Abstract
BACKGROUND
Progressive familial intrahepatic cholestasis (PFIC) encompasses a group of autosomal recessive disorders with high morbidity and mortality. Variants in the gene encoding tight junction protein-2 (TJP2) have been linked to PFIC type 4 (PFIC4), which predominantly presents in childhood. However, there are only limited data from adults with TJP2-related PFIC4. We report a family with an autosomal recessive disorder with a novel variant in the TJP2 gene in adults with very variable expression of PFIC4.
CASE SUMMARY
The index patient presented at 19 years old with liver cirrhosis and variceal bleeding and was treated with endoscopic banding and beta-blockers. In 2018, he developed primary liver cancer that was treated with radiofrequency ablation followed by liver transplantation in 2019. Genetic testing revealed a novel homozygous TJP2 variant causing PFIC4 (TJP2([NM_004817.3]:c.[3334C>T]; [3334C>T])). The consanguineous family consists of the father and mother (both heterozygous) and their 12 children, of which five carry the variant in a homozygous state; however, these five siblings have highly variable expression of PFIC4. Two homozygous brothers had cirrhosis and portal hypertension at diagnosis at the ages of 19 and 36. Two other homozygous brothers, age 23 and 19, and the homozygous sister, age 21, have elevated liver enzymes but presently no cirrhosis, which may suggest an age-dependent penetrance. In addition, five sisters had severe and mild intrahepatic cholestasis of pregnancy and carry the TJP2 variant in a homozygous and heterozygous state, respectively.
CONCLUSION
This novel TJP2 variant is associated with PFIC4 causing severe liver disease with cirrhosis and primary liver cancer in adolescents/adults.
Keywords: Progressive familial intrahepatic cholestasis, Tight junction protein 2, Genetic variants, Liver cirrhosis, Liver cancer, Case report
Core tip: We report a family with progressive familiar intrahepatic cholestasis type-4 (PFIC4) with a novel variant in the tight junction protein-2 gene. The five homozygous children have highly variable expression of PFIC4. Two brothers presented with cirrhosis, portal hypertension and hepatocellular carcinoma at ages 19 and 36. Two other homozygous brothers and a sister (age 19-23) have elevated liver enzymes without cirrhosis. Furthermore, five sisters had severe and mild intrahepatic cholestasis of pregnancy and carry the tight junction protein-2 variant in homozygous and heterozygous states, respectively. Close monitoring for cirrhosis and hepatocellular carcinoma development is warranted in PFIC4 syndrome.
INTRODUCTION
Progressive familial intrahepatic cholestasis (PFIC) encompasses a group of autosomal recessive disorders with high mortality and morbidity in childhood. There are few reports of the exact incidence of PFIC, but it is estimated to be 1/50000 to 1/100000[1]. Variants in the ATP-transporter genes ATP8B1, ABCB11, and ABCB4 are responsible for PFIC types 1, 2 and 3, respectively, and recently, the gene encoding tight junction protein-2 (TJP2) has been linked to PFIC type 4 (PFIC4), which predominantly presents in childhood[2]. However, there are only limited data from adults with TJP2-related PFIC4.
The TJP2 gene was first discovered by Gumbiner et al[3] in 1991. The TJP2 gene is located on chromosome 9q21.11 and has a total of 80932 base pairs and includes 23 exons[4]. The TJP2 gene product is the tight junction protein 2, also called zona occludens 2 (ZO-2), which belongs to the membrane-associated guanylate cyclase family[5]. TJP2 stabilizes tight junctions by binding to the cytoplasmic C termini of junctional transmembrane proteins, such as claudins, and linking them to the actin cytoskeleton[6]. The hepatocyte membranes adjacent to each other at the junction of the bile duct canaliculi and the hepatocyte basal membrane surface form a tight junction essential for the separation of bile from plasma[7,8]. Loss-of-function variants in the TJP2 gene can cause defects in tight junction function, leading to severe cholestatic liver disease, along with extrahepatic manifestations[9].
Here, we report a family with a novel TJP2 variant. The index patient, a 20-year-old male, was diagnosed with cirrhosis and portal hypertension in 2015 due to unexplained intrahepatic cholestasis. Based on the family history, we performed genetic screening of the index patient, eight of his eleven siblings and his parents. We discovered a novel TJP2 gene variant (TJP2 [NM_004817.3]:c.[3334C>T];[3334C>T] (p.(Gln1112*)) causing liver disease when both alleles were affected. Here, we report the clinical history.
CASE PRESENTATION
Clinical and biochemical data of the index patient and family
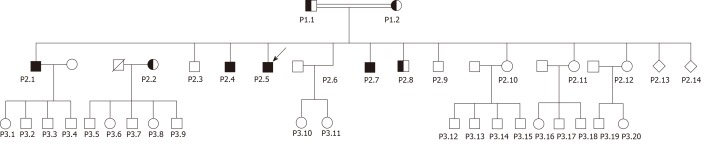
Basic information: The family consists of the parents (P1.1-P1.2), 12 live siblings (P2.1-P2.12) and two siblings who died at birth (P2.13-2.14); and 20 grandchildren (P3.1-P3. 20) as depicted in Figure 1.
Figure 1.
The family pedigree of the tight junction protein 2 variant.
Case 1: Index patient (P2.5)
Chief complaints: In 2015, the index patient (P2.5), a 20-year-old male, presented at our department with decompensated liver cirrhosis with previous variceal bleeding and complaints of tiredness.
History of present illness: At age 19, he had variceal bleeding treated with endoscopic banding and beta-blocker as secondary prophylaxis. In 2018 he developed biopsy-proven hepatocellular carcinoma (HCC) treated with radiofrequency ablation, and in 2019, he had a liver transplantation.
Physical examination: At first visit the patient was in good performance status and had normal BMI. He had clinical signs of splenomegaly and mild icterus but otherwise a normal physical examination with no signs of ascites.
Laboratory examinations: He presented with elevated bilirubin, alkaline phosphatase, decreased serum albumin (ALB) and normal alanine aminotransferase (ALT) as shown in Table 1.
Table 1.
Clinical and biochemical parameters and FibroScan data of tested family members on the day of the first presentation
| TJP2 status |
P1.1 |
P1.2 |
P2.1 |
P2.2 |
P2.3 |
P2.4 |
P2.5 |
P2.6 |
P2.7 |
P2.8 |
P2.9 |
P2.10-12 |
| +/wt | +/wt | +/+ | +/wt | wt/wt | +/+ | +/+ | +/+ | +/+ | +/wt | +/+ | NA | |
| Gender | M | F | M | F | M | M | M | F | M | M | M | F, F, F |
| Age in 2018 (yr) | 60 | 55 | 36 | 32 | 31 | 25 | 23 | 21 | 19 | 16 | 15 | NA |
| ALAT (< 45 female; < 70 male; (U/L) | 16 | 19 | 114 | 15 | 10 | 43 | 37 | 44 | 97 | 21 | 22 | NA |
| Bilirubin (5-25 μmol/L) | 8 | 4.7 | 29 | 4.4 | 14 | 12 | 45 | 10 | 17 | 14 | 11 | NA |
| Alkaline phosphatase (35-105 U/L) | 101 | 104 | 120 | 65 | 50 | 112 | 212 | 117 | 115 | 104 | 121 | NA |
| GGT (10-80 U/L) | 72 | 21 | 61 | 14 | 12 | 44 | 156 | 55 | 86 | 12 | 15 | NA |
| Coagulation II, VII, X/INR | 1.0 | 1.2 | 1.2 | 0.81 | 1 | 1.2 | 1.7 | 1.1 | 0.73 | 0.75 | 0.72 | NA |
| Albumin (36-48 g/L) | 44 | 41 | 36 | 37 | 44 | 46 | 29 | 37 | 27 | 44 | 47 | NA |
| Creatinine (60-105 μmol/L) | 68 | 40 | 71 | 49 | 62 | 68 | 66 | 37 | 71 | 67 | 53 | NA |
| Cholesterol (< 5.0 mmol/L) | 5.7 | 6.5 | 5 | 4.3 | 3.4 | 3.8 | 3.2 | 4.4 | NA | 3.3 | 4.7 | NA |
| HDL cholesterol (> 1.0 mmol/L) | 0.91 | 1.1 | 1.8 | 2.0 | 0.92 | 2 | 1.7 | 1.9 | NA | 1.1 | 1.3 | NA |
| LDL cholesterol (< 3 mmol/L) | 3.3 | 3.7 | 2.7 | 1.9 | 2.2 | 1.4 | 1.1 | 2.2 | NA | 1.8 | 3 | NA |
| Triglyceride (< 2 mmol/L) | 3.3 | 3.5 | 1.1 | 0.9 | 1.1 | 1 | 1 | 0.7 | NA | 0.9 | 1 | NA |
| Leucocytes (3.5-10.0 10^9/L) | 11.7 | 4.72 | 4.19 | 6.96 | 5.8 | 7.03 | 4.25 | 6.44 | 5.78 | 7.8 | 5.7 | NA |
| Hemoglobin (> 7.3 female; > 8.3 male, mmol/L) | 9.4 | 7.8 | 9.3 | 7.5 | 9.9 | 10.1 | 7.8 | 8 | 9.9 | 10.3 | 9.6 | NA |
| Platelets (165-400, × 109/L) | 335 | 405 | 77 | 266 | 229 | 218 | 64 | 191 | 210 | 379 | 339 | NA |
| sCD163 (0.69-3.86 mg/L) | 2.32 | 1.94 | NA | 1.90 | 2.95 | 3.96 | 5.23 | 6.03 | 3.91 | 1.31 | 1.75 | NA |
| FibroScan (kPa) | 4.4 | 3.3 | 75 | 4-7 | 6.6 | 8.1 | 24 | 9.1 | 5.9 | 5.7 | 7.8 | NA |
TJP2 status is indicated by +/+ (homozygous) +/WT (heterozygous), and wt/wt indicating wild type. NA: Not applicable; WT: Wild type.
In 2015, a liver biopsy showed established cirrhosis that was etiologically nonspecific but may include cirrhosis due to a cholestatic liver disease. In the fibrous septae, there were findings of original bile ducts and some bile duct proliferation accompanied by light to moderate etiologically nonspecific lymphocytic inflammation with only a few plasma cells. There were no granulomas and only very mild focal interphase hepatitis. A liver biopsy in 2018 confirmed the HCC diagnosis found on CT scanning.
Imaging examinations: A CT scan at first visit showed liver cirrhosis, splenomegaly, and portosystemic collateral blood flow. A CT scan in 2018 showed a 3 cm HCC, which was treated with RFA and with no signs of recurrence on follow-up CT scans.
Liver vein catherization showed a hepatic venous pressure gradient of 12 mmHg on beta-blocker treatment consistent with significant portal hypertension.
History of past illness: He had been told of transient icterus a few months after birth after an uncomplicated pregnancy. The remaining childhood was uneventful with normal development and milestones.
FINAL DIAGNOSIS
The patient was diagnosed with the PFIC4 syndrome of liver cirrhosis and portal hypertension with variceal bleeding and later HCC caused by a mutation in the TJP2 gene.
TREATMENT
The portal hypertension and varices were treated with endoscopic band ligation and beta-blocker treatment. The HCC was initially treated with radiofrequency ablation and finally the patient received a successful liver transplantation.
OUTCOME AND FOLLOW-UP
The patient is in good performance status and health, and in follow-up after successful liver transplantation.
Family history
This Syrian family immigrated to Denmark in 2015. Both parents and nine of their 12 children live in Denmark, while three children live in the Middle East. The three siblings living outside Denmark are apparently healthy with no liver-related symptoms, but all three have a history of mild pruritus during pregnancy (G4+G3+G2), suggesting intrahepatic cholestasis of pregnancy (ICP). However, the information is limited, and no genetic, clinical or biochemical information is available. All 20 children of the third generation (all below the age of 18) have no apparent liver-related symptoms.
Case 2-3: Parents (P1.1-P1.2): Consanguineous, unspecified.
P1.1: The father, 60 years old in 2018, and heterozygous for the TJP2 variant, has no liver-related complaints and normal liver biochemistry. He has slightly elevated cholesterol and triglyceride levels.
P1.2: The mother, 55 years old in 2018, and heterozygous for the TJP2 variant, has no liver-related symptoms and normal liver biochemistry. She has slightly elevated cholesterol and triglyceride levels. No history of intrahepatic cholestasis or pruritus during 14 pregnancies. Twelve children are alive, and two died in the perinatal period (P2.13-2.14) without any information on liver disease.
Case 4-11: Siblings to the index case. The index case (P2.5) has 11 living siblings (P2.1-P2.4 and P2.6-P2.12), and two siblings (P2.13-P2.14) who died at birth. Three of the 11 live siblings (P2.10-P2.12) do not live in Denmark and could not be studied. The remaining siblings were studied.
P2.1: Male, 36-year-old, homozygous for the TJP2 variant, presented in 2018 with abdominal pain and pruritus, elevated liver parameters, bile acids and pruritus (Table 1). Ultrasound showed splenomegaly. Due to clinical decompensation with ascites and worsening liver function with increasing ALT 224 U/L, alkaline phosphatase 191 U/L, bilirubin 434 μmol/L and INR 2.6, he was diagnosed with severe acute-on-chronic liver failure (ACLF) and received successful liver transplantation. The patient is married and has 4 children, all apparently healthy but heterozygous for the variant.
Biopsy of the explanted liver showed established cirrhosis with regeneration noduli with bile duct proliferation and inflammation in the fibrous septae. There was pronounced canalicular cholestasis. There were no granulomas.
P2.2: Female, 32-year-old in 2018, heterozygous for the TJP2 variant. She has no liver-related symptoms and normal liver biochemistry. During all five uncomplicated pregnancies, she suffered from mild pruritus with no evidence of elevated liver enzymes and received no treatment with ursodeoxycholic acid. She has five healthy children.
P2.3: Male, 31 years old in 2018, carries two wild-type TJP2 alleles and has no liver-related symptoms and normal liver biochemistry.
P2.4: Male, 25-year-old in 2018, homozygous for the TJP2-variant. He had neonatal icterus, but apart from that otherwise healthy. He has slightly elevated alkaline phosphatase and normal ALT and bilirubin.
Liver biopsy 2018 showed normal liver histology with no signs of cholestatic liver disease.
P2.6: Female, 18-year-old in 2016, homozygous for the TJP2-variant. In 2016, she was asymptomatic but had elevated liver alkaline phosphatase (117 U/L). At age 21, she was pregnant, and in pregnancy week 10, she had slightly elevated alkaline phosphatase. From pregnancy week 23, she developed pruritus and increased ALT (91 U/L), alkaline phosphatase (235 U/L), and bile acid levels (57 mmol/L) but normal bilirubin. She was treated with ursodeoxycholic acid from pregnancy week 29 (250 mg t.i.d. increased to 500 mg b.i.d.) with effect on pruritus and bile acids. After spontaneous delivery at gestational week 37+4 of a healthy girl, the liver parameters normalized.
In August 2018, a new pregnancy was complicated with pruritus and elevated alkaline phosphatase (129 U/L) and bile acids (35 µmol/L) from week 17 but normal ALT and bilirubin levels. She was treated with ursodeoxycholic acid (500 mg t.i.d.) and rimactan (300 mg/day) for severe pruritus until labor was induced at week 38, and she delivered a healthy daughter with no signs of cholestatic liver disease.
A liver biopsy in 2017 between two pregnancies showed minimal unspecific changes with minor lymphocytic infiltration and no ductopenia. She has elevated sCD163 (6.03 mg/L) and a FibroScan of 9.1 kPa.
P2.7: Male, 19-year-old in 2018, homozygous for the TJP2 variant, has no symptoms of liver disease. He has slightly elevated liver enzymes and a FibroScan of 5.9 kPa and sCD163 3.91 mg/L.
P2.8: Male, 16-year-old in 2018, heterozygous for the TJP2 variant, has no symptoms of liver disease and normal liver enzymes and FibroScan.
P2.9: Male, 15-year-old male in 2018, carries two wild-type TJP2 alleles, has no symptoms of liver disease and normal liver enzymes and a FibroScan of 7.8 kPa.
Case 12-34: P2.10-P2.12: Living outside Denmark and are apparently without symptoms of liver disease; however, all three women with 4, 3 and 2 pregnancies have reported pruritus in their pregnancies. Blood samples and genetic testing have not been performed.
P3.1-P3.20: Apparently without symptoms of liver disease but limited information available.
Other gene variants
As we used a gene panel approach, in addition to the TJP2 variants, we also discovered a number of other variants in genes potentially involved in genetic cholestatic liver diseases, ABCB11, ABCG5, ABCC2 and UGT1A1, as shown in Table 2.
Table 2.
Genetic variations discovered in the family using the gene panel approach
| Patient ID | Age (yr) | Gender | TJP2 variation | TJP2 genotype | Other gene variations | Phenotype |
| P2.1 | 36 | M | TJP2 [NM_004817.3]:c.[3334C>T];[3334C>T] | Homozygous | ABCB11[NM_003742.2]:c.[1331T>C];[=] | PFIC4 with cirrhosis, portal hypertension and acute-on-chronic liver failure; Liver transplantation |
| ABCC2[NM_000392.3]:c.[3563T>A];[4544G>A]1 | ||||||
| ABCG5[NM_022436.2]:c.[148C>T];[=] | ||||||
| UGT1A1[NM_000463.2]C.-53TA[6];[7] | ||||||
| P2.5 | 23 | M | TJP2 [NM_004817.3]:c.[3334C>T];[3334C>T] | Homozygous | ABCB11[NM_003742.2]:c.[1331T>C];[1331T>C]2 | PFIC4 with cirrhosis, portal hypertension. Primary liver cancer; Liver transplantation |
| ABCC2[NM_000392.3]:c.[3563T>A];[4544G>A]1 | ||||||
| ABCG5[NM_022436.2]:c.[148C>T];[=] | ||||||
| P2.4 | 25 | M | TJP2 [NM_004817.3]:c.[3334C>T];[3334C>T] | Homozygous | ABCB11[NM_003742.2]:c.[1331T>C];[1331T>C]2 | Elevated liver enzymes |
| ABCC2[NM_000392.3]:c.[3563T>A];[4544G>A]1 | ||||||
| ABCG5[NM_022436.2]:c.[148C>T];[=] | ||||||
| P2.6 | 21 | F | TJP2 [NM_004817.3]:c.[3334C>T];[3334C>T] | Homozygous | ABCB11[NM_003742.2]:c.[1331T>C];[=] | Elevated liver enzymes; Severe cholestasis in pregnancy |
| ABCC2[NM_000392.3]:c.[3563T>A];[4544G>A]1 | ||||||
| ABCG5[NM_022436.2]:c.[148C>T];[=] | ||||||
| UGT1A1[NM_000463.2]C.-53TA[6];[7] | ||||||
| P2.7 | 19 | M | TJP2 [NM_004817.3]:c.[3334C>T];[3334C>T] | Homozyg-ous | ABCB11[NM_003742.2]:c.[1331T>C];[=] | Elevated liver enzymes |
| ABCC2[NM_000392.3]:c.[3563T>A];[4544G>A]1 | ||||||
| ABCG5[NM_022436.2]:c.[148C>T];[=] | ||||||
| UGT1A1[NM_000463.2]C.-53TA[6];[7] | ||||||
| P1.1 | 60 | M | TJP2 [NM_004817.3]:c.[3334C>T];[3334=] | Heterozygous | ABCB11[NM_003742.2]:c.[1331T>C];[1331T=] | Normal |
| ABCC2[NM_000392.3]:c.[3563T>A];[4544G>A]1 | ||||||
| UGT1A1[NM_000643.2]C.-53TA[6];[7] | ||||||
| P1.2 | 55 | F | TJP2 [NM_004817.3]:c.[3334C>T];[3334=] | Heterozygous | ABCB11[NM_003742.2]:c.[1331T>C];[=] | Normal |
| ABCG5[NM_022436.2]:c.[148C>T];[=] | ||||||
| P2.2 | 32 | F | TJP2 [NM_004817.3]:c.[3334C>T];[3334=] | Heterozygous | ABCB11[NM_003742.2]:c.[1331T>C];[=] | Mild cholestasis in pregnancy |
| ABCG5[NM_022436.2]:c.[148C>T];[=] | ||||||
| P2.8 | 16 | M | TJP2 [NM_004817.3]:c.[3334C>T];[=] | Heterozygous | ABCB11[NM_003742.2]:c.[1331T>C];[1331T=]1 | Normal |
| ABCG5[NM_022436.2]:c.[148C>T];[=] | ||||||
| UGT1A1[NM_000463.2]C.-53TA[6];[7] | ||||||
| P2.3 | 31 | M | TJP2 [NM_004817.3]:c.[3334=];[3334=] | Wild type | ABCG5[NM_022436.2]:c.[148C>T];[=] | Normal |
| P2.9 | 15 | M | TJP2 [NM_004817.3]:c.[3334=];[3334=] | Wild type | ABCB11[NM_003742.2]:c.[1331T>C];[1331T>C]2 | Normal |
| ABCC2[NM_000392.3]:c.[3563T>A];[4544G>A]1 | ||||||
| P2.10 | - | F | - | - | Mild cholestasis, 4 pregnancies? | |
| P2.11 | - | F | - | - | Mild cholestasis, 3 pregnancies? | |
| P2.12 | - | F | - | - | Mild cholestasis, 2 pregnancies? |
Compound heterozygosity for the ABCC2 gene variant coding for multidrug resistance protein 2 (MRP2) may be associated with pruritus in patients with primary biliary cholangitis.
Homozygosity for ABCB11 gene variant coding for bile salt export pump (BSEP) protein is associated with cholestasis in pregnancy and drug-induced cholestasis. M: Male; F: Female.
Genetic testing
DNA from the index patient and his family members was screened for variants in genes known to be involved in a spectrum of liver and cystic diseases using an in-house-targeted gene panel. The following genes were analyzed: ATP8B1, ABCB11, ABCB4, ABCG5, ABCC2, JAG1, NOTCH2, UGT1A1 and TJP2.
Genomic DNA was isolated from peripheral blood leukocytes using the magnetic bead-based automated Chemagic MSM1 instrument, following the manufacturer’s instructions (PerkinElmer Chemagen, Baesweiler, Germany). A library for Illumina paired-end sequencing was constructed from 1 µg of genomic DNA using the KAPA HTP Library Preparation Kit according to the manufacturer’s instructions (KAPA Biosystems Inc., Wilmington, MA). The generated libraries were enriched for regions of interest using a customized targeting probe set (SeqCap EZ Choise, Roche Nimblegen, Inc., Madison, WI) and sequenced on a MiSeq Desktop Sequencer (Illumina, San Diego, CA). The reads obtained from sequencing were aligned to the human genome (hg19), and variants were called using Biomedical Genomics Workbench v.2 (CLC Bio-Qiagen, Aarhus, Denmark).
DISCUSSION
In this case report, we present a novel variant in the TJP2 gene in a large family of consanguineous parents using a gene panel approach. This TJP2 variant caused liver disease in the homozygous state, while heterozygous individuals were unaffected except for a high rate of ICP in pregnancies. In contrast to previously published case reports, our family presented with liver disease and HCC during adolescence or in adulthood. For TJP2 and for this variant in particular, there seems to be an age-dependent and high penetrance, including increased risk of liver cancer. In addition to this novel TJP2 variant, the family also harbored variants in other genes involved in cholestatic liver diseases, which may explain differences in genotype-phenotype presentations.
The novel TJP2 variant [NM_004817.3]:c.[3334C>T];[3334C>T] (p.(Gln1112*)) is a nonsense-variant that likely induces nonsense-mediated decay and degradation of the TJP2-protein and therefore mimics TJP2 haploinsufficiency. TJP2 haploinsufficiency may present with different phenotypes, including typical PFIC4 with rapid progression of cholestatic liver disease mainly in children[6], or unexplained cholestasis with pruritis; with some extrahepatic features as hearing loss[10], or as ICP[11]. Previously described TJP2 variants are also most likely protein truncating with alteration of the reading frame resulting in generation of premature stop codons. This is followed by the absence of the protein, as demonstrated by both Western ligand blotting and immunohistochemistry[6]. Normally, the TJP2 protein binds to junctional transmembrane proteins on the C-terminus of cytoplasmic domains and belongs to a family of membrane-associated guanylate kinase homologs[12]. The TJP2 protein participates in functional junctions in epithelial and endothelial cells[13], where it plays an important role in orientating parts of these paracellular structures interacting with cytoskeletal proteins and integral membrane proteins[4,6]. In the absence of TJP2, the normal compactness of the tight junctions is impaired, resulting in leakage of bile and biliary substances into the liver parenchyma, which may elicit an inflammatory response, including macrophage activation and fibrosis formation.
In previous studies, several variants have been described in TJP2 and associated with liver as well as other diseases. We performed a literature search for publications regarding TJP2 variants as presented in Table 3 with genetic, clinical, biochemical, and histological data. The majority of studies report severe liver disease in children homozygous for a variant in TJP2. These children have elevated liver enzymes and bile acids and congenital hepatic fibrosis at age 0-12 years, and phenotypes also include HCC development and need for liver transplantation[6,14-17]. Children with the variant in a heterozygous state presented with normal or slightly elevated liver enzymes, bile acids and normal FibroScans[2,18]. Regarding other diseases, Wang et al[10]. described autosomal dominant nonsyndromic hearing impairment (ADNSHHI) at the age of 21-68 years in patients with a missense variant in TJP2 (c.2081G>A (p.G694E)). Carlton et al[19] described familial hypercholesterolemia age of <1 to 8 years in children homozygous for TJP2 variants.
Table 3.
Literature review of findings of genetically verified tight junction protein 2 variants
| Ref. | TJP2 gene | Age | Liver enzymes | Bile acids | Fibrosis | Pruritus | ICP | HCC | Liver failure | TJP2 mutation | TJP2/Included |
| Vitale et al[2] | Heterozygous | 37-51 yr | Elevated | Elevated | Normal FibroScan | 1 with obvious symptoms | 1 with DIC and ICP | NK | NK | p.[T62M]; [=]p.[I875T]; [=] | 10/48 |
| Sambrotta et al[6] | 12 children Homozyg-ous | 1-3 mo | Low or normal | Elevated | Cirrhosis and portal hypertension age 4 and 7 | No | No | Not described | 9 liver transplantations | c.766_769delGCCT, c.885delC | 12/12 |
| 1.5-4 years age | c.782delA, c.1361delC, c.1992-2A>G, c.953-735_2356-249del | ||||||||||
| 1 died 13 months | c.3408-?_3573+?del and c.1894C>T | ||||||||||
| Wang et al[10] | 21-68 yr | NK | NK | NK | NK | NK | NK | NK | c.2081G>A(p.G694E) | 8/21 Hearing loss | |
| Dixon et al[11] | Heterozygous | NK | Elevated | Elevated | NK | Yes | All confirmed ICP | NK | NK | p.Thr62Met; p.Thr626Ser | 3/26 |
| 1 stillbirth | c.1877C > G. p.Thr626Aer | ||||||||||
| Ge et al[14] | Compound Heterozygous | 23 mo | Elevated | Elevated | NK | Alleviated after treatment | - | NK | NK | c.2448 + 1G > C | 1/1 |
| c.2639delC (p.T880Sfs*12) | |||||||||||
| Zhou et al[15] | Compound Heterozygous | 26 mo | Elevated | NK | Both with cirrhosis | NK | No | Yes | Yes | 2668-1G>T /c.2438dupT (p.Asn814Glnfs | 2/2 |
| Homozyg-ous | 6 mo | Elevated | Yes, age 2 years | NK | c.817delG (p.A273fs | ||||||
| Vij et al[16] | Homozyg-ous | 7 yr | Normal | Elevated | Cirrhotic explant liver and high-grade dysplastic nodule | Yes | No | Yes, early well- differentiated | No | c.(2659+1_2660-1)/(2760+1_2761-1) | 1/1 |
| Shagrani et al[17] | Heterozygous | 0-12 yr | Elevated | Normal | 1 with congenital hepatic fibrosis | No | No | No | NK | c.2038delA:p.R680fs | 12/37 |
| Homozyg-ous | But 4 patients required liver transplantation | c.1012C>T:p.R338X | |||||||||
| c.1012C>T:p.R338X | |||||||||||
| c.1013delG:p.R338fs | |||||||||||
| c.1190C>T:p.P397L | |||||||||||
| c.1373delC:p.A458fs | |||||||||||
| c.1373delC:p.A458fs | |||||||||||
| Chen et al[18] | Heterozygous | > 1 yr | NK | Elevated but no detailed data | NK | No | No | No | NK | c.2174G>A, | 4/33 |
| c.343A>G/c.1377T>G | |||||||||||
| c.343A>G/c.1377T>G | |||||||||||
| c.925C>T | |||||||||||
| Carlton et al[19] | Homozyg-ous | 1-8 yr | Almost normal | Almost normal or NK | NK | Yes | NK | NK | NK | 143C/143C | 11 individuals with familial hypercholanemia in 8 families |
Intrahepatic cholestasis of pregnancy (ICP) and stillbirth have been described in women heterozygous for another variant in the TJP2 gene[2,11] Similarly, the women in the present family heterozygous or homozygous for the TJP2 variant presented with mild and severe ICP, respectively, and the latter needed medical treatment of pruritus and induction of labor (week 38).
In contrast to previous reports on TJP2 variants, homozygous members of this family presented with liver disease in late adolescence or adulthood, including cirrhosis at age 19 (P2.5) and cirrhosis and ACLF at age 35 (P2.1). Two other homozygous brothers, age 23 and 19 (P2.4, P27) and homozygous sister age 21 (P2.6), have only had mild disease with no fibrosis on liver biopsies thus far. However, they all have high FibroScans and sCD163 levels as a marker of macrophage activation involved in liver inflammation and fibrosis.
The basic pathological sign in intrahepatic cholestasis is capillary bile duct occlusion with accumulation of bile in capillary bile ducts, along with zone 3 hepatocytes and Kupffer cells accompanied by hepatocyte degeneration, foam cells, fibrosis formation, and finally cirrhosis[20]. In children with early stage PFIC4, capillary bile duct cholestasis and mild hepatocyte degeneration can be observed with hepatocyte giant cell transformation (more common in infancy, gradually recovering with age) and ballooning of hepatocytes. Infant biliary tract injury is mild, but with aging, biliary tract injury progress is accompanied by bile duct paucity. As described above, macrophages seem to be involved in the progression of liver disease with fibrosis and cirrhosis. Macrophage activation can be determined by sCD163 levels, and we have previously demonstrated elevated levels in relation to liver disease severity (e.g., inflammation, fibrosis, portal hypertension) and prognosis[21,22]. In support of this, we also showed elevated sCD163 levels in this PFIC4 family in patients with severe liver disease and carrying the variant in a homozygous state compared to family members heterozygous for the variant or wild-type subjects with normal liver parameters. We propose that sCD163 levels and FibroScan in addition to standard biochemistry (alkaline phosphatase, bilirubin, ALAT, INR, GGT, and bile acids) can be used as follow-up in these patients prospectively. In support of this, sCD163 and FibroScan values were especially high in patients with cirrhosis.
Since we used a gene panel approach for the genetic investigation in this family, we discovered some other genetic variants in addition to the TJP2 variant, as shown in Table 2. Of interest in relation to liver diseases were variants in the ABCB11, ABCG5, ABCC2 and the UGT1A1 genes.
Variants in ABCB11 encoding the BSEP protein result in a variety of cholestatic diseases, such as PFIC2, benign recurrent intrahepatic cholestasis type 2 (BRIC2), drug-induced cholestasis, and intrahepatic cholestasis of pregnancy[11,23]. There are some commonly known characteristics of progressive familial intrahepatic cholestasis associated with different genes, as shown[24,25] in Table 4. Of the nine tested siblings, the two TJP2-homozygous patients with liver cirrhosis were heterozygous (P2.1) and homozygous (P2.5) for a common disease-associated polymorphism ABCB11, respectively; the latter also presented with HCC. This might contribute to the phenotype suggesting that homozygosity for variants in both TJP2 and ABCB11 could be a significant risk factor for severe liver disease, including HCC development. Similarly, of the two female siblings presenting with mild (P2.2) and severe (P2.6) ICP, the latter was homozygous for the variants in both TJP2 and ABCB11. This patient may also be at increased risk for HCC, and this warrants close follow-up and monitoring, especially if progression to cirrhosis is observed.
Table 4.
Common known phenotypes of the genes included in the panel including characteristics of progressive familial intrahepatic cholestasis[24,25]
| Mutated gene (deficiency) | Disease association (autosomal recessive) | Disease association (autosomal dominant) |
| ATP8B1(FIC1) | PFIC1(2009) BRIC1 | ICP |
| ABCB11(BSEP) | PFIC2(2009) BRIC2 | ICP Drug-induced cholestasis |
| ABCB4 (MDR3) | PFIC3 | ICP LPAC Drug-induced cholestasis |
| TJP2(TJP2) | PFIC4 | ICP |
| ABCC2(MRP2) | Dubin-Johnson | ICP |
| ABCG5(ABCG5) | Sitosterolemia | Cholelithiasis |
| UGT1A1(UGT1A1) | Crigler-Najjar syndrome type 1; Crigler-Najjar syndrome type 2; Gilbert syndrome (promotor regions) | |
| NR1H4(FXR) | Intralobular cholestasis | Posttransplant hepatic steatosis |
| MYO5B(MYO5B) | Microvillus inclusion disease (MVID); Cholestasis without MVID |
ICP: Intrahepatic cholestasis during pregnancy; LPAC: Low phospholipid-associated cholelithiasis; PFIC: Progressive familial intrahepatic cholestasis; BRIC: Benign recurrent intrahepatic cholestasis.
The ABCC2 (ATP Binding Cassette Subfamily C Member 2) gene is associated with Dubin-Johnson syndrome[26] and pruritus in patients with biliary cirrhosis[27]. In this family, the father (P1.1) and six siblings harbored two ABCC2 variants on the same allele, and this combination was associated with more severe liver disease, as indicated in the patients with cirrhosis (P2.1 and P2.5); however, younger patients were less affected apart from ICP (P2.6).
CONCLUSION
In conclusion, we report an adult PFIC4 family with a novel variant in the TJP2 gene (TJP2 [NM_004817.3]:c.[3334C>T];[3334C>T] p.(Gln1112*)). This variant has not been previously linked to liver disease. In the same family, patients homozygous or heterozygous for this TJP2 variant have different manifestations and severity of disease, which may be explained by age-dependent penetrance of disease and/or the other genetic variants detected by the gene panel approach (e.g., ABCB11 and ABCC2 variants). This novel TJP2 variant is associated with adult PFIC4 liver disease with the development of liver cirrhosis and HCC. Patients who are homozygous for this variant should have close follow-up and monitoring of liver disease progression, including HCC screening by ultrasound, standard biochemistry, macrophage activation marker sCD163, and FibroScan.
Footnotes
Informed consent statement: Written informed consent was obtained from the index patient and all individuals of the family in Denmark for the genetic study and publication of this case report.
Conflict-of-interest statement: All other authors declare that they have no conflicts of interest.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Manuscript source: Invited manuscript
Peer-review started: November 18, 2019
First decision: December 23, 2019
Article in press: January 19, 2020
Specialty type: Gastroenterology and hepatology
Country of origin: Denmark
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ali FEM, Jin C, Mizuguchi T, Norton PA, Zhu YY S-Editor: Ma YJ L-Editor: A E-Editor: Ma YJ
Contributor Information
Chun-Shan Wei, Department of Clinical Medicine - Department of Hepatology and Gastroenterology, Aarhus University Hospital, Aarhus N DK-8200, Denmark; Department of Hepatology, Shenzhen Traditional Chinese Medicine Hospital, The Fourth Clinical Medical College of Guangzhou University of Chinese Medicine, Shenzhen 518033, Guangdong Province, China.
Naja Becher, Department of Clinical Genetics, Aarhus University Hospital, Aarhus N DK-8200, Denmark.
Jenny Blechingberg Friis, Department of Clinical Genetics, Aarhus University Hospital, Aarhus N DK-8200, Denmark.
Peter Ott, Department of Clinical Medicine - Department of Hepatology and Gastroenterology, Aarhus University Hospital, Aarhus N DK-8200, Denmark.
Ida Vogel, Department of Clinical Genetics, Aarhus University Hospital, Aarhus N DK-8200, Denmark.
Henning Grønbæk, Department of Clinical Medicine - Department of Hepatology and Gastroenterology, Aarhus University Hospital, Aarhus N DK-8200, Denmark. henngroe@rm.dk.
References
- 1.Nicastro E, D'Antiga L. Bergamo, Italy: Springer Nature Switzerland AG; 2019. Genetic cholestatic disorders. Paediatric hepatology, gastroenterology and transplantation; p. 228. [Google Scholar]
- 2.Vitale G, Gitto S, Raimondi F, Mattiaccio A, Mantovani V, Vukotic R, D'Errico A, Seri M, Russell RB, Andreone P. Cryptogenic cholestasis in young and adults: ATP8B1, ABCB11, ABCB4, and TJP2 gene variants analysis by high-throughput sequencing. J Gastroenterol. 2018;53:945–958. doi: 10.1007/s00535-017-1423-1. [DOI] [PubMed] [Google Scholar]
- 3.Gumbiner B, Lowenkopf T, Apatira D. Identification of a 160-kDa polypeptide that binds to the tight junction protein ZO-1. Proc Natl Acad Sci USA. 1991;88:3460–3464. doi: 10.1073/pnas.88.8.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chlenski A, Ketels KV, Korovaitseva GI, Talamonti MS, Oyasu R, Scarpelli DG. Organization and expression of the human zo-2 gene (tjp-2) in normal and neoplastic tissues. Biochim Biophys Acta. 2000;1493:319–324. doi: 10.1016/s0167-4781(00)00185-8. [DOI] [PubMed] [Google Scholar]
- 5.Jesaitis LA, Goodenough DA. Molecular characterization and tissue distribution of ZO-2, a tight junction protein homologous to ZO-1 and the Drosophila discs-large tumor suppressor protein. J Cell Biol. 1994;124:949–961. doi: 10.1083/jcb.124.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sambrotta M, Strautnieks S, Papouli E, Rushton P, Clark BE, Parry DA, Logan CV, Newbury LJ, Kamath BM, Ling S, Grammatikopoulos T, Wagner BE, Magee JC, Sokol RJ, Mieli-Vergani G University of Washington Center for Mendelian Genomics, Smith JD, Johnson CA, McClean P, Simpson MA, Knisely AS, Bull LN, Thompson RJ. Mutations in TJP2 cause progressive cholestatic liver disease. Nat Genet. 2014;46:326–328. doi: 10.1038/ng.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blau BJ, Miki T. The role of cellular interactions in the induction of hepatocyte polarity and functional maturation in stem cell-derived hepatic cells. Differentiation. 2019;106:42–48. doi: 10.1016/j.diff.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Treyer A, Müsch A. Hepatocyte polarity. Compr Physiol. 2013;3:243–287. doi: 10.1002/cphy.c120009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sticova E, Jirsa M, Pawłowska J. New Insights in Genetic Cholestasis: From Molecular Mechanisms to Clinical Implications. Can J Gastroenterol Hepatol. 2018;2018:2313675. doi: 10.1155/2018/2313675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang HY, Zhao YL, Liu Q, Yuan H, Gao Y, Lan L, Yu L, Wang DY, Guan J, Wang QJ. Identification of Two Disease-causing Genes TJP2 and GJB2 in a Chinese Family with Unconditional Autosomal Dominant Nonsyndromic Hereditary Hearing Impairment. Chin Med J (Engl) 2015;128:3345–3351. doi: 10.4103/0366-6999.171440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dixon PH, Sambrotta M, Chambers J, Taylor-Harris P, Syngelaki A, Nicolaides K, Knisely AS, Thompson RJ, Williamson C. An expanded role for heterozygous mutations of ABCB4, ABCB11, ATP8B1, ABCC2 and TJP2 in intrahepatic cholestasis of pregnancy. Sci Rep. 2017;7:11823. doi: 10.1038/s41598-017-11626-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laura RP, Ross S, Koeppen H, Lasky LA. MAGI-1: a widely expressed, alternatively spliced tight junction protein. Exp Cell Res. 2002;275:155–170. doi: 10.1006/excr.2002.5475. [DOI] [PubMed] [Google Scholar]
- 13.Sambrotta M, Thompson RJ. Mutations in TJP2, encoding zona occludens 2, and liver disease. Tissue Barriers. 2015;3:e1026537. doi: 10.1080/21688370.2015.1026537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ge T, Zhang X, Xiao Y, Wang Y, Zhang T. Novel compound heterozygote mutations of TJP2 in a Chinese child with progressive cholestatic liver disease. BMC Med Genet. 2019;20:18. doi: 10.1186/s12881-019-0753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou S, Hertel PM, Finegold MJ, Wang L, Kerkar N, Wang J, Wong LJ, Plon SE, Sambrotta M, Foskett P, Niu Z, Thompson RJ, Knisely AS. Hepatocellular carcinoma associated with tight-junction protein 2 deficiency. Hepatology. 2015;62:1914–1916. doi: 10.1002/hep.27872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vij M, Shanmugam NP, Reddy MS, Sankaranarayanan S, Rela M. Paediatric hepatocellular carcinoma in tight junction protein 2 (TJP2) deficiency. Virchows Arch. 2017;471:679–683. doi: 10.1007/s00428-017-2204-1. [DOI] [PubMed] [Google Scholar]
- 17.Shagrani M, Burkholder J, Broering D, Abouelhoda M, Faquih T, El-Kalioby M, Subhani SN, Goljan E, Albar R, Monies D, Mazhar N, AlAbdulaziz BS, Abdelrahman KA, Altassan N, Alkuraya FS. Genetic profiling of children with advanced cholestatic liver disease. Clin Genet. 2017;92:52–61. doi: 10.1111/cge.12959. [DOI] [PubMed] [Google Scholar]
- 18.Chen HL, Li HY, Wu JF, Wu SH, Chen HL, Yang YH, Hsu YH, Liou BY, Chang MH, Ni YH. Panel-Based Next-Generation Sequencing for the Diagnosis of Cholestatic Genetic Liver Diseases: Clinical Utility and Challenges. J Pediatr. 2019;205:153–159.e6. doi: 10.1016/j.jpeds.2018.09.028. [DOI] [PubMed] [Google Scholar]
- 19.Carlton VE, Harris BZ, Puffenberger EG, Batta AK, Knisely AS, Robinson DL, Strauss KA, Shneider BL, Lim WA, Salen G, Morton DH, Bull LN. Complex inheritance of familial hypercholanemia with associated mutations in TJP2 and BAAT. Nat Genet. 2003;34:91–96. doi: 10.1038/ng1147. [DOI] [PubMed] [Google Scholar]
- 20.Mourad MM, Algarni A, Liossis C, Bramhall SR. Aetiology and risk factors of ischaemic cholangiopathy after liver transplantation. World J Gastroenterol. 2014;20:6159–6169. doi: 10.3748/wjg.v20.i20.6159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Møller HJ, Kazankov K, Rødgaard-Hansen S, Nielsen MC, Sandahl TD, Vilstrup H, Moestrup SK, Grønbæk H. Soluble CD163 (sCD163): Biomarker of Kupffer Cell Activation in Liver Disease. In: Preedy VR, editor. Biomarkers in Liver Disease. In: Preedy VR, editor. Dordrecht: Springer Netherlands; 2016. pp. 1–28. [Google Scholar]
- 22.Grønbaek H, Sandahl TD, Mortensen C, Vilstrup H, Møller HJ, Møller S. Soluble CD163, a marker of Kupffer cell activation, is related to portal hypertension in patients with liver cirrhosis. Aliment Pharmacol Ther. 2012;36:173–180. doi: 10.1111/j.1365-2036.2012.05134.x. [DOI] [PubMed] [Google Scholar]
- 23.Davit-Spraul A, Fabre M, Branchereau S, Baussan C, Gonzales E, Stieger B, Bernard O, Jacquemin E. ATP8B1 and ABCB11 analysis in 62 children with normal gamma-glutamyl transferase progressive familial intrahepatic cholestasis (PFIC): phenotypic differences between PFIC1 and PFIC2 and natural history. Hepatology. 2010;51:1645–1655. doi: 10.1002/hep.23539. [DOI] [PubMed] [Google Scholar]
- 24.Bull LN, Thompson RJ. Progressive Familial Intrahepatic Cholestasis. Clin Liver Dis. 2018;22:657–669. doi: 10.1016/j.cld.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Aamann L, Ørntoft N, Vogel I, Grønbaek H, Becher N, Vilstrup H, Ott P, Lildballe DL. Unexplained cholestasis in adults and adolescents: diagnostic benefit of genetic examination. Scand J Gastroenterol. 2018;53:305–311. doi: 10.1080/00365521.2017.1422800. [DOI] [PubMed] [Google Scholar]
- 26.Huynh MT, Chrétien Y, Grison S, Delaunay JL, Lascols O, Tran CT, Goria O, Ramond MJ, Barbu V. Novel compound heterozygous ABCC2 variants in patients with Dubin-Johnson syndrome and intrahepatic cholestasis of pregnancy. Clin Genet. 2018;94:480–481. doi: 10.1111/cge.13420. [DOI] [PubMed] [Google Scholar]
- 27.Kojima H, Nies AT, König J, Hagmann W, Spring H, Uemura M, Fukui H, Keppler D. Changes in the expression and localization of hepatocellular transporters and radixin in primary biliary cirrhosis. J Hepatol. 2003;39:693–702. doi: 10.1016/s0168-8278(03)00410-0. [DOI] [PubMed] [Google Scholar]