Abstract
Aim
Our objective was to identify preventable adverse drug events and factors contributing to their development.
Methods
We performed a retrospective chart review combining data from three prospective multicentre observational studies that assessed emergency department patients for adverse drug events. A clinical pharmacist and physician independently reviewed the charts, extracted data and rated the preventability of each adverse drug event. A third reviewer adjudicated all discordant or uncertain cases. We calculated the proportion of adverse drug events that were deemed preventable, performed multivariable logistic regression to explore the characteristics of patients with preventable events, and identified contributing factors.
Results
We reviewed the records of 1 356 adverse drug events in 1 234 patients. Raters considered 869 (64.1%) of adverse drug events probably or definitely preventable. Patients with mental health diagnoses (OR 1.8; 95% CI 1.3–2.5) and diabetes (OR 1.7; 95% CI 1.2–2.4) were more likely to present with preventable events. The medications most commonly implicated in preventable events were warfarin (9.4%), hydrochlorothiazide (4.5%), furosemide (4.0%), insulin (3.9%) and acetylsalicylic acid (2.7%). Common contributing factors included inadequate patient instructions, monitoring and follow‐up, and reassessments after medication changes had been made.
Conclusions
Our study suggests that patients with mental health conditions and diabetes require close monitoring. Efforts to address the identified contributing factors are needed.
Keywords: adverse drug events, contributing factors, medication safety, preventability
What is already known about the subject
Adverse drug events represent a major burden on the healthcare system.
Efforts to reduce adverse drug events should focus on preventable events and consider factors that contribute to their development.
What this study adds
More than two‐thirds of adverse drug events were rated as probably or definitely preventable.
Patients with mental health conditions, diabetes, and those receiving high‐risk medications (i.e., warfarin, hydrochlorothiazide, furosemide, insulin and acetylsalicylic acid) were at increased risk of experiencing a preventable adverse drug event.
Contributing factors commonly included inadequate patient instructions, monitoring and follow‐up, and reassessments after medication changes had been made.
1. INTRODUCTION
Adverse drug events, the unintended and harmful events associated with medications, represent a major burden on the healthcare system.1, 2, 3 They are a leading cause of unplanned hospital admissions and emergency department visits.2, 4, 5 Developing effective strategies to prevent adverse drug events has become an international research priority.6, 7 Current prevention efforts focus on vulnerable populations such as the elderly, and those taking medications that are commonly associated with clinically significant and measurable harms.7 However, a focus on preventability is key to strategic planning, as policies that do not target factors contributing to preventable events cannot be expected to reduce adverse drug events.
Prospective studies indicate that between 28 and 80% of adverse drug events are preventable.8, 9, 10, 11, 12, 13, 14, 15 Most studies describe the type and severity of preventable events,8, 10, 15, 16, 17, 18, 19 without identifying high‐impact patient populations, system‐level targets for intervention, or the contexts in which preventable events occur.2, 7, 15, 17, 20, 21, 22, 23 Some preventability assessment methods have made broad assumptions in assessing preventability.24 For example, one algorithm automatically assumes that all adverse drug events due to medications amenable to monitoring using laboratory testing can be averted by increasing the frequency of laboratory monitoring.24 However, it is possible that increasing the frequency of routine laboratory monitoring without understanding contextual or contributing factors risks being ineffective while unnecessarily increasing resource utilization.
Our main objective was to describe the characteristics of preventable adverse drug events experienced by patients presenting to emergency departments with an adverse drug event. Our secondary objective was to highlight factors that contributed to their development.
2. METHODS
This was a multi‐centre retrospective research and/or medical record review conducted in four Canadian acute care hospitals. The institutional review board of the University of British Columbia and of each participating hospital approved the protocol for this study. The need for patient consent was waived during ethics review. The Drug Safety and Effectiveness Network, in collaboration with Health Canada and the Canadian Institutes of Health Research, supported this study. None of the funding organizations participated in the conceptualization of the study, the development of the research protocol, or in the collection, analysis or interpretation of data. The study authors wrote the manuscript.
2.1. Population
We reviewed the medical and/or research records of all patients who had been diagnosed with one or more medication‐related problems or adverse drug events in one of three prospective multi‐centre parent studies conducted by our research group (Appendix A).4, 5, 25, 26 The first parent study, conducted in 2008–2009, derived a clinical decision rule to identify patients at high‐risk of adverse drug events from 1 591 patients presenting to the emergency departments of two tertiary care hospitals, Vancouver General (VGH) and St. Paul's Hospitals (SPH), in Vancouver, British Columbia, Canada.5 In this study, 226 of the enrolled patients were diagnosed with an adverse drug event. This parent study was approved by the UBC Clinical Ethics Board. The second parent study, conducted in 2011–2013, evaluated the impact of pharmacist‐led medication review on health outcomes from 10 807 patients presenting to the emergency departments of VGH, Lions Gate Hospital (LGH), an urban community hospital in North Vancouver, British Columbia, and Richmond General Hospital (RGH), an urban community hospital in Richmond, British Columbia.4 In this study, patients at high risk of adverse drug events based on the clinical decision rule derived and validated in the other two parent studies were systematically selected for medication review (Appendix A). Of these patients, 2862 were diagnosed with medication‐related problems. The need for ethics review for this study was waived as this was a quality improvement project. The third parent study, conducted in 2014–2015, validated the previously derived clinical decision rules using 1 529 patients presenting to the emergency departments of VGH, LGH and the Ottawa Civic Hospital (OCH), an urban tertiary care hospital in Ottawa, Ontario, Canada.25 In this study, 240 of the enrolled patients were diagnosed with an adverse drug event. This parent study was approved by the UBC Clinical Ethics Board. All three studies systematically enrolled patients presenting to an emergency department (Appendix B). In the three parent studies, clinical pharmacists and physicians evaluated all enrolled patients at the point‐of‐care, and documented medication‐related problems or adverse drug events in research and medical records. All cases in which the clinical pharmacist and physician diagnoses were concordant were considered final. An independent committee adjudicated all cases in which their assessments were discordant or uncertain.
The current study was conceived after the completion of these three prior studies, as the parent studies had allowed us to compile a large database of prospectively identified adverse drug events. We reviewed the research records and/or medical records of the hospital that all participants diagnosed with a medication‐related problem or adverse drug event presented to in the primary studies, and used charts to exclude patients no longer meeting our case definition of adverse drug event for the current study (Appendix C), or who had been diagnosed with an alternative diagnosis since the conclusion of the parent study. We excluded patients whose research and medical records could not be retrieved, and those with illegible records.4, 5, 25, 26
2.2. Definitions
We defined adverse drug events as harm caused by a drug or the inappropriate use of a drug, consistent with its effective definition in clinical practice.27 Adverse drug events included adverse drug reactions, undesirable effects to drugs occurring within the normal therapeutic range,28, 29 drug interactions, supra‐ and sub‐therapeutic doses, events due to non‐adherence or inappropriate drug withdrawal, and cases in which the patient was on an ineffective or on no drug despite previous documentation of an indication for and lack of contraindication to the drug (e.g., a patient presenting with an ischaemic stroke with a previously documented history of atrial fibrillation and a high stroke risk but no anticoagulation).19 For events presenting with abnormal vital signs, we defined cut‐offs a priori, excluding all other cases (Appendix B). For events involving laboratory abnormalities, we used the reference values of each participating hospital. We categorized severity as mild when the adverse drug event required no change in medical management, moderate when it required a change in medical management, and severe when it was either the primary reason for hospitalization, caused permanent disability, or proved fatal.
Based on our findings from previous work, we considered adverse drug events as preventable when there was “lack of adherence to best medical practice, including inappropriate drug, dosage, route or frequency of administration of a drug for the patient's clinical condition, age, weight or renal function; administration of a drug despite a known allergy, a previous adverse reaction to, or a drug interaction; non‐adherence; laboratory monitoring not or inappropriately performed; prescribing or dispensing errors, or errors in drug administration.”30
We defined contributing factors as any patient‐, provider‐ or system‐level factors that our reviewers deemed instrumental to the development of the event.
2.3. Chart review methods
After excluding cases that did not meet our case definition, a clinical pharmacist (S.W.) and physician (one of C.H., D.V. or F.S.) reviewed the medical and/or research records of all patients diagnosed with one or more adverse drug events, and independently assessed each event's preventability. Events were categorized as definitely, probably or not preventable.30 If preventability ratings were discordant, reviewers discussed the case until reaching consensus, and a third reviewer adjudicated all cases in which consensus was not easily achieved. One clinical pharmacist (S.W.) entered up to five culprit drugs for each adverse drug event and identified any number of potential contributing factors based on clinical judgement by reviewing the patient's research and/or medical record in detail.
2.4. Statistical analysis
We conducted descriptive analyses on baseline demographics for all included patients. We calculated the proportion of preventable adverse drug events as the number of definitely and probably preventable events, divided by the overall number of adverse drug events. We determined the frequency and proportion of preventable vs non‐preventable adverse drug events at different levels of severity and patient harm. We reported all odds ratios with 95% confidence intervals (95% CI). We tabulated the number and frequency of the different adverse drug event types and the most common culprit drugs among preventable and non‐preventable events. We dichotomized all multi‐level variables using dummy coding (i.e., present vs absent) and reported P‐values from chi‐square tests comparing the proportions of each adverse drug event‐level characteristic between preventable and non‐preventable events to indicate instances where a particular adverse drug event characteristic was significantly more common among one group compared to the other. We calculated the number and proportion of preventable and non‐preventable adverse drug events in which one or more contributing factors were identified. We determined the frequency at which contributing factors were identified among preventable adverse drug events and grouped them thematically. We used multivariable logistic regression to identify factors associated with patients diagnosed with a preventable adverse drug event vs patients without any preventable events. We included all measured patient‐level variables, including age, gender, hospital and major comorbidities in the model. We adjusted all inferential statistics (odds ratios and P‐values) to account for the effects of clustering by adverse drug event, by patient and/or by hospital.
3. RESULTS
We reviewed the charts of 3 202 patients, of whom 1 234 were diagnosed with at least one adverse drug event (Figure 1). Among these patients, 809 (65.6%) were diagnosed with one or more preventable events (Table 1). Patients with preventable adverse drug events were mostly female (56%), with a median age of 71 years (Interquartile Range (IQR) 33.7) and took a median of eight medications (IQR 8). The most common comorbidities among patients with preventable adverse drug events were hypertension (47.8%), diabetes (24.1%) and mental health diagnosis (22.2%). Patients with one or more preventable adverse drug events were more likely to have a mental health diagnosis (OR 1.8; 95% CI 1.3–2.5) or diabetes (OR 1.7; 95% CI 1.2–2.4) compared with patients without any preventable adverse drug events (Table 2).
Figure 1.
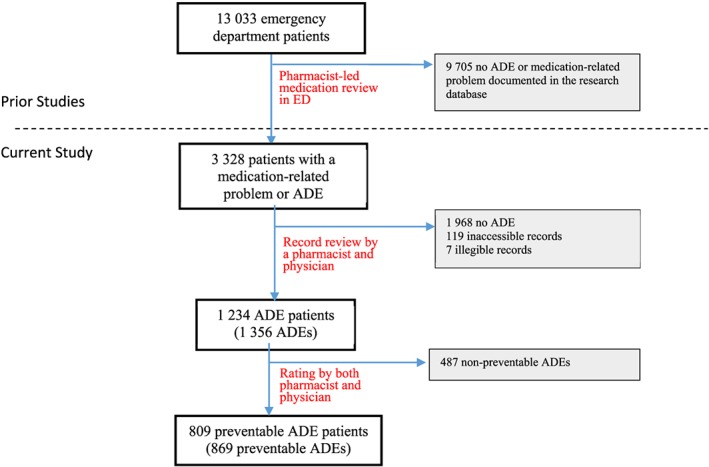
Flow diagram of patients through the study
Table 1.
Characteristics of 1 234 ADE patients by preventability
| Patient characteristics | Patients with ≥1 preventable ADEs (N = 809) | Patients with only non‐preventable ADEs (N = 425) |
|---|---|---|
| Median age, y (IQR) | 71.0 (33.7) | 66.5 (34.1) |
| Number ≥ 80 years, n (%) | 284 (35.1) | 124 (29.2) |
| Female, n (%) | 452 (55.9) | 244 (57.4) |
| Enrolling hospital, n (%) | ||
| VGH | 612 (75.6) | 324 (76.2) |
| LGH | 139 (17.2) | 65 (15.3) |
| SPH | 25 (3.1) | 13 (3.1) |
| OCH | 33 (4.1) | 23 (5.4) |
| Comorbidites, n (%) | ||
| Diabetes | 195 (24.1) | 63 (14.8) |
| Chronic heart failure | 95 (11.7) | 45 (10.6) |
| Atrial fibrillation | 166 (20.5) | 90 (21.2) |
| Renal failure | 91 (11.2) | 31 (7.3) |
| Dementia | 62 (7.7) | 21 (4.9) |
| Hypertension | 387 (47.8) | 169 (39.8) |
| Mental health diagnosis | 180 (22.2) | 63 (14.8) |
| Other condition | 717 (88.6) | 354 (83.3) |
Table 2.
Multivariable associations between patient factors and preventable adverse drug events (≥1) versus patients with only non‐preventable adverse drug events (N = 1 234)
| Independent variables | Patients with preventable ADEs | Patients with ADE | Patients with preventable events (%) | ORa (95% CI) | P‐value |
|---|---|---|---|---|---|
| Age ≤ 80 y | 525 | 826 | 63.6 | 1.00 | |
| Age > 80 y | 284 | 408 | 69.6 | 1.2 (0.9–1.6) | 0.26 |
| Gender | |||||
| Male | 357 | 538 | 66.4 | 1.00 | |
| Female | 452 | 696 | 64.9 | 0.9 (0.7–1.1) | 0.39 |
| Hospital | |||||
| VGH | 612 | 936 | 65.4 | 1.00 | |
| LGH | 139 | 204 | 68.1 | 1.2 (0.9–1.7) | 0.24 |
| OCH | 33 | 56 | 58.9 | 0.7 (0.4–1.3) | 0.24 |
| SPH | 25 | 38 | 65.8 | 1.1 (0.5–2.1) | 0.81 |
| Comorbidities | |||||
| Diabetesa | 195 | 258 | 75.6 | 1.7 (1.2–2.4) | <0.01 |
| Chronic heart failurea | 95 | 140 | 67.9 | 1.0 (0.6–1.5) | 0.86 |
| Atrial fibrillationa | 166 | 256 | 64.8 | 0.9 (0.6–1.2) | 0.37 |
| Renal failurea | 91 | 122 | 74.6 | 1.3 (0.8–2.1) | 0.24 |
| Dementiaa | 62 | 83 | 74.7 | 1.5 (0.8–2.5) | 0.19 |
| Hypertensiona | 387 | 556 | 69.6 | 1.3 (1.0–1.7) | 0.11 |
| Mental health diagnosisa | 180 | 243 | 74.1 | 1.8 (1.3–2.5) | <0.01 |
Dummy coded so that patients without the health condition are the reference category.
The 1 234 patients included in the study experienced 1 356 adverse drug events. Of the 1 356 events reviewed, reviewers considered 869 (64.1%) to have been preventable. Among preventable events, non‐adherence (28.2%), adverse drug reactions (23.8%) and supratherapeutic dosing (13.6%) were most common, while among non‐preventable events adverse drug reactions (54.8%), low dose (11.9%) and ineffective drugs (11.7%) were more common (Table 3). Adverse drug events due to non‐adherence were more likely to be considered preventable (28.2%) vs non‐preventable (3.1%; P < 0.01; Table 3). In contrast, a lower proportion of the preventable ADEs were adverse drug reactions (23.8%) compared with the non‐preventable ADEs (54.8%; P < 0.01; Table 3). Severe adverse drug events and those resulting in permanent harm or death were equally common between preventable and non‐preventable events, but preventable events had a higher proportion of patients who suffered no harm compared to non‐preventable events (Table 3).
Table 3.
Comparison of preventable with non‐preventable adverse drug events
| Preventable ADEs (N = 869) | Non‐preventable ADEs (N = 487) | P‐value | |
|---|---|---|---|
| ADE type, n (%) | |||
| Non‐adherence | 245 (28.2) | 15 (3.1) | <0.01 |
| Adverse drug reaction | 207 (23.8) | 267 (54.8) | <0.01 |
| High dose | 118 (13.6) | 41 (8.4) | <0.01 |
| Needs additional drug/untreated indication | 117 (13.5) | 36 (7.4) | <0.01 |
| Low dose | 92 (10.6) | 58 (11.9) | 0.46 |
| Othera | 56 (6.4) | 13 (2.7) | <0.01 |
| Ineffective drug | 34 (3.9) | 57 (11.7) | <0.01 |
| ADE severity, n (%) | |||
| Mild | 22 (2.6) | 26 (5.3) | <0.01 |
| Moderate | 573 (65.9) | 312 (64.1) | 0.49 |
| Severe/fatal | 274 (31.5) | 149 (30.6) | 0.72 |
| Outcomes, n (%) | |||
| No harm | 153 (17.6) | 62 (12.8) | 0.02 |
| Temporary harm | 692 (79.6) | 414 (85.0) | 0.01 |
| Permanent harm/death | 24 (2.8) | 11 (2.3) | 0.58 |
Includes drug interactions, drug withdrawals, and drug transcription/dispensing/administration errors.
The most common medications associated with preventable adverse drug events were warfarin (9.4%), hydrochlorothiazide (4.5%), furosemide (4.0%), insulin (3.9%) and acetylsalicylic acid (2.7%; Table 4). The top medication classes associated with preventable events included coumarin derivatives (9.4%), opiate agonists (8.5%), atypical antipsychotics (5.2%), thiazide diuretics (4.5%) and loop diuretics (4.1%). Of these medication classes, only atypical antipsychotics and thiazide diuretics were more common among preventable (5.2% and 4.5%, respectively) compared with non‐preventable events (1.4% and 2.2%, respectively; P < 0.01 and P = 0.02; Table 4).
Table 4.
Comparison of medications implicated in preventable and non‐preventable adverse drug events
| Preventable ADE drugs (N = 1 166)a | Non‐preventable ADE drugs (N = 635)a | P‐value | |
|---|---|---|---|
| Top culprit medications, n (%) a | |||
| Warfarin | 110 (9.4) | 57 (9.0) | 0.75 |
| Hydrochlorothiazide | 52 (4.5) | 14 (2.2) | 0.02 |
| Furosemide | 47 (4.0) | 24 (3.8) | 0.80 |
| Insulin (human) | 45 (3.9) | 11 (1.7) | 0.02 |
| Acetylsalicylic acid | 32 (2.7) | 23 (3.6) | 0.30 |
| Top culprit medication classes, n (%) a | |||
| Coumarin derivatives | 110 (9.4) | 57 (9.0) | 0.75 |
| Opiate agonists | 99 (8.5) | 67 (10.6) | 0.15 |
| Atypical antipsychotics | 61 (5.2) | 9 (1.4) | <0.01 |
| Thiazide diuretics | 52 (4.5) | 14 (2.2) | 0.02 |
| Loop diuretics | 48 (4.1) | 24 (3.8) | 0.73 |
There were often multiple drugs implicated per ADE. We used the total number of drugs for each class of ADE as the denominator for each column.
Multiple factors contributed to 262 (30.1%) preventable adverse drug events. Frequently identified contributing factors were related to medication prescribing (45.8%) and monitoring (35.5%), barriers to adherence (23.5%), problems with communication (11.5%), social factors (10.7%) and inadequate access to appropriate care (9.1%) (Table 5). The most frequently identified contributing factor among preventable adverse drug events was deemed to be inadequate patient education or instructions (23.8%). Common prescribing issues included delays to or inadequate clinical reassessments after medication changes (15.6%) and inadequate laboratory monitoring (15.3%; Table 5). Common barriers to adherence included mental health illness (6.8%) and patient preference (6.3%; Table 5).
Table 5.
Most frequently identified factors that contributed to the development of preventable events (N = 782)
| Contributing factors identified among 782 preventable ADEsa | n (%) | |
|---|---|---|
| Communication problem | Lack of communication between a healthcare provider and a patient | 58 (7.4) |
| Lack of communication between physicians | 18 (2.3) | |
| Lack of communication between nurses | 6 (0.8) | |
| Lack of communication between physician and pharmacist | 3 (0.4) | |
| Lack of communication between physician and a nurse | 3 (0.4) | |
| Lack of communication between pharmacists | 2 (0.3) | |
| Drug delivery/labelling/packaging/storage problem | Drug name, label or packaging problem | 1 (0.1) |
| Drug storage or delivery problem | 1 (0.1) | |
| EMS problem | Ambulance did not transport hypoglycaemic patient to hospital | 3 (0.4) |
| Error | Provider error in drug administration | 1 (0.1) |
| Inadequate monitoring | Delay in or inadequate clinical reassessment after medication change | 122 (15.6) |
| Insufficient laboratory monitoring | 120 (15.3) | |
| Too aggressive medical therapy for patient's condition/age | 36 (4.6) | |
| Mental health illness/social problem‐related | Non‐adherence associated with mental health illness | 53 (6.8) |
| Patient confusion/dementia | 16 (2.0) | |
| Substance misuse | 15 (1.9) | |
| Missing information | Critical information missing that could have prevented or mitigated the ADE | 31 (4.0) |
| Non‐adherence | Patient preference to not take medications | 49 (6.3) |
| Prior ADE leading to patient non‐adherence | 38 (4.9) | |
| Patient self‐titrating medications inappropriately | 30 (3.8) | |
| No compliance aid when required | 20 (2.6) | |
| Patient non‐adherence due to financial/lack of coverage | 18 (2.3) | |
| Regimen too complex (e.g., high number of daily doses) | 11 (1.4) | |
| Patient missed doses (forgetfulness/intoxication) | 10 (1.2) | |
| Patient error in administration | 7 (0.9) | |
| Patient hearing problem | 1 (0.1) | |
| Prior ADE | Missed/misdiagnosed previous ADE | 3 (0.4) |
| Provider‐level problem | Provided inadequate patient education or instructions | 186 (23.8) |
| Provider non‐adherence with current treatment guidelines | 101 (12.9) | |
| Lack of staff education | 43 (5.5) | |
| Medication prescribed inappropriately because patient insisted | 18 (2.3) | |
| Prescribed despite lack of clear indication for the culprit drug | 10 (1.2) | |
| Systems level problem | Environmental problem | 4 (0.5) |
| Lack of quality control or independent check systems | 4 (0.5) | |
| Unable to access care | Patient unable to access a prescription refill | 44 (5.6) |
| Patient unable to access GP for appointment | 15 (1.9) | |
| Patient unable to access specialist for appointment | 8 (1.0) | |
| Patient unable to access appropriate level of care | 4 (0.5) | |
| aThere were 87 preventable adverse drug events with no identified contributing factors | ||
4. DISCUSSION
We sought to describe preventable adverse drug events from outpatient medications among adults presenting to emergency departments. Almost two‐thirds of adverse drug events diagnosed in the emergency department were preventable. Patients with mental health conditions and diabetes were more likely to experience preventable events, while older patients were not. Atypical antipsychotics and thiazide diuretics were more commonly associated with preventable events, while other medication classes commonly targeted in preventative efforts, such as coumarin derivatives and anti‐infectives, were not associated with preventable events.31, 32 Insufficient education and monitoring commonly contributed to preventable events.
Current efforts in adverse drug event prevention often target specific patient populations, such as the elderly, or those taking medication classes such as anticoagulants that are commonly associated with adverse drug events, regardless of their preventability.32, 33, 34 Many adverse drug events experienced by older adults may not be preventable, given the complexities of their medication regimens.35 This is in keeping with systematic reviews that have indicated that medication reviews in older patients may increase adherence and drug knowledge, without resulting in improvements in patient‐oriented or system‐level outcomes such as reduced hospitalizations and emergency department visits.26, 36 Developing strategies that specifically target patients and medications associated with preventable events may be an effective alternative.
Understanding contributing factors to preventable events can provide insight into why the adverse drug events occurred. Previous studies have recommended steps to improve patient adherence, encourage provider adherence to guidelines, increase diligence in monitoring patients, and optimize communication between providers and patients.19, 23, 31, 37 Others have highlighted inappropriate prescribing practices and insufficient monitoring of medications as contributing factors.38, 39 In a systematic review, identifying over‐treated patients with diabetes and de‐intensifying therapy was associated with reduced hypoglycaemic events without compromising glycaemic control.40 Our findings support these conclusions, and highlighted the importance of enhanced monitoring for patients on thiazide diuretics and insulin, as these medications were commonly implicated in preventable events. Specifying medications at highest risk of preventable adverse drug events may assist in developing more specific recommendations to enhance the feasibility of and reduce the resource utilization associated with enhanced monitoring. Similarly, focusing resources to promote better education and monitoring for high‐risk non‐adherent patients may be advantageous.41, 42 For example, additional resources for mental health patients may include access to intense case management, a team‐based model of care providing educational and environmental support to help patients remain adherent to medications.42, 43
Our study is not without limitations. While our study was multi‐centre and conducted in two provinces, most cases were enrolled in British Columbian urban hospitals. Our results may therefore not be representative of the types of adverse drug events seen in other jurisdictions or contexts.
Our retrospective assessments of preventability and contributing factors were limited by the availability of the information documented in research records and/or paper‐based and electronic medical records. It was often difficult to assess the preventability of an event without knowing the exact circumstances of the care that had been provided, or the patient's perspective. Individual and professional biases may have affected how reviewers perceived the preventability of an event.30, 44 Not all contributing factors were explicitly documented in the research or medical records, and therefore, undoubtedly, our clinical judgement, the patient's prior history, and documentation of the event's resolution informed our assessments. We assumed that if a clinician had addressed the contributing factor prior to the event, the event would have been prevented. To increase the thoroughness and reduce the subjectivity of our preventability assessments, a second reviewer independently assessed the preventability of an event to produce a more robust assessment. However, this was not possible for the assessment of contributing factors, due to budgetary and time constraints.
Many contributing factors could be attributed to multiple non‐exclusive domains. Often, the charts provided insufficient information to attribute a contributing factor to any one single domain, and might be attributable to more than one domain (e.g., communication could be a provider‐level, patient‐level or system‐level issue). Given that prospective assessment and study of contributing factors is not feasible (it is not ethical to identify and observe contributing factors without intervening), this is a limitation inherent to this body of work.
In conclusion, efforts to reduce adverse drug events should focus on preventable adverse drug events and address common contributing factors. Our work suggests that close monitoring of patients with mental health conditions and diabetes is warranted. In addition, enhanced laboratory monitoring is indicated specifically for patients on insulin and thiazide diuretics.
COMPETING INTERESTS
The authors have no competing of interest to declare.
CONTRIBUTORS
C.H., S.W., A.C. and M.W. conceived of and designed the study. S.W., C.H., F.S. and D.V. collected data. A.C., J.H. and M.W. cleaned and analysed the quantitative data. All authors contributed to, read, and approved of the final manuscript.
ACKNOWLEDGEMENTS
This study was funded by the Canadian Institutes for Health Research. We would like to acknowledge the contributions of Christine Ackerley, Kane Larson, Caleb Roda, and Puneet Vashisht who organized and prepared prior research records for data collection. This study was funded through by Health Canada and the Canadian Institutes of Health Research (CIHR) through the Drug Safety and Effectiveness Network (DSEN).
Appendix A. Characteristics of the original studies from which patients with suspected adverse drug events were enrolled into the current study44
| Study title | Cohort 1[5] | Cohort 2[4, 27] | Cohort 3[26] |
|---|---|---|---|
| Design | Prospective observational | Controlled clinical trial | Prospective observational |
| Primary study objective | To derive a clinical decision instrument to identify patients at high‐risk of presenting with an adverse drug event | To evaluate the impact of emergency department‐based pharmacist‐led medication review on health outcomes. | To validate clinical decision instruments to identify patients at high‐risk of presenting with an adverse drug event |
| Study period | 2008–2009 | 2011–2013 | 2014–2015 |
| Hospitals | VGH, SPH | VGH, LGH, RGH | VGH, LGH, OCH |
| Participants |
Included • >19 years • used ≥1 prescription or OTC medication in the past 2 weeks • spoke English or translator available Excluded • violent behaviour • intentional self‐poisoning • scheduled revisit • previously enrolled • transferred directly to an admitting service • left AMA |
Included • >19 years • used ≥1 prescription or OTC medication in the past 2 weeks • spoke English or translator available • high‐risk based on ADE decision rule5, 26 Excluded • required immediate resuscitation (CTAS = 1).45 • multisystem trauma • scheduled re‐visit • sexual assault • surgical complication • pregnancy complication • social problem |
Included • >19 years • used ≥1 prescription or OTC medication in the past 2 weeks • spoke English or translator available Excluded • violent behaviour • intentional self‐poisoning • scheduled revisit • previously enrolled • needle stick injury • sexual assault • triaged to fast track zone where time to disposition too rapid for enrolment • transferred directly to admitting service • left AMA |
| Enrolment (Appendix B) | Random selection of first patient with subsequent systematic selection | Random selection of first patient with subsequent systematic selection | Random selection of first patient with subsequent systematic selection |
| ADE definition | “Untoward and unintended event arising from the use of prescription or OTC medications.”28, 30 | Same as in cohort 1. Medication‐related problems were captured and documented in addition to ADEs. | “Untoward and unintended event arising from the appropriate or inappropriate use of a prescription or OTC medication.” |
| Data collection process (Appendix C) | Trained clinical pharmacist and treating physician independently evaluated enrolled patients for ADEs. An independent committee adjudicated all discordant and uncertain cases by record review. | Clinical pharmacists working in the ED and treating physician independently evaluated enrolled patients for ADEs. Clinical pharmacists and physicians discussed uncertain or discordant cases in ED. | Trained clinical pharmacist and treating physician independently evaluated enrolled patients for ADEs. An independent committee adjudicated all discordant and uncertain cases by record review. |
| Follow‐up duration | Until ED/hospital discharge, and by telephone follow‐up if required | Until ED/hospital discharge, linkage with administrative database for health outcomes | Until ED/hospital discharge, and by telephone follow‐up if required |
| No. participants | 1591 | 10 807 | 1529 |
| No. participants considered for enrolment in current study | 226 | 2862 | 240 |
VGH, Vancouver General Hospital, a tertiary care hospital in Vancouver, BC, Canada; SPH, Saint Paul's Hospital, a tertiary care hospital in Vancouver, BC, Canada; LGH, Lions Gate Hospital, an urban community hospital in North Vancouver, BC, Canada; RGH, Richmond General Hospital, an urban community hospital in Richmond, BC, Canada; OCH, Ottawa Civic Hospital, a tertiary care hospital in Ottawa, ON, Canada; ADE, adverse drug event; AMA, against medical advice; OTC, over‐the‐counter; CTAS, Canadian Triage Acuity Score; ED, emergency department
Appendix B. Patient enrolment algorithm

Appendix C. Current study definitions
Adverse drug event (ADE):
“Unintended and harmful symptoms, signs or abnormal laboratory values arising from the appropriate or inappropriate use of medications (prescription, over‐the‐counter or complimentary and alternative medications).”
“Harm caused by the use of a drug.”
The sign(s) or symptom(s) are generally not deemed to have an alternate explanation by the pharmacist and the treating physician. In cases where alternative causes are possible (e.g., fall), the event should only be considered a suspect adverse drug event, if it is likely that the symptom would not have been as severe or not have occurred without the patient being on the drug (e.g., intracranial haemorrhage).
- Abnormal vital signs may constitute an adverse drug event, if the abnormal vital signs meet the cut‐offs below, and treating the adverse drug event is medically appropriate according to the treating physician:
-
○HR ≤ 50 and associated with symptoms (e.g., pre‐syncope/syncope, prolonged sinus pauses on ECG)
-
○HR ≥ 120 and associated with symptoms (e.g., pre‐syncope/syncope, chest pain or palpitations)
-
○BP ≥ 180 SBP or ≥ 100 DBP
-
○
Abnormal laboratory tests can be considered an adverse drug event if they are outside of the hospital's reference range, and treating the adverse drug event is medically appropriate according to the treating physician.
Harm caused by the use of alcohol or illicit drugs does not constitute an adverse drug event.
ADE classification:
Adverse drug reaction (ADR): “A response to a drug that is noxious and unintended, and occurs at doses normally used in man for the prophylaxis, diagnosis or therapy of disease”.
Drug interaction.
Dosage Too High.
Dosage Too Low.
Non‐adherence.
Inappropriate Drug Withdrawal.
Ineffective Drug.
Needs Additional Drug/Untreated Indication: This categorization should be reserved for cases in which there is clear previous documentation (i.e., before the index ED visit) in the patient's records about a hard indication for the drug, and lack of contra‐indication for treatment.
Transcription/Dispensing/Administration Error.
ADE severity:
Fatal: The adverse event resulted in death.
Severe: The adverse drug event was the primary reason for the patient's hospitalization, caused permanent disability or was life‐threatening.
Moderate: The adverse event required a change in medical management (medical therapy, a diagnostic procedure or consultation).
Mild: No change in medical therapy, including no adjustment of medications required.
ADE preventability:
Preventable if: there is a lack of adherence to best medical practice, including inappropriate drug, dosage, route or frequency of administration of a drug for the patient's clinical condition, age, weight or renal function; administration of a drug despite a known allergy, a previous adverse reaction to, or a drug interaction; non‐adherence; laboratory monitoring not or inappropriately performed; prescribing or dispensing errors, or errors in drug administration.
Woo SA, Cragg A, Wickham ME, et al. Preventable adverse drug events: Descriptive epidemiology. Br J Clin Pharmacol. 2020;86:291–302. 10.1111/bcp.14139
Principal Investigator: The authors confirm that the Principal Investigator for this paper is Dr. Corinne Hohl and that she had direct clinical responsibility for patients.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med. 1991;324(6):370‐376. [DOI] [PubMed] [Google Scholar]
- 2. Budnitz DS, Pollock DA, Weidenbach KN, Mendelsohn AB, Schroeder TJ, Annest JL. National surveillance of emergency department visits for outpatient adverse drug events. JAMA. 2006;296(15):1858‐1866. [DOI] [PubMed] [Google Scholar]
- 3. Institute of Medicine . To err is human: building a safer health system. Washington, DC: National Academy Press; 2000. [Google Scholar]
- 4. Hohl CM, Partovi N, Ghement I, et al. Impact of early in‐hospital medication review by clinical pharmacists on health services utilization. PLoS One. 2017;12(2):e0170495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hohl CM, Yu E, Hunte GS, et al. Clinical decision rules to improve the detection of adverse drug events in emergency department patients. Acad Emerg Med. 2012;19(6):640‐649. [DOI] [PubMed] [Google Scholar]
- 6. WHO Research Priority Setting Working Group . Global Priorities for Research in Patient Safety. Geneva, Switzerland: World Health Organization; 2008. [Google Scholar]
- 7. Harris Y, Hu DJ, Lee C, Mistry M, York A, Johnson TK. Advancing medication safety: establishing a National Action Plan for adverse drug event prevention. Jt Comm J Qual Patient Saf. 2015;41(8):351‐360. [DOI] [PubMed] [Google Scholar]
- 8. Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA. 1995;274(1):29‐34. [PubMed] [Google Scholar]
- 9. Bates DW, Leape LL, Petrycki S. Incidence and preventability of adverse drug events in hospitalized adults. J Gen Intern Med. 1993;8(6):289‐294. [DOI] [PubMed] [Google Scholar]
- 10. Classen DC, Pestotnik SL, Evans RS, Burke JP. Computerized surveillance of adverse drug events in hospital patients. JAMA. 1991;266(20):2847‐2851. [PubMed] [Google Scholar]
- 11. Franceschi M, Scarcelli C, Niro V, et al. Prevalence, clinical features and avoidability of adverse drug reactions as cause of admission to a geriatric unit: a prospective study of 1756 patients. Drug Saf. 2008;31(6):545‐556. [DOI] [PubMed] [Google Scholar]
- 12. Gholami K, Shalviri G. Factors associated with preventability, predictability, and severity of adverse drug reactions. Ann Pharmacother. 1999;33(2):236‐240. [DOI] [PubMed] [Google Scholar]
- 13. Gurwitz JH, Field TS, Avorn J, et al. Incidence and preventability of adverse drug events in nursing homes. Am J Med. 2000;109(2):87‐94. [DOI] [PubMed] [Google Scholar]
- 14. Lagnaoui R, Moore N, Fach J, Longy‐Boursier M, Begaud B. Adverse drug reactions in a department of systemic diseases‐oriented internal medicine: prevalence, incidence, direct costs and avoidability. Eur J Clin Pharmacol. 2000;56(2):181‐186. [DOI] [PubMed] [Google Scholar]
- 15. Tafreshi MJ, Melby MJ, Kaback KR, Nord TC. Medication‐related visits to the emergency department: a prospective study. Ann Pharmacother. 1999;33(12):1252‐1257. [DOI] [PubMed] [Google Scholar]
- 16. Courtman BJ, Stallings SB. Characterization of drug‐related problems in elderly patients on admission to a medical ward. Canadian Journal Hosp Pharm. 1995;48(3):161‐166. [PubMed] [Google Scholar]
- 17. Dequito AB, Mol PG, van Doormaal JE, et al. Preventable and non‐preventable adverse drug events in hospitalized patients: a prospective chart review in the Netherlands. Drug Saf. 2011;34(11):1089‐1100. [DOI] [PubMed] [Google Scholar]
- 18. Gandhi TK, Weingart SN, Borus J, et al. Adverse drug events in ambulatory care. N Engl J Med. 2003;348(16):1556‐1564. [DOI] [PubMed] [Google Scholar]
- 19. Zed PJ, Abu‐Laban RB, Balen RM, et al. Incidence, severity and preventability of medication‐related visits to the emergency department: a prospective study. CMAJ. 2008;178(12):1563‐1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365(21):2002‐2012. [DOI] [PubMed] [Google Scholar]
- 21. Gurwitz JH, Field TS, Judge J, et al. The incidence of adverse drug events in two large academic long‐term care facilities. Am J Med. 2005;118(3):251‐258. [DOI] [PubMed] [Google Scholar]
- 22. Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324(6):377‐384. [DOI] [PubMed] [Google Scholar]
- 23. van der Hooft CS, Dieleman JP, Siemes C, et al. Adverse drug reaction‐related hospitalisations: a population‐based cohort study. Pharmacoepidemiol Drug Saf. 2008;17(4):365‐371. [DOI] [PubMed] [Google Scholar]
- 24. Schumock GT, Thornton JP. Focusing on the preventability of adverse drug reactions. Hosp Pharm. 1992;27:538. [PubMed] [Google Scholar]
- 25. Hohl CM, Badke K, Zhao A, et al. Prospective validation of clinical criteria to identify emergency department patients at high risk for adverse drug events. Acad Emerg Med. 2018;25(9):1015‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hohl CM, McGrail K, Sobolev B. The effect of pharmacist‐led medication review in high‐risk patients in the emergency department: an evaluation protocol. CMAJ Open. 2015;3(1):e103‐e110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nebeker J, Barach P, Samore M. Clarifying adverse drug events: a clinician's guide to terminology, documentation, and reporting. Ann Intern Med. 2004;140(10):795‐801. [DOI] [PubMed] [Google Scholar]
- 28. Government of Canada . Adverse Reaction Information 2012 [online]. Available at: http://www.hc-sc.gc.ca/dhp-mps/medeff/advers-react-neg/index-eng.php. Accessed 4 December 2019.
- 29. World Health Organization . International drug monitoring: the role of the hospital. Report of a WHO meeting. Geneva, Switzerland: World Health Organization; 1969. [PubMed]
- 30. Woo SA, Cragg A, Wickham ME, et al. Methods for evaluating adverse drug event preventability in emergency department patients. BMC Med Res Methodol. 2018;18(1):160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zed PJ. Drug‐related visits to the emergency department. J Pharm Pract. 2005;18(5):329‐335. [Google Scholar]
- 32. Office of Disease Prevention and Health Promotion . National Action Plan for Adverse Drug Event Prevention. Washington, DC: US Department of Health and Human Services; 2014.
- 33. Cresswell KM, Fernando B, McKinstry B, Sheikh A. Adverse drug events in the elderly. Br Med Bull. 2007;83(1):259‐274. [DOI] [PubMed] [Google Scholar]
- 34. Trivalle C, Cartier T, Verny C, et al. Identifying and preventing adverse drug events in elderly hospitalised patients: a randomised trial of a program to reduce adverse drug effects. J Nutr Health Aging. 2010;14(1):57‐61. [DOI] [PubMed] [Google Scholar]
- 35. Canadian Institute for Health Information . Highlights of 2010‐2011 Inpatient Hospitalizations and Emergency Department Visits; 2012.
- 36. Holland R, Desborough J, Goodyer L, Hall S, Wright D, Loke YK. Does pharmacist‐led medication review help to reduce hospital admissions and deaths in older people? A systematic review and meta‐analysis. Br J Clin Pharmacol. 2007;65(3):303‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hohl CM, Dankoff J, Colacone A, Afilalo M. Polypharmacy, adverse drug‐related events, and potential adverse drug interactions in elderly patients presenting to an emergency department. Ann Emerg Med. 2001;38(6):666‐671. [DOI] [PubMed] [Google Scholar]
- 38. Levinson DR. Adverse events in hospitals: national incidence among Medicare beneficiaries. Available at: https://oig.hhs.gov/oei/reports/oei-06-09-00090.pdf. Accessed 4 December 2019.
- 39. Thomsen LA, Winterstein AG, Sondergaard B, Haugbolle LS, Melander A. Systematic review of the incidence and characteristics of preventable adverse drug events in ambulatory care. Ann Pharmacother. 2007;41(9):1411‐1426. [DOI] [PubMed] [Google Scholar]
- 40. Abdelhafiz AH, Sinclair AJ. Deintensification of hypoglycaemic medications—use of a systematic review approach to highlight safety concerns in older people with type 2 diabetes. J Diabetes Complications. 2018;32(4):444‐450. [DOI] [PubMed] [Google Scholar]
- 41. Al Hamarneh YN, Charrois T, Lewanczuk R, Tsuyuki RT. Pharmacist intervention for glycaemic control in the community (the RxING study). BMJ Open. 2013;3(9):e003154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kane JM, Kishimoto T, Correll CU. Non‐adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategies. World Psychiatry. 2013;12(3):216‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Marshall M, Lockwood A. Assertive community treatment for people with severe mental disorders. Cochrane Database Syst Rev. 2000;((2)):CD001089. [DOI] [PubMed] [Google Scholar]
- 44. Hohl CM, Woo SA, Cragg A, et al. Repeat adverse drug events associated with outpatient medications: a descriptive analysis of 3 observational studies in British Columbia, Canada. CMAJ Open. 2019;7(3):E446‐E453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Manos D, Petrie DA, Beveridge RC, Walter S, & Ducharme J. Inter‐observer agreement using the Canadian Emergency Department Triage and Acuity Scale CJEM 2002, 4:(1)16 22 [DOI] [PubMed] [Google Scholar]
- 46. Edwards IR Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet, 2000. 356:(9237)1255‐1259 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are not publicly available due to privacy or ethical restrictions.


