Abstract
Aims
To summarise the effectiveness of interventions on appropriate opioid use for noncancer pain among hospital inpatients.
Methods
Two reviewers independently searched 6 databases up to March 2018 original research articles reporting on quantitative outcomes of interventions on appropriate opioid use among hospital inpatients. Appropriate opioid use was measured by changes in prescribing, such as the lowest effective opioid dose and duration, or clinical outcomes such as adequate pain control. Quality and intervention complexity assessments were performed by 2 independent reviewers. The full methodological approach was published on PROSPERO (ID: CRD42019145947).
Results
Of 398 full‐text articles assessed for eligibility, 37 articles were included in the review. Most articles had a moderate or high risk of bias (27 of 37 studies). Thirty‐one articles primarily addressed appropriate opioid use and 6 articles targeted opioid safety as a secondary outcome. A multifaceted approach was the most common primary intervention (16 studies) and adequate pain control was the main outcome measured (14 studies). Health provider education, reinforced by hard‐copy material and feedback, was associated with a 13.0 to 29.5% increase in the proportion of opioid prescriptions written in concordance with local guidelines and reduced pain scores ranging from 7.0 to 34.5%. Interventions to improve opioid safety in patient‐controlled analgesia reduced medication errors by up to 89.1%.
Conclusion
Interventions involving academic detailing and education, especially when reinforced by feedback, show positive effects on appropriate opioid use among hospital inpatients. Future studies investigating the impact of administrative interventions on opioid use and related outcomes are warranted.
Keywords: hospital, inpatients, intervention, opioid, pain management
What is already known about this subject
Harms related to opioid analgesics among hospital inpatients are well documented.
A range of interventions have been trialled to improve the appropriate use of opioids, however, they have yielded mixed findings.
No review has been conducted to summarise the effect of interventions addressing opioid use in the hospital inpatient setting.
What this study adds
We identified 37 studies using 4 main types of interventions to optimise opioid use, ranging from opioid‐targeted strategies such as multifaceted interventions, decision‐making tools, and pain‐monitoring, to broader medication safety interventions.
Interventions involving education for health care providers and patients, particularly when reinforced by performance feedback or hard‐copy material, contribute towards improved prescribing and clinical outcomes related to opioid use among hospital inpatients.
1. INTRODUCTION
Opioid analgesics are commonly used to manage moderate to severe acute pain among hospital inpatients.1 The National Institute for Health and Care Excellence identified opioids as a high‐risk medicine2 as they account for up to 16.1% of hospital adverse drug events (ADEs).3, 4, 5 Opioid‐related ADEs include constipation, nausea, sedation and respiratory depression. The association between opioid‐related ADEs and adverse clinical outcomes, including increased length of hospital stay, costs, rate of readmission and even death, are well documented.6, 7, 8, 9
Recent attention has been placed upon optimising opioid use and reducing related harms through the development of clinical prescribing guidelines. Guidelines for the management of acute noncancer pain recommend initial nonopioid analgesia, and if insufficient, the addition of the lowest effective dose of immediate‐release opioid analgesia for <3–5 days.10 Although the introduction of these guidelines was associated with some reduction in unnecessary opioid use, further improvements may be observed when linked with additional interventions to reinforce the implementation of these recommendations into clinical practice.11, 12 Therefore, numerous systems‐level interventions to promote appropriate opioid prescribing have been implemented internationally in a variety of settings.13, 14
A 2017 Cochrane review investigated the effectiveness of such interventions to reduce opioid use in the outpatient setting.15 The interventions reported in the review predominantly involved adjuvant therapies, such as cognitive behavioural therapy. However, these therapies were targeted to address opioid use in the management of chronic pain in the primary care setting, which have limited evidence for treating acute pain among hospital inpatients. Moreover, the overall findings of the review were mixed. Currently, no systematic review has been conducted to investigate the effect of interventions on the appropriate use of opioids in the hospital setting. Therefore, the aim of this systematic review was to investigate the effectiveness of interventions on opioid appropriateness during inpatient admission for noncancer pain.
2. METHODS
This review was performed in adherence to the Preferred Reporting Items for Systematic reviews and Meta‐Analyses (PRIMSA) guidelines.16 The protocol for this systematic review was published on PROSPERO on 27 November 2019 (ID: CRD42019145947).
2.1. Inclusion and exclusion criteria
Inclusion criteria included original peer‐reviewed research articles that reported quantitative outcomes of interventions on appropriate opioid use for noncancer pain during inpatient stay. A significant proportion of hospitalised patients are prescribed opioid analgesics in the emergency department (ED),1 thus interventions conducted in this setting were included in the review. Given the paucity of clinical trials available in this area, observational studies were also included to capture data relevant to this review. Appropriate use could be measured by changes in prescribing practices, such as using the lowest effective opioid dose and duration or guideline adherence, or clinical outcomes such as reduced pain intensity or length of stay (LOS). We excluded studies which exclusively reported on opioid use related to palliative care, oncology or opioid‐substitution therapy as they were outside the scope of this review. Studies focusing on discharge opioid use were excluded as they often included interventions in the primary care setting. We also excluded studies involving participants below the age of 18 years; case reports/series, conference abstracts, expert opinion or literature reviews; or studies written in languages other than English.
2.2. Search strategy
A systematic search was conducted using 6 electronic databases. This search was applied to Medline (1960–Present) and adapted for Scopus (1960–Present), Embase (1969–Present), Cochrane Central Register of Controlled Trials (1995–Present), International Pharmaceutical Abstracts (1970–Present) and PsycINFO (1963–Present). The last search was run on 21 March 2018.
The search terms applied to all electronic databases were developed with a clinical librarian and integrated 3 key themes: opioid analgesics, by exploding the terms Analgesics, Opioid or Narcotics and listing individual opioid names; interventions that affect prescribing, by using entry term headings describing interventions in adjacency with prescribing; and the hospital inpatient setting, by exploding the entry term Hospital Departments and applying multiple field searches for inpatients, hospital and acute care. Appropriate syntax and subject headings were applied to the same key terms across all databases and the full search strategy is available in A.3.. References of relevant articles were also screened to identify any other potential studies not identified by the search strategy.
2.3. Data extraction
After the removal of duplicates, 2 authors (S.L. and J.N.) independently screened articles by title and abstract for potentially eligible studies. Full‐text articles were then assessed to confirm eligibility. Any discrepancies were brought to a third author (J.P.) for consensus to be made. We extracted author, country and year of the study conducted, study size, design and follow‐up period, intervention performed, outcomes and potential sources of bias. Authors were contacted to request additional data where necessary.
2.4. Quality assessment
Two authors (S.L. and J.N.) independently performed a quality assessment for all included studies using the Cochrane Risk of Bias Assessment Tool 17 for randomised controlled trials and the Risk Of Bias In Non‐randomised Studies of Interventions tool18 for nonrandomised studies. Seven standard allocation criteria categorised studies as possessing low, moderate, high or unclear risk of bias for randomised controlled trials and as possessing low, moderate, serious, critical risk of bias or no information for nonrandomised studies (Appendix 2).
2.5. Data synthesis
The complexity of each primary intervention was graded according to the Cochrane Intervention Complexity Assessment Tool for Systematic Reviews (iCAT_SR) 19 (Appendix 3) by 2 authors (S.L. and J.N.) independently. The overall intervention complexity was calculated by assigning each criteria a score of 0–4, indicating a low or high degree of complexity, and then averaging the total score across all of the criteria. Assessment criteria included the number of active intervention components, behaviour of recipients to which the intervention was targeted, organisational levels targeted, the degree of tailoring required to apply the intervention and the level of skill required to deliver and receive the intervention.
Studies were arranged together by: (i) interventions which addressed appropriate opioid use as a primary or secondary focus (e.g. medication safety interventions in which opioid use was a secondary outcome); (ii) the intervention type (e.g. multifaceted interventions, decision‐making tools, pain monitoring). Intervention types were ordered by overall complexity using aggregated iCAT_SR scores.
Heterogeneity between studies was assessed by comparing study design, intervention approach and outcomes. Due to heterogeneity in interventions and reported outcomes between studies, a meta‐analysis could not be performed.
3. RESULTS
The search strategy generated a total of 9424 articles, of which 398 full‐text articles were assessed for eligibility. Refinement using the exclusion and inclusion criteria resulted in 36 studies included in the review. One additional article was obtained by manual search of the reference list of identified articles, resulting in a total of 37 articles (Figure 1). Appropriate opioid use was addressed as the primary intervention focus in 31 articles. Six studies involved medication safety interventions and reported on changes in opioid use as a secondary outcome.
Figure 1.
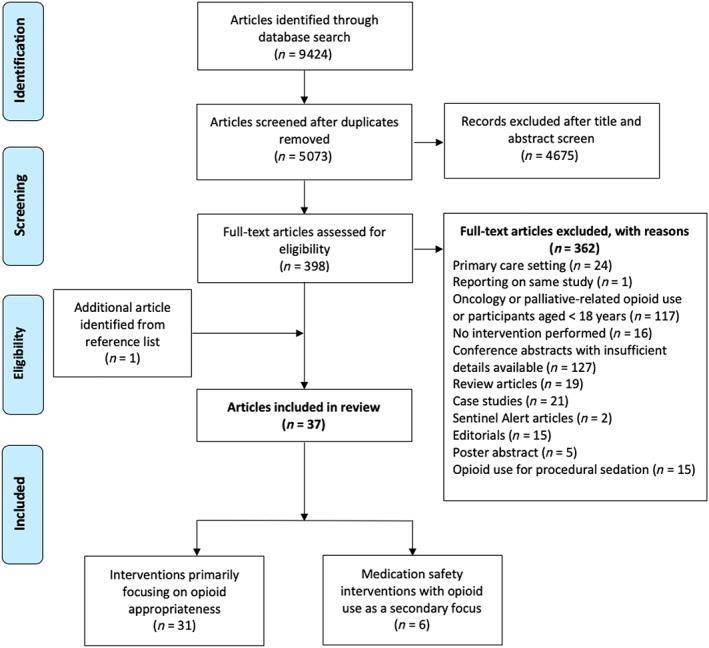
Study inclusion and exclusion criteria flow diagram
3.1. Study characteristics
Of the 37 articles, there were 4 randomised controlled trials (RCTs), 8 cohort studies, 23 pre–post intervention studies and 2 cross‐sectional studies.
Fifteen interventions involved surgical inpatients,20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34 3 were implemented in the intensive care unit,35, 36, 37 7 were conducted in the ED,38, 39, 40, 41, 42, 43, 44 5 involved geriatric inpatients (≥65 years),45, 46, 47, 48, 49 and the remaining 7 studies were conducted in all inpatients,50, 51, 52 and those receiving patient‐controlled analgesia (PCA)53, 54, 55 or transdermal fentanyl.56
The interventions were performed in various countries worldwide, with the majority, 14, conducted in the USA (Tables 1, 2, 3, 4).20, 21, 22, 23, 25, 29, 31, 40, 41, 50, 52, 54, 55, 56 Due to variability in study type and interventions employed, we summarised the review according to intervention type.
Table 1.
Summary of multifaceted interventions primarily aimed at prescribing in the hospital inpatient setting (n = 16)
| Author, year, country | Study size (n; I = intervention, C = standard care) | Study design | Study population Follow‐up duration | Interventiona | Outcomes | ||
|---|---|---|---|---|---|---|---|
| Health care professional education | Other | Prescribing | Clinical | ||||
| Educational interventions to manage pain mainly using opioid analgesics | |||||||
| Taylor et al., 2015, Australia38 | 1317(I: 0 mo = 2323 mo = 2476 mo = 238C: 0 mo = 2013 mo = 1996 mo = 200) | Multicentre, cluster‐randomised, controlled intervention trial | ED6 mo | ‐ Multidisciplinary face‐to‐face education to reduce pain by increasing provision of adequate analgesia | ‐ Audit and feedback (daily) | No change in opioid analgesic use | ↑ Patient satisfaction with pain management (42.9–53.9%*) |
| ‐ Patient education | |||||||
| ↑ Patient education (80.5% vs 86.1%*) | |||||||
| ‐ Hard copy and email material | |||||||
| ‐ Multiple choice self‐assessment | |||||||
| ‐ Online module | |||||||
| ‐ Pain posters | |||||||
| Morisson et al., 2009, USA20 | 217(I: 129C: 88) | Controlled prospective propensity score‐matched clinical trial | Surgical6 mo | ‐ Multidisciplinary face‐to‐face health professional education to improve pain management according to local guidelines | ‐ Protocols for analgesia, opioid tapering and treatment of opioid‐related adverse events | ↑ Regular opioid analgesic use (98.0 vs 48.0%*) | ↓ No or mild pain at rest (66.0 vs 51.0%*) and activity (52.0 vs 38.0%*) |
| ↑ Concurrent laxatives prescribed (92.0 vs 83.0%*) | No change in opioid‐related adverse effects | ||||||
| ‐ Audit and feedback (monthly) | |||||||
| ‐ NRS pain evaluation | |||||||
| Bingle et al., 1991, USA21 | 296(I: 147C: 149) | Pre–post intervention study | Surgical 6 mo | ‐ Academic detailing education for physicians encouraging pethidine (meperidine) use | ↑ Adherence of pethidine prescriptions to appropriateness criteria (30.0 vs 43.0%*) | ||
| ‐ Pen and hard‐copy material (Handbook on the Rational Use of Medication for Pain by Gerald M. Aronoff and Wayne O. Evans; pethidine dose <75 mg and administration interval longer than 3‐hourly defined to be inadequate) | |||||||
| Boothby et al., 2003, USA50 | (60I: 30C: 30) | Pre–post intervention study | All inpatient3 mo | ‐ Academic detailing education for physicians discouraging pethidine use | ‐ Opinion leader | ↓ Pethidine use by 29.5% (95% CI 1.97–2.88*) ↑ Pethidine switch to morphine or hydromorphone and nonopioid analgesicsIn 85.0% of orders. | ↓ Opioid‐related ADEs (53.0 vs 23.0%*) |
| ‐ Policy change to replace pethidine with alternative opioids | |||||||
| ‐ Hard copy pocket cards for physicians and pharmacists | |||||||
| ‐ VAS pain evaluation‐ Bulletin and table‐top card material | ‐ Removal of pethidine from | ||||||
| PCA | |||||||
| ‐ Pharmacist medication review | |||||||
| Neitzel et al., 1999, USA22 | 118 (I: 61 C: 57) | Pre–post intervention study | Surgical8 mo | ‐ Physician, pharmacist and nurse 8‐h face‐to‐face education to increase evidence‐based pain management (Pain Awareness: Provider Information, Patient Needs syllabus developed by Pain Guidelines Implementation Team 1996) including systematic pain assessment, opioid analgesia, care plan communication | ‐ Opinion leader | ↑ Hydromorphone use by 18.0% | No change in pain intensity↓ Pethidine use by 48.0%* No change in opioid‐related ADEs↓ Hospital LOS from 5.9 to 5.1 ds* |
| ‐ Patient education | |||||||
| No change in morphine use | |||||||
| ‐ Hard‐copy material | |||||||
| Shaw et al., 2003, Australia51 | 336(I: Pre = 46, Post = 128C: Pre = 116Post = 46) | Pre–post intervention study | All inpatients 6 mo | ‐ Academic detailing education for physicians to increase appropriate opioid prescribing | ↓ Prescription error rate for drugs of addiction (41.0 vs 24.0%*) | ||
| ‐ Hard copy (bookmark) material summarising prescribing guidelines | |||||||
| VanGulik et al., 2010, Netherlands35 | 190 (I: 130 C: 60) | Pre–post intervention study | ICU 3 mo | ‐ Multidisciplinary face‐to‐face education to increase pain assessment (NRS) and treatment with opioid analgesics | ‐ CPOE | ↑ Morphine use (22.6 vs 29.3 mg/d*) | ↓ Pain intensity (OR 2.54, 95% CI 1.22–5.65*) |
| No change in ICU LOS or MV time | |||||||
| ‐ Email and bulletin material | |||||||
| Akce et al., 2014, USA52 | 150 (I: 75 C: 75) | Retrospective serial cross‐sectional study | All inpatients 12 mo | ‐ Physician face‐to‐face education through group discussion of opioid case scenarios | ‐ CPOE | No change in opioid use | No change in pain intensity |
| ‐ Hard copy pocket cards for physicians | |||||||
| Educational interventions to manage pain mainly using nonopioid analgesics | |||||||
| Titsworth et al., 2016, USA23 | 96 (I: 48 C: 48) | Prospective, interrupted time‐series trial | Surgical 10 mo | ‐ Physician and nurse face‐to‐face education to increase pain assessment and use of nonopioid analgesia (paracetamol, NSAIDs, ketamine, gabapentin) ‐ NRS pain documentation | ‐ Acute pain service support ‐ Clinical rounds ‐ OME calculation tool‐ Patient education‐ Analgesia protocol | No change in morphine use on first postoperative d↓ Morphine use (3rd postoperative d: 72.3 ± 70.7 vs 39.2 ± 36.5 mg/d ± SD*)↑ Paracetamol use (56.3 vs 75.0%*)↑ NSAID use (8.3% vs 31.3%*) ↑ Gabapentin use (12.5 vs 33.3%*) No change in hospital LOS | ↓ Pain intensity (NRS: 4.31 vs 2.94*) |
| Benditz et al., 2016, Germany24 | 367 | Prospective cohort study | Surgical24 mo | ‐ Physician and nurse face‐to‐face education to reduce pain by using nonpharmacological methods, nonopioid and opioid analgesics (German Guidelines of Pain Management in Nursing)‐ NRS pain evaluation | ‐ Adverse drug event evaluation‐ Internal benchmarking ‐ Monthly audit and feedback‐ Patient education | ↓ Pain intensity by 24.4%*No change in opioid‐related ADEs of nausea, dizziness or tiredness | |
| Auyong et al., 2015, USA25 | 252 (I: 126 C: 126) | Pre–post intervention study | Surgical 1 mo | ‐ Multidisciplinary face‐to‐face education regarding updated Enhanced Recovery After Orthopedic Surgery Pathway‐Electronic and hard‐copy material ‐ NRS pain evaluation | ‐ Multimodal analgesia protocol including regular oral paracetamol, NSAIDs and gabapentin and when‐required oxycodone.‐ Physical therapy‐ Patient education | ↓ Morphine use on postoperative d 1 (IV equivalent 32.0 vs 23.5 mg*) and d 2 (IV equivalent 23.3 vs 15.9 mg*) | ↓ Hospital LOS (76.6 vs 56.1 h*)↓ Opioid‐related nausea on postoperative d 1 (37.3 vs 25.4%*) |
| Chan et al., 2018, Hong Kong26 | 642 (I: 332 C: 310) | Pre–post intervention study | Surgical 8 mo | ‐Physician and nurse face‐to‐face education to encourage safe opioid use, nonopioid analgesia and nurse education to improve PCA safety‐ NRS pain evaluation | ‐ Audit and feedback (every 6 mo)‐ Patient education | ↑ Morphine use (88.6 vs 99.3%*) ↑ Diclofenac use (1.0 vs 96.9%) | ↓ Pain intensity (VAS > 7; 55.5 vs 21.0%*)↑ Pruritus incidence (12.4 vs 26.5%*) |
| Humphries et al., 1997, UK27 | 242 (I: 122 C: 120) | Pre–post intervention study | Surgical12 mo | ‐ Posters and hard‐copy material outlining opioid guidelines (Victoria Hospital Blackpool Acute Pain Service) for physician and nursing staff | ‐ Analgesia protocol | ↑ Adherence of opioid prescriptions to British National Formulary (41.0 vs 61.0%*) and acute pain service (16.0 vs 26.0%*) | |
| Juhl et al., 1996, Denmark28 | 317 (I: 126 C: 191) | Pre–post intervention study | Surgical 12 mo | ‐ Physician and nurse face‐to‐face 1‐d compulsory education to encourage routine use of nonopioid analgesia‐ Bedside tuition continued until all nurses familiar with new procedures | ‐ Performance feedback ‐ Nurse‐administered morphine | ↑ Non‐opioid analgesic use (15.0 vs 94.0%*) | ↓ Pain intensity (93.0 vs 86.0*) |
| Majumder et al., 2016, USA29 | 200 (I: 100 C: 100) | Pre–post intervention study | Surgical 3 mo | ‐ Physician face‐to‐face education to minimise the use of opioid analgesics and paralytic agents | ‐ Multimodal analgesia (IV hydromorphone, oxycodone, oral gabapentin, paracetamol, NSAIDs)‐ Patient education | ↑ Switch from IV to oral opioid analgesia (2.2 vs 3.6 d after opioid initiation*) | ↓ Hospital LOS (4.0 vs 6.1 d*)↓ Hospital 90‐d readmission (16.0 vs 4.0%*) |
| Usichenko et al., 2012, Germany30 | 520 (I: 251 C: 269) | Pre–post intervention questionnaire study | Surgical 14 mo | ‐ Multidisciplinary face‐to‐face education to increase nonopioid analgesic use ‐ Procedure‐specific, multifaceted analgesia protocol (PROSPECT and German Society of Anaesthesiology and Intensive Care Guidelines)‐ NRS pain evaluation | ‐ 24 h acute pain service‐ Audit and feedback (every 6 mo)‐ ADE treatment protocol ‐ Patient education | ↓ Pain intensity by 25.0–30.0% on visual rating scale* ↓ Nausea incidence (40.0 vs 17.0%*)↓ Vomiting incidence (25.0 vs 11.0%*) ↓ Tiredness incidence (76.0 vs 30.0%*)↑ Patient satisfaction with pain treatment*↑ QOL* | |
Sorted in descending order of intervention complexity, as assessed by the iCAT_SR Tool,19 and subclassified in descending order of study design.68
Denotes statistical significance (P < .05).
ED = emergency department; ICU = intensive care unit; VAS = visual analogue scale; NRS = numeric rating scale; CPOE = computerised physician order entry; PCA = patient controlled analgesia; NSAID = nonsteroidal anti‐inflammatory drug; LOS = length of stay; ADE = adverse drug event; MV = mechanical ventilation; OR = odds ratio; CI = confidence interval; OME = oral morphine equivalents; IQR = interquartile range; QOL = quality of life; IV = intravenous; PROSPECT = procedure specific postoperative pain management
Table 2.
Summary of decision‐making tools to guide opioid prescribing in the hospital inpatient setting (n = 5)
| Author, year, country | Study size (n; I = intervention, C = standard care) | Study design | Study population, Follow‐up duration | Interventiona | Outcomes | ||
|---|---|---|---|---|---|---|---|
| Health care professional education | Other | Prescribing | Clinical | ||||
| Pain assessment tool | |||||||
| Moustafa et al., 2016, France39 | 434 (I: 214 C: 220) | Pre–post intervention study | ED patients ≥75 years 6 mo | ‐ Physician and nurse face‐to‐face training for Algoplus tool | ‐ Algoplus pain assessment tool for physicians and nurses | No change in opioid analgesic use | |
| Prescription drug monitoring program data | |||||||
| Baehren et al., 2010, USA40 | 179 | Prospective quasi‐experimental study | ED 1 mo | ‐ PDMP data provision to physicians (Ohio Automated Rx Reporting System; mandatory recording of all controlled substance prescription, ED inclusive) | ↓ Opioids prescribed in 25.1% (45 of 179) cases than initially intended | ||
| McAllister et al., 2015, USA41 | 710 (I: 356 C: 354) | Pre–post intervention study | ED 12 mo | ‐ PDMP data provision to physicians (The Electronic‐Florida Online Reporting of Controlled Substances Evaluation; nonmandatory recording of controlled drug use and emergency departments exempt) | No changes in number of controlled substance prescriptions | ||
| Computerised physician order entry | |||||||
| Netherton et al., 2014, Canada42 | 436 (I: 230 C: 206) | Pre–post intervention study | ED 12 mo | ‐ Online CPOE training | ‐ CPOE to increase ketorolac | ↑ Fentanyl use (9.7 vs 16.7%*)No change in morphine use ↑ Ketorolac use (65.6 vs 76.5%*) | |
| McEvoy et al., 2014, USA56 | 270 (I: 120 C: 150) | Pre–post intervention study | Inpatients receiving transdermal fentanyl 9 mo | ‐ Online and face‐to‐face CPOE training | ‐ CPOE to restrict transdermal fentanyl transdermal to indications such as persistent, moderate to severe pain in opioid‐tolerant patients (US Food and Drug Administration and Joint Commission sentinel event alert) | ↑ Adherence of transdermal fentanyl prescriptions to guidelines (48.7–85.0%*) | ↓ ADE incidence overall (34.7 vs 23.3%*) ↓ Respiratory depression (16.7 vs 8.3%*) |
Sorted in descending order of intervention complexity, as assessed by the iCAT_SR Tool,19 and subclassified in descending order of study design.68
Denotes statistical significance (P < .05).
ED = Emergency department; PDMP = prescription drug monitoring program; CPOE = computerised physician order entry; ADE = adverse drug event
Table 3.
Summary of interventions to improve patient monitoring in the hospital inpatient setting (n = 10)
| Author, year, country | Study size (n; I = intervention, C = standard care) | Study design | Study population, Follow‐up duration | Interventiona | Outcomes | ||
|---|---|---|---|---|---|---|---|
| Health care professional education | Other | Prescribing | 2Clinical | ||||
| Pain monitoring | |||||||
| Muntlin et al., 2010, Sweden43 | 200 (I: 100 C: 100) | Quasi‐experimental study | ED 4 mo | ‐ Optional face‐to‐face nurse education to increase opioid analgesic use‐ Analgesic protocol‐ NRS pain evaluation | ↑ Analgesic use (46.0–65.0%*) | ↓ Pain intensity (NRS: 3.8 vs 2.9*) ↑ Patient satisfaction with pain management (Out of 10: 2.3 vs 3.6*)No changes in opioid‐related ADEs | |
| Chanques et al., 2006, France36 | 230 (I: 130 C: 100) | Pre–post intervention study | ICU 4 mo | ‐ Face‐to‐face nurse education to evaluate pain as ‘vital sign’ ‐ BPS and NRS pain evaluation | No change in morphine use↑ Tramadol use (15.0 vs 27.0%*) | ↓ Pain incidence (63.0 vs 42.0%*) and intensity (NRS > 6, 36.0 vs 16.0%*)↓ MV duration (120 [IQR 48–312] vs 65 [IQR 24–192] h*)No change in ICU LOS or mortality | |
| Duncan & Pozehl, 2000, USA31 | 240 (I: 121 C: 119) | Pre–post intervention study | Surgical 15 mo | ‐ Face‐to‐face nurse education of opioid use as ‘cornerstone’ pain treatment (according to Acute Pain Management: Operative or Medical Procedures and Trauma: Clinical Practice Guideline (Agency for Health Care Policy & Research. 1992)‐ NRS pain evaluation | ‐ Audit and feedback (daily) | ↑ Morphine use (OME: 12.70 vs 14.54 mg/d*) | ↓ Pain intensity (NRS: 3.58 vs 3.16*) |
| Olsen et al., 2016, Norway37 | 650 (I: 398 C: 252) | Pre–post intervention study | ICU 22 weeks | ‐ Face‐to‐face nurse education to encourage analgesic and nonpharmacologic pain management‐ Analgesia protocol ‐ NRS and BPS pain evaluation | ↑ Fentanyl use as epidural (median 223 vs 310 μg*) | ↓ Agitation events (3.0 vs 6.0%*) ↓ MV duration (median 46 vs 79 h*)↓ ICU LOS (median 2.6 vs 3.0 d, P = 0.04) | |
| Pierik et al., 2016, Netherlands44 | 660 (I: 156 C: 504) | Pre–post intervention study | ED 6 mo | ‐ Education to encourage nonopioid use before opioid analgesia (Pain management for trauma patients in the chain of emergency care) ‐ Analgesia protocol‐ NRS pain evaluation ‐ Leaflet material | ↑ Analgesic use (95% CI 11.5–30.9*) ↓ Time to opioid administration (37 vs 15 min*) | ↑ Pain relief (95% CI 1.8–18.5*)No changes in ED LOS or patient satisfaction | |
| Cui et al., 2017, China32 | 148 (I: 71 C: 77) | Separate sample pre–post intervention study | Surgical 24 mo | ‐ Mandatory nurse education encouraging nonpharmacological pain management‐ Analgesic protocol‐ Hard copy guidelines (Acute Pain Management Handbook for Nurses developed by Professor Ramani Vijayan, Department of Anaesthiology, University of Malaya, Malaysia)‐ NRS pain evaluation | ↑ Nonopioids and opioid combination use (38.8 vs 66.2%*)No change in the opioid use alone↑ Nonpharmacological pain management (60.0 vs 96.0%*) | ↑ Pain relief (53.0 vs 78.0%*) | |
| Manias et al., 2011, Australia45 | 192 (I: 96 C: 96) | Non‐equivalent control pre–post intervention study | Geriatric ≥65 years 3 mo | ‐ Face‐to‐face nurse education to appropriately manage pain using nonpharmacological and pharmacological methods ‐ Pain evaluation using 8 scales (including NRS and VAS) | ‐ Audit and feedback (daily)‐ Case study discussion | ↓ Oxycodone use (Odds reduction by 1.2 times at baseline, 4 times following intervention*) | ↓ Pain intensity both at rest and on movement (VASrest 6.05 vs 4.40* and VASmovement 7.42 vs 5.27*) |
| Patient controlled analgesia safety monitoring | |||||||
| Paul et al., 2010, Canada53 | 25 198(I: 12 193 C: 13 005) | Longitudinal cohort study | Inpatients receiving PCA36 mo | ‐ Nurse face‐to‐face PCA education for safe use and ADE monitoring | ‐ Mandatory computerised incident reporting‐ Policy change requiring review of PCA settings during shifts and upon handover‐ Purchase of new PCA pumps | ↓ PCA error rate (OR: 0.28 [95% CI = 0.14, 0.53] vs 0.05 [95% CI = 0.001, 0.30]*) | |
| Ferguson et al., 2010, USA54 | 3732 (I: 1979 C: 1753) | Pre–post intervention study | Inpatients receiving PCA 4 mo | ‐ Nurse face‐to‐face PCA education for safe use and ADE monitoring‐ Practical competency demonstration‐ Online module | ↓ PCA error rate (0.46 vs 0.05%*) | ||
| Whipple et al., 1994, USA55 | 4669 | Retrospective cross‐sectional study | Inpatients receiving PCA | ‐ Computerised PCA ADE monitoring | ↓ PCA overdose events (11 vs 6 of 294 potential events detected) | ||
Sorted in descending order of intervention complexity, as assessed by the iCAT_SR Tool,19 and subclassified in descending order of study design.68
Denotes statistical significance (P < 0.05).
ED = Emergency department; ICU = intensive care unit; VAS = visual analogue scale; NRS = numeric rating scale; BPS = Behavioural pain scale; PCA = patient‐controlled analgesia; LOS = length of stay; ADE = adverse drug event; MV = mechanical ventilation; OR = odds ratio; CI = confidence interval; IQR = interquartile range
Table 4.
Summary of medication safety interventions involving opioid use as a secondary outcome in the hospital inpatient setting (n = 6)
| Author, year, country | Study size (n; I = intervention, C = standard care) | Study design | Study population | Interventiona | Outcomes | |
|---|---|---|---|---|---|---|
| Prescribing | Clinical | |||||
| Adverse event monitoring | ||||||
| O'Sullivan et al., 2015, Switzerland46 | 737 (I: 361 C: 376) | Cluster randomised controlled trial | Geriatric ≥65 years | ‐ Clinical pharmacist medication review ‐ Computerised decision support | ↓ Opioid‐related ADEs (50.5 vs 31.9%*) | |
| Kjeldsen et al., 2013, Denmark33 | 1782(I: 775C: 1007) | Controlled, prospective intervention study | Surgical | ‐ Clinical pharmacist medication review | Opioids involved in 59.0% of clinical pharmacist recommendations (620/1059) |
No changes in opioid‐related ADEs No changes in 6‐mo readmission rate, mortality, number of laboratory tests, or hospital LOS |
| Bos et al., 2016, The Netherlands34 | 11 651 I: 5711C: 5940 | Prospective, multicentre, open pre–post intervention study | Surgical | ‐ Clinical pharmacist medication review‐ Prescriber face‐to‐face education‐ Guideline dissemination ‐ CPOE | ↓ Opioid use (40.3–37.0%*) | ↓ Tramadol and anticholinergic‐related ADEs (48–20*)↓ Overall mortality, hospital LOS and 30‐d readmission (1. vs 1.1%*) |
| Assessment of prescription appropriateness | ||||||
| Dalleur et al., 2014, Belgium47 | 146 (I: 74C: 72) | Double‐blind randomised controlled trial | Geriatric ≥75 years | ‐ STOPP criteria review to reduce PIMs | ↓ PIM rate (19.3 vs 39.7%*)No significant changes in opioid use | |
| O'Connor et al., 2016, Ireland48 | 732 (I = 360C = 372) | Single‐blind cluster randomised controlled trial | Geriatric ≥65 years | ‐ STOPP‐START criteria review to reduce PIMs | Opioids accounted for 7.0% of intervention group ADEs | ↓ Patients experiencing ≥1 ADE (21.0 vs 11.7%; (absolute RR = 9.3%, NNT = 11) |
| Urfer et al., 2016, Swtizerland49 | 900(I: 450C: 450) | Single‐centre, quasi‐experimental pre–post intervention study | Geriatric ≥65 years | ‐ 5‐point checklist for physicians to reduce PIMS and polypharmacy‐ STOPP‐START criteria to review PIMs | No change in opioid use | |
Sorted in descending order of study design.68
Denotes statistical significance (P < .05).
CPOE = computerised physician order entry; LOS = length of stay; ADE = adverse drug event; STOPP = Screening Tool of Older Person's Prescriptions; START = Screening Tool to Alert doctors to Right Treatment; PIM = potentially inappropriate medication; RR = relative risk; NNT = numbers needed to treat
3.2. Quality assessment
Risk of bias assessment was performed for 4 RCTs and 33 nonrandomised studies (A.2.). Out of the 31 articles in which opioid use was evaluated as a primary outcome, 24 studies were graded as having either a moderate or serious risk of bias, largely due to confounding or selection of participants into the study.21, 22, 23, 24, 26, 27, 28, 30, 31, 32, 35, 36, 37, 39, 40, 41, 42, 43, 44, 50, 52, 54, 55, 56 A moderate or serious risk of bias was found in 3 of 6 studies in which opioid use was addressed as a secondary outcome.33, 34, 48 Selective reporting of outcomes and other risk of bias were unclear in most studies. Due to a significant degree of heterogeneity between intervention approach and outcome measures, a meta‐analysis was not deemed suitable.
3.3. Interventions primarily targeting appropriate opioid use
Thirty‐one studies examined the effect of interventions to improve prescribing or clinical outcomes related to opioid use. Interventions used to improve appropriate opioid use for analgesia included 16 articles which employed a multifaceted intervention to educate health providers (such as prescribers, nurses, pharmacists) and patients to change opioid use,20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 35, 38, 50, 51, 52 5 studies using decision‐making tools to guide opioid prescribing39, 40, 41, 42, 56 and 10 that targeted pain monitoring and response to analgesic treatment.31, 32, 36, 37, 43, 44, 45, 53, 54, 55 Predominant prescribing outcomes included the quantity of opioids prescribed and compliance to published guidelines, while clinical outcomes included pain intensity, opioid‐related ADEs and patient satisfaction with adequate pain control. To some extent, greater intervention complexity was associated with effectiveness on improving patterns of opioid use (A.1.).
3.3.1. Multifaceted interventions primarily aimed at prescribing
Sixteen studies examined the effect of multifaceted interventions involving education to change patterns of opioid use.20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 35, 38, 50, 51, 52 Of these, 8 interventions focused on educating health professionals to improve pain control, primarily through the increased use of opioid analgesia.20, 21, 22, 35, 38, 50, 51, 52 An RCT conducted by Taylor et al. employed an educational intervention for health care professionals (doctors and nurses) and patients to achieve adequate analgesia, defined by a reduction in numerical rating scores (NRS; range 0–10) of at least 2 points and to a target score of <4, clinically representative of mild pain. The use of both nonopioid and opioid analgesics was supported to achieve satisfactory pain control. Education was reinforced by physical and electronic dissemination of material and daily audit and feedback provided by site investigators. Patient education was also provided to facilitate communication of the patient's concerns in pain management. While no significant changes in analgesic use were observed in the intervention group, an 11.0% increase in patient education was associated with patient improved satisfaction with pain control (42.9–53.9%; P = .001).38 Interventions involving health professional education reinforced by hard‐copy material to improve pain control were used in 7 studies,21, 22, 35, 38, 50, 51, 52 although the definition of prescription appropriateness varied between studies. A study by Bingle et al. (1991), conducted in the context of addressing inadequate analgesia, used academic detailing (1‐on‐1 face‐to‐face educational outreach) to encourage the prescription of pethidine (meperidine) above a defined dose and frequency.21 In contrast, in a 2003 study funded by Abbot Pharmaceuticals, Boothby et al. implemented academic detailing to discourage the use of pethidine in favour of other analgesics with superior safety‐efficacy profiles.50 Nevertheless, the use of academic detailing was associated with reduced prescription error rates (41.0% vs 24.0%; P < .05)51 and increased compliance of prescribed opioids to predetermined recommendations.21, 50 The provision of patient education to aid shared healthcare decision‐making was associated with a significant reduction in LOS from 5.9 to 5.1 days (P < .05), despite no changes in morphine use or pain intensity.22 Prescriber education through case scenario discussion alone, without further feedback, was not associated with changes in pain intensity or opioid use.52
Eight interventions involved health professional education to manage pain by encouraging the use of nonpharmacological measures and nonopioid analgesics, reserving opioid analgesia for stronger pain.23, 24, 25, 26, 27, 28, 29, 30 While these studies shared key education messages and mainly targeted physicians and nurses, distinctions existed between the types of additional strategies used, such as reinforcement with audit and feedback, or patient involvement in decision‐making. The use of performance feedback conducted every 1–6 months to reinforce health professional education was associated with significant improvements in the primary outcome of all the studies in which it was applied, including patient satisfaction and reduced pain intensity.24, 26, 28, 30 Additionally, 2 studies using performance feedback reported 79.0–95.9% increased nonopioid analgesic use26, 28 and 1 study showed a reduction in opioid‐related ADEs such as nausea (40.0 vs 17.0%; P < .05).30 Of 3 studies using an analgesia protocol to guide pain treatment,23, 25, 27 1 study demonstrated significantly increased use of nonopioid analgesia23 and 2 studies reported reduced morphine use ranging from 26.6 to 45.8%.23, 25 Patient education was linked to significant improvements in patient satisfaction and quality of life.30
3.3.2. Decision‐making tools to guide opioid prescribing
Five studies used tools to guide the appropriate prescribing of opioids.39, 40, 41, 42, 56 A pain assessment tool was used in 1 study, which did not report a significant difference in the quantity of opioids used.39
Two studies, which assessed whether the provision of prescription drug monitoring programme (PDMP) data influenced prescribers' decisions about the risk of opioid misuse and subsequent analgesic prescription, produced mixed results.40, 41 The study conducted by Baehren et al., in which PDMP reporting for outpatient and ED prescriptions was mandatory, reported a 25.0% reduction in opioids prescribed,40 whereas nonmandatory PDMP use from which the ED was exempt was not associated with significant differences in the prescription of controlled substances.41
Two studies used computerised physician order entry to guide prescribing. Both interventions provided online training for prescribers and showed significantly increased compliance of prescribed opioids to regulatory statements such as the Joint Commission Sentinel Event Alert on safe opioid use.42, 56 In addition to online education, 1 study also used face‐to‐face training to support computerised order entry and reported a reduction in overall ADEs (34.7 vs 23.3%, P = .043) and respiratory depression (16.7 vs 8.3%, P = .043).56
3.3.3. Patient monitoring
Ten studies assessed the impact of increased patient monitoring on analgesic use, pain intensity and PCA‐related ADEs.31, 32, 36, 37, 43, 44, 45, 53, 54, 55 Seven studies assessed pain intensity by implementing scales such as the numeric rating scale and visual analogue scale.31, 32, 36, 37, 43, 44, 45 All interventions were performed primarily by nurses. Of these, 3 interventions focused on pain evaluation as a vital sign and treatment using opioid analgesics.31, 36, 43 These studies reported significantly increased use of morphine by 12.7% (P < .05)28 and tramadol by 12.0% (P < .05),31 as well as reduced pain intensity; however, no changes in opioid‐related ADEs or LOS were shown. In contrast, 4 studies used pain scales to assess pain severity and provide treatment using multimodal strategies including nonpharmacological and nonopioid methods.32, 37, 44, 45 The use of opioid analgesics varied in these studies, however, significantly increased use of nonpharmacological pain management (60.0 vs 96.0%; P < 0.05) and nonopioid analgesia (38.8 vs 66.2%; P < 0.05) were reported.32 These changes were associated with improved clinical outcomes including reduced pain intensity ranging from 27.2 to 38.5%,32, 45 reduced duration of mechanical ventilation (median 46 vs 79 h; P < .06), and a nonsignificant reduction in LOS.37
Three studies evaluated the impact of increased ADE monitoring to improve PCA safety.53, 54, 55 Interventions to increase nursing assessment of patients' response to PCA and competency demonstration were introduced in 2 studies, both of which demonstrated relative reductions of 82.1–89.1% in the frequency of PCA errors.53, 54 In the remaining study, a computer‐based system to monitor PCA errors reported 11 of 294 potential overdose events compared to 6 detected through standard practice.55
3.4. Medication safety intervention studies
Six studies involved medication safety interventions, in which opioid use was addressed as a secondary focus.33, 34, 46, 47, 48, 49 Of these, 3 studies examined the effects of ADE monitoring33, 34, 46 and 3 used specific criteria to assess prescription appropriateness.47, 48, 49
Clinical pharmacist review of medications was used in all 3 studies which monitored for ADEs.33, 34, 46 Medication review alone was not associated with changes in opioid ADEs.33 However, 2 studies which included additional interventions such as computerised physician order entry and dissemination of drug safety guidelines reported significant reductions in opioid use (40.3–37.0%; P < .05) and opioid‐related ADEs by 18.6–28.0%.34, 46
Three studies implemented the Screening Tool of Older Person's Prescriptions and Screening Tool to Alert doctors to Right Treatment (STOPP/START) criteria to evaluate the prevalence of potentially inappropriate prescriptions compared to usual care.47, 48, 49 One RCT using STOPP/START criteria reported reduced rates of inappropriate prescriptions by 20.4% (39.7 vs 19.3%; P < .05).47 However, the use these of criteria did not change opioid use.47, 48, 49
4. DISCUSSION
4.1. Principal findings
To our knowledge, this is the first systematic review to evaluate the effectiveness of interventions on appropriate opioid use in the hospital inpatient setting. Multifaceted interventions involving academic detailing and education, particularly when reinforced by audit and feedback and hard‐copy material demonstrated improvements on the appropriate use of opioids, which contributed towards improved clinical outcomes. Patient education was linked to increased patient satisfaction and quality of life. Pain monitoring to determine appropriate treatment was associated with the increased use of nonopioid analgesia and reduced opioid‐related ADEs, while still maintaining adequate pain control. Increased concordance of opioid prescribing to published guidelines was reported after the introduction of computerised physician order entry systems. To some extent, increased intervention complexity was associated with increased effectiveness on improving appropriate opioid use. Opioid safety interventions integrating multiple strategies, such as clinical pharmacist review, computerised physician order entry and dissemination of published guidelines, facilitated greater improvements in prescribing and clinical outcomes compared to a single‐component approach. However, due to the high risk of bias in many of these studies, these results require further investigation.
4.2. Comparison with other studies
No other review has been conducted that systematically evaluates the effectiveness of interventions on appropriate opioid use in the hospital inpatient setting. Although there is a Cochrane review (2017) that evaluates the effectiveness of interventions to reduce opioid use in the management of chronic pain, its included studies were all conducted in the primary care setting.15 The majority of the interventions included in the Cochrane review involved adjuvant therapies such as acupuncture and cognitive behavioural therapy. These interventions may also be useful in the hospital setting; however, the evidence supporting their use for acute pain is limited. Few studies implemented adjuvant therapies in this review. Further research on the impact of nonpharmacological pain management on opioid use in the hospital setting is required.
4.3. Strengths and limitations
The main strength of this review was the rigorous approach taken in its systematic search. Two independent reviewers determined each study's eligibility and performed quality and complexity assessments. A broad search strategy was developed with a clinical librarian and applied to 6 databases to capture an extensive amount of the available literature. Also, this search was not limited by publication year, which allowed shifts in the clinical focus of opioid use to be captured over time.
However, this review had several limitations. Firstly, a meta‐analysis could not be performed due to substantial heterogeneity in intervention approach and outcomes (e.g. LOS), limiting the generalisability of our results. Moreover, the majority of studies in the present review involved follow‐up periods under 24 months, therefore the long‐term sustainability of these interventions is unknown. Finally, some relevant studies may have been omitted. Although we employed a comprehensive search strategy and manually searched reference lists to include all relevant studies, only articles from the designated databases published in English were included. We did not search grey literature, thus introducing a level of publication bias.
4.4. Policy and research implications
Our findings highlight that the majority of studies were performed in the context of managing patients' pain through the provision of opioid analgesia, whereas fewer addressed patient safety and opioid‐related harm. Interventions aimed at increasing the use of opioids reflect recommendations by the American Pain Society in 1995 to evaluate pain as a vital sign, contributing to the subsequent rise in the use of opioids to reduce pain.57 The emphasis on pain treatment may be further attributable to its influence on patient satisfaction, a parameter often linked to physician and institution reimbursement in the USA.58 However, evidence suggests that the emphasis on reducing pain and under‐representation of opioid‐related harms have contributed to the present overuse of opioid analgesics, particularly in the management of both acute and chronic noncancer pain.58, 59 Moreover, growing evidence of opioid‐related harms, including increased morbidity and mortality, puts into question the safety of extensive opioid use.60, 61, 62 Very few interventions specifically assessed the therapeutic benefit of ongoing analgesic treatment or monitored for cases in which harms related to opioid use outweighed the benefits. Therefore, the implementation of policies to reinforce the equal weighting of opioid‐related risks and benefits are warranted to facilitate a balanced approach to pain management.
Additionally, few studies reported the application of best‐available evidence to guide the de‐escalation of opioids once initiated. Recent literature suggest that a significant proportion of opioids prescribed during hospital admission are continued postdischarge, which may contribute to an increased risk of dependence and unintended harm.63 Interventions to deprescribe potentially inappropriate medications in older hospitalised adults have been shown to improve prescribing and clinical outcomes.64 However, limited evidence exists that applies similar principles to assess the ongoing need for opioid analgesia. Hence, there is scope for the development of policies to guide opioid deprescribing in cases where the risks of therapy outweigh the benefits to further contribute towards the safe and efficacious use of opioids.
This review supports the use of certain interventions to improve the safe and efficacious use of opioids, although the definition of opioid appropriateness varied, depending on the context in which the study was conducted. Earlier interventions conducted in the context of inadequate pain treatment encouraged opioid use as effective analgesic agents, before the focus of appropriate use shifted to potential opioid‐related harms.65, 66, 67 No consensus could be established from the reviewed articles on the definition of opioid appropriateness. Thus, further studies are required to develop an internationally accepted definition of appropriate opioid use.
Overall, the quality of evidence was low. No definitive conclusions could be drawn on efficacy on appropriate opioid use by intervention type. Although preliminary data suggest that interventions to improve appropriate opioid use may reduce hospital costs linked to opioid‐related harms, further studies are needed to assess their net cost‐effectiveness. Intervention complexity appeared to contribute to an extent towards effectiveness on improving patterns of opioid use. However, intervention efficacy may also be influenced by additional factors including retrospective study design, challenges in translating changes in practice to clinical outcome, and intrinsic methods of the interventions. There was a paucity of evidence to inform the feasibility of administrative‐level involvement to influence hospital opioid use. Thus, further research is required to address the implementability of executive‐level interventions to improve safe and efficacious opioid use in the hospital setting.
5. CONCLUSION
Interventions involving academic detailing and education, especially when reinforced by performance feedback, show positive effects on appropriate opioid use for hospital inpatients. The development of policies to guide the deprescribing of opioids in cases where opioid‐related harms outweigh the benefits are warranted. Future studies on appropriate opioid use for hospital inpatients should focus on the effectiveness of interventions performed at the organisation‐level to inform enhanced acute pain management using opioid analgesics.
CONFLICT OF INTEREST
None declared.
STATEMENT OF ORIGINALITY
This work is submitted for publication in the British Journal of Clinical Pharmacology. The authors declare that this review has not been and, if accepted, will not be published in whole or in part in any other journal. All authors have read and approved the full manuscript in its submitted form.
CONTRIBUTORS
J.P. and D.G. conceived the study and designed the study protocol in collaboration with S.L. S.L. performed data collection and analysis. All authors contributed to its revision. S.L. drafted the manuscript and all authors read and approved the final manuscript.
FUNDING
D.G. is supported by the National Health and Medical Research Council (NHMRC) Dementia Leadership Fellowship. This had no role in the design of the study, data collection and analysis, or preparation of the manuscript.
COMPETING INTERESTS
None declared.
ETHICS APPROVAL
None required.
ACKNOWLEDGEMENTS
The authors would like to acknowledge support provided by Edward Luca, the University of Sydney during search strategy development.
Appendix A.
A.1. Database search terms
MEDLINE (1960 to Present) (OvidSP).
1. exp Analgesics, Opioid/or exp Narcotics/.β
2. (acetyldihydrocodeine or alfentanil or allylprodine or alphamethylfentanyl or alphaprodine or benzylmorphine or betaprodine or buprenorphine or butorphanol or bremazocine or codeine or contin or dextromoramide or dextropropoxyphene or dezocine or diacetylmorphine or diamorphine or dihydrocodeine or dihydromorphine or dihydromorphone or diphenoxylate or dipipanone or enadoline or ethylketazocine or ethylmorphine or etonitazene or etorphine or fentanyl or heroin or hydrocodone or hydromorphin* or hydromorphone or ketazocine or ketobemidone or lefetamine or levomethadon or levomethadyl or levomethorphan* or levorphanol or loperamide or meperidine or meptazinol or methadone or methadyl or methylmorphine or morphin* or nalbuphine or narcotic* or nicocodeine or nicomorphine or normorphine or noscapin* or ohmefentanyl or opiate* or opioid* or opium or oripavine or oxycodone or oxycontin or oxymorphone or papaveretum or papaverin or pentazocine or percocet or peronine or pethidine or phenazocine or phencyclidine or pholcodine or piritramid* or prodine or promedol or propoxyphene or remifentanil or sufentanil or tapentadol or thebaine or tilidine).tw.
3. 1 or 2.
4. INPATIENTS/or inpatient*.mp. or (inpatient* adj2 hospital*).mp. or (inpatient* adj2 setting*).mp.
5. hospital.mp. or Hospitals/or (hospital* adj2 setting*).mp.
6. exp Hospital Departments/.
7. (acute adj2 care).mp. or acute disease/
8. emergency service, hospital/or trauma centers/or (emergency adj2 department).mp.
9. 4 or 5 or 6 or 7 or 8.
10. ((prescrib* adj2 interven*) or (approp* adj2 prescrib*)).mp.
11. medication errors/or inappropriate prescribing/or pharmacy service, hospital/.
12. Medication Therapy Management/.
13. “Drug Utilization Review”/or drug utili?ation review.mp. or Drug Utilization/or stewardship.mp.
14. drug monitor*.mp. or Drug Monitoring/.
15. Medication Systems, Hospital/
16. intervention*.ti. or (intervention* adj6 (clinician* or collaborat* or design* or doctor* or educa* or impact* or improve* or individuali* or interdisciplin* or multicomponent or multi‐component or multidisciplin* or multi‐disciplin* or multifacet* or multi‐facet* or multimodal* or multi‐modal* or pharma* or physician* or practitioner* or prescrib* or professional* or provider* or tailor* or target* or usual care)).ti,ab.
17. (adherence or alert* or benchmark* or (change adj3 treatment) or computer assist* or support or compute* or clinical decision* or dosing or formulary or guidance or guideline* or impact or justification or overuse or over‐prescrib* or overprescrib* or under‐prescrib* or underprescrib* or pathway* or program* or programme* or (quality adj3 improv*) or reminder* or restriction* or unnecessary).ti.
18. 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17.
19. 3 and 9 and 18.
20. limit 19 to (English language and humans).
A.2. Appendix (i)
Quality assessment summary of included randomised controlled trials using the Cochrane Risk of Bias Assessment Tool 17 (n = 4).
| Study authors | Dalleur et al.47 | |
|---|---|---|
| Bias | Judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk of bias | Selection through “simple randomisation using drawing of lots” |
| Allocation concealment (selection bias) | Low risk of bias | “The IGCT nurse provided the evaluator with a list of the patients included in the study, which did not specify allocation group. The evaluator gathered data on the primary outcome.” |
| Blinding of participants and personnel | Low risk of bias | “The attending ward physician (who is responsible for prescriptions during hospitalisation and at discharge), the evaluator (OD), and the patients were blinded to group assignment.” |
| Blinding of outcome assessment (detection bias) | Low risk of bias | Blinding of outcome assessment described in article. |
| Incomplete outcome data (attrition bias) | Unclear risk of bias | Attrition is described but differences between patients who completed and did not complete the trial was not analysed |
| Selective reporting (reporting bias) | Low risk of bias | Comprehensive reporting of outcomes. The study protocol is available and all study outcomes are prespecified. |
| Other bias | N/A | N/A |
| Overall quality assessment | Low risk of bias | |
| Study authors | O'Connor et al.48 | |
|---|---|---|
| Bias | Judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk of bias | “It was not a double‐blinded study in which participants and researchers were blinded to the group randomization of each participant and the end points being assessed by a blinded assessor. Similarly, the intervention participants' attending doctors could not be blinded to their randomization group, because they had to decide whether to accept or reject individual STOPP/START criteria” |
| Allocation concealment (selection bias) | High risk of bias | Allocation not concealed. |
| Blinding of participants and personnel | High risk of bias | “The intervention could not be double‐ blinded (because of its nature)” |
| Blinding of outcome assessment (detection bias) | High risk of bias | Outcome assessment not blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk of bias | Attrition is described but differences between patients who completed and did not complete the trial was not analysed |
| Selective reporting (reporting bias) | Low risk of bias | Comprehensive reporting of outcomes. The study protocol is available and all study outcomes are prespecified. |
| Other bias | N/A | N/A |
| Overall quality assessment | High risk of bias | |
| Study authors | O'Sullivan et al.46 | |
|---|---|---|
| Bias | Judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk of bias | “We cluster‐randomised the admitting consultants and their teams into 2 groups prior to study initiation, i.e. intervention or control consultants. At admission, we allocated patients to 1 of 2 groups, i.e. (1) usual pharmaceutical care (control group) or (2) the CDSS‐supported SPRM intervention designed to optimise geriatric pharmaceutical care (intervention group), based on the particular consultant with primary responsibility for the patient's care during the index hospital admission. To avoid potentially biased selection of subjects into either arm of the study, we approached prospective trial patients in the order of their admission to the hospital to assess their eligibility for the trial.” |
| Allocation concealment (selection bias) | High risk of bias | Allocation of participants and investigators was not concealed or randomised. |
| Blinding of participants and personnel | High risk of bias | “Due to the nature of the intervention, it was not possible to blind participating attending doctors …It was not possible for the intervention to be double‐ blinded due to its nature;” |
| Blinding of outcome assessment (detection bias) | Low risk of bias | “The primary researcher recorded all documented new symptoms and clinical phenomena from every patient's medical records electronically and these were cross‐referenced with the trigger list, thereby minimising (but not abolishing) potential observer bias. In addition, we sought to further attenuate observer bias by including only those ADRs corroborated by the medically trained ADR assessor who was blinded to the group allocation of each patient in the trial.” |
| Incomplete outcome data (attrition bias) | Low risk of bias | “34 patients (17 intervention and 17 control patients) died during their index hospital admission; we included these patients in the final analysis on the basis of adherence to the intention‐to‐treat principle.” |
| Selective reporting (reporting bias) | Low risk of bias | Comprehensive reporting of outcomes. The study protocol is available and all study outcomes are prespecified. |
| Other bias | N/A | N/A |
| Overall quality assessment | Low risk of bias | |
| Study authors | Taylor et al.38 | |
|---|---|---|
| Bias | Judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk of bias | The study authors acknowledge that “patients were not individually randomised. However, the EDs were cluster randomised to early (5 EDs) and late (4 EDs) intervention clusters, using a computer‐generated random number function. During periods when study staff were available (usually 0800–1800, 7 days/week), consecutive patients who met the study entrance criteria were recruited.” |
| Allocation concealment (selection bias) | Unclear risk of bias | Not described |
| Blinding of participants and personnel | Low risk of bias | As reported in the article, “patients were only advised of the study at follow up. This was deliberate in order to minimise the Hawthorne effect. Had patients been aware that they were enrolled in a pain management study, this may have affected their follow‐up responses.” |
| Blinding of outcome assessment (detection bias) | Low risk of bias | “Patients were either telephoned or visited in the ward by a site investigator who was blinded to the data that was collected in the ED, including whether the patient had received ‘adequate analgesia’” |
| Incomplete outcome data (attrition bias) | Low risk of bias | “In total, 1527 patients were recruited although follow‐up data were not available for 210 (13.8%). Patients lost to follow up differed only in that fewer were administered any type of analgesia (76.7 vs 82.8%, P = 0.04)” |
| Selective reporting (reporting bias) | High risk of bias | The study authors acknowledge that “satisfaction is highly subjective, affected by a range of confounding variables and difficult to measure accurately. At patient follow up, as our data collectors were aware of the study hypothesis and the intervention status of their ED, measurement bias may have been introduced.” |
| Other bias | N/A | N/A |
| Overall quality assessment | Low risk of bias | |
A.3. Appendix (ii)
Quality assessment summary of included nonrandomised controlled studies using the Risk Of Bias In Non‐randomised Studies of Interventions tool18 (n = 33).
| Study authors | Akce et al.52 | |
|---|---|---|
| Bias | Judgement | Support for judgement |
| Bias due to confounding | Moderate risk of bias | The study authors acknowledge the potential for confounding factors; however, these have not been controlled for by study design or statistical analyses. |
| Bias in selection of participants into the study | Low risk of bias | Randomised selection of participants into the study. |
| Bias in classification of interventions | No information | Classification of interventions not explicitly described. |
| Bias due to deviations from intended interventions | No information | Deviation from intended interventions of the study is not adequately described. |
| Bias due to missing data | Serious risk of bias | Attrition of data is described but not analysed. |
| Bias in measurement of outcomes | Low risk of bias | Double data abstraction and data entry to minimise bias in measurement of outcomes. |
| Bias in selection of the reported result | Serious risk of bias | Retrospective data recorded. |
| Overall quality assessment | Moderate risk of bias | |
| Study authors | Auyong et al.25 | |
|---|---|---|
| Bias | Judgement | Support for judgement |
| Bias due to confounding | Low risk of bias | Potential risk of bias due to confounding accounted for by statistical analysis including logistic regression models |
| Bias in selection of participants into the study | Low risk of bias | Consecutive selection of participants into the study |
| Bias in classification of interventions | Low risk of bias | Intervention groups clearly defined |
| Bias due to deviations from intended interventions | No information | Potential deviations from intended interventions not described. |
| Bias due to missing data | Low risk of bias | No missing data reported. |
| Bias in measurement of outcomes | Moderate risk of bias | Outcome assessors not blinded to intervention status. |
| Bias in selection of the reported result | Moderate risk of bias | Multiple logistic regression models used to compare binary secondary outcomes which may increase the risk of bias arising from selective reporting of results. |
| Overall quality assessment | Low risk of bias | |
| Study authors | Baehren et al.40 | |
|---|---|---|
| Bias | Judgement | Support for judgement |
| Bias due to confounding | Serious risk of bias | Potential confounding factors not controlled for by study design or statistical analyses. |
| Bias in selection of participants into the study | Serious risk of bias | The study authors acknowledge that “Enrolment was based on a convenience sample “ |
| Bias in classification of interventions | Low risk of bias | Clear classification of intervention groups. |
| Bias due to deviations from intended interventions | Serious risk of bias | As reported in the article, “the number of patients treated by each physician is not the same.” |
| Bias due to missing data | No information | Attrition of data not reported. |
| Bias in measurement of outcomes | Low risk of bias | “Subjects were identified only by the research assistants who reviewed the triage information patients were assigned a sequential number” |
| Bias in selection of the reported result | Moderate risk of bias | Primary outcomes clearly reported. Multiple outcome measurements within secondary outcome domains. |
| Overall quality assessment | Moderate risk of bias | |
| Study authors | Benditz et al.24 | |
|---|---|---|
| Bias | Judgement | Support for judgement |
| Bias due to confounding | Serious risk of bias | Potential confounding factors not addressed by experimental design or statistical analyses. Patients informed of study but all received same intervention; health care professionals had increased experience with protocol over time. |
| Bias in selection of participants into the study | Low risk of bias | The study authors state “no patients were available on Mondays, which may also represent some kind of selection bias. …Wards to be visited were randomized daily by drawing a number to prevent selection bias.” |
| Bias in classification of interventions | Moderate risk of bias | Classification of interventions not clearly defined. |
| Bias due to deviations from intended interventions | No information | No information to describe potential deviations from intended interventions. |
| Bias due to missing data | Serious risk of bias | As acknowledged in the article, “We have no information about the excluded patients.” |
| Bias in measurement of outcomes | Low risk of bias | The study authors state “To avoid any interviewer–patient interaction bias, the nurse informed the patients that she was working independently from the health care team, that all information or judgment given in the interview would be treated confidentially, and that participation was voluntary. Data were anonymized after the interview.” |
| Bias in selection of the reported result | Low risk of bias | Primary and secondary outcome measurements reported in article tables. |
| Overall quality assessment | Moderate risk of bias | |
| Study authors | Bingle et al.21 | |
|---|---|---|
| Bias | Judgement | Support for judgement |
| Bias due to confounding | Serious risk of bias | Potential confounding factors not addressed by experimental design or statistical analyses |
| Bias in selection of participants into the study | Serious risk of bias | Potential bias introduced by selection of participants into the study not controlled in the study. |
| Bias in classification of interventions | No information | Limited description of the differences between the 3 detailing interventions |
| Bias due to deviations from intended interventions | Moderate risk of bias | Intervention groups moderately defined, which may introduce a certain degree of bias due to deviations of intended interventions. |
| Bias due to missing data | No information | Attrition data not described. |
| Bias in measurement of outcomes | Serious risk of bias | Retrospective chart audit, limited description of process. Pre and post screening performed by different people. |
| Bias in selection of the reported result | Low risk of bias | Primary and secondary outcome measurements reported in article tables. |
| Overall quality assessment | Serious risk of bias | |
| Study authors | Boothby et al.50 | |
|---|---|---|
| Bias | Judgement | Support for judgement |
| Bias due to confounding | Serious risk of bias | Potential confounding factors not addressed by experimental design or statistical analyses |
| Bias in selection of participants into the study | Serious risk of bias | Selection of participants into the study done by convenience sampling. |
| Bias in classification of interventions | Low risk of bias | Intervention groups clearly defined. |
| Bias due to deviations from intended interventions | No information | No information to describe potential deviations from intended interventions. |
| Bias due to missing data | No information | Attrition data not described. |
| Bias in measurement of outcomes | Low risk of bias | The study authors report that “data were normalized with regard to the varying hospital census”. |
| Bias in selection of the reported result | Low risk of bias | Primary and secondary outcome measurements reported in article tables. |
| Overall quality assessment | Moderate risk of bias | |
| Study authors | Bos et al.34 | |
|---|---|---|
| Bias | Judgement | Support for judgement |
| Bias due to confounding | Serious risk of bias | Co‐morbidities e.g. Charlson's and polypharmacy not accounted for as can increase risk of ADRs—although age, sex etc. were |
| Bias in selection of participants into the study | Serious risk of bias | Not just index admission, re‐admissions were included—can skew data as some can be predisposed to drug related problems; could not be adjusted for in analysis |
| Bias in classification of interventions | Low risk of bias | Clear classification of intervention groups, pre and post groups separated by 3 mo. |
| Bias due to deviations from intended interventions | No information | No information to describe potential deviations from intended interventions. |
| Bias due to missing data | No information | No attrition of data described. |
| Bias in measurement of outcomes | Low risk of bias | The authors of the study report that “By blinding all case record forms with respect to the study period before assessment by the experts and by correcting for confounders, the probability of bias was minimized.” |
| Bias in selection of the reported result | Low risk | Primary and secondary outcome measurements reported in article tables. |
| Overall quality assessment | Serious risk of bias | |
| Study authors | Chan et al.26 | |
|---|---|---|
| Bias | Judgement | Support for judgement |
| Bias due to confounding | Serious risk of bias |
Postop D2–3 may be insufficient to titrate PRN agents to optimal analgesia so pain ratings may not reflect full potential of the intervention regimen Also unclear if patients aware of involvement in active intervention aimed at improving pain—may have placebo if patients were aware |
| Bias in selection of participants into the study | Serious risk of bias | Patients not randomised; recruitment periods were different lengths |
| Bias in classification of interventions | Low risk of bias | Fully prospective study |
| Bias due to deviations from intended interventions | No information | Not described. |
| Bias due to missing data | Serious risk of bias | The authors acknowledge that “there are some missing data in both surveys, especially the second questionnaire.” 29.5% missing data in the intervention group (Group B). |
| qBias in measurement of outcomes | Serious risk of bias | Surveys completed by patients; risk of deviation or subjective interpretation. Outcome assessors were not blinded. |
| Bias in selection of the reported result | No information | Not described. |
| Overall quality assessment | Serious risk of bias | |
| Study authors | Chanques et al.36 | |
|---|---|---|
| Bias | Judgement | Support for judgement |
| Bias due to confounding | Low risk of bias | Accounted for delirium and intensive care unit handicaps in communicating pain. Healthcare professionals blinded to results of assessments in control phase. |
| Bias in selection of participants into the study | Low risk of bias | Prospective, consecutive recruitment of participants into the study. |
| Bias in classification of interventions | Low risk of bias | Clear classification of interventions. |
| Bias due to deviations from intended interventions | No information | Strategies to address potential deviations from intended interventions not adequately described. |
| Bias due to missing data | Moderate risk of bias | Attrition data described, but no analysis was performed. |
| Bias in measurement of outcomes | Serious risk of bias | Potential for subjective measurement of pain scores. Duration of intervention period was 8 weeks longer; bigger sample size (but interrater reliability established). Cannot blind healthcare professionals who administer RASS and BPS |
| Bias in selection of the reported result | No information | Not described. |
| Overall quality assessment | Moderate risk of bias | |
| Study authors | Cui et al.32 | |
|---|---|---|
| Bias | Judgement | Support for judgement |
| Bias due to confounding | Serious risk of bias | Postop main medication assessed by may be swayed as surgeons informed of intervention; cannot guarantee that they did not change practices per protocol—which nurses may have picked up during their rounds together |
| Bias in selection of participants into the study | Serious risk of bias | Selection of participants into the study by convenience sampling. |
| Bias in classification of interventions | Low risk of bias | Clear classification of interventions. |
| Bias due to deviations from intended interventions | No information | Strategies to address potential deviations from intended interventions not adequately described. |
| Bias due to missing data | Low risk of bias | 16 and 15 nurses completed, though unclear how many patients were recruited then excluded due to missing data or other reasons |
| Bias in measurement of outcomes | Serious risk of bias | Questionnaire measurement of outcomes; potential for subjective interpretation and response. |
| Bias in selection of the reported result | No information | Not described. |
| Overall quality assessment | Moderate risk of bias | |
| Study authors | Duncan and Pozehl31 | |
|---|---|---|
| Bias | Judgement | Support for judgement |
| Bias due to confounding | Serious risk of bias | Potential confounding factors not addressed by experimental design or statistical analyses |
| Bias in selection of participants into the study | Moderate risk of bias | Retrospective audit of patient medical records. |
| Bias in classification of interventions | Low risk of bias | Clear classification of interventions. |
| Bias due to deviations from intended interventions | No information | Strategies to address potential deviations from intended interventions not adequately described. |
| Bias due to missing data | Moderate risk of bias | Attrition data described, but no analysis was performed |
| Bias in measurement of outcomes | Serious risk of bias | Self‐reported pain intensity ratings; potential for subjective measurement. |
| Bias in selection of the reported result | No information | Not described. |
| Overall quality assessment | Moderate risk of bias | |
| Study authors | Ferguson et al.54 | |
|---|---|---|
| Bias | Judgement | Support for judgement |
| Bias due to confounding | Serious risk of bias | Confounding factors acknowledged but could not be controlled for due to the nature of the hospital setting. |
| Bias in selection of participants into the study | Low risk of bias | All patient‐controlled analgesia (PCA) errors in hospital over set period. |
| Bias in classification of interventions | Low risk of bias | Intervention groups clearly defined. |
| Bias due to deviations from intended interventions | Serious risk of bias | Acknowledged potential for deviations from intended interventions. |
| Bias due to missing data | No information | Missing data not reported. |
| Bias in measurement of outcomes | Low risk of bias | Data PCA errors were objective and carried a lower potential for subjective interpretation. |
| Bias in selection of the reported result | No information | Not described. |
| Overall quality assessment | Moderate risk of bias | |
| Study authors | Humphries et al.27 | |
|---|---|---|
| Bias | Judgement | Support for judgement |
| Bias due to confounding | Serious risk of bias | No adjustments made to account for potential confounding factors. |
| Bias in selection of participants into the study | Low risk of bias | All eligible patients included in analysis. |
| Bias in classification of interventions | Low risk of bias | Intervention group clearly defined. |
| Bias due to deviations from intended interventions | No information | Potential deviations from intended interventions not described. |
| Bias due to missing data | Low risk of bias | Study authors report no attrition of data. |
| Bias in measurement of outcomes | Moderate risk of bias | Outcome assessors not blinded to intervention status. |
| Bias in selection of the reported result | No information | |
| Overall quality assessment | Moderate risk of bias | |
| Study authors | Juhl et al.28 | |
|---|---|---|
| Bias | Judgement | Support for judgement |
| Bias due to confounding | Serious risk of bias | Measurement of pain scores depends on the day which postoperative patients were interviewed; was not consistent between patients nor between pre–post intervention periods. |
| Bias in selection of participants into the study | Moderate risk of bias | Prospective and consecutive patient inclusion. However, nurses' participation was voluntary, which is susceptible to volunteer bias. |
| Bias in classification of interventions | Low risk of bias | Prospective study and clear pre–post periods. |
| Bias due to deviations from intended interventions | Low risk | Intervention groups clearly defined to minimise deviation from intended interventions. |
| Bias due to missing data | Moderate risk of bias | Attrition described but no analysis performed. |
| Bias in measurement of outcomes | Moderate risk of bias | Nurses who assessed subjective pain could not be blinded; good that McGill Pain Questionnaire translated by 3 qualified people |
| Bias in selection of the reported result | Moderate risk of bias | Reporting of anaesthetists' vs surgeons' prescribing patterns were nonconsistent (converse figures reported) |
| Overall quality assessment | Moderate risk of bias | |
| Study authors | Kjeldsen et al.33 | |
|---|---|---|
| Bias | Judgement | Support for judgement |
| Bias due to confounding | Serious risk of bias | Diff number of patients treated with NSAIDs (one of the risk drugs) which can affect (opioid) analgesia requirements and possibly subsequent recs (Table 1) |
| Bias in selection of participants into the study | Low risk of bias | Controlled prospective study design, analysis performed on 2 groups to ensure similar cohorts. |
| Bias in classification of interventions | Low risk of bias | “To ensure standardized interventions, an information sheet was developed for each of the risk medications” |
| Bias due to deviations from intended interventions | No information | Strategies to address potential deviations from intended interventions not adequately described. |
| Bias due to missing data | Moderate risk of bias | Reasons for exclusion; differs slightly between the 2 groups |
| Bias in measurement of outcomes | Low risk of bias | Comprehensive reporting of outcomes |
| Bias in selection of the reported result | Low risk of bias | Selection of reported results addressed |
| Overall quality assessment | Serious risk of bias | |
| Study authors | Majumder et al.29 | |
|---|---|---|
| Bias | Judgement | Support for judgement |
| Bias due to confounding | Moderate risk of bias | Confounding variables are addressed to an extent by study design (e.g. restriction of included patients). |
| Bias in selection of participants into the study | Low risk of bias | Consecutive selection of participants into the study. |
| Bias in classification of interventions | Low risk of bias | Intervention groups clearly defined. |
| Bias due to deviations from intended interventions | No information | Potential deviations from intended interventions not described. |
| Bias due to missing data | Low risk of bias | Study authors report no attrition of data. |
| Bias in measurement of outcomes | Moderate risk of bias | Outcome assessors not blinded to intervention status of the participant. |
| Bias in selection of the reported result | Low risk of bias | Reported results reflect predefined primary and secondary outcome measures. |
| Overall quality assessment | Low risk of bias | |
| Study authors | Manias et al.45 | |
|---|---|---|
| Bias | Judgement | Support for judgement |
| Bias due to confounding | Moderate risk of bias | Potential confounding factors addressed to an extent by study design. |
| Bias in selection of participants into the study | Low risk of bias | Consecutive patient recruitment into the study. |
| Bias in classification of interventions | Low risk of bias | Clear classification of interventions. |
| Bias due to deviations from intended interventions | No information | Strategies to address potential deviations from intended interventions not adequately described. |
| Bias due to missing data | Low risk of bias | No missing data reported. |
| Bias in measurement of outcomes | Low risk of bias | The study authors report “The research assistant who collected data from both groups was blinded to group assignment. In addition, nurses in each hospital were not informed about their allocated group.” |
| Bias in selection of the reported result | No information | Not described. |
| Overall quality assessment | Low risk of bias | |
| Study authors | McAllister et al.41 | |
|---|---|---|
| Bias | Judgement | Support for judgement |
| Bias due to confounding | Serious risk of bias | Potential confounding factors not addressed by experimental design or statistical analyses |
| Bias in selection of participants into the study | Low risk of bias | All eligible participants included into the study. |
| Bias in classification of interventions | Low risk of bias | Clear classification of interventions. |
| Bias due to deviations from intended interventions | No information | Strategies to address potential deviations from intended interventions not adequately described. |
| Bias due to missing data | No information | Missing data not described. |
| Bias in measurement of outcomes | Low risk of bias | Clear documentation of outcome measurement. |
| Bias in selection of the reported result | No information | Not described |
| Overall quality assessment | Moderate risk of bias | |
| Study authors | McEvoy et al.56 | |
|---|---|---|
| Bias | Judgement | Support for judgement |
| Bias due to confounding | Serious risk of bias | Potential confounding factors not addressed by experimental design or statistical analyses |
| Bias in selection of participants into the study | Low risk of bias | Retrospective analysis of electronic medication orders. |
| Bias in classification of interventions | Low risk of bias | Clear classification of intervention groups. |
| Bias due to deviations from intended interventions | No information | Strategies to address potential deviations from intended interventions not adequately described. |
| Bias due to missing data | Low risk of bias | Study authors reported no missing data. |
| Bias in measurement of outcomes | Low risk of bias | Clear documentation of outcome measurement. |
| Bias in selection of the reported result | Serious risk of bias | The authors acknowledge that “the duration of postintervention assessment and sample size were not the same as the preintervention assessment. This may have affected the overall results due to variance in prescribing patterns.” |
| Overall quality assessment | Moderate risk of bias | |
| Study authors | Morisson et al.20 | |
|---|---|---|
| Bias | Judgement | Support for judgement |
| Bias due to confounding | Low risk of bias | Study design involved propensity score matching with control participants. Statistical analyses to control for confounding factors included logistic regression models. |
| Bias in selection of participants into the study | Low risk of bias | All eligible participants were enrolled in the study. |
| Bias in classification of interventions | Low risk of bias | Intervention groups clearly defined. |
| Bias due to deviations from intended interventions | No information | Potential deviations from intended interventions not described. |
| Bias due to missing data | Low risk of bias | Details of subject enrolment clearly outlined and no other missing data reported. |
| Bias in measurement of outcomes | Low risk of bias | The study was double‐blinded. Participants were not informed as to whether they were enrolled in the intervention or control unit and outcome assessors were blinded to participant group. |
| Bias in selection of the reported result | Low risk of bias | Primary and secondary outcome measurements reported in article tables. |
| Overall quality assessment | Low risk of bias | |
| Study authors | Moustafa et al.39 | |
|---|---|---|
| Bias | Judgement | Support for judgement |
| Bias due to confounding | Serious risk of bias | Potential confounding factors not addressed by experimental design or statistical analyses |
| Bias in selection of participants into the study | Low risk of bias | Prospective, consecutive selection of participants into the study. |
| Bias in classification of interventions | Moderate risk of bias | Classification of interventions not explicitly described. |
| Bias due to deviations from intended interventions | No information | Strategies to address potential deviations from intended interventions not adequately described. |
| Bias due to missing data | Low risk of bias | No attrition of data (100% pain assessment performed) |
| Bias in measurement of outcomes | Serious risk of bias | The study authors acknowledge “ED personnel were aware (in the second phase only) of the main outcome of the study and it is likely that the Hawthorne effect had an impact on pain assessment, but it did not so much on analgesics treatment.” |
| Bias in selection of the reported result | No information | Not described. |
| Overall quality assessment | Serious risk of bias | |
| Study authors | Muntlin et al.43 | |
|---|---|---|
| Bias | Judgement | Support for judgement |
| Bias due to confounding | Serious risk of bias | Potential confounding factors not addressed by experimental design or statistical analyses |
| Bias in selection of participants into the study | Serious risk of bias | The authors report “Patients were approached to take part in the study 24 h a day, weekdays and weekends until the desired number of patients had been included.” Convenience sampling may introduce bias into the study. |
| Bias in classification of interventions | Serious risk of bias | Clear classification of interventions, however, phases B and A2 were not as explicitly described as A1. |
| Bias due to deviations from intended interventions | No information | No information to describe potential deviations from intended interventions. |
| Bias due to missing data | Moderate risk of bias | Excluded data is described, however, differences between included and excluded patients were not analysed |
| Bias in measurement of outcomes | Serious risk of bias | Questionnaire reporting of outcomes; potential for discrepancies between researchers |
| Bias in selection of the reported result | No information | Not described. |
| Overall quality assessment | Serious risk of bias | |
| Study authors | Neitzel et al.22 | |
|---|---|---|
| Bias | Judgement | Support for judgement |
| Bias due to confounding | Serious risk of bias | Potential confounding factors not addressed by experimental design or statistical analyses. Patient pain experience may affect length of stay. |
| Bias in selection of participants into the study | Serious risk of bias | Selection of participants into the study by convenience sampling. |
| Bias in classification of interventions | Low risk of bias | Intervention groups clearly described. |
| Bias due to deviations from intended interventions | No information | Strategies to address potential deviations from intended interventions not adequately described. |
| Bias due to missing data | Low risk of bias | Reasonably complete data collection |
| Bias in measurement of outcomes | Moderate risk of bias | Outcome assessors not blinded to the intervention status of the participants. Provider survey is potentially subjective. |
| Bias in selection of the reported result | No information | Not described |
| Overall quality assessment | Moderate risk of bias | |
| Study authors | Netherton et al.42 | |
|---|---|---|
| Bias | Judgement | Support for judgement |
| Bias due to confounding | Serious risk of bias | The authors acknowledge “We have not been able to adjust for confounders; so the increase in ketorolac use seen in Calgary EDs may be due to secular changes, that is, physician knowledge about ketorolac use for acute pain, or due to an influx of newly trained ED physicians who may have been familiar with its use in their residency training programs” |
| Bias in selection of participants into the study | Low risk of bias | Random selection of participants into the study. |
| Bias in classification of interventions | Low risk of bias | Clear classification of interventions. |
| Bias due to deviations from intended interventions | No information | Not described. |
| Bias due to missing data | Moderate risk of bias | Attrition data described, but no analysis performed; “Excluding patients arriving by Emergency Medical Service may have led to the exclusion of the most severe presentations of renal colic.” |
| Bias in measurement of outcomes | Moderate risk of bias | Outcome assessors were not blinded to the intervention status of the study participants. |
| Bias in selection of the reported result | No information | Not described. |
| Overall quality assessment | Moderate risk of bias | |
| Study authors | Olsen et al.37 | |
|---|---|---|
| Bias | Judgement | Support for judgement |
| Bias due to confounding | Serious risk of bias | The authors acknowledge “A pre–post intervention design has weaknesses, as confounding variables could influence the outcome, but differences in baseline variables between groups were controlled, using regression analysis” |
| Bias in selection of participants into the study | Serious risk of bias | Non‐consecutive recruitment of eligible patients into the study. |
| Bias in classification of interventions | Low risk of bias | Clear classification of interventions. |
| Bias due to deviations from intended interventions | No information | Potential deviation from intended interventions not adequately described. |
| Bias due to missing data | Moderate risk of bias | Attrition data described, but no analysis was performed. |
| Bias in measurement of outcomes | Serious risk of bias | Potential for subjective pain assessment; “we did not have independent observers assessing pain in the control group” |
| Bias in selection of the reported result | Serious risk of bias | No results re pain events or even any of the 3 pain tools; high risk of selective reporting |
| Overall quality assessment | Serious risk of bias | |
|
Study authors |
Paul et al.53 | |
|---|---|---|
| Bias | Judgement | Support for judgement |
| Bias due to confounding | Low risk of bias | The authors report that potential confounding factors were addressed. “Cases where the cause of the critical incident was attributed to patient factors were excluded from this study” |
| Bias in selection of participants into the study | Low risk of bias | “Two reviewers (B.B., A.M.) obtained and examined all Hamilton Health Sciences Acute Pain Service (HHS APS) critical incident reports (n = 642) dating from 1 February 2002 to 28 February 2009. Each report was reviewed to determine whether the event involved a PCA setup, programming, or administration error. Any discrepancies were resolved through consensus or discussion with a third reviewer” |
| Bias in classification of interventions | Low risk of bias | Classification of interventions between intervention and control groups explicitly described. |
| Bias due to deviations from intended interventions | No information | Potential deviation from intended interventions not adequately described. |
| Bias due to missing data | No information | Missing data not described. |
| Bias in measurement of outcomes | Moderate risk of bias | Outcome assessors not blinded to the intervention status of participants. |
| Bias in selection of the reported result | No information | Not described |
| Overall quality assessment | Low risk of bias | |
| Study authors | Pierik et al.44 | |
|---|---|---|
| Bias | Judgement | Support for judgement |
| Bias due to confounding | Serious risk of bias | The study authors acknowledge that “There are a number of important potential confounding factors, e.g. severity of injury, knowledge and experience of pain management, which were not measured and may have differed in both periods No adjustments could be made.” |
| Bias in selection of participants into the study | Low risk of bias | Consecutive patient recruitment of participants into the study. |
| Bias in classification of interventions | Low risk of bias | Clear classification of interventions. |
| Bias due to deviations from intended interventions | No information | Potential deviations from intended interventions not adequately described. |
| Bias due to missing data | Low risk of bias | No attrition of data. |
| Bias in measurement of outcomes | Serious risk of bias | The authors report “The percentage of patients who were actually administered analgesics might be underestimated. Even though the ED staff was instructed to list all medications, some may have neglected to do so, especially for over‐the‐counter analgesics.” |
| Bias in selection of the reported result | Low risk of bias | Objective selection of reported results described. |
| Overall quality assessment | Moderate risk of bias | |
| Study authors | Shaw et al.51 | |
|---|---|---|
| Bias | Judgement | Support for judgement |
| Bias due to confounding | Serious risk of bias | Potential confounding factors not addressed by experimental design or statistical analyses |
| Bias in selection of participants into the study | Low risk of bias | Analysis of all eligible prescription error rates according to predefined criteria. |
| Bias in classification of interventions | Low risk of bias | Clear intervention methodology and classification described. |
| Bias due to deviations from intended interventions | Low risk of bias | Hard copy material provided to study participants, unlikely to produce deviation across participants. |
| Bias due to missing data | No information | Missing data not described. |
| Bias in measurement of outcomes | Low risk of bias | Quantitative analysis of prescription error rates. |
| Bias in selection of the reported result | Unclear | Not described |
| Overall quality assessment | Low risk of bias | |
| Study authors | Titsworth et al.23 | |
|---|---|---|
| Bias | Judgement | Support for judgement |
| Bias due to confounding | Serious risk of bias | Potential confounding factors not addressed by experimental design or statistical analyses. Patient questionnaire performed, no blinding of patients described, potential for patients to answer more positively due to nonanonymity |
| Bias in selection of participants into the study | Low risk of bias | “Systematic random sampling was used to identify every 10th postoperative neurosurgical patient admitted preintervention and every 17th patient postintervention” |
| Bias in classification of interventions | Low risk of bias | Explicit classification of interventions. |
| Bias due to deviations from intended interventions | Low risk of bias | Intended interventions were clearly documented to minimise risk of bias introduced by deviation from intervention. |
| Bias due to missing data | Moderate risk of bias | Excluded data is described, however, differences between included and excluded patients were not analysed |
| Bias in measurement of outcomes | Serious risk of bias | “Nurses collected pain scores and were aware of the initiative; this could have potentially influenced patient‐reporting practices, resulting in response bias.” |
| Bias in selection of the reported result | No information | Not described. |
| Overall quality assessment | Moderate risk of bias | |
| Study authors | Urfer et al.49 | |
|---|---|---|
| Bias | Judgement | Support for judgement |
| Bias due to confounding | Low risk of bias | Potential confounding factors addressed by restriction of participant sample to patients aged 65 years and above. |
| Bias in selection of participants into the study | Low risk of bias | Consecutive selection of participants into the study. |
| Bias in classification of interventions | Low risk of bias | Clear distinction between use and no use of 5‐point checklist. |
| Bias due to deviations from intended interventions | No information | Potential deviation from intended interventions not adequately described. |
| Bias due to missing data | Moderate risk of bias | Excluded data is described, however, differences between included and excluded patients were not analysed |
| Bias in measurement of outcomes | Low risk of bias | “Data were recorded on a standardized case report form and anonymized before statistical analysis.” |
| Bias in selection of the reported result | Moderate risk of bias | “it was not possible to blind the 2 investigators assessing medication appropriateness, because charts used for chart review contained hospitalization dates, dates of laboratory examinations etc.” |
| Overall quality assessment | Low risk of bias | |
| Study authors | Usichenko et al.30 | |
|---|---|---|
| Bias | Judgement | Support for judgement |
| Bias due to confounding | Serious risk of bias | History of opioid use and opioid tolerance may affect pain experience and pain scores. Potential confounding factor(s) not adjusted for. |
| Bias in selection of participants into the study | Low risk of bias | Prospective, consecutive recruitment of participants into the study |
| Bias in classification of interventions | Low risk of bias | Clear classification of intervention groups. |
| Bias due to deviations from intended interventions | Low risk of bias | Interventions clearly defined and no deviation from intended interventions reported. |
| Bias due to missing data | Low risk of bias | 91% and 85% survey completion rate; proportions similar across groups |
| Bias in measurement of outcomes | Moderate risk of bias | Potential for subjective reporting of pain in surveys using visual rating scale |
| Bias in selection of the reported result | Moderate risk of bias | Power calculations done |
| Overall quality assessment | Moderate risk of bias | |
| Study authors | VanGulik et al.35 | |
|---|---|---|
| Bias | Judgement | Support for judgement |
| Bias due to confounding | Serious risk of bias | Nurses not blinded to intervention; bedside manner or attention to detail/pain may become more astute as they gain more experience with pain medication especially over 15 mo. |
| Bias in selection of participants into the study | Unclear risk of bias | Unclear if patient selection was random or consecutive for total knee arthroplasty patients. |
| Bias in classification of interventions | Low risk of bias | Clear classification of intervention groups, prospective study design. |
| Bias due to deviations from intended interventions | Low risk of bias | The study authors report “the patients were neither aware of nor trained in the pain management programme and they were asked exactly the same question in both phases, though by different persons” |
| Bias due to missing data | No information | Only 1.3% of records missing from retrospective audit. |
| Bias in measurement of outcomes | Serious risk of bias | The study authors report “The pain scores in the control group were asked when patients were at rest, not undergoing interventions, which may have led to relatively low pain levels at that particular moment … .during the intervention phase, nurses were trained to be more alert of high NRS levels compared to the control phase” |
| Bias in selection of the reported result | No information | Not described |
| Overall quality assessment | Moderate risk of bias | |
| Study authors | Whipple et al.55 | |
|---|---|---|
| Bias | Judgement | Support for judgement |
| Bias due to confounding | Serious risk of bias | Potential confounding factors not addressed by experimental design or statistical analyses |
| Bias in selection of participants into the study | Serious risk of bias | Retrospective review of PCA errors. No strategies to minimise selection bias reported. |
| Bias in classification of interventions | Low risk of bias | Clear classification of intervention groups. |
| Bias due to deviations from intended interventions | Low risk of bias | Objective review of PCA errors using defined criteria. |
| Bias due to missing data | Low risk of bias | No attrition of data due to the nature of the study. |
| Bias in measurement of outcomes | No information | Measures to reduce bias in outcome measurement not described |
| Bias in selection of the reported result | No information | Not described. |
| Overall quality assessment | Moderate risk of bias | |
A.4. Appendix
Intervention complexity assessment.
Summary of complexity assessment of interventions primarily addressing appropriate opioid use opioids in the hospital inpatient setting (n = 31).
| Intervention type (n) | Primary author | Shania's iCAT Score* | Average iCAT Score |
|---|---|---|---|
| Multifaceted interventions primarily aimed at prescribing (16) | Taylor38 | 9.0 | 10.4 |
| Morisson20 | 9.0 | ||
| Bingle21 | 9.0 | ||
| Boothby50 | 13.0 | ||
| Neitzel22 | 13.0 | ||
| Shaw51 | 9.0 | ||
| VanGulik35 | 10.0 | ||
| Akce52 | 9.0 | ||
| Titsworth23 | 14.0 | ||
| Benditz24 | 14.0 | ||
| Auyong25 | 10.0 | ||
| Chan26 | 8.0 | ||
| Humphries27 | 8.0 | ||
| Juhl28 | 10.0 | ||
| Majumder29 | 12.0 | ||
| Usichenko30 | 10.0 | ||
| Decision‐making tools to guide opioid prescribing (5) | Moustafa39 | 8.0 | 7.4 |
| Baehren40 | 7.0 | ||
| McAllister41 | 7.0 | ||
| Netherton42 | 8.0 | ||
| McEvoy56 | 7.0 | ||
| Interventions to improve patient monitoring (10) | Muntlin43 | 8.0 | 7.3 |
| Chanques36 | 8.0 | ||
| Duncan31 | 7.0 | ||
| Olsen37 | 7.0 | ||
| Pierik44 | 8.0 | ||
| Cui32 | 7.0 | ||
| Manias45 | 8.0 | ||
| Paul53 | 7.0 | ||
| Ferguson54 | 7.0 | ||
| Whipple55 | 6.0 |
Overall intervention complexity was calculated by assigning each criteria a score of 0 to 4, indicating a low or high degree of complexity using the Cochrane Intervention Complexity Assessment Tool for Systematic Reviews (iCAT_SR).19
Liu S, Gnjidic D, Nguyen J, Penm J. Effectiveness of interventions on the appropriate use of opioids for noncancer pain among hospital inpatients: A systematic review. Br J Clin Pharmacol. 2020;86:210–243. 10.1111/bcp.14203
REFERENCES
- 1. Volkow N. Characteristics of Opioid Prescriptions in 2009. JAMA. 2011;305(13):1299‐1301. 10.1001/jama.2011.401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. National Institute for Health and Care Excellence . Pain management: Initial opioid prescriptions and likelihood of long‐term opioid use. London: NICE; 2017. [Google Scholar]
- 3. Shafi S, Collinsworth A, Copeland L. Association of Opioid‐Related Adverse Drug Events With Clinical and Cost Outcomes Among Surgical Patients in a Large Integrated Health Care Delivery System. JAMA Surg. 2018;153(8):782‐782. 10.1001/jamasurg/2018.2792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Davies E, Green C, Taylor S, Williamson P, Mottram D, Pirmohamed M. Adverse Drug Reactions in Hospital In‐Patients: A Prospective Analysis of 3695 Patient‐Episodes. PLoS ONE. 2009;4(2):e4439 10.1371/journal.pone.0004439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Herzig S, Rothberg M, Cheung M, Ngo L, Marcantonio E. Opioid utilization and opioid‐related adverse events in nonsurgical patients in US hospitals. J Hosp Med. 2013;9(2):73‐81. 10.1002/jhm.2102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. American Geriatrics Society Panel on the Pharmacological Management of Persistent Pain in Older Persons . Pharmacologic Management of Persistent Pain in Older Persons. J Am Geriatr Soc. 2009;57(8):1331‐1346. [DOI] [PubMed] [Google Scholar]
- 7. Cerdá M, Ransome Y, Keyes K, et al. Prescription opioid mortality trends in New York City, 1990–2006: Examining the emergence of an epidemic. Drug Alcohol Depend. 2013;132(1–2):53‐62. 10.1016/j.drugalcdep.2012.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oderda G, Evans R, Lloyd J, et al. Cost of Opioid‐Related Adverse Drug Events in Surgical Patients. J Pain Symptom Manage. 2003;25(3):276‐283. 10.1016/s0885-3924(02)00691-7 [DOI] [PubMed] [Google Scholar]
- 9. Pizzi L, Toner R, Foley K, et al. Relationship Between Potential Opioid‐Related Adverse Effects and Hospital Length of Stay in Patients Receiving Opioids After Orthopedic Surgery. Pharmacotherapy. 2012;32(6):502‐514. 10.1002/j.1875-9114.2012.01101 [DOI] [PubMed] [Google Scholar]
- 10. The Society of Hospital Medicine . Reducing Adverse Drug Events Related to Opioids Implementation Guide 2015. https://www.calhospital.org/sites/main/files/file-attachments/radeo_implementation_guide.pdf. Accessed July 5, 2018.
- 11. Franklin G, Mai J, Turner J, Sullivan M, Wickizer T, Fulton‐Kehoe D. Bending the prescription opioid dosing and mortality curves: Impact of the Washington State opioid dosing guideline. Am J Ind Med. 2011;55(4):325‐331. 10.1002/ajim.21998 [DOI] [PubMed] [Google Scholar]
- 12. Penm J, MacKinnon N, Mashni R, et al. Statewide cross‐sectional survey of emergency departments' adoption and implementation of the Ohio opioid prescribing guidelines and opioid prescribing practices. BMJ Open. 2018;8(6):e020477 10.1136/bmjopen-2017-020477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pomerleau A, Nelson L, Hoppe J, Salzman M, Weiss P, Perrone J. The Impact of Prescription Drug Monitoring Programs and Prescribing Guidelines on Emergency Department Opioid Prescribing: A Multi‐Center Survey. Pain Med. 2016; 18(5):889–897. 10.1093/pm/pnw032 [DOI] [PubMed] [Google Scholar]
- 14. Tournebize J, Gibaja V, Muszczak A, Kahn J. Are Physicians Safely Prescribing Opioids for Chronic Noncancer Pain? A Systematic Review of Current Evidence. Pain Pract. 2015;16(3):370‐383. 10.1111/papr.12289 [DOI] [PubMed] [Google Scholar]
- 15. Eccleston C, Fisher E, Thomas K, et al. Interventions for the reduction of prescribed opioid use in chronic non‐cancer pain. Cochrane Database Syst Rev. 2017; 41(3):329‐330. 10.1002/14651858.cd010323.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev. 2015;4(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from: https://www.handbook.cochrane.org.
- 18. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomized studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lewin S, Hendry M, Chandler J, et al. Assessing the complexity of interventions within systematic reviews: development, content and use of a new tool (iCAT_SR). BMC Med Res Methodol. 2017;17(1):76 10.1186/s12874-017-0349-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morrison RS, Flanagan S, Fischberg D, Cintron A, Siu AL. A novel interdisciplinary analgesic program reduces pain and improves function in older adults after orthopedic surgery. J Am Geriatr Soc. 2009. Jan;57(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bingle GJ, O'Connor TP, Evans WO, Detamore S. The effect of 'detailing' on physicians' prescribing behavior for postsurgical narcotic analgesia. Pain. 1991;45(2):171‐173. [DOI] [PubMed] [Google Scholar]
- 22. Neitzel JJ, Miller EH, Shepherd MF, Belgrade M. Improving pain management after total joint replacement surgery. Orthop Nurs. 1999;18(4):37‐45. [PubMed] [Google Scholar]
- 23. Titsworth WL, Abram J, Guin P, et al. A prospective time‐series quality improvement trial of a standardized analgesia protocol to reduce postoperative pain among neurosurgery patients. J Neurosurg. 2016;125(6):1523‐1532. [DOI] [PubMed] [Google Scholar]
- 24. Benditz A, Greimel F, Auer P, et al. Can consistent benchmarking within a standardized pain management concept decrease postoperative pain after total hip arthroplasty? A prospective cohort study including 367 patients. J Pain Res. 2016;9:1205‐1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Auyong DB, Allen CJ, Pahang JA, Clabeaux JJ, MacDonald KM, Hanson NA. Reduced length of hospitalization in primary total knee arthroplasty patients using an updated enhanced recovery after orthopedic surgery (ERAS) pathway. J Arthroplasty. 2015. Oct 1;30(10):1705‐1709. [DOI] [PubMed] [Google Scholar]
- 26. Chan RPL, Chan WS. Outcome following introduction of a procedure specific pain management programme for caesarean section. Sri Lankan J Anaesth. 2018;26(1):39‐44. [Google Scholar]
- 27. Humphries CA, Counsell DJ, Pediani RC, Close SL. Audit of opioid prescribing: the effect of hospital guidelines. Anaesthesia. 1997. Jul;52(8):745‐749. [DOI] [PubMed] [Google Scholar]
- 28. Juhl IU, Bülow HH, Nielsen PR, Videbæk B, Sonnenschein C. Management of postoperative pain. An intervention study. Acta Anaesthesiol Scand. 1996;40(7):852‐857. [DOI] [PubMed] [Google Scholar]
- 29. Majumder A, Fayezizadeh M, Neupane R, Elliott HL, Novitsky YW. Benefits of multimodal enhanced recovery pathway in patients undergoing open ventral hernia repair. J Am Coll Surg. 2016. Jun 1;222(6):1106‐1115. [DOI] [PubMed] [Google Scholar]
- 30. Usichenko TI, Röttenbacher I, Kohlmann T, et al. Implementation of the quality management system improves postoperative pain treatment: A prospective pre−/post‐interventional questionnaire study. Br J Anaesth. 2013;110(1):87‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Duncan K, Pozehl B. Effects of performance feedback on patient pain outcomes. Clin Nurs Res. 2000;9(4):379‐397;98–401 [DOI] [PubMed] [Google Scholar]
- 32. Cui C, Wang LX, Li Q, Zaslansky R, Li L. Implementing a pain management nursing protocol for orthopaedic surgical patients: Results from a PAIN OUT project. J Clin Nurs. 2018;27(7–8):1684‐1691. [DOI] [PubMed] [Google Scholar]
- 33. Kjeldsen LJ, Clemmensen MH, Kronborg C, et al. Evaluation of a controlled, national collaboration study on a clinical pharmacy service of screening for risk medications. Int J Clin Pharmacol. 2014;36(2):368‐376. [DOI] [PubMed] [Google Scholar]
- 34. Bos JM, Bemt PM, Kievit W, et al. A multifaceted intervention to reduce drug‐related complications in surgical patients. Br J Clin Pharmacol. 2017;83(3):664‐677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Van Gulik L, Ahlers SJ, Brkic Z, et al. Improved analgesia after the realisation of a pain management programme in ICU patients after cardiac surgery. Eur J Anaesthesiol. 2010;27(10):900‐905. [DOI] [PubMed] [Google Scholar]
- 36. Chanques G, Jaber S, Barbotte E, et al. Impact of systematic evaluation of pain and agitation in an intensive care unit. Crit Care Med. 2006;34(6):1691‐1699. [DOI] [PubMed] [Google Scholar]
- 37. Olsen BF, Rustoen T, Sandvik L, Jacobsen M, Valeberg BT. Results of implementing a pain management algorithm in intensive care unit patients: The impact on pain assessment, length of stay, and duration of ventilation. J Crit Care. 2016;36:207‐211. [DOI] [PubMed] [Google Scholar]
- 38. Taylor DM, Fatovich DM, Finucci DP, et al. Best‐practice pain management in the emergency department: A cluster‐randomised, controlled, intervention trial. Emerg Med Australas. 2015;27(6):549‐557. [DOI] [PubMed] [Google Scholar]
- 39. Moustafa F, Macian N, Giron F, Schmidt J, Pereira B, Pickering G. Intervention study with Algoplus: A pain behavioral scale for older patients in the emergency department. Pain Pract. 2017;17(5):655‐662. [DOI] [PubMed] [Google Scholar]
- 40. Baehren DF, Marco CA, Droz DE, Sinha S, Callan EM, Akpunonu P. A Statewide Prescription Monitoring Program Affects Emergency Department Prescribing Behaviors. Ann Emerg Med. 2010;56:19‐23.e3. 10.1016/j.annemergmed.2009.12.011 [DOI] [PubMed] [Google Scholar]
- 41. McAllister MW, Aaronson P, Spillane J, et al. Impact of prescription drug‐monitoring program on controlled substance prescribing in the ED. Am J Emerg Med. 2015;33(6):781‐785. [DOI] [PubMed] [Google Scholar]
- 42. Netherton SJ, Lonergan K, Wang D, McRae A, Lang E. Computerized physician order entry and decision support improves ED analgesic ordering for renal colic. Am J Emerg Med. 2014;32(9):958‐961. 10.1016/j.ajem.2014.05.002 [DOI] [PubMed] [Google Scholar]
- 43. Muntlin Å, Carlsson M, Säfwenberg U, Gunningberg L. Outcomes of a nurse‐initiated intravenous analgesic protocol for abdominal pain in an emergency department: A quasi‐experimental study. Int J Nurs Stud. 2011;48(1):13‐23. [DOI] [PubMed] [Google Scholar]
- 44. Pierik JG, Berben SA, IJzerman MJ, et al. A nurse‐initiated pain protocol in the ED improves pain treatment in patients with acute musculoskeletal pain. Int Emerg Nurs. 2016;27:3‐10. [DOI] [PubMed] [Google Scholar]
- 45. Manias E, Gibson SJ, Finch S. Testing an Educational Nursing Intervention for Pain Assessment and Management in Older People. Pain Med. 2011;12(8):1199‐1215. [DOI] [PubMed] [Google Scholar]
- 46. O'Sullivan D, O'Mahony D, O'Connor MN, et al. Prevention of Adverse Drug Reactions in Hospitalised Older Patients Using a Software‐Supported Structured Pharmacist Intervention: A Cluster Randomised Controlled Trial. Drugs Aging. 2016;33(1):63‐73. [DOI] [PubMed] [Google Scholar]
- 47. Dalleur O, Boland B, Losseau C, et al. Reduction of potentially inappropriate medications using the STOPP criteria in frail older inpatients: A randomised controlled study. Drugs Aging. 2014;31(4):291‐298. [DOI] [PubMed] [Google Scholar]
- 48. O'Connor MN, O'Sullivan D, Gallagher PF, Eustace J, Byrne S, O'Mahony D. Prevention of Hospital‐Acquired Adverse Drug Reactions in Older People Using Screening Tool of Older Persons' Prescriptions and Screening Tool to Alert to Right Treatment Criteria: A Cluster Randomized Controlled Trial. J Am Geriatr Soc. 2016;64(8):1558‐1566. [DOI] [PubMed] [Google Scholar]
- 49. Urfer M, Elzi L, Dell‐Kuster S, Bassetti S. Intervention to improve appropriate prescribing and reduce polypharmacy in elderly patients admitted to an internal medicine unit. PLoS ONE. 2016;11(11):e0166359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Boothby LA, Wang LJ, Mayhew S, Chestnutt L. Academic detailing of meperidine at a teaching hospital. Hosp Pharm. 2003;38(1):30‐35. [Google Scholar]
- 51. Shaw J, Harris P, Keogh G, Graudins L, Perks E, Thomas PS. Error reduction: academic detailing as a method to reduce incorrect prescriptions. Eur J Clin Pharmacol. 2003;59(8–9):697‐699. [DOI] [PubMed] [Google Scholar]
- 52. Akce M, Suneja A, Genord C, Singal B, Hopper JA. A multifactorial intervention for hospital opioid management. J Opioid Manag. 2014;10(5):337‐344. [DOI] [PubMed] [Google Scholar]
- 53. Paul JE, Bertram B, Antoni K, et al. Impact of a comprehensive safety initiative on patient‐controlled analgesia errors. Anesthesiology. 2010;113(6):1427‐1432. [DOI] [PubMed] [Google Scholar]
- 54. Ferguson R, Williams ML, Beard B. Combining quality improvement and staff development efforts to decrease patient‐controlled analgesia pump errors. J Nurses Staff Dev. 2010;26(5):E1‐E4. [DOI] [PubMed] [Google Scholar]
- 55. Whipple JK, Quebbeman EJ, Lewis KS, Gaughan LM, Gallup EL, Ausman RK. Identification of patient‐controlled analgesia overdoses in hospitalized patients: computerized method of monitoring adverse events. Ann Pharmacother. 1994;28(May):655‐658. [DOI] [PubMed] [Google Scholar]
- 56. McEvoy T, Moore J, Generali J. Original article: Inpatient prescribing and monitoring of fentanyl transdermal systems: Adherence to safety regulations. Hosp Pharm. 2014;49(10):942‐949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. American Pain Society Quality of Care Committee . Quality improvement guidelines for the treatment of acute pain and cancer pain. JAMA. 1995;274:1874‐1880. [DOI] [PubMed] [Google Scholar]
- 58. Zgierska A, Miller M, Rabago D. Patient Satisfaction, Prescription Drug Abuse, and Potential Unintended Consequences. JAMA. 2012;307(13):1377‐1378. 10.1001/jama.2012.419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sullivan MD, Ballantyne JC. Must we reduce pain intensity to treat chronic pain? Pain. 2016;157(1):65‐69. [DOI] [PubMed] [Google Scholar]
- 60. King N, Fraser V, Boikos C, Richardson R, Harper S. Determinants of Increased Opioid‐Related Mortality in the United States and Canada, 1990–2013: A Systematic Review. Am J Public Health. 2014;104(8):e32‐e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Beaudoin F, Merchant R, Janicki A, McKaig D, Babu K. Preventing Iatrogenic Overdose: A Review of In–Emergency Department Opioid‐Related Adverse Drug Events and Medication Errors. Ann Emerg Med. 2015;65(4):423‐431. [DOI] [PubMed] [Google Scholar]
- 62. Reitan J, Moleski R, Van Breda A, Adamson R, Lew I. Cost and Quality Implications of Opioid‐Based Postsurgical Pain Control in Total Abdominal Hysterectomy: A Study of Cost Outliers and Opioid‐Related Adverse Events. Hosp Pharm. 2012;47(11):855‐862. [Google Scholar]
- 63. Hoppe JA, Kim H, Heard K. Association of emergency department opioid initiation with recurrent opioid use. Ann Emerg Med. 2015;65(5):493‐499. e494 [DOI] [PubMed] [Google Scholar]
- 64. Thillainadesan J, Gnjidic D, Green S, Hilmer S. Impact of Deprescribing Interventions in Oldxer Hospitalised Patients on Prescribing and Clinical Outcomes: A Systematic Review of Randomised Trials. Drugs Aging. 2018;35(4):303‐319. [DOI] [PubMed] [Google Scholar]
- 65. Foy R, Leaman B, McCrorie C, et al. Prescribed opioids in primary care: cross‐sectional and longitudinal analyses of influence of patient and practice characteristics. BMJ Open. 2016;6(5):e010276 10.1136/bmjopen-2015-010276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Boudreau D, Von Korff M, Rutter CM, et al. Trends in long‐term opioid therapy for chronic non‐cancer pain. Pharmacoepidemiol Drug Saf. 2009;18(12):1166‐1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Leong M, Murnion B, Haber PS. Examination of opioid prescribing in Australia from 1992 to 2007. Intern Med J. 2009;39(10):676‐681. [DOI] [PubMed] [Google Scholar]
- 68. National Health and Medical Research Council Australia . Levels of Evidence 2008. https://www.mja.com.au/sites/default/files/NHMRC.levels.of.evidence.2008-09.pdf. Accessed December 5, 2018.


