Abstract
Medicines are a major component of modern healthcare delivery, both in resource consumption and as drivers of innovation. The ever‐increasing application of digitalisation within day‐to‐day living and as part of our healthcare systems—with the resultant data generation—presents the opportunity to better define the populations exposed to medicines, and their benefits and harm in real world settings. This article outlines the development of the Scottish National Prescribing Information System (PIS) and describes how this capability is being used to support the safe and effective use of medicines, both nationally and internationally. Since 2009, PIS has included e‐prescribed/e‐dispensed and reimbursed medicines data, now totalling 976 million prescriptions, with codified structured data on dose instructions. A literature review, covering the period from January 2009 to March 2019, identified 40 full publications using PIS, the first occurring in 2014. The majority involved pharmacoepidemiology/drug‐use studies (50%) in cancer and cardiovascular disease. Measuring the value and impact of PIS was extended beyond publication quantification by illustrating the translation of PIS outputs into the learning health system at scale. The developing Scottish capabilities add breadth and depth to the wider evolving international environment, and offer the potential to contribute collegiately to the global effort on medicine safety and effectiveness, including support for the World Health Organisation Global Patient Safety Challenge: Medication Without Harm.
Keywords: drug utilisation, medication safety, pharmacoepidemiology, prescribing, public health
1. INTRODUCTION
Medicines are among the most frequently used interventions for the prevention and treatment of illness, and a major contributor to driving innovation in modern healthcare. Accordingly, medicines are now among the largest expenditures in healthcare, accounting for on average 16% of total healthcare expenditure observed among Organisation for Economic Cooperation and Development countries in 2015,1 increasing to a mean of 23–30% in low and middle income countries.2, 3 The result is mounting pressure on public resources, and rising scrutiny by health policy makers worldwide. Drivers of this growth in expenditure include ageing populations with increasing prevalence of chronic disease; growing patient expectations; tighter therapeutic management targets; and the launch of premium priced medicines. The situation is intensified by political and public demands for accelerated access to new medicines within publicly funded healthcare systems, and in part is compounded through the move of regulatory bodies towards the concept of adaptive licensing—recognising that drug development, licensing, reimbursement, utilisation, and monitoring of outcomes should be viewed as a continuum.4 Therefore, the challenge for health policy makers, Health Technology Assessment bodies and clinicians delivering care is a diminishing evidence base and, consequently, increasing uncertainty as to the value of a medicine at market entry. Furthermore, medicines are not without harm, with UK studies estimating that 6.5% of hospital admissions are attributable to adverse effects of medicines.5 Hence, in 2017, the World Health Organisation launched the Medication Without Harm Patient Safety Challenge, calling for a 50% reduction in severe, avoidable medication‐related harm globally over the next 5 years.6
In this changing landscape, the importance of improved medicines intelligence capabilities using real world data, captured as a by‐product of routine care, is recognised by multiple stakeholders.7, 8, 9 The growth in electronic health records integrated in and/or aligned to digital solutions for medicines prescribing, dispensing and administration, underpinned by transformative digital health policies, has enabled the creation of large, accessible data repositories. This availability of reliable real‐world data (RWD) may allow for the early adoption of promising medicines, followed by real‐world determination of value through the study of medicine safety and effectiveness in more diverse populations, beyond the relatively small populations exposed within the clinical trials submitted to licensing authorities. Such data repositories are also central to examining the quality of prescribing and the impact of interventions designed to promote the rational use of medicines.
Pharmacoepidemiology and drug utilisation research, embracing the study of the use and effects/side effects of medicines in populations, are the main scientific domains positioned to take a leadership role in exploiting these evolving data capabilities.2, 10 These disciplines have built on strong foundations within some regions—notably the Nordic countries, having invested in developing their individual level national medicines registries and record linkage capabilities to understand the burden and outcome of medicines use.11, 12, 13 In 2007, the combined Nordic registries contained data from 17 million prescription users (68% of the total population), and now—in 2019—have a minimum of 14 years of history (earliest Finland starting in 1994, latest Sweden since 2005).12 However, to realise the potential of these Big Data within healthcare moving forward, there is a need to embrace the wider data science/artificial intelligence communities.8 The future benefits, in a medicines context, will be: generation of new clinical evidence to inform more precise clinical decision support; better understanding of inappropriate medicines use; and intelligence to inform pharmaceutical/health policy.
This review aims to outline the development of the Scottish Prescribing Information System (PIS), quantify the published scientific outputs, and describe a number of applications of the PIS resource across Scotland which have been adopted to drive improvements in prescribing practice.
1.1. The Scottish National PIS
1.1.1. Data provisioning at scale
Scotland has a strong international profile in health informatics and a history of using linked health data for research, embedded within a rigorous governance framework.9, 14, 15 This is founded on the adoption of a unique person identifier—the Community Health Index (CHI) number—allocated to all residents when registering with NHS Scotland health services.16 Each time a patient interacts with the health system this identifier is used, providing the opportunity to describe a patient's pathway through the healthcare system. From 2009 onwards, the routinely used digital prescribing (e‐prescribing) and dispensing (e‐dispensing) systems across primary care in Scotland have mandated the inclusion of the CHI identifier. These digital prescriptions represent almost 900 million of the 976 million prescriptions within PIS that have been dispensed and reimbursed since 2009; 96.5% of all prescriptions have a valid CHI number attached. For the first time, this has provided the ability to track medicines exposure over time at an individual level, and facilitated linking this to other health and social care records to assess intended and any unintended consequences of medicines use in the primary care/community setting for the entire Scottish population (5.4 million people, £1.14 billion medicines expenditure in 2017/2018).17, 18 The data variables accessible through PIS cover 4 broad areas: patient; medicine; prescriber; and dispenser. A detailed account of these variables is reported elsewhere.17
Historically, researchers and NHS users seeking to quantify individual drug exposure across populations faced spending significant time transforming data into a usable format, often with variable and poorly documented methods. The present PIS resource includes unstructured free‐text dose instructions as part of the electronic prescribing message; using a natural language processing algorithm which takes a zero assumptions approach, structured output from these free‐text instructions (estimated at 100 million items annually) is efficiently generated to allow users maximum flexibility to derive drug exposure appropriate to the area(s) of study. A detailed account of the development of the natural language processing tool is reported elsewhere.19
1.1.2. Use of PIS in research
Materials and methods
To understand the potential scientific contribution of the PIS, we undertook 2 tasks. Firstly, an assessment of the number of approvals for using PIS, requested by researchers/other parties through the national electronic Data Research and Innovation Service (eDRIS)20; for the period 2014–2018, we were able to quantify all applications approved nationally through our information governance systems. And secondly, a review of the literature covering the time frame from 2009 to 2019, using the search terms (“Prescribing Information System” AND “Scotland”) in EMBASE, MEDLINE and SCOPUS, as well as searching for “Prescribing Information System” in Google Scholar. In addition, article reference lists were used to identify further studies.
Key findings
Between 2014 and 2018, 124 national approvals (ranging from 15–36 per annum) were given for using PIS (Figure 1A), record linked to other data repositories and provisioned through the Safe Haven network.21 This number is, however, likely to be an underestimation of total PIS use for 3 reasons: any request before 2014 was not easily retrievable through the eDRIS documentation system, although anticipated to be small in number; PIS has been made available at a regional level in Scotland through the federated Safe Haven network to support more localised studies; and within the NHS, various national health and social care programmes—including the national Infection Intelligence Platform22 and the National Therapeutics Indicators programme23—have also used PIS data. In addition, those seeking to use PIS data only (i.e. without any linkage to other datasets) can approach the NHS National Services Scotland Information Services Division directly through the information request process.
Figure 1.
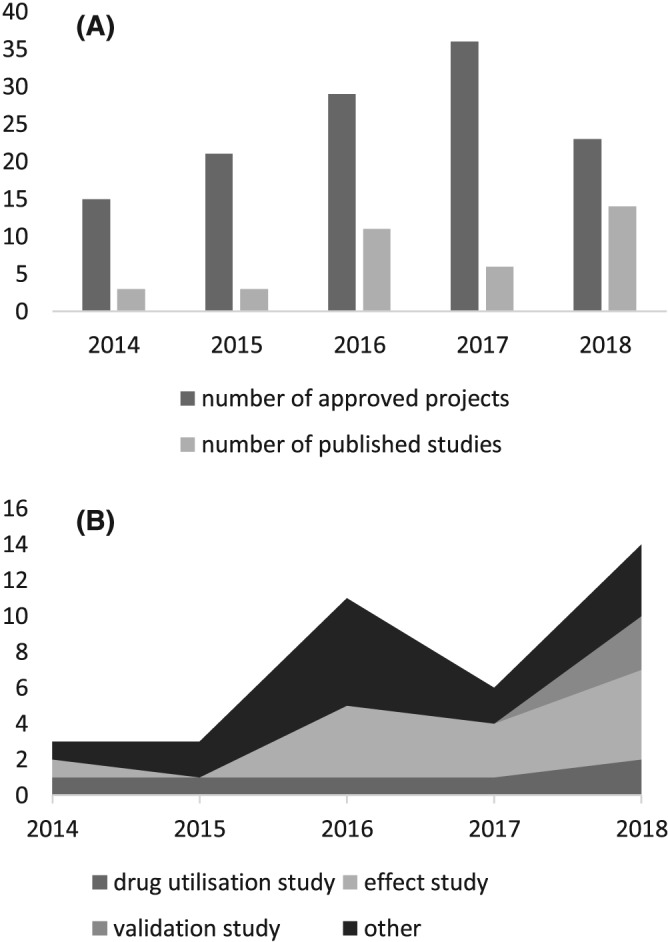
(A) Approvals to use Prescribing Information System (PIS) and publications based on research using PIS, 2014–2018. (B) Number and type of studies conducted using PIS, 2014–2018
A total of 267 publications were identified over the period January 2009 to March 2019. After screening titles and abstracts, and removing duplicates and conference abstracts, 40 full publication articles were included and reviewed.19, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62 The number of articles published by year for the period 2014–2018, totalling 37, is presented in Figure 1A; a further 3 studies were published in the first months of 2019.
Of the 40 full publications, 14 (35.0%) were pharmacoepidemiological studies assessing drug effects, while 6 studies (15.0%) analysed drug utilisation; 3 studies were validation studies. The effect studies were all retrospective cohort studies focusing on a select patient cohort having been exposed to specific drugs (based on, e.g. specific diagnoses including invasive breast cancer or atrial fibrillation, or residency/population characteristics such as having attended a Scottish school within a set time frame), 1 of which was a long‐term follow‐up study of clinical trials previously conducted in Scotland. In contrast, the drug utilisation studies were quite diverse in terms of both study design/methodology and study aim—from cross‐sectional studies describing prescribing practices of individual drugs to cohort studies analysing patient adherence to medication. Among the remaining publications, the majority were studies using prescribing data as proxy for either comorbidities or disease outcomes (n = 13, 32.5%). PIS has also been used for economic and/or policy analyses (n = 4, 10.0%). Commonly studied therapeutic areas included cancer, cardiovascular disease, and mental health; however, drug utilisation and effect studies focused particularly on the cardiovascular system and antibacterial drugs. Study types for the period 2014 to 2018 are presented in Figure 1B.
Overall, 82.5% of studies (n = 33) included record linkage: the most commonly linked datasets were hospital in‐patient records (Scottish Morbidity Records, in‐patient and day‐case dataset/SMR01), death records (National Records of Scotland), and the Scottish Cancer Registry (SMR06),63 although a wide range of other data sources have been used—from laboratory test results to educational resources such as the School Leaver database. For details regarding study type, record linkage and therapeutic areas, see Table 1.
Table 1.
Type of study, record linkage and therapeutic area (n = 40)
| Publications n (%) | |
|---|---|
| Study type | |
| Validation study | 3 (7.5) |
| Drug utilisation study | 6 (15.0) |
| Effect study | 14 (35.0) |
| Other a | 17 (42.5) |
| Record linkage | |
| Yes | 33 (82.5) |
| Death records (National Records of Scotland) | 23 (57.5) |
| Outpatient clinic attendance (SMR00) | 2 (5.0) |
| General acute in‐patient (SMR01) | 28 (70.0) |
| Maternity in‐patient (SMR02) | 2 (5.0) |
| Mental health in‐patient (SMR04) | 4 (10.0) |
| Cancer registry (SMR06) | 10 (25.0) |
| Survey data | 5 (12.5) |
| Other b | 11 (27.5) |
| Therapeutic area c | |
| Cancer | 9 (22.5) |
| Cardiovascular disease | 7 (17.5) |
| Mental health | 4 (10.0) |
| Respiratory disease | 3 (7.5) |
| Diabetes | 2 (5.0) |
| Infectious diseases | 2 (5.0) |
| Drug classification d | |
| Cardiovascular system | 11 (55.0) |
| Central nervous system | 5 (25.0) |
| Infections | 4 (20.0) |
SMR, Scottish Morbidity Records.
includes policy analyses, economic evaluations, and studies using prescribing data as proxy for comorbidities and/or disease outcomes.
includes e.g. laboratory data, accident and emergency records, Care Home Census, School Leaver Database etc.
includes all studies, regardless of study type.
includes only studies where the focus is drug use (i.e. drug utilisation/effect studies; n = 20); drug classification based on British National Formulary.
1.1.3. A learning health system at scale: application of PIS to improve prescribing practice
Measuring the value and impact of PIS in driving improvements in prescribing practice can in part be quantified by publications, as publications are often the genesis for changing practice. Nevertheless, practice impact is not always well captured through traditional publication routes. Hence, this section outlines illustrations of how PIS is being used and applied at scale across the Scottish healthcare system, informing and influencing the global landscape.
Antimicrobial stewardship
The scale of antimicrobial resistance (AMR) and the threat this poses to public health is well described and internationally recognised.64, 65 The strategic aims of the UK 5‐year AMR strategy 2013–2018 included optimising prescribing practice through implementation of antimicrobial stewardship programmes and better access to/use of surveillance data, and a call for conservation of current antibiotics66; the optimisation of antibiotic use remains a key area of focus in the most recent UK 2019–2024 action plan.67
In Scotland, antimicrobial stewardship is coordinated by the Scottish Antimicrobial Prescribing Group (SAPG).68 As 80% of antibiotic use in humans occurs within a community setting,69 the national surveillance data on antibiotic use held in PIS has been a powerful tool for SAPG and NHS Boards to plan, prioritise, and evaluate the impact of interventions. By example, PIS data has facilitated the reporting of trends in total antibiotic use/proportion of population use to evaluate the impact of efforts to reduce prescribing for self‐limiting infections; furthermore, PIS data have enabled the investigation of antibiotic use in specific groups, e.g. children and the elderly, and specific settings, such as nursing/care home institutions.26, 70, 71
To maximise the utility of nationally available data so as to minimise both the threat from AMR and the harm from Community and Healthcare Associated Infections, the PIS resource has been integrated within a national Infection Intelligence Platform (IIP) enabling the linkage of PIS with microbiology, hospital activity and deaths data to allow rapid enquiry, knowledge generation, and feedback to clinicians and policy makers. Since 2013, the SAPG has used this intelligence capability to answer important clinical questions.22 One illustration involved a call from clinicians to understand whether reductions in prescribing antibiotics in primary care had included patients who should have received antibiotics. The IIP allowed rapid identification of patients with severe upper respiratory tract infections presenting to hospitals across Scotland, and an examination of their prior antibiotic exposure in the community via PIS. This study demonstrated that despite overall reductions in primary care antibiotic use, prescribing rates among patients with these infections had increased, while mortality decreased.72 Findings provided reassurances to the clinical community that the SAPG strategy had not adversely affected patients with serious infections requiring treatment.
Furthermore, the characterisation of patient risk factors (including prior antibiotic use) for individual infections including Clostridium difficile and multidrug resistant urinary infections has been an increasing use of the IIP.37, 52 Outputs from these national risk association studies are currently being used to inform the development of digital decision support tools to inform clinician treatment choice.
High‐risk medicines stewardship
UK studies estimate that 6.5% of hospital admissions are attributable to adverse effects of high‐risk medicines, including warfarin and nonsteroidal anti‐inflammatory drugs5—equivalent to approximately 61,000 admissions annually in Scotland.
The impact of using PIS data at scale for providing routine feedback to clinicians at a GP practice level on high‐risk medicines in their practice was demonstrated through the EFIPPS study, a cluster randomised trial across 262 practices located in 3 NHS Boards.35 This study included generation of 6 validated prescribing safety measures, deliverable through PIS, and demonstrated the effectiveness of a scalable, low intensity intervention.
Translation of these safety prescribing measures into routine healthcare systems across Scotland was achieved by inclusion into national polypharmacy guidance,73 and integration within the established Scottish National Therapeutic Indicators programme.23 Starting in 2012/2013, this programme has provided feedback on prescribing activity to all GP practices and Health Boards across Scotland to drive improvement in prescribing quality—initially using aggregate GP practice level data, but the addition of individual level data through PIS presented the opportunity to include measures that directly addressed patient safety issues, particularly regarding the prescribing of drug combinations known to increase the risk of adverse drug events. Since 2017, the EFIPPS study measures have fully been adopted within the National Therapeutic Indicators programme, following initial designation as additional prescribing measures. 23
Additional evolving use of PIS data (national/international)
Several new initiatives by various research communities within Scotland and elsewhere—frequently informed by the NHS—to drive wider application of PIS data are emerging, including:
Scottish Atlas of Variation: The Atlas of Variation is a public‐facing interactive data dashboard that utilises PIS data to count numbers of treated patients, and presents these as standardised rates to show high‐level variation across the healthcare system.74 The Atlas of Variation currently includes prescribing measures relating to cardiovascular disease, with further areas under development. Complimentary and more detailed information is available to authorised users (NHS staff) within other dashboard products, such as the Discovery Dashboard. 75 These dashboard developments use multiple data sources and help in understanding patterns of medicines use within the wider health and care context.
Impact of regulatory guidance on primary care prescribing: PIS data linked to other health records is being used to assess the impact of various guidelines, including the European Medicines Agency (EMA) guidance on valproate prescribing and harm reduction strategies for females of childbearing potential within NHS Scotland; and EMA regulatory label changes on the use of systemic diclofenac products in a multicountry study covering Scotland, England, Denmark and the Netherlands.
- Development of data science methods, applied to RWD assessment of new medicines: Using direct oral anticoagulants as an example, activities include:
-
○Applying various data science algorithms, including advanced statistical modelling and novel machine learning techniques, to build predictive models of treatment outcomes and adverse events using increasingly deeply phenotyped data. The aim is to move beyond single‐variable analysis and develop methods capable of analysing multiple outcomes simultaneously.
-
○Identifying and testing multiple measures that quantify patient adherence using PIS data. The outcome is a proposed resource toolkit for researchers seeking to better understand patients' medicines management behaviour using PIS.46
-
○
2. DISCUSSION
The increasing burden on health and social care services requires new and sustainable ways of providing the support people need. The growing use of digital solutions more widely in society, including in the health and wellbeing domains, is starting to reshape interactions between health practitioners and patients. Digital solutions, generating new data streams from sensors and devices, coupled with the growth and accessibility of genomics/metabolomics data within healthcare systems, present an opportunity to gain greater insight into patient care pathways and the potential reasons for outcome variation—including the use and effect of medicines.
Exploitation of the advancing volume and depth of these RWD capabilities in healthcare has been signalled as the next step change to improving patient outcomes, delivering more efficient and effective healthcare, and driving innovation in clinical research. Most recently in the UK, this has been galvanised through the implementation of the Health Data Research UK (HDR UK) Institute, with the vision for “[…] large scale data and advanced analytics to benefit every patient interaction, clinical trial, and biomedical discovery and to enhance public health.”8 Through HDR UK, the plan is to: grow the next generation of data scientists with the technical expertise to work with data from varied sources, often messy and unstructured; apply novel artificial intelligence techniques at scale to inform and shape clinical research programmes; and build novel decision support systems for use within the healthcare setting.
In Scotland, the comprehensive health care system affords the opportunity to capture whole system exposure to medicines for an entire population of 5.4 million. The medicines intelligence toolkit capabilities to date have focused on exploiting the individual primary care (community) prescribing data as illustrated in this article, to inform and improve a learning health system. Building on the experiences of others and collaborating with colleagues, particularly the Nordic countries where similar resources are available,11, 12, 13 growing capabilities at scale will enable Scotland to maximise the learning from diverse exposed populations across different health systems. Given the ever‐increasing precision therapeutics agenda, this scalability agenda crossing multiple jurisdictions will become ever more important if we are to have the numbers to ascertain benefits and harms from new, advancing therapies that are often focused on increasingly specific patient groups.
Building on the PIS experience, the present focus is the hospital sector, which is currently underrepresented in real‐world research, initially prioritising cancer treatment. Scotland, based on the Beating Cancer: Ambition and Action plan,76 has invested in the Cancer Medicines Outcomes Programme to create a national resource to measure clinical and patient reported outcomes of cancer medicines in the real world, and to provide rapid feedback to inform clinical practice.77 Scotland's ambition is to extend electronic prescribing and administration systems across the full inpatient and outpatient hospital setting, envisaged to be accomplished by 2023.78 This will complete the medicine treatment pathway, facilitating much richer understanding of drug exposure and its correlation to patient benefits and harm. Remaining areas that require attention when working with RWD in Scotland include: absence of diagnosis on prescriptions, necessitating triangulation through other data sources; and national laboratory data (comprising e.g. biochemistry and haematology) to better define patient phenotypes and assess the effect of medicines on disease measures.79
More internationally, from a medicines intelligence perspective, RWD use is being supported by organisations such as the International Society of Pharmacoepidemiology80 and the EMA coordinated European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP) initiative,81 amongst others. Such bodies represent forums to stimulate discussion on evolving data capabilities and methodological approaches, and produce guidance, standards and resources for the community to be used for uncovering new evidence. Further debate is, however, needed to clarify how real world studies can support and complement randomised clinical trials (RCTs), instead of being seen as a threat to—or even potential replacement of—RCTs.82 Delivery of more real‐world RCTs, representative of the complex, multimorbid populations being treated in clinical practice, should be enabled and supported through digital health advancements; we will be stronger in our ambitions to understand medicines by adding to our research toolkit, rather than depleting it.10 In addition, even though numerous studies using prescribing resources similar to PIS particularly from the Nordic countries have been published, thus far there is a scarcity of publications describing how prescribing resources are impacting and/or are being integrated into routine health care systems.
Our collective vision must be to shape how our ever‐extending real‐world Big Data can contribute effectively across the health and care policy spectrum to improve medicine safety and effectiveness. Key research avenues should include:
Driving the availability and use of RWD by medicines regulators, Health Technology Assessment and pharmaceutical industry decision makers.
Developing advanced analytics and visualisation techniques to generate new, accessible evidence to populate novel clinical decision support tools, promoting shared clinician‐patient treatment choice.
Exploiting and embracing the use of new digital health and lifestyle data streams to gain new insights into how medicines are impacting patients' lives, and a deeper understanding of lifestyle behaviours and their potential to inform tailored approaches to health interventions involving the choice/use of medicines.
Making high quality data easily discoverable so that our advancing, diverse data science community can engage with established communities to foster technical expertise and insight.
The collective benefits of these efforts will be more effective patient care; more efficient healthcare delivery; and improved value for money for scarce public resources.
3. CONCLUSION
Scotland has grasped the opportunity to invest in national prescribing capabilities to generate novel intelligence in order to improve understanding of medicines use in routine clinical care, and to inform and drive improvement in a learning health system to assure equitable and safe use of medicines. Nevertheless, we need to recognise—both in Scotland and elsewhere—that the next major gain will be in building and strengthening international collaborations, as precision therapeutics and stratified medicines are evolving to shape the future of healthcare.
COMPETING INTERESTS
M.B., W.M. and S.M. are employees of the Scottish National Health Service. There are no potential conflicts of interest to declare.
ACKNOWLEDGEMENTS
We thank Elizabeth Nicholson, Senior Information Analyst at eDRIS/National Services Scotland, for providing data for this project. We acknowledge the funding support from The Farr Institute @ Scotland. The Farr Institute @ Scotland is supported by a 10‐funder consortium: Arthritis Research UK, the British Heart Foundation, Cancer Research UK, the Economic and Social Research Council, the Engineering and Physical Science Research Council, the Medical Research Council, the National Institute of Health Research, the National Institute for Social Care and Health Research (Welsh Assembly Government), the Chief Scientist Office (Scottish Government Health Directorates), and the Wellcome Trust (MRC Grant No: MR/K007017/1).
Bennie M, Malcolm W, McTaggart S, Mueller T. Improving prescribing through big data approaches—Ten years of the Scottish Prescribing Information System. Br J Clin Pharmacol. 2020;86:250–257. 10.1111/bcp.14184
There is no principal investigator for this study.
REFERENCES
- 1. OECD . Health at a Glance 2017: OECD Indicators. Paris: OECD Publishing; 2017. [Google Scholar]
- 2. Elseviers M, Wettermark B, Almarsdóttir AB, et al. Drug utilization research methods and applications. Wiley Blackwell: Chichester; 2016. [Google Scholar]
- 3. Lu Y, Hernandez P, Abegunde D, Edejer T. The World Medicines Situation 2011: Medicines Expenditure. Geneva: WHO; 2011. [Google Scholar]
- 4. EMA . European Medicines Agency Guidance for applicants seeking access to PRIME scheme. London, UK: European Medicines Agency; 2018. Contract No.: 13.08. [Google Scholar]
- 5. Pirmohamed M, James S, Meakin S, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329(7456):15‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. WHO . Medication Without Harm ‐ Global Patient Safety Challenge on Medication Safety. Geneva: World Health Organisation; 2017. [Google Scholar]
- 7. abpi . The vision for real world data ‐ harnessing the opportunities in the UK. London: The Association of the British Pharmaceutical Industry; 2011. [Google Scholar]
- 8. HDR UK . One Institute Strategy 2019/2020. London: Health Data Research UK; 2019. [Google Scholar]
- 9. Scottish Government . Building capability to assess real‐world benefits, risks and value of medicines: Towards a Scottish Medicines Intelligence Unit. Edinburgh: Scottish Government, Data Scopting Taskforce; 2018. [Google Scholar]
- 10. Strom BL, Kimmel SE, Hennessy S. Textbook of pharmacoepidemiology. Chichester, West Sussex [England]; Hoboken, NJ: Wiley Blackwell; 2013. [Google Scholar]
- 11. Furu K, Wettermark B, Andersen M, Martikainen JE, Almarsdottir AB, Sørensen HT. The Nordic countries as a cohort for Pharmacoepidemiological research. Basic Clin Pharmacol Toxicol. 2010;106(2):86‐94. [DOI] [PubMed] [Google Scholar]
- 12. Wettermark B, Zoëga H, Furu K, et al. The Nordic prescription databases as a resource for pharmacoepidemiological research—a literature review. Pharmacoepidem Dr S. 2013;22(7):691‐699. [DOI] [PubMed] [Google Scholar]
- 13. Wallerstedt SM, Wettermark B, Hoffmann M. The first decade with the Swedish prescribed drug register – a systematic review of the output in the scientific literature. Basic Clin Pharmacol Toxicol. 2016;119(5):464‐469. [DOI] [PubMed] [Google Scholar]
- 14. SHIP . A blueprint for Health Records research in Scotland. Scottish Health Informatics Programme: Edinburgh; 2012. [Google Scholar]
- 15. Scottish Government . A Health and Biomedical Informatics research strategy for Scotland: Enhancing research capability in Health Informatics for patient and public benefit 2015–2020. Scottish Government: Edinburgh; 2015. [Google Scholar]
- 16. ISD Scotland . Data dictionary A‐Z: CHI number. NHS National Services Scotland. https://www.ndc.scot.nhs.uk/Dictionary-A-Z/Definitions/index.asp?Search=C&ID=128&Title=CHI%20Number. Updated 2019. Accessed August 14, 2019.
- 17. Alvarez‐Madrazo S, McTaggart S, Nangle C, Nicholson E, Bennie M. Data resource profile: the Scottish National Prescribing Information System (PIS). Int J Epidemiol. 2016;45(3):714‐715f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. ISD Scotland . Scottish Health Service Costs ‐ Year ended March 2018. Edinburgh: NHS National Services Scotland; 2018. [Google Scholar]
- 19. McTaggart S, Nangle C, Caldwell J, Alvarez‐Madrazo S, Colhoun H, Bennie M. Use of text‐mining methods to improve efficiency in the calculation of drug exposure to support pharmacoepidemiology studies. Int J Epidemiol. 2018;47(2):617‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. ISD Scotland . Products and Services: eDRIS. NHS National Services Scotland. https://www.isdscotland.org/Products-and-Services/eDRIS/. Published 2010. Accessed August 14, 2019.
- 21. NHS Research Scotland . Research in Scotland: Data Safe Haven. NHS Scotland. https://www.nhsresearchscotland.org.uk/research-in-scotland/data/safe-havens. Updated 2019. Accessed August 14, 2019.
- 22. Bennie M, Malcolm W, Marwick CA, Kavanagh K, Sneddon J, Nathwani D. Building a national infection intelligence platform to improve antimicrobial stewardship and drive better patient outcomes: the Scottish experience. J Antimicrob Chemother. 2017;72(10):2938‐2942. [DOI] [PubMed] [Google Scholar]
- 23. NHS Scotland . National Therapeutic Indicators 2018. NHS Scotland: Edinburgh; 2018. [Google Scholar]
- 24. Bell S, Davey P, Nathwani D, et al. Risk of AKI with gentamicin as surgical prophylaxis. J am Soc Nephrol. 2014;25(11):2625‐2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bergman BP, Mackay DF, Pell JP. Acute myocardial infarction in Scottish military veterans: a retrospective cohort study of 57,000 veterans and 173,000 matched nonveterans. Am J Epidemiol. 2014;179(12):1434‐1441. [DOI] [PubMed] [Google Scholar]
- 26. Covvey JR, Johnson BF, Elliott V, Malcolm W, Mullen AB. An association between socioeconomic deprivation and primary care antibiotic prescribing in Scotland. J Antimicrob Chemother. 2014;69(3):835‐841. [DOI] [PubMed] [Google Scholar]
- 27. Black M, Bhattacharya S, Philip S, Norman JE, McLernon DJ. Planned Cesarean delivery at term and adverse outcomes in childhood health. JAMA. 2015;314(21):2271‐2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chin RF, Ajetunmobi O, Weir CJ, Wood R. Prescription of emergency antiepileptic medication after a first childhood seizure: analysis of routine administrative data. Epileptic Disord. 2015;17(2):172‐176. [DOI] [PubMed] [Google Scholar]
- 29. Ness V, Malcolm W, McGivern G, Reilly J. Growth in nurse prescribing of antibiotics: the Scottish experience 2007–13. J Antimicrob Chemother. 2015;70(12):3384‐3389. [DOI] [PubMed] [Google Scholar]
- 30. Bergman BP, Mackay DF, Morrison D, Pell JP. Smoking‐related cancer in military veterans: retrospective cohort study of 57,000 veterans and 173,000 matched non‐veterans. BMC Cancer. 2016;16(1):311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cardwell CR, Pottegård A, Vaes E, et al. Propranolol and survival from breast cancer: a pooled analysis of European breast cancer cohorts. Breast Cancer Res. 2016;18(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gao L, Dimitropoulou P, Robertson JR, McTaggart S, Bennie M, Bird SM. Risk‐factors for methadone‐specific deaths in Scotland's methadone‐prescription clients between 2009 and 2013. Drug Alcohol Depend. 2016;167:214‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gibb FW, Teoh WL, Graham J, Lockman KA. Risk of death following admission to a UK hospital with diabetic ketoacidosis. Diabetologia. 2016;59(10):2082‐2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gray RT, Coleman HG, Hughes C, Murray LJ, Cardwell CR. Statin use and survival in colorectal cancer: results from a population‐based cohort study and an updated systematic review and meta‐analysis. Cancer Epidemiol. 2016;45:71‐81. [DOI] [PubMed] [Google Scholar]
- 35. Guthrie B, Kavanagh K, Robertson C, et al. Data feedback and behavioural change intervention to improve primary care prescribing safety (EFIPPS): multicentre, three arm, cluster randomised controlled trial. BMJ. 2016;354:i4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Johnson CF, Frei C, Downes N, McTaggart SA, Akram G. Benzodiazepine and z‐hypnotic prescribing for older people in primary care: a cross‐sectional population‐based study. Br J Gen Pract. 2016;66(647):e410‐e415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kavanagh K, Pan J, Marwick C, et al. Cumulative and temporal associations between antimicrobial prescribing and community‐associated Clostridium difficile infection: population‐based case–control study using administrative data. J Antimicrob Chemother. 2016;72(4):1193‐1201. [DOI] [PubMed] [Google Scholar]
- 38. Mc Menamin ÚC, Murray LJ, Hughes CM, Cardwell CR. Statin use and breast cancer survival: a nationwide cohort study in Scotland. BMC Cancer. 2016;16(1):600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mole DJ, Gungabissoon U, Johnston P, et al. Identifying risk factors for progression to critical care admission and death among individuals with acute pancreatitis: a record linkage analysis of Scottish healthcare databases. BMJ Open. 2016;6(6):e011474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mukherjee M, Stoddart A, Gupta RP, et al. The epidemiology, healthcare and societal burden and costs of asthma in the UK and its member nations: analyses of standalone and linked national databases. BMC Med. 2016;14(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cardwell CR, Spence AD, Hughes CM, Murray LJ. Statin use after esophageal cancer diagnosis and survival: a population based cohort study. Cancer Epidemiol. 2017;48:124‐130. [DOI] [PubMed] [Google Scholar]
- 42. Fleming M, Fitton CA, Steiner MFC, et al. Educational and health outcomes of children treated for attention‐deficit/hyperactivity disorder. JAMA Pediatr. 2017;171(7):e170691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Katikireddi SV, Whitley E, Lewsey J, Gray L, Leyland AH. Socioeconomic status as an effect modifier of alcohol consumption and harm: analysis of linked cohort data. Lancet Public Health. 2017;2(6):e267‐e276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. MacBride‐Stewart S, Marwick C, Houston N, Watt I, Patton A, Guthrie B. Evaluation of a complex intervention to improve primary care prescribing: a phase IV segmented regression interrupted time series analysis. Br J Gen Pract. 2017;67(658):e352‐e360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mc Menamin ÚC, Cardwell CR, Hughes CM, Murray LJ. Low‐dose aspirin use and survival in breast cancer patients: a nationwide cohort study. Cancer Epidemiol. 2017;47:20‐27. [DOI] [PubMed] [Google Scholar]
- 46. Mueller T, Alvarez‐Madrazo S, Robertson C, Bennie M. Use of direct oral anticoagulants in patients with atrial fibrillation in Scotland: applying a coherent framework to drug utilisation studies. Pharmacoepidemiol Dr S. 2017;26(11):1378‐1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bergman BP, Mackay DF, Pell JP. Peripheral arterial disease in Scottish military veterans: a retrospective cohort study of 57 000 veterans and 173 000 matched non‐veterans. J Public Health. 2018;41(1):e9‐e15. [DOI] [PubMed] [Google Scholar]
- 48. Burton JK, Papworth R, Haig C, et al. Statin use is not associated with future long‐term care admission: extended follow‐up of two randomised controlled trials. Drugs Aging. 2018;35(7):657‐663. [DOI] [PubMed] [Google Scholar]
- 49. Gray RT, Coleman HG, Hughes C, Murray LJ, Cardwell CR. Low‐dose aspirin use and survival in colorectal cancer: results from a population‐based cohort study. BMC Cancer. 2018;18(1):228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hafferty JD, Campbell AI, Navrady LB, et al. Self‐reported medication use validated through record linkage to national prescribing data. J Clin Epidemiol. 2018;94:132‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Johnson CF, Nassr OA, Harpur C, Kenicer D, Thom A, Akram G. Benzodiazepine and z‐hypnotic prescribing from acute psychiatric inpatient discharge to long‐term care in the community. Pharm Pract (Granada). 2018;16(3):1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Malcolm W, Fletcher E, Kavanagh K, et al. Risk factors for resistance and MDR in community urine isolates: population‐level analysis using the NHS Scotland infection intelligence platform. J Antimicrob Chemother. 2018;73(1):223‐230. [DOI] [PubMed] [Google Scholar]
- 53. Scott D, Fletcher E, Kane H, et al. Risk of infection following semi‐invasive ultrasound procedures in Scotland, 2010 to 2016: a retrospective cohort study using linked national datasets. Ultrasound. 2018;26(3):168‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shah ASV, Anand A, Strachan FE, et al. High‐sensitivity troponin in the evaluation of patients with suspected acute coronary syndrome: a stepped‐wedge, cluster‐randomised controlled trial. Lancet. 2018;392(10151):919‐928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Song J, Elliot E, Morris AD, et al. A case study in distributed team science in research using electronic health records. Int J Popul Data Sci. 2018;3(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Spence AD, Busby J, Hughes CM, Johnston BT, Coleman HG, Cardwell CR. Statin use and survival in patients with gastric cancer in two independent population‐based cohorts. Pharmacoepidemiol Dr S. 2018;28(4):460‐470. [DOI] [PubMed] [Google Scholar]
- 57. Spence AD, Busby J, Johnston BT, et al. Low‐dose aspirin use does not increase survival in 2 independent population‐based cohorts of patients with Esophageal or gastric cancer. Gastroenterology. 2018;154(4):849‐860.e1. [DOI] [PubMed] [Google Scholar]
- 58. Torrance N, Mansoor R, Wang H, et al. Association of opioid prescribing practices with chronic pain and benzodiazepine co‐prescription: a primary care data linkage study. Brit J Anaesth. 2018;120(6):1345‐1355. [DOI] [PubMed] [Google Scholar]
- 59. Williams AJ, Henley W, Frank J. Impact of abolishing prescription fees in Scotland on hospital admissions and prescribed medicines: an interrupted time series evaluation. BMJ Open. 2018;8(12):e021318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Devereux G, Craig L, Seaton A, Turner S. Maternal vitamin D and E intakes in pregnancy and asthma to age 15 years: a cohort study. Pediatr Pulmonol. 2019;54(1):11‐19. [DOI] [PubMed] [Google Scholar]
- 61. Gao L, Robertson JR, Bird SM. Non drug‐related and opioid‐specific causes of 3262 deaths in Scotland's methadone‐prescription clients, 2009–2015. Drug Alcohol Depen. 2019;197:262‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mueller T, Alvarez‐Madrazo S, Robertson C, Wu O, Bennie M. Comparative safety and effectiveness of direct oral anticoagulants in patients with atrial fibrillation in clinical practice in Scotland. Brit J Clin Pharm. 2019;85(2):422‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. ISD Scotland . National Data Catalogue: National Datasets. NHS National Services Scotland. https://www.ndc.scot.nhs.uk/National-Datasets/. Updated 2019. .
- 64. WHO . Global Action Plan on Antimicrobial Resistance. Geneva: World Health Organisation; 2019. [Google Scholar]
- 65.Review on antimicrobial resistance. Tackling drug‐resistant infections globally: Final report and recommendations. London: Review on antimicrobial resistance; 2016.
- 66. UK Government . UK Five Year Antimicrobial Resistance Strategy 2013 to 2018. London: Department of Health; 2013. [Google Scholar]
- 67. UK Government . Tackling antimicrobial resistance 2019–2024: The UK's five‐year national action plan. London: Department of Health; 2019. [DOI] [PubMed] [Google Scholar]
- 68. Healthcare Improvement Scotland . The Scottish Antimicrobial Prescribing Group. NHS Scotland. https://www.sapg.scot/about-us/. Accessed August 14, 2019.
- 69. Health Protection Scotland . Scottish One Health Antimicrobial Use and Antimicrobial Resistance Report in 2017. Glasgow: Health Protection Scotland; 2018. [Google Scholar]
- 70. Health Protection Scotland . Scottish One Health Antimicrobial Use and Antimicrobial Resistance Report in 2016. Glasgow: Health Protection Scotland; 2017. [Google Scholar]
- 71. Health Protection Scotland & ISD Scotland . Scottish Antimicrobial Use and Resistance in Humans in 2015. Edinburgh/Glasgow: Health Protection Scotland & ISD Scotland; 2016. [Google Scholar]
- 72. McGivern G, Malcolm W, Marwick C, Kavangh K, Sneddon J, MacDonald A, Reid N. Measuring potential unintended consequences of reducing primary care antibiotic prescribing using NHS Scotland's Infection Intelligence Platform. Poster presented to the Scottish Antimocrobial Prescribing Group/NHS Scotland 2015.
- 73. Scottish Government . Polypharmacy Guidance: Realistic presribing 3rd Edition, 2018. Edinburgh: NHS Scotland; 2018. [Google Scholar]
- 74. ISD Scotland . Scottish Atlas of Healthcare Variation (Beta): Exploring geographical variation in the health & care system in Scotland. NHS Scotland. 2019 https://www.isdscotland.org/products-and-services/scottish-atlas-of-variation/. Published 2019. .
- 75. ISD Scotland . NSS Discovery. NHS National Services Scotland. https://www.isdscotland.org/Products-and-Services/Discovery/. Published 2010. .
- 76. Scottish Government . Beating cancer: ambition and action. Edinburgh: Scottish Government; 2016. [Google Scholar]
- 77. Scottish Government . Making medicine more effective: £300,000 for Cancer Medicines Outcome Programme. Scottish Government. https://news.gov.scot/news/making-medicine-more-effective. Published 2017. Accessed August 14, 2019.
- 78. Scottish Government . Scotland's Digital Health and Care Strategy: enabling, connecting and empowering. Edinburgh: Scottish Government; 2018. [Google Scholar]
- 79. Scottish Government . Review of Access to New Medicines: Report from the Data Scoping Taskforce. Edinburgh: Scottish Government; 2018. [Google Scholar]
- 80. ISPE . About ISPE. International Society of Pharmacoepidemiology. https://www.pharmacoepi.org/about-ispe/overview/. Updated 2019. Accessed August 14, 2019.
- 81. ENCePP . What is ENCePP? European Medicines Agency. http://www.encepp.eu/structure/index.shtml. Updated 2019. Accessed August 14, 2019.
- 82. Gerstein HC, McMurray J, Holman RR. Real‐world studies no substitute for RCTs in establishing efficacy. Lancet. 2019;393(10168):210‐211. [DOI] [PubMed] [Google Scholar]


