Abstract
OBJECTIVE:
Subjective memory complaints (SMCs) are associated with MCI and dementia, but are understudied in African Americans (AA). We compared SMC endorsement in White and AA participants and evaluated predictors of diagnostic progression.
METHODS:
Initial visit variables, including SMC and memory performance, were compared within a cognitively normal race-matched sample of White and AA participants (Ntotal = 912; 456each race) to assess the presence and predictors of SMC, the predictors of future diagnostic progression, and the change in memory performance over time.
RESULTS:
More White (32.9%) than AA (24.3%) participants reported SMC (P < .01, phi = −.10). SMC was predicted by memory performance (B=−.03, SE=.013, OR=.968, P<.05) and race (B=−.99, SE=.080, OR=.373, P<.001). SMCs and memory performance were associated with progression, χ2 (3, n = 912) = 102.37, P < .001. AA race (−2.05 ± 0.24 SE) and SMC (−0.45 ± 0.21 SE) were associated with worse memory performance at baseline and over time, (χ2 (3) = 13.54, P < 0.01).
CONCLUSIONS:
In contrast to previous research, our study found that SMC is associated with diagnostic progression and objective memory declines in both White and AA participants.
Keywords: subjective memory complaints, diagnostic progression, African American
1. INTRODUCTION
Initial signs and symptoms of disease that are noticed by affected individuals may be the first motivators for initiating contact with a healthcare provider. Subjective memory complaints (SMCs) are common during the aging process and may portend cognitive decline in an individual, with increased numbers of SMCs associated with an increased risk for cognitive impairment.1 At an initial clinic visit, individuals with SMCs may have a five-times greater risk of developing future dementia2, and highly educated individuals with subjective memory impairments may be at an even higher risk of subsequently converting to Alzheimer’s disease (AD).3 SMCs are associated with worse performance on neuropsychological measures of both episodic and working memory.4,5 In addition, SMCs are correlated with Alzheimer’s brain pathology, including elevated amyloid deposition,6 decreased whole brain volume, and decreased volume in the entorhinal cortex.7,8
The presence and predictive utility of SMCs within African Africans is under-researched. Few studies examine SMCs in African Americans, and fewer still have contrasted differences in clinical presentation between African Americans and Whites. Of the existing research examining SMC endorsement, differences in sample, methodological approach, and cognitive tests have resulted in a lack of consensus about the frequency of endorsement by race and the relationship between cognitive complaints and disease progression.9 There are also study differences in the use and treatment of SMCs versus the broader category of subjective cognitive concerns, which may be contributing to confusion in the literature.10 While some samples show roughly equal rates of subjective cognitive concern between Caucasian and African American participants11, other studies suggest that African Americans are more likely to view AD as an inevitable part of aging12 and less likely to endorse SMCs to their providers.13,14 Knowledge about AD may be lower among minority samples.15 African Americans may utilize and receive healthcare differently than their White counterparts, resulting in different symptom reporting patterns.16,17
The majority of previous research evaluating the efficacy of subjective complaints within African Americans has found little or no evidence of a relationship between subjective complaints and either cognitive performance or diagnosis. In a cross-sectional analysis of clinically normal Caucasian and African American participants, subjective cognitive concerns were associated with objective memory performance in Caucasians, but not African Americans.11 Similarly, memory complaint at baseline was associated with declines in cognitive function for Whites, but not Blacks within a racially mixed community sample.13 Within an exclusively African American sample from Baltimore, subjective memory was predicted by psychological well-being, but was not related to neuropsychological performance scores on measures of verbal episodic memory or working memory.18 These findings suggest that SMCs may not be useful when evaluating African American patients.
In the current study, we evaluated predictors of SMCs in cognitively normal White and African American participants at their initial visit to NIA funded Alzheimer’s Disease Centers (ADCs). To help clarify the uncertain relationship between race and SMCs, we chose to examine SMCs within both cross-sectional and longitudinal analyses within a national sample. We created a race-matched sample to decrease the influence of demographic factors such as age, sex, and education, on symptom reporting and performance. We also considered multiple cognitive and psychological measures of functioning. Consistent with previous literature, we hypothesized that a higher proportion of White participants would report SMCs. We also examined the relationship between SMCs in White and African American participants at initial visit and later cognitive impairment, as measured by cognitive diagnosis at the third annual visit. We hypothesized that endorsement of SMCs would be associated with diagnostic progression in White, but not African American participants. Finally, we evaluated memory performance scores over time as a function of both race and SMCs. We hypothesized that SMC endorsement would be associated with memory declines in White, but not African American participants.
2. METHODS
We obtained archival data from the National Alzheimer’s Coordinating Center (NACC).19 Variables were identified from the NACC Uniform Data Set (UDS), which includes relevant neuropsychological, behavioral, medical, and health history information necessary for the longitudinal diagnosis and tracking of neurodegenerative diseases.20 Participant written consent was obtained using forms approved by the institutional review boards at each ADC.
2.1. Participants and sample selection
We requested data for White and African American participants diagnosed as cognitively normal at initial visit by their ADC clinicians and for whom there were at least three consecutive annual visits. A diagnosis of ‘normal cognition’ requires that neuropsychological test scores are within expectation for age and that persons are able to independently perform instrumental activities of daily living. The present sample participated in an initial visit between June 2005 and February 2013 and completed three total annual visits prior to the NACC data freeze in December 2016. Only participants whose primary language was English were included. A change in the NACC neuropsychological battery involving the replacement of several tests and the addition of others was implemented in spring of 2015 with UDS Version 3. To minimize the effects of changes in the neuropsychological battery, only those participants with data from UDS Version 2 were included. A sample of 3,232 individuals, with 2,751 White and 481 African American participants, met the above criteria. This sample contained data from 31 ADCs.
Because of a greater number of White participants in NACC, we utilized a race-matched sample to evaluate the significance and relative contributions of predictor variables within each race, independent of sample size. To control for factors associated with diagnostic progression, race was treated as the primary exposure variable with African American and White participants matched on age, sex, and education. Creation of the matched sample was performed with the Case Control Matching function in SPSS Version 24,21 which iteratively matches at random eligible cases from the larger original sample according to a pre-specified tolerance level. For sex, tolerance was set to 0, requiring matched pairs to represent identical matches; for age and education, tolerance was set to 1, allowing matched pairs to differ by a value of 1, if necessary, on both continuous variables. The matching procedure resulted in a selected sample (N = 912) that contained 456 participants of each race. Figure 1 depicts participant flow into the study sample.
Figure 1.
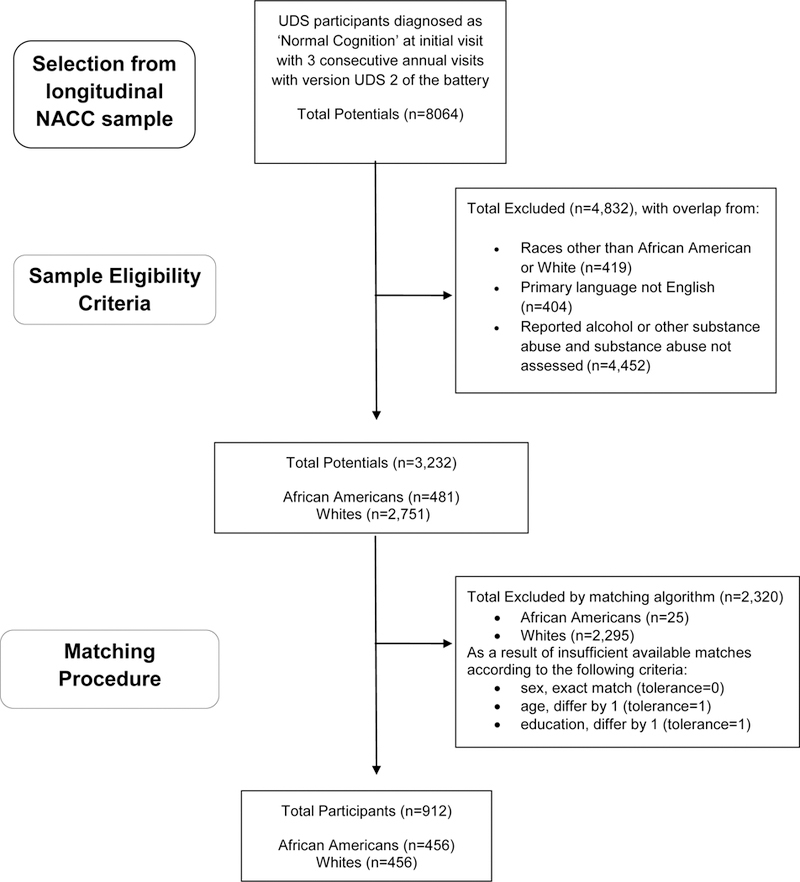
Sample selection from larger available NACC sample.
2.2. Measures
2.2.1. Subjective Memory Complaint (SMC).
At each annual visit to an ADC, participants are asked about their subjective experience of memory decline, relative to previous abilities, as part of the Clinician Judgment of Symptoms form within the UDS.
Self - Does the subject report a decline in memory (relative to previously attained abilities)?
2.2.2. Model Predictors.
Objective memory performance was assessed through the Logical Memory Delayed Recall (LM) score taken from the Wechsler Memory Scale-III,22 as well as through the Mini-Mental State Exam (MMSE), a 30-point global cognitive screening measure.23 Other model predictors included the Geriatric Depression Scale - 15 (GDS), a self-report measure of depression symptomatology,24 and the Hachinski Ischemia Scale (HIS), a clinical rating assessing cerebrovascular disease burden.25
2.3. Data analysis
Data analyses were completed in IBM SPSS Statistics Version 2421 and R Version 3.4.1.26 Frequencies of SMC at initial visit were evaluated with a chi-square test of independence, including phi for effect size. We then utilized separate conditional logistic regression models to examine the predictors of participant SMC at initial visit, and the predictors, including SMC, of cognitive diagnosis at third annual visit. The presence of cognitive impairment at the third visit was derived from a categorical diagnostic severity variable provided by NACC with four classifications: 1) Normal Cognition; 2) Impaired-not-MCI, for those “judged to be cognitively impaired…but presentation, tests, symptoms, and clinical evaluation are not consistent with MCI”; 3) MCI; and 4) Dementia.27 Criteria for diagnostic groups, as outlined in the ADC guidebook, follow the 2005 Petersen criteria28. A new dichotomous variable representing stability of diagnosis was created for each participant, with stable cognitively normal diagnosis = 0 and diagnostic progression to impairment = 1. Finally, using longitudinal data for three consecutive annual visits, we evaluated the effects of race and SMC endorsement on the LM performance trajectories over time within and between matched pairs. Using a model comparison framework for linear mixed effects modeling with the lme4 package in R29, we evaluated increasingly complex models to account for the fixed main effects of race and SMC, as well as their interactions with time. In this approach, models representing alternative theories of the data are built and compared to one another. If subsequent models provide superior fit and result in a significant log likelihood difference, then the more complex model is adopted. This process confirms that a final model will adequately fit the data and possess statistical significance.30 In the simplest model, Model 1, we evaluated only the fixed main effects of race and SMC and allowed the slopes of each matched pair to vary. In Model 2, we added interaction effects of race with time and SMC with time, given that previous research has suggested that both race and SMC endorsement may influence performance trajectories.12,13 In Model 3, we maintained the predictors from Model 2, but accounted for the nesting of individual participants within matched pairs by allowing the slope of individual participants to vary from their matched counterparts.
3. RESULTS
3.1. Participant characteristics
The race-matched sample is characterized in Table 1 on demographic features as well as through mean scores on the model predictors, LM, MMSE, GDS and HIS. African American participants performed significantly worse than White participants on the two cognitive predictors, LM and the MMSE. African American participants had higher HIS scores, indicative of greater vascular burden; the groups did not significantly differ in self-rated depression scores.
Table 1.
Participant Characteristics of the Race-Matched Sample at Initial Visit
| White (N=456) |
African American (N=456) |
Test Statistic |
P | Effect Size |
|
|---|---|---|---|---|---|
| Matched Characteristics | M(SD) | M(SD) | |||
| Age | 72.71 (8.10) | 72.74 (8.09) | t=−.06 | .95 | η 2< .001 |
| Gender (% female) | 347 (76.1%) | 347 (76.1%) | Χ2 =.00 | .53 | ϕ < .001 |
| Education | 14.60 (2.72) | 14.45 (2.81) | t=.80 | .42 | η 2< .001 |
| Model Predictors | |||||
| Hachinski Ischemic Scale+ | .71 (1.01)* | 1.00 (1.17)* | t=−4.03 | <.001 | η 2= .02 |
| Geriatric Depression Scale+ | 1.30 (2.02) | 1.21 (1.92) | t=0.65 | .51 | η 2< .001 |
| Mini-Mental State Exam | 29.06 (1.26)* | 28.15 (1.86)* | t=8.58 | <.001 | η 2= .08 |
| Logical Memory Delay | 11.77 (4.06)* | 9.75 (4.02)* | t=7.46 | <.001 | η 2= .06 |
Groups are significantly different
Higher scores indicate worse performance or greater symptom burden
3.2. Frequency and predictors of subjective memory complaint at initial visit
A chi-square test for independence was performed to determine the frequency of SMC endorsement by race. There was a significant association between race and participant SMC (χ2(1, n = 912) = 7.75, P < .01, phi = −.10), with SMC significantly greater for White participants (32.9%) than African American participants (24.3%). Conditional logistic regression evaluated initial visit predictors of participant SMC, including race (White vs. African American), GDS, HIS, MMSE, and LM as well as the interactions of race with MMSE and race with LM. The full model was significant, with only race and LM as significant predictors. Self-reported depression, overall cognitive status as measured by the MMSE, and vascular burden were not related to SMC. Interaction effects between race and MMSE and race and LM were also non-significant. A final model including only the significant predictors accounted for 17.8 – 23.7% (varying estimates of pseudo R2) of the variance in SMC, χ2(2, n=912) = 178.67, P < .001 (Table 2). African Americans were less likely to report SMC during initial visit; however, worse verbal episodic memory performance as measured through LM was a significant predictor of SMC for both races.
Table 2.
Full Model Statistics for Conditional Logistic Regression Predicting Initial Visit Presence of Subjective Memory Complaint and Third Annual Visit Diagnostic Conversion
| Subjective Memory Complaint | B | SE | OR | 95% CI |
| Race | −.99 | .080 | .373** | [.319, .436] |
| Logical Memory Delayed Recall | −.03 | .013 | .968* | [.945, .993] |
| Diagnostic Conversion | B | SE | OR | 95% CI |
| Patient Subjective Memory Complaint | .74 | .220 | 2.089* | [1.36, 3.22] |
| Logical Memory Delayed Recall | −.07 | .027 | .932* | [0.89, 0.98] |
| Race | −1.34 | .173 | .262** | [0.18, 0.37] |
Abbreviations: CI, confidence interval; OR, odds ratio; SE, standard error.
P < .001
P < .05
3.3. Relationship of subjective memory complaint at initial visit to cognitive diagnosis at third annual visit
Conditional logistic regression evaluated the following initial visit predictors of diagnostic progression at third annual visit: race, SMC, HIS, GDS, MMSE and LM performance as well as the interaction between race and SMC. As with the above model, the full model was significant and most predictors did not significantly predict progression. To simplify the final model, non-significant predictors were eliminated, which included the GDS, HIS, MMSE, and the interaction of race with SMC. Table 2 shows the full final model statistics, including odds ratios and confidence intervals. The final model predicting cognitive diagnosis at third annual visit was significant, χ2 (3, n = 912) = 102.37, P < .001, and accounted for approximately 31.3 – 41.7% of the variance in the stability of diagnosis, by varying estimates of pseudo R2. African American participants were less likely to convert to an impaired diagnosis (B = −1.34, P < .001); SMC was associated with a two-fold higher odds of progression (B = .74, P < .01); and better performance on LM was associated with decreased odds of progression (B = −.07, P < .01). Frequencies of cognitive diagnosis within each race did not differ overall, χ2 (3, n = 912) = 2.69, P = .44, phi = .05; both groups had similar sample proportions of progression to MCI and dementia at the third annual visit (see Table 3). If a diagnosis of impaired cognition was made, ADC clinicians indicated the presumed etiology (e.g., Alzheimer’s disease, cerebrovascular disease, etc.). These findings are shown in Table 3.
Table 3.
Rates and Presumed Etiology of Diagnostic Conversion at Third Annual Visit for White and African American Participants
| White (N=456) |
African American (N=456) |
|
|---|---|---|
| N (% of sample) | N (% of sample) | |
| Diagnosis | ||
| Normal Cognition (no conversion) | 378 (82.9) | 384 (84.2) |
| Impaired Scores – Not MCI | 21 (4.6) | 12 (2.6) |
| MCI | 47 (10.3) | 48 (10.5) |
| Dementia | 10 (2.2) | 12 (2.6) |
| Etiology of Decline+ | N = 78 (17.1) | N = 72 (15.8) |
| Alzheimer’s disease | 43 (9.4) | 41 (9.0) |
| Cerebrovascular disease | 6 (1.3) | 6 (1.3) |
| FTD-Behavioral subtype | 1 (.2) | 0 (0) |
| Lewy Body Dementia | 3 (.7) | 1 (.2) |
| Mixed Etiology | 3 (.7) | 7 (1.5) |
| All Others ++ | 22 (4.8) | 17 (3.7) |
If a diagnosis of impaired cognition is made, ADC clinicians indicate the suspected etiology
All Others includes all other neurodegenerative conditions, including additional subtypes of FTD, such as primary progressive aphasia, as well as corticobasal degeneration, progressive supranuclear palsy, posterior cortical atrophy, Parkinson’s disease, multiple system atrophy, Huntington’s disease, prion disease, Down Syndrome, and all other incidental causes such as neoplasm, TBI, and normal pressure hydrocephalus.
Abbreviations: ADC, Alzheimer’s Disease Center; MCI, mild cognitive impairment; FTD, frontotemporal dementia; TBI, traumatic brain injury
3.4. Memory performance over time as a function of race and SMC between and within matched pairs
Linear mixed effects modeling evaluated the influence of race and SMC on LM performance over time within and between matched pairs of participants. The full final model demonstrated that African American race and SMC were associated with lower baseline performance scores as well as worse performance trajectories over time on the measure of delayed memory, (χ2 (3)=13.54, P < 0.01). Increasingly sophisticated models accounting for both fixed and random effects were tested and compared to one another through likelihood tests and model fit indices29 (see Table 4). Although overall model fit was not superior for Model 3 versus Model 2, the log likelihood difference was significant. In addition, Model 3 more accurately accounted for the structure of the nested data, and so it was adopted as the final model. Our full final Model 3 included significant random effects representing the slopes of matched pairs as well as the individual participant slopes nested within those matched pairs. There was greater variance within the race-matched pairs than between pairs, consistent with the pattern of fixed effects. Significant fixed effects (see Table 5) indicated the presence of different baseline performance scores as a result of participant race and SMC endorsement. African Americans were, on average, about two points lower in delayed recall performance at their baseline visit (−2.05 ± 0.24 SE), as compared to White participants. Similarly, those who endorsed SMC had a roughly half point lower score on delayed recall at baseline (−0.45 ± 0.21 SE) as compared to those who did not endorse SMC. The overall slope of delayed recall scores over time was slightly positive (0.60 ± 0.10 SE), indicating the presence of practice effects as a result of repeated exposures to the same measure at every annual visit. However, both African American race (−0.27 ± 0.12 SE) and SMC endorsement (−0.49 ± 0.15 SE) weakened the positive slope over time, suggesting subtle, but measureable declines in performance. White participants with SMC improved by 0.11 units per visit while African Americans with SMC exhibited a decline of −0.16 units per visit.
Table 4.
Model Comparisons
| Model | Predictors & (Slope) | df | AIC | BIC | Deviance | Test | X2 | df | p |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Race + SMC | 7 | 12216 | 12256 | 12202 | -- | -- | -- | -- |
| (Matched pairs) | |||||||||
| 2 | Race + SMC + RacexTime + SMCxTime | 10 | 12180 | 12238 | 12160 | 1 vs. 2 | 41.34 | 3 | < .001 |
| (Matched Pairs) | |||||||||
| 3 | Race + SMC + RacexTime + SMCxTime | 13 | 12173 | 12247 | 12147 | 2 vs. 3 | 13.54 | 3 | < .01 |
| (Participants nested in matched Pairs) | |||||||||
Abbreviations: AIC, akaike information criterion; BIC, Bayesian information criterion; SMC, subjective memory complaint
Table 5.
Fixed effects parameter estimates
| Predictor | b | SE | t-value | 95% CI |
|---|---|---|---|---|
| Intercept | 11.89 | 0.20 | 59.62 | 11.50, 12.28 |
| Race | −2.05 | 0.24 | −8.46 | −2.53, −1.58 |
| SMC endorsement | −0.45 | 0.21 | −2.12 | −0.87, −0.04 |
| Time | 0.60 | 0.10 | 6.07 | 0.41, 0.79 |
| Race x Time | −0.27 | 0.12 | −2.25 | −0.51, −0.04 |
| SMC x Time | −0.49 | 0.15 | −3.24 | −0.78, −0.19 |
Abbreviations: CI, confidence interval; SE, standard error; SMC, subjective memory complaint
4. DISCUSSION
Despite the widespread use of SMCs within both research and clinical practice, few studies have focused on the presence and predictive ability of SMCs within African Americans. Of the studies that have included or focused on African American samples, an interaction with race and SMC typically suggests the absence of a relationship between SMC and cognitive decline or diagnosis in African Americans. The present paper’s primary purpose was to examine the presence and diagnostic efficacy of SMCs in cognitively normal White and African American participants within a race-matched sample, controlling for the contributions of age, education, and sex. According to our results, SMCs are one aspect of clinical presentation that differs by racial group, despite comparable rates of later progression to dementia within the present sample and disproportionate disease prevalence.31–33 Contrary to previous research, the present analyses support the relationship between SMCs and objective memory decline as well as future cognitive diagnosis in both White and African American participants, but as hypothesized, African American participants were less likely to endorse SMCs, even in the presence of lower neuropsychological test scores.
Despite group differences in both cognitive performance and SMC rates at initial visit, the significant predictors of SMC endorsement were the same within both races. Objective memory performance, as measured by the delayed recall of prose passages (LM), was predictive of SMC at initial visit. In addition, cognitive diagnosis at the third annual visit was predicted by initial visit SMCs and LM performance for both White and African American participants. SMC at initial visit was associated with two-fold higher odds of progression to impairment two years later. For each unit increase in LM performance, indicating better recall ability, there was a 7% decrease in the odds of progression. These relationships held for both races and resulted in the same proportions of diagnostic progression to MCI and dementia.
Our hypothesis that the relationship between SMC and memory decline would be stronger among Whites than African Americans was not supported. In fact, the presence of both African American race and SMC endorsement was associated with a decline in memory performance over time, relative to the weakened positive slope that was present for White participants endorsing SMC. The results of Model 3, which included interaction effects with time and random slopes for participants nested within race-matched pairs, demonstrated non-zero effects of SMC endorsement and race on performance trajectories – even when demographic factors were controlled through matching.
The racial difference in the endorsement of SMCs between African American and White participants warrants further investigation, particularly because there is little evidence that the higher rate of endorsement by White participants is related to higher rates of disease. White participants endorsed SMCs more so than their African American counterparts in the sample, despite scoring higher on all measures of neuropsychological functioning (through unadjusted score comparisons). Given the research evidence of higher AD incidence within African Americans as compared to non-Hispanic Whites, differences in symptom reporting may be a critical contributing factor to non-equivalent evaluations and access to treatment.31–33 Racial disparities in symptom reporting by participants should be further evaluated to establish whether and how cultural factors and systematic diagnostic bias may influence the characterization of cognitive change, particularly at early stages of the disease process when declines may be subtle and harder to detect.
The contribution of cultural factors in African American symptom reporting patterns, disease understanding, and feelings toward research is understudied. African Americans are less likely to participate in clinical trials as a result of perceived study risks, informant requirements, and study location34 and may be less likely to report declines in functioning relevant to diseases that are not well understood.35 Knowledge about AD may be lower among minority samples,15 but educational interventions targeted to African Americans have achieved success when a community-based approach was utilized, incentives and compensation for time were provided, and outcomes were tailored.36
Among older adult participants deemed cognitively normal, roughly 25 – 33% believed they declined in memory functioning. Among cognitively normal older adults of both races, those with SMCs were more likely to convert than those without memory concerns and were less likely to exhibit practice effects from repeated testing. While neuropsychological testing is currently the gold standard for determining the severity of disease across the continuum of clinical manifestations,37 its use in the very early stages of disease detection may be limited, particularly in those individuals for whom lower performance scores are deemed common, as may be the case with demographically adjusted tests within African American participants or those with lower levels of education.38 There is a need for more nuanced measures of performance during initial characterization. Current research utilizing performance features such as the serial position effect,39 domain based factor scores,40 and multimodal measures of reserve/decline41 hold promise for the detection of cognitive change. Evidence of diminished practice effects also offer increased diagnostic sensitivity, particularly in early and even preclinical phases of disease.42,43
There are diverse measures of subjective complaints that are currently utilized in the research literature. Additionally, sample characteristics, including demographics, health factors, genetic risk, and protective lifestyle factors contribute to disparate findings. SMCs are strongly associated with poor psychological well-being44, poor health, depression, and anxiety.44,45 Individuals are more likely to report comorbid symptoms of anxiety or depression when presenting with SMCs.46 SMCs are difficult to quantify across time and participants and individuals with normal cognition tend to overestimate their decline, while individuals with objective cognitive decline tend to underestimate their cognitive difficulties.47 Given these factors, it is critical to evaluate the predictors of subjective complaints across multiple groups in addition to their presence and the associated future disease outcomes. Adherence to research criteria for SMCs in future studies, including detailed descriptions of samples, harmonization of measures across projects, and use of standardized cut-off scores for participant classification offer promise for replication across studies and generalizability of results.48
4.1. Limitations
The use of SMCs within research can be problematic. SMCs are used by clinicians during the diagnostic decision making process; therefore, there is a risk of criterion contamination when analyses consider both the complaints themselves as well as the outcome diagnosis. Our logistic regression analyses may therefore inflate the significant relationship between SMCs and progression or the overall rate of progression. However, the results of the linear mixed effects model support a similar interpretation of the data, in which SMCs are associated with lower performance scores at baseline and more decline in performance over time. The ideal statistical approach to these questions would utilize an autopsy-confirmed diagnostic sample; however, racially diverse autopsy-confirmed samples are not readily available.
As with most observational cohorts, NACC participants are not nationally representative. Our sample is therefore determined by both convenience sampling and statistical matching, which may limit the generalizability of our results. Previous epidemiological research has noted significantly higher rates of depression, lower socioeconomic status, lower quality of education, and higher rates of comorbid cardiovascular disease and diabetes within African Americans.31 The present sample differs from other community-based samples of African American participants as a result of higher educational attainment, lower rates of vascular burden,49 and lower reported depression.50 An independent sample evaluating the relationships among SMCs, cognitive performance, and cardiovascular and psychological symptoms found stronger associations between SMCs and symptoms of depression and anxiety. In the absence of psychological symptoms, it was cardiovascular risk factors rather than objective memory performance that predicted the presence of SMCs.51 While the results of our analyses may not be generalizable to community samples of participants with vascular risk and psychological comorbidities, we believe they are useful for identifying and confirming potential protective factors against decline (e.g., education, vascular and psychological health) and for understanding the relationships between SMC and decline among those who are generally healthy.
We chose to analyze initial visit data in cognitively normal participants to more closely replicate the circumstances of initial visit in clinical practice. Unfortunately, this choice relies on the accuracy of the initial visit diagnosis in NACC, which may itself underestimate the presence of disease in the sample. Similarly, our sample examined three annual visits, a decision meant to maximize our sample of African American participants; however, a longer time interval with more annual evaluations would have allowed us to confirm the stability and accuracy of initial diagnoses. Finally, the NACC sample, because it includes diagnostic information, may be subject to center-wide variability in coding or classification practices. Our analysis of longitudinal LM performance does not suffer from the same interpretative constraints and supports the results from our first set of analyses.
4.2. Future directions
While secondary data analysis and the publicly available data from the NACC UDS provide a great opportunity for evaluating rates and patterns, future research should investigate more detailed aspects of SMCs by African American participants within clinical settings.48 Research is needed to identify the barriers to SMC endorsement in African American participants so that interventions can target the relationships among symptom reporting, diagnosis, and treatment outcomes. The distinction between subjective memory complaints and subjective cognitive complaints is another fruitful area of study. Recent research suggests that subjective cognitive decline is associated with neuropsychological performance across multiple neuropsychological domains as well as future progression.52
Acknowledgments
Funding/Support
The NACC database is funded by NIA/NIH Grant U01 AG016976. NACC data are contributed by the NIA-funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG005131 (PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), P50 AG047270 (PI Stephen Strittmatter, MD, PhD).
This work was also supported by an NIH Center Grant for the Emory Goizueta Alzheimer’s Disease Research Center (P50 AG025688).
A version of these analyses was first presented at the annual meeting of the National Academy of Neuropsychology, Boston, Massachusetts, in October 2017.
Footnotes
Declaration of Conflicting Interests
The author(s) have no conflicts of interest to disclose with respect to the research, authorship, and/or publications of this article.
REFERENCES
- 1.Amariglio RE, Townsend MK, Grodstein F, Sperling RA, Rentz DM. Specific subjective memory complaints in older persons may indicate poor cognitive function. J Am Geriatr Soc. 2011;59(9):1612–1617. doi: 10.1111/j.1532-5415.2011.03543.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reid LM, MacLullich AMJ. Subjective memory complaints and cognitive impairment in older people. Dement Geriatr Cogn Disord. 2006;22(5–6):471–485. doi: 10.1159/000096295 [DOI] [PubMed] [Google Scholar]
- 3.van Oijen M, de Jong FJ, Hofman A, Koudstaal PJ, Breteler MMB. Subjective memory complaints, education, and risk of Alzheimer’s disease. Alzheimer’s Dement. 2007;3(2):92–97. doi: 10.1016/j.jalz.2007.01.011 [DOI] [PubMed] [Google Scholar]
- 4.Amariglio RE, Becker JA, Carmasin J, et al. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia. 2012;50(12):2880–2886. doi: 10.1016/j.neuropsychologia.2012.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schultz SA, Oh JM, Koscik RL, et al. Subjective memory complaints , cortical thinning , and cognitive dysfunction in middle-age adults at risk of AD. Alzheimer’s Dement Diagnosis, Assess Dis Monit. 2015;1(1):33–40. doi: 10.1016/j.dadm.2014.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnes LL, Schneider JA, Boyle PA, Bienias JL, Bennett DA. Memory complaints are related to Alzheimer disease pathology in older persons. Neurology. 2006;67(9):1581–1585. doi: 10.1212/01.wnl.0000242734.16663.09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kryscio RJ, Abner EL, Cooper GE, et al. Self-reported memory complaints: Implications from a longitudinal cohort with autopsies. Neurology. 2014;83(15):1359–1365. doi: 10.1212/WNL.0000000000000856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jessen F, Feyen L, Freymann K, et al. Volume reduction of the entorhinal cortex in subjective memory impairment. Neurobiol Aging. 2006;27(12):1751–1756. doi: 10.1016/j.neurobiolaging.2005.10.010 [DOI] [PubMed] [Google Scholar]
- 9.Arvanitakis Z, Leurgans SE, Fleischman DA, et al. Memory Complaints, Dementia, and Neuropathology in Older Blacks and Whites. 2018. doi: 10.1002/ana.25189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molinuevo JL, Rabin LA, Amariglio R, et al. Implementation of subjective cognitive decline criteria in research studies. Alzheimer’s Dement. 2017. doi: 10.1016/j.jalz.2016.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson JD, Rentz DM, Aghjayan SL, et al. Subjective cognitive concerns are associated with objective memory performance in Caucasian but not African-American persons. Age Ageing. 2017;46(6):988–993. doi: 10.1093/ageing/afx077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chin AL, Negash S, Hamilton R. Diversity and disparity in dementia: the impact of ethnoracial differences in Alzheimer disease. Alzheimer Dis Assoc Disord. 2011;25(3):187–195. doi: 10.1097/WAD.0b013e318211c6c9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blazer DG, Hays JC, Fillenbaum GG, Gold DT. Memory Complaints as a Predictor of Cognitive Decline: A comparison of African American and White Elders.pdf. J Aging Health. 1997;9(2):171–184. [DOI] [PubMed] [Google Scholar]
- 14.Roberts JS, Connell CM, Cisewski D, Hipps YG, Demissie S, Green RC. Differences Between African Americans and Whites in Their Perceptions of Alzheimer Disease. Alzheimer Dis Assoc Disord. 2003;17(1):19–26. [DOI] [PubMed] [Google Scholar]
- 15.Fornazzari L, Fischer C, Hansen T, Ringer L. Knowledge of Alzheimer’s disease and subjective memory impairment in Latin American seniors in the Greater Toronto Area. Int Psychogeriatrics. 2009;21(05):966. doi: 10.1017/S1041610209990433 [DOI] [PubMed] [Google Scholar]
- 16.Husaini BA, Sherkat DE, Moonis M, Levine R, Holzer C, Cain VA. Racial differences in the diagnosis of dementia and in its effects on the use and costs of health care services. Psychiatr Serv. 2003;54(1):92–96. [DOI] [PubMed] [Google Scholar]
- 17.Shadlen MF, Larson EB, Gibbons L, McCormick WC, Teri L. Alzheimer’s disease symptom severity in blacks and whites. J Am Geriatr Soc. 1999;47(4):482–486. [DOI] [PubMed] [Google Scholar]
- 18.Sims RC, Whitfield KE, Ayotte BJ, Gamaldo AA, Edwards CL, Allaire JC. Subjective memory in older African Americans. Exp Aging Res. 2011;37(2):220–240. doi: 10.1080/0361073X.2011.555640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beekly DL, Ramos EM, Lee WW, et al. The National Alzheimer’s Coordinating Center (NACC) Database: The Uniform Data Set. [DOI] [PubMed] [Google Scholar]
- 20.Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20(4):210–216. doi: 10.1097/01.wad.0000213865.09806.92 [DOI] [PubMed] [Google Scholar]
- 21.IBM SPSS Statistics for Windows. 2016.
- 22.Wechsler D WMS-III: Wechsler Memory Scale Administration and Scoring Manual. Psychological Corporation; 1997. [Google Scholar]
- 23.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi:0022-3956(75)90026-6[pii] [DOI] [PubMed] [Google Scholar]
- 24.Yesavage JA, Sheikh JI. 9/Geriatric Depression Scale (GDS) recent Evidence and Development of a Shorter Version. Clin Gerontol. 1986;5(1–2):165–173. [Google Scholar]
- 25.Hachinski VC, Iliff LD, Zilhka E, et al. Cerebral blood flow in dementia. Arch Neurol. 1975;32(C):632–637. [DOI] [PubMed] [Google Scholar]
- 26.R Core Team. R: A language and enviorment for statistical computing. 2017. https://www.r-project.org/. [Google Scholar]
- 27.Clinical ADC, Force T, Morris JC, et al. NACC Uniform Data Set ( UDS ) CODING GUIDEBOOK for Initial Visit Packet. Natl Alzheimer Coord Counc. 2008;(February). [Google Scholar]
- 28.Petersen RC, Morris JC. Mild Cognitive Impairment as a Clinical Entity and Treatment Target. Arch Neurol. 2005;62(7):1160–1163. doi: 10.1001/archneur.62.7.1160 [DOI] [PubMed] [Google Scholar]
- 29.Bates D, Maechler M, Bolker B, Walker S. Fitting Linear mixed-effects models using lme4. J Stat Softw. 2015;67(1):1–48. doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 30.Bliese PD, Ployhart RE. Growth Modeling Using Random Coefficient Models: Model Building, Testing, and Illustrations. Organ Res Methods. 2002;5(4):362–387. doi: 10.1177/109442802237116 [DOI] [Google Scholar]
- 31.Steenland K, Goldstein FC, Levey A, Wharton W. A Meta-Analysis of Alzheimer’s Disease Incidence and Prevalence Comparing African-Americans and Caucasians. J Alzheimer’s Dis. 2015;50(1):71–76. doi: 10.3233/JAD-150778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Demirovic J, Prineas R, Loewenstein D, et al. Prevalence of dementia in three ethnic groups: The South Florida program on aging and health. Ann Epidemiol. 2003;13(6):472–478. doi: 10.1016/S1047-2797(02)00437-4 [DOI] [PubMed] [Google Scholar]
- 33.2017 Alzheimer’s Disease Facts and Figures Vol 13; 2017. doi: 10.1016/j.jalz.2017.02.001 [DOI] [Google Scholar]
- 34.Zhou Y, Elashoff D, Kremen S, Teng E, Karlawish J, Grill JD. African Americans are less likely to enroll in preclinical Alzheimer’s disease clinical trials. Alzheimer’s Dement Transl Res Clin Interv. 2017;3(1):57–64. doi: 10.1016/j.trci.2016.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDougall GJ Jr, Holston EC. Black and White Men at Risk for Memory Impairment. Nurs Res. 2003;52(1):42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gardois P, Booth A, Goyder E, Ryan T. Health promotion interventions for increasing stroke awareness in ethnic minorities: a systematic review of the literature. BMC Public Health. 2014;14(1):409. doi: 10.1186/1471-2458-14-409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.John SE, Gurnani AS, Bussell C, Saurman JL, Griffin JW, Gavett BE. The effectiveness and unique contribution of neuropsychological tests and the δ latent phenotype in the differential diagnosis of dementia in the uniform data set. Neuropsychology. 2016;30(8):946–960. doi: 10.1037/neu0000315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manly JJ. Deconstructing race and ethnicity: implications for measurement of health outcomes. Med Care. 2006;44(11 Suppl 3):S10–6. doi: 10.1097/01.mlr.0000245427.22788.be [DOI] [PubMed] [Google Scholar]
- 39.Kasper E, Brueggen K, Grothe MJ, et al. Neuronal correlates of serial position performance in amnestic mild cognitive impairment. Neuropsychology. 2016;30(8):906–914. doi: 10.1037/neu0000287 [DOI] [PubMed] [Google Scholar]
- 40.Crane PK, Carle A, Gibbons LE, et al. Development and assessment of a composite score for memory in the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Brain Imaging Behav. 2012. doi: 10.1007/s11682-012-9186-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hohman TJ, McLaren DG, Mormino EC, et al. Asymptomatic Alzheimer disease: Defining resilience. Neurology. 2016. doi: 10.1212/WNL.0000000000003397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hassenstab J, Ruvolo D, Jasielec M, Xiong C, Grant E, Morris JC. Absence of practice effects in preclinical Alzheimer’s disease. Neuropsychology. 2015;29(6):940–948. doi: 10.1037/neu0000208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duff K, Foster NL, Hoffman JM. Practice effects and amyloid deposition: Preliminary data on a method for enriching samples in clinical trials. Alzheimer Dis Assoc Disord. 2014;28(3):247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benito-Leon J, Mitchell AJ, Bermejo-Pareja F. A Population-Based Study of Cognitive Function in Older People with Subjective Memory Complaints. J Alzheimer’s Dis. 2010;22:159–170. doi: 10.3233/JAD-2010-100972 [DOI] [PubMed] [Google Scholar]
- 45.Comijs HC, Deeg DJH, Dik MG, Twisk JWR, Jonker C. Memory complaints; the association with psycho-affective and health problems and the role of personality characteristics A 6-year follow-up study. J Affect Disord. 2002;72:157–165. [DOI] [PubMed] [Google Scholar]
- 46.Yates JA, Clare L, Woods RT. Subjective memory complaints, mood and MCI: a follow-up study. Aging Ment Health. 2017;21(3):313–321. doi: 10.1080/13607863.2015.1081150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edmonds EC, Delano-Wood L, Galasko DR, Salmon DP, Bondi MW. Subjective cognitive complaints contribute to misdiagnosis of mild cognitive impairment. J Int Neuropsychol Soc. 2014;20(8):836–847. doi: 10.1017/S135561771400068X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Molinuevo JL, Rabin LA, Amariglio R, et al. Implementation of subjective cognitive decline criteria in research studies. Alzheimer’s Dement. 2017. doi: 10.1016/j.jalz.2016.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clark R, Anderson NB, Clark VR, Williams DR. Racism as a stressor for African Americans: A biopsychosocial model. Am Psychol. 1999;54(10):805–816. doi: 10.1037/0003-066X.54.10.805 [DOI] [PubMed] [Google Scholar]
- 50.Davis S, Liu Y, Quarells R, R D-D. Stress-related racial discrimination and hypertension likelihood in a population-based sample of African Americans: The Metro Atlanta Heart Disease Study. Ethn Dis. 2005;15(4):585–593. [PubMed] [Google Scholar]
- 51.Sperling SA, Tsang S, Williams IC, Park MH, Helenius IM, Manning CA. Subjective Memory Change, Mood, and Cerebrovascular Risk Factors in Older African Americans. J Geriatr Psychiatry Neurol. 2017. doi: 10.1177/0891988717732153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kielb S, Rogalski E, Weintraub S, Rademaker A. Objective features of subjective cognitive decline in a United States national database. Alzheimer’s Dement. 2017. doi: 10.1016/j.jalz.2017.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]


