Abstract
With this study, we aim to test the hypothesis that the effect of cannabidiol on drop‐seizure frequency in patients with Lennox–Gastaut syndrome and Dravet syndrome could be attributed to a drug–drug interaction with clobazam. We performed clinical trial simulations for the effect of 20 mg/kg/day cannabidiol on drop‐seizure frequency in patients with Lennox–Gastaut syndrome. We assumed that patients taking 10 or 20 mg clobazam would have a 2‐ to 7‐fold increase in N‐desmethylclobazam exposure, whereas patients not taking clobazam would have a median reduction in drop‐seizure frequency and a variability in the percent reduction similar to the placebo group. The results show that the effect of cannabidiol on the median reduction in drop‐seizure frequency in patients with Lennox–Gastaut syndrome may be explained by a drug–drug interaction with clobazam. This may have important implications for the use of cannabidiol and its Food and Drug Administration registration.
Keywords: cannabinoids, drug interactions, epilepsy, modelling and simulation
What is already known about this subject
Effects of cannabidiol on drop‐seizure frequency in 3 randomised controlled trials in patients with Dravet and Lennox–Gastaut syndromes led to the Food and Drug Administration approval of cannabidiol as an antiepileptic drug.
Clobazam and N‐desmethylclobazam reduce the drop‐seizure frequency in patients with Lennox–Gastaut syndrome.
Cannabidiol increases the exposure of N‐desmethylclobazam, suggesting a drug–drug interaction effect between cannabidiol and clobazam.
What this study adds
This simulation study demonstrates that the reduction in drop‐seizure frequency observed in patients taking cannabidiol may be explained by a drug–drug interaction with clobazam.
1. INTRODUCTION
Cannabidiol appears to be the most promising nonintoxicating and nonpsychoactive ingredient of cannabis. Its anticonvulsant properties and minimal neurotoxicity nominate cannabidiol as a potential treatment agent of seizures and epilepsy in children and adults.1, 2, 3, 4
In June 2018 cannabidiol was approved by the US Food and Drug Administration (FDA) as the add‐on antiepileptic drug Epidiolex in patients aged 2 years and older with Lennox–Gastaut and Dravet syndromes. The drug is currently under regulatory review with the European Medicines Agency and a decision is expected in the first quarter of 2019.5
Three phase 3 double‐blind, randomised controlled trials with cannabidiol in patients with Lennox–Gastaut and Dravet syndromes were pivotal to the FDA approval.6, 7, 8 The results of the 2 trials in patient with Lennox–Gastaut syndrome (GWPCARE3 and GWPCARE4) showed that 20 mg/kg/day of cannabidiol induced a median percent reduction in drop‐seizure frequency of 41.9% as compared to 17.2% in the placebo group (GWPCARE3)6 or of 43.9% as compared to 21.8% in the placebo group in the GWPCARE4 trial.8 A reduction in convulsive seizure frequency of 38.9% compared to 13.3% was observed in the placebo group in the GWPCARE1 trial in patients with Dravet syndrome.7 The FDA‐approved maintenance dose of cannabidiol was 10 mg/kg/day with a maximum recommended dose of 20 mg/kg/day.
The use of cannabidiol as an approved medicine has been subject of ongoing discussion, because of its unique history of recreational use and the evolved consideration that cannabidiol could be part of a routine medical treatment. “Science lags behind the proposed uses,” was noted by Martin and Cranswick in the special issue of the British Journal of Clinical Pharmacology, summarising the current knowledge and limitations of cannabinoids (volume 84, issue 11).9 In this issue, several authors addressed the limited availability of pharmacokinetic and pharmacodynamic data regarding the use of cannabidiol.10, 11, 12, 13 Special attention was given to the unknown effects of cannabidiol on addiction and mental health when given to children compared to adults,14 and to the pharmacodynamic interactions that may occur if cannabis is administered with other central nervous system depressant drugs.10 Still, insufficient data are available that support the efficacy of cannabidiol as (adjunctive) treatment for epilepsy and underlying pharmacodynamics interactions.15
Previous studies identified significant drug–drug interactions between cannabidiol and other antiepileptic drugs, such as topiramate, eslicarbazepine, zonisamide, rufinamide and N‐desmethylclobazam, the active metabolite of clobazam.16, 17, 18 Cannabidiol doses of 20 mg/kg/day (or 1500 mg/day) increased the exposure of N‐desmethylclobazam with a mean of 6‐fold (95% CI of the median fold‐increase of 1.9–7.01 at week 4 and 2.17–6.33 at week 8) in children with refractory epilepsy despite clobazam dose reductions,17 with 3‐fold in adults with epilepsy18 and with 3.4‐fold in healthy subjects.19 Cannabidiol doses of 5, 10 and 20 mg/kg/day resulted in a mean increase of N‐desmathylclobazam of 3.6, 2.7 and 3.3‐fold, respectively, in children with Dravet syndrome.20 Data from the 2 trials in patients with Lennox–Gastaut and 1 trial in patients with Dravet syndrome moreover showed that respectively 49%, 48% and 66% of the patients taking cannabidiol during the studies, were taking clobazam as a concomitant antiepileptic drug.
We therefore hypothesise that the reported effect of cannabidiol on drop‐seizure frequency in the 3 trials in patients with Lennox–Gastaut and Dravet syndromes could be attributed solely to the drug–drug interaction with clobazam. To evaluate this hypothesis, we conducted clinical trial simulations with emphasis on the GWPCARE3 trial in patients with Lennox–Gastaut syndrome.6
Clinical trial simulations are valuable tools in pharmacology investigations to complement real‐world clinical trials and give insight into e.g. improved trial planning and interpretation of trial results.21, 22 In the special issue in the British Journal of Clinical Pharmacology, Liu and Martin also emphasised the relevance of pharmacokinetic modelling to inform dosing of cannabidiol in special groups, such as children.11 In a recent publication, simulations were performed to assess the impact of drug–drug interactions between several antiepileptic drugs, including clobazam but not cannabidiol, and to evaluate the need for dose adjustments in patients requiring combination therapy.23
2. METHODS
We performed clinical trial simulations for the effect of 20 mg/kg/day cannabidiol on drop‐seizure frequency in patients with Lennox–Gastaut syndrome with the assumptions that patients taking clobazam (47%) would have a 2‐ to 7‐fold increase in N‐desmethylclobazam exposure, whereas patients not taking clobazam (53%) would have a median reduction in drop‐seizure frequency and a variability in the percent reduction similar to the placebo group. The dose level of clobazam used by patients in GWPCARE3 trial was not reported by Devinsky et al,6 but was set to 10 and 20 mg, based on a brief survey among neurologists in the Netherlands treating patients with Lennox–Gastaut syndrome. The GWPCARE3 trial was chosen due to the availability of an exposure–response model for the effect of clobazam on drop‐seizure in patients with Lennox–Gastaut syndrome.
2.1. Clobazam and N‐desmethylclobazam concentrations
Clobazam and N‐desmethylclobazam exposure were simulated with a population pharmacokinetic model published by Tolbert et al,24, 25 from which the individual average concentration of clobazam (pi) and N‐desmethylclobazam (mi) were calculated using equations [A] and [B] with individual parameters as defined in [C] (see Table 1 for the used equations, parameter definitions and estimates).
Table 1.
Equations, parameter definitions and estimates used for simulations
| Equations | |||
|---|---|---|---|
|
[A]
|
[D] | ||
|
[B]
|
[E]
|
||
|
[C] (CL/F)i = (CL/F) · exp(ηCL/Fi) K40i = K40 · exp(ηK40i)
|
[F]
|
||
| Definitions and parameter estimates | |||||
|---|---|---|---|---|---|
| Parameter | Definition | Estimates | |||
| Unit | Value | SE | Source | ||
| Cavg, p | Average concentration of clobazam | μg/mL | |||
| Cavg, m | Average concentration of N‐desmethylclobazam | μg/mL | |||
| Dose | Total daily dose | Mg | |||
| Fm | Fraction of clobazam metabolised to N‐desmethylclobazam | ||||
| η | Random effect; normally distributed with mean 0 and variance ω2 | ||||
| Eff (DDI, Peff) | Exposure–response relationship | ||||
| 1‐Peff | Maximum effect attributed to the drug | ||||
| Effscaled | Rescaled effect, taking into account clobazam treatment prior to trial | ||||
| Eff (1,0) | Reduction in seizure frequency by clobazam prior to initiation of the trial | ||||
| CL/F | Apparent total clobazam clearance | L/h | 2.8 | 0.137 | 24, 25 |
| Pm | Logit of Fm | −3.15 | 0.092 | 24, 25 | |
| K40 | Individual clearance of N‐desmethylclobazam | 1/h | 0.019 | 0.0001 | 24, 25 |
| EC50p | Exposure of clobazam | μg/mL | 0.303 | 0 | 25 |
| EC50m | Exposure of N‐desmethylclobazam | μg/mL | 0.899 | 0 | 25 |
| Peff | Placebo effect | 0.172 | 0a | 6 | |
| DDI | Drug–drug interaction effect | 2–7 | 17, 18, 19, 20 | ||
| ω2 CL/F | Variance for random effect of CL/F | 0.361 | 0.049 | 24, 25 | |
| ω2 Pm | Variance for random effect of Pm | 1 | 0.105 | 24, 25 | |
| ω2 K40 | Variance for random effect of K40 | 0.021 | 0.016 | 24, 25 | |
| ω2 eff b | Variance for random effect of reduction in drop‐seizure frequency | 0.08 | 0.0144 | 25 | |
Placebo effect was fixed to the level observed in the GWPCARE3 trial.
Value was increased from 0.0563 to 0.08 to obtain a similar variability in the percent reduction in drop‐seizure frequency as the placebo group in the GWPCARE3 trial. The SE was hereafter calculated based on a relative standard error of 18% as reported in.25
2.2. Drop‐seizure frequency
The effect of clobazam and N‐desmethylclobazam on drop‐seizure frequency in patients with Lennox–Gastaut syndrome was simulated with a population pharmacokinetic/pharmacodynamic model accounting for competitive agonism between clobazam and N‐desmethylclobazam25 with the addition of a drug–drug interaction effect resulting in an increase in N‐desmethylclobazam average exposure (see equation [D] and Table 1 for the used equation, parameter definitions and estimates).
To account for the fact that patients were already on clobazam treatment prior to initiation of the trial, the exposure–response relationship was rescaled by equation [E]. Eff(1,0) was the reduction in seizure frequency by clobazam prior to initiation of the trial as calculated by equation [D] with the assumption of no placebo effect, i.e. maximum achievable effect is 1, and no drug–drug interaction effect. The effect per individual, i, was hereafter simulated using equation [F].
2.3. Simulations
For simulation of the median reduction in seizure frequency, 50 000 subjects were simulated using the values of the parameter estimates as presented in Table 1. First, the placebo effect was simulated and the variability of the effect was changed in an iterative manner until simulations and observations in the placebo group of the GWPCARE3 trial were similar. For simulations of the confidence interval around the median, 5000 trials of 76 subjects per dose group were simulated with for each trial a unique set of parameter values drawn from normal distributions with mean values and standard errors given in Table 1. For each trial, patients on clobazam (n = 36) and not on clobazam (n = 40) were randomly sampled. The 90% prediction interval was obtained as the 5th and 95th percentiles of the simulated median values. Additionally the proportion of subjects with ≤0%, >0–<25%, ≥25–<50%, ≥50–75% and ≥75% reduction in drop‐seizure frequency from baseline were summarised for placebo and 20 mg/kg/day of cannabidiol based on 50000 simulated subjects from each treatment group, and the results were compared to those from the GWPCARE3 trial.25
2.4. Nomenclature of target and ligands
Key protein targets and ligands in this article were hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/2018.26
3. RESULTS
The clinical trial simulations show that the effect of cannabidiol on the median reduction in drop‐seizure frequency may be explained entirely by the drug–drug interaction given a clobazam dose of 20 mg and a 6‐fold increase in N‐desmethylclobazam exposure (Figure 1). The simulations also suggest that an increase in N‐desmethylclobazam of at least 3‐fold could result into the outcome observed in the GWPCARE3 trial in patients with Lennox–Gastaut syndrome (Figure 1, prediction interval contains 41.9%). The median predicted effect on drop‐seizure frequency attributed to the drug–drug interaction is also substantial for lower increases of N‐desmethylclobazam, namely a 35% reduction for a 3‐fold increase in N‐desmethylclobazam as compared to an observed reduction of 41.9%.
Figure 1.
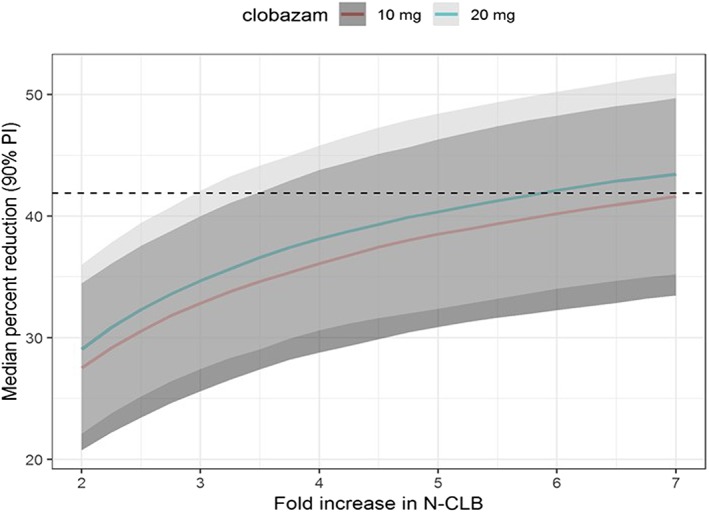
Effect of cannabidiol on drop‐seizure frequency explained by drug–drug interaction with clobazam according to clinical trial simulations. Clinical trial simulations of a population for which 47% are taking clobazam at doses of 10 or 20 mg show that the effect of cannabidiol on the median reduction in drop‐seizure frequency may be explained entirely by the drug–drug interaction given a clobazam dose of 20 mg and a 6‐fold increase in N‐desmethylclobazam (N‐CLB) exposure. The outcome of the GWPCARE3 trial is included as a reference line (black dotted line). Median (full line) and 90% prediction interval (PI) of the median (shaded area) percent reduction in drop‐seizure frequency from baseline
The results of the clinical trial simulations in relation to the secondary outcomes of the GWPCARE3 trial are shown in Figure 2. The simulated proportion of patients with ≤0%, >0–<25%, ≥25–<50%, ≥50–<75% and ≥75% reduction in drop‐seizure frequency from baseline for the placebo group is similar to the observations in the trial in patients with Lennox–Gastaut syndrome (Figure 2 top). A 4‐fold increase in N‐desmethylclobazam levels was sufficient to explain the secondary outcomes in the 20 mg/kg/day cannabidiol dose group of the GWPCARE3 trial in patients with Lennox–Gastaut syndrome (Figure 2 bottom), whereas the proportion of patients with ≥75% reduction in drop‐seizure frequency was under‐predicted when assuming <4‐fold increase in N‐desmethylclobazam levels (figure not shown).
Figure 2.
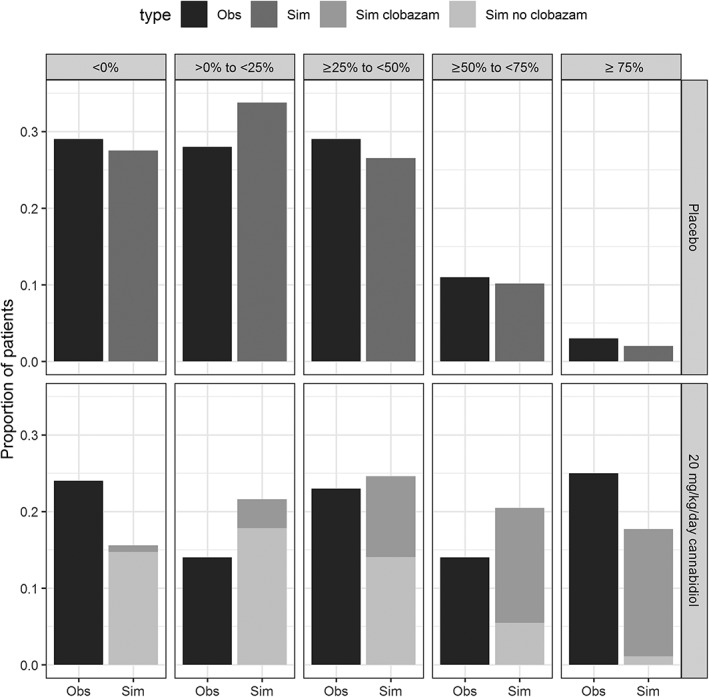
Observed (Obs) variability in drop‐seizure frequency captured by clinical trial simulations (Sim, top) and 4‐fold increase in N‐desmethylclobazam levels explained the reduction in drop‐seizure frequency from baseline for the 20 mg/kg/day cannabidiol dose group of the GWPCARE3 trial (bottom). Clinical trial simulations show that the proportion of patients with ≤0%, >0–<25%, ≥25–<50%, ≥50–<75% and ≥75% reduction in drop‐seizure frequency in the GWPCARE3 trial (black) and according to the simulation (grey) for the placebo group was similar (top). A 4‐fold increase in N‐desmethylclobazam levels was sufficient to explain the reduction in drop‐seizure frequency from baseline in the 20 mg/kg/day cannabidiol dose group (bottom)
4. DISCUSSION
Through clinical trial simulations, we demonstrate that the reduction in drop‐seizure frequency observed in the cannabidiol groups is likely to be largely explained by a drug–drug interaction with clobazam. If the effects of cannabidiol on seizure frequency in Lennox–Gastaut patients to a large extent can be explained through elevation of plasma concentrations of clobazam, then this means that cannabidiol in itself may not have any, or at best limited antiepileptic efficacy. We believe this may have important implications for the use of cannabidiol as an antiepileptic drug in Lennox–Gastaut syndrome. Based on the results of this simulation study, we hypothesise that this drug–drug interaction may be similar in patients with Dravet syndrome, which is a reasonable assumption knowing that 60% of the patients with Dravet syndrome used clobazam in the Dravet study.
Less than 50% of all patients in the 20 mg/kg/day dose group in the GWPCARE3 trial were on clobazam. Therefore, assumptions regarding the reduction in drop‐seizure frequency and variability of this reduction for the subjects not on clobazam are essential for a correct prediction of the median reduction. Our results illustrate that the pharmacokinetic/pharmacodynamic model correctly characterises variability in placebo patients and thus also in patients not on clobazam. The simulations suggest that patients showing >50% reduction in drop‐seizure frequency are most likely to be patients on clobazam, whereas patients with <25% reduction are expected not to take clobazam.
To our knowledge, there is no population pharmacokinetic model available to model the interaction between cannabidiol and clobazam on an individual level. Therefore, we could not account for clobazam dose reduction on an individual level, which occurred in 23.7% of the 20 mg/kg/day dose group in the GWPCARE3 trial. On a population level (in the drug–drug interaction study published by Geffrey et al.16), a 6‐fold increase in N‐desmethylclobazam concentrations was observed, even though 77% of the subjects treated with cannabidiol experienced clobazam dose reduction.
There were some limitations to our analyses. Simulation validity may be limited, as we did not utilise real‐world data in our clinical trial simulations.27 It is unknown how well our pharmacodynamics/pharmacokinetics model reflected real‐world drug and patient characteristics, e.g. drug compliance and disease progression, as clinical trial simulations approximate drug behaviour based on mathematical models and numerical methods. In addition, we concentrated on drop‐seizure frequency and had to make several assumptions related to the exact increase in concentrations of N‐desmethylclobazam, and related to the dose of clobazam used by 49% of all patients with Lennox–Gastaut syndrome in the 2018 study published in the New England Journal of Medicine.
A recently published study in patients with treatment‐resistant epilepsy suggests that the effect of cannabidiol on the frequency of all seizures may not be dependent on the drug–drug interaction with clobazam, although the authors recognise that the finding may be a consequence of the open‐label flexible treatment design.28 A comparison with the outcomes of the GWPCARE1–4 trial series and our clinical trial simulations are difficult due to the difference in study population and design, as well as the different outcome measure and data analytic model presented in the paper by Gaston et al.28 Furthermore, no information is provided on the number of subjects in each responder category (≥50%, ≥75% etc).
In conclusion, the clinical trial simulations described here offer an alternative explanation for the reduction in seizures observed in Lennox–Gastaut syndrome when treated with cannabidiol, namely a pharmacokinetic interaction between cannabidiol and clobazam. If cannabidiol has any antiepileptic effects of its own, then these would appear to be considerably smaller than the effect that elevated levels of N‐desmethylclobazam exert on seizure frequency. To what extent elevation of plasma concentrations of N‐desmethyl clobazam contribute to the antiepileptic effects observed of cannabidiol can be easily appreciated if the authors of the papers and GW Pharmaceuticals, the producer of Epidiolex, make all data of the trials of cannabidiol in Lennox–Gastaut syndrome publicly available, so that our conclusions can be strengthened or rejected.
CONTRIBUTORS
K.R.B., K.B. and G.J.G. designed the study and were involved in the execution of the study, provided substantial contributions to the analysis of the data, discussed the results, commented on the manuscript and approved the final paper.
COMPETING INTERESTS
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf. K.R.B., K.B. and G.J.G. declare no support from any organisation; no financial relationships with any organisations that might have an interest in the submitted work; no other relationships or activities that could have influenced the submitted work.
Bergmann KR, Broekhuizen K, Groeneveld GJ. Clinical trial simulations of the interaction between cannabidiol and clobazam and effect on drop‐seizure frequency. Br J Clin Pharmacol. 2020;86:380–385. 10.1111/bcp.14158
The authors confirm that the Principal Investigator for this paper is Geert Jan Groeneveld.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Iannotti FA, Hill CL, Leo A, et al. Nonpsychotropic plant cannabinoids, cannabidivarin (CBDV) and cannabidiol (CBD), activate and desensitize transient receptor potential vanilloid 1 (TRPV1) channels in vitro: potential for the treatment of neuronal hyperexcitability. ACS Chem Nerosci. 2014;5(11):1131‐1141. [DOI] [PubMed] [Google Scholar]
- 2. Bih CI, Chen T, Nunn AV, Bazelot M, Dallas M, Whalley BJ. Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics. 2015;12(4):699‐730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Matagne A, Klitgaard H. Validation of corneally kindled mice: a sensitive screening model for partial epilepsy in man. Epilepsy Res. 1998;31(1):59‐71. [DOI] [PubMed] [Google Scholar]
- 4. Pertwee RG, Howlett A, Abood ME, et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev. 2010;62(4):588‐631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pharmaceuticals G . GW Pharmaceuticals announces the European Medicines Agency (EMA) accepts Epidiolex (R) (cannabidiol) marketing authorization application (MAA) for review 2018. Available from: https://www.gwpharm.com/about/news/gw-pharmaceuticals-announces-european-medicines-agency-ema-accepts-epidiolexr.
- 6. Devinsky O, Patel AD, Cross JH, et al. Effect of Cannabidiol on drop seizures in the Lennox–Gastaut syndrome. N Engl J Med. 2018;378(20):1888‐1897. [DOI] [PubMed] [Google Scholar]
- 7. Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug‐resistant seizures in the Dravet syndrome. New Eng J Med. 2017;376(21):2011‐2020. [DOI] [PubMed] [Google Scholar]
- 8. Thiele EA, Marsh ED, French JA, et al. Cannabidiol in patients with seizures associated with Lennox‐Gastaut syndrome (GWPCARE4): a randomised, double‐blind, placebo‐controlled phase 3 trial. The Lancet. 2018;391(10125):1085‐1096. [DOI] [PubMed] [Google Scholar]
- 9. Martin JH, Cranswick N. Care and concern with cannabinoids used therapeutically. Br J Clin Pharmacol. 2018;84(11):2455‐2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lucas CJ, Galettis P, Schneider J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br J Clin Pharmacol. 2018;84(11):2477‐2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu Z, Martin JH. Gaps in predicting clinical doses for cannabinoids therapy: overview of issues for pharmacokinetics and pharmacodynamics modelling. Br J Clin Pharmacol. 2018;84(11):2483‐2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dryburgh LM, Bolan NS, Grof CP, et al. Cannabis contaminants: sources, distribution, human toxicity and pharmacologic effects. Br J Clin Pharmacol. 2018;84(11):2468‐2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bonomo Y, Souza JDS, Jackson A, Crippa JAS, Solowij N. Clinical issues in cannabis use. Br J Clin Pharmacol. 2018;84(11):2495‐2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Newton‐Howes G. The challenges of ‘medical cannabis’ and mental health: a clinical perspective. Br J Clin Pharmacol. 2018;84(11):2499‐2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Verrotti A, Castagnino M, Maccarrone M, Fezza F. Plant‐derived and endogenous cannabinoids in epilepsy. Clin Drug Investig. 2016;36(5):331‐340. [DOI] [PubMed] [Google Scholar]
- 16. Gaston TE, Bebin EM, Cutter GR, Liu Y, Szaflarski JP, Program UC. Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia. 2017;58(9):1586‐1592. [DOI] [PubMed] [Google Scholar]
- 17. Geffrey AL, Pollack SF, Bruno PL, Thiele EA. Drug–drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia. 2015;56(8):1246‐1251. [DOI] [PubMed] [Google Scholar]
- 18. ClinicalTrials.gov identifier NCT02565108 GW Research Ltd: A Randomized Controlled Trial to Investigate Possible Drug‐drug Interactions Between Clobazam and Cannabidiol 2015. Available from: https://clinicaltrials.gov/ct2/show/NCT02565108.
- 19. Morrison G, Crockett J, Blakey G, Sommerville K. A phase 1, open‐label, pharmacokinetic trial to investigate possible drug‐drug interactions between Clobazam, Stiripentol, or valproate and Cannabidiol in healthy subjects. Clin Pharmacol Drug Dev. 2019;8(8):1009‐1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Devinsky O, Patel AD, Thiele EA, et al. Randomized, dose‐ranging safety trial of cannabidiol in Dravet syndrome. Neurology. 2018;90(14):e1204‐e1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Holford N, Ma S, Ploeger B. Clinical trial simulation: a review. Clinical Pharmacology & Therapeutics. 2010;88(2):166‐182. [DOI] [PubMed] [Google Scholar]
- 22. EFPIA M , Marshall S, Burghaus R, et al. Good practices in model‐informed drug discovery and development: practice, application, and documentation. CPT Pharmacometrics Syst Pharmacol. 2016;5(3):93‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dijkman SC, Rauwé WM, Danhof M, Della PO. Pharmacokinetic interactions and dosing rationale for antiepileptic drugs in adults and children. Br J Clin Pharmacol. 2018;84(1):97‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tolbert D, Bekersky I, Chu HM, Ette EI. Drug‐metabolism mechanism: knowledge–based population pharmacokinetic approach for characterizing clobazam drug‐drug interactions. J Clin Pharmacol. 2016;56(3):365‐374. [DOI] [PubMed] [Google Scholar]
- 25. Center for Drug Evaluation and Research F . Application number 202067Orig1s000, clinical pharmacology and biopharmaceutics review.
- 26. Alexander SP, Kelly E, Marrion NV, et al. The concise guide to PHARMACOLOGY 2017/18: transporters. Br J Pharmacol. 2017;174:S360‐S446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kimko H, Lee K. Improving realism in clinical trial simulations via real‐world data. CPT Pharmacometrics Syst Pharmacol. 2017;6(11):727‐729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gaston TE, Bebin EM, Cutter GR, et al. Drug–drug interactions with cannabidiol (CBD) appear to have no effect on treatment response in an open‐label expanded access Program. Epilepsy Behav. 2019;98(Pt A):201‐206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


