Abstract
Objective:
To evaluate determinants of willingness to accept a treatment to return memory to normal among persons with cognitive impairment who received an amyloid PET scan and their care partner and discordance in risk taking.
Methods:
Using data from CARE-IDEAS (n=1,872 dyads), a supplement of the Imaging Dementia–Evidence for Amyloid Scanning study, we predicted scan recipient’s willingness to accept a risky treatment, the risk care partners believed their care recipient would accept, and discordance in these perceptions.
Results:
Scan recipients were willing to accept a treatment with a 27.94% (SD=34.36) risk of death. Care partners believed their care recipient would accept a 29.68% (SD=33.74) risk of death; thus, overestimating risk acceptance by 1.74 (SD=41.88) percentage points. A positive amyloid PET scan was associated with willingness to accept greater risk. Poorer functioning of the care recipient was associated with care partners believing their care recipient would accept more risk. The amyloid PET scan result was not significantly associated with discordance, but poorer functioning of the care recipient resulted in care partners overestimating risk.
Conclusions:
Scan recipients were willing to accept a treatment with a high risk of death. Discordance was affected by scan recipient’s having poorer functioning.
Keywords: dementia, family caregiving, risk taking
INTRODUCTION
Alzheimer’s disease and related dementias (ADRD) are neurodegenerative diseases that result in diminished cognitive and functional abilities.[1, 2] Due to cognitive and functional decline, persons with ADRD often must rely on care partners (e.g., a spouse) to serve as their health care proxy.[3-8]
Care partners must accurately understand and represent the preferences of the person with ADRD.[9] Empirical evaluations have identified important discrepancies in how care partners assess care preferences of their patients.[10-13] For example, randomized trials have demonstrated that care partners incorrectly interpret advance care directives of their patients and are unable to predict the preferences of an ill patient.[14, 15] Among those with cognitive impairment or ADRD, evidence suggests that care partners do not fully understand the preferences of the person with ADRD.[16, 17] This discrepancy may be due to care partners assuming that persons with ADRD are unable to state their preferences.[18, 19] This perception exists despite evidence that persons with mild to moderate cognitive impairment or ADRD are able to state their care preferences and want to participate in their own care.[20, 21]
To facilitate person-centered care, it is vital that care partners and persons with ADRD discuss care preferences.[22, 23] There is increasing enthusiasm that early diagnosis of ADRD can facilitate care preference discussions between persons with ADRD, care partners, and providers.[24] Mounting evidence supports the use of amyloid PET scans as a tool to improve ADRD diagnostic confidence.[25-27] In 2013, the Centers for Medicare and Medicaid Services provided coverage for amyloid PET scans as part of the Imaging Dementia Evidence for Amyloid Scanning (IDEAS) study.[28, 29] The goal of the IDEAS Study is to examine how amyloid PET scans guide physicians in diagnosing ADRD in a person with cognitive impairment. CARE-IDEAS is an approved supplemental study that introduces the voice of the person with cognitive impairment (i.e., scan recipient/patient) and their care partner using open-ended questions and standardized survey items on decision-making and cognitive status.
First, we used CARE-IDEAS data to evaluate scan recipients’ risk tolerance to accept a risk of death for a hypothetical treatment that could return their memory to normal. Second, we evaluated scan recipients’ risk tolerance as perceived by the care partner. Third, we evaluated discordance in risk taking between scan recipients’ risk tolerance as perceived by the care partner and the risk tolerance as stated by the scan recipient. Finally, we explored the association between risk tolerance and discordance in risk taking and an amyloid PET scan result, the scan recipient’s cognition, and the scan recipient’s everyday functioning.
METHODS
Study Setting and Participants
Details of the IDEAS Study are reported elsewhere.[28, 29] In short, the IDEAS Study recruited 18,295 Medicare beneficiaries aged ≥65 with progressive mild cognitive impairment and/or dementia of uncertain cause from 592 dementia practices over 22 months. Referring doctors believed that an amyloid PET scan could help to guide their patients’ care.
The IDEAS Study transferred the contact information of all IDEAS patients who agreed to be contacted for supplemental studies (n=12,474) to TrialMatch®, the Alzheimer’s Association’s clinical studies matching service. Trained TrialMatch® agents called these participants and described the approved IDEAS supplemental studies, including CARE-IDEAS.
Of the 3,717 IDEAS Study patients who agreed to be contacted by CARE-IDEAS, 2,228 of them and 1,872 of their care partners completed the baseline telephone interview; 1,872 dyads had an IDEAS patient (i.e., scan recipient) and care partner who both completed the baseline interview. The median time between the date of the amyloid PET scan and the CARE-IDEAS baseline interview was 4.5 months. All CARE-IDEAS recruitment and enrollment protocols and documents were approved by the Institutional Review Board at Brown University on 8/8/2016 (#1606001534).
Measures
Scan recipient and care partner sociodemographic characteristics
Both members of the dyad reported sociodemographic characteristics including date of birth, gender, race, educational attainment, and relationship to care partner/scan recipient.
Risk taking
Scan recipients were asked the following stated preference question:
“Suppose there is a new technology that can return your memory to normal but has a risk of death. What is the highest risk of death–if any–that you would be willing to accept for this treatment? The number you give can be anywhere between 0% and 100%.”
Care partners were asked to assess the risk they believed the scan recipient would accept.
In addition to evaluating total risk as stated by scan recipients and care partners, we calculated dyadic discordance operationalized as the difference between each scan recipient’s risk tolerance as perceived by their care partner and the risk tolerance expressed by the scan recipient. Positive scores indicate the care partner believes the scan recipient is willing to take on more risk than he or she reported while negative values indicate the care partner believes the scan recipient is willing to take on less risk than he or she reported.
Amyloid PET scan result
As part of the IDEAS Study, all patients received an amyloid PET scan. A radiologist at each site interpreted each patient’s scan result as positive for cortical beta-amyloid, negative for cortical beta-amyloid, or uninterpretable/technically inadequate. If the result was positive or negative, the radiologist provided a level of confidence (low, intermediate, or high) of interpretation. Disclosure of the amyloid PET scan was considered part of clinical care and not mandated or tracked as part of the IDEAS study protocol. IDEAS clinicians were encouraged to disclose the result of the amyloid PET scan in person, with a care partner present, and as soon as possible.
Cognition and Everyday Function
Cognitive performance was evaluated in the CARE-IDEAS interview by an abbreviated version of the modified Telephone Interview Cognitive Status (TICS-M) [30, 31], which was developed for telephone use in large-scale epidemiological studies; higher TICS-M scores indicate better cognition (scale range 0–41).
Additionally, the 12-item Everyday Cognition scale (ECog-12),[32] a shortened version of the 39-item version [33] was administered to care partners, to collect information about the scan recipient’s everyday functioning. The ECog-12 is designed to collect information about the level of impairment in everyday cognition and functioning. Higher ECog-12 scores indicate poorer function in everyday activities (scale range 1–4).
Planning for the person with cognitive impairments future
Both members of the dyad were asked to identify the individual most involved in helping the scan recipient make decisions about their health care in the future (care partner or someone else). In addition, both members of the dyad were asked if they have discussed the care preferences of the scan recipient were he or she to become unable to speak for himself or herself.
Statistical Analysis
We restricted the sample to all dyads with complete data on the variables of interest, and then descriptively evaluated the sample stratified by amyloid PET scan result (positive or negative). We examined the distribution of risk stated by scan recipients and care partners, as well as risk-taking discordance.
We estimated a regression model to examine the effect of the amyloid PET scan result, scan recipient’s cognitive function, and scan recipient’s everyday functioning on willingness to take risk. We estimated a two-part model to account for the large proportion of scan recipients that indicated they would accept 0% risk of death. In the first part, we estimated a logistic regression to predict if scan recipients were willing to accept >0% risk. In the second part, we estimated a linear regression to predict scan recipients’ stated risk tolerance among scan recipients willing to accept >0% risk. Both parts of the model included main effects for the amyloid PET scan result, cognitive function (the scan recipient’s TICS-M) and everyday function (ECog-12 as reported by the care partner about the person with cognitive impairment). We also controlled for scan recipients’ self-reported sociodemographic characteristics (age, gender, marital status, education, and race). We evaluated the model’s conditional and unconditional marginal effects.
We estimated a second two-part regression model to examine the association between the amyloid PET scan result and scan recipients’ cognitive function and everyday function on the risk care partners believed the care recipients would accept. We also controlled for care partners’ sociodemographic characteristics (age, gender, education, race, and relationship to scan recipient). Finally, we included an indicator for whether the care partner is the individual most involved in helping make decisions about the scan recipient’s health, and an indicator for whether the scan recipient has discussed their preferences for who should make health decision if they are no longer able to. We again evaluated the model’s conditional and unconditional marginal effects.
We estimated a third linear regression model to examine the effect of the amyloid PET scan result and scan recipients’ cognitive function and everyday function on dyadic discordance. This model also controlled for care partners’ sociodemographic characteristics, if the care partner is involved in helping make decisions about the scan recipient’s health, and if the scan recipient has discussed their preferences for their health care proxy.
All analyses were conducted using Stata version 14.2 (Stata, College Station, TX).
RESULTS
Sample Characteristics
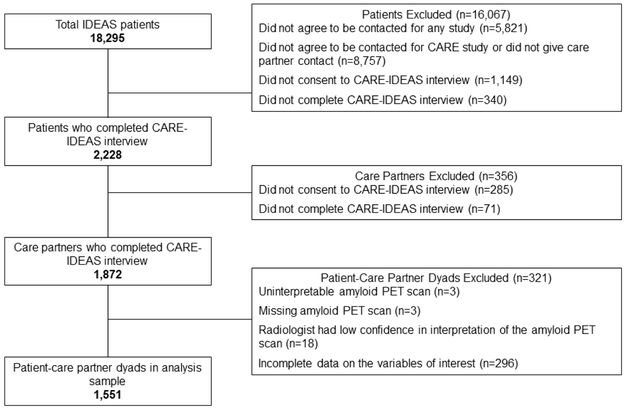
Of the 1,872 cases in which both dyads completed the baseline interview, we excluded 4 dyads in which the amyloid PET scan was uninterpretable, 3 dyads in which the amyloid PET scan result was missing, 18 dyads in which the radiologist had low confidence in the interpretation of the amyloid PET scan, and 296 dyads with incomplete data on the variables of interest (Figure 1). The analysis sample consisted of 1,551 dyads. Scan recipients excluded from the analysis had a significantly lower TICS-M score (eTable 1). There were no other significant differences between included and excluded dyads.
Figure 1.
Sample Selection
The mean age of scan recipients was 74.47 (SD = 5.55) (Table 1). Most scan recipients were male (61%), married (91%), completed college or had an advanced degree (59%), and were White (96%). Scan recipients had a mean TICS-M score of 20.77 (SD = 6.03). Scan recipients who had a positive amyloid PET scan were significantly older, had a lower TICS-M score indicating greater cognitive impairment, and were more likely to identify as White than those who had a negative scan. There were no other significant sociodemographic differences between scan recipients based on amyloid PET scan result.
Table 1.
Characteristics of Dyads by Amyloid PET Scan Result
| Total (n=1,551 dyads) |
Positive (n=1,050 dyads) |
Negative (n=501 dyads) |
Pa | |
|---|---|---|---|---|
| Scan recipient characteristics (reported by scan recipient) | ||||
| Mean risk willing to accept (SD) | 27.94 (34.36) | 29.85 (34.76) | 23.96 (33.19) | 0.002 |
| Mean age (SD), y | 74.47 (5.55) | 74.77 (5.5) | 73.85 (5.6) | 0.002 |
| Mean TICS-M (SD) | 20.77 (6.03) | 19.87 (6) | 22.65 (5.67) | <0.001 |
| Female, n (%) | 601 (38.75) | 417 (39.71) | 184 (36.73) | 0.259 |
| Marital status, n (%) | 0.232 | |||
| Married/living with a partner | 1415 (91.23) | 961 (91.52) | 454 (90.62) | |
| Separated/divorced | 49 (3.16) | 34 (3.24) | 15 (2.99) | |
| Widowed | 71 (4.58) | 48 (4.57) | 23 (4.59) | |
| Never married | 16 (1.03) | 7 (0.67) | 9 (1.8) | |
| Education, n (%) | 0.193 | |||
| High school graduate or less | 243 (15.67) | 174 (16.57) | 69 (13.77) | |
| Some college | 395 (25.47) | 252 (24) | 143 (28.54) | |
| Bachelor's degree | 364 (23.47) | 251 (23.9) | 113 (22.55) | |
| Advanced degree | 549 (35.4) | 373 (35.52) | 176 (35.13) | |
| Race, n (%) | 0.021 | |||
| White | 1491 (96.13) | 1013 (96.48) | 478 (95.41) | |
| African American | 27 (1.74) | 12 (1.14) | 15 (2.99) | |
| Other | 33 (2.12) | 25 (2.38) | 8 (1.58) | |
| Care partner characteristics (reported by care partner) | ||||
| Mean risk believe scan recipient will accept (SD) | 29.68 (33.74) | 32.23 (33.98) | 24.31 (32.62) | <0.001 |
| Mean age (SD), y | 69.99 (9.48) | 70.21 (9.49) | 69.52 (9.45) | 0.179 |
| Mean care partner reported ECog-12 for scan recipient (SD) | 2.21 (0.71) | 2.26 (0.7) | 2.11 (0.71) | <0.001 |
| Female, n (%) | 1060 (68.34) | 713 (67.9) | 347 (69.26) | 0.591 |
| Marital status, n (%) | 0.012 | |||
| Married/living with a partner | 1475 (95.1) | 1007 (95.9) | 468 (93.41) | |
| Separated/divorced | 29 (1.87) | 17 (1.62) | 12 (2.4) | |
| Widowed | 16 (1.03) | 5 (0.48) | 11 (2.2) | |
| Never married | 31 (2) | 21 (2) | 10 (2) | |
| Education, n (%) | 0.529 | |||
| High school graduate or less | 222 (14.31) | 155 (14.76) | 67 (13.37) | |
| Some college | 434 (27.98) | 295 (28.1) | 139 (27.74) | |
| Bachelor's degree | 427 (27.53) | 295 (28.1) | 132 (26.35) | |
| Advanced degree | 468 (30.17) | 305 (29.05) | 163 (32.53) | |
| Race, n (%) | <0.003 | |||
| White | 1490 (96.07) | 1017 (96.86) | 473 (94.41) | |
| African American | 25 (1.61) | 9 (0.86) | 16 (3.19) | |
| Other | 36 (2.32) | 24 (2.29) | 12 (2.4) | |
| Relationship to scan recipient, n (%) | 0.227 | |||
| Spouse | 1378 (88.85) | 931 (88.67) | 447 (89.22) | |
| Child | 121 (7.8) | 88 (8.38) | 33 (6.59) | |
| Other | 52 (3.35) | 31 (2.95) | 21 (4.19) | |
| Out of all the scan recipient’s family and friends who is the one person most involved in helping make decisions about their health care if they are no longer able, n (%) | 0.471 | |||
| Care partner | 1485 (95.74) | 1008 (96) | 477 (95.21) | |
| Someone else | 66 (4.26) | 42 (4) | 24 (4.79) | |
| The scan recipient has discussed their preferences for who should make health care decision if they are no longer able to make it, n (%) | 0.269 | |||
| Yes | 1370 (88.33) | 934 (88.95) | 436 (87.03) | |
| No | 181 (11.67) | 116 (11.05) | 65 (12.97) | |
Comparison between positive and negative amyloid PET scan
The mean age of care partners was 69.99 (SD = 9.48) (Table 1). Care partners were predominately female (68%), married (95%), completed college or had an advanced degree (57%), White (96%), and were the scan recipient’s spouse (89%). In addition, care partners identified as the individual most involved with the scan recipient’s care (95%), and most care partners had spoken to their care recipient about his or her care preferences (88%). Care partners reported a mean ECog-12 of 2.21 (SD = 0.71) for the scan recipient. Care partners of scan recipients’ with a positive amyloid PET scan reported a significantly higher ECog-12 (i.e., poorer everyday function) than care partners of scan recipients’ who had a negative amyloid PET scan. In addition, care partners of scan recipients who had a positive amyloid PET scan were more likely to be married and White than care partners of scan recipients who had a negative amyloid PET scan. There were no other significant sociodemographic differences between care partners based on amyloid PET scan result.
Scan Recipients’ Willingness to Accept Risky Treatment
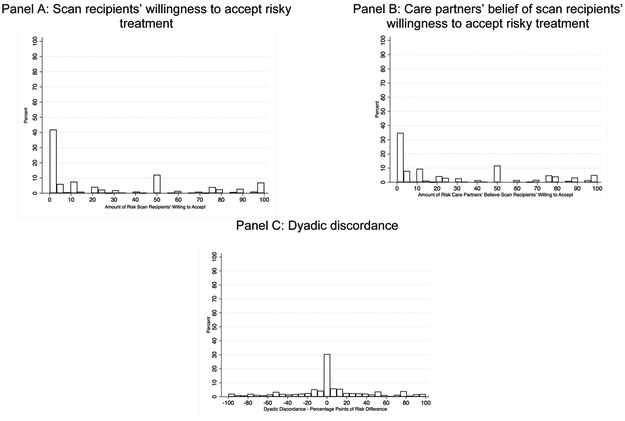
There was variation in the amount of risk scan recipients were willing to accept with 36% of scan recipients indicating they would accept 0% risk of death, 43% indicating they would accept >0–50% risk, and 21% indicating they would accept >50% risk (Figure 2 Panel A). Scan recipients on average were willing to accept a treatment that carried a 27.94% (SD = 34.36) risk of death. Scan recipients that were willing to accept >0% risk of death on average accepted a treatment that carried a 43.52% (SD = 34.07) risk of death (median risk of death = 50%).
Figure 2.
Distribution of responses to risky treatment question
In the first part of the two-part model, a positive amyloid PET scan and a higher TICS-M score were significantly associated with an increased likelihood of a scan recipient being willing to accept the risky treatment (Table 2). In the second part of the two-part model, a positive amyloid PET scan and a decrease in the TICS-M were significantly associated with greater risk taking. The unconditional marginal effects indicated that having a positive amyloid PET scan was associated with scan recipients willing to take 5.62 (95% CI: 1.98, 9.25) more percentage points of a risk of death than someone with a negative scan. Furthermore, the unconditional marginal effects indicated that cognition (−0.19, 95% CI: −0.51, 0.13) and everyday function (1.64, 95% CI: −1.03, 4.32) were not significantly associated with the scan recipient’s self-assessed risk taking.
Table 2.
Marginal Effects of Amyloid PET Scan, Cognition, and Everyday Function on Risk Taking (n=1,551 dyads)
| Model 1: Scan recipient willingness to accept risk |
Model 2: Risk care partner believes scan recipient will accept |
Model 3: Risk taking Discordancea (n=1,551) |
|||||
|---|---|---|---|---|---|---|---|
| Part 1: Probability willing to accept >0 risk (n=1,551) |
Part 2: Amount of risk conditional on willing to accept >0 (n=996) |
Unconditional Marginal Effect (95% CI) |
Part 1: Probability willing to accept >0 risk (n=1,551) |
Part 2: Amount of risk conditional on willing to accept >0 (n=1,130 |
Unconditional Marginal Effect (95% CI) |
Marginal Effect (95% CI) |
|
| Conditional Marginal Effect (95% CI) |
Conditional Marginal Effect (95% CI) |
Conditional Marginal Effect (95% CI) |
Conditional Marginal Effect (95% CI) |
||||
| Amyloid PET scan positive (ref = negative) | 0.05*
(0.01, 0.10) |
4.97*
(0.37, 9.57) |
5.62**
(1.98, 9.25) |
0.12***
(0.07, 0.17) |
4.01 (−0.33, 8.34) |
7.62***
(4.07, 11.18) |
2.58 (−2.00, 7.17) |
| Scan recipient characteristics (reported by scan recipient) | |||||||
| TICS-M | 0.01**
(0.00, 0.01) |
−0.73***
(−1.12, −0.33) |
−0.19 (−0.51, 0.13) |
0.00 (0.00, 0.01) |
−0.05 (−0.41, 0.31) |
0.05 (−0.27, 0.36) |
0.21 (−0.18, 0.60) |
| Age, y | 0.00 (−0.01, 0.00) |
0.29 (−0.10, 0.69) |
−0.01 (−0.32, 0.31) |
||||
| Female | −0.10***
(−0.16, −0.05) |
4.89*
(0.17, 9.61) |
−1.52 (−5.30, 2.49) |
||||
| Marital status (ref = married/living with partner) | |||||||
| Separated/divorced | 0.16**
(0.04, 0.27) |
−14.37*
(−25.60, −3.14) |
−4.15 (−13.68, 5.39) |
||||
| Widowed | 0.10 (−0.01, 0.20) |
−3.14 (−13.37, 7.10) |
2.21 (−6.46, 10.87) |
||||
| Never married | 0.09 (−0.12, 0.30) |
−9.78 (−29.89, 10.33) |
−2.93 (−19.29, 13.43) |
||||
| Education (ref = high school graduate or less) | |||||||
| Some college | 0.10*
(0.02, 0.17) |
−0.58 (−7.66, 6.50) |
4.22 (−1.44, 9.88) |
||||
| Bachelor's degree | 0.08*
(0.00, 0.16) |
−3.86 (−11.09, 3.37) |
1.49 (−4.22, 7.21) |
||||
| Advanced degree | 0.07 (0.00, 0.15) |
−7.41*
(−14.31, −0.50) |
−1.36 (−6.74, 4.01) |
||||
| Race (ref = White) | |||||||
| African American | 0.08 (−0.10, 0.25) |
−4.90 (−20.05, 10.24) |
0.06 (−12,89, 13.01) |
||||
| Other | 0.07 (−0.09, 0.23) |
3.29 (−10.26, 16.83) |
5.39 (−6.82, 17.60) |
||||
| Care partner characteristics (reported by care partner) | |||||||
| ECog-12 reported for person with cognitive impairment | 0.03 (−0.01, 0.06) |
0.71 (−2.57, 3.98) |
1.64 (−1.03, 4.32) |
0.06***
(0.02, 0.09) |
3.99*
(0.91, 7.07) |
5.20***
(2.56, 7.83) |
3.42*
(0.11, 6.72) |
| Age, y | −0.01*
(−0.01, 0.00) |
0.57***
(0.30, 0.85) |
0.28*
(0.04, 0.52) |
0.28 (−0.02, 0.58) |
|||
| Female | −0.02 (−0.07, 0.03) |
4.09 (−0.15, 8.32) |
2.24 (−1.38, 5.87) |
3.54 (−1.08, 8.16) |
|||
| Education (ref = high school graduate or less) | |||||||
| Some college | 0.04 (−0.03, 0.12) |
−2.77 (−9.11, 3.56) |
0.00 (−5.57, 5.57) |
−3.32 (−10.09, 3.46) |
|||
| Bachelor's degree | 0.01 (−0.06, 0.09) |
−7.74*
(−14.12, −1.36) |
−4.83 (−10.34, 0.68) |
−7.16*
(−13.96, −0.37) |
|||
| Advanced degree | 0.06 (−0.01, 0.13) |
−10.51*** (−16.77, −4.25) |
−5.23 (−10.67, 0.22) |
−3.32 (−10.04, 3.41) |
|||
| Race (ref = White) | |||||||
| African American | −0.03 (−0.21, 0.15) |
12.17 (−3.63, 27.98) |
7.28 (−7.12, 21.68) |
10.20 (−6.50, 26.91) |
|||
| Other | 0.02 (−0.13, 0.16) |
1.75 (−10.82, 14.31) |
1.96 (−9.19, 13.12) |
3.18 (−10.69, 17.05) |
|||
| Relationship to scan recipient (ref = spouse) | |||||||
| Child | −0.09 (−0.22, 0.03) |
2.11 (−7.41, 11.63) |
−2.53 (−10.62, 5.57) |
−1.07 (−11.41, 9.27) |
|||
| Other | 0.08 (−0.07, 0.14) |
10.35 (−0.15, 20.84) |
11.51*
(1.28, 21.74) |
13.69*
(1.52, 25.85) |
|||
| Someone else is the one person most involved in helping make decisions about the scan recipient’s health care if they are no longer able (ref = care partner) | 0.03 (−0.07, 0.14) |
8.42 (−0.48, 17.33) |
|||||
| Yes, the scan recipient has discussed their preferences for who should make health care decision if they are no longer able to make it (ref = no) | 0.06 (−0.01, 0.13) |
9.30 (−0.24, 18.84) |
0.58 (−4.68, 5.84) |
11.28*
(0.71, 21.84) |
|||
CI: Confidence interval
risk-taking discordance = care partner risk – scan recipient risk
p<0.05;
p<0.01;
p<0.001
Care Partners’ Belief of Scan Recipients’ Willingness to Accept Risky Treatment
As with scan recipients, there was variation in the risk care partners believed their care recipient would accept (Figure 2 Panel B). Approximately 27% of care partners believed their care recipient would accept 0% risk of death, 50% believed their care recipient would accept >0–50% risk, and 23% believed their care recipient would accept >50% risk. On average, care partners believed their care recipient would accept a treatment that carried a 29.68% (SD = 33.74) risk of death. Among care partners that believed their care recipient would accept >0% risk, they believed their care recipient would accept a treatment that carried a 40.73% (SD = 33.35) risk of death (median risk of death = 30%).
In the first part of the two part-model, a positive amyloid PET scan and a higher ECog-12 score were significantly associated with an increased likelihood of care partners believing their care recipient would be being willing to accept the risky treatment (Table 2). In the second part of the two-part model, the amyloid PET scan and the scan recipients’ cognition were not significantly associated with care partners’ perception of their care recipient’s willingness to take risk. However, a higher ECog-12, which indicates poorer everyday functioning, was significantly associated with care partners believing their care recipient would accept additional risk. The unconditional marginal effects indicated that having a positive amyloid PET scan was associated with care partners believing their care recipient would accept 7.62 (95% CI: 4.07, 11.18) additional percentage points of a risk of death than care partners of care recipients with a negative scan. In addition, a one-point increase on the ECog-12 was associated with care partners believing the care recipient would accept even more mortality risk (5.20 percentage points higher; 95% CI: 2.56, 7.07). Scan recipients’ cognition (0.05, 95% CI: −0.27, 0.36) was not significantly associated with perceived risk taking.
Dyadic Discordance
Approximately 24% of dyads agreed precisely on the amount risk that would be accepted, 35% of care partners underestimated the amount of risk their care recipient would accept, and 41% of care partners overestimated the amount of risk their care recipient would accept (Figure 2 Panel C). Nearly 23% of care partners overestimated the amount of risk their care recipient would accept by ≥25 percentage points. Within dyads, care partners believed scan recipients would accept on average 1.74 (SD = 41.88) more percentage points of risk (a 6% increase over the scan recipient’s self-assessed risk) than was actually the case.
The amyloid PET scan result (2.58, 95% CI: −2.00, 7.17) and TICS-M (0.21, 95% CI: −0.18, 0.60) were not significantly associated with risk-taking discordance (Table 2). However, worse functioning based on the ECog-12 was associated with care partners overestimating the scan recipient’s mortality risk acceptance by 3.42 (95% CI: 0.11, 6.27) percentage points, which represents a 12% increase in risk relative to the sample mean.
DISCUSSION
To our knowledge, our study is the first to understand the association between receiving substantial evidence in support of a diagnosis of Alzheimer’s disease and willingness to undergo a risky treatment. Our study supports conclusions in the literature that there is variation in care partners’ understanding of their care recipients’ preferences.[16, 17, 34, 35] On average, care partners only overestimated their care recipients’ risk taking by 1.74 percentage points; 35% of care partners underestimated their care recipients’ risk taking and 41% of care partners over estimated their care recipients’ risk taking, many substantially (SD=41.88) with ~23% of care partners overestimating risk taking by ≥25 percentage points. Discordance in risk taking was substantially greater when care partners perceived their care recipients having greater limitations in performing everyday tasks. As care partners serve as a link between the person with cognitive impairment and the health system, discordance in understanding preferences can have significant implications beyond risk taking for hypothetical treatments.
While scan recipients were willing to accept a treatment that carried a high absolute risk of death (27.94%), this risk is likely magnitudes larger than the risk of adverse events that would be associated with any actual intervention. Our findings on the total risk of death are similar to other studies that have evaluated risk for treatments that prevent cognitive impairment.[34] In general, risk taking to prevent cognitive decline or ADRD is higher than for many other chronic conditions. For example, patients with Crohn’s disease and multiple sclerosis both indicate they are willing to accept <1% risk of death for a treatment to prevent disease progression.[36, 37] The willingness of persons with cognitive impairment to accept a large risk to restore memory is likely due to the progressive nature of cognitive decline combined with loss of independence.[1, 2]
From a clinical perspective, an amyloid PET scan increases diagnostic confidence and influences clinical practice.[25-27] We were unable to determine how providers conveyed the meaning of the amyloid PET scan result to families. Among multiple factors, the method of delivering the scan result (via phone or in person and by clinical or non-clinical staff), and the information accompanying the scan result (e.g., describing management strategies) would likely influence scan recipients and care partners stated risk taking. It appears, as evidenced by a willingness to accept greater risk, both scan recipients and their care partners understand that a positive amyloid PET scan is associated with greater confidence that the Alzheimer’s disease diagnosis is definitive.
For scan recipients, cognition and the ability to perform everyday functional tasks were not significantly associated with risk taking. However, poorer everyday functioning was significantly associated with care partners believing their patient would accept greater risk and with care partners overestimating the care recipient’s willingness to take risk. When care partners believe their care recipient has more functional limitations then care partners may also believe that their care recipients are unable to state their own preferences.[16, 17, 21, 38]
As persons with cognitive impairment often rely on care partners to communicate their preferences to the health care system, the effects of discordance between a patient and care partner could be far reaching.[7] In terms of care, our results suggest that care partners are more aggressive with treatment that carriers a risk of death compared to care recipients. Care partners advocate for even more aggressive care when they perceive their patient has greater everyday functional limitations. This may also have implications for clinical research in which patients may be nudged by care partners and providers to volunteer for trials in which risks are not well understood.
Our study has several limitations. First, we asked individuals to assess risk taking in the context of a hypothetical treatment. Actual risk taking would likely differ if interventions were available due price, adverse events, and method of delivery. Second, our findings may not be generalizable to more severe stages of cognitive impairment. The sample TICS-M and ECog-12 scores both indicated scan recipients had mild cognitive impairment or mild dementia. Third, this is a cross-sectional study so we cannot identify the causal effect of receiving an amyloid PET scan on change in total risk taking and change in discordance in risk taking following a scan. Fourth, our sample is not diverse, and most participants were white (96%) and highly educated (58% had a bachelors or advanced degree). Whites are generally more risk adverse than African Americans; however, those with greater levels of education are on average willing to accept more risk.[37] In the context of our study, it is unclear whether the estimates of risk taking, which are large in absolute terms, are smaller or larger than expected in the general population of persons with progressive mild cognitive impairment and/or dementia.
In conclusion, many persons with cognitive impairment appear to be willing to accept a substantial amount of risk for a hypothetical treatment that could return their memory to normal. Approximately 21% of scan recipients indicated they would accept >50% chance of risk for a treatment that could return their memory to normal. A positive amyloid PET scan was significantly associated with greater risk taking while merely having poor cognition was not. Discordance in risk taking between care partners and patients was not affected by diagnostic results but was affected by a scan recipient’s having poorer everyday functioning. Amyloid PET scans have the potential to serve as a tool to facilitate care preference discussions, but the delivery of scan results should also include assessments and discussions that provide a thorough understanding of the patient’s functional abilities.
Supplementary Material
Acknowledgements:
The authors acknowledge Faye Dvorchak and Kathleen Nye for their support in managing the CARE-IDEAS study. The authors also acknowledge helpful comments and advice from Dr. Reed Johnson, Dr. Brian McGarry, and the participants at ASHEcon 2018, Clemson. Finally, the authors acknowledge the caregivers and patients affiliated with the Duke Bryan Center for helping us pilot test the risky treatment questions and the participants in the CARE-IDEAS Study. This study, entitled Caregivers’ Reactions and Experiences: Imaging Dementia-Evidence for Amyloid Scanning (CARE IDEAS) was funded by National Institute on Aging, NIH, 1R56AG053934-01, National Institute on Aging, NIH, 5R01AG053934-02. Van Houtven received support from the Center for Innovation to Accelerate Discovery and Practice Transformation, at the Durham VA Healthcare System (CIN 13–410). We have deposited specific information regarding Stata code used in the analyses in an electronic repository as a guide for investigators who obtain the requisite data use agreement for the relevant data sources and want to replicate our study (https://repository.library.brown.edu/studio/item/bdr:847518/).
This study was supported by a grant from National Institute on Aging (Grant # 1R01AG053934–01A1)
References
- 1.Jutkowitz E, MacLehose RF, Gaugler JE, et al. Risk Factors Associated With Cognitive, Functional, and Behavioral Trajectories of Newly Diagnosed Dementia Patients. J Gerontol A Biol Sci Med Sci. 2017;72:251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alzheimer's Association. 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 2016;12:459–509. [DOI] [PubMed] [Google Scholar]
- 3.Wolff JL, Feder J, Schulz R. Supporting Family Caregivers of Older Americans. N Engl J Med. 2016;375:2513–2515. [DOI] [PubMed] [Google Scholar]
- 4.Wolff JL, Spillman BC, Freedman VA, et al. A National Profile of Family and Unpaid Caregivers Who Assist Older Adults With Health Care Activities. JAMA Intern Med. 2016;176:372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brodaty H, Donkin M. Family caregivers of people with dementia. Dialogues Clin Neurosci. 2009;11:217–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jutkowitz E, Kane RL, Gaugler JE, et al. Societal and Family Lifetime Cost of Dementia: Implications for Policy. J Am Geriatr Soc. 2017;65:2169–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elliott BA, Gessert CE, Peden-McAlpine C. Family decision-making in advanced dementia: narrative and ethics. Scand J Caring Sci. 2009;23:251–258. [DOI] [PubMed] [Google Scholar]
- 8.Hirschman KB, Xie SX, Feudtner C, et al. How does an Alzheimer’s disease patient’s role in medical decision making change over time? J Geriatr Psychiatry Neurol. 2004;17:55–60. [DOI] [PubMed] [Google Scholar]
- 9.Kitwood T Dementia reconsidered: The person comes first. Buckingham, England: Open University Press Buckingham; 1997. [Google Scholar]
- 10.Carpenter BD, Lee M, Ruckdeschel K, et al. Adult children as informants about parent’s psychosocial preferences. Fam Relat. 2006;55:552–563. [Google Scholar]
- 11.Roberto KA. Making critical health care decisions for older adults: Consensus among family members. Fam Relat. 1999;48:167–175. [Google Scholar]
- 12.Mattimore TJ, Wenger NS, Desbiens NA, et al. Surrogate and physician understanding of patients’ preferences for living permanently in a nursing home. J Am Geriatr Soc. 1997;45:818–824. [DOI] [PubMed] [Google Scholar]
- 13.Heid AR, Bangerter LR, Abbott KM, et al. Do Family Proxies Get It Right? Concordance in Reports of Nursing Home Residents’ Everyday Preferences. J Appl Gerontol. 2017;36:667–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fagerlin A, Ditto PH, Hawkins NA, et al. The Use of Advance Directives in End-of-life Decision Making, Problems and Possibilities. Am Behav Sci. 2016;46:268–283. [Google Scholar]
- 15.Ditto PH, Danks JH, Smucker WD, et al. Advance directives as acts of communication: a randomized controlled trial. Arch Intern Med. 2001;161:421–430. [DOI] [PubMed] [Google Scholar]
- 16.Reamy AM, Kim K, Zarit SH, et al. Understanding discrepancy in perceptions of values: individuals with mild to moderate dementia and their family caregivers. Gerontologist. 2011;51:473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitlatch CJ, Piiparinen R, Feinberg LF. How well do family caregivers know their relatives’ care values and preferences? Dementia. 2009;8:223–243. [Google Scholar]
- 18.Feinberg LF, Whitlatch CJ. Are persons with cognitive impairment able to state consistent choices? Gerontologist. 2001;41:374–382. [DOI] [PubMed] [Google Scholar]
- 19.Menne HL, Whitlatch CJ. Decision-making involvement of individuals with dementia. Gerontologist. 2007;47:810–819. [DOI] [PubMed] [Google Scholar]
- 20.Hirschman KB, Joyce CM, James BD, et al. Do Alzheimer’s Disease Patients Want to Participate in a Treatment Decision, and Would Their Caregivers Let Them? Gerontologist. 2005;45:381–388. [DOI] [PubMed] [Google Scholar]
- 21.Clark PA, Tucke SS, Whitlatch CJ. Consistency of information from persons with dementia: An analysis of differences by question type. Dementia. 2008;7:341–358. [Google Scholar]
- 22.Miller LM, Whitlatch CJ, Lyons KS. Shared decision-making in dementia: A review of patient and family carer involvement. Dementia (London). 2016;15:1141–1157. [DOI] [PubMed] [Google Scholar]
- 23.Brechling BG, Schneider CA. Preserving autonomy in early stage dementia. J Gerontol Soc Work. 1993;20:17–33. [Google Scholar]
- 24.Borson S, Frank L, Bayley PJ, et al. Improving dementia care: the role of screening and detection of cognitive impairment. Alzheimers Dement. 2013;9:151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ossenkoppele R, Prins ND, Pijnenburg YA, et al. Impact of molecular imaging on the diagnostic process in a memory clinic. Alzheimers Dement. 2013;9:414–421. [DOI] [PubMed] [Google Scholar]
- 26.Sánchez-Juan P, Ghosh PM, Hagen J, et al. Practical utility of amyloid and FDG-PET in an academic dementia center. Neurology. 2014;82:230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Wilde A, van der Flier WM, Pelkmans W, et al. , Association of Amyloid Positron Emission Tomography With Changes in Diagnosis and Patient Treatment in an Unselected Memory Clinic Cohort: The ABIDE Project. JAMA Neurol. 2018;75:1062–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.iDEAS: Imaging Dementia--Evidence for Amyloid Scanning: Curious about the status of the IDEAS Study? 2018. Available at: https://www.ideas-study.org/. Accessed July 31, 2018.
- 29.Rabinovici GD, et al. , Association of Amyloid Positron Emission Tomography With Subsequent Change in Clinical Management Among Medicare Beneficiaries With Mild Cognitive Impairment or Dementia. JAMA, 2019. 321(13): p. 1286–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallo JJ, Breitner JC. Alzheimer’s disease in the NAS–NRC Registry of aging twin veterans, IV. Performance characteristics of a two-stage telephone screening procedure for Alzheimer’s dementia. Psychol Med. 1995;25:1211–1219. [DOI] [PubMed] [Google Scholar]
- 31.Welsh KA, Breitner JCS, Magruder-Habib KM. Detection of Dementia in the Elderly Using Telephone Screening of Cognitive Status. Neuropsychiatry Neuropsychol Behav Neurol. 1993;6:103–110. [Google Scholar]
- 32.Tomaszewski Farias S, Mungas D, Harvey DJ, et al. The measurement of everyday cognition: development and validation of a short form of the Everyday Cognition scales. Alzheimers Dement. 2011;7:593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Farias ST, Mungas D, Reed BR, et al. The measurement of everyday cognition (ECog): scale development and psychometric properties. Neuropsychology. 2008;22:531–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hauber AB, Johnson FR, Fillit H, et al. Older Americans’ risk-benefit preferences for modifying the course of Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23:23–32. [DOI] [PubMed] [Google Scholar]
- 35.Oremus M, Tarride JE, Pullenayegum E, et al. Caregivers’ willingness-to-pay for Alzheimer’s disease medications in Canada. Dementia (London). 2015;14:63–79. [DOI] [PubMed] [Google Scholar]
- 36.Johnson FR, Ozdemir S, Mansfield C, et al. Crohn’s Disease Patients’ Risk-Benefit Preferences: Serious Adverse Event Risks Versus Treatment Efficacy. Gastroenterology. 2007;133:769–779. [DOI] [PubMed] [Google Scholar]
- 37.Johnson FR, et al. , Multiple sclerosis patients’ benefit-risk preferences: serious adverse event risks versus treatment efficacy. J Neurol, 2009. 256(4): p. 554–62. [DOI] [PubMed] [Google Scholar]
- 38.Loewenstein DA, Argüelles S, Bravo M, et al. Caregivers’ judgments of the functional abilities of the Alzheimer’s disease patient: a comparison of proxy reports and objective measures. J Gerontol B Psychol Sci Soc Sci. 2001;56:P78–P84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.