Purine bases, released by the hydrolytic and phosphorolytic degradation of nucleic acids and nucleotides, can be salvaged and recycled. The hypoxanthine-guanine phosphoribosyltransferase (HGPRT), which catalyzes the formation of guanosine-5′-monophosphate from guanine and inosine-5′-monophosphate from hypoxanthine, represents a potential target for specific inhibitor development. Deletion of the HGPRT gene (Δhgprt) in the model organism Mycobacterium smegmatis confirmed that this enzyme is not essential for M. smegmatis growth. Prodrugs of acyclic nucleoside phosphonates (ANPs), originally designed against HGPRT from Mycobacterium tuberculosis, displayed anti-M. smegmatis activities comparable to those obtained for M. tuberculosis but also inhibited the Δhgprt M. smegmatis strain. These results confirmed that ANPs act in M. smegmatis by a mechanism independent of HGPRT.
KEYWORDS: purine salvage pathway, hypoxanthine-guanine phosphoribosyltransferase, guanine, hypoxanthine, Mycobacterium smegmatis, inhibitors
ABSTRACT
Purine metabolism plays a ubiquitous role in the physiology of Mycobacterium tuberculosis and other mycobacteria. The purine salvage enzyme hypoxanthine-guanine phosphoribosyltransferase (HGPRT) is essential for M. tuberculosis growth in vitro; however, its precise role in M. tuberculosis physiology is unclear. Membrane-permeable prodrugs of specifically designed HGPRT inhibitors arrest the growth of M. tuberculosis and represent potential new antituberculosis compounds. Here, we investigated the purine salvage pathway in the model organism Mycobacterium smegmatis. Using genomic deletion analysis, we confirmed that HGPRT is the only guanine and hypoxanthine salvage enzyme in M. smegmatis but is not required for in vitro growth of this mycobacterium or survival under long-term stationary-phase conditions. We also found that prodrugs of M. tuberculosis HGPRT inhibitors displayed an unexpected antimicrobial activity against M. smegmatis that is independent of HGPRT. Our data point to a different mode of mechanism of action for these inhibitors than was originally proposed.
IMPORTANCE Purine bases, released by the hydrolytic and phosphorolytic degradation of nucleic acids and nucleotides, can be salvaged and recycled. The hypoxanthine-guanine phosphoribosyltransferase (HGPRT), which catalyzes the formation of guanosine-5′-monophosphate from guanine and inosine-5′-monophosphate from hypoxanthine, represents a potential target for specific inhibitor development. Deletion of the HGPRT gene (Δhgprt) in the model organism Mycobacterium smegmatis confirmed that this enzyme is not essential for M. smegmatis growth. Prodrugs of acyclic nucleoside phosphonates (ANPs), originally designed against HGPRT from Mycobacterium tuberculosis, displayed anti-M. smegmatis activities comparable to those obtained for M. tuberculosis but also inhibited the Δhgprt M. smegmatis strain. These results confirmed that ANPs act in M. smegmatis by a mechanism independent of HGPRT.
INTRODUCTION
Mycobacterium tuberculosis is an opportunistic pathogen that caused 1.2 million deaths among HIV-negative people worldwide in 2018 and an additional 251,000 deaths among people with HIV (1). The evolution of M. tuberculosis strains with resistance to multiple first- and second-line drugs (2) has led to an urgent need for new types of antituberculosis compounds. Purine metabolism plays a ubiquitous role in the physiology of mycobacteria, which are able to both synthesize purines de novo and scavenge them via the salvage pathway (3–5). Inhibitors targeting several enzymes implicated in purine metabolism can suppress M. tuberculosis growth at micromolar concentrations (6–12).
Hypoxanthine-guanine phosphoribosyltransferase (HGPRT; EC 2.4.2.8), the key enzyme in the purine salvage pathway, catalyzes the synthesis of inosine- or guanosine-5′-monophosphate via replacement of the 1-pyrophosphate group in phosphoribosyl pyrophosphate with a corresponding free nucleobase. Its precise role in M. tuberculosis physiology remains unclear due to a lack of sufficient experimental data; however, based on random saturation insertional mutagenesis analysis, HGPRT has been proposed to be essential for M. tuberculosis growth in vitro (13, 14). A detailed enzymatic mechanism and oligomerization analysis revealed that M. tuberculosis HGPRT belongs to the type I phosphoribosyltransferase family (15, 16). The arrangement of the sequentially unique mobile loop in the M. tuberculosis HGPRT molecule is responsible for its distinct kinetic properties and quaternary structure organization compared to its human counterpart (12, 15). In the M. tuberculosis HGPRT structure, these loops are located between the subunits of tetramers, whereas in the human HGPRT structure, the loops are at the extremities of the tetramer. This difference enabled the design of acyclic nucleoside phosphonate (ANP) inhibitors—analogues of natural nucleotides (17) with high selectivity for M. tuberculosis HGPRT over its human counterpart. The corresponding cell membrane-permeable phosphoramidate prodrugs inhibited M. tuberculosis growth in vitro at micromolar concentrations (12). However, the detailed mechanism of antibacterial activity of these prodrugs has not been studied in detail.
Mycobacterium smegmatis is a fast-growing saprophytic bacteria often used as a model in mycobacterial research because it shares many basic features with M. tuberculosis, such as cell wall biogenesis, adaptation to low oxygen conditions, dormancy, and stress response (18). Locus MSMEG_6110 in the M. smegmatis genome encodes a HGPRT that shares 85% primary sequence homology with its M. tuberculosis counterpart. Conservation of amino acid residues involved in the binding of substrates and ANP-based inhibitors suggests similar modes of action for the M. tuberculosis and M. smegmatis HGPRT homologues (12).
In this study, we examined the role of HGPRT in M. smegmatis and found that M. smegmatis growth is unexpectedly sensitive to treatment with ANP phosphoramidate prodrugs independently on HGPRT.
RESULTS
HGPRT is not essential for M. smegmatis growth.
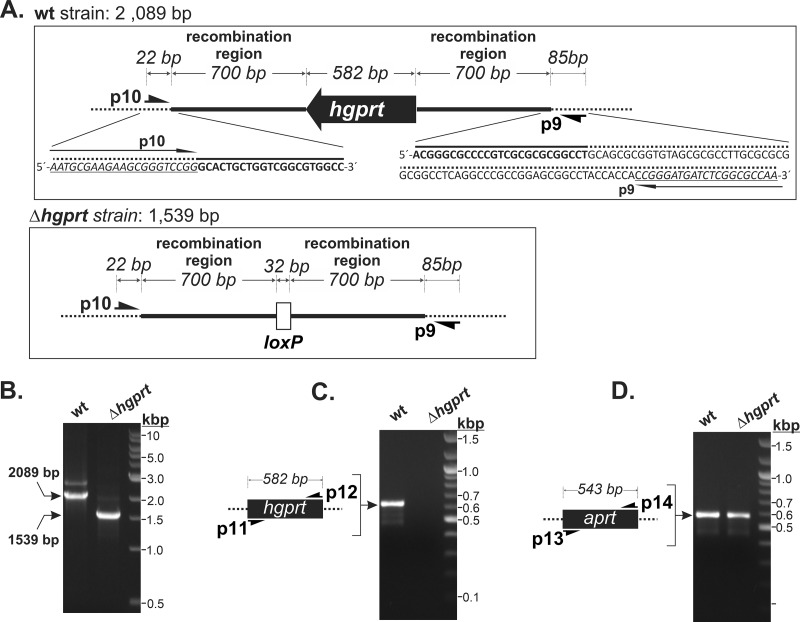
To analyze the importance of HGPRT for M. smegmatis growth, we deleted the HGPRT coding sequence (Δhgprt) using the method described by Shenkerman et al. (2014) (19). Briefly, this involved Chec9 DNA recombinase-directed gene knockout with a pYS2 plasmid-derived deletion Hygr cassette flanked by 32-bp loxP sites, which allows precise recombination of DNA sequences of interest and subsequent excision of the cassette from the chromosome by a Cre recombinase mediated by loxP sites. Colonies of recombinants, selected on agar medium with hygromycin, were visible after 3 days of cultivation. The resulting genetic background of the M. smegmatis Δhgprt strain was verified by PCR using specific primers that anneal close to the upstream and downstream 700-bp recombination regions (Fig. 1A). PCR with the wild-type (wt) strain, used as a reference, yielded an amplicon of 2,089 bp (Fig. 1B), corresponding to the HGPRT coding sequence and upstream and downstream regions (Fig. 1A). The Δhgprt strain amplicon was 1,539 bp (Fig. 1B), indicating that the 582-bp HGPRT coding sequence had been replaced with the 32-bp loxP site (Fig. 1A). DNA sequencing of the 1,539-bp amplicon confirmed the expected recombination process. We also carried out a control PCR using primers specific for the HGPRT gene to confirm the absence of the HGPRT coding sequence in different genome positions of the Δhgprt strain. We used primers specific for the adenine phosphoribosyltransferase (APRT) gene as a positive control. Both HGPRT and APRT amplicons were generated in PCRs with the reference wt strain, while only the APRT amplicon was present in reactions with the Δhgprt strain (Fig. 1C and D).
FIG 1.
PCR screening of the HGPRT coding sequence deletion. (A) Schematic showing the HGPRT gene region in the wt strain (top panel) and its replacement with the 32-bp loxP site in the Δhgprt strain (bottom panel). The bold line corresponds to the 700-bp upstream and downstream HGPRT gene regions used for homologous recombination with the hygromycin cassette (Hygr). Positions of screening primers p9 and p10 are indicated (B to D). Chromosomal DNA from wt and Δhgprt strains was isolated and used as the templates for independent PCR experiments using the following primer pairs: p9/p10, which anneal in the boundaries of the recombined region (B); p11/p12, which are specific for the HGPRT coding sequence (C); and p13/p14, which are specific for the APRT coding sequence (used as a positive control) (D). Samples were separated on 1% agarose gels.
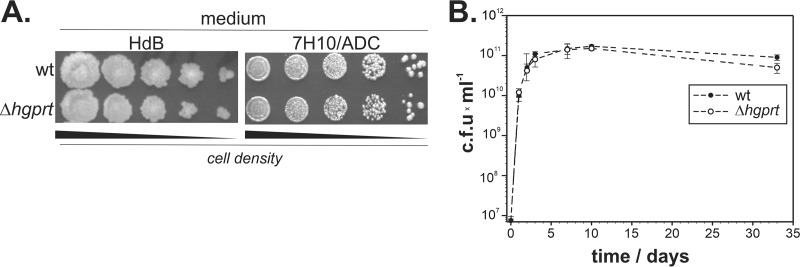
To assess the Δhgprt strain phenotype, the wt and mutated strain were grown on solid minimal Hartmans-De Bont (HdB) medium or Middlebrook 7H10–albumin-dextrose complex (7H10-ADC) medium. Growth (Fig. 2A) and survival under the stationary phase (Fig. 2B) were comparable for the wt and Δhgprt strains, confirming that HGPRT is not essential for M. smegmatis growth. The data also suggest that HGPRT does not contribute to adaptation to long-term starvation conditions (Fig. 2B).
FIG 2.
Influence of HGPRT deletion on M. smegmatis growth. (A) Growth of the wt and Δhgprt strains on minimal HdB and 7H10-ADC (28) solid media; (B) bacterial survival during the stationary phase in 7H9-ADC medium.
Guanine/hypoxanthine salvage is catalyzed exclusively by HGPRT in M. smegmatis.
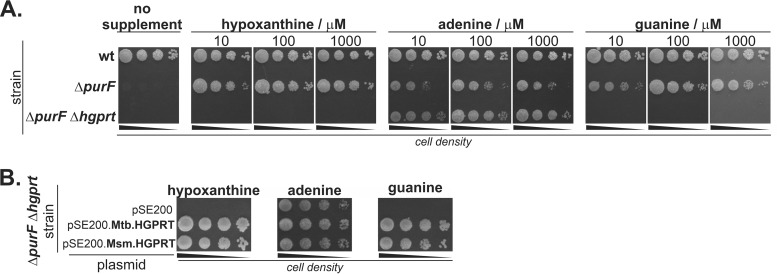
We next tested the importance of HGPRT for the salvage of adenine, guanine, and hypoxanthine. To impair de novo biosynthesis of purine bases and direct purine metabolism through the salvage pathway, we knocked out amidophosphoribosyltransferase (PurF) (20, 21), which catalyzes the first step of de novo purine synthesis. Additionally, we prepared the ΔpurF Δhgprt double mutant. We compared the growth of the wt, Δhgprt, ΔpurF, and ΔpurF Δhgprt strains on solid medium in the presence of hypoxanthine, adenine, and guanine. As was reported previously (21), the ΔpurF M. smegmatis strain was unable to grow in the absence of a purine supplement, and its growth was restored in the presence of hypoxanthine, adenine, or guanine at concentration higher than 10 μM (Fig. 3A). Growth of the ΔpurF Δhgprt mutant, with simultaneously inactivated de novo purine biosynthesis and HGPRT activity, was observed only in the presence of adenine (Fig. 3A). To confirm the role of HGPRT in M. smegmatis purine biosynthesis and to assess whether M. tuberculosis HGPRT has similar properties, we performed complementation experiments in the M. smegmatis ΔpurF Δhgprt strain using plasmids expressing M. smegmatis and M. tuberculosis HGPRTs. Growth of the strain was restored by both M. smegmatis and M. tuberculosis HGPRT enzymes (Fig. 3B). Our results indicate that HGPRT is the exclusive salvaging enzyme for guanine and hypoxanthine in M. smegmatis and suggest that M. tuberculosis HGPRT behaves similarly.
FIG 3.
Role of HGPRT in the M. smegmatis purine salvage pathway. (A) Effect of inactivation of the purF coding sequence on wt and Δhgprt M. smegmatis purine auxotrophy. M. smegmatis strains were grown on 7H10-ADC medium with or without purine supplement at indicated concentrations for 3 days at 37°C. (B) Plasmid-based complementation of HGPRT activity in the ΔpurF Δhgprt strain. Bacteria transformed with empty vector (pSE200) or vectors carrying M. tuberculosis/M. smegmatis HGPRT coding sequences were cultivated on 7H10-ADC with 25 μg/ml kanamycin and 100 μM purine supplement for 3 days at 37°C.
ANP-based inhibitors of M. tuberculosis HGPRT arrest M. smegmatis growth but do not target M. smegmatis HGPRT.
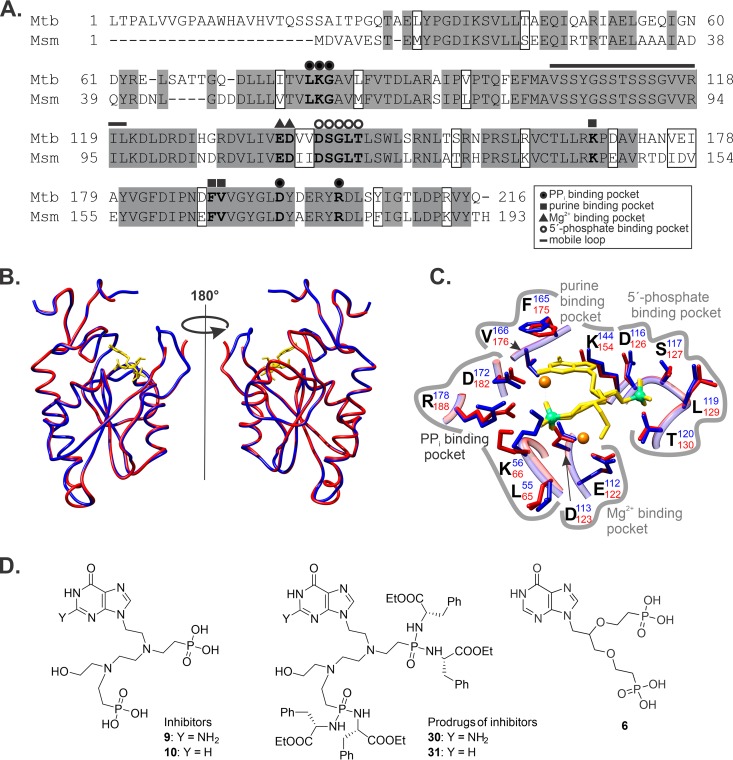
The amino acid sequences of HGPRT from M. smegmatis and M. tuberculosis share 72% identity and 85% similarity, and the residues forming binding pockets for purines, pyrophosphate, and the Mg2+ cation are conserved (Fig. 4A). We built a model of the M. smegmatis HGPRT structure (Fig. 4B) using the structure of M. tuberculosis HGPRT in complex with inhibitor 9 (Fig. 4D), a guanine-derived double-branched ANP, as the template (12). The overall model (Fig. 4B) and detail of the active site (Fig. 4C) suggest identical binding of this compound to M. smegmatis HGPRT.
FIG 4.
Similarity of M. smegmatis and M. tuberculosis HGPRTs. (A) M. tuberculosis and M. smegmatis HGPRT primary structure alignment. Identical and similar amino acid residues are in gray and white boxes, respectively. Key amino acid residues involved in binding of double-branched ANPs are in bold. (B) Structural model of M. smegmatis HGPRT (blue) aligned with M. tuberculosis HGPRT in complex with compound 9 (red; PDB code 4RHX), which was used as a template. Compound 9 is shown in yellow. (C) Active site of M. tuberculosis HGPRT (red) and the M. smegmatis HGPRT model (blue) in complex with compound 9 (yellow). Key amino acid residues involved in the binding are shown. (D) Structures of ANP inhibitors and their prodrugs (12).
Eng et al. (12) previously reported that membrane-permeable phosphoramidate prodrugs, derived from corresponding acyclic nucleoside phosphonates, selectively inhibit purified M. tuberculosis HGPRT and arrest M. tuberculosis growth. We tested two prodrugs from a series previously prepared in our laboratory, the tetra-phosphoramidate compound 30 (prodrug of 9; MIC50 of 4.5 μM for M. tuberculosis strain H37Rv) (Fig. 4D) and the corresponding hypoxanthine derivative 31 (prodrug of 10, MIC50 of 8 μM for M. tuberculosis), for growth arrest of wt and mutant M. smegmatis (Table 1). Both compounds had MIC50 values for wt M. smegmatis comparable to those obtained for M. tuberculosis, but surprisingly, they also comparably inhibited the Δhgprt strain and the ΔpurF strain, which are dependent on HGPRT activity and can grow only in the presence of purines. Because the solubility of guanine in the cultivation media used in this study is limited, we cultivated the ΔpurF strain in the presence of hypoxanthine (Table 1). Overexpression of both M. tuberculosis and M. smegmatis HGPRT enzymes in the wt and ΔpurF Δhgprt M. smegmatis strains did not change the MIC50 value. These unexpected results indicate that both inhibitors target another protein, the function of which is crucial for M. smegmatis viability.
TABLE 1.
MIC50 values of prodrugs of acyclic nucleoside phosphonate M. tuberculosis HGPRT inhibitors
| Species and strain | Plasmid | MIC50 ± SD (μM) of compound: |
|
|---|---|---|---|
| 30 | 31 | ||
| M. tuberculosisa H37Rv | None | 4.5 | 8.0 |
| M. smegmatis | |||
| wt | None | 8.1 ± 0.4 | 15.6 ± 0.9 |
| None | 8.1 ± 0.6 (Hpx)b | 16.1 ± 1.8 (Hpx) | |
| pSE200-Mtb.HGPRT | 8.2 ± 0.9 | 13.9 ± 1.7 | |
| pSE200-Msm.HGPRT | 8.3 ± 0.6 | 13.6 ± 1.9 | |
| Δhgprt strain | None | 8.0 ± 0.9 | 15.6 ± 1.1 |
| ΔpurF strain | None | 8.9 ± 1.2 (Hpx) | 22.2 ± 2.4 (Hpx) |
| ΔpurF Δhgprt strain | pSE200-Mtb.HGPRT | 8.7 ± 0.7 (Hpx) | 20.1 ± 2.2 (Hpx) |
| pSE200-Msm.HGPRT | 8.8 ± 0.5 (Hpx) | 19.2 ± 3 (Hpx) | |
Published in reference 12.
Hpx, in the presence of 100 μM hypoxanthine.
DISCUSSION
In this study, we showed that HGPRT is the only guanine/hypoxanthine salvage enzyme in M. smegmatis, but its activity is not required for viability under standard growth conditions. We also found that prodrugs derived from ANP-based inhibitors designed for the highly similar M. tuberculosis HGPRT do not target M. smegmatis HGPRT but display antimicrobial activity against M. smegmatis. This finding raises questions about the selectivity of known M. tuberculosis HGPRT-targeted compounds in bacterial cells (12). A random genome phage mutagenesis screen of M. tuberculosis (13, 14) identified HGPRT as an essential gene for M. tuberculosis; however, the phenotype of the Δhgprt M. tuberculosis strain has not been analyzed. Previous characterizations of M. tuberculosis HGPRT emphasized the necessity of evaluating the Δhgprt M. tuberculosis phenotype to elucidate the exact role of HGPRT in M. tuberculosis physiology and metabolic changes during the transition to hypoxia (16).
Eng and coworkers presented the first crystal structures of M. tuberculosis HGPRT in complex with ANP-based compounds that inhibit the enzyme at micromolar concentrations (PDB codes 4RHU, 4RHX, and 4RHY), revealing that these compounds fit well in the active site (12). These micromolar inhibitors did not display any significant antimicrobial activities because their highly polar character does not allow them to cross cell membranes. However, using a prodrug approach (22) to mask the charges of the phosphonate moieties by attaching hydrophobic groups via a phosphoramidate bond facilitated penetration of these compounds into M. tuberculosis and improved their antimicrobial activities. Notably, protein target analysis of synthesized prodrugs in M. tuberculosis has not been performed. Interestingly, the MIC50 values of these compounds for M. tuberculosis growth were in the 4.5 to 15 μM range, even for compound 6 (Fig. 4D), which displayed very low inhibitory activity in enzymatic tests (Ki, ≥50 μM) (12). Our aim was not to test ANPs for in vitro inhibition of an isolated M. smegmatis enzyme but to use the constructed M. smegmatis HGPRT knockout mutants to analyze the selectivity of HGPRT inhibitors in bacterial cells. The model of the complex of compound 9 with M. smegmatis HGPRT (Fig. 4B), based on the structure of M. tuberculosis HGPRT with this inhibitor (Ki, 1.6 μM), showed enzyme-inhibitor interactions identical to those observed in M. tuberculosis HGPRT (Fig. 4C), suggesting similar inhibition activities. Assays of the antimicrobial activity of guanine- and hypoxanthine-containing prodrugs (compounds 30 and 31, respectively) against M. smegmatis resulted in MIC50 values comparable to those obtained for M. tuberculosis. However, we obtained comparable MIC50 values for the Δhgprt, ΔpurF, and ΔpurF Δhgprt M. smegmatis strains in the presence of overexpressed M. smegmatis HGPRT, indicating thatj the antimicrobial activity of ANP prodrugs is not attributable to inhibition of HGPRT.j Rather, the compounds act by a different mechanism than originally supposed. The unchanged MIC50 values of 30 and 31 in wt M. smegmatis in the presence of overexpressed M. tuberculosis HGPRT suggest that a similar mechanism also functions in M. tuberculosis.
We cannot rule out differences in purine metabolism and the role of HGPRT in M. smegmatis and M. tuberculosis, as purine salvage pathways and the biosynthesis of nucleic acids have not been investigated in detail in both species. Furthermore, adaptation to different ecological niches could be responsible for many metabolic differences (23). Our data, however, show that inhibitors designed and tested against particular intracellular mycobacterial enzymes can have a different-than-expected mode of action, suggesting that their mechanisms in bacterial cells must be evaluated in more detail.
MATERIALS AND METHODS
Construction of plasmids.
pYS2-based deletion plasmids (19) were constructed as follows: regions of amplified sequences and corresponding primers used to construct pYS2-based deletion plasmids are listed in Table S1 in the supplemental material. Upstream (ups) and downstream (dns) regions of the hgprt and purF coding sequences were amplified by PCR using Q5 DNA polymerase (New England BioLabs) with M. smegmatis chromosomal DNA as the template. ups regions were inserted into pYS2 via SpeI and SwaI sites using T4 DNA ligase and were sequenced. Similarly, the corresponding dns region sequences were inserted into the pYS2 intermediates via PacI and NsiI sites. The pSE200-based constitutive expression plasmids (21) were constructed as follows: M. tuberculosis Mtb.hgprt and M. smegmatis Msm.hgprt were PCR amplified from corresponding template chromosomal DNA using primer pairs 15/16 and 17/18, respectively. PCR fragments were inserted into SwaI-linearized pSE200 using the In-Fusion approach. All prepared constructs were verified by sequencing. M. smegmatis strains were transformed as previously described (24). Primers are listed in Table S1.
Gene deletion.
Gene disruption using a pYS2 deletion plasmid has been described previously (19). Briefly, M. smegmatis carrying the temperature-sensitive replicating expression plasmid pYS1 encoding Chec9 DNA recombinase under the control of the acetamidase promoter was inoculated in 7H9-ADC medium containing 25 μg/ml kanamycin and 0.25% acetamide to an initial optical density at 600 nm (OD600) of 0.001. The culture was grown at 34°C and 220 rpm until the OD600 reached 0.8. The culture was then used for preparation of competent cells, which were transformed with a PacI-linearized pYS2 deletion plasmid (24). Gene disruptants were selected on 7H10-ADC medium containing 150 μg/ml hygromycin at 42°C to cure out of the pYS1 vector. To remove the Hygr cassette, gene disruptants were next transformed with the pML2714 vector, which constitutively expresses Cre recombinase. In the resulting gene disruptants, the deleted gene was replaced with a 32-bp loxP site. Deletions were screened by PCR using Q5 polymerase and primer pairs that anneal at the boundaries of the deleted region (Table S1 and Fig. 1). The resulting PCR product was sequenced. Constructed M. smegmatis deletion strains are listed in Table 2.
TABLE 2.
List of M. smegmatis strains used in this study
| Strain genotype | Parental strain/genotype | Reference |
|---|---|---|
| wt | mc2 155/wt | 29 |
| Δhgprt | mc2 155/hgprt∷loxP | This study |
| ΔpurF | mc2 155/purF∷Hygr | This study |
| Δhgpr ΔpurF | Δhgprt/hgprt∷loxP purF∷Hygr | This study |
Synthesis of ANP phosphoramidate prodrugs 30 and 31.
Prodrugs of ANP-based inhibitors were prepared as previously described (12). Characterization of synthetic compounds is described in the supplemental material. The samples tested on M. smegmatis were identical to those tested previously on M. tuberculosis.
Determination of antibacterial activities of ANP prodrugs.
M. smegmatis strains were propagated in 7H9-ADC medium containing 0.5% (vol/vol) glycerol and 0.05% tyloxapol to the mid-exponential phase (OD600 of 0.5). When required, 100 μM hypoxanthine was additionally present. Strains carrying pSE200-based expression vectors were cultivated in the presence of 25 μg/ml kanamycin. This culture was used to test the antimicrobial activity of ANP prodrugs with a modified resazurin microplate assay (25). Serially diluted ANP prodrugs in 7H9-ADC medium containing 0.05% tyloxapol and 0.5% glycerol were pipetted in 100-μl aliquots into a 96-well plate, and the final concentration of dimethyl sulfoxide (DMSO) was adjusted to 0.5%. M. smegmatis culture at an OD600 of 0.005 (100 μl) was added. Plates were incubated at 37°C for 24 h, 30 μl of a 0.02% resazurin solution in phosphate-buffered saline (PBS) was added, and the plate was incubated for an additional 24 h. Sample fluorescence was determined with a Tecan Infinite M1000 microplate reader with an excitation wavelength of 530 nm and emission read at 580 nm. Growth was calculated as follows:
where FL(positive control) represents a M. smegmatis culture sample, without ANPs and FL(blank) represents cultivation without cells.
Stationary-phase survival assay.
M. smegmatis was inoculated into 100 ml of 7H9-ADC medium with 0.5% (vol/vol) glycerol and 0.05% tyloxapol to an OD600 of 0.005 in a 500-ml Erlenmeyer flask. Cultures were cultivated at 37°C and 180 rpm. Aliquots (1 ml) were collected at 0, 1, 3, 5, 7, 10, and 31 days of cultivation and serially diluted. A 30-μl aliquot of each dilution was spotted onto 7H10-ADC plates. Plates were incubated for 3 days at 37°C, and CFUs were determined from spots with 10 to 40 M. smegmatis colonies.
M. smegmatis cultivation on agar media.
M. smegmatis strains were cultivated in 7H9-ADC medium with medium containing 0.5% (vol/vol) glycerol and 0.05% tyloxapol until cultures reached the mid-exponential growth phase. When required, cultures were supplemented with 100 μM hypoxanthine or 25 μg/ml kanamycin. Cells were pelleted at 3,000 × g for 5 min and washed twice with PBS. The final OD600 was adjusted to 0.1, and three serial 10-fold dilutions were prepared. Fractions from these dilutions (5 μl) were spotted onto 7H10-ADC medium containing 0.5% glycerol; 3 mM asparagine; and 200 μM adenine, guanine, or hypoxanthine. Growth was compared with that on purine-free medium.
To test complementation of HGPRT deficiency in the ΔpurF Δhgprt strain, the bacteria were transformed with pSE200.Mtb.HGPRT, pSE200.Msm.HGPRT, or empty pSE200 and selected on 7H10-ADC medium containing 25 μg/ml kanamycin, 100 μM adenine, and 3 mM asparagine. Resulting transformants were spotted onto 7H10-ADC containing 25 μg/ml kanamycin and 100 μM adenine, hypoxanthine, or guanine.
Sequence alignment and 3D modeling.
Alignment of M. tuberculosis and M. smegmatis HGPRT amino acid sequences was performed with Clustal W. The structural model of M. smegmatis HGPRT was built with UCSF Chimera with the Modeller software extension (26, 27) using the M. tuberculosis HGPRT X-ray structure as a template (PDB 4RHX) (12).
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Ministry of Education of the Czech Republic (NPU I LO 1302).
We thank Dagmar Grundová for excellent technical support.
We declare that we have no conflict of interest.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.World Health Organization. 2019. Global tuberculosis report 2019. World Health Organization, Geneva, Switzerland: https://www.who.int/tb/publications/global_report/en/. [Google Scholar]
- 2.Daley CL, Caminero JA. 2018. Management of multidrug-resistant tuberculosis. Semin Respir Crit Care Med 39:310–324. doi: 10.1055/s-0038-1661383. [DOI] [PubMed] [Google Scholar]
- 3.Wheeler PR. 1987. Biosynthesis and scavenging of purines by pathogenic mycobacteria including Mycobacterium leprae. J Gen Microbiol 133:2999–3011. doi: 10.1099/00221287-133-11-2999. [DOI] [PubMed] [Google Scholar]
- 4.Malathi VG, Ramakrishnan T. 1966. Biosynthesis of nucleic acid purines in Mycobacterium tuberculosis H37Rv. Biochem J 98:594–597. doi: 10.1042/bj0980594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ducati RG, Breda A, Basso LA, Santos DS. 2011. Purine salvage pathway in Mycobacterium tuberculosis. Curr Med Chem 18:1258–1275. doi: 10.2174/092986711795029627. [DOI] [PubMed] [Google Scholar]
- 6.Parker WB, Long MC. 2007. Purine metabolism in Mycobacterium tuberculosis as a target for drug development. Curr Pharm Des 13:599–608. doi: 10.2174/138161207780162863. [DOI] [PubMed] [Google Scholar]
- 7.Makowska-Grzyska M, Kim Y, Gorla SK, Wei Y, Mandapati K, Zhang M, Maltseva N, Modi G, Boshoff HI, Gu M, Aldrich C, Cuny GD, Hedstrom L, Joachimiak A. 2015. Mycobacterium tuberculosis IMPDH in complexes with substrates, products and antitubercular compounds. PLoS One 10:e0138976. doi: 10.1371/journal.pone.0138976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Usha V, Gurcha SS, Lovering AL, Lloyd AJ, Papaemmanouil A, Reynolds RC, Besra GS. 2011. Identification of novel diphenyl urea inhibitors of Mt-GuaB2 active against Mycobacterium tuberculosis. Microbiology 157:290–299. doi: 10.1099/mic.0.042549-0. [DOI] [PubMed] [Google Scholar]
- 9.Park Y, Pacitto A, Bayliss T, Cleghorn LAT, Wang Z, Hartman T, Arora K, Ioerger TR, Sacchettini J, Rizzi M, Donini S, Blundell TL, Ascher DB, Rhee K, Breda A, Zhou N, Dartois V, Jonnala SR, Via LE, Mizrahi V, Epemolu O, Stojanovski L, Simeons F, Osuna-Cabello M, Ellis L, MacKenzie CJ, Smith ARC, Davis SH, Murugesan D, Buchanan KI, Turner PA, Huggett M, Zuccotto F, Rebollo-Lopez MJ, Lafuente-Monasterio MJ, Sanz O, Diaz GS, Lelièvre J, Ballell L, Selenski C, Axtman M, Ghidelli-Disse S, Pflaumer H, Bösche M, Drewes G, Freiberg GM, Kurnick MD, Srikumaran M, Kempf DJ, Green SR, Ray PC, Read K, Wyatt P, Barry CE, Boshoff HI. 2017. Essential but not vulnerable: indazole sulfonamides targeting inosine monophosphate dehydrogenase as potential leads against Mycobacterium tuberculosis. ACS Infect Dis 3:18–33. doi: 10.1021/acsinfecdis.6b00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keough DT, Hockova D, Rejman D, Spacek P, Vrbkova S, Krecmerova M, Eng WS, Jans H, West NP, Naesens LMJ, de Jersey J, Guddat LW. 2013. Inhibition of the Escherichia coli 6-oxopurine phosphoribosyltransferases by nucleoside phosphonates: potential for new antibacterial agents. J Med Chem 56:6967–6984. doi: 10.1021/jm400779n. [DOI] [PubMed] [Google Scholar]
- 11.Eng WS, Rejman D, Pohl R, West NP, Woods K, Naesens LMJ, Keough DT, Guddat LW. 2018. Pyrrolidine nucleoside bisphosphonates as antituberculosis agents targeting hypoxanthine-guanine phosphoribosyltransferase. Eur J Med Chem 159:10–22. doi: 10.1016/j.ejmech.2018.09.039. [DOI] [PubMed] [Google Scholar]
- 12.Eng WS, Hockova D, Spacek P, Janeba Z, West NP, Woods K, Naesens LMJ, Keough DT, Guddat LW. 2015. First crystal structures of Mycobacterium tuberculosis 6-oxopurine phosphoribosyltransferase: complexes with GMP and pyrophosphate and with acyclic nucleoside phosphonates whose prodrugs have antituberculosis activity. J Med Chem 58:4822–4838. doi: 10.1021/acs.jmedchem.5b00611. [DOI] [PubMed] [Google Scholar]
- 13.Griffin JE, Gawronski JD, DeJesus MA, Ioerger TR, Akerley BJ, Sassetti CM. 2011. High-resolution phenotypic profiling defines genes essential for mycobacterial growth and cholesterol catabolism. PLoS Pathog 7:e1002251. doi: 10.1371/journal.ppat.1002251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeJesus MA, Gerrick ER, Xu WZ, Park SW, Long JE, Boutte CC, Rubin EJ, Schnappinger D, Ehrt S, Fortune SM, Sassetti CM, Ioerger TR. 2017. Comprehensive essentiality analysis of the Mycobacterium tuberculosis genome via saturating transposon mutagenesis. mBio 8:e02133-16. doi: 10.1128/mBio.02133-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eng WS, Keough DT, Hockova D, Winzor DJ, Guddat LW. 2017. Oligomeric state of hypoxanthine-guanine phosphoribosyltransferase from Mycobacterium tuberculosis. Biochimie 135:6–14. doi: 10.1016/j.biochi.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 16.Patta PC, Martinelli LKB, Rotta M, Abbadi BL, Santos DS, Basso LA. 2015. Mode of action of recombinant hypoxanthine-guanine phosphoribosyltransferase from Mycobacterium tuberculosis. RSC Adv 5:74671–74683. doi: 10.1039/C5RA14918E. [DOI] [Google Scholar]
- 17.Keough DT, Hockova D, Holy A, Naesens LMJ, Skinner-Adams TS, de Jersey J, Guddat LW. 2009. Inhibition of hypoxanthine-guanine phosphoribosyltransferase by acyclic nucleoside phosphonates: a new class of antimalarial therapeutics. J Med Chem 52:4391–4399. doi: 10.1021/jm900267n. [DOI] [PubMed] [Google Scholar]
- 18.Tyagi JS, Sharma D. 2002. Mycobacterium smegmatis and tuberculosis. Trends Microbiol 10:68–69. doi: 10.1016/s0966-842x(01)02296-x. [DOI] [PubMed] [Google Scholar]
- 19.Shenkerman Y, Elharar Y, Vishkautzan M, Gur E. 2014. Efficient and simple generation of unmarked gene deletions in Mycobacterium smegmatis. Gene 533:374–378. doi: 10.1016/j.gene.2013.09.082. [DOI] [PubMed] [Google Scholar]
- 20.Keer J, Smeulders MJ, Williams HD. 2001. A purF mutant of Mycobacterium smegmatis has impaired survival during oxygen-starved stationary phase. Microbiology 147:473–481. doi: 10.1099/00221287-147-2-473. [DOI] [PubMed] [Google Scholar]
- 21.Knejzlik Z, Herkommerova K, Pichova I. 2019. Catabolism of 8-oxo-purines is mainly routed via the guanine to xanthine interconversion pathway in Mycobacterium smegmatis. Tuberculosis (Edinb) 119:101879. doi: 10.1016/j.tube.2019.101879. [DOI] [PubMed] [Google Scholar]
- 22.Hockova D, Janeba Z, Naesens L, Edstein MD, Chavchich M, Keough DT, Guddat LW. 2015. Antimalarial activity of prodrugs of N-branched acyclic nucleoside phosphonate inhibitors of 6-oxopurine phosphoribosyltransferases. Bioorg Med Chem 23:5502–5510. doi: 10.1016/j.bmc.2015.07.038. [DOI] [PubMed] [Google Scholar]
- 23.Banuls A-L, Sanou A, Anh NTV, Godreuil S. 2015. Mycobacterium tuberculosis: ecology and evolution of a human bacterium. J Med Microbiol 64:1261–1269. doi: 10.1099/jmm.0.000171. [DOI] [PubMed] [Google Scholar]
- 24.Goude R, Roberts DM, Parish T. 2015. Electroporation of mycobacteria. Methods Mol Biol 1285:117–130. doi: 10.1007/978-1-4939-2450-9_7. [DOI] [PubMed] [Google Scholar]
- 25.Blanco-Ruano D, Roberts DM, Gonzalez-Del-Rio R, Álvarez D, Rebollo MJ, Pérez-Herrán E, Mendoza A. 2015. Antimicrobial susceptibility testing for Mycobacterium sp. Methods Mol Biol 1285:257–268. doi: 10.1007/978-1-4939-2450-9_15. [DOI] [PubMed] [Google Scholar]
- 26.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. 2004. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 27.Šali A, Blundell TL. 1993. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol 234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 28.Berney M, Weimar MR, Heikal A, Cook GM. 2012. Regulation of proline metabolism in mycobacteria and its role in carbon metabolism under hypoxia. Mol Microbiol 84:664–681. doi: 10.1111/j.1365-2958.2012.08053.x. [DOI] [PubMed] [Google Scholar]
- 29.Snapper SB, Melton RE, Mustafa S, Kieser T, Jacobs WR Jr.. 1990. Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol Microbiol 4:1911–1919. doi: 10.1111/j.1365-2958.1990.tb02040.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.