blends of all formulations were evaluated for true, bulk and tapped densities and results were found in the range of 1.40–1.47, 0.44–0.52 and 0.51–0.63 g/cm3 respectively. Flow properties of all formulations were assessed by using Hausner’s ratio and angle of repose and their respective values were found to be 1.13–1.24, and 33.43–38.31o respectively, indicating better flow properties (Table 2). Formulation blends that failed to meet the acceptable limits of micromeritics characterization and excessive weight than the target formulation were excluded from study (i.e. F11, F12, and F13). Mannitol causes more adhesion problems during compression and this could be overcome by using it in combination with Avicel PH102 which facilitates the compression. After micromeritic evaluation remaining formulations were subjected to compression by direct compression method using manual hydraulic press.
Table 2. Micromeritic properties of selected Aceclofenac fast dispersible tablet formulations.
| Form Codes | True density ρt (g/cm3) | Bulk density ρb (g/ cm3) | Tapped density ρtapp (g/ cm3) | Hausner's ratio | Angle of repose (θ) |
|---|---|---|---|---|---|
| FA | 1.47 | 0.46 | 0.52 | 1.13 | 34.53 |
| FB | 1.40 | 0.44 | 0.51 | 1.17 | 33.43 |
| FC | 1.47 | 0.50 | 0.60 | 1.20 | 38.31 |
| FD | 1.44 | 0.49 | 0.61 | 1.24 | 37.09 |
| FE | 1.46 | 0.52 | 0.63 | 1.23 | 36.17 |
| FF | 1.47 | 0.51 | 0.59 | 1.15 | 34.67 |
Disintegration time
All formulations except F13, and F15 showed acceptable disintegration time ranging from 18–45 sec. Formulations F13 and F15 contained 0.5% and 0.1% superdisintegrant respectively, therefore, failed to meet the disintegration time of fast dispersible tablets. Ac-di-sol in the concentration of 1.75% indicated acceptable disintegration time but the formulation F9 having the same amount of superdisintegrant presented disintegration time of 4min which is beyond the requirement of fast dispersible tablets. This difference might be due to the presence of lesser concentration of mannitol (7.63%) which has more water solubility and thus facilitate disintegration process.
On the basis of the weight of tablets closer to target formulation and disintegration time, six formulations were selected for further evaluation. These formulations were assessed by different quality tests and results were found to be inadequate limits. The quality attributes of compressed tablets are mentioned in Table 3.
Table 3. Quality attributes of different Aceclofenac fast dispersible tablet formulations at different compressional pressures.
| Test parameters | FA | FB | FC | FD | FE | FF |
|---|---|---|---|---|---|---|
| Weight (mg) | 204.1 ± 1.912 | 212.1 ± 1.790 | 207.4 ± 1.955 | 214.2 ± 2.097 | 204.3 ± 2.311 | 212.2 ± 2.529 |
| Diameter (mm) | 8.468 ± 0.007 | 8.482 ± 0.010 | 8.49 ± 0.007 | 8.47 ± 0.006 | 8.47 ± 0.021 | 8.47 ± 0.014 |
| Thickness (mm) | 2.72 ± 0.055 | 2.76 ± 0.047 | 2.72 ± 0.081 | 2. 77 ± 0.062 | 2.75 ± 0.087 | 2.73 ± 0.086 |
| Hardness (N) | 33.58 ± 8.00 | 35.40 ± 6.93 | 34.61 ± 10.46 | 40.68 ± 9.90 | 53.42 ± 11.80 | 43.99 ± 6.25 |
| Tensile strength (MPa) | 0.932 ± 0.239 | 0.9628 ± 0.203 | 0.958 ± 0.311 | 1.108 ± 0.292 | 1.468 ± 0.362 | 1.213 ± 0.207 |
| Relative density (g/cm3) | 0.93 ± 0.01 | 0.95 ± 0.01 | 0.92± 0.02 | 0.93±0.02 | 0.92 ± 0.03 | 0.92 ± 0.02 |
| Friability (%) | 0.50 | 0.68 | 0.30 | 0.32 | 0.34 | 0.29 |
| Disintegration time (sec) | 18 | 21 | 40 | 36 | 45 | 40 |
| Assay (%) | 99.95 ± 1.34 | 100.10 ± 0.68 | 99.63 ± 0.48 | 100.58 ±1.68 | 98.63 ± 0.80 | 99.56 ± 1.23 |
| Dissolution (%) | 99.42 ± 0.52 | 100.38 ± 0.71 | 99.05 ± 0.90 | 100.35 ± 0.77 | 99.10 ± 1.04 | 100.10 ± 1.44 |
RSM plot and ANOVA summary
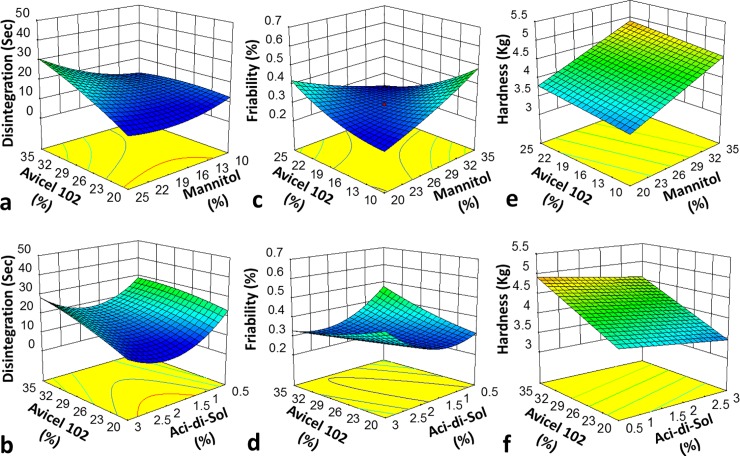
It is indicated in the RSM plot that disintegration time was increased with increased concentration of avicel PH102 as shown in Fig 1A and 1B. The ANOVA summary for the first response (disintegration) indicated that the model F value was 4.54 and the Probability value was less than 0.05 indicating that the quadratic model was significant. The “Adeq Precision” was 8.196 which indicated adequate signal-to-noise ratio and the design space could be navigated by the model. RSM plot Fig 1C and 1D indicated that friability was decreased with increased concentration of avicel PH 102 and mannitol. For the second response friability, F value, Probability and Adeq Precision were 10.18, < 0.05 and 11.265 respectively indicating quadratic model was valuable with the satisfactory signal. RSM plot Fig 1E and 1F presented that hardness of fast dispersible aceclofenac tablets was increased with higher concentration of avicel PH102 and mannitol. F value, Probability and Adeq Precision for third response hardness were 10.18, < 0.05 and 11.265 respectively showing model terms and linear model were acceptable with adequate signal. If A = Avicel PH102, B = Mannitol, and C = Ac-di-sol then the final equations in terms of coded factors for disintegration, friability and hardness are given below:
Fig 1. 3D Response surface plots of different fast dispersible Aceclofenac tablet formulations presenting effect of independent variables on (a & b) disintegration time, (c & d) Friability and (e & f) Hardness.
Tensile strength and hardness
Mechanical strength is another important parameter that also represents the inter-particulate bonding. For tablet dosage form it is recommended to determine the mechanical properties of dominant ingredient so as to predict the overall compressional behavior of tablets [32]. Mechanical properties were estimated by measuring the tensile strength which was in the range of 0.932± 0.239–1.468 ± 0.362 MN/m2. Tablet hardness of selected formulations was found to be in the range from 33.58 ± 8.00–53.42 ± 11.80N. Results of tensile strength and hardness indicated sufficient mechanical strength of all formulations due to the development of strong inter-particle bonds of powder blends and % friability of the tablets was 0.29–0.68%.
Compressional behavior analysis
For evaluating compressional behavior, ten tablets from each formulation were prepared by applying different compressional pressure 7.72, 23.16, 30.88, 38.0, 46.32, 54.04, 61.76, 69.48 and 77.2 MN/m2. Heckel and Kawakita equations were employed for estimating the compressional behavior of fast dispersible Aceclofenac tablets. Previously it was reported that both these compaction equations are suitable for describing the compression process of powder materials based on Avicel PH 102 (24). Different parameters of Heckel and Kawakita equations were derived from these plots i.e. Do, DA, DB, PY, Dl and PK and reported in Table 4. Heckel and Kawakita plots are presented in Figs 2 and 3 respectively.
Table 4. Compressional parameters obtained from heckel and kawakita equations of formulation blends.
| Formulation Codes | Heckel Parameters | Kawakita Parameters | ||||
|---|---|---|---|---|---|---|
| Do | DA | DB | PY (MN/m2) | DI = 1-a | Pk = 1/b (MN/m2) | |
| FA | 0.313 | 0.892 | 0.579 | 86.950 | 0.325 | 1.712 |
| FB | 0.314 | 0.922 | 0.608 | 59.520 | 0.319 | 1.040 |
| FC | 0.343 | 0.870 | 0.527 | 75.750 | 0.357 | 4.180 |
| FD | 0.340 | 0.883 | 0.543 | 64.930 | 0.349 | 4.350 |
| FE | 0.355 | 0.869 | 0.514 | 66.660 | 0.367 | 6.560 |
| FF | 0.339 | 0.863 | 0.524 | 67.560 | 0.350 | 7.690 |
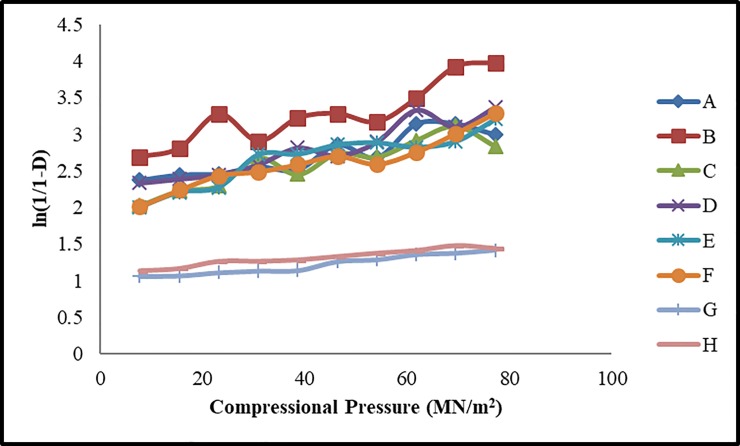
Fig 2. Heckel plots of fast dispersible Aceclofenac tablets.
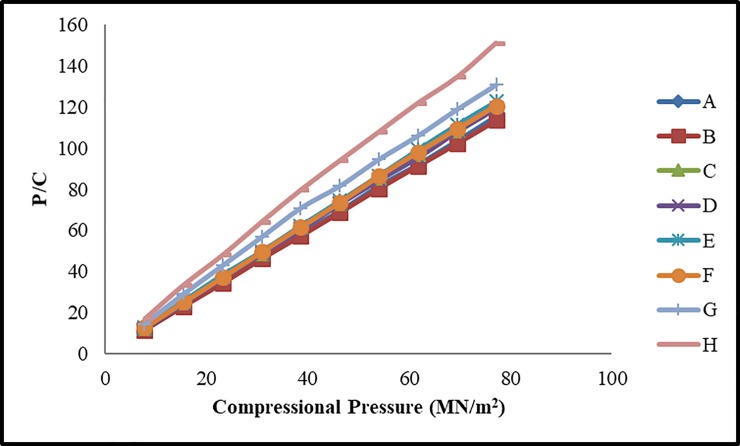
Fig 3. Kawakita plots of fast dispersible Aceclofenac tablets.
It was reported that DO values for different aceclofenac formulations were increased as the concentration of binder was increased. This phenomenon indicates that at die filling the initial packing of the formulations increases with the higher concentration of binder [32]. In formulations FC, FD, FE and FF, the amount of avicel PH 102 was high (27.5–35%) which resulted in increased hardness, tensile strength and less percentage friability (Table 3). Scientists also found that compaction behavior of powder bed mostly dependent upon the deformation properties of the blends and the applied processing methods [32].
Heckel analysis
From Heckel plots, it was found that the initial packing of powder blends (Do) in FE was found to be high i.e. Do = 0.355 as FE contained increased concentration of avicel PH102 (35%). The DA values at zero and low pressures presented the total degree of packing. Generally formulations with increased concentration of avicel PH102 exhibited the lowest values of DA. The DA value which showed the degree of packing at low compressional pressure was found lowest in FE (0.869) and FF (0.863). Values of DA for different formulations observed in the presented sequence: FF<FE<FC<FD<FA<FB.
The DB values presented the densification of powder bed at low pressure which shows the rearrangement of the particles by applying compressional pressure leading to the particle fragmentation. This fragmentation could be plastic or elastic. It was observed that formulations containing low amount of avicel PH102 i.e. FA (0.568) and FB (0.579) showed decreased values of DB. The sequence is FE<FF<FC<FD<FA<FB. Amin et al., 2012 stated that each powder has its own compressional characteristics. In the form of powder blend these characteristics get significantly changed which affects tablet stability [32]. It was observed that all formulations yielded low values of Do than DB due to particle fragmentation and particle rearrangement in the die at reduced pressure. Generally, increased porosity of powder blend at zero pressure yields low values of Do.
Another parameter ‘PY’ (mean yield pressure) is the measure of the plasticity of the material. Plastic material is desirable for compression while elastic material creates problems during compression due to elastic recovery. Formulations having greater tendency to deform plastically usually have low values of PY. In this study FB (59.52 MN/m2) and FD (64.93 MN/m2) showed the lowest ‘PY’ value and the highest plasticity (Table 4). It means that as the amount of avicel PH 102 affects the plasticity of the formulation and facilitates the manufacturing process.
Kawakita analysis
The Kawakita plot was also constructed to evaluate the compressional behavior of formulations (Fig 2). Kawakita plots presented a linear relationship at different compressional pressures with a correlation coefficient above 99% for formulations FA-FF. From the slope and intercept of Kawakita plots the values of a and ab were determined respectively. Values of 1-a indicated initial relative density (DI) of the formulations. By using reciprocal of b values, the inverse measurement of plasticity (Pk) was estimated as given in Table 4. It was observed that initial relative density of formulations (DI) was decreased in formulations containing low concentration of avicel PH102. The initial relative density (DI) of all formulations was in the range of 0.319–0.367. Results indicated that higher DI values were observed as compared to the corresponding values of DO, which has been previously reported by other researches [32]. Furthermore the PK values of these formulations were also higher.
It was observed in the present study that as the concentration of avicel PH102 was increased the PK value was also increased (1.04–7.69 MN/m2) which is indicative of elasticity of material. Formulations FE and FF had the highest PK values and FA and FB had the lowest PK values. It was found from Heckel and Kawakita parameters analysis that FE and FF showed the fastest onset of plastic deformation whereas FA and FB showed maximum plastic deformation in combination with aceclofenac and other excipients. However no clear cut variation pattern of Heckel and Kawakita parameters was observed.
In vitro dissolution
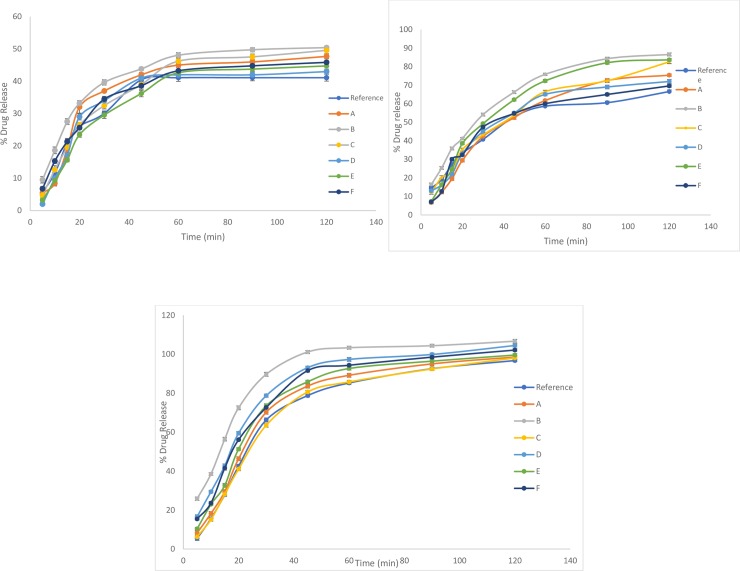
In the present study in vitro drug release profiles of newly developed and optimized aceclofenac formulations were determined in different dissolution media as shown in Fig 4A–4C. All formulations demonstrated maximum drug release in phosphate buffer pH 6.8. Different kinetic models were used to analyze the release behavior of formulations FA-FF. Results indicated that all formulations followed First-order and Weibull model in different dissolution media with highest r2 values found in phosphate buffer pH 6.8 i.e. 0.946–0.954 and 0.989–0.996 respectively as shown in Table 5.
Fig 4.
Drug release kinetics of Aceclofenac fast dispersible tablets in 900 ml of (a) pH 1.2 (b) phosphate buffer pH 4.5 (c) phosphate buffer pH 6.8.
Table 5. Release kinetics of fast dispersible Aceclofenac (100 mg) tablets at different pH.
| Formulation Codes | First Order | Higuchi | Hixson-Crowell | Weibull model | |||||
|---|---|---|---|---|---|---|---|---|---|
| r2 | K | r2 | KH | r2 | KHC | r2 | α | Β | |
| (hr-1) | (hr-1/2) | (hr-1/3) | |||||||
| 0.1N HCI | |||||||||
| Reference | 0.374 | 0.007 | 0.765 | 4.622 | 0.645 | 0.001 | 0.893 | 9.695 | 0.378 |
| FA | 0.353 | 0.009 | 0.768 | 5.046 | 0.664 | 0.001 | 0.963 | 8.065 | 0.367 |
| FB | 0.408 | 0.009 | 0.796 | 5.107 | 0.696 | 0.002 | 0.939 | 8.181 | 0.379 |
| FC | 0.323 | 0.009 | 0.756 | 5.043 | 0.653 | 0.001 | 0.925 | 7.764 | 0.359 |
| FD | 0.349 | 0.009 | 0.760 | 5.106 | 0.657 | 0.001 | 0.925 | 7.812 | 0.364 |
| FE | 0.324 | 0.009 | 0.751 | 5.031 | 0.646 | 0.001 | 0.919 | 7.803 | 0.359 |
| FF | 0.330 | 0.009 | 0.760 | 5.089 | 0.659 | 0.001 | 0.925 | 7.745 | 0.362 |
| pH 4.5 | |||||||||
| Reference | 0.681 | 0.041 | 0.868 | 6.480 | 0.809 | 0.002 | 0.950 | 9.179 | 0.483 |
| FA | 0.948 | 0.021 | 0.943 | 8.357 | 0.944 | 0.004 | 0.985 | 17.192 | 0.733 |
| FB | 0.946 | 0.022 | 0.945 | 8.418 | 0.950 | 0.004 | 0.988 | 17.079 | 0.736 |
| FC | 0.950 | 0.022 | 0.950 | 8.391 | 0.951 | 0.004 | 0.991 | 16.625 | 0.726 |
| FD | 0.954 | 0.022 | 0.946 | 8.474 | 0.950 | 0.004 | 0.989 | 17.511 | 0.744 |
| FE | 0.964 | 0.022 | 0.953 | 8.527 | 0.958 | 0.005 | 0.987 | 20.903 | 0.785 |
| FF | 0.950 | 0.021 | 0.943 | 8.359 | 0.941 | 0.004 | 0.982 | 17.076 | 0.730 |
| pH 6.8 | |||||||||
| Reference | 0.948 | 0.044 | 0.781 | 11.553 | 0.969 | 0.012 | 0.996 | 41.658 | 1.267 |
| FA | 0.946 | 0.051 | 0.736 | 11.438 | 0.950 | 0.012 | 0.991 | 27.069 | 1.223 |
| FB | 0.954 | 0.052 | 0.733 | 11.339 | 0.951 | 0.012 | 0.992 | 21.981 | 1.144 |
| FC | 0.948 | 0.051 | 0.713 | 11.351 | 0.944 | 0.012 | 0.996 | 29.015 | 1.248 |
| FD | 0.952 | 0.051 | 0.741 | 11.398 | 0.953 | 0.012 | 0.993 | 21.695 | 1.111 |
| FE | 0.951 | 0.050 | 0.728 | 11.240 | 0.948 | 0.012 | 0.992 | 20.114 | 1.080 |
| FF | 0.949 | 0.050 | 0.735 | 11.374 | 0.950 | 0.012 | 0.989 | 24.857 | 1.165 |
Limitation of the study
This study based on the results generated on manual tablet press and not on the large scale rotary compression machine.
Conclusion
In this study, fast dispersible aceclofenac tablets were prepared and the effect of avicel PH102 was examined on compressional, mechanical and release properties of fast dispersible aceclofenac formulations. Using the Heckel and Kawakita equations, the compressional behavior was observed. The concentration of avicel PH102 exhibited a significant impact on the compressional, mechanical and release properties of the Aceclofenac fast dispersible formulations. Formulation FB having avicel PH102 (20%), mannitol (25%) and ac-di-sol (3%) exhibited excellent compactional strength with rapid disintegration and quick drug release. Hence a suitable selection of excipient with appropriate concentration is important at formulation development stage to ensure stable, elegant, and palatable dosage form for the patient.
Supporting information
(XLSX)
(XLSX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Fukami J, Yonemochi E, Yoshihashi Y, Terada K. Evaluation of rapidly disintegrating tablets containing glycine and carboxymethylcellulose. International journal of pharmaceutics. 2006;310(1–2):101–9. 10.1016/j.ijpharm.2005.11.041 [DOI] [PubMed] [Google Scholar]
- 2.Schiermeier S, Schmidt PC. Fast dispersible ibuprofen tablets. European journal of pharmaceutical sciences. 2002;15(3):295–305. 10.1016/s0928-0987(02)00011-8 [DOI] [PubMed] [Google Scholar]
- 3.Martin TP, Hayes P, Collins DM. Tablet dispersion as an alternative to formulation of liquid dosage forms. Australian Journal of Hospital Pharmacy. 1993;23(6):378–86. [Google Scholar]
- 4.Setty CM, Prasad D, Gupta V, Sa B. Development of fast dispersible aceclofenac tablets: effect of functionality of superdisintegrants. Indian journal of pharmaceutical sciences. 2008;70(2):180 10.4103/0250-474X.41452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parfitt K, Martindale. Martindale:. The Complete Drug Reference. 36th ed Massachusetts, London, UK, 2009: RPS Publishing Britian; 2009. [Google Scholar]
- 6.Thoorens G, Krier F, Leclercq B, Carlin B, Evrard B. Microcrystalline cellulose, a direct compression binder in a quality by design environment—A review. International journal of pharmaceutics. 2014;473(1):64–72. 10.1016/j.ijpharm.2014.06.055. [DOI] [PubMed] [Google Scholar]
- 7.Chang R-K, Guo X, Burnside BA, Couch RA. Fast-dissolving tablets. Pharmaceutical technology. 2000;24(6):52–. [Google Scholar]
- 8.Bala R, Khanna S, Pawar PK. Formulation and optimization of fast dissolving intraoral drug delivery system for clobazam using response surface methodology. Journal of advanced pharmaceutical technology & research. 2013;4(3):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panigrahi R, Behera SP, Panda CS. A review on fast dissolving tablets. 2010. [Google Scholar]
- 10.Mor J, Chauhan P, Jalwal P. Development and Evaluation of Oral Fast Dissolving Tablets of Lornoxicam using Superdisintegrants-A Comparative Study. Cellulose. 2016;100(100):100. [Google Scholar]
- 11.Kumar V. Formulation and Evaluation of Meclizine Hcl Orally Dispersible Tablets by using Natural Super Disintegrants. Int J Pharm Sci & Scient Res. 2016;2:1–53. [Google Scholar]
- 12.Mor J, Chauhan P, Jalwal P. Development and evaluation of oral fast dissolving tablets of Lornoxicam using superdisintegrants-A comparative study. The Pharma Innovation. 2016;5(7, Part A):1. [Google Scholar]
- 13.Nicklasson F, Alderborn G. Analysis of the compression mechanics of pharmaceutical agglomerates of different porosity and composition using the Adams and Kawakita equations. Pharmaceutical research. 2000;17(8):949–54. 10.1023/a:1007575120817 [DOI] [PubMed] [Google Scholar]
- 14.Lin C-W, Cham T-M. Compression behavior and tensile strength of heat-treated polyethylene glycols. International journal of pharmaceutics. 1995;118(2):169–79. [Google Scholar]
- 15.Odeku O, Awe O, Popoola B, Odeniyi M, Itiola O. Compression and mechanical properties of tablet formulations: containing corn, sweet potato, and cocoyam starches as binders. Pharmaceutical technology. 2005;29(4):82–90. [Google Scholar]
- 16.Fell J, Newton J. Determination of tablet strength by the diametral-compression test. Journal of pharmaceutical sciences. 1970;59(5):688–91. 10.1002/jps.2600590523 [DOI] [PubMed] [Google Scholar]
- 17.Eur.Pharm. European Pharmacopoeia. In: European Department for the Quality of Medicines SS, editor. 3rd ed2001.
- 18.Bhardwaj S, Jain V, Jat R, Mangal A, Jain S. Formulation and evaluation of fast dissolving tablet of aceclofenac. International journal of drug delivery. 2010;2(1). [Google Scholar]
- 19.Sharma AJ, A.; Purohit A.; Jatav R.; Sheorey R. Formulation and evaluation of aceclofenac fast dissolving tablets. International Journal Of Pharmacy & Life Sciences. 2011;4(2):681–6. [Google Scholar]
- 20.Mustapha MA, Igwilo CI, Silva BO. Influence of Concentration of Modified Maize Starch on Compaction Characteristics and Mechanical Properties of Paracetamol Tablet Formulations. Medical Journal of Islamic World Academy of Sciences. 2013;109(893):1–7. [Google Scholar]
- 21.Mohammed B, Isah A, Ibrahim M. Influence of compaction pressures on modified cassava starch as a binder in paracetamol tablet formulations. Nigerian Journal of Pharmaceutical Sciences. 2009;8(1):80–8. [Google Scholar]
- 22.Mahmoodi F, Alderborn G, Frenning G. An experimental evaluation of an effective medium based compaction equation. European Journal of Pharmaceutical Sciences. 2012;46(1–2):49–55. 10.1016/j.ejps.2012.02.006 [DOI] [PubMed] [Google Scholar]
- 23.Denny P. Compaction equations: a comparison of the Heckel and Kawakita equations. Powder Technology. 2002;127(2):162–72. [Google Scholar]
- 24.Adetunji OA, Odeniyi MA, Itiola OA. Compression, mechanical and release properties of chloroquine phosphate tablets containing corn and trifoliate yam starches as binders. Tropical journal of pharmaceutical research. 2006;5(2):589–96. [Google Scholar]
- 25.Kawakita K, Lüdde K-H. Some considerations on powder compression equations. Powder technology. 1971;4(2):61–8. [Google Scholar]
- 26.Adetunji OA, Odeniyi MA, Itiola OA. Compression, mechanical and release properties of chloroquine phosphate tablets containing corn and trifoliate yam starches as binders. Tropical journal of pharmaceutical research. 2007;5(2):589–96. [Google Scholar]
- 27.Costa P, Lobo JMS. Modeling and comparison of dissolution profiles. European journal of pharmaceutical sciences. 2001;13(2):123–33. 10.1016/s0928-0987(01)00095-1 [DOI] [PubMed] [Google Scholar]
- 28.Vudathala GK, Rogers JA. Dissolution of fludrocortisone from phospholipid coprecipitates. Journal of pharmaceutical sciences. 1992;81(3):282–6. 10.1002/jps.2600810318 [DOI] [PubMed] [Google Scholar]
- 29.Dash S, Murthy PN, Nath L, Chowdhury P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm. 2010;67(3):217–23. [PubMed] [Google Scholar]
- 30.Shoaib MH, Siddiqi SAS, Yousuf RI, Zaheer K, Hanif M, Rehana S, et al. Development and evaluation of hydrophilic colloid matrix of famotidine tablets. Aaps Pharmscitech. 2010;11(2):708–18. 10.1208/s12249-010-9427-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higuchi T. Rate of release of medicaments from ointment bases containing drugs in suspension. Journal of pharmaceutical sciences. 1961;50(10):874–5. [DOI] [PubMed] [Google Scholar]
- 32.Amin MCIM, Albawani SM, Amjad MW. A comparative study of the compaction properties of binary and bilayer tablets of direct compression excipients. Tropical Journal of Pharmaceutical Research. 2012;11(4):585–94. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.