Abstract
Oral anticoagulation (OAC) for stroke prevention in patients with atrial fibrillation is underutilised. One of the impediments to warfarin therapy is the frequent monitoring required, usually at a specialised warfarin clinic. The advent of direct oral anticoagulants (DOACs) facilitates OAC therapy without an onerous monitoring regimen. This benefit may result in the more significant adoption of DOACs in areas without a warfarin clinic. This study analysed national administrative data for reimbursed pharmacy claims to assess OAC prescribing from 2010 to 2017 and compared the use of DOACs in areas with warfarin clinics compared to those without. Over the study period, the number of patients on OAC increased by 84%, due to a rapid increase in DOAC prescribing. The findings demonstrate that DOACs have resulted in an increase in the overall uptake of OAC therapy in Ireland. However, the increased utilisation was not evidently related to populations underserved by warfarin clinics.
Keywords: anticoagulation, atrial fibrillation, direct oral anticoagulant, novel oral anticoagulant, warfarin
What is already known about this subject
Oral anticoagulation (OAC) is underutilised in patients for stroke prevention. Direct oral anticoagulants (DOACs) overcome some issues inherent in the use of warfarin therapy, such as the necessity for frequent monitoring, often in a warfarin clinic. DOACs have been integrated into international guidelines and implemented in patient care.
What this study adds
The use of DOACs has resulted in an increase in the overall uptake of OAC therapy in Ireland. The increased utilisation was not evidently related to populations underserved by warfarin clinics. Further research to identify populations undertreated with OAC is required to target quality improvement interventions
1. INTRODUCTION
Atrial fibrillation (AF) is a frequent cardiovascular presentation and the most common cardiac arrhythmia.1 Its prevalence has previously been estimated to be 0.5–3% in the general population, increasing substantially in the elderly.1, 2, 3 The primary concern for those with this condition is a risk of thromboembolic stroke. It is thought that at least 15% of strokes are due to AF and these strokes are often more detrimental to the patient's subsequent functional ability compared to those of other aetiologies.3
There is robust evidence to suggest patients with AF and a high stroke risk derive a benefit from oral anticoagulation (OAC).4, 5 Despite this evidence, the appropriate use of OAC has been inadequate, exposing large numbers of patients with AF to an excess risk of ischaemic stroke. Estimates of this undertreatment in the UK suggest that 45% of patients do not receive appropriate OAC treatment, and, of those admitted to UK hospitals with a stroke and AF in early 2013, only 36% were on OAC.6 These findings have been replicated in other populations.7, 8, 9, 10 Warfarin therapy has been the gold standard OAC for the prevention of stroke and venous thromboembolism (VTE) as well as the treatment of VTE.
Direct oral anticoagulants (DOACs) have been added to the armamentarium following ‐scale randomised trials demonstrating noninferiority to warfarin for stroke prevention in the setting of AF.11, 12, 13, 14 DOACs have been integrated into practice in recent years. This is reflected by updated guidelines on the treatment of AF with subsequent increases in the overall uptake of OAC.15, 16, 17, 18, 19, 20, 21 DOACs offer an alternative to warfarin in the setting of AF and VTE with a requirement for less intensive monitoring due to their pharmacological profiles. DOACs are therefore more convenient for both the prescriber and the patient.
However, real‐world evidence suggests that despite this medical advance, OACs are still under‐utilised.20, 22, 23 Why is this the case? The treating physician's overestimation of the risk of a bleeding event and underestimation of the benefit of OAC have been described as barriers to treatment.24 Issues from the patient's perspective include the fear of bleeding events, as well as poor adherence due to the chronic nature of OAC treatment.25 Factors that are less often described are the inconvenience of warfarin monitoring via international normalised ratio (INR) testing and frequently changing dosing regimens.26, 27 The unavailability of a local warfarin clinic adds to this inconvenience, thus affecting those living in rural locations disproportionally.28, 29 However, the relationship of OAC prescribing trends, particularly with the advent of DOAC therapy, and the availability of anticoagulation monitoring services has not been studied. Doing so may improve the targeting of interventions to improve OAC uptake in other health systems.
The aims of this work are to: (i) report the contemporary OAC utilisation trends in Ireland following the introduction of DOACs; and (ii) identify if there was a greater uptake of DOACs in areas not served by a warfarin clinic.
2. METHODS
2.1. Health Services Executive–Primary Care Reimbursement Service
The Health Services Executive–Primary Care Reimbursement Service (HSE‐PCRS) General Medical Services (GMS) scheme is a means‐tested system in Ireland providing prescribed medicines for a small co‐payment per item. The scheme covers approximately 1/3 of the population, but over‐represents females and the elderly.30 The HSE‐PCRS pharmacy claims database used for this study contains reimbursement records of medicines dispensed by pharmacies to patients in primary care. Medicines were identified by their 7‐digit World Health Organisation anatomical therapeutic chemical classification code.31
2.2. OAC utilisation in Ireland
Monthly patient numbers were extracted from the HSE‐PCRS database. These figures represented the number of patients dispensed an OAC in each calendar month which was reimbursed under the GMS scheme. The reimbursement figures are considered a surrogate for patients prescribed OAC. These figures may more accurately reflect the actual population treated with OAC as noninitiation of treatment is eliminated. The patients were identified by anatomical therapeutic chemical codes for warfarin and 4 DOACs; apixaban, dabigatran, edoxaban, rivaroxaban. The number of patients eligible in each calendar year was identified from the HSE‐PCRS annual reports.32 The monthly figures were adjusted for the number of GMS eligible patients prior to analysis and graphing.
Following a review of the data trends, a transition point was chosen at the beginning of 2013. This divided the study period in 2 segments; 2010 to 2012 when DOAC were seldom prescribed and 2013 to 2017, when DOAC utilisation increased rapidly. Segmented regression analyses were used to analyse the PCRS data over the study period using the chosen cut‐off.33 The effect of DOAC prescribing was quantified in terms of the slope change (β coefficient) for the periods above, in terms of both DOACs and total OACs. As patients may repeat prescriptions monthly, the Durbin–Watson (DW) statistic was applied to test for first‐order autocorrelation in the residuals from the statistical regression analysis. Analysis was performed using Microsoft Excel (Microsoft Corporation, Seattle, USA) and SPSS software version 24 (IBM Corp, Armonk, NY, USA).
2.3. Analysis of proximity to a hospital‐based warfarin clinic and OAC utilisation
Spatial analysis techniques were employed to explore geographical patterns in GMS OAC prescribing at a local authority level relative to hospital‐based warfarin clinics. The reimbursement figures were for the period of January to December 2017. The presence of a warfarin clinic was established from HSE directories and confirmed by contacting the hospitals. Tableau Personal Desktop version 10.3 was utilised for all mapping and spatial analysis. Polygon shapefiles of Local Health Office (LHO) boundaries and point shapefiles of hospital‐based warfarin clinics were used to assist with spatial analysis and to create interactive visualisations. Proportions of patients on warfarin by LHO were compared using χ2 tests.
2.4. Ethics
Ethical approval for the PCRS data was not required as all data were anonymised.
3. RESULTS
The number of patients on oral anticoagulation increased from 28 445 in January 2010 to 52 119 in December 2017 (Figure 1). This represented an increase of 84% when adjusted for patients eligible for the GMS reimbursement. The period after the 2013 cut‐off had a 67% increase in patients on OAC when adjusted for the eligible population, which was much greater than the 10% increase evident for the period prior to 2013. The statistical analysis of the reimbursement trends corroborated this finding with the change in slope for OAC was significant after the 2013 cut‐off point (β 0.19, 95% confidence interval = 0.16, 0.22; P < .001).
Figure 1.
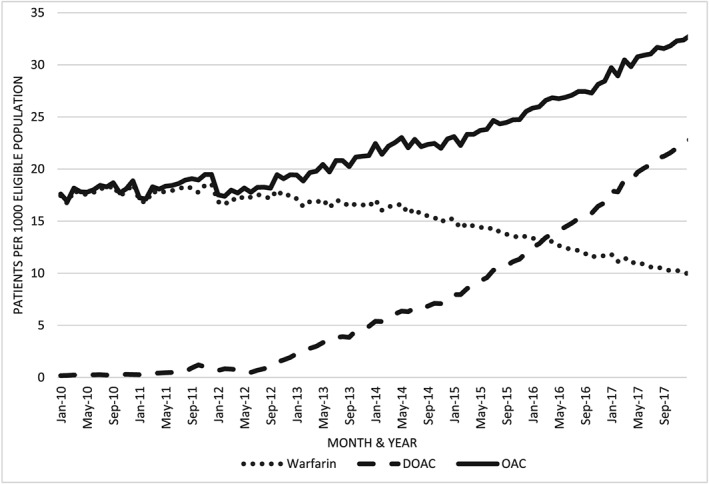
Anticoagulant prescribing trends 2010–2017 for general medical scheme eligible patients
This increase in patients treated with OAC was not associated with an increase in patients on warfarin, which showed a 42% decrease over the study period. The rate of patients on warfarin was unchanged from January 2010 to December 2012, 17.43 patients per 1000 eligible compared to 17.53. Therefore, the decrease was entirely in the second period (2013 to 2017).
The increase in patients on OAC was due to the prescribing of DOACs. As a percentage of all patients on OAC, those prescribed DOACs increased from 1 to 69% over the study period. As evident in Figure 1, there was a significant change in the rate of patients prescribed DOACs after the 2013 cut‐off (β coefficient 0.31, 95% confidence interval 0.28, 0.34; P < .001). The DW statistic indicated no significant autocorrelation in the residuals for either the OAC (P = .64) or DOAC (P = .94) regression analysis.
Analysis of the regional data showed an even divide for areas with at least 1 hospital‐based warfarin clinic present (green squares in Figure 2), with 59% having a DOAC predominance (areas with warfarin <40% or 40–50% in Figure 2). For areas without a warfarin clinic (red squares on Figure 2), 50% had a DOAC predominance. The percentage of patients on DOACs was not different for the areas with a warfarin clinic versus those without (χ2 = 0.04; P = .84). Of areas with a warfarin clinic, 18% had DOAC‐prescribing rates of >60%, which was not different to those without a warfarin clinic at 20% (χ2 = 0.02; P = .90).
Figure 2.
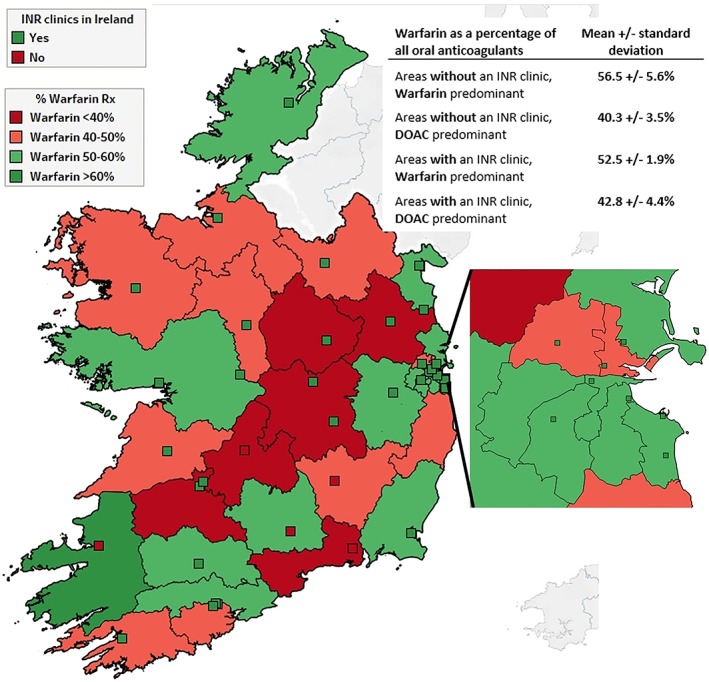
Anticoagulant prescribing patterns relative to warfarin clinic location. % Warfarin Rx, percentage of warfarin in terms of total oral anticoagulants prescriptions reimbursed; INR, international normalised ratio. Green squares represent hospitals with a warfarin clinic while red squares represent those without a warfarin clinic. An enlarged map of the Dublin area is inserted to the right. The inserted table gives warfarin as a percentage of total oral anticoagulation prescribed for local health areas grouped by OAC‐prescribing predominance and presence/absence of a warfarin clinic. ©OpenStreet map
4. DISCUSSION
This study found an increasing trend in the use of OAC in Ireland. This increase was driven by patients treated with DOACs, particularly from 2013 onwards. The rapid adoption of this new anticoagulant class was probably multifactorial. However, on examination of OAC‐prescribing trends in local health office areas served by hospital‐based warfarin clinics, there was no relationship observed. Therefore, the absence of a warfarin clinic did not appear to influence the instigation of DOAC treatment.
The underutilisation of OAC is a substantial public health issue.34 While it is difficult to assess the extent of adverse events accurately due to this issue, the number of patients presenting to hospital with a stroke and AF while not anticoagulated is well described.22, 35 It is, therefore, of paramount importance to identify why OAC is underutilised, including whether availability of specialised clinics is a factor. The use of warfarin is complicated by the requirement for frequent monitoring and the difficulty maintaining patients within its narrow therapeutic window.36, 37 DOACs surmount many of these difficulties and this may explain the increasing trends in OAC usage seen in our study.
The most recent US guidelines for the management of AF recommend DOACs over warfarin for nonvalvular AF.38 Therefore, the increasing use of DOAC therapy might be expected to continue. It is unclear whether adherence to OAC may be improved by use of DOACs with conflicting evidence.31, 39 There is still a concern that less frequent interaction with healthcare professionals, in a setting such as a warfarin clinic, may have unintended health consequences for a largely elderly population on OAC. For example, monitoring of renal function is recommended by guidelines,16 but this may not occur in the hectic setting of primary care. Indeed, patients have expressed a desire to access specialist anticoagulation services following a switch to DOAC therapy which may reflect their concern regarding bleeding events and a wish for closer monitoring while on OAC.31
The inconvenience and cost of frequent INR monitoring is greater for those distant to a warfarin clinic.40 It may be reasonable to expect that the absence of a local service to manage warfarin therapy would lead to the more significant adoption of DOAC therapy. However, this was not borne out by the prescribing patterns seen in this study. It is possible that the greater convenience afforded by DOACs is widely applicable, not just to those living in more remote locations. While studies referred to in this paper suggest rural residence as a factor in OAC underuse, a Canadian population‐based study did not validate this association.41 Therefore, the influence of both warfarin clinics and DOAC adoption are likely to be health system dependent.
4.1. Strengths and limitations
Strengths of the study include the use of reliable administrative data to assess OAC prescribing patterns. As OAC is a frequently required therapy, the large numbers of patients included in the study allow clear trends to be visualised and assessed. A limitation of the study is that PCRS data does not include an indication for OAC use. While it is expected that most OAC is prescribed for stroke prevention in the setting of AF, a proportion of the patients will be treated for VTE. As mentioned previously, the GMS data over‐represents elderly and female patients. Patients' warfarin treatment may also be managed by point of care (POC) testing in a primary care setting but POC was not widely funded by the health service in Ireland, thus limiting its utilisation. Also, there are no reliable data on extent of INR POC testing in Ireland. Another limitation is the assumption that a clinic only cared for those in its local health office area. Some clinics are in close proximity to the local health office area boundaries and patients may attend the warfarin clinics in a neighbouring local health office area, thereby contaminating the results.
5. CONCLUSION
The introduction of DOACs has resulted in an increase in the overall uptake of OAC therapy in Ireland. The increased utilisation was not evidently related to populations underserved by warfarin clinics. Further research into the pharmaco‐epidemiology of oral anticoagulation use may identify patient cohorts for targeted interventions to improve appropriate prevention of stroke.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
The authors confirm that the Principal Investigator for this paper is Dr Cormac Kennedy and that he had direct responsibility for the data.
CONTRIBUTORS
Concept and study design: C.K., C.N.C., K.B., S.C., M.B. Analysis: C.K., C.N.C., K.B. Preparation of manuscript: C.K. Manuscript review and revision: S.C., K.B., C.C., M.B.
ACKNOWLEDGEMENTS
Ms Niamh Geragthy, the HSE Health Intelligence Unit for providing the LHO shapefiles, and the HSE Medicines Management Program for sourcing information on the warfarin clinics in Ireland. K.B. is funded by the Health Research Board (RL‐15‐1579).
Kennedy C, Ni Choitir C, Clarke S, Bennett K, Barry M. Direct oral anticoagulants uptake and an oral anticoagulation paradox. Br J Clin Pharmacol. 2020;86:392–397. 10.1111/bcp.14171
REFERENCES
- 1. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the anticoagulation and risk factors in atrial fibrillation (atria) study. JAMA. 2001;285(18):2370‐2375. [DOI] [PubMed] [Google Scholar]
- 2. Hu D, Sun Y. Epidemiology, risk factors for stroke, and Management of Atrial Fibrillation in China. J am Coll Cardiol. 2008;52(10):865‐868. [DOI] [PubMed] [Google Scholar]
- 3. Zoni‐Berisso M, Lercari F, Carazza T, Domenicucci S. Epidemiology of atrial fibrillation: European perspective. Clin Epidemiol. 2014;6:213‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Petersen P, Godtfredsen J, Boysen G, Andersen ED, Andersen B. Placebo‐controlled, randomised trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation: the Copenhagen AFASAK study. The Lancet. 1989;333(8631):175‐179. [DOI] [PubMed] [Google Scholar]
- 5. Stroke Prevention in Atrial Fibrillation Investigators . Stroke prevention in atrial fibrillation study. Final results. Circulation. 1991;84(2):527‐539. [DOI] [PubMed] [Google Scholar]
- 6. Royal College of Physicians Clinical Effectiveness and Evaluation Unit . Sentinel Stroke National Audit Programme (SSNAP). Clinical audit first pilot public report, 2013.
- 7. Abdul‐Rahim AH, Wong J, McAlpine C, Young C, Quinn TJ. Associations with anticoagulation: a cross‐sectional registry‐based analysis of stroke survivors with atrial fibrillation. Heart. 2014;100(7):557‐562. 10.1136/heartjnl-2013-305267 [DOI] [PubMed] [Google Scholar]
- 8. Holt TA, Hunter TD, Gunnarsson C, Khan N, Cload P, Lip GYH. Risk of stroke and oral anticoagulant use in atrial fibrillation: a cross‐sectional survey. Br J Gen Pract. 2012;62(603):e710‐e717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kassianos G, Arden C, Hogan S, Dew R, Fuat A. Current management of atrial fibrillation: an observational study in NHS primary care. BMJ Open. 2013;3(11):e003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hsu JC, Maddox TM, Kennedy KF, et al. Oral anticoagulant therapy prescription in patients with atrial fibrillation across the spectrum of stroke risk: insights from the ncdr pinnacle registry. JAMA Cardiol. 2016;1(1):55‐62. [DOI] [PubMed] [Google Scholar]
- 11. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139‐1151. [DOI] [PubMed] [Google Scholar]
- 12. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883‐891. [DOI] [PubMed] [Google Scholar]
- 13. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981‐992. [DOI] [PubMed] [Google Scholar]
- 14. Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093‐2104. [DOI] [PubMed] [Google Scholar]
- 15. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893‐2962. [DOI] [PubMed] [Google Scholar]
- 16. Steffel J, Verhamme P, Potpara TS, et al. The 2018 European heart rhythm association practical guide on the use of non‐vitamin K antagonist oral anticoagulants in patients with atrial fibrillation: executive summary. EP Europace. 2018;20(8):1231‐1242. [DOI] [PubMed] [Google Scholar]
- 17. National Institute for Health and Care Excellence . Atrial fibrillation: the management of atrial fibrillation. London: National Institute for Health and Care Excellence; 2014. [Google Scholar]
- 18. Link MS. Important changes for practicing physicians in the focused atrial fibrillation guideline update. Circulation. 2019;xx(xx):xx‐xx. [DOI] [PubMed] [Google Scholar]
- 19. Maura G, Billionnet C, Drouin J, Weill A, Neumann A, Pariente A. Oral anticoagulation therapy use in patients with atrial fibrillation after the introduction of non‐vitamin K antagonist oral anticoagulants: findings from the French healthcare databases, 2011–2016. BMJ Open. 2019;9(4):e026645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Apenteng PN, Gao H, Hobbs FR, Fitzmaurice DA, UK GARFIELD‐AF Investigators and GARFIELD‐AF Steering Committee . Temporal trends in antithrombotic treatment of real‐world UK patients with newly diagnosed atrial fibrillation: findings from the GARFIELD‐AF registry. BMJ Open. 2018;8(1):e018905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brown JD, Shewale AR, Dherange P, Talbert JC. A comparison of Oral anticoagulant use for atrial fibrillation in the pre‐ and post‐DOAC eras. Drugs Aging. 2016;33(6):427‐436. [DOI] [PubMed] [Google Scholar]
- 22. Xian Y, O'Brien EC, Liang L, et al. Association of preceding antithrombotic treatment with acute ischemic stroke severity and in‐hospital outcomes among patients with atrial fibrillation. JAMA. 2017;317(10):1057‐1067. [DOI] [PubMed] [Google Scholar]
- 23. Marzec LN, Wang J, Shah ND, et al. Influence of direct Oral anticoagulants on rates of Oral anticoagulation for atrial fibrillation. J am Coll Cardiol. 2017;69(20):2475‐2484. [DOI] [PubMed] [Google Scholar]
- 24. Sen S, Dahlberg KW. Physician's fear of anticoagulant therapy in nonvalvular atrial fibrillation. Am J Med Sci. 2014;348(6):513‐521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Raparelli V, Proietti M, Cangemi R, Lip GY, Lane DA, Basili S. Adherence to oral anticoagulant therapy in patients with atrial fibrillation. Focus on non‐vitamin K antagonist oral anticoagulants. Thromb Haemost. 2017;117(2):209‐218. [DOI] [PubMed] [Google Scholar]
- 26. Dantas GC, Thompson BV, Manson JA, Tracy CS, Upshur REG. Patients' perspectives on taking warfarin: qualitative study in family practice. BMC Fam Pract. 2004;5(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zimetbaum PJ, Thosani A, Yu H‐T, et al. Are atrial fibrillation patients receiving warfarin in accordance with stroke risk? Am J Med. 2010;123(5):446‐453. [DOI] [PubMed] [Google Scholar]
- 28. Gage BF, Boechler M, Doggette AL, et al. Adverse outcomes and predictors of underuse of antithrombotic therapy in medicare beneficiaries with chronic atrial fibrillation. Stroke. 2000;31(4):822‐827. [DOI] [PubMed] [Google Scholar]
- 29. Jackson SL, Peterson GM, House M, Bartlett T. Point‐of‐care monitoring of anticoagulant therapy by rural community pharmacists: description of successful outcomes. Aust J Rural Health. 2004;12(5):197‐200. [DOI] [PubMed] [Google Scholar]
- 30. Sinnott S‐J, Bennett K, Cahir C. Pharmacoepidemiology resources in Ireland—an introduction to pharmacy claims data. Eur J Clin Pharmacol. 2017;73(11):1449‐1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bartoli‐Abdou JK, Patel JP, Crawshaw J, et al. Exploration of adherence and patient experiences with DOACs one year after switching from vitamin‐K antagonists‐ insights from the switching study. Thromb Res. 2018;162:62‐68. [DOI] [PubMed] [Google Scholar]
- 32. PCRS PCRS Annual Reports. In: Health Services Executive; [Google Scholar]
- 33. Wagner AK, Soumerai SB, Zhang F, Ross‐Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299‐309. [DOI] [PubMed] [Google Scholar]
- 34. Nieuwlaat R, Schwalm J‐D, Khatib R, Yusuf S. Why are we failing to implement effective therapies in cardiovascular disease? Eur Heart J. 2013;34(17):1262‐1269. [DOI] [PubMed] [Google Scholar]
- 35. Darkow T, Vanderplas AM, Lew KH, Kim J, Hauch O. Treatment patterns and real‐world effectiveness of warfarin in nonvalvular atrial fibrillation within a managed care system. Curr Med Res Opin. 2005;21(10):1583‐1594. [DOI] [PubMed] [Google Scholar]
- 36. O'Brien EC, Holmes DN, Ansell JE, et al. Physician practices regarding contraindications to oral anticoagulation in atrial fibrillation: findings from the outcomes registry for better informed treatment of atrial fibrillation (ORBIT‐AF) registry. Am Heart J. 2014;167(4):601, e1‐609. [DOI] [PubMed] [Google Scholar]
- 37. Ogilvie IM, Newton N, Welner SA, Cowell W, Lip GYH. Underuse of Oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010;123(7):638, e4‐645. [DOI] [PubMed] [Google Scholar]
- 38. January CT, Wann L, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the Management of Patients with atrial fibrillation. Circulation. 2019;140:e125‐e151. [DOI] [PubMed] [Google Scholar]
- 39. Burn J, Pirmohamed M. Direct oral anticoagulants versus warfarin: is new always better than the old? Open Heart. 2018;5(1):e000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jowett S, Bryan S, Mahé I, et al. A multinational investigation of time and traveling costs in attending anticoagulation clinics. Value Health. 2008;11(2):207‐212. [DOI] [PubMed] [Google Scholar]
- 41. Wu C, McMurtry MS, Sandhu RK, et al. Impact of rural residence on warfarin use and clinical events in patients with non‐Valvular atrial fibrillation: a Canadian population based study. PloS One. 2015;10(10):e0140607. [DOI] [PMC free article] [PubMed] [Google Scholar]


