Abstract
Socioeconomic status (SES), defined as the ability to access desired resources, is associated with behaviors that may affect vitamin D status. Most studies of the effect of vitamin D status on outcomes do not account for individual-level SES. The ability to adjust for SES in epidemiologic studies, when data on conventional SES measures have not been obtained, would be advantageous. We identified all serum 25(OH)D measurements in adults age 18 years and older residing in Olmsted County, MN, a mixed urban-rural setting, between January 1, 2005 and December 31, 2011, through the Rochester Epidemiology Project. The first 25(OH)D measurement was considered the index measurement for each subject. SES was determined for each subject by the HOUsing-based SocioEconomic Status (HOUSES) index, derived from real property data. The HOUSES index is an aggregated z-score of assessed housing value, area of living space, number of bedrooms, and number of bathrooms, with higher scores indicating higher SES. Multivariable analyses were adjusted for age, BMI, sex, race, season of 25(OH)D measurement, and Charlson comorbidity index. HOUSES was matched for 10,378 of 11,002 subjects (94%) with 25(OH)D measurements available. The mean (SD) age was 54.3 (17.1) years with 26.9% ≥65 years; 77.3% were women, and 12.1% were non-white. The mean 25(OH)D concentration was 30.0 (12.9) ng/mL, and 598 (5.8%) had a 25(OH)D value ˂12 ng/mL. The mean (SD) HOUSES was −1.55 (3.09),−0.97 (3.34), 0.14 (3.52), 0.24 (3.51) for serum 25(OH)D categories of <12, 12–19, 20–50, and >50 ng/mL, respectively (P=0.12 for trend). 25(OH)D increased by 0.43 (95% CI 0.36–0.50) ng/mL for each unit increase in HOUSES in univariate analysis and by 0.28 (0.21–0.35; P<0.001) ng/mL in multivariable analysis. This represents a change of 4 ng/mL across the entire range of observed HOUSES, an effect similar in magnitude to the seasonal variation of 25(OH)D values. SES was independently associated with serum 25(OH)D concentrations in a dose-response manner after adjustment for important covariates. HOUSES is a useful tool to assess the role of individual-level SES in health outcomes when other SES measures are unavailable and to control for confounding by SES in examining the effect of 25(OH)D on clinical and metabolic outcomes.
Keywords: vitamin D, epidemiology, mortality, nutrition, health disparities
1. Introduction
Socioeconomic status (SES) is defined as one’s ability to access to desired resources,1 which may be associated with behaviors that affect vitamin D status. These behaviors can affect vitamin D status through diet, exercise, sun exposure, and supplement use. Serum 25-hydroxyvitamin-D [25(OH)D] concentrations are used to assess vitamin D status. Epidemiologic studies have examined the effects of 25(OH)D values on clinically important outcomes like mortality, cardiovascular disease, fractures, and cancer incidence. SES is associated with these outcomes and may be an important confounder in epidemiologic studies. Furthermore, SES has an impact on pathways underlying health disparities from biological effects to social policy.2
Most studies of the effect of vitamin D status on outcomes do not account for individual-level socioeconomic status (SES).3–11 Studies based on the Third National Health and Nutrition Examination Survey (NHANES III) data assessed SES with the ratio of a family’s income to the poverty threshold of a family of the same size (poverty:income ratio),12 which was an important covariate in adjustment for the effect of 25(OH)D on mortality. A lower poverty:income ratio was associated with 25(OH)D<10 ng/mL (25 nmol/L).12 When this ratio was dichotomized, with low SES defined as 200% of the poverty index or lower, low SES was associated with a higher risk of vitamin D deficiency in unadjusted analysis but lower risk in an adjusted analysis that included race and physical activity.13 Others have used education as a proxy for SES.14 While these measures may reflect individual level SES, they could be subject to social desirability, recall bias from self-report, and within-group heterogeneity (ie, variability of earnings in each educational category),15,16 which could be reason for inconsistent results in the literature. Additionally, large retrospective studies (e.g., registry or electronic health record-based studies), without survey data for SES, generally lack individual level SES. Accurately adjusting for individual level SES in epidemiologic studies of vitamin D is necessary to evaluate the independent effect of vitamin D status on outcomes.
The HOUsing-based SocioEconomic Status (HOUSES) index is a validated individual level SES measure for large-scale clinical and epidemiological studies, associated with numerous health outcomes, health behaviors, health care access and care quality in adults and children.17–20 A patient’s address information, available in medical records, is directly linked to publicly available real property data, reflecting a patient’s current wealth or income, thus conceptually it can be used as an objective SES measure. It does not require completion of questionnaires by study subjects to obtain socioeconomic data, and socioeconomic status can be calculated through simple linkage to the subject’s address. Our objective was to determine the relationship of vitamin D status with socioeconomic status as measured by HOUSES index.
2. Methods
2.1. Olmsted County and the Rochester Epidemiology Project
We selected study subjects from the Rochester Epidemiology Project (REP) database, a population-based medical record linkage system that includes health care utilization, diagnostic and laboratory data for virtually all medical care within Olmsted County, Minnesota, covering 98% of all health care services provided for Olmsted County residents.21,22 Over 95% of the Olmsted County population has granted authorization for their records to be used for research.23,24 Olmsted County, MN is a mixed urban-rural setting located in the Upper-Midwestern United States with limited sun exposure in winter (44° north latitude). The Olmsted County population was 148,700 in 2011, and in the 2010 census, the proportions of Olmsted County residents classified as white, black, Asian and Hispanic were 86%, 4.8%, 5.5%, and 4.2%, respectively. Compared with the entire U.S. 2010 population, the county is less ethnically diverse (72% versus 86% white), more educated (85% versus 94% high school graduates) and wealthier ($51,914 versus $64,090 median household income). However, characteristics of the population are very similar to the overall population of the Upper-Midwestern United States.24
2.2. Patient Selection and Outcomes
We identified all initial serum 25(OH)D measurements in persons age 18 years and older residing in Olmsted County, MN between 01/01/2005 and 12/31/2011. We collected data on age, sex, race, Charlson Comorbidity Index (CCI), smoking status, and body mass index (BMI) at the time of initial 25(OH)D measurement. Diagnoses of hypertension, hyperlipidemia, and osteoporosis in the medical record prior to the date of 25(OH)D measurement were recorded.
2.3. HOUSES Index and Psychometric Properties
We used HOUSES index data to assess individual SES at the time of initial 25(OH)D measurement. For generating the HOUSES index, we retrieved subject addresses from the REP, which collects and maintains all historical individual addresses during residence in Olmsted County. The HOUSES index is a robust individual measure of SES represented by a single factor made up of four items (number of bedrooms, number of bathrooms, square footage of the unit, and estimated building value of the unit) ascertained from the county Assessor’s office.25
Variables were aggregated into an overall z-score such that a higher HOUSES score indicated higher SES. HOUSES index has demonstrated criterion validity with moderate to good correlations with education, income, Hollingshead Index, and Nakao-Treas Index (NT) in Olmsted County, MN and Jackson County, MO.25 HOUSES index has construct validity, predicting a broad range of health behavior (e.g. smoking exposure) and outcomes (e.g. obesity, preterm birth, and general health), conceptually and empirically associated with SES.25 While HOUSES has moderate to good correlation with other conventional SES measures, it better predicts health outcomes than other SES measures.25–27
2.4. Laboratory Methods
All 25(OH)D tests ordered in Olmsted County during the study interval were measured at the Mayo Clinic Laboratory using isotope-dilution liquid chromatography tandem mass spectrometry (LC-MS/MS).28 For this study, 25(OH)D refers to the sum of 25(OH)D2 and 25(OH)D3. Interassay coefficients of variation (CV) for 25(OH)D2 were 6–14% and for 25(OH)D3 were 6–13% in control samples. The recovery of analyte spiked into patient samples was 82–115% (mean, 102%) of predicted for 25(OH)D2 and 88–115% (mean, 103%) for 25(OH)D3. The limit of detection was 4 ng/mL. Throughout the study interval, Mayo Clinic Laboratory participated in the CDC Vitamin D standardization program. The relationship between serum 25(OH)D and all-cause and cause-specific mortality in this cohort are reported elsewhere.10
2.5. Statistical Analysis
All analyses were performed with SAS version 9.4 (SAS Institute Inc., Cary, NC). Serum 25(OH)D was examined as a continuous variable and as a categorical variable using predetermined ranges of interest: 25(OH)D values <12, 12–19, 20–50 (reference category), and >50 ng/mL. The association between serum 25(OH)D and SES was assessed using bivariate and multivariable linear regression. Non-linear models were tested but did not provide substantial improvement in fit. Multivariable analysis was adjusted for age, sex, race, month of 25(OH)D measurement, and Charlson comorbidity index at the time of 25(OH)D measurement. These variables were selected to provide the simplest model that maintained the maximum R2. Local regression was used to create a smoothed fit of the data to help us determine if linear regression was appropriate for this data.
3. Results
3.1. Study Subjects
HOUSES was successfully geocoded for 10,378 of 11,002 subjects (94%) with 25(OH)D measurements between 2005 and 2011. The mean (±SD) serum 25(OH)D concentration for the population was 30.0±12.9 ng/mL with a mean age of 54.3±17.1 y (Table 1). A total of 598 (5.7%) had 25(OH)D values <12 ng/mL. The cohort was mostly female (77.3%) and white (87.9%). In comparison with the overall population of Olmsted County (Table 2), those who had 25(OH)D measurements were more likely older, female, white, smokers, and have hypertension,
Table 1.
Characteristics of study subjects.
| 25(OH)D Categories (ng/mL) | |||||
|---|---|---|---|---|---|
| Total (N=10378) | <12 (N=598) | 12–19 (N=1514) | 20–50 (N=7749) | >50 (N=517) | |
| 25(OH)D, Mean (SD), ng/mL | 30.01 (12.86) | 8.48 (2.06) | 15.85 (2.28) | 32.36 (7.65) | 61.07 (15.81) |
| Age, mean (SD), years | 54.28 (17.09) | 51.09 (18.09) | 51.77 (16.97) | 54.91 (16.91) | 55.91 (17.82) |
| Age groups, n (%) | |||||
| 18–49 yr | 3986 (38.4%) | 293 (49.0%) | 682 (45.0%) | 2831 (36.5%) | 180 (34.8%) |
| 50–64 yr | 3603 (34.7%) | 179 (29.9%) | 519 (34.3%) | 2733 (35.3%) | 172 (33.3%) |
| ≥65 yr | 2789 (26.9%) | 126 (21.1%) | 313 (20.7%) | 2185 (28.2%) | 165 (31.9%) |
| Sex, n (%) | |||||
| Female | 8019 (77.3%) | 436 (72.9%) | 1112 (73.4%) | 6010 (77.6%) | 461 (89.2%) |
| Male | 2359 (22.7%) | 162 (27.1%) | 402 (26.6%) | 1739 (22.4%) | 56 (10.8%) |
| Race, n (%) | |||||
| White | 9120 (87.9%) | 393 (65.7%) | 1149 (75.9%) | 7081 (91.4%) | 497 (96.1%) |
| Non-white | 1257 (12.1%) | 205 (34.3%) | 365 (24.1%) | 667 (8.6%) | 20 (3.9%) |
| Season, n (%) | |||||
| Winter, Dec–Feb | 2596 (25.0%) | 210 (35.1%) | 461 (30.4%) | 1825 (23.6%) | 100 (19.3%) |
| Spring, Mar–May | 2713 (26.1%) | 201 (33.6%) | 478 (31.6%) | 1891 (24.4%) | 143 (27.7%) |
| Summer, Jun–Aug | 2355 (22.7%) | 80 (13.4%) | 231 (15.3%) | 1911 (24.7%) | 133 (25.7%) |
| Fall, Sept–Nov | 2714 (26.2%) | 107 (17.9%) | 344 (22.7%) | 2122 (27.4%) | 141 (27.3%) |
| Charlson index, mean (SD) | 3.38 (3.40) | 3.73 (4.12) | 3.24 (3.47) | 3.37 (3.34) | 3.52 (3.22) |
| BMI, mean (SD) | 28.83 (7.47) | 31.69 (10.03) | 31.13 (8.30) | 28.37 (6.98) | 25.68 (6.03) |
| HOUSES Z, mean (SD) | −0.12 (3.51) | −1.55 (3.09) | −0.98 (3.35) | 0.14 (3.52) | 0.24 (3.50) |
| Ever Smoked | |||||
| Missing | 697 | 44 | 110 | 520 | 23 |
| No | 4918 (50.8%) | 244 (44.0%) | 649 (46.2%) | 3768 (52.1%) | 257 (52.0%) |
| Yes | 4763 (49.2%) | 310 (56.0%) | 755 (53.8%) | 3461 (47.9%) | 237 (48.0%) |
| Hypertension | |||||
| No | 5977 (57.6%) | 300 (50.2%) | 846 (55.9%) | 4518 (58.3%) | 313 (60.5%) |
| Yes | 4401 (42.4%) | 298 (49.8%) | 668 (44.1%) | 3231 (41.7%) | 204 (39.5%) |
| Hyperlipidemia | |||||
| No | 4768 (45.9%) | 309 (51.7%) | 724 (47.8%) | 3517 (45.4%) | 218 (42.2%) |
| Yes | 5610 (54.1%) | 289 (48.3%) | 790 (52.2%) | 4232 (54.6%) | 299 (57.8%) |
| Osteoporosis | |||||
| No | 8265 (79.7%) | 511 (85.6%) | 1291 (85.3%) | 6089 (78.7%) | 374 (72.6%) |
| Yes | 2102 (20.3%) | 86 (14.4%) | 223 (14.7%) | 1652 (21.3%) | 141 (27.4%) |
Table 2.
Comparison of subject characteristics between those who had 25(OH)D measurements and the entire Olmsted County population, ages 18 years and older on January 1, 2010.
| Characteristic | 25(OH)D Cohort (N=11,002) | Olmsted (N=106,869) | P value |
|---|---|---|---|
| Age groups, n (%) | <0.001 | ||
| 18–49 yr | 4235 (38.5) | 62400 (58.4) | |
| 50–64 yr | 3779 (34.3) | 26483 (24.8) | |
| ≥65 yr | 2988 (27.2) | 17986 (16.8) | |
| Sex, n (%) | <0.001 | ||
| Women | 8482 (77.1) | 57472 (53.8) | |
| Men | 2520 (22.9) | 49397 (46.2) | |
| Race, n (%) | <0.001 | ||
| White | 9637 (87.6) | 91587 (85.7) | |
| Non-white | 1365 (12.4) | 15282 (14.3) | |
| Ever Smoked, n (%) | <0.001 | ||
| No | 5204 (50.8) | 62839 (58.8) | |
| Yes | 5050 (49.2) | 44030 (41.2) | |
| Hypertension, n (%) | <0.001 | ||
| No | 6344 (57.7) | 76304 (71.4) | |
| Yes | 4658 (42.3) | 30565 (28.6) |
3.2. Association of HOUSES with Subject Characteristics
In bivariate regression analysis, greater HOUSES Index was associated with younger age, male sex, and white race (Table 3). Health risk factors, including Charlson Comorbidity Index, greater BMI, smoking, hypertension, hyperlipidemia, and osteoporosis, were associated with lower HOUSES Index. Lower serum 25(OH)D values were associated with lower HOUSES Index.
Table 3:
Bivariate Linear Regression Analysis of Subject Characteristics Associated with HOUSES Index.
| Characteristic | Estimate (95% CI) | R2 | p-value |
|---|---|---|---|
| Age (per decade) | −0.15 (−0.19, −0.11) | 0.005 | <0.001 |
| Sex (M vs F) | 0.30 (0.14, 0.46) | 0.001 | <0.001 |
| Race (White vs Non-White) | 1.02 (0.81, 1.22) | 0.01 | <0.001 |
| Charlson Comorbidity Index (per unit) | −0.14 (−0.16, −0.12) | 0.02 | <0.001 |
| BMI (per unit) | −0.07 (−0.08, −0.07) | 0.03 | <0.001 |
| Ever Smoked (Y vs N) | −1.10 (−1.24, −0.96) | 0.02 | <0.001 |
| Hypertension (Y vs N) | −0.93 (−1.07, −0.80) | 0.02 | <0.001 |
| Hyperlipidemia | −0.30 (−0.44, −0.16) | 0.002 | <0.001 |
| Osteoporosis | −0.58 (−0.75, −0.41) | 0.004 | < 0.001 |
| Serum 25(OH)D (per 10 ng/mL) | 0.32 (0.27, 0.37) | 0.01 | < 0.001 |
| Serum 25(OH)D (ng/mL) | 0.02 | ||
| 20 – 50 | Reference | ||
| <12 | −1.69 (−1.98, −1.40) | <0.001 | |
| 12 – 19 | −1.11 (−1.31, −0.92) | <0.001 | |
| > 50 | 0.10 (−0.21, 0.41) | 0.52 |
3.3. Association of 25(OH)D with HOUSES and Subject Characteristics
Serum 25(OH)D values were positively associated with advancing age, female sex, white race, Charlson Comorbidity Index, hyperlipidemia, osteoporosis, and greater HOUSES Index (Table 4). Greater BMI, smoking, and hypertension were associated with lower serum 25(OH)D values.
Table 4.
Bivariate Linear Regression Analysis of Subject Characteristics Associated with Serum 25(OH)D Concentrations.
| Characteristic | Estimate (95% CI) | R2 | p-value |
|---|---|---|---|
| Age (per decade) | 0.76 (0.62, 0.90) | 0.01 | <0.001 |
| Sex (M vs F) | −3.42 (−3.99, −2.85) | 0.01 | <0.001 |
| Race (White vs Non-White) | 8.55 (7.84, 9.27) | 0.05 | <0.001 |
| Charlson Comorbidity Index (per unit) | 0.11 (0.04, 0.18) | 0.001 | 0.003 |
| BMI (per unit) | −0.37 (−0.40, −0.34) | 0.05 | <0.001 |
| Ever Smoked (Y vs N) | −1.26 (−1.76, −0.76) | 0.002 | <0.001 |
| Hypertension (Y vs N) | −0.52 (−1.00, −0.03) | 0.000 | 0.038 |
| Hyperlipidemia | 0.96 (0.48, 1.44) | 0.001 | <0.001 |
| Osteoporosis | 3.56 (2.97, 4.15) | 0.01 | <0.001 |
| Houses Z | 0.43 (0.36, 0.50) | 0.01 | <0.001 |
A multivariable analysis was performed to identify the optimal model of subject characteristics independently associated with serum 25(OH)D concentrations (Table 5). In the final model, advancing age, female sex, white race, and greater HOUSES Index were associated with greater serum 25(OH)D values, while Charlson Comorbidity Index, BMI, and winter season were associated with lower 25(OH)D values. Greater HOUSES Index remained positively associated with serum 25(OH)D values in the model controlling for these other characteristics (R2=0.13 for complete model). The variables not in the final model were smoking, hypertension, hyperlipidemia, and osteoporosis. The multivariable model including these additional variables had the same R2=0.13 and had minimal effect on the coefficient (0.25) or significance of the HOUSES Z.
Table 5.
Multivariable Linear Regression Analysis of Characteristics Associated with 25(OH)D concentrations (ng/mL). R2=0.13 for complete model.
| Characteristic | Estimate (95% CI) | p-value |
|---|---|---|
| Age (per decade) | 0.82 (0.64, 1.01) | <0.001 |
| Sex (M vs F) | −3.02 (−3.58, −2.46) | <0.001 |
| Race (White vs Non-White) | 7.98 (7.25, 8.70) | <0.001 |
| Charlson Comorbidity Index (per unit) | −0.16 (−0.26, −0.07) | <0.001 |
| BMI (per unit) | −0.34 (−0.37, −0.31) | <0.001 |
| Season | ||
| Winter | Reference | |
| Spring | 0.98 (0.33, 1.63) | 0.003 |
| Summer | 3.86 (3.19, 4.54) | <0.001 |
| Fall | 2.98 (2.33, 3.63) | <0.001 |
| Houses Z | 0.28 (0.21, 0.35) | <0.001 |
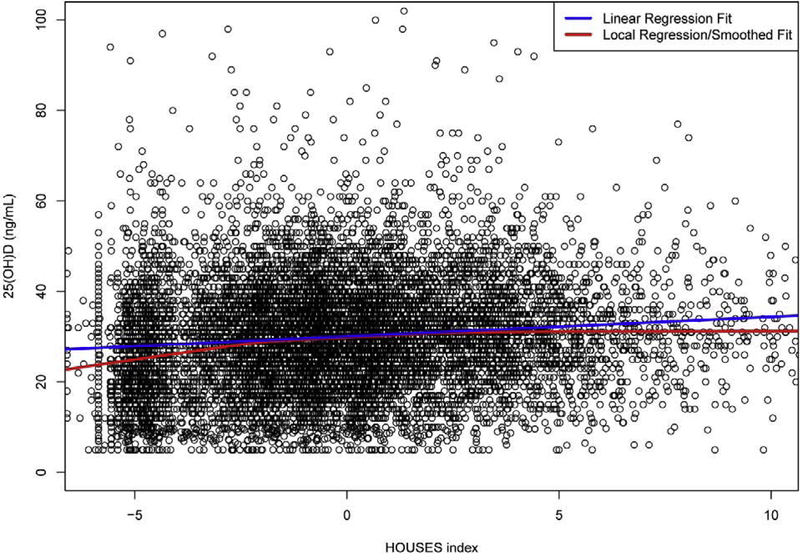
SES was independently associated with serum 25(OH)D concentrations in a dose-response manner. Serum 25(OH)D increased by 0.28 (0.21–0.35; P<0.001) ng/mL per unit change in HOUSES in multivariable analysis. This represents a change of 4 ng/mL over the range of observed HOUSES indices, which is similar in magnitude to the effect of summer season compared with winter, based on adjusted seasonal data from our cohort (Table 5). A local regression/smoothed fit analysis demonstrated greater decrease in serum 25(OH)D values with HOUSES Index below −1.0 than with the linear regression fit (Figure 1).
Figure 1.
Relationship of Serum 25(OH)D with HOUSES Index.
4. Discussion
We found that serum 25(OH)D values were positively associated with individual-level SES as assessed by the HOUSES Index. This positive relationship remained significant after adjusting for important covariates affecting 25(OH)D values, and the magnitude of the effect over the range of observed SES statuses was similar to the seasonal variation of 25(OH)D values. The clinical significance of this magnitude of variation needs to be determined in the context of subgroups and outcomes of interest to be examined in the future.
Other studies have assessed the relationship of vitamin D status with aspects of socioeconomic status in varying populations. Among healthy schoolgirls in Delhi, stratified by upper or lower socioeconomic status, those in the upper socioeconomic stratum had lower 25(OH)D levels and lower sun exposure.29 In a study of socioeconomic factors associated with vitamin D deficiency in young adults (18–25 years old) in Copenhagen, Denmark, less exercise, greater BMI, alcohol abstinence, and smoking were modifiable risk factors independently associated with vitamin D deficiency.30 We observed a magnitude of seasonal variation in 25(OH)D values (3.9 ng/mL) that was similar to values reported in other studies, ranging from 2.6 to 6.9 ng/mL, with differences based on sex, age, and geographic location.31–33
The prevalence of 25(OH)D values <20 ng/mL in our cohort was 20.4%. This is similar to a representative sample of the Canadian population (20%), which has similar racial/ethnic diversity to Olmsted County, MN.32 The prevalence is lower than the United States sample from the 2001–2006 NHANES survey (32%).34 However, the NHANES report included a much higher proportion of non-white patients (30.2%, compared to 12.1% in our study), which likely accounts for this difference.
Many observational studies and meta-analyses have assessed the association of vitamin D status with various outcomes, including mortality, fractures, cardiovascular disease, and cancers.3–11,13 These studies generally confirm an association of low vitamin D status with mortality, fractures, cardiovascular disease, and cancer. However, vitamin D deficiency may be a marker of disease risk but not causally related to the risk of these events.
It is difficult to assess SES without collecting additional data through subject questionnaires, which makes retrospective cohort studies difficult to perform if SES data were not previously collected. HOUSES has the advantage of permitting determination of SES using only the subject’s address and publically available individual-level real property data, and unlike neighborhood level data, it can be determined at the individual level. HOUSES is useful for overcoming the absence of conventional SES measures in commonly used datasets in epidemiological research. SES is associated with many important outcomes, like mortality and cancer. Adjustment for SES may help overcome some of the limitations of observational studies by capturing the effects of some unmeasured variables associated with both vitamin D status and outcomes.
This study had several limitations. The specific behaviors that were associated with SES and vitamin D status were not identified. We did not obtain data regarding behaviors related to vitamin D status, like dietary intake of vitamin D, supplement use, exercise, and sun exposure, which likely differ by SES. The relationship of SES with vitamin D status is likely explained in part by these behaviors. The sample is not a true population sample, as it included only those who presented for care and had 25(OH)D measured, and women are overrepresented. Women are at greater risk of osteoporosis than men, likely leading to more 25(OH)D measurements in women. Osteoporosis was associated with greater 25(OH)D values, likely due to vitamin D supplementation. Although we found a relationship of 25(OH)D with SES in those with indications for 25(OH)D measurement, this relationship may not be generalizable to the population that did not have 25(OH)D measured. This sampling bias would be unlikely to directly affect the relationship of 25(OH)D with SES, but we do not have data in those without 25(OH)D measurements to conclusively demonstrate this. The population was representative of the upper Midwest, but the findings may be difficult to generalize more broadly to other socioeconomic contexts and ethnic groups. Another limitation is that HOUSES has not been validated outside of the United States.
We conclude that HOUSES is a useful tool for assessing the role of individual-level SES in health outcomes, especially when other SES measures are not available, and could be used to control for confounding by SES in examining the effect of 25(OH)D on clinical and metabolic outcomes.
Highlights.
Socioeconomic factors are associated with behaviors that may affect vitamin D status.
Socioeconomic status (SES) measured with HOUSES was independently associated with serum 25(OH)D in a dose-response manner, with an effect similar in magnitude to the seasonal variation of 25(OH)D values.
HOUSES is a useful tool to assess the role of individual-level SES in health outcomes when other SES measures are unavailable and to control for confounding by SES in examining the effect of 25(OH)D on clinical and metabolic outcomes.
Financial support and disclosure
This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute of Aging of the National Institutes of Health (NIH) under Award Number R01AG034676. The study was also supported by the Mayo Clinic CTSA through grant number UL1 TR002377 from the National Center for Advancing Translational Sciences (NCATS), a component of the NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Dr. Tom Thacher is a consultant for ERT, Ultragenyx Pharmaceutical Inc., and Kyowa Kirin Pharmaceutical Development Inc. The other authors have nothing to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Oakes JM, Rossi PH. The measurement of SES in health research: current practice and steps toward a new approach. Soc Sci Med 2003;56:769–84. [DOI] [PubMed] [Google Scholar]
- 2.Warnecke RB, Oh A, Breen N, et al. Approaching health disparities from a population perspective: the National Institutes of Health Centers for Population Health and Health Disparities. Am J Public Health 2008;98:1608–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Degerud E, Nygard O, de Vogel S, et al. Plasma 25-Hydroxyvitamin D and Mortality in Patients With Suspected Stable Angina Pectoris. J Clin Endocrinol Metab 2018;103:1161–70. [DOI] [PubMed] [Google Scholar]
- 4.Iannuzzo G, Forte F, Lupoli R, Di Minno MND. Association of Vitamin D deficiency with peripheral arterial disease: a meta-analysis of literature studies. J Clin Endocrinol Metab 2018. [DOI] [PubMed] [Google Scholar]
- 5.Durup D, Jorgensen HL, Christensen J, Schwarz P, Heegaard AM, Lind B. A reverse J-shaped association of all-cause mortality with serum 25-hydroxyvitamin D in general practice: the CopD study. J Clin Endocrinol Metab 2012;97:2644–52. [DOI] [PubMed] [Google Scholar]
- 6.Gaksch M, Jorde R, Grimnes G, et al. Vitamin D and mortality: Individual participant data meta-analysis of standardized 25-hydroxyvitamin D in 26916 individuals from a European consortium. PloS One 2017;12:e0170791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cauley JA, Danielson ME, Boudreau R, et al. Serum 25-hydroxyvitamin D and clinical fracture risk in a multiethnic cohort of women: the Women’s Health Initiative (WHI). J Bone Miner Res 2011;26:2378–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson JL, May HT, Horne BD, et al. Relation of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am J Cardiol 2010;106:963–8. [DOI] [PubMed] [Google Scholar]
- 9.Zhang R, Li B, Gao X, et al. Serum 25-hydroxyvitamin D and the risk of cardiovascular disease: dose-response meta-analysis of prospective studies. Am J Clin Nutr 2017;105:810–9. [DOI] [PubMed] [Google Scholar]
- 10.Dudenkov DV, Mara KC, Petterson TM, Maxson JA, Thacher TD. Serum 25-Hydroxyvitamin D Values and Risk of All-Cause and Cause-Specific Mortality: A Population-Based Cohort Study. Mayo Clin Proc 2018;93:721–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gandini S, Boniol M, Haukka J, et al. Meta-analysis of observational studies of serum 25-hydroxyvitamin D levels and colorectal, breast and prostate cancer and colorectal adenoma. Int J Cancer 2010. May 6 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 12.Ginde AA, Scragg R, Schwartz RS, Camargo CA Jr. Prospective study of serum 25-hydroxyvitamin D level, cardiovascular disease mortality, and all-cause mortality in older U.S. adults. J Am Geriatr Soc 2009;57:1595–603. [DOI] [PubMed] [Google Scholar]
- 13.Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med 2008;168:1629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schottker B, Haug U, Schomburg L, et al. Strong associations of 25-hydroxyvitamin D concentrations with all-cause, cardiovascular, cancer, and respiratory disease mortality in a large cohort study. Am J Clin Nutr 2013;97:782–93. [DOI] [PubMed] [Google Scholar]
- 15.Nederhof AJ. Methods of coping with social desirability bias: A review. European Journal of Social Psychology 1985;15:263–80. [Google Scholar]
- 16.Coughlin SS. Recall bias in epidemiologic studies. J Clin Epidemiol 1990;43:87–91. [DOI] [PubMed] [Google Scholar]
- 17.Butterfield MC, Williams AR, Beebe T, et al. A two-county comparison of the HOUSES index on predicting self-rated health. J Epidemiol Community Health 2011;65:254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wi CI, St Sauver JL, Jacobson DJ, et al. Ethnicity, Socioeconomic Status, and Health Disparities in a Mixed Rural-Urban US Community-Olmsted County, Minnesota. Mayo Clin Proc 2016;91:612–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryu E, Juhn YJ, Wheeler PH, et al. Individual housing-based socioeconomic status predicts risk of accidental falls among adults. Ann Epidemiol 2017;27:415–20 e2. [DOI] [PubMed] [Google Scholar]
- 20.Bjur KA, Wi CI, Ryu E, et al. Socioeconomic Status, Race/Ethnicity, and Health Disparities in Children and Adolescents in a Mixed Rural-Urban Community-Olmsted County, Minnesota. Mayo Clin Proc 2019;94:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melton LJ 3rd. History of the Rochester Epidemiology Project. Mayo Clin Proc 1996;71:266–74. [DOI] [PubMed] [Google Scholar]
- 22.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ 3rd. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc 2012;87:1202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobsen SJ, Xia Z, Campion ME, et al. Potential effect of authorization bias on medical record research. Mayo Clin Proc 1999;74:330–8. [DOI] [PubMed] [Google Scholar]
- 24.St Sauver JL, Grossardt BR, Leibson CL, Yawn BP, Melton LJ 3rd, Rocca WA. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc 2012;87:151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Juhn YJ, Beebe TJ, Finnie DM, et al. Development and initial testing of a new socioeconomic status measure based on housing data. J Urban Health 2011;88:933–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson MD, Urm SH, Jung JA, et al. Housing data-based socioeconomic index and risk of invasive pneumococcal disease: an exploratory study. Epidemiol Infect 2013;141:880–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris MN, Lundien MC, Finnie DM, et al. Application of a novel socioeconomic measure using individual housing data in asthma research: an exploratory study. NPJ Prim Care Respir Med 2014;24:14018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh RJ, Taylor RL, Reddy GS, Grebe SK. C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. J Clin Endocrinol Metab 2006;91:3055–61. [DOI] [PubMed] [Google Scholar]
- 29.Puri S, Marwaha RK, Agarwal N, et al. Vitamin D status of apparently healthy schoolgirls from two different socioeconomic strata in Delhi: relation to nutrition and lifestyle. Br J Nutr 2008;99:876–82. [DOI] [PubMed] [Google Scholar]
- 30.Tonnesen R, Hovind PH, Jensen LT, Schwarz P. Determinants of vitamin D status in young adults: influence of lifestyle, sociodemographic and anthropometric factors. BMC Public Health 2016;16:385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levis S, Gomez A, Jimenez C, et al. Vitamin D deficiency and seasonal variation in an adult South Florida population. J Clin Endocrinol Metab 2005;90:1557–62. [DOI] [PubMed] [Google Scholar]
- 32.Greene-Finestone LS, Berger C, de Groh M, et al. 25-Hydroxyvitamin D in Canadian adults: biological, environmental, and behavioral correlates. Osteoporos Int 2011;22:1389–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Degerud E, Hoff R, Nygard O, et al. Cosinor modelling of seasonal variation in 25-hydroxyvitamin D concentrations in cardiovascular patients in Norway. Eur J Clin Nutr 2016;70:517–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ganji V, Zhang X, Tangpricha V. Serum 25-hydroxyvitamin D concentrations and prevalence estimates of hypovitaminosis D in the U.S. population based on assay-adjusted data. J Nutr 2012;142:498–507. [DOI] [PubMed] [Google Scholar]