Abstract
Background
Walking is a volitional behavior that requires planning and initiation before a step is observed. Following a signal to begin walking, studies of gait initiation in specialized labs have identified three phases that occur during the transition from a standing position via anticipatory postural adjustment (APA) to the first step. Routine instrumented gait testing outside of the laboratory setting focuses on gait execution and does not include gait initiation measures.
Research question
Can a single IMU sensor be used for performing gait initiation evaluations outside the lab?
Methods
We recorded walking in young (N=41) and older (N=26) adults using an instrumented gait mat while they were wearing a 3D accelerometer on their lower back. Subjects were instructed to begin walking following an auditory signal. An algorithm was developed to extract the following measures from the acceleration signal: gait Initiation time, measured from the start of the auditory cue to begin walking and ends at the heel-strike of the swing leg, time-to-APA (reaction time), APA duration and swing time (execution of the first step).
Results
Intraclass correlation coefficient analysis showed good to excellent agreement between gait initiation metrics obtained with the gait mat and the wearable sensor (mean 0.88, range [0.75-0.96]). Except for swing time, all measures were longer in the older subjects, compared to the young adults (p<0.01).
Significance
Extracting gait initiation measures from routine instrumented gait testing may facilitate studies that can better determine the extent to which impaired gait planning and execution contribute to mobility impairments.
Keywords: accelerometer, inertial measurement units, gait, aging
INTRODUCTION
While many non-neurologic systems affect mobility, intact complex central nervous system (CNS) function is crucial for the planning and initiation of all volitional movements. These neural control systems begin in the brain and extend throughout the entire CNS to regulate musculoskeletal structures in the periphery, the final effectors of all movement. Prior studies in specialized laboratories have advanced our knowledge about the complexity of gait planning and its initiation [1–4]. The duration of gait initiation has been divided into three phases to capture the complex physiologic changes that occur during the transition following a cue to move from a quiet standing posture to the first step. The first phase is best conceptualized as a form of reaction time, beginning with the cue to begin moving and ending when the anticipatory postural adjustment (APA) begins. Gait preparation is the second phase [2,5–8]; this phase extends from the beginning to the end of APA which includes demonstrable shifts of body weight crucial for stabilizing the body to prevent falls as walking begins. The third phase is the gait execution [2,8–10]; this phase extends from the end of APA to the completion of the first step. Measuring all three phases is necessary to capture the total duration of gait initiation from its earliest planning to the first observable step. In addition, the evaluation of all three phases of gait initiation is crucial to elucidate the extent to which mobility impairments are related to impaired planning, postural adjustments, or execution alone.
The gait initiation terminology is not well defined in the literature and has been measured differently by various investigators. Rosin et al [2] defined gait initiation as the time from the cue to the end of the execution phase. Yiou et al. [3] measured gait initiation starting from the beginning of the APA. Rajachandrakumar et al. [4] define gait initiation as the execution phase (i.e., from the end of the APA to the end of the initiation step). In the current work, we use the term gait initiation to refer to all three phases from the cue and until the end of the execution phase.
Earlier studies of gait initiation have employed force-plates, 3D cameras, instrumented treadmills, and instrumented gait mats, limiting studies to specialized gait labs [11–15]. Since these approaches cannot be employed in the community setting, there is a paucity of data about the contributions of gait planning and initiation to mobility impairments across the full health spectrum of older adults. Recent work has used inertial measurement units (IMUs) to evaluate gait initiation and the APA. Martinez-Mendez et al. [16] proposed an accelerometer and gyroscope-based system with a sensor on the lower back to detect the APA duration. Subjects were instructed to stand barefoot with their feet 2 cm apart. Using tailored filtering and a threshold that were optimally adapted to their data set, they determined the beginning and end of the APA. Mancini et al. [8] introduced an approach to APA detection using a full IMU system placed on the lower back and on each of the shanks. Subjects were positioned with a fixed distance of 10 cm between their feet and a threshold to determine the timing of the APA. Bonora et al. [17] used full IMU system placed on the lower back and one on the shank. The distance between the legs was not controlled and subjects were only asked to stand quietly for 10 seconds before the beginning of the trial. The process of identifying the various components of the APA and the initiation step involved a calibration process that used data collected from trials of a validation group that were performed on a force-plate with the IMUs. While fairly successful, these approaches are limited because they require the study participants to stand in a pre-specified manner, because they use multiple sensors, or because they require relatively extensive calibration, thus restricting the usage outside the lab.
To address this gap, we developed and applied a new algorithm to gait initiation recordings, based on signals taken from young and older adults, from a single triaxial accelerometer worn on the lower back. Metrics that quantify the total duration of gait initiation and temporal measures of the three key phases of gait planning and initiation were extracted from these recordings and used for validation of the algorithm. As a secondary question and a form of clinical validation,we compared these measures in young and older adults with the expectation that they will be prolonged in the older adults [10,18]. The gait initiation measures were also correlated with the gait speed of the subjects. Gait speed is a widely used predictor of multiple adverse outcomes in older adults [19–21]. We aimed to examine if the gait initiation metrics reflect gait speed or they provide additional information distinct from gait speed.
METHODS
Participants
A convenience sample of 41 healthy young adults and 26 older female adults (see Table 1) was used in this study. The young adults were between 20 and 45 years of age, free of any disease likely to impact their gait and balance. The older adults were recruited as part of a study of the effects of non-invasive brain stimulation on gait in older adults. Their age was above 65, they had a score of 24 or above on the Montreal Cognitive Assessment (MoCA) [22], and they reported at least 2 falls in the past year (idiopathic fallers). All the measures that are used in this validation study were taken from the subject’s first visit, prior to any intervention. All subjects provided informed written consent, as approved by the local human studies committee, prior to their participation in this study.
Table 1:
Subject characteristics
| Healthy young adults (n=41) | Healthy young adults women only (n=21) | Older adults (n=26) | P value: young vs older adults (women only) | |
|---|---|---|---|---|
| Age (years) | 31.2±5.0 | 30.9±4.8 | 75.4±5.5 | <0.01 |
| Sex (women/men) | 21/20 | 21/0 | 26/0 | |
| Dominant Leg (% right) | 85.7% | 100% | 84.6% | 0.06 |
| Years of education | 18.0±2.4 | 18.6±2.1 | 14.5±2.5 | <0.01 |
| Gait speed (cm/sec) | 144.2±15.5 | 145.7±10.9 | 106.4±18.1 | 0.02 |
| Mass (Kg) | 67.2±14.2 | 57.1±9.3 | 66.5±9.7 | 0.01 |
| Height (cm) | 168.6±10.4 | 160.2±6.1 | 160.3±6.9 | 0.42 |
Experimental Set Up:
The triaxial acceleration sensor (Opal by APDM) was placed on the lower back at L4-L5 using an elastic strap. It provided raw 3D acceleration data at a sample rate of 128 Hz. The Opal system was synchronized to a Zeno walkway (ProtoKinetics, Havertown, PA, USA) with embedded pressure sensors over an active area of 61cm x 792cm, spatial resolution of ±1.27 cm and a sampling rate of 120 Hz, that was used as the “gold standard” reference for the validation [23]. The Zeno walkway system also contains a remote control which was used for generating the auditory cue with a random delay between 1 to 5 seconds. Foot dominance for each subject was determined using the Waterloo Footedness Questionnaire [24].
Gait Recordings:
Subjects stood at the beginning of the walkway and were instructed to start walking when they heard an auditory cue. In the first 3 trials, the subjects were not instructed about which leg to begin walking with (random condition). In a second set of 3 trials, subjects were instructed to start walking with the right leg and in a third set of 3 trials, with the left leg. In addition, the subjects performed one trial that included walking at a self-selected, comfortable gait speed on the Zeno walkway.
Initial Processing of Gait Recording:
Data from the Zeno walkway was recorded and initial processing and quality control was conducted using ProtoKinetics Movement Analysis Software (PKMAS) ver 5.09. Data was then exported to Matlab for further processing to extract gait initiation metrics (described below).
Algorithm Extraction of Gait Initiation Metrics
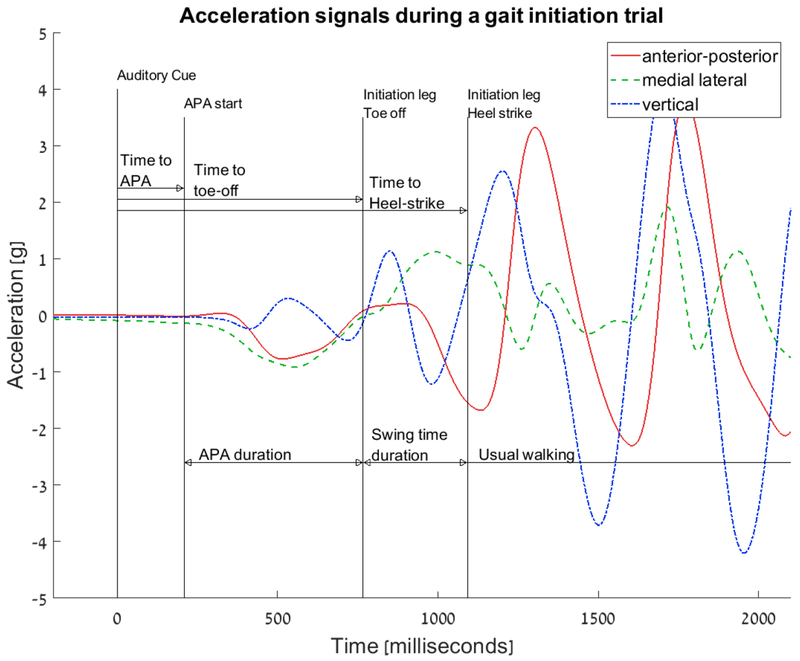
Following the auditory cue to begin walking, the algorithm detected three key points in the acceleration signals recorded with the wearable body fixed sensor. As illustrated in Figure 1, these points included: 1) beginning of the APA phase, 2) end of the APA based on the initiation leg toe-off, and 3) the initiation leg heel-strike. Based on the timing for these points, five gait initiation measures were computed:
Time-to-APA – time from the auditory cue to the beginning of the APA.
Time-to-toe-off - time from the auditory cue to the end of the APA. The APA end is detected as the toe-off event of the initiation leg.
Time-to-heel-strike – the time from the auditory cue to the heel-strike of the first step.
APA duration – the time from the beginning to the end of the APA waveform.
Swing time duration – the time from the toe-off to the heel-strike of the first step.
The algorithm development and data processing were performed using MATLAB software (MathWorks, Natick, MA). A more detailed description of the algorithm is provided in the supplementary methods section.
Figure 1:
Example of an acceleration signal during a gait initiation trial and the parameters that are extracted by the algorithm. The first vertical line at time 0 represents the beginning of the trial at which the auditory cue was played. The next 3 vertical lines are the points derived by the algorithm: 1) APA start 2) Initiation leg toe-off, which also marks the end of the APA, and 3) Initiation leg heel-strike, which also represents the end of the gait initiation. The time measured from the auditory cue to these 3 points define the measures Time to APA, Time-to-toe-off and Time-to-Heel-strike and is also depicted in the figure. Two additional measures are derived from the 3 times: the APA duration and the Swing time duration.
Statistical Analyses
Validation of Gait Initiation:
Comparisons of the gait initiation measures between the algorithm-derived and gold standard measures were performed using Bland-Altman and intraclass correlation coefficients (ICC) analysis (Two way mixed, absolute, single measures). Bland-Altman plots ICC results were interpreted using published guidelines [25]. Measures extracted using the algorithm were also grouped into the 3 different conditions, i.e., trials with the instruction to start with the dominant leg, trials that started with the non-dominant leg, or trials with no instruction at which they started with a leg of their choice (random). Group Comparisons for Age: To investigate the age-related differences in the gait initiation metrics, only data acquired from the women were used to avoid bias that might be caused by sex. Mann-Whitney tests were used for the group comparisons due to non-normal distribution of the time points identified by the algorithm. Within group comparisons (e.g., for dominant leg vs non-dominant leg) were performed using the Wilcoxon signed rank. Statistical analyses were performed using IBM SPSS statistics version 25.
RESULTS
Validation of body-fixed sensor gait initiation metrics
In total, 563 trials of gait initiation were recorded. Fourteen of 563 trials (2.5%) from 11 of 67 subjects were removed due to cases in which the subjects did not perform quiet standing before the cue (8 cases) or the algorithm detected very short APA duration(<200ms, 6 cases) [26]. This left 549 of 563 trials (97.5%) for validation of the acceleration derived gait initiation metrics.
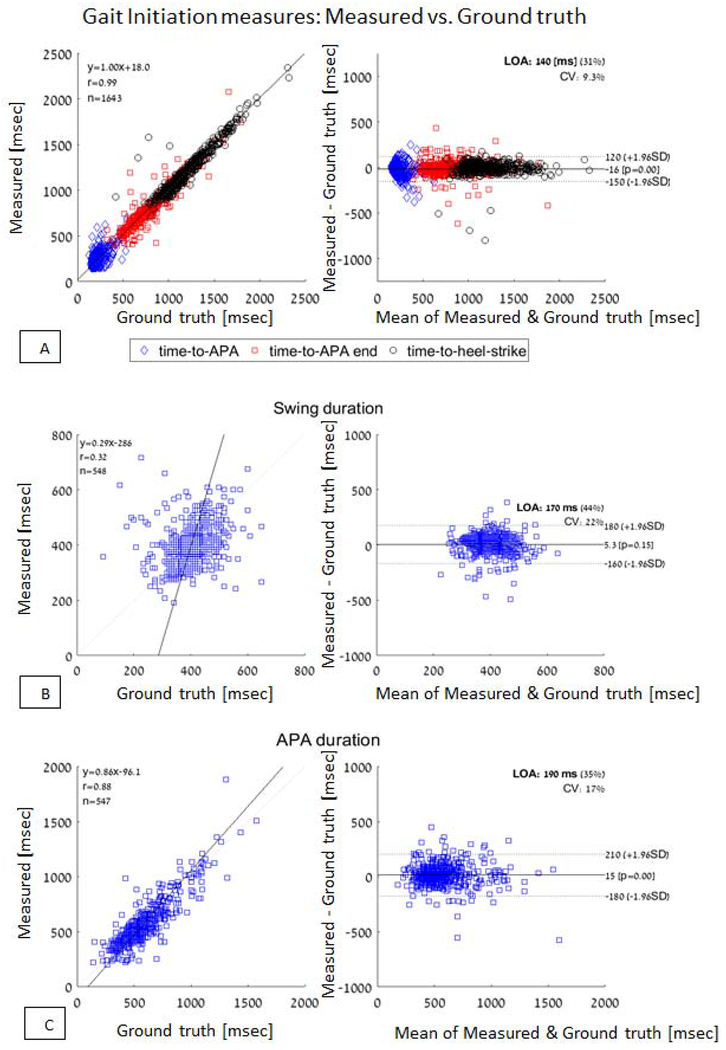
Figure 1 depicts an example of an acceleration signal during a gait initiation trial and the computed parameters (see also supplementary material). Table 2 presents the results of the ICC analyses. The first analysis was performed on all the gait initiation trials and additional analysis was performed after averaging the results of each leg condition (i.e., dominant, non-dominant and random) for each subject. All the parameters, except for initiation leg swing time duration, showed good to excellent agreement between the measurements made using the two systems. Bland-Altman plots of the time-to-APA, time-to-APA-end, and time-to-heel-strike that were extracted by comparing the acceleration-based to the Zeno walkway-based measures are depicted in Figure 2a. Bland-Altman plots of APA duration and swing time duration are depicted in Figure 2b and 2c. Consistent with the ICC values, there was generally good agreement between the two methods.
Table 2:
Intraclass Correlation Coefficients of Gait Initiation Metrics Extracted from the 3D Accelerometer and Zeno Walkway
| Single measures | Single measures Average on each leg condition: dominant, non-dominant and random per subject |
|||||||
|---|---|---|---|---|---|---|---|---|
| 95% Confidence Interval | 95% Confidence Interval | |||||||
| ICC | Lower Bound | Upper Bound | Cronbach’s Alpha | ICC | Lower Bound | Upper Bound | Cronbach’s Alpha | |
| Time-to-APA | 0.75 | 0.64 | 0.83 | 0.88 | 0.76 | 0.50 | 0.87 | 0.90 |
| Time-to-toe-off | 0.94 | 0.90 | 0.93 | 0.96 | 0.97 | 0.95 | 0.97 | 0.98 |
| APA duration | 0.88 | 0.82 | 0.87 | 0.92 | 0.92 | 0.90 | 0.94 | 0.96 |
| Time-to-heel-strike | 0.96 | 0.91 | 0.93 | 0.96 | 0.97 | 0.96 | 0.98 | 0.97 |
| Swing time duration | 0.38 | 0.24 | 0.39 | 0.48 | 0.47 | 0.35 | 0.57 | 0.64 |
Figure 2:
Bland-Altman plots of A: time-to-APA, time-to-APA end, and time-to-heel-strike, B: APA duration and C: swing time duration.
Gait initiation metrics in young and older women
Results of the parameters that were extracted by the algorithm for the young and older adults are summarized in Table 3. In this comparison, 181 trials from 21 healthy young women (30.9±4.8 yrs) were compared with 201 trials from 26 older women (75.4±5.7 yrs). Between the groups, all of the measures were significantly different (p<0.05) in the dominant, non-dominant, and random conditions, except for the swing time durations, and the time-to-APA and APA-duration in the random condition trials. Within each group, there were no significant differences between the dominant and non-dominant conditions, however, among the young adults, the measures in these two conditions differed from those obtained during the random condition for several of the measures as seen in Table 3.
Table 3:
Gait initiation parameters in young adult women and older adult women grouped by testing condition
| Young adults (n=21) | Older adults (n=26) | |||||
|---|---|---|---|---|---|---|
| Dominant | Non-Dominant | Random | Dominant | Non-Dominant | Random | |
| Time-to-APA [msec] | 223.7±65.6x | 215.2±38.0 x | 237.3±64.4 | 268.4±85.5 x | 270.4±72.6 x | 280.2±114.1 |
| Time-to-toe-off [msec] | 732.0±179.3*x | 725.9±148.8*x | 808.7±185.1 x | 896.4±193.7 x | 896.4±193.6x | 883.5.3±193.6x |
| Time-to-heel-strike [msec] | 1130.0±189.8* x | 1132.5±177.8* x | 1205.8±200.7 x | 1286.8±197.0 x | 1282.2±183.7 x | 1303.0±231.2 x |
| APA duration [msec] | 508.3±152.5* x | 510.3±106.1* x | 571.4±161.2 | 627.5±183.9 x | 613.5±184.1 x | 634.1±222.1 |
| Swing time duration [msec] | 398.0±64.8 | 406.6±62.0 | 397.0±64.8 | 390.5±78.5 | 398.7±77.8 | 388.7±71.0 |
Within the same group, parameters of dominant or non-dominant conditions that are significantly different from the random condition (Wilcoxon rank) are marked with an asterisk. These differences were only significant among the young adults.
parameters that are significantly different between the groups
Associations among the gait initiation metrics with self-selected comfortable gait speed
Among all subjects, gait speed was weakly to modestly correlated with Time-to-APA (r=−0.27), Time-to-toe-off (r=−0.34; p<0.01), APA duration (r=− 0.25; p<0.01), Time-to-heel-strike (r=−0.36; p<0.01) and Swing time duration (r=−0.07; p=0.12). When we stratified by age, the correlations between gait initiation metrics and gait speed were strongest for Time-to-heel-strike in the older adults group (r=−0.34; p<0.01).
DISCUSSION
In this study, we validated a new algorithm that extracts key measures of gait initiation from recordings obtained with a single fixed-body wearable sensor by comparing these results with a conventional, instrumented gait mat. The sensor-based results from the gait initiation trials show that gait planning and initiation metrics are prolonged in older women compared to their younger counterparts, demonstrating an expected slowing of gait initiation with age. In addition, gait initiation metrics are only modestly related to comfortable gait speed, suggesting that they capture an important additional dimension of mobility. These encouraging initial results offer the potential to expand instrumented gait testing in the community-settings so that both clinicians and investigators can systematically examine the inter-relationships between gait planning and initiation difficulties and movement performance in older adults.
Studies using a single IMU to quantify diverse portable instrumented gait testing paradigms have demonstrated the utility of a single sensor configuration and led to improved characterization of spatial-temporal characteristics of movement [27–29]. Nonetheless, previous work using a single sensor configuration focused on quantifying different facets of gait and balance. Only a small number of studies [8,16,17] attempted to use wearable sensors for quantifying the various phases of gait initiation.
The current study extends prior work in several ways. Using a single IMU and minimal requirement of only quiet standing (i.e., no requirement about the spacing of the feet) prior to the beginning of the gait initiation trial creates a simple setup that is easy to apply outside the lab. We described the development and initial technical and clinical validation results of an algorithm that measures various components of gait initiation from the 3D acceleration signals from the lower back. The comparison between the algorithm and the gold standard, as evaluated using ICC, showed good to excellent agreement in all the measures except for the swing time duration. Indeed, except for swing duration, these correlation results are better than those achieved by previously reported methods [8,16,17]. Averaging the results of three trials from each condition (i.e., dominant, non-dominant and random) only slightly improved the ICC results which suggests that a single trial per condition may be sufficient to capture gait initiation performance.
When applying the algorithm to the young and older adults, it managed to provide interesting insights within each group and also to age-related differences between the groups. The reaction time, measured by the time from the auditory cue to the beginning of the APA was longer in the older adults, as might be expected. The APA duration was also longer in the older adults and, correspondingly, the time to complete the entire gait initiation process to the first heel-strike was also longer. Nonetheless, these findings differ somewhat from those seen in the past [30,31]. The differences are possibly caused by the way the gait initiation trials were performed. In Lue at al. [30], the subjects were instructed to stand in their preferred stance, as in our protocol, but they initiated the gait at any time of their choice after a cue. On the other hand, in the study by Halliday et al. [31], subjects were asked to begin to move after the cue, but unlike in our protocol, subjects were instructed to stand with their feet side by side and parallel at pelvic width. This may suggest that the gait initiation results are very sensitive to the posture and instructions that are given to the subjects. Differences in inclusion and exclusion criteria may have also contributed to the disparities across the studies. With a tool and algorithm that can now be widely applied, we are better positioned to sort out these issues.
Interestingly, there were no significant differences between gait initiation parameters extracted from trials in which the subjects were instructed to start with their dominant leg as compared to those extracted from trials with instruction to start with their non-dominant leg. This may suggest that when conducting a gait initiation trial that includes an instruction to start with a specific leg, it is not critical if the subjects start with their dominant or non-dominant leg. In the random trials, time-to-APA, which reflects the reaction time, was not different between the dominant and non-dominant trials, however a significant difference was observed in the young adults group in the duration of the APA and the time to complete the gait initiation. These findings in the young adults are consistent with previously reported results [3]. One interpretation is that the older adults had a kind of ceiling effect on their time to complete the APA, whereas the younger adults were capable of performing it faster when primed with the instruction on which leg to begin with rather than selecting it independently. Perhaps the time to choose and decide which leg to use increased the initiation time as explained by Hick’s law [32,33], which states that decisions and reaction times are influenced by the number of choices that are available. In the future, it would be interesting to see if these differences are affected by neurologic disease or other factors.
Using sensors on the shank, as suggested by other approaches [8,16,17], may provide more sensitive information about the movement of the leg and increase the accuracy of the swing time detection. On the other hand, adding more sensors will make the data collection system more complex to use. The simplicity of the suggested system will make it less prone to data collection and operational problems even if it used outside the lab by non-technical personnel.
The suggested algorithm can detect the Time-to-toe-off and Time-to-heel-strike without information about the auditory cue timing in the recording. However, the information about the auditory cue is currently used for the detection of the APA start, and more importantly, to detect if the subject was at a quiet standing position during the cue. In the future, it will help to detect the APA start without the requirement of information on the auditory cue. If so, perhaps the described approach could be applied to daily-living measures to evaluate how certain gait initiation parameters change throughout the day. Perhaps additional improvements to the algorithm and minimizing outliers that are seen in the Bland-Altman plot in the detection of the end of the APA points will also improve the results of the swing duration and provide better agreement with the gold-standard reference values. The current work set the stage for these future avenues.
The present study has several limitations. A major limitation of the algorithm is its imprecise measure of the initiation leg swing time. Another limitation is that the algorithm provides only temporal measures of gait initiation and not information regarding the displacement of the COP or the APA magnitude. We also did not randomize the order of testing (i.e., left, right, random), nonetheless since both groups followed the same order, the impact on the group differences is likely to be minimal. Finally, while we showed age-associated differences between young adult women and older adult women, we did not compare young men and older adult men. Future development of the algorithm can build on the present work to address these limiations, to develop these additional measures, and further examine the factors that contribute to changes in gait initiation.
In summary, this acceleration-based algorithm shows promising results compared to a gold standard reference, enabling gait initiation testing outside of the lab with only a single, lower-back sensor. The algorithm also managed to demonstrate differences between young women and older adult women in both the planning and execution of gait initiation. The mild to moderate correlations between the gait initiation measures and the gait speed show that these measures present additional information to that obtained from just walking at their comfortable speed. Further examination of the cognitive and motor interactions might provide important information and aid with the early identification of cognitive and motor impairments of aging that are related to speed of processing.
Supplementary Material
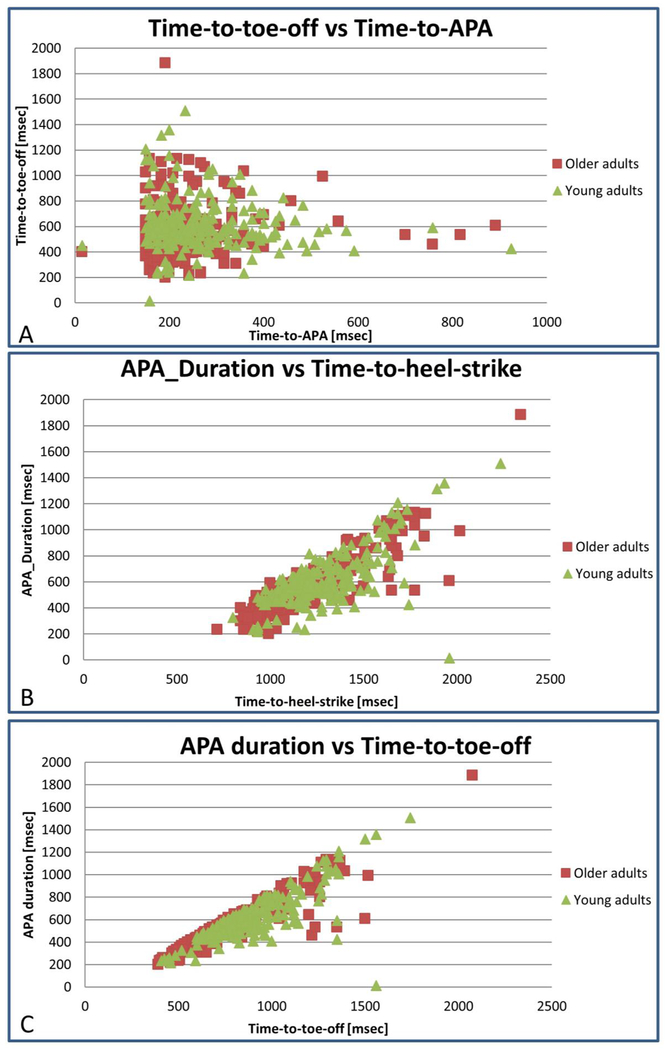
Figure 3:
Correlations examples between gait initiation metrics. Time-to-APA and Time-to-toe-off are not correlated as seen in a. The correlations between APA duration and Time-to-heel-strike and to Time-to-toe-off are depicted in B and C.
Highlights.
Gait testing outside of the lab typically does not include gait initiation.
A method was developed to extract gait initiation measures from a 3D accelerometer.
The approach is based on a wearable device, rather than force platforms or cameras.
This new method may enable widespread assessment of gait initiation and mobility.
ACKNOWLEDGEMENTS
This work was supported by NIH [R01AG17917, RF1AG22018, R01NS78009, R01AG56352] the Illinois Department of Public Health; and the Robert C. Borwell Endowment Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
Each of the authors has read and concurs with the content in the final manuscript. The material within has not been and will not be submitted for publication elsewhere except as an abstract. All authors made substantial contributions to all of the following: (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, (3) final approval of the version to be submitted.
REFERENCES
- [1].Day BL, Bancroft MJ, Voluntary steps and gait initiation, Handb. Clin. Neurol 159 (2018) 107–118. doi: 10.1016/B978-0-444-63916-5.00006-9. [DOI] [PubMed] [Google Scholar]
- [2].Rosin R, Topka H, Dichgans J, Gait initiation in Parkinson’s disease, Mov. Disord 12 (1997) 682–690. doi: 10.1002/mds.870120509. [DOI] [PubMed] [Google Scholar]
- [3].Yiou E, Do MC, Control of mediolateral stability during rapid step initiation with preferred and non-preferred leg: Is it symmetrical?, Gait Posture. 32 (2010) 145–147. doi: 10.1016/j.gaitpost.2010.03.018. [DOI] [PubMed] [Google Scholar]
- [4].Rajachandrakumar R, Fraser JE, Schinkel-lvy A, Inness EL, Biasin L, Brunton K, Mcllroy WE, Mansfield A, Atypical anticipatory postural adjustments during gait initiation among individuals with sub-acute stroke, Gait Posture. 52 (2017) 325–331. doi: 10.1016/j.gaitpost.2016.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mickelborough J, Van Der Linden ML, Tallis RC, Ennos AR, Muscle activity during gait initiation in normal elderly people, Gait Posture. 19 (2004) 50–57. doi: 10.1016/S0966-6362(03)00016-X. [DOI] [PubMed] [Google Scholar]
- [6].Melzer I, Shtilman I, Rosenblatt N, Oddsson LIE, Reliability of voluntary step execution behavior under single and dual task conditions, J. Neuroeng. Rehabil 4 (2007). doi: 10.1186/1743-0003-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rocchi L, Mancini M, Chiari L, Cappello A, Dependence of anticipatory postural adjustments for step initiation on task movement features: A study based on dynamometric and accelerometric data, Annu. Int. Conf. IEEE Eng. Med. Biol. - Proc. (2006) 1489–1492. doi: 10.1109/IEMBS.2006.260731. [DOI] [PubMed] [Google Scholar]
- [8].Mancini M, Chiari L, Holmstrom L, Salarian A, Horak FB, Validity and reliability of an IMU-based method to detect APAs prior to gait initiation, Gait Posture. 43 (2016) 125–131. doi: 10.1016/j.gaitpost.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Delval A, Dujardin K, Tard C, Devanne H, Willart S, Bourriez JL, Derambure P, Defebvre L, Anticipatory postural adjustments during step initiation: Elicitation by auditory stimulation of differing intensities, Neuroscience. 219 (2012) 166–174. doi: 10.1016/j.neuroscience.2012.05.032. [DOI] [PubMed] [Google Scholar]
- [10].Melzer I, Oddsson LIE, The Effect of a Cognitive Task on Voluntary Step Execution in Healthy Elderly and Young Individuals, Changes. 52 (2004) 1255–1262. [DOI] [PubMed] [Google Scholar]
- [11].Martin K, Blizzard L, Garry M, Thomson R, McGinley J, Srikanth V, Gait initiation in older people-Time to first lateral movement may be the measure of choice, Gait Posture. 34 (2011) 374–378. doi: 10.1016/j.gaitpost.2011.06.004. [DOI] [PubMed] [Google Scholar]
- [12].Melzer I, Kurz I, Shahar D, Levi M, Oddsson L, Application of the voluntary step execution test to identify elderly fallers, Age Ageing. 36 (2007) 532–537. doi: 10.1093/ageing/afm068. [DOI] [PubMed] [Google Scholar]
- [13].Hass CJ, Gregor RJ, Waddell DE, Oliver A, Smith DW, Fleming RP, Wolf SL, The influence of Tai Chi training on the center of pressure trajectory during gait initiation in older adults, Arch. Phys. Med. Rehabil 85 (2004) 1593–1598. doi: 10.1016/j.apmr.2004.01.020. [DOI] [PubMed] [Google Scholar]
- [14].Okada Y, Fukumoto T, Takatori K, Nagino K, Hiraoka K, Abnormalities of the first three steps of gait initiation in patients with Parkinson’s disease with freezing of gait, Parkinsons. Dis 2011 (2011) 202937. doi: 10.4061/2011/202937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Boyd S, Drennan S, Ferguson A, Millar J, Wilcox M, Kerr A, P 018 - Comparison of gait initiated on a treadmill (cued and uncued) and overground in healthy individuals, Gait Posture. 65 (2018) 260. doi: 10.1016/j.gaitpost.2018.06.160. [DOI] [Google Scholar]
- [16].Martinez-Mendez R, Sekine M, Tamura T, Detection of anticipatory postural adjustments prior to gait initiation using inertial wearable sensors, J. Neuroeng. Rehabil 8 (2011). doi: 10.1186/1743-0003-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bonora G, Carpinella I, Cattaneo D, Chiari L, Ferrarin M, A new instrumented method for the evaluation of gait initiation and step climbing based on inertial sensors: A pilot application in Parkinson’s disease, J. Neuroeng. Rehabil 12 (2015). doi: 10.1186/sl2984-015-0038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lewis RD, Brown JMM, Influence of muscle activation dynamics on reaction time in the elderly, Analysis. 69 (1994) 344–349. [DOI] [PubMed] [Google Scholar]
- [19].Abellan Van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, Cesari M, Donini LM, Gillette-Guyonnet S, Inzitari M, Nourhashemi F, Onder G, Ritz P, Salva A, Visser M, Vellas B, Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) task force, J. Nutr. Heal. Aging 13 (2009) 881–889. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- [20].Viccaro LJ, Perera S, Studenski SA, Is timed up and go better than gait speed in predicting health, function, and falls in older adults?, J. Am. Geriatr. Soc 59 (2011) 887–892. doi: 10.1111/j.1532-5415.2011.03336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ostir GV, Berges IM, Ottenbacher KJ, Fisher SR, Barr E, Hebei JR, Guralnik JM, Gait Speed and Dismobility in Older Adults, Arch. Phys. Med. Rehabil 96 (2015) 1641–1645. doi: 10.1016/j.apmr.2015.05.017. [DOI] [PubMed] [Google Scholar]
- [22].Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H, The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment, J. Am. Geriatr. Soc 53 (2005) 695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- [23].Lynall RC, Zukowski LA, Plummer P, Mihalik JP, Reliability and validity of the protokinetics movement analysis software in measuring center of pressure during walking, Gait Posture. 52 (2017) 308–311. doi: 10.1016/j.gaitpost.2016.12.023. [DOI] [PubMed] [Google Scholar]
- [24].Elias LJ, Bryden MP, Bulman-Fleming MB, Footedness is a better predictor than is handedness of emotional lateralization, Neuropsychologia. 36 (1998) 37–43. doi: 10.1016/S0028-3932(97)00107-3. [DOI] [PubMed] [Google Scholar]
- [25].Koo TK, Li MY, A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research, J. Chiropr. Med 15 (2016) 155–163. doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hiraoka K, Hatanaka R, Nikaido Y, Jono Y, Nomura Y, Tani K, Chujo Y, Asymmetry of anticipatory postural adjustment during gait initiation, J. Hum. Kinet 42 (2014) 7–14. doi: 10.2478/hukin-2014-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hausdorff JM, Hillel I, Shustak S, Del Din S, Bekkers EMJ, Pelosin E, Nieuwhof F, Rochester L, Mirelman A, Everyday stepping quantity and quality among older adult fallers with and without mild cognitive impairment: Initial evidence for new motor markers of cognitive defcits?, Journals Gerontol. - Ser. A Biol. Sci. Med. Sci 73 (2018) 1078–1082. doi: 10.1093/gerona/glx187. [DOI] [PubMed] [Google Scholar]
- [28].Del Din S, Godfrey A, Rochester L, Validation of an Accelerometer to Quantify a Comprehensive Battery of Gait Characteristics in Healthy Older Adults and Parkinson’s Disease: Toward Clinical and at Home Use, IEEE J. Biomed. Heal. Informatics. 20 (2016) 838–847. doi: 10.1109/JBHI.2015.2419317. [DOI] [PubMed] [Google Scholar]
- [29].Weiss A, Herman T, Giladi N, Hausdorff JM, Objective assessment of fall risk in Parkinson’s disease using a body-fixed sensor worn for 3 days, PLoS One. 9 (2014). doi: 10.1371/journal.pone.0096675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lu C, Amundsen Huffmaster SL, Harvey JC, MacKinnon CD, Anticipatory postural adjustment patterns during gait initiation across the adult lifespan, Gait Posture. 57 (2017) 182–187. doi: 10.1016/j.gaitpost.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Halliday SE, Winter DA, Frank JS, Patla AE, Prince F, The initiation of gait in young, elderly, and Parkinson’s disease subjects, Gait Posture. 8 (1998) 8–14. doi: 10.1016/S0966-6362(98)00020-4. [DOI] [PubMed] [Google Scholar]
- [32].Schneider Darryl W.; Anderson John R., A Memory-Based Model of Hick’s Law, Cogn. Psychol 62 (2011) 193–222. doi: 10.1002/0471142905.hg1504s82.ENU. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hick WE, On the Rate of Gain of Information, Q. J. Exp. Psychol (1952) 11–26. doi: 10.1080/17470215208416600. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.